Naphthalene-Based Polymers as Catalytic Supports for Suzuki Cross-Coupling
Abstract
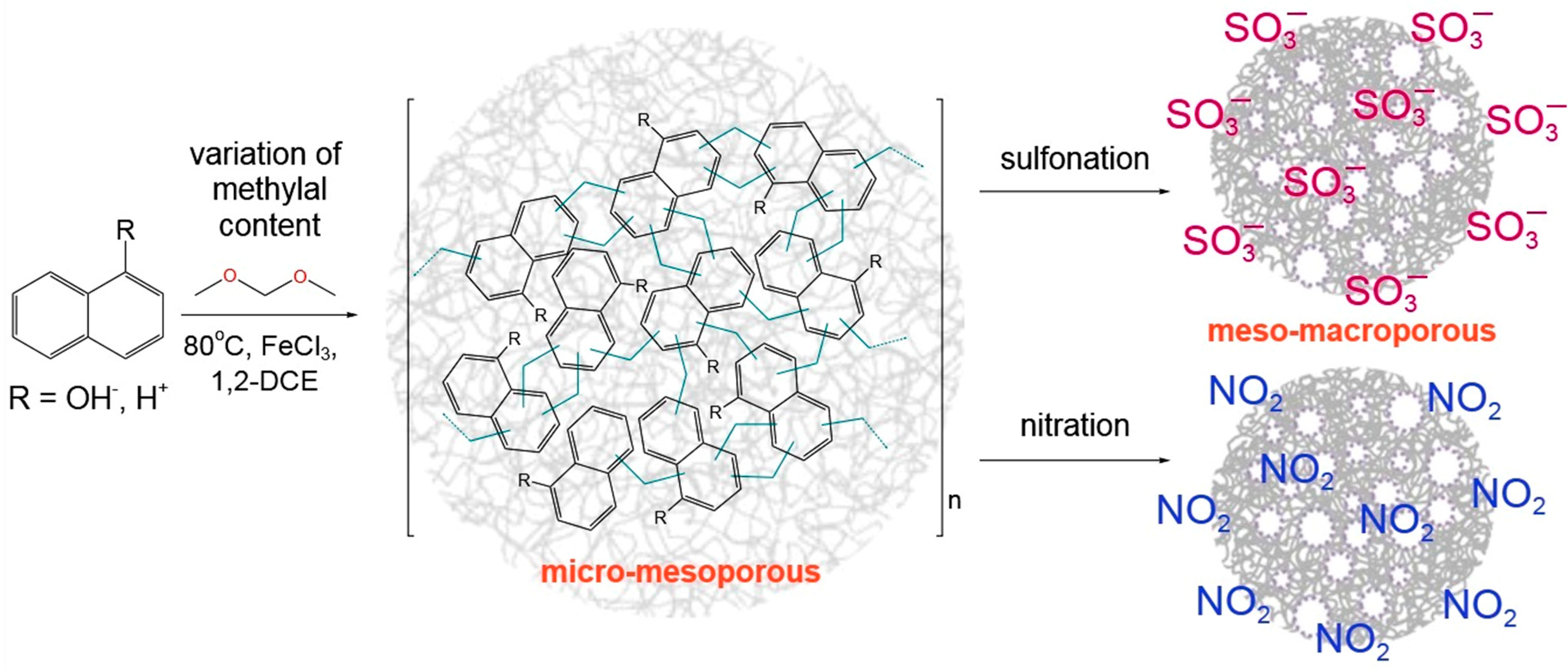
1. Introduction
2. Results
2.1. Characterization of the Polymers
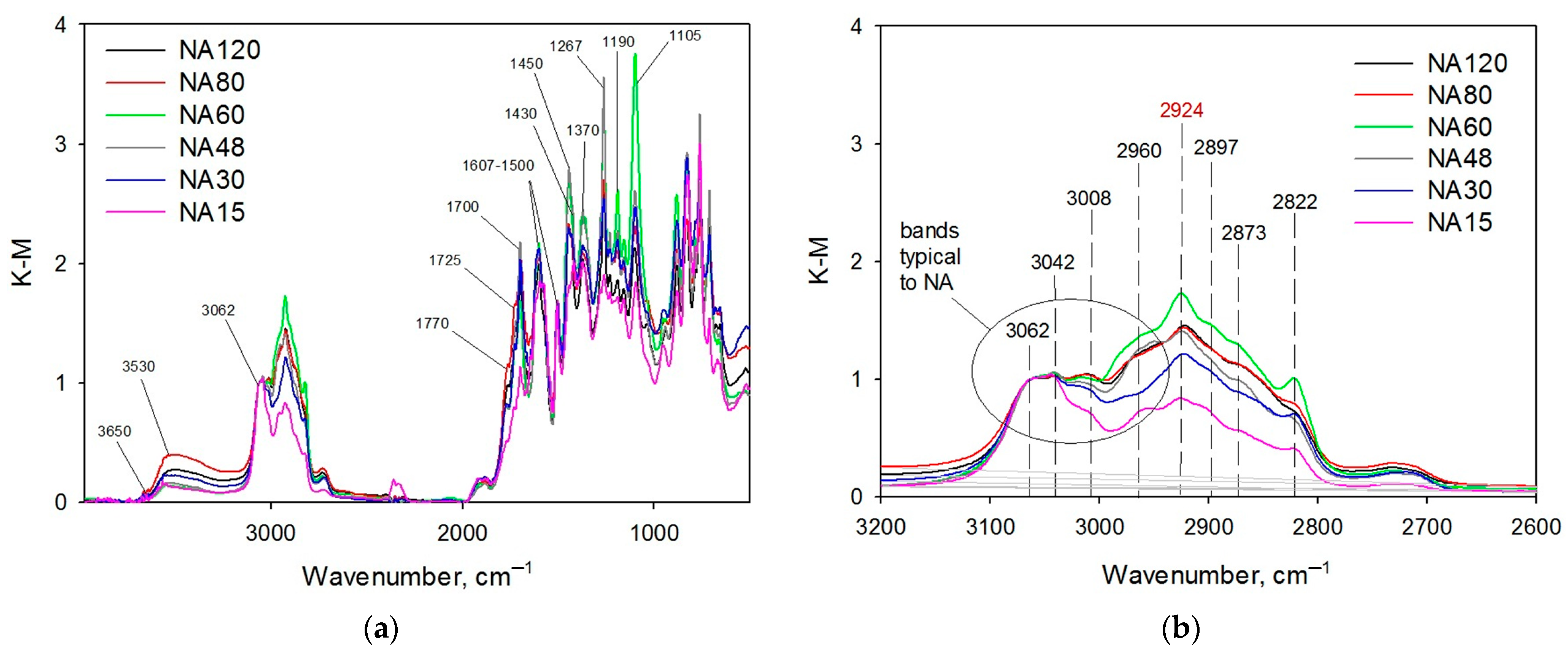
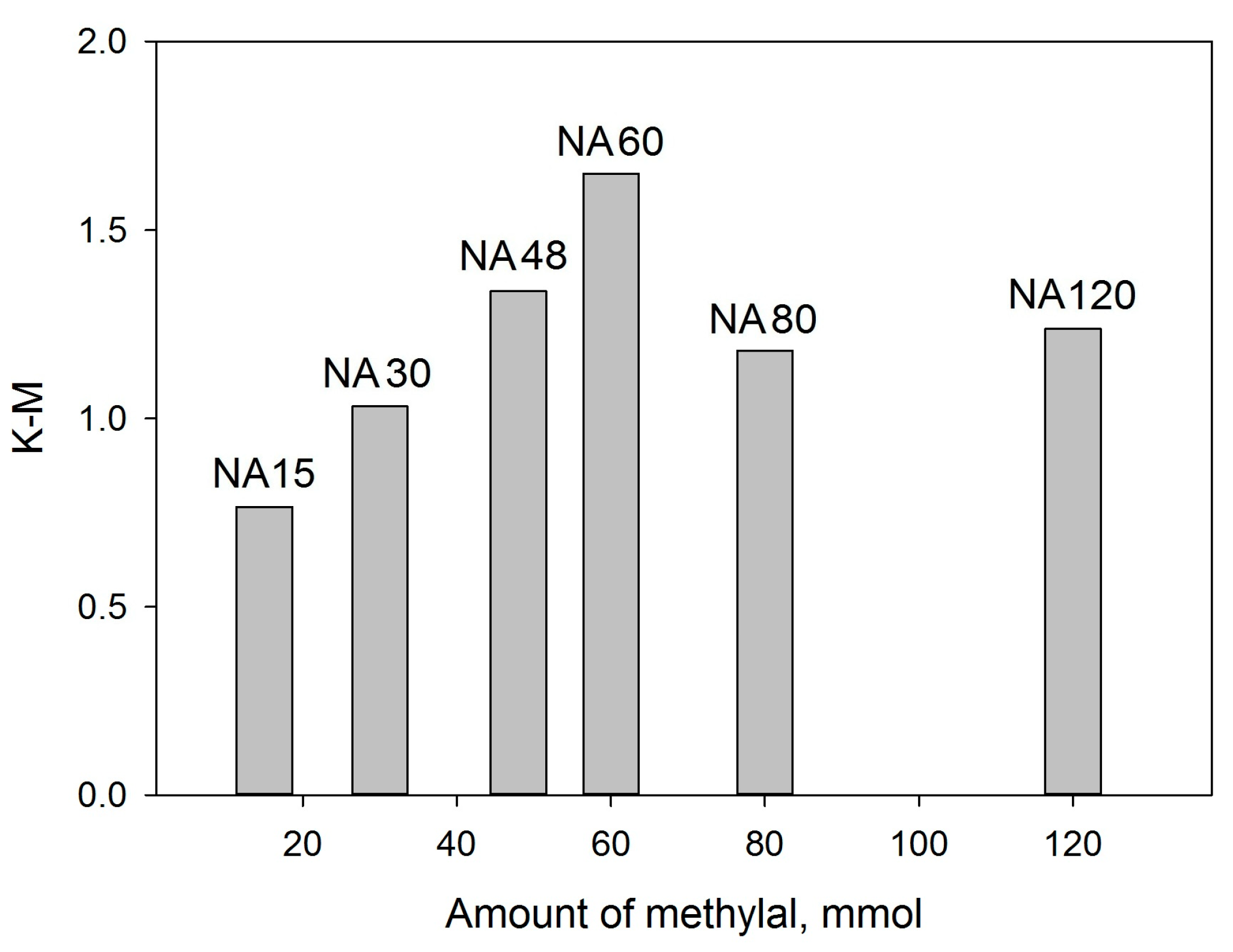
2.1.1. Liquid Nitrogen Physisorption and DRIFTS
2.1.2. TGA
2.1.3. XPS
2.2. Characterization of the Catalysts
2.3. Suzuki Cross-Coupling
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterization of Polymers
4.3. Modification of Polymers with Niro- and Sulfo-Groups
4.4. Catalyst Synthesis and Characterization
4.5. Reaction Procedure and Analysis of the Reaction Mixture
4.6. Catalysts Separation from the Reaction Mixture for Reuse
4.7. Procedure of Hot-Filtration of the Reaction Mixture
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| BP | biphenyl |
| BrAB | 4-bromoaminobenzene |
| BrAL | 4-bromobenzaldehyde |
| BrAN | 4-bromoanisole |
| BrNB | 4-bromonitrobenzene |
| BrTL | 4-bromotoluene |
| 1,2-DCE | 1,2-dichloroethan |
| DMF | N,N-dimethylformamide |
| DPA | diphenylamine |
| DVB | divinylbenzene |
| EtOH | ethanol |
| HAP | hyper-crosslinked aromatic polymer |
| HPS | hyper-cross-linked polystyrene |
| NA | naphthalene |
| NL | naphthol |
| NNA | nitrated NA |
| NP | nanoparticle |
| MBP | 4-methoxybiphenyl |
| MeOH | methanol |
| i-PrOH | isopropanol |
| PS | polystyrene |
| SNA | sulfonated NA |
| SSA | specific surface area |
References
- Biffis, A.; Centomo, P.; Zotto, A.D.; Zecca, M. Pd Metal Catalysts for Cross-Couplings and Related Reactions in the 21st Century: A Critical Review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Astruc, D. Development of the Applications of Palladium on Charcoal in Organic Synthesis. Adv. Synth. Catal. 2018, 360, 3426–3459. [Google Scholar] [CrossRef]
- De Tovar, J.; Rataboul, F.; Djakovitch, L. Heterogenization of Pd(II) Complexes as Catalysts for the Suzuki-Miyaura Reaction. Appl. Catal. A 2021, 627, 118381. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, W.; Tang, W. Palladium-Catalyzed Cross-Couplings in the Synthesis of Agrochemicals. Adv. Agrochem. 2022, 1, 125–138. [Google Scholar] [CrossRef]
- Dong, D.-Q.; Yang, H.; Zhou, M.-Y.; Wei, Z.-H.; Wu, P.; Wang, Z.-L. Recent Advances in Palladium-Catalyzed Reactions in Water. Curr. Opin. Green Sustain. Chem. 2023, 40, 100778. [Google Scholar] [CrossRef]
- Somorjai, G. On the Move. Nature 2004, 430, 730. [Google Scholar] [CrossRef] [PubMed]
- Kann, N. Recent Applications of Polymer Supported Organometallic Catalysts in Organic Synthesis. Molecules 2010, 15, 6306–6331. [Google Scholar] [CrossRef]
- Fiore, A.M.; Varvaro, G.; Agostinelli, E.; Mangone, A.; De Giglio, E.; Terzano, R.; Allegretta, I.; Dell’Anna, M.M.; Fiore, S.; Mastrorilli, P. Synthesis and Use in Catalysis of Hematite Nanoparticles Obtained from a Polymer Supported Fe(III) Complex. Eur. J. Inorg. Chem. 2022, 2022, e202100943. [Google Scholar] [CrossRef]
- Kashin, A.S.; Ananikov, V.P. Catalytic C-C and C-Heteroatom Bond Formation Reactions: In Situ Generated or Preformed Catalysts? Complicated Mechanistic Picture Behind Well-Known Experimental Procedures. J. Org. Chem. 2013, 78, 11117–11125. [Google Scholar] [CrossRef]
- Eremin, D.B.; Ananikov, V.P. Understanding Active Species in Catalytic Transformations: From Molecular Catalysis to Nanoparticles, Leaching, “Cocktails” of Catalysts and Dynamic Systems. Coord. Chem. Rev. 2017, 346, 2–19. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Z.; Wang, L.; Chen, X.; Zhang, Y.; Yang, X.; Pang, D.; Kang, J.; Guo, L. Highly Efficient and Recyclable Amorphous Pd(II)/Crystal Pd(0) Catalyst for Boosting Suzuki Reaction in Aqueous Solution. Nano Res. 2022, 15, 1193–1198. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.; Zhu, G. Multifunctional Porous Aromatic Frameworks: State of the Art and Opportunities. Energy Chem. 2020, 2, 100037. [Google Scholar] [CrossRef]
- Tan, L.; Tan, B. Hypercrosslinked Porous Polymer Materials: Design, Synthesis, and Applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef]
- Cai, X.; Nie, J.; Yang, G.; Wang, F.; Ma, C.; Lu, C.; Chen, Z. Phosphorus-Rich Network Polymer Supported Ruthenium Nanoparticles for Nitroarene Reduction. Mater. Lett. 2019, 240, 80–83. [Google Scholar] [CrossRef]
- Germain, J.; Fréchet, J.M.J.; Svec, F. Nanoporous Polymers for Hydrogen Storage. Small 2009, 5, 1098. [Google Scholar] [CrossRef]
- Dell’Anna, M.M.; Romanazzi, G.; Mastrorilli, P. Polymer Supported Catalysts Obtained from Metal-Containing Monomers. Curr. Org. Chem. 2013, 17, 1236–1273. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Mishra, D.K.; Hwang, J.-S. Selective Hydrogenation of D-Glucose Using Amine Functionalized Nanoporous Polymer Supported Ru Nanoparticles Based Catalyst. Catal. Today 2016, 265, 163–173. [Google Scholar] [CrossRef]
- Wang, K.; Jia, Z.; Yang, X.; Wang, L.; Gu, Y.; Tan, B. Acid and Base Coexisted Heterogeneous Catalysts Supported on Hypercrosslinked Polymers for One-Pot Cascade Reactions. J. Catal. 2017, 348, 168–176. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Davankov, V.A. Porous Structure of Hypercrosslinked Polystyrene: State-of-the-Art Mini-Review. React. Funct. Polym. 2006, 66, 768–779. [Google Scholar] [CrossRef]
- Sulman, E.M.; Nikoshvili, L.Z.; Matveeva, V.G.; Tyamina, I.Y.; Sidorov, A.I.; Bykov, A.V.; Demidenko, G.N.; Stein, B.D.; Bronstein, L.M. Palladium Containing Catalysts Based on Hypercrosslinked Polystyrene for Selective Hydrogenation of Acetylene Alcohols. Top. Catal. 2012, 55, 492–497. [Google Scholar] [CrossRef]
- Bakhvalova, E.S.; Pinyukova, A.O.; Mikheev, A.V.; Demidenko, G.N.; Sulman, M.G.; Bykov, A.V.; Nikoshvili, L.Z.; Kiwi-Minsker, L. Noble Metal Nanoparticles Stabilized by Hyper-Cross-Linked Polystyrene as Effective Catalysts in Hydrogenation of Arenes. Molecules 2021, 26, 4687. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, L.; Gao, S.; Wang, H.; He, Z.; Xu, Y.; Huang, K. In Situ Encapsulated Ultrafine Pd Nanoparticles in Nitrogen-Doped Porous Carbon Derived from Hyper-Crosslinked Polymers Effectively Catalyse Hydrogenation. J. Catal. 2021, 396, 342–350. [Google Scholar] [CrossRef]
- Yuan, M.; Yang, R.; Wei, S.; Hu, X.; Xu, D.; Yang, J.; Dong, Z. Ultra-Fine Pd Nanoparticles Confined in a Porous Organic Polymer: A Leaching-and-Aggregation-Resistant Catalyst for the Efficient Reduction of Nitroarenes by NaBH4. J. Colloid Interface Sci. 2019, 538, 720–730. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Jia, X.; Fan, X.; Zhang, B.; Zhang, A.; Zhang, Q. Hydroxyl-Based Hypercrosslinked Microporous Polymers and Their Excellent Performance for CO2 Capture. Ind. Eng. Chem. Res. 2018, 57, 17259–17265. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Chen, G.; Li, D.; Zhou, Y.; Yang, X.; Wang, J. Hypercrosslinked Organic Polymer Based Carbonaceous Catalytic Materials: Sulfonic acid Functionality and Nano-Confinement Effect. Appl. Catal. B 2015, 718, 176–177. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, J.; Luo, W.; Su, L.; Huang, Z. Catalytic Conversion of Carbohydrates into 5-Hydroxymethyl Furfural over Sulfonated Hyper-Cross-Linked Polymer in DMSO. Chem. Eng. J. 2018, 334, 1055–1064. [Google Scholar] [CrossRef]
- Minato, H.; Higosaki, N.; Isobe, C. Polymerization of Naphthalene and Reactions of Polynaphthalene. Bull. Chem. Soc. Jpn. 1969, 42, 779–781. [Google Scholar] [CrossRef]
- Teng, D.-G.; Wei, X.-Y.; Yang, Z.; Zhu, Q.-J.; Gao, H.-S.; Li, J.-H.; Zhang, M.; Zong, Z.-M. Investigation on Naphthalene and Its Derivatives-Based Microporous Organic Hyper-Cross-Linked Polymers via Different Methodologies. Macromol. Chem. Phys. 2020, 221, 1900302. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Affolter, C. Structure Determination of Organic Compounds: Tables of Spectral Data; Springer: Berlin/Heideberg, Germany, 2000. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; Jonson Willey & Sons Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Tsyurupa, M.P.; Blinnikova, Z.K.; Davidovich, Y.A.; Lyubimov, S.E.; Naumkin, A.V.; Davankov, V.A. On the Nature of “Functional Groups” in Non-Functionalized Hypercrosslinked Polystyrenes. React. Funct. Polym. 2012, 72, 973–982. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST). X-ray Photoelectron Spectroscopy Database, Version 4.1. 2012. Available online: https://srdata.nist.gov/xps/ (accessed on 21 November 2020).
- Dalla Valle, C.; Zecca, M.; Rastrelli, F.; Tubaro, C.; Centomo, P. Effect of the Sulfonation on the Swollen State Morphology of Styrenic Cross-Linked Polymers. Polymers 2020, 12, 600. [Google Scholar] [CrossRef]
- Martins, C.R.; Ruggeri, G.; De Paoli, M.-A. Synthesis in Pilot Plant Scale and Physical Properties of Sulfonated Polystyrene. J. Braz. Chem. Soc. 2003, 14, 797–802. [Google Scholar] [CrossRef]
- Sułkowski, W.W.; Nowak, K.; Sułkowska, A.; Wolińska, A.; Bajdur, W.M.; Pentak, D.; Mikuła, B. Study of the Sulfonation of Expanded Polystyrene Waste and of Properties of the Products Obtained. Pure Appl. Chem. 2009, 81, 2417–2424. [Google Scholar] [CrossRef]
- Holländer, A.; Kröpke, S. Polymer Surface Treatment with SO2-Containing Plasmas. Plasma Process. Polym. 2010, 7, 390–402. [Google Scholar] [CrossRef]
- Wu, T.; Kaden, W.E.; Kunkel, W.A.; Anderson, S.L. Size-Dependent Oxidation of Pdn (n ≤ 13) on Alumina/NiAl(110): Correlation with Pd Core Level Binding Energies. Surf. Sci. 2009, 603, 2764–2770. [Google Scholar] [CrossRef]
- Pekridis, G.; Kaklidis, N.; Konsolakis, M.; Iliopoulou, E.F.; Yentekakis, I.V.; Marnellos, G.E. Correlation of Surface Characteristics with Catalytic Performance of Potassium Promoted Pd/Al2O3 Catalysts: The Case of N2O Reduction by Alkanes or Alkenes. Top. Catal. 2011, 54, 1135. [Google Scholar] [CrossRef]
- Sapunov, V.N.; Nikoshvili, L.Z.; Bakhvalova, E.S.; Sulman, M.G.; Matveeva, V.G. Kinetic Aspects of Suzuki Cross-Coupling Using Ligandless Pd Nanoparticles Embedded in Aromatic Polymeric Matrix. Processes 2023, 11, 878. [Google Scholar] [CrossRef]
- Nikoshvili, L.; Bakhvalova, E.S.; Bykov, A.V.; Sidorov, A.I.; Vasiliev, A.L.; Matveeva, V.G.; Sulman, M.G.; Sapunov, V.N.; Kiwi-Minsker, L. Study of Deactivation in Suzuki Reaction of Polymer-Stabilized Pd Nanocatalysts. Processes 2020, 8, 1653. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, J.; Snyder, S.A.; Morris, A.J.; Turner, S.R. Nanoporous Highly Crosslinked Polymer Networks with Covalently Bonded Amines for CO2 Capture. Polymer 2018, 154, 55–61. [Google Scholar] [CrossRef]
- Li, B.; Gong, R.; Wang, W.; Huang, X.; Zhang, W.; Li, H.; Hu, C.; Tan, B. A New Strategy to Microporous Polymers: Knitting Rigid Aromatic Building Blocks by External Cross-Linker. Macromolecules 2011, 44, 2410–2414. [Google Scholar] [CrossRef]
- Philippides, A.; Budd, P.M.; Price, C.; Cuncliffe, A.V. The Nitration of Polystyrene. Polymer 1993, 34, 3509–3513. [Google Scholar] [CrossRef]
- Coughlin, J.E.; Reisch, A.; Markarian, M.Z.; Schlenoff, J.B. Sulfonation of polystyrene: Toward the “ideal” polyelectrolyte. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2416–2424. [Google Scholar] [CrossRef]
- Ngadiwiyana; Ismiyarto; Gunawan; Purbowatiningrum, R.S.; Prasetya, N.B.A.; Kusworo, T.D.; Susanto, H. Sulfonated Polystyrene and Its Characterization as a Material of Electrolyte Polymer. J. Phys. Conf. Ser. 2018, 1025, 012133. [Google Scholar] [CrossRef]
- Nikoshvili, L.; Nemygina, N.; Bykov, A.; Sidorov, A.; Matveeva, V.; Tyamina, I.; Sulman, M.; Sulman, E.; Stein, B. Synthesis of 4-methoxybiphenyl using Pd-containing catalysts based on polymeric matrix of functionalized hypercrosslinked polystyrene. Bull. Chem. React. Eng. Catal. 2015, 10, 256–265. [Google Scholar] [CrossRef]
- Pentsak, E.O.; Eremin, D.B.; Gordeev, E.G.; Ananikov, V. P. Phantom Reactivity in Organic and Catalytic Reactions as a Consequence of Microscale Destruction and Contamination-Trapping Effects of Magnetic Stir Bars. ACS Catal. 2019, 9, 3070–3081. [Google Scholar] [CrossRef]

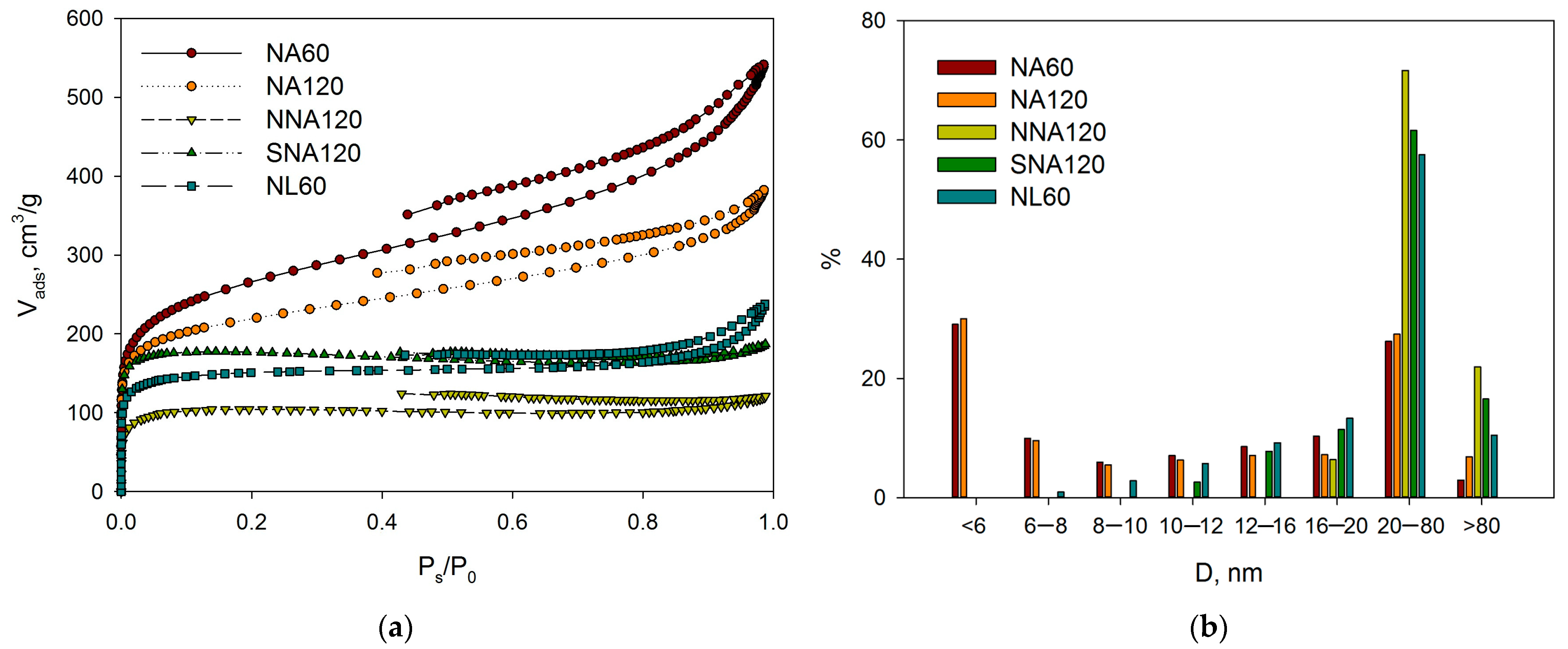


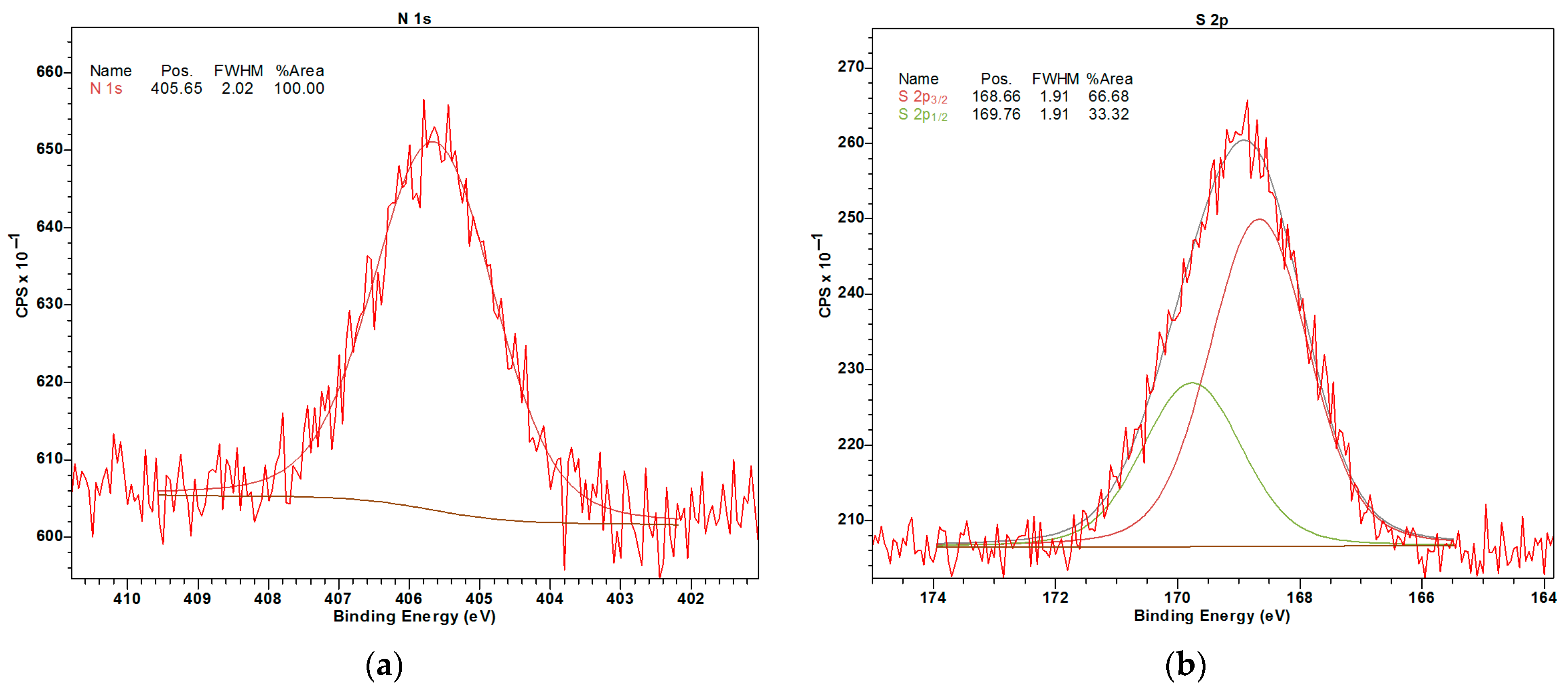

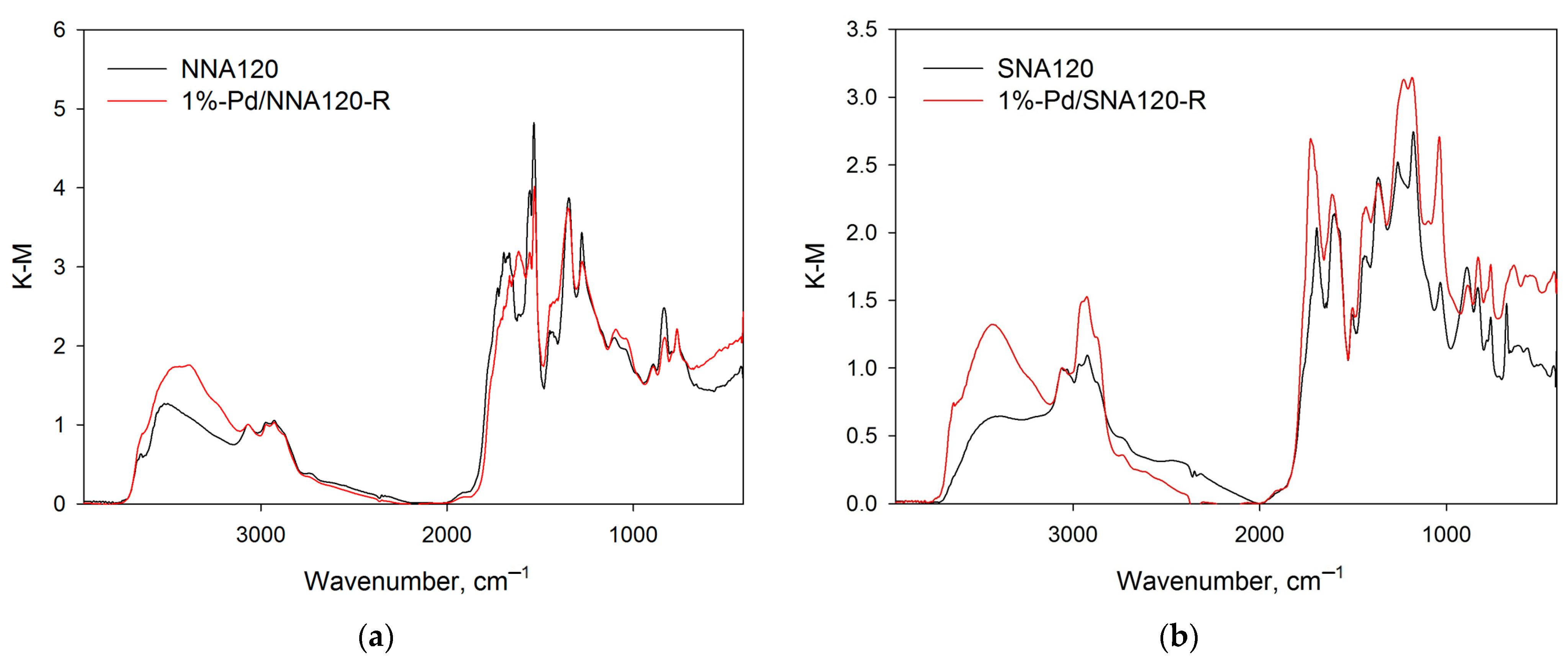
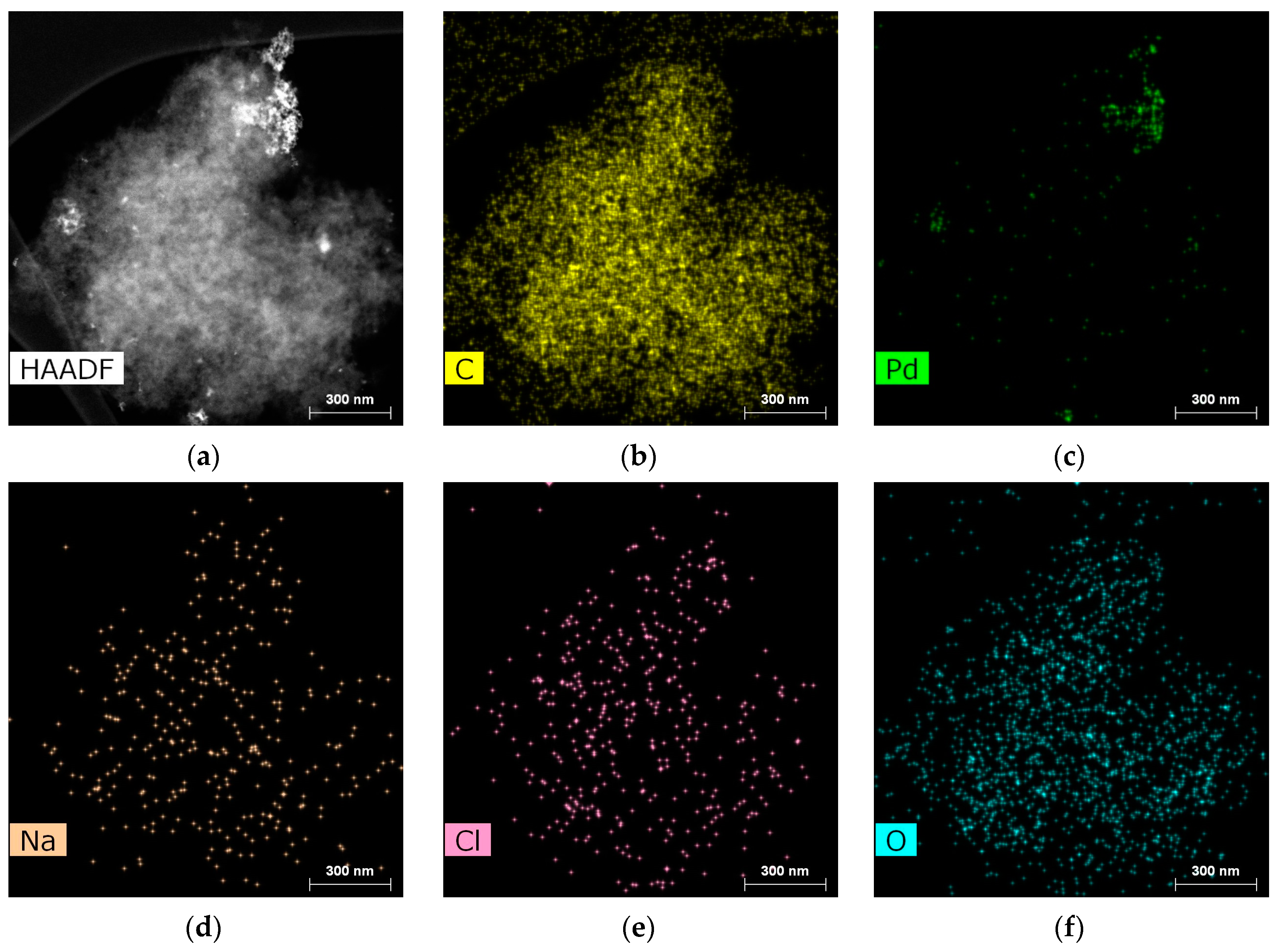
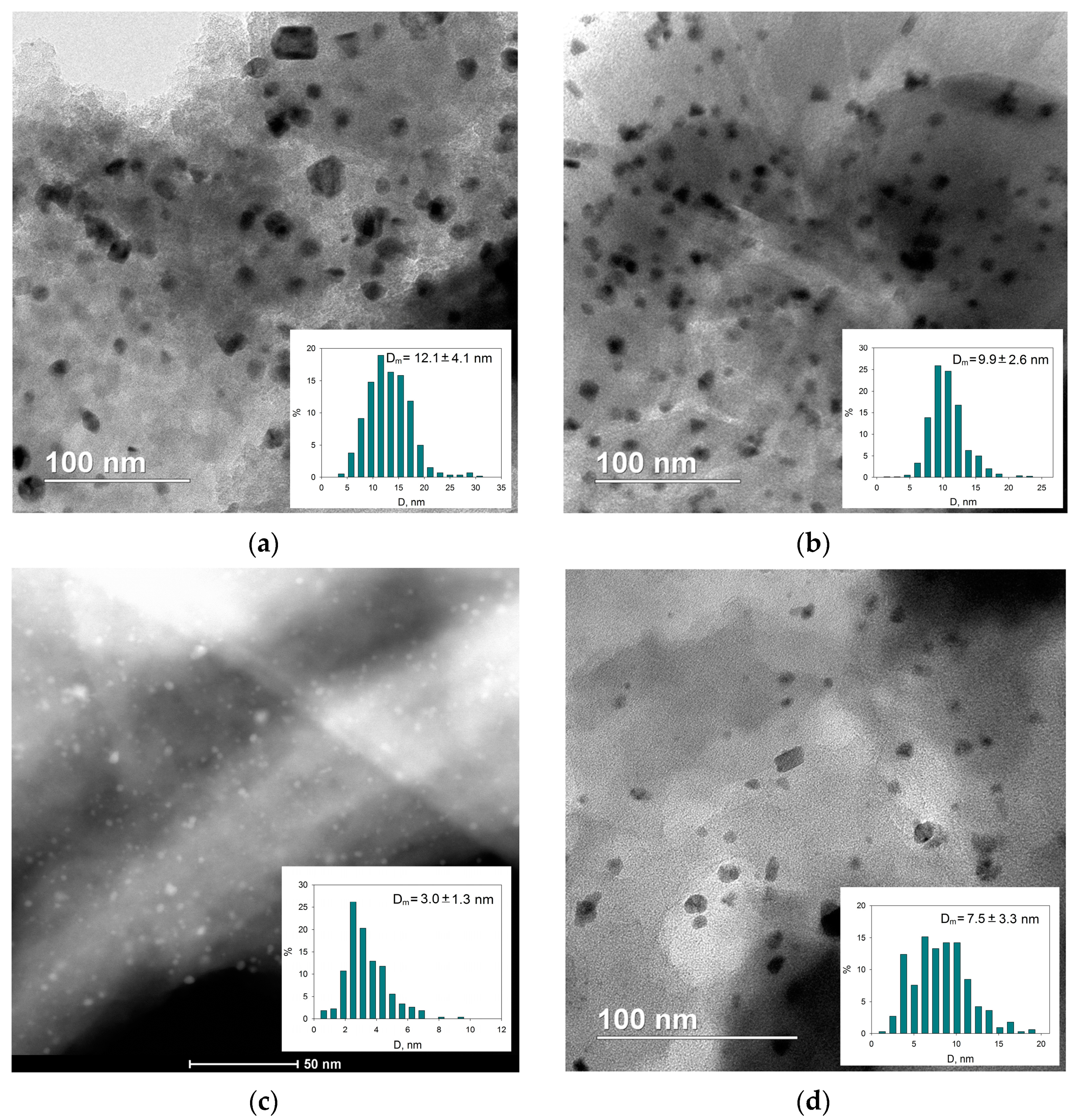


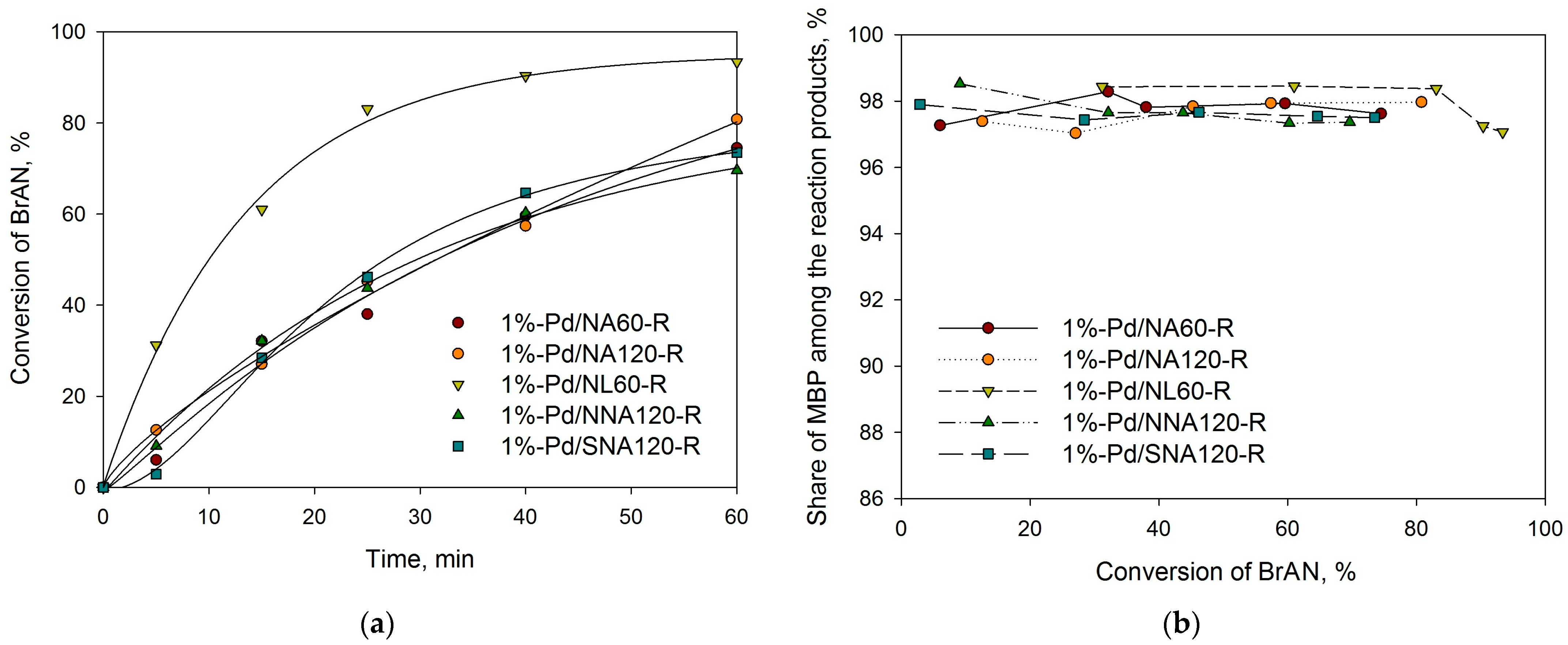
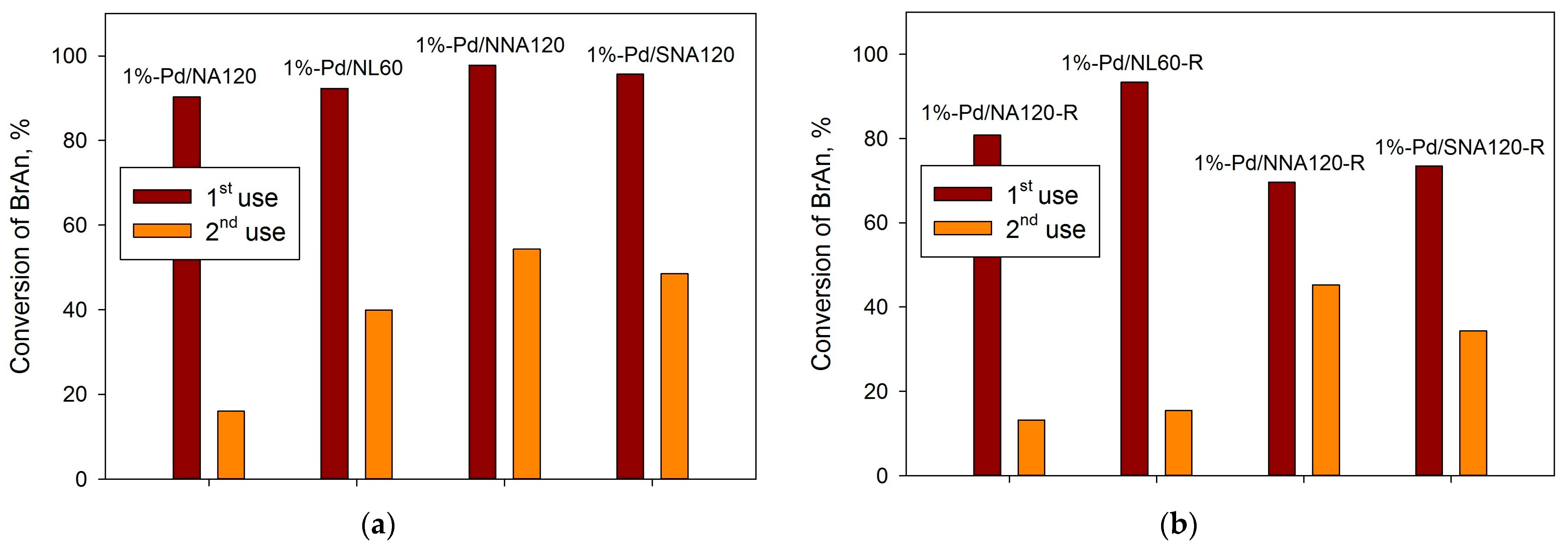
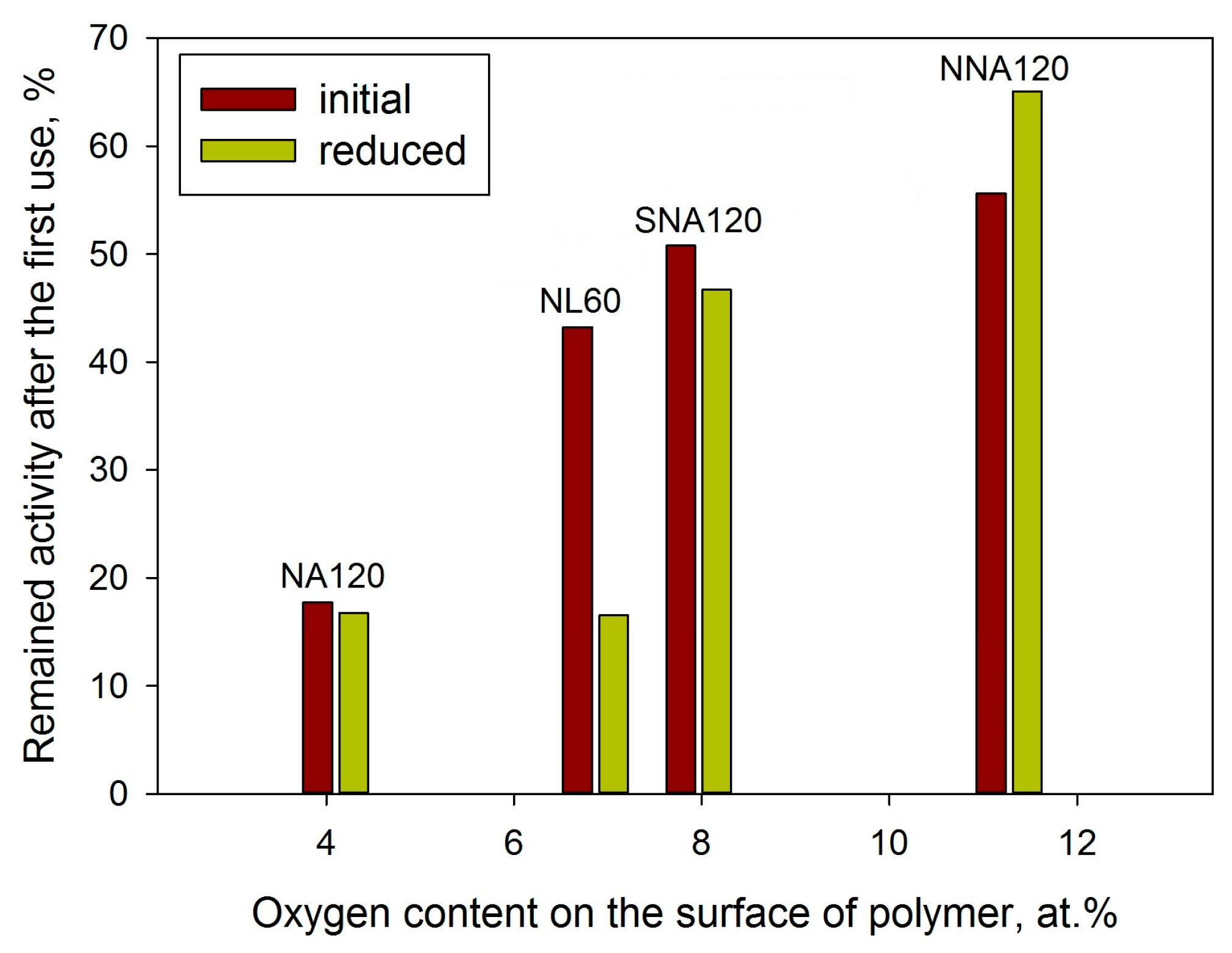

| Sample | SSABET, m2/g | SSAt-plot, m2/g | Vmicropore, mL/g |
|---|---|---|---|
| NA120 | 757 | 253 1; 524 2 | 0.234 |
| NA80 | 896 | 365 1; 553 2 | 0.238 |
| NA60 | 943 | 400 1; 544 2 | 0.246 |
| NA48 | 998 | 281 1; 743 2 | 0.331 |
| NA30 | 1065 | 603 1; 462 2 | 0.204 |
| NA15 | 915 | 360 1; 555 2 | 0.254 |
| NNA120 | 361 | N/A | N/A |
| SNA120 | 608 | N/A | N/A |
| NL60 | 519 | 94 1; 425 2 | 0.191 |
| Sample | Conversion of BrAN, % | Yield of MBP, % | R0, molBrAN/(molPd·min) |
|---|---|---|---|
| 1%-Pd/NA60 | 96.3 | 90.7 | 89.2 |
| 1%-Pd/NA120 | 90.3 | 86.9 | 14.9 |
| 1%-Pd/NL60 | 92.3 | 88.5 | 41.1 |
| 1%-Pd/NNA120 | 97.7 | 93.4 | 77.0 |
| 1%-Pd/SNA120 | 95.6 | 91.6 | 64.0 |
| 1%-Pd/NA60-R | 74.5 | 72.7 | 9.3 |
| 1%-Pd/NA120-R | 80.8 | 79.1 | 12.6 |
| 1%-Pd/NL60-R | 93.4 | 90.6 | 31.2 |
| 1%-Pd/NNA120-R | 69.6 | 67.9 | 10.7 |
| 1%-Pd/SNA120-R | 73.5 | 71.7 | 9.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhvalova, E.S.; Bykov, A.V.; Markova, M.E.; Lugovoy, Y.V.; Sidorov, A.I.; Molchanov, V.P.; Sulman, M.G.; Kiwi-Minsker, L.; Nikoshvili, L.Z. Naphthalene-Based Polymers as Catalytic Supports for Suzuki Cross-Coupling. Molecules 2023, 28, 4938. https://doi.org/10.3390/molecules28134938
Bakhvalova ES, Bykov AV, Markova ME, Lugovoy YV, Sidorov AI, Molchanov VP, Sulman MG, Kiwi-Minsker L, Nikoshvili LZ. Naphthalene-Based Polymers as Catalytic Supports for Suzuki Cross-Coupling. Molecules. 2023; 28(13):4938. https://doi.org/10.3390/molecules28134938
Chicago/Turabian StyleBakhvalova, Elena S., Alexey V. Bykov, Mariia E. Markova, Yury V. Lugovoy, Alexander I. Sidorov, Vladimir P. Molchanov, Mikhail G. Sulman, Lioubov Kiwi-Minsker, and Linda Z. Nikoshvili. 2023. "Naphthalene-Based Polymers as Catalytic Supports for Suzuki Cross-Coupling" Molecules 28, no. 13: 4938. https://doi.org/10.3390/molecules28134938
APA StyleBakhvalova, E. S., Bykov, A. V., Markova, M. E., Lugovoy, Y. V., Sidorov, A. I., Molchanov, V. P., Sulman, M. G., Kiwi-Minsker, L., & Nikoshvili, L. Z. (2023). Naphthalene-Based Polymers as Catalytic Supports for Suzuki Cross-Coupling. Molecules, 28(13), 4938. https://doi.org/10.3390/molecules28134938








