Chemical Synthesis, Safety and Efficacy of Antihypertensive Candidate Drug 221s (2,9)
Abstract
1. Introduction
2. Results and Discussion
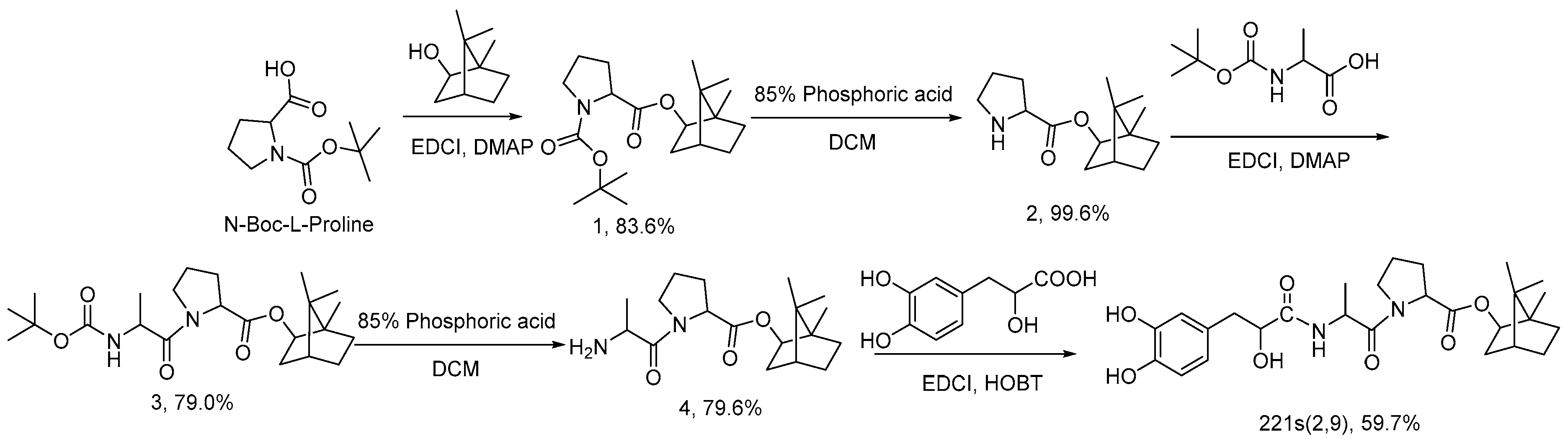
2.1. Synthesis of 221s (2,9)
2.2. Analysis of 221s (2,9)
2.3. Acute Toxicity Test of 221s (2,9)
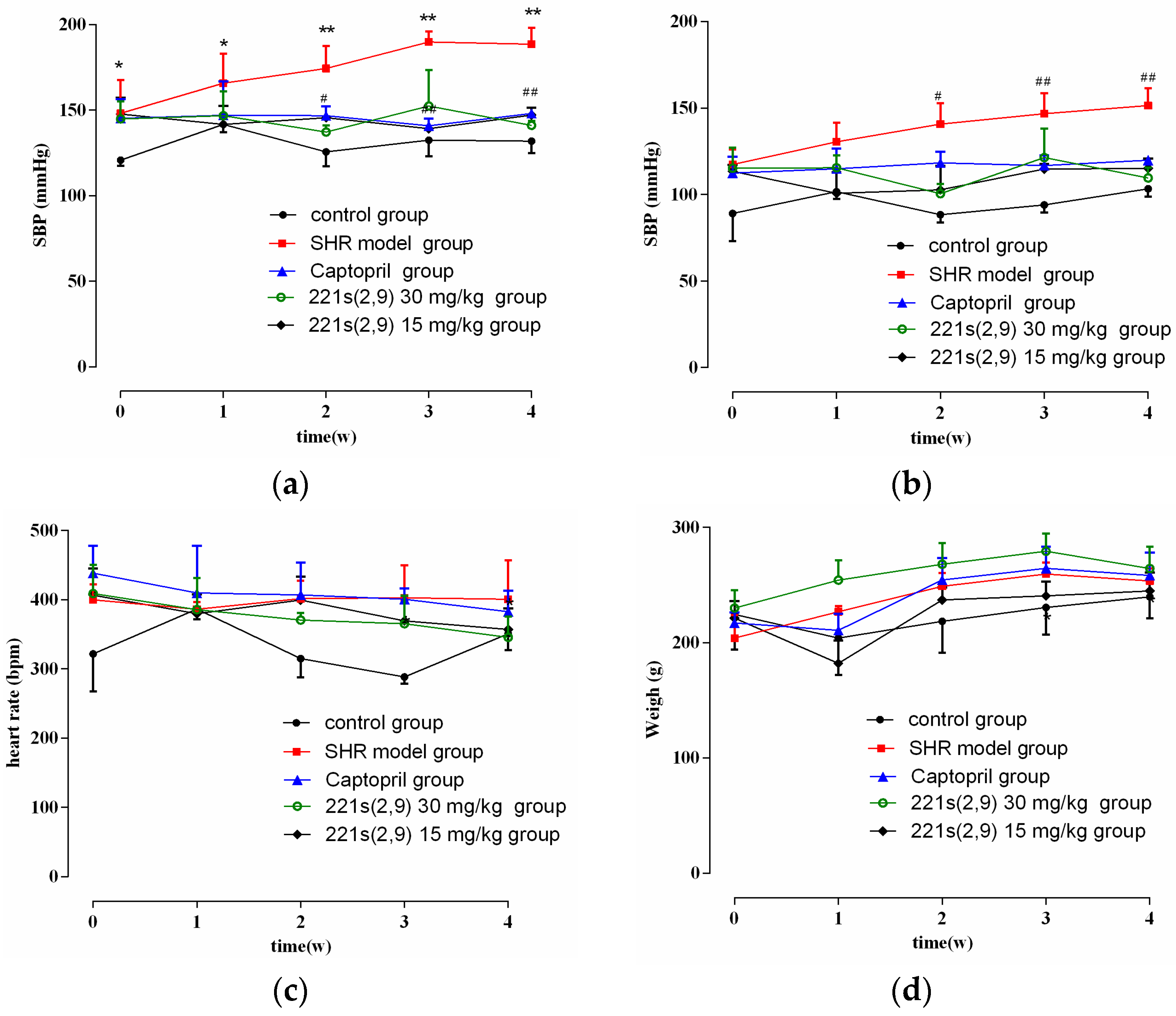
2.4. The Effect of 221s (2,9) on Blood Pressure, Heart Rate and Body Weight of Rats
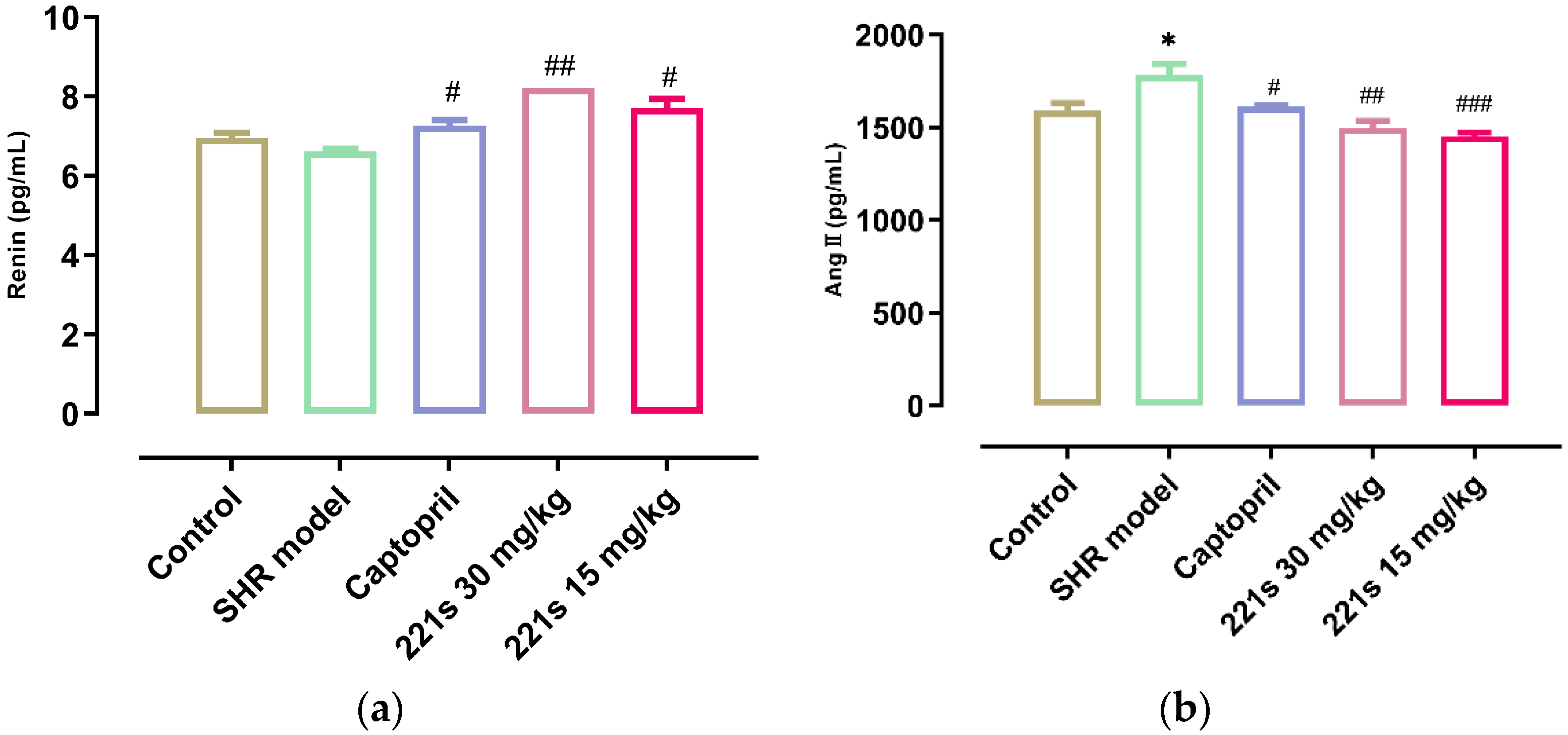
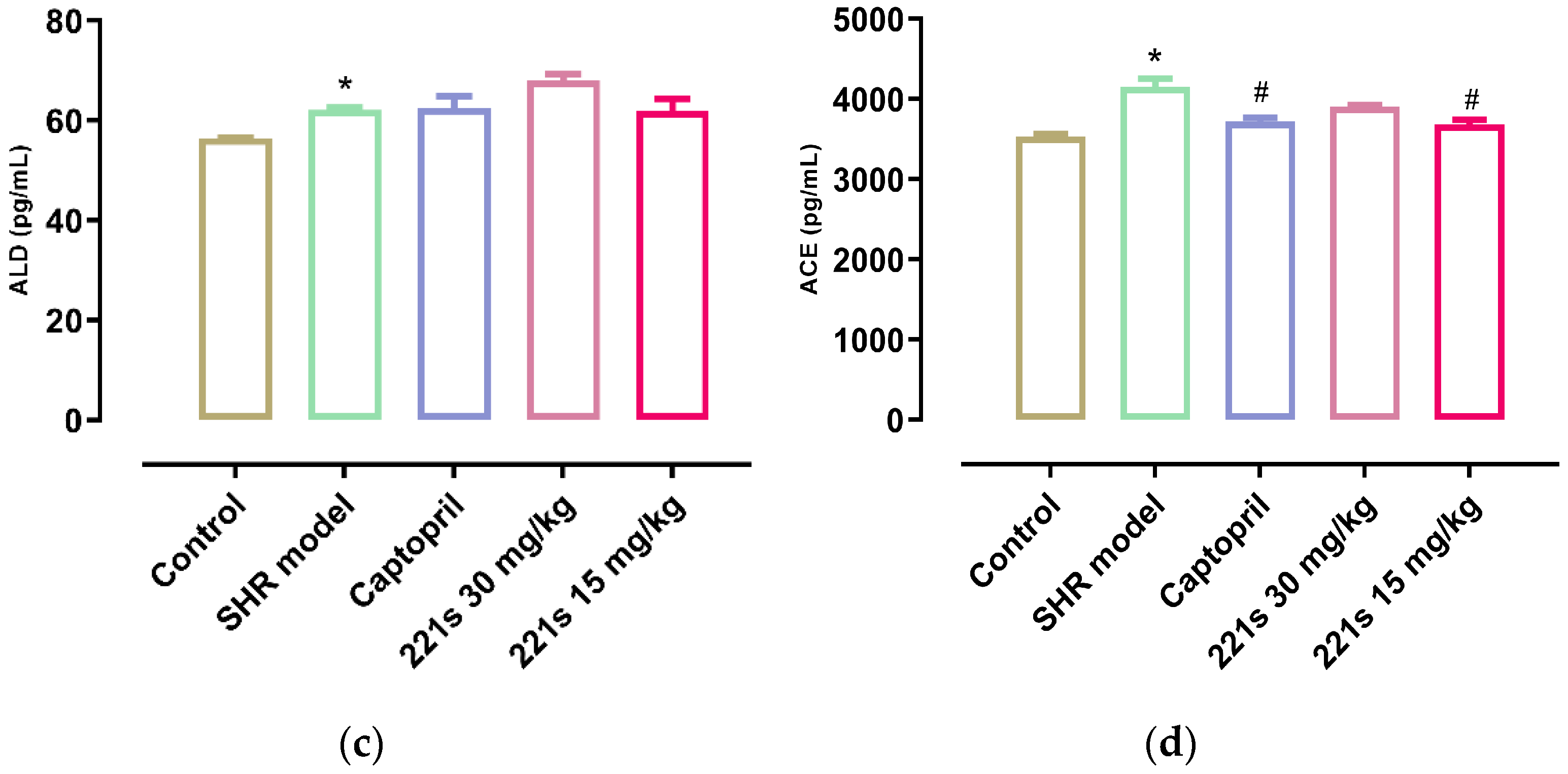
2.5. Measurement of REN, AngII, ALD, and ACE in Serum
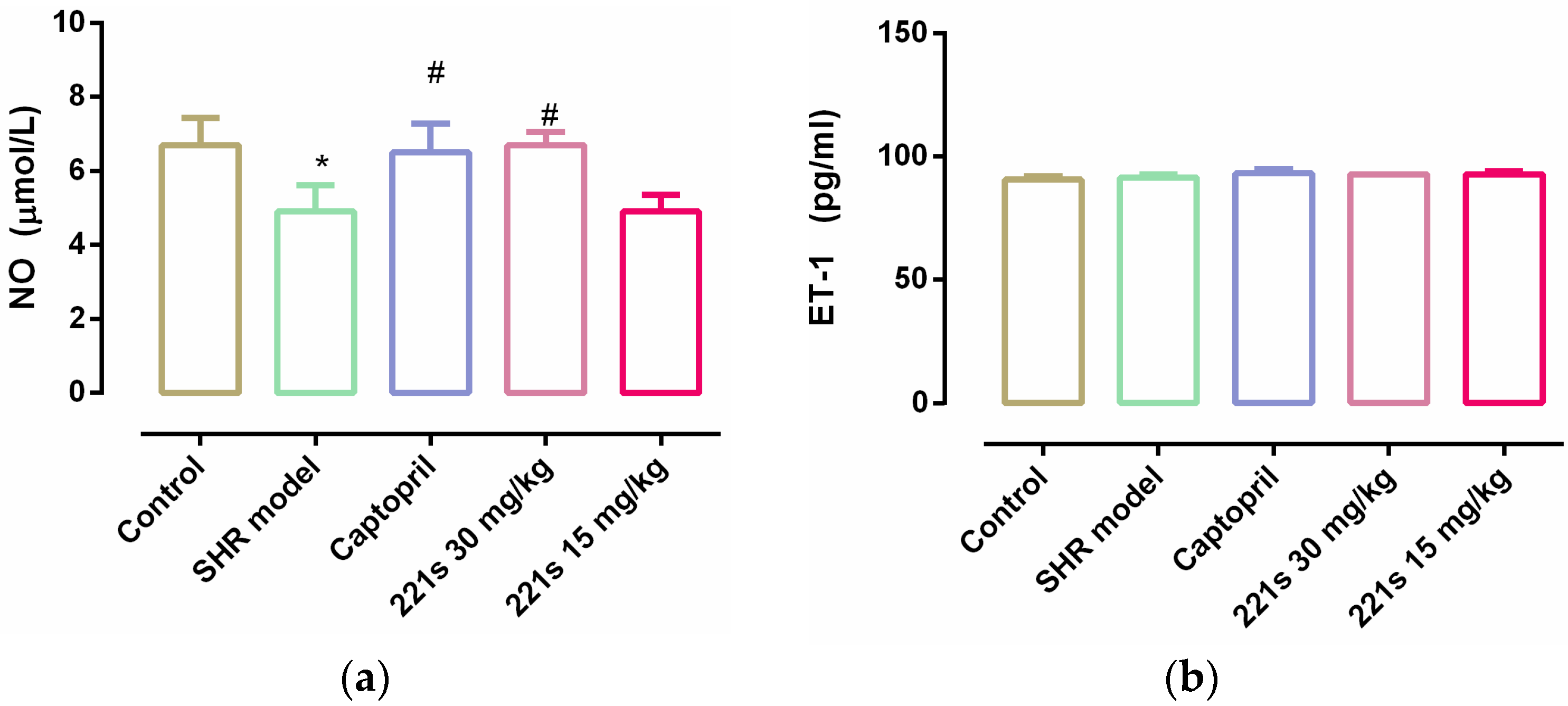
2.6. Measurement of NO and ET-1 in Serum
2.7. HE Staining
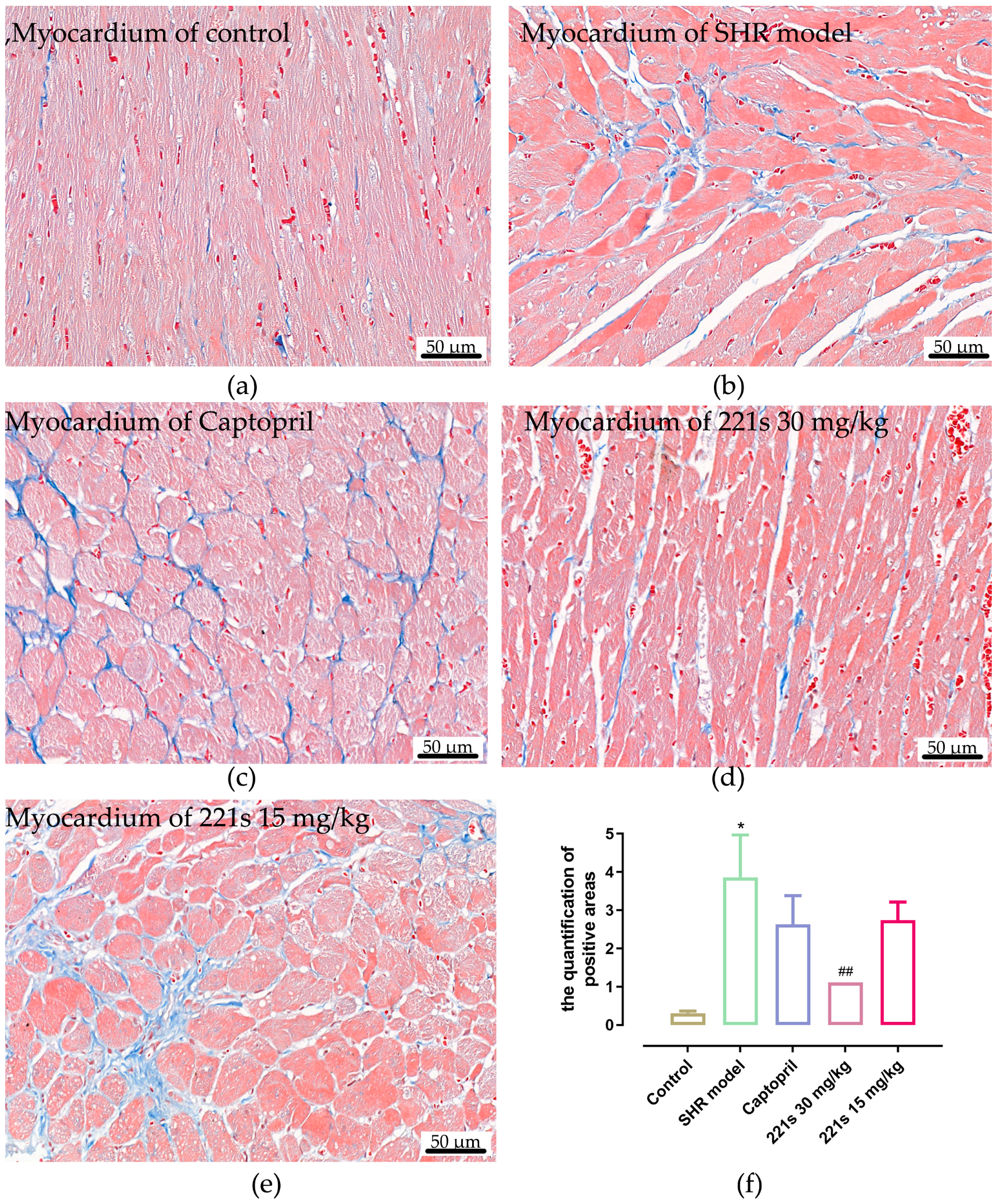
2.8. Masson Staining
3. Experimental Section
3.1. Reagent
3.2. Experimental Animal
3.3. Experimental Apparatus
3.4. Synthesis of 221s (2,9)
3.4.1. Synthesis of Compound 1
3.4.2. Synthesis of Compound 2
3.4.3. Synthesis of Compound 3
3.4.4. Synthesis of Compound 4
3.4.5. Synthesis of 221s (2,9)
3.5. Determination of Purity
3.5.1. HPLC
3.5.2. Thermal Analysis
3.5.3. The Safety Evaluation of 221s (2,9)
3.6. The Hypotensive Effect and the Underlying Mechanism of 221s (2,9) on RAAS in SHR Rats
3.6.1. Animals and Animal Care
3.6.2. Measurement of Blood Pressure, Heart Rate and Body Weight
3.6.3. Measurement of REN, AngII, ALD, NO and ET-1 in Serum
3.6.4. Histopathological Study
3.6.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Beckett, N.S.; Peters, R.; Fletcher, A.E.; Staessen, J.A.; Liu, L.; Dumitrascu, D.; Stoyanovsky, V.; Antikainen, R.L.; Nikitin, Y.; Anderson, C.; et al. Treatment of hypertension in patients 80 years of age or older. N. Engl. J. Med. 2008, 358, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Slany, J. Non-drug treatment of hypertension including alternative methods. J. Fur. Hyperton. 2013, 17, 152–156. [Google Scholar]
- Safar, M.E.; Rudnichi, A.; Asmar, R. Drug treatment of hypertension. J. Hypertens. 2000, 18, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, N.M.; Carnegie, A.; Raskin, P.; Heller, J.A.; Simmons, M. Potassium supplementation in hypertensive patients with diuretic-induced hypokalemia. N. Engl. J. Med. 1985, 312, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.R.; Odendaal, H.J. The addition of a diuretic to anti-hypertensive therapy for early severe hypertension in pregnancy. Int. J. Gynecol. Obstet. 1998, 60, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Massie, B.M. Effect of diuretic therapy on hypertensive left ventricular hypertrophy. Eur. Heart J. 1992, 13, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Vischer, A.S.; Kuster, G.M.; Twerenbold, R.; Pfister, O.; Zhou, Q.; Villiger, A.; Poglitsch, M.; Krähenbühl, S.; Mayr, M.; Osswald, S.; et al. Influence of Antihypertensive Treatment on RAAS Peptides in Newly Diagnosed Hypertensive Patients. Cells 2021, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Opie, L.H. Renoprotection by angiotensin-receptor blockers and ACE inhibitors in hypertension. Lancet 2001, 358, 1829–1831. [Google Scholar] [CrossRef]
- Neutel, J.M. Complementary Mechanisms of Angiotensin Receptor Blockers and Calcium Channel Blockers in Managing Hypertension. Postgrad. Med. 2009, 121, 40–48. [Google Scholar] [CrossRef]
- Pacanowski, M.A.; Gong, Y.; Cooper-Dehoff, R.M.; Schork, N.J.; Shriver, M.D.; Langaee, T.Y.; Pepine, C.J.; Johnson, J.A. Beta-adrenergic receptor gene polymorphisms and beta-blocker treatment outcomes in hypertension. Clin. Pharmacol. Ther. 2008, 84, 715–721. [Google Scholar] [CrossRef]
- Ligueros, M.; Unwin, R.; Wilkins, M. Selective α1-adrenoreceptor blockers in the treatment of hypertension: Should we be using them more? Clin. Auton. Res. 1991, 1, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, A.; Sessa, M.; Scavone, C.; De Angelis, A.; Vitale, C.; Berrino, L.; Rossi, F.; Rosano, G.; Capuano, A. New and old roles of the peripheral and brain renin-angiotensin-aldosterone system (RAAS): Focus on cardiovascular and neurological diseases. Int. J. Cardiol. 2017, 227, 734–742. [Google Scholar] [CrossRef]
- Kitakaze, M.; Minamino, T.; Node, K.; Komamura, K.; Shinozaki, Y.; Mori, H.; Kosaka, H.; Inoue, M.; Hori, M.; Kamada, T. Beneficial effects of inhibition of angiotensin-converting enzyme on ischemic myocardium during coronary hypoperfusion in dogs. Circulation 1995, 92, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Kitakaze, M.; Asanuma, H.; Funaya, H.; Node, K.; Takashima, S.; Sanada, S.; Asakura, M.; Ogita, H.; Kim, J.; Hori, M. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers synergistically increase coronary blood flow in canine ischemic myocardium: Role of bradykinin. J. Am. Coll. Cardiol. 2002, 40, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Berdeaux, A.; Bonhenry, C.; Giudicelli, J.F. Effects of four angiotensin I converting enzyme inhibitors on regional myocardial blood flow and ischemic injury during coronary artery occlusion in dogs. Fundam. Clin. Pharmacol. 1987, 1, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Omoro, S.A.; Majid, D.S.; El-Dahr, S.S.; Navar, L.G. Kinin influences on renal regional blood flow responses to angiotensin-converting enzyme inhibition in dogs. Am. J. Physiol. 1999, 276, F271–F277. [Google Scholar] [CrossRef]
- Overlack, A. ACE inhibitor-induced cough and bronchospasm. Incidence, mechanisms and management. Drug Saf. 1996, 15, 72–78. [Google Scholar] [CrossRef]
- Umemura, K.; Nakashima, M.; Saruta, T. Thromboxane A2 synthetase inhibition suppresses cough induced by angiotensin converting enzyme inhibitors. Life Sci. 1997, 60, 1583–1588. [Google Scholar] [CrossRef]
- Messerli, F.H.; Bavishi, C.; Bangalore, S. Why Are We Still Prescribing Angiotensin-Converting Enzyme Inhibitors? Circulation 2022, 145, 413–415. [Google Scholar] [CrossRef]
- Wilkerson, R.G.; Winters, M.E. Angiotensin-Converting Enzyme Inhibitor-Induced Angioedema. Emerg. Med. Clin. N. Am. 2022, 40, 79–98. [Google Scholar] [CrossRef]
- Reardon, L.C.; Macpherson, D.S. Hyperkalemia in outpatients using angiotensin-converting enzyme inhibitors. How much should we worry? Arch. Intern. Med. 1998, 158, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Salik, J.R.; Golas, S.B.; McCoy, T.H. A comparative study assessing the incidence and degree of hyperkalemia in patients on angiotensin-converting enzyme inhibitors versus angiotensin-receptor blockers. J. Hum. Hypertens. 2022, 36, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Sleight, P. The renin-angiotensin system: A review of trials with angiotensin-converting enzyme inhibitors and angiotensin receptor blocking agents. Eur. Heart J. Suppl. 2002, 4, A53–A57. [Google Scholar] [CrossRef]
- Hodsman, G.P.; Isles, C.G.; Murray, G.D.; Usherwood, T.P.; Webb, D.J.; Robertson, J.I. Factors related to first dose hypotensive effect of captopril: Prediction and treatment. BMJ 1983, 286, 832–834. [Google Scholar] [CrossRef]
- Nicolaou, P.A. Sex differences in heart failure medications targeting the renin-angiotensin-aldosterone system. Eur. J. Pharmacol. 2021, 897, 173961. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Junot, C.; Ezan, E.; Ménard, J. Angiotensin I-converting enzyme and metabolism of the haematological peptide N-acetyl-seryl-aspartyl-lysyl-proline. Clin. Exp. Pharmacol. Physiol. 2001, 28, 1066–1069. [Google Scholar] [CrossRef]
- Stillwell, W.; Carman, J.K.; Bell, L.; Horning, M.G. The metabolism of safrole and 2′,3′-epoxysafrole in the rat and guinea pig. Drug Metab. Dispos. 1974, 2, 489–498. [Google Scholar]
- Lam, F.F.; Yeung, J.H.; Chan, K.M.; Or, P.M. Relaxant effects of danshen aqueous extract and its constituent danshensu on rat coronary artery are mediated by inhibition of calcium channels. Vascul. Pharmacol. 2007, 46, 271–277. [Google Scholar] [CrossRef]
- Wu, L.; Qiao, H.; Li, Y.; Li, L. Protective roles of puerarin and Danshensu on acute ischemic myocardial injury in rats. Phytomedicine 2007, 14, 652–658. [Google Scholar] [CrossRef]
- Chan, K.; Chui, S.H.; Wong, D.Y.; Ha, W.Y.; Chan, C.L.; Wong, R.N. Protective effects of Danshensu from the aqueous extract of Salvia miltiorrhiza (Danshen) against homocysteine-induced endothelial dysfunction. Life Sci. 2004, 75, 3157–3171. [Google Scholar] [CrossRef]
- Jia, S.; Chen, Q.; Wu, J.; Yao, X.; Shao, J.; Cheng, X.; Zhang, C.; Cen, D.; Wang, Y.; Shen, Z.; et al. Danshensu derivative ADTM ameliorates CCl(4)-induced acute liver injury in mice through inhibiting oxidative stress and apoptosis. Pathol. Res. Pract. 2021, 228, 153656. [Google Scholar] [CrossRef]
- Li, H.; Xie, Y.H.; Yang, Q.; Wang, S.W.; Zhang, B.L.; Wang, J.B.; Cao, W.; Bi, L.L.; Sun, J.Y.; Miao, S.; et al. Cardioprotective effect of paeonol and danshensu combination on isoproterenol-induced myocardial injury in rats. PLoS ONE 2012, 7, e48872. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, D.; Wu, J.; Ou, Y.; Mu, C.; Han, B.; Zhang, Q. Improved blood-brain barrier distribution: Effect of borneol on the brain pharmacokinetics of kaempferol in rats by in vivo microdialysis sampling. J. Ethnopharmacol. 2015, 162, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Goyal, N.; Hopkinson, B. Preparation of L-proline based aeruginosin 298-A analogs: Optimization of the P1-moiety. Bioorg. Med. Chem. Lett. 2009, 19, 3798–3803. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kawasaki, S.; Oda, N.; Yagi, S.; El Bialy, S.A.A.; Uenishi, J.i.; Yamauchi, M.; Ikeda, M. Diastereoselective synthesis of 2-alkylated 4-silyloxyproline esters. J. Chem. Soc. Perkin Trans. 1 2001, 23, 2623–2631. [Google Scholar] [CrossRef]
- Lundt, B.F.; Johansen, N.L.; Vølund, A.; Markussen, J. Removal of t-butyl and t-butoxycarbonyl protecting groups with trifluoroacetic acid. Mechanisms, biproduct formation and evaluation of scavengers. Int. J. Pept. Protein. Res. 1978, 12, 258–268. [Google Scholar] [CrossRef]
- Gilbertson, S.R.; Chang, C.W.T. Synthesis of phosphino oxazoline ligand libraries from amino acid and phosphino carboxylate building blocks. J. Org. Chem. 1998, 63, 8424–8431. [Google Scholar] [CrossRef]
- Doi, T.; Hoshina, Y.; Mogi, H.; Yamada, Y.; Takahashi, T. Solid-phase combinatorial synthesis of aeruginosin derivatives and their biological evaluation. J. Comb. Chem. 2006, 8, 571–582. [Google Scholar] [CrossRef]
- Bavishi, C.; Bangalore, S.; Messerli, F.H. Renin Angiotensin Aldosterone System Inhibitors in Hypertension: Is There Evidence for Benefit Independent of Blood Pressure Reduction? Prog. Cardiovasc. Dis. 2016, 59, 253–261. [Google Scholar] [CrossRef]
- Umar, A.; Imam, G.; Yimin, W.; Kerim, P.; Tohti, I.; Berke, B.; Moore, N. Antihypertensive effects of Ocimum basilicum on blood pressure in renovascular hypertensive rats. Fundam. Clin. Pharmacol. 2010, 24, 26. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, B.; Yu, L.; Wang, R.; Tang, Y.; Chen, Y.; Wang, N.; Zhang, Y.; Tan, X.; Yang, K.; Zhang, B.; et al. Chemical Synthesis, Safety and Efficacy of Antihypertensive Candidate Drug 221s (2,9). Molecules 2023, 28, 4975. https://doi.org/10.3390/molecules28134975
Qin B, Yu L, Wang R, Tang Y, Chen Y, Wang N, Zhang Y, Tan X, Yang K, Zhang B, et al. Chemical Synthesis, Safety and Efficacy of Antihypertensive Candidate Drug 221s (2,9). Molecules. 2023; 28(13):4975. https://doi.org/10.3390/molecules28134975
Chicago/Turabian StyleQin, Bei, Lili Yu, Rong Wang, Yimei Tang, Yunmei Chen, Nana Wang, Yixin Zhang, Xiong Tan, Kuan Yang, Bo Zhang, and et al. 2023. "Chemical Synthesis, Safety and Efficacy of Antihypertensive Candidate Drug 221s (2,9)" Molecules 28, no. 13: 4975. https://doi.org/10.3390/molecules28134975
APA StyleQin, B., Yu, L., Wang, R., Tang, Y., Chen, Y., Wang, N., Zhang, Y., Tan, X., Yang, K., Zhang, B., He, M., Zhang, Y., & Hu, Y. (2023). Chemical Synthesis, Safety and Efficacy of Antihypertensive Candidate Drug 221s (2,9). Molecules, 28(13), 4975. https://doi.org/10.3390/molecules28134975





