Advances in Controllable Release Essential Oil Microcapsules and Their Promising Applications
Abstract
1. Introduction
2. Antimicrobial Mechanism of EOs
3. Preparation of Controllable-Release EOs Microcapsules
3.1. Single-Layer Microcapsules
3.2. Multilayer Microcapsules
3.3. Stimuli-Responsive Microcapsules
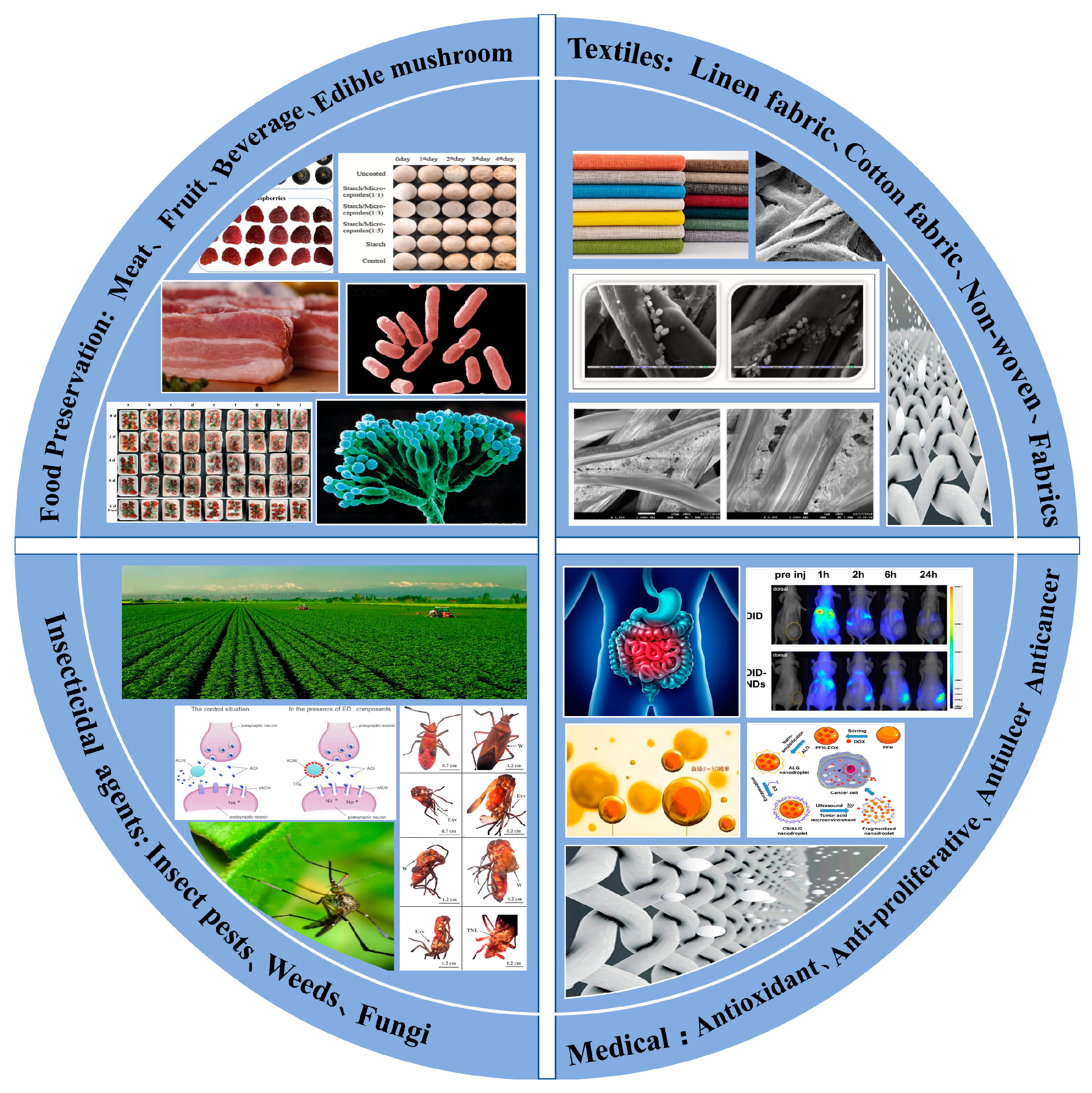
4. Application of Controllable Release EOs Microcapsules
4.1. Food Industry
4.2. Textiles
4.3. Agriculture Field
4.4. Medical Field
5. Conclusions and Prospect
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Bonilla, J.; Poloni, T.; Lourenço, R.V.; Sobral, P.J.A. Antioxidant potential of eugenol and ginger essential oils with gelatin/chitosan films. Food Biosci. 2018, 23, 107–114. [Google Scholar] [CrossRef]
- Pierini, G.D.; Bortolato, S.A.; Robledo, S.N.; Alcaraz, M.R.; Fernández, H.; Goicoechea, H.C.; Zon, M.A. Second-order electrochemical data generation to quantify carvacrol in oregano essential oils. Food Chem. 2022, 368, 130840. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, A.N.R.; Weimer, P.; Rossi, R.C.; Hoffmann, J.F.; Koester, L.S.; Suyenaga, E.S.; Ferreira, C.D. Lime and orange essential oils and d-limonene as a potential COVID-19 inhibitor: Computational, in chemico, and cytotoxicity analysis. Food Biosci. 2023, 51, 102348. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casaniana, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Kouamé, K.J.E.; Bora, A.F.M.; Li, X.; Sun, Y.; Liu, L. Novel trends and opportunities for microencapsulation of flaxseed oil in foods: A review. J. Funct. Foods 2021, 87, 104812. [Google Scholar] [CrossRef]
- Meng, Q.; Zhong, S.; Gao, Y.; Cui, X. Advances in polysaccharide-based nano/microcapsules for biomedical applications: A review. Int. J. Biol. Macromol. 2022, 220, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, A.; Hou, Y.; Jin, Y.; Xu, X.; Han, J.; Liu, W. Microcapsule delivery systems of functional ingredients in infant formulae: Research progress, technology, and feasible application of liposomes. Trends Food Sci. Tech. 2022, 119, 36–44. [Google Scholar] [CrossRef]
- Álvarez-Henao, M.V.; Saavedra, N.; Medina, S.; Cartagena, C.J.; Alzate, L.M.; Londonõ-Londonõ, J. Microencapsulation of lutein by spray-drying: Characterization and stability analyses to promote its use as a functional ingredient. Food Chem. 2018, 256, 181–187. [Google Scholar] [CrossRef]
- Baysal, G.; Olcay, H.S.; Lumberjack, B.; Özpinar, H. The antioxidant and antibacterial properties of chitosan encapsulated with the bee pollen and the apple cider vinegar. J. Biomater. Sci. Polym. Ed. 2022, 33, 995–1011. [Google Scholar] [CrossRef]
- Mehran, M.; Masoum, S.; Memarzadeh, M. Microencapsulation of Mentha spicata essential oil by spray drying: Optimization, characterization, release kinetics of essential oil from microcapsules in food models. Ind. Crops Prod. 2020, 154, 112694. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; Bhandari, B.; He, Y. Nano-particle encapsulation of fish oil by spray drying. Food Res. Int. 2008, 41, 172–183. [Google Scholar] [CrossRef]
- Domadia, P.; Swarup, S.; Bhunia, A.; Sivaraman, J.; Dasgupta, D. Inhibition of bacterial cell division protein FtsZ by cinnamaldehyde. Biochem. Pharmacol. 2007, 74, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Swain, S.S.; Paidesetty, S.K.; Padhy, R.N.; Hussain, T. Nano-technology platforms to increase the antibacterial drug suitability of essential oils: A drug prospective assessment. OpenNano 2023, 9, 100115. [Google Scholar] [CrossRef]
- Pauli, A. Antimicrobial properties of essential oil constituents. Int. J. Aromather. 2001, 11, 126–133. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Encapsulation strategies to enhance the antibacterial properties of essential oils in food system. Food Control 2021, 123, 107856. [Google Scholar] [CrossRef]
- Delaquis, P.J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef]
- Ju, J.; Chen, X.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of essential oil as a sustained release preparation in food packaging. Trends Food Sci. Tech. 2019, 92, 22–32. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Saei-Dehkordi, S.S.; Tajik, H.; Moradi, M.; Khalighi-Sigaroodi, F. Chemical composition of essential oils in Zataria multiflora Boiss. from different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem. Toxicol. 2010, 48, 1562–1567. [Google Scholar] [CrossRef]
- Sandri, G.; Zacaria, J.; Fracaro, F.; Delamare, A.P.L.; Echeverri-garay, S. Antimicrobial activity of the essential oils of Brazilian species of the genus Cunila against foodborne pathogens and spoiling bacteria. Food Chem. 2007, 103, 823. [Google Scholar] [CrossRef]
- Meenua, M.; Padhanb, B.; Patel, M.; Patel, R.; Xu, B. Antibacterial activity of essential oils from different parts of plants against Salmonella and Listeria spp. Food Chem. 2023, 404, 134723. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, C.; Shi, C.; Aliakbarlu, J.; Cui, H.; Lin, L. Antibacterial mechanisms of clove essential oil against Staphylococcus aureus and its application in pork. Int. J. Food Microbiol. 2022, 380, 109864. [Google Scholar] [CrossRef] [PubMed]
- Costa, W.K.; Oliveira, A.M.; Santos, I.B.S.; Silva, V.B.G.; Silva, E.K.C.; Alves, J.V.O.; Silva, A.P.S.; Lima, V.L.M.; Correia, M.T.S.; Silva, M.V. Antibacterial mechanism of Eugenia stipitata McVaugh essential oil and synergistic effect against Staphylococcus aureus. S. Afr. J. Bot. 2022, 147, 724–730. [Google Scholar] [CrossRef]
- He, Q.; Zhang, L.; Yang, Z.; Ding, T.; Ye, X.; Liu, D.; Guo, M. Antibacterial mechanisms of thyme essential oil nanoemulsions against Escherichia coli O157:H7 and Staphylococcus aureus: Alterations in membrane compositions and characteristics. Innov. Food Sci. Emerg. 2022, 75, 102902. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Sun, Y.; Abdel-Samie, M.A.; Lin, L. Antibacterial activity and mechanism of Chuzhou chrysanthemum essential oil. J. Funct. Foods 2018, 48, 159–166. [Google Scholar] [CrossRef]
- Wang, F.; Wei, F.; Song, C.; Jiang, B.; Tian, S.; Yi, J.; Yu, C.; Song, Z.; Sun, L.; Bao, Y.; et al. Dodartia orientalis L. essential oil exerts antibacterial activity by mechanisms of disrupting cell structure and resisting biofilm. Ind. Crops Prod. 2017, 109, 358–366. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; Fu, X.; Chen, W.; Yang, J.; Chen, Z.; Wang, Z.; Zhuan, S.; Xiang, X.; Feng, J.; et al. Preparation of peppermint oil nanoemulsions: Investigation of stability, antibacterial mechanism and apoptosis effects. Colloids Surf. B 2021, 201, 111626. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Miao, X.; Lin, Z.; Xiu, Y.; Shi, L.; Zhang, Q.; Liang, D.; Lin, S.; He, B. Disruption of metabolic function and redox homeostasis as antibacterial mechanism of Lindera glauca fruit essential oil against Shigella flexneri. Food Control 2021, 130, 108282. [Google Scholar] [CrossRef]
- Zhou, C.; Li, C.; Siva, S.; Cui, H.; Lin, L. Chemical composition, antibacterial activity and study of the interaction mechanisms of the main compounds present in the Alpinia galanga rhizomes essential oil. Ind. Crops Prod. 2021, 165, 113441. [Google Scholar] [CrossRef]
- Barbosa, L.N.; Alves, F.C.B.; Andrade, B.F.M.T.; Albano, M.; Rall, V.L.M.; Fernandes, A.A.H.; Buzalaf, M.A.R.; Leite, A.L.; Pontes, L.G.; Santos, L.D.; et al. Proteomic analysis and antibacterial resistance mechanisms of Salmonella Enteritidis submitted to the inhibitory effect of Origanum vulgare essential oil, thymol and carvacrol. J. Proteom. 2020, 214, 103625. [Google Scholar] [CrossRef]
- Yang, Z.; He, Q.; Ismail, B.; Hu, Y.; Guo, M. Ultrasonication induced nano-emulsification of thyme essential oil: Optimization and antibacterial mechanism against Escherichia coli. Food Control 2022, 133, 108609. [Google Scholar] [CrossRef]
- Dai, J.; Li, C.; Cui, H.; Lin, L. Unraveling the anti-bacterial mechanism of Litsea cubeba essential oil against E. coli O157:H7 and its application in vegetable juices. Int. J. Food Microbiol. 2021, 338, 108989. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial mechanism of oregano essential oil. Ind. Crops Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Hu, W.; Li, C.; Dai, J.; Cui, H.; Lin, L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind. Crops Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
- Huang, J.; Qian, C.; Xu, H.; Huang, Y. Antibacterial activity of Artemisia asiatica essential oil against some common respiratory infection causing bacterial strains and its mechanism of action in Haemophilus influenzae. Microb. Pathog. 2018, 114, 470–475. [Google Scholar] [CrossRef]
- Meng, X.; Li, D.; Zhou, D.; Wang, D.; Liu, Q.; Fang, S. Chemical composition, antibacterial activity and related mechanism of the essential oil from the leaves of Juniperus rigida Sieb. et Zucc against Klebsiella pneumoniae. J. Ethnopharmacol. 2016, 194, 698–705. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Y.; Liu, L.; Liu, Y.; Niu, X. Incorporation of clove essential oil nanoemulsion in chitosan coating to control Burkholderia gladioli and improve postharvest quality of fresh Tremella fuciformis. LWT 2022, 1770, 114059. [Google Scholar] [CrossRef]
- Zhan, J.; He, F.; Cai, H.; Wu, M.; Xiao, Y.; Xiang, F.; Yang, Y.; Ye, C.; Wang, S.; Li, S. Composition and antifungal mechanism of essential oil from Chrysanthemum morifolium cv. Fubaiju. J. Funct. Foods 2021, 87, 104746. [Google Scholar] [CrossRef]
- Zhang, R.; Cui, Y.; Cheng, M.; Guo, Y.; Wang, X.; Wang, J. Antifungal activity and mechanism of cinnamon essential oil loaded into mesoporous silica nanoparticles. Ind. Crops Prod. 2021, 171, 113846. [Google Scholar] [CrossRef]
- Hu, Z.; Yuan, K.; Zhou, Q.; Lu, C.; Du, L.; Liu, F. Mechanism of antifungal activity of Perilla frutescens essential oil against Aspergillus flavus by transcriptomic analysis. Food Control 2021, 123, 107703. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, P.P.; Prakash, B. Unravelling the antifungal and anti-aflatoxin B1 mechanism of chitosan nanocomposite incorporated with Foeniculum vulgare essential oil. Carbohyd. Polym. 2020, 236, 116050. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, R.; Gassem, M.A.; Athinarayanan, J.; Periyasamy, V.S.; Prasad, S.; Alshatwi, A.A. Antifungal activity of nanoemulsion from Cleome viscosa essential oil against food-borne pathogenic Candida albicans. Saudi J. Biol. Sci. 2021, 28, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Sourirajan, A.; Sharma, P.; Kumar, A.; Upadhyay, N.K.; Shukla, R.K.; Dev, K.; Krishnakuma, B.; Singh, M.; Bose, D. Bioenhancer potential of Aegle marmelos (L.) Corrêa essential oil with antifungal drugs and its mode of action against Candida albicans. Biocatal. Agric. Biotechnol. 2023, 48, 102647. [Google Scholar] [CrossRef]
- Hendges, C.; Stangarlin, J.R.; Zamban, V.C.; Mascaro, M.H.N.; Carmelo, D.B. Antifungal activity and control of the early blight in tomato through tea tree essential oil. Crop Prot. 2021, 148, 105728. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, J.; Ma, X.; Jia, C.; Han, J.; Song, C.; Liu, Y.; Wei, D.; Xu, H.; Qin, J.; et al. Nano-emulsification essential oil of Monarda didyma L. to improve its preservation effect on postharvest blueberry. Food Chem. 2023, 417, 135880. [Google Scholar] [CrossRef]
- Chen, X.; Lu, L.X.; Qiu, X.L.; Tang, Y.L. Advances in Mechanism Research on Controlled Release Antimicrobial Food Packaging Films. J. Food Sci. Biotechnol. 2020, 39, 1–7. [Google Scholar]
- Khounvilay, K.; Estevinho, B.N.; Sittikijyothin, W. Citronella oil microencapsulated in carboxymethylated tamarind gum and its controlled release. Eng. J. 2019, 23, 217–227. [Google Scholar] [CrossRef]
- Dima, C.; Pătraşcu, L.; Cantaragiu, A.; Alexe, P.; Dima, Ş. The kinetics of the swelling process and the release mechanisms of Coriandrum sativum L. essential oil from chitosan/alginate/inulin microcapsules. Food Chem. 2016, 195, 39–48. [Google Scholar] [CrossRef]
- Campelo-Felix, P.H.; Souza, H.J.B.; Figueiredo, J.A.; Fernandes, R.V.B.; Botrel, D.A.; Oliveira, C.R.; Yoshida, M.I.; Borges, S.V. Prebiotic Carbohydrates: Effect on Reconstitution, Storage, Release, and Antioxidant Properties of Lime Essential Oil Microparticles. J. Agric. Food Chem. 2017, 65, 445–453. [Google Scholar] [CrossRef]
- Bajac, J.; Nikolovski, B.; Lončarević, I.; Petrović, J.; Bajac, B.; Đurović, S.; Petrović, L. Microencapsulation of juniper berry essential oil (Juniperus communis L.) by spray drying: Microcapsule characterization and release kinetics of the oil. Food Hydrocoll. 2022, 125, 107430. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, J.; Zhong, Z. Preparation and characterization of long-term antibacterial and pH-responsive Polylactic acid/Octenyl succinic anhydride-chitosan @ tea tree oil microcapsules. Int. J. Biol. Macromol. 2022, 220, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Sheikh, J. Novel Chitosan-Gelatin microcapsules containing rosemary essential oil for the preparation of bioactive and protective linen. Ind. Crops Prod. 2022, 178, 114549. [Google Scholar] [CrossRef]
- Xu, L.; Xu, X.; Xu, Y.; Huang, M.; Li, Y. Fabrication and immediate release characterization of UV responded oregano essential oil loaded microcapsules by chitosan-decorated titanium dioxide. Food Chem. 2023, 400, 133965. [Google Scholar] [CrossRef]
- Gao, M.; Ji, M.; He, Y.; Pan, X.; Wang, X.; Si, T.; Sun, Y. Construction of consumer-friendly essential oil microcapsules with viscous cores to provide extra long-lasting release. Powder Technol. 2023, 413, 118040. [Google Scholar] [CrossRef]
- Noghabi, M.S.; Molaveisi, M.; Dehnad, D. Development of Persian gum-based microcapsules to speed up the release of cinnamon essential oil in the simulated saliva conditions. LWT Food Sci. Technol. 2023, 183, 114802. [Google Scholar] [CrossRef]
- Zhao, B.; Ni, Y.; Chen, K.; Lin, Z.; Jia, Z.; Qiu, H. Double-shell lignin microcapsules were prepared by one-step method for fabric coatings with UV resistance and durable antibacterial activity. Prog. Org. Coat. 2023, 179, 107518. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Wang, Y.; Zhang, Z.; Wang, Q. Preparation, characterization and release kinetics of a multilayer encapsulated Perilla frutescens L. essential oil hydrogel bead. Int. J. Biol. Macromol. 2023, 124776. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J.A. simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Heydari, M.K.; Assadpour, E.; Jafari, S.M.; Javadian, H. Encapsulation of rose essential oil using whey protein concentrate-pectin nanocomplexes: Optimization of the effective parameters. Food Chem. 2021, 356, 129731. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Adhikari, B.; Chang, L. Microencapsulation of rose essential oil in mung bean protein isolate-apricot peel pectin complex coacervates and characterization of microcapsules. Food Hydrocoll. 2022, 124, 107366. [Google Scholar] [CrossRef]
- Das, S.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Upadhyay, N.; Singh, P.; Sharam, S.; Dubey, N.K. Encapsulation in chitosan-based nanomatrix as an efficient green technology to boost the antimicrobial, antioxidant and in situ efficacy of Coriandrum sativum essential oil. Int. J. Biol. Macromol. 2019, 133, 294–305. [Google Scholar] [CrossRef]
- Hasheminejad, N.; Khodaiyan, F. The effect of clove essential oil loaded chitosan nanoparticles on the shelf life and quality of pomegranate arils. Food Chem. 2020, 309, 125520. [Google Scholar] [CrossRef]
- Zhang, W.; Shu, C.; Chen, Q.; Cao, J.; Jiang, W. The multilayer film system improved the release and retention properties of cinnamon EOs and its application as coating in inhibition to penicillium expansion of apple fruit. Food Chem. 2019, 299, 125109. [Google Scholar] [CrossRef]
- Chang, Y.; Choi, I.; Cho, A.R.; Han, J. Reduction of Dickeya chrysanthemi on fresh-cut iceberg lettuce using antimicrobial sachet containing microencapsulated oregano essential oil. LWT Food Sci. Technol. 2017, 82, 361–368. [Google Scholar] [CrossRef]
- Copado, C.N.; Julio, L.M.; Diehl, B.W.K.; Ixtaina, V.Y.; Tomás, M.C. Multilayer microencapsulation of chia seed oil by spray-drying using electrostatic deposition technology. LWT 2021, 152, 112206. [Google Scholar] [CrossRef]
- Yang, L.; Guo, Z.; Li, W.; Gou, Q.; Han, L.; Yu, Q. The impact of lemon seeds oil microcapsules based on a bilayer macromolecule carrier on the storage of the beef jerky. Food Packag. Shelf Life 2022, 32, 100838. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Wang, X.; Zhang, X.; Li, Y.; Zhang, S. Microencapsulation of essential oils by complex coacervation method: Preparation, thermal stability, release properties and applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 1363–1382. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Xu, Y.; Cen, K.; Gao, C.; Feng, X.; Tang, X. Nanoemulsion of cinnamon essential oil Co-emulsi ed with hydroxypropyl-β-cyclodextrin and Tween-80: Antibacterial activity, stability and slow release performance. Food Biosci. 2021, 43, 101232. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Bhandari, B.; Bai, B. Fennel essential oil loaded porous starch-based microencapsulation as an efficient delivery system for the quality improvement of ground pork. Int. J. Biol. Macromol. 2021, 172, 464–474. [Google Scholar] [CrossRef]
- Ozdemir, N.; Bayrak, A.; Tat, T.; Altay, F.; Kiralan, M.; Kurt, A. Microencapsulation of basil essential oil: Utilization of gum arabic/whey protein isolate/maltodextrin combinations for encapsulation efficiency and in vitro release. J. Food Meas. Charact. 2021, 15, 1865–1876. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Ju, R.; Bhandari, B.; Liu, K. Antibacterial mechanisms of star anise essential oil microcapsules encapsulated by rice protein-depolymerized pectin electrostatic complexation and its application in crab meatballs. Int. J. Food Microbiol. 2023, 384, 109963. [Google Scholar] [CrossRef]
- Jiménez-Martín, E.; Gharsallaoui, A.; Pérez-Palacios, T.; Carrascal, J.R.; Rojas, T.A. Suitability of using monolayered and multilayered emulsions for microencapsulation of ω-3 fatty acids by spray drying: Effect of Storage at Different Temperatures. Food Bioprocess Technol. 2015, 8, 100–111. [Google Scholar] [CrossRef]
- Lu, L.X.; Xu, J.; Pan, L. Research progress on stimulus-responsive microcapsules. J. Food Sci. Biotechnol. 2021, 40, 8–17. [Google Scholar]
- Niu, Y.; Wu, J.; Kang, Y.; Zhao, Q.; Xiao, Z.; Zhao, D. Encapsulation technique and application progress of mechanical stimuli-responsive microcapsules. Prog. Org. Coat. 2023, 176, 107390. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, Q.; Cao, J.; Shi, Y.; Wang, J.; Ma, H.; Sun, Y.; Song, Y. Bifunctional alginate/chitosan stabilized perfluorohexane nanodroplets as smart vehicles for ultrasound and pH responsive delivery of anticancer agents. Int. J. Biol. Macromol. 2021, 191, 1068–1078. [Google Scholar] [CrossRef]
- Coimbra, A.; Ferreira, S.; Duarte, A.P. Biological properties of Thymus zygis essential oil with emphasis on antimicrobial activity and food application. Food Chem. 2022, 393, 133370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, D.; Lv, J.; Li, Q.; Kong, C.; Luo, Y. Effect of cinnamon essential oil on bacterial diversity and shelf-life in vacuum-packaged common carp (Cyprinus carpio) during refrigerated storage. Int. J. Food Microbiol. 2017, 249, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zhang, G.; Wang, J.; Li, C.; Cui, H.; Lin, L. Application of glycyrrhiza polysaccharide nanofibers loaded with tea tree essential oil/gliadin nanoparticles in meat preservation. Food Biosci. 2021, 43, 101270. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Rhim, J.; Cao, J.; Jiang, W. Effective strategies of sustained release and retention enhancement of EOs in active food packaging films/coatings. Food Chem. 2022, 367, 130671. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Yu, J.; Chen, H.; Gao, H. Development of microcapsule bioactive paper loaded with cinnamon essential oil to improve the quality of edible fungi. Food Packag. Shelf Life 2021, 27, 100617. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Y.; Wang, M.; Li, R.; Dai, J.; Yan, J.; Qin, W.; Liu, Y. 3D printing of essential oil/β-cyclodextrin/popping candy modified atmosphere packaging for strawberry preservation. Carbohyd. Polym. 2022, 297, 120037. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, L.; Liu, J.; Yu, K.; Yu, W.; Jiang, H.; Zhong, J.; Zou, L.; Liu, W. Effect of sustained-release tea tree essential oil solid preservative on fresh-cut pineapple storage quality in modified atmospheres packaging. Food Chem. 2023, 417, 135898. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Li, L.; Deng, W.; Liu, M.; Hu, J. Biodegradable starch-based packaging films incorporated with polyurethane-encapsulated essential-oil microcapsules for sustained food preservation. Int. J. Biol. Macromol. 2023, 235, 123889. [Google Scholar] [CrossRef]
- Chatha, S.A.S.; Asgher, M.; Asgher, R.; Hussain, A.I.; Iqbal, Y.; Hussain, S.M.; Bilal, M.; Saleem, F.; Iqbal, H.M.N. Environmentally responsive and anti-bugs textile nishes-Recent trends, challenges, and future perspectives. Sci. Total Environ. 2019, 690, 667–682. [Google Scholar] [CrossRef]
- Yinggam, B.; Kacha, W.; Rungseevijitprapa, W.; Sudta, P.; Prasitpuriprecha, C.; Brantner, A. Response surface optimization of spray-dried citronella oil microcapsules with reduced volatility and irritation for cosmetic textile uses. Powder Technol. 2019, 355, 372–385. [Google Scholar] [CrossRef]
- Specos, M.M.M.; García, J.J.; Tornesello, J.; Marino, P.; Vecchia, M.D.; Tesoriero, M.V.D.; Hermida, L.G. Microencapsulated citronella oil for mosquito repellent finishing of cotton textiles. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 653–658. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Liu, Y.; Chen, M.; Hu, Y.; Yang, Z. Study on the grafting of chitosan–gelatin microcapsules onto cotton fabrics and its antibacterial effect. Colloids Surf. B 2013, 109, 103–108. [Google Scholar] [CrossRef]
- Chen, K.; Xu, C.; Zhou, J.; Zhao, R.; Gao, Q.; Wang, C. Multifunctional fabric coatings with slow-releasing fragrance and UV resistant properties from ethyl cellulose/silica hybrid microcapsules. Carbohydr. Polym. 2020, 232, 115821. [Google Scholar] [CrossRef]
- Hammoud, Z.; Abada, M.B.; Greige-Gerges, H.; Elaissari, A.; Jemâa, J.M.B. Insecticidal effects of natural products in free and encapsulated forms: An overview. J. Nat. Pestic. Res. 2022, 1, 100007. [Google Scholar] [CrossRef]
- López, A.; Castro, S.; Andina, M.J.; Ures, X.; Munguía, B.; Llabot, J.M.; Elder, H.; Dellacassa, E.; Palma, S.; Domínguez, L. Insecticidal activity of microencapsulated Schinus molle essential oil. Ind. Crops Prod. 2014, 53, 209–216. [Google Scholar] [CrossRef]
- Ahsaei, S.M.; Rodríguez-Rojo, S.; Salgado, M.; Cocero, M.J.; Talebi-Jahromi, K.; Amoabediny, G. Insecticidal activity of spray dried microencapsulated essential oils of Rosmarinus officinalis and Zataria multiflora against Tribolium confusum. Crop Prot. 2020, 128, 104996. [Google Scholar] [CrossRef]
- Lv, H.; Huo, S.; Zhao, L.; Zhang, H.; Liu, Y.; Liu, S.; Tani, A.; Wang, R. Preparation and application of cinnamon-Litsea cubeba compound essential oil microcapsules for peanut kernel postharvest storage. Food Chem. 2023, 415, 135734. [Google Scholar] [CrossRef]
- Kim, J.; Jang, M.; Shin, E.; Kim, J.; Lee, S.H.; Park, C.G. Fumigant and contact toxicity of 22 wooden essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae). Pestic. Biochem. Physiol. 2016, 33, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ngamekaue, N.; Chitprasert, P. Effects of beeswax-carboxymethyl cellulose composite coating on shelf-life stability and intestinal delivery of holy basil essential oil-loaded gelatin microcapsules. Int. J. Biol. Macromol. 2019, 135, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Thuekeaw, S.; Angkanaporn, K.; Chirachanchai, S.; Nuengjamnong, C. Dual pH responsive via double-layered microencapsulation for controlled release of active ingredients in simulated gastrointestinal tract: A model case of chitosan-alginate microcapsules containing basil oil (Ocimum basilicum Linn.). Polym. Degrad. Stab. 2021, 191, 109660. [Google Scholar] [CrossRef]
| EOs | Bacteria | Antibacterial Mechanism |
|---|---|---|
| Clove EOs [26] | Listeria monocytogenes | |
| Clove EOs [26] Eugenia stipitata EOs [27] Thyme EOs [28] Chuzhou chrysanthemum EOs [29] Dodartia orientalis L. EOs [30] Peppermint EOs [31] | Staphylococcus aureus |
|
| Lindera glauca fruit EOs [32] | Shigella flexneri |
|
| Chuzhou chrysanthemum EOs [29] Dodartia orientalis L. EOs [30] Peppermint EOs [31] Alpinia galanga rhizomes EOs [33] Thyme EOs [34,35] Litsea cubeba EOs [36] | Escherichia coli |
|
| Dodartia orientalis L. EOs [30] Origanum vulgare EOs [34] | Salmonella Enteritidis |
|
| Oregano EOs [37] Litsea cubeba EOs [38] | Methicillin-resistant Staphylococcus aureus |
|
| Artemisia asiatica EOs [39] | Haemophilus influenzae |
|
| Juniperus rigida EOs [40] | Klebsiella pneumoniae |
|
| Clove EOs [41] | Burkholderia gladioli |
| EOs | Fungus | Antifungal Mechanism |
|---|---|---|
| Chrysanthemum morifolium cv. Fubaiju EOs [42] | C. albicans; C. glabrata; C. tropicalis; S. cerevisiae; D. hansenii; Z. parabailii | |
| Cinnamon EOs [43] | Mucor sp. FJ09; Mucor circinelloides CNRMA 03.0371 |
|
| Perilla frutescens EOs [44] Foeniculum vulgare EOs [45] | Aspergillus flavus Aflatoxin B1 |
|
| Cleome viscosa EOs [46] Aegle marmelos L. Corrêa EOs [47] | Candida albicans |
|
| Tea tree EOs [48] Monarda didyma L. EOs [49] | Alternaria solani |
|
| Monarda didyma L. EOs [49] | Colletotrichum sp. |
|
| Core Materials | Wall Materials | Microcapsule Method | Release Model | Release Mechanism | References |
|---|---|---|---|---|---|
| Coriander EOs | Chitosan/Chitosan-Alginate/ | Spray-drying | Mt/M∞ = ktn | Anomalous diffusion | [52] |
| Coriander EOs | Alginate/Chitosan-Inulin | Spray-drying | Mt/M∞ = ktn | Diffusion swelling | [52] |
| Lime EOs | whey protein | Spray-drying | Mt/M∞ = ktn | Fickian diffusion | [53] |
| Lime EOs | whey protein-inulin | Spray-drying | Mt/M∞ = ktn | Anomalous diffusion | [53] |
| Lime EOs | whey protein-oligofructose | Spray-drying | Mt/M∞ = ktn | Anomalous diffusion | [53] |
| Spearmint EOs | Inulin-Gum Arabic | Spray-drying | Mt/M∞ = k1tm + k2t2m | Fickian diffusion | [12] |
| Juniper berry EOs | Gum arabic/Gum arabic-Maltodextrin/Sodium Alginate | Spray-drying | Mt/M∞ = k1tm + k2t2m | Fickian diffusion | [54] |
| Tea tree EOs | Polylactic acid/Octenyl succinic anhydride chitosan | Double emulsion and solvent evaporation method | Mt/M∞ = k1tm + k2t2m | Fick diffusion and skeletal dissolution | [55] |
| Rosemary EOs | Chitosan-Gelatin | Spray drying | Mt/M∞ = ktn | Fickian diffusion | [56] |
| Oregano EOs | Chitosan-decorated Titanium Dioxide | Ion-exchange-mediated self-assembly technique | Mt/M∞ = ktn | Fickian diffusion | [57] |
| Caprylic/capric triglyceride | Melamine-formaldehyde | Crosslinked method | Mt/M∞ = kt | Burst release | [58] |
| Caprylic/capric triglyceride | Polystyrene | Crosslinked method | Mt/M∞ = ktn | Diffusion | [58] |
| Cinnamon EOs | Persian gum-maltodextrin | Spray-drying | Mt/M∞ = k1tm + k2t2m | Fickian diffusion | [59] |
| Cinnamon EOs | Persian gum-maltodextrin | Spray-drying | Mt/M∞ = ktn | Fickian diffusion | [59] |
| Lemon EOs | Polyurethane-lignin | A joint method of interfacial polymerization with free radical copolymerization | Mt/M∞ = kt1/2 | Diffusion | [60] |
| Perilla frutescens L. EOs | Starch sodium octylsuccinate/sodium alginate/chitosan | Coacervation methods | Mt/M∞ = k1tm + k2t2m | Fick diffusion | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, Y.; Zhang, Z.; Li, H. Advances in Controllable Release Essential Oil Microcapsules and Their Promising Applications. Molecules 2023, 28, 4979. https://doi.org/10.3390/molecules28134979
Zhao Y, Wang Y, Zhang Z, Li H. Advances in Controllable Release Essential Oil Microcapsules and Their Promising Applications. Molecules. 2023; 28(13):4979. https://doi.org/10.3390/molecules28134979
Chicago/Turabian StyleZhao, Yana, Yanbo Wang, Zhijun Zhang, and Huizhen Li. 2023. "Advances in Controllable Release Essential Oil Microcapsules and Their Promising Applications" Molecules 28, no. 13: 4979. https://doi.org/10.3390/molecules28134979
APA StyleZhao, Y., Wang, Y., Zhang, Z., & Li, H. (2023). Advances in Controllable Release Essential Oil Microcapsules and Their Promising Applications. Molecules, 28(13), 4979. https://doi.org/10.3390/molecules28134979





