Characterization of Antioxidant and Antimicrobial Activity and Phenolic Compound Profile of Extracts from Seeds of Different Vitis Species
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolic Content
2.2. Antioxidant Activity
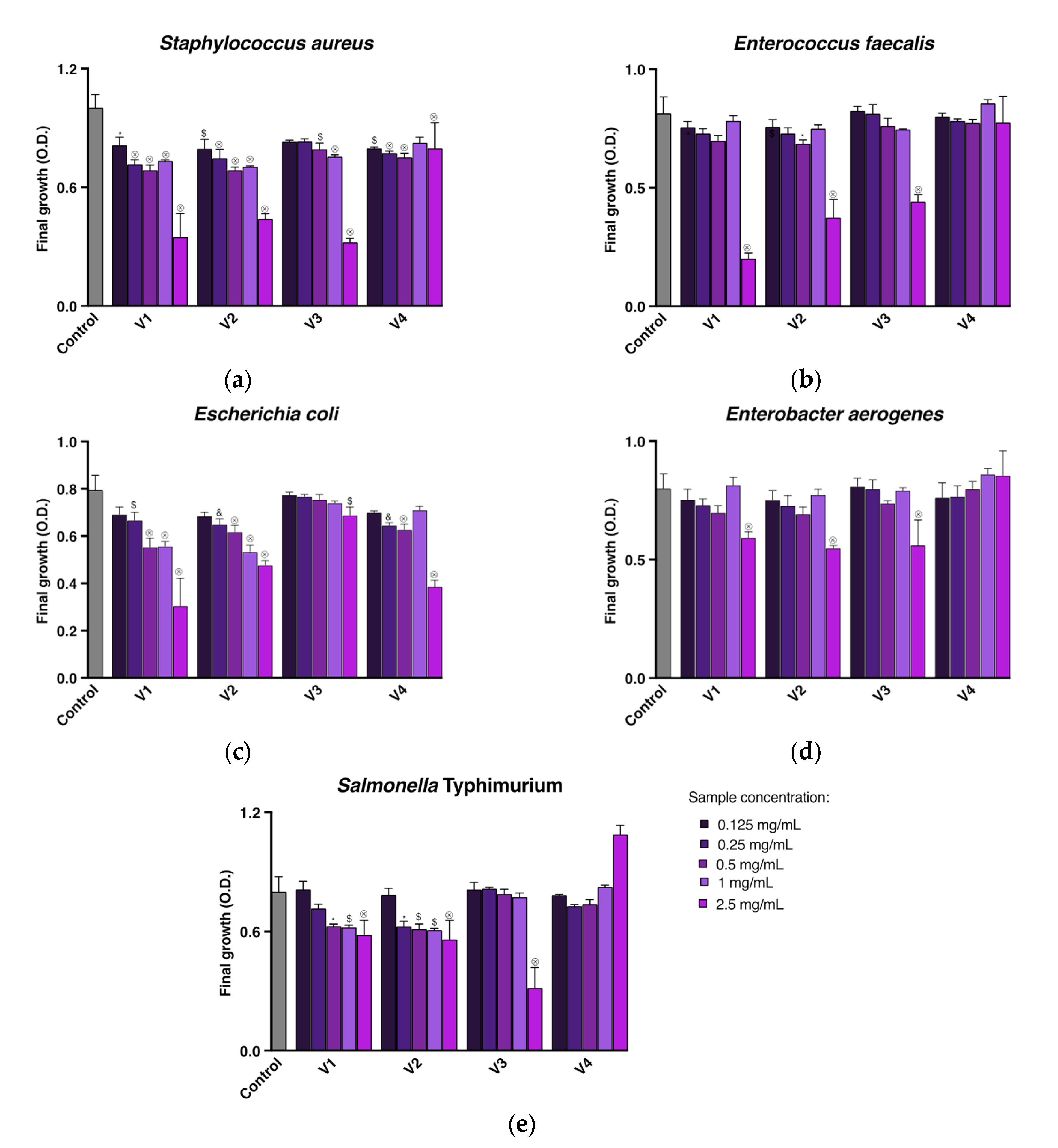
2.3. Antimicrobial Activity
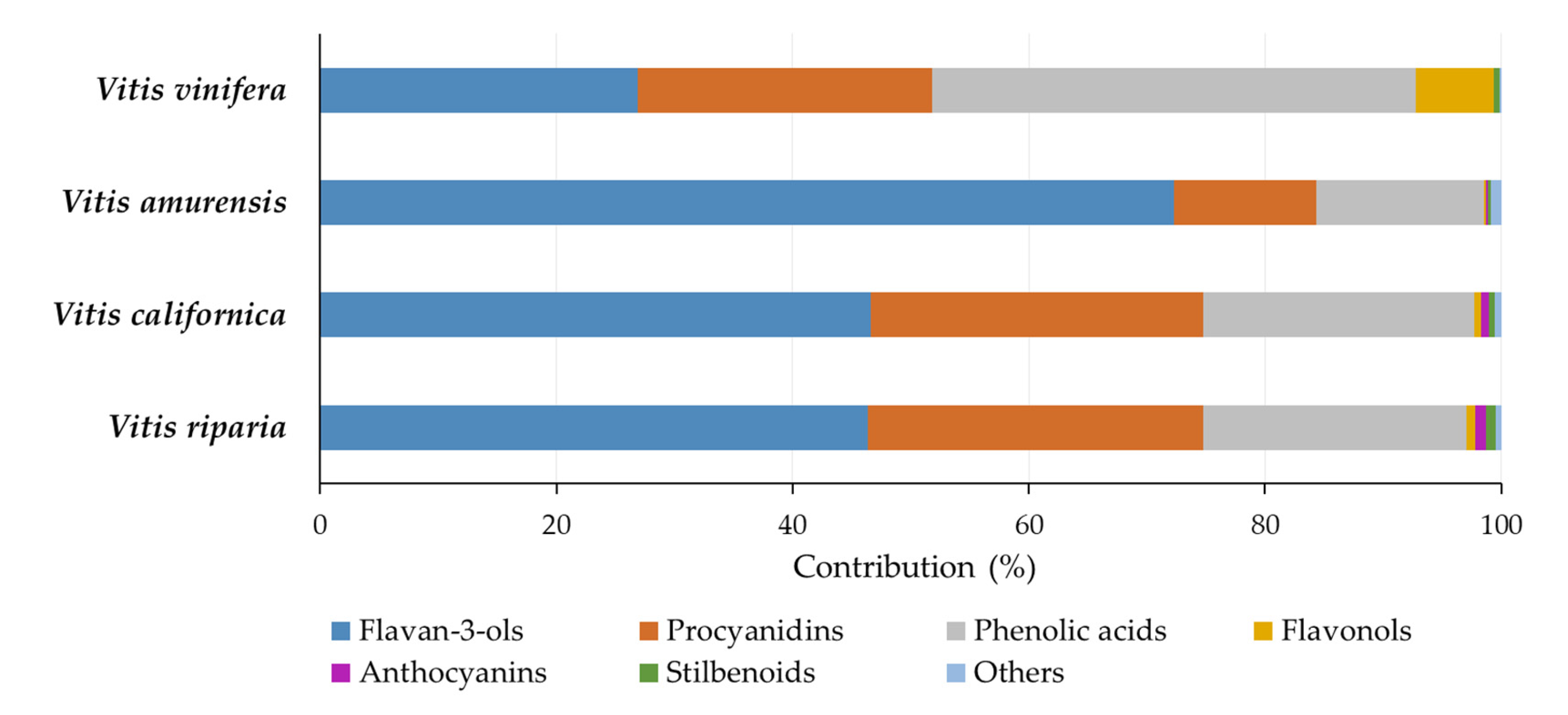
2.4. Phenolic Compound Profile of Seed Extracts
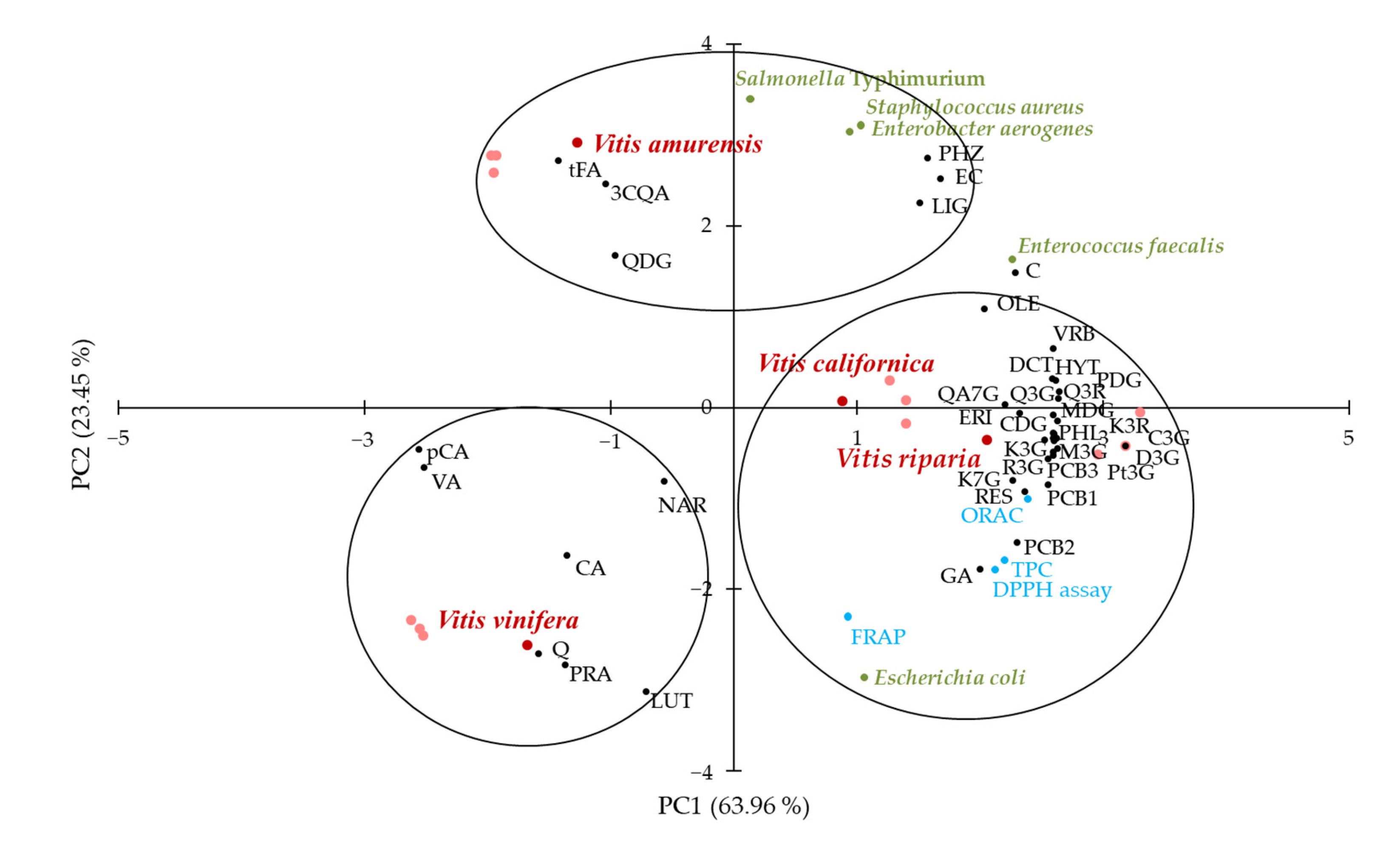
2.5. Overall Rate of Results with Principal Component Analysis
3. Materials and Methods
3.1. Plant Material, Chemicals and Reagents
3.2. Extract Preparation
3.3. Determination of the Total Phenolic Content
3.4. In Vitro Antioxidant Activity Assays
3.5. Determination of the Antimicrobial Activity
3.6. Determination of Phenolic Profile of the Extracts
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rao, M.; Bast, A.; de Boer, A. Valorized Food Processing By-Products in the EU: Finding the Balance between Safety, Nutrition, and Sustainability. Sustainability 2021, 13, 4428. [Google Scholar] [CrossRef]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C.; Caponio, F. Bioactive Compounds from Vine Shoots, Grape Stalks, and Wine Lees: Their Potential Use in Agro-Food Chains. Foods 2021, 10, 342. [Google Scholar] [CrossRef]
- Maier, T.; Schieber, A.; Kammerer, D.R.; Carle, R. Residues of Grape (Vitis vinifera L.) Seed Oil Production as a Valuable Source of Phenolic Antioxidants. Food Chem. 2009, 112, 551–559. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Heuzé, V.; Tran, G.; Grape Seeds and Grape Seed Oil Meal. Feedipedia, a Programme by INRAE, CIRAD, AFZ and FAO. Available online: https://www.feedipedia.org/node/692 (accessed on 21 March 2023).
- Lutterodt, H.; Slavin, M.; Whent, M.; Turner, E.; Yu, L. Fatty Acid Composition, Oxidative Stability, Antioxidant and Antiproliferative Properties of Selected Cold-Pressed Grape Seed Oils and Flours. Food Chem. 2011, 128, 391–399. [Google Scholar] [CrossRef]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s New in Biopotential of Fruit and Vegetable By-Products Applied in the Food Processing Industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- Kapcsándi, V.; Lakatos, E.H.; Sik, B.; Linka, L.Á.; Székelyhidi, R. Antioxidant and Polyphenol Content of Different Vitis vinifera Seed Cultivars and Two Facilities of Production of a Functional Bakery Product. Chem. Pap. 2021, 75, 5711–5717. [Google Scholar] [CrossRef]
- Kuchtová, V.; Kohajdová, Z.; Karovičová, J.; Lauková, M. Physical, Textural and Sensory Properties of Cookies Incorporated with Grape Skin and Seed Preparations. Pol. J. Food Nutr. Sci. 2018, 68, 309–317. [Google Scholar] [CrossRef]
- Kaynarca, G.B.; Kamer, D.D.A.; Yucel, E.; Gümüş, T. Proposed Use of a Polyvinyl Alcohol with Grape Pomace Extract as an Edible Coating for Strawberries. Pol. J. Food Nutr. Sci. 2023, 73, 151–162. [Google Scholar] [CrossRef]
- Vostrejs, P.; Adamcová, D.; Vaverková, M.D.; Enev, V.; Kalina, M.; Machovsky, M.; Šourková, M.; Marova, I.; Kovalcik, A. Active Biodegradable Packaging Films Modified with Grape Seeds Lignin. RSC Adv. 2020, 10, 29202–29213. [Google Scholar] [CrossRef]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape Pomace as a Source of Phenolic Compounds and Diverse Bioactive Properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Jungfer, E.; Ritter, C.; Santiago-Schübel, B.; Thiele, B.; Fett, R.; Galensa, R. Characterization of Flavan-3-ols in Seeds of Grape Pomace by CE, HPLC-DAD-MSn and LC-ESI-FTICR-MS. Food Res. Int. 2012, 48, 848–855. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; García-Villanova, B.; Guerra-Hernández, E.; Verardo, V. Grape Seeds Proanthocyanidins: An Overview of in Vivo Bioactivity in Animal Models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef]
- Pugajeva, I.; Perkons, I.; Górnaś, P. Identification and Determination of Stilbenes by Q-TOF in Grape Skins, Seeds, Juice and Stems. J. Food Compos. Anal. 2018, 74, 44–52. [Google Scholar] [CrossRef]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical Composition, Antioxidant and Antimicrobial Activity of Phenolic Compounds Extracted from Wine Industry By-Products. Food Control 2018, 92, 516–522. [Google Scholar] [CrossRef]
- Gengaihi, S.E.; Ella, F.M.A.; Emad, M.H.; Shalaby, E.; Doha, H. Antioxidant Activity of Phenolic Compounds from Different Grape Wastes. J. Food Process. Technol. 2014, 5, 296. [Google Scholar] [CrossRef]
- Tang, G.Y.; Zhao, C.N.; Liu, Q.; Feng, X.L.; Xu, X.Y.; Cao, S.Y.; Meng, X.; Li, S.; Gan, R.Y.; Li, H.B. Potential of Grape Wastes as a Natural Source of Bioactive Compounds. Molecules 2018, 23, 2598. [Google Scholar] [CrossRef]
- Terra, X.; Pallarés, V.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L.; Blay, M. Modulatory Effect of Grape-Seed Procyanidins on Local and Systemic Inflammation in Diet-Induced Obesity Rats. J. Nutr. Biochem. 2011, 22, 380–387. [Google Scholar] [CrossRef]
- Pascual-Serrano, A.; Bladé, C.; Suárez, M.; Arola-Arnal, A. Grape Seed Proanthocyanidins Improve White Adipose Tissue Expansion during Diet-Induced Obesity Development in Rats. Int. J. Mol. Sci. 2018, 19, 2632. [Google Scholar] [CrossRef]
- Soto, M.U.R.; Brown, K.; Ross, C.F. Antioxidant Activity and Consumer Acceptance of Grape Seed Flour-Containing Food Products. Int. J. Food Sci. Technol. 2012, 47, 592–602. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sineiro, J.; Amado, I.R.; Franco, D. Influence of Natural Extracts on the Shelf Life of Modified Atmosphere-Packaged Pork Patties. Meat Sci. 2014, 96, 526–534. [Google Scholar] [CrossRef]
- Libera, J.; Latoch, A.; Wójciak, K.M. Utilization of Grape Seed Extract as a Natural Antioxidant in the Technology of Meat Products Inoculated with a Probiotic Strain of LAB. Foods 2020, 9, 103. [Google Scholar] [CrossRef]
- Blakeney, M. Food Loss and Food Waste: Causes and Solutions; Edward Elgar Publishing: Cheltenham, UK, 2019; pp. 1–225. [Google Scholar]
- Gomomo, Z.; Fanadzo, M.; Mewa-Ngongang, M.; Hoff, J.W.; van der Rijst, M.; Okudoh, V.I.; Kriel, J.; du Plessis, H.W. Control of Mould Spoilage on Apples Using Yeasts as Biological Control Agents. Pol. J. Food Nutr. Sci. 2022, 72, 119–128. [Google Scholar] [CrossRef]
- Lianou, A.; Panagou, E.Z.; Nychas, G.J.E. Microbiological Spoilage of Foods and Beverages. In The Stability and Shelf Life of Food, 2nd ed.; Subramaniam, P., Ed.; Woodhead Publishing: Duxford, UK, 2016; pp. 3–42. [Google Scholar]
- Kowalska, J.; Maćkiw, E.; Korsak, D.; Postupolski, J. Characteristic and Antimicrobial Resistance of Bacillus Cereus Group Isolated from Food in Poland. Pol. J. Food Nutr. Sci. 2022, 72, 297–304. [Google Scholar] [CrossRef]
- Kirk, M.D.; Angulo, F.J.; Havelaar, A.H.; Black, R.E. Diarrhoeal Disease in Children due to Contaminated Food. Bull. World Health Organ. 2017, 95, 233–234. [Google Scholar] [CrossRef]
- Mattos, G.N.; Tonon, R.V.; Furtado, A.A.L.; Cabral, L.M.C. Grape By-Product Extracts against Microbial Proliferation and Lipid Oxidation: A Review. J. Sci. Food Agric. 2017, 97, 1055–1064. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Igrejas, G.; Gaivão, I.; Aires, A.; Klibi, N.; Dapkevicius, M.d.L.E.; Valentão, P.; Falco, V.; Poeta, P. Valorization of Winemaking By-Products as a Novel Source of Antibacterial Properties: New Strategies to Fight Antibiotic Resistance. Molecules 2021, 26, 2331. [Google Scholar] [CrossRef]
- Chen, Q.; Diao, L.; Song, H.; Zhu, X. Vitis amurensis Rupr: A Review of Chemistry and Pharmacology. Phytomedicine 2018, 49, 111–122. [Google Scholar] [CrossRef]
- Narduzzi, L.; Stanstrup, J.; Mattivi, F. Comparing Wild American Grapes with Vitis vinifera: A Metabolomics Study of Grape Composition. J. Agric. Food Chem. 2015, 63, 6823–6834. [Google Scholar] [CrossRef]
- Howard, J.L. Vitis californica. In Fire Effects Information System; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer): Missoula, MT, USA, 1993; Available online: https://www.fs.usda.gov/database/feis/plants/vine/vitcal/all.html (accessed on 15 June 2023).
- Liang, Z.; Yang, Y.; Cheng, L.; Zhong, G.Y. Characterization of Polyphenolic Metabolites in the Seeds of Vitis Germplasm. J. Agric. Food Chem. 2012, 60, 1291–1299. [Google Scholar] [CrossRef]
- Weidner, S.; Rybarczyk, A.; Karamać, M.; Król, A.; Mostek, A.; Grębosz, J.; Amarowicz, R. Differences in the Phenolic Composition and Antioxidant Properties between Vitis coignetiae and Vitis vinifera Seeds Extracts. Molecules 2013, 18, 3410–3426. [Google Scholar] [CrossRef]
- Weidner, S.; Powałka, A.; Karamać, M.; Amarowicz, R. Extracts of Phenolic Compounds from Seeds of Three Wild Grapevines—Comparison of Their Antioxidant Activities and the Content of Phenolic Compounds. Int. J. Mol. Sci. 2012, 13, 3444–3457. [Google Scholar] [CrossRef]
- Zhu, L.; Li, X.; Hu, X.; Wu, X.; Liu, Y.; Yang, Y.; Zang, Y.; Tang, H.; Wang, C.; Xu, J. Quality Characteristics and Anthocyanin Profiles of Different Vitis amurensis Grape Cultivars and Hybrids from Chinese Germplasm. Molecules 2021, 26, 6696. [Google Scholar] [CrossRef]
- Poudel, P.R.; Tamura, H.; Kataoka, I.; Mochioka, R. Phenolic Compounds and Antioxidant Activities of Skins and Seeds of Five Wild Grapes and Two Hybrids Native to Japan. J. Food Compos. Anal. 2008, 21, 622–625. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Dabić Zagorac, D.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Ž.L.; Natić, M.M. Identification and Quantification of Phenolic Compounds in Berry Skin, Pulp, and Seeds in 13 Grapevine Varieties Grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Selvi, T.; Sakariah, K.K. Antibacterial and Antioxidant Activities of Grape (Vitis vinifera) Seed Extracts. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B. Phenol-Based Antioxidants and the in Vitro Methods Used for Their Assessment. Compr. Rev. Food Sci. Food Saf. 2012, 11, 148–173. [Google Scholar] [CrossRef]
- Krasteva, D.; Ivanov, Y.; Chengolova, Z.; Godjevargova, T. Antimicrobial Potential, Antioxidant Activity, and Phenolic Content of Grape Seed Extracts from Four Grape Varieties. Microorganisms 2023, 11, 395. [Google Scholar] [CrossRef]
- Radulescu, C.; Buruleanu, L.C.; Nicolescu, C.M.; Olteanu, R.L.; Bumbac, M.; Holban, G.C.; Simal-Gandara, J. Phytochemical Profiles, Antioxidant and Antibacterial Activities of Grape (Vitis vinifera L.) Seeds and Skin from Organic and Conventional Vineyards. Plants 2020, 9, 1470. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in Grape Seeds—Biochemistry and Functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef]
- Karamać, M. In-Vitro Study on the Efficacy of Tannin Fractions of Edible Nuts as Antioxidants. Eur. J. Lipid Sci. Technol. 2009, 111, 1063–1071. [Google Scholar] [CrossRef]
- Bauza-Kaszewska, J.; Żary-Sikorska, E.; Gugolek, A.; Ligocka, A.; Kosmala, M.; Karlińska, E.; Fotschki, B.; Juśkiewicz, J. Synergistic Antimicrobial Effect of Raspberry (Rubus idaeus L., Rosaceae) Preparations and Probiotic Bacteria on Enteric Pathogens. Pol. J. Food Nutr. Sci. 2021, 71, 51–59. [Google Scholar] [CrossRef]
- Orak, H.H.; Karamać, M.; Amarowicz, R.; Orak, A.; Janiak, M.A.; Tenikecier, H.S. Variations of Genotypes of Vicia Species as Influenced by Seed Phenolic Compounds and Antioxidant Activity. Zemdirb. Agric. 2022, 109, 35–42. [Google Scholar] [CrossRef]
- Gai, F.; Janiak, M.A.; Sulewska, K.; Peiretti, P.G.; Karamać, M. Phenolic Compound Profile and Antioxidant Capacity of Flax (Linum usitatissimum L.) Harvested at Different Growth Stages. Molecules 2023, 28, 1807. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Oxidants and Antioxidants Part A; Packer, L., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Boudjou, S.; Oomah, B.D.; Zaidi, F.; Hosseinian, F. Phenolics Content and Antioxidant and Anti-Inflammatory Activities of Legume Fractions. Food Chem. 2013, 138, 1543–1550. [Google Scholar] [CrossRef]
- Pozzo, L.; Russo, R.; Frassinetti, S.; Vizzarri, F.; Árvay, J.; Vornoli, A.; Casamassima, D.; Palazzo, M.; Della Croce, C.M.; Longo, V. Wild Italian Prunus spinosa L. Fruit Exerts In Vitro Antimicrobial Activity and Protects against In Vitro and In Vivo Oxidative Stress. Foods 2020, 9, 5. [Google Scholar] [CrossRef]
- Raffaelli, A.; Saba, A. Ion Scanning or Ion Trapping: Why Not Both? Mass Spectrom. Rev. 2021, 42, 1152–1173. [Google Scholar] [CrossRef]
| Grapevine Species | TPC (mg GAE/g) | FRAP (mg TE/g) | DPPH Assay (mg TE/g) | ORAC (mg TE/g) |
|---|---|---|---|---|
| Vitis riparia | 121 ± 12 a | 40.5 ± 2.7 a | 232 ± 21 a | 262 ± 20 a |
| Vitis californica | 97.2 ± 7.3 a,b | 35.5 ± 1.4 a,b | 221 ± 17 a | 252 ± 12 a |
| Vitis amurensis | 46.6 ± 3.0 c | 22.4 ± 2.0 b | 55.2 ± 5.6 c | 141.1 ± 6.2 b |
| Vitis vinifera | 78.7 ± 4.4 b,c | 35.4 ± 1.4 a,b | 154 ± 14 b | 174 ± 13 b |
| E. coli | S. aureus | S. Typhimurium | E. aerogenes | E. faecalis | TPC | FRAP | DPPH Assay | |
|---|---|---|---|---|---|---|---|---|
| S. aureus | −0.617 | |||||||
| S. Typhimurium | −0.838 | 0.926 | ||||||
| E. aerogenes | −0.643 | 0.963 | 0.895 | |||||
| E. faecalis | −0.021 | 0.772 | 0.515 | 0.711 | ||||
| TPC | 0.812 | −0.103 | −0.447 | −0.135 | 0.518 | |||
| FRAP | 0.641 | −0.492 | −0.652 | −0.460 | −0.028 | 0.624 | ||
| DPPH assay | 0.730 | −0.151 | −0.476 | −0.147 | 0.381 | 0.867 | 0.568 | |
| ORAC | 0.601 | 0.093 | −0.261 | 0.082 | 0.637 | 0.926 | 0.554 | 0.853 |
| Compound Name | Acronym | Vitis riparia | Vitis californica | Vitis amurensis | Vitis vinifera |
|---|---|---|---|---|---|
| Gallic acid | GA | 166 ± 32 a | 137 ± 18 a | 44.6 ± 3.3 b | 105.8 ± 5.1 a,b |
| Protocatechuic acid | PRA | 5.75 ± 0.38 b | 6.13 ± 0.13 b | 4.22 ± 0.18 b | 39.93 ± 0.83 a |
| 3-O-Caffeoylquinic acid | 3CQA | 0.102 ± 0.034 b | 0.0574 ± 0.0071 b | 0.207 ± 0.024 a | 0.0838 ± 0.0079 b |
| Caffeic acid | CA | 0.0864 ± 0.0063 a | 0.137 ± 0.026 a | 0.115 ± 0.023 a | 0.220 ± 0.090 a |
| Vanillic acid | VA | 1.418 ± 0.074 c | 1.56 ± 0.16 c | 3.33 ± 0.27 b | 4.25 ± 0.21 a |
| p-Coumaric acid | pCA | 0.495 ± 0.039 c | 0.537 ± 0.016 c | 0.835 ± 0.019 b | 0.960 ± 0.023 a |
| trans-Ferulic acid | tFA | 0.225 ± 0.058 b | 0.239 ± 0.049 b | 0.607 ± 0.036 a | 0.267 ± 0.034 b |
| 2,3-Dicaffeoyl-tartaric acid | DCT | 0.0423 ± 0.0029 a | 0.0280 ± 0.0041 b | 0.0111 ± 0.0033 c | 0.0037 ± 0.0005 c |
| ∑ Phenolic acids | 174.45 | 145.40 | 53.89 | 151.48 | |
| Quercetin | Q | 3.76 ± 0.36 b | 2.03 ± 0.17 b | 0.215 ± 0.026 b | 23.8 ± 2.9 a |
| Quercetin 3-O-glucoside | Q3G | 0.0752 ± 0.0023 a | 0.0600 ± 0.0036 b | 0.0383 ± 0.0037 c | 0.0355 ± 0.0036 c |
| Quercetin 3-O-rutinoside | Q3R | 0.705 ± 0.020 a | 0.446 ± 0.011 b | 0.1710 ± 0.0080 c | 0.0865 ± 0.0047 d |
| Quercetin 3,4-O-diglucoside | QDG | 0.0667 ± 0.0094 a | 0.0910 ± 0.0084 a | 0.0932 ± 0.0085 a | 0.0766 ± 0.0043 a |
| Quercetagetin 7-O-glucoside | QA7G | 0.471 ± 0.010 b | 0.695 ± 0.022 a | 0.120 ± 0.017 c | 0.0785 ± 0.0033 c |
| Kaempferol 7-O-glucoside | K7G | 0.1046 ± 0.0096 a | 0.1099 ± 0.0082 a | 0.0646 ± 0.0040 b | 0.0747 ± 0.0027 b |
| Kaempferol 3-O-glucoside | K3G | 0.312 ± 0.011 a | 0.217 ± 0.014 b | 0.0414 ± 0.0026 c | 0.0539 ± 0.0025 c |
| Kaempferol 3-O-rutinoside | K3R | 0.098 ± 0.013 a | 0.0679 ± 0.0044 a | 0.0057 ± 0.0012 b | 0.0054 ± 0.0011 b |
| ∑ Flavonols | 5.59 | 3.72 | 0.75 | 24.20 | |
| Cyanidin 3-O-glucoside | C3G | 0.1360 ± 0.0033 a | 0.0699 ± 0.0065 b | 0.0058 ± 0.0003 c | 0.0038 ± 0.0003 c |
| Cyanidin 3,5-O-diglucoside | CDG | 0.809 ± 0.020 a | 0.381 ± 0.018 b | 0.0166 ± 0.0029 c | 0.0024 ± 0.0005 c |
| Delphinidin 3-O-glucoside | D3G | 0.433 ± 0.027 a | 0.3411 ± 0.0090 b | 0.0163 ± 0.0025 c | 0.0242 ± 0.0033 c |
| Peonidin 3,5-O-diglucoside | PDG | 1.128 ± 0.088 a | 0.719 ± 0.014 b | 0.184 ± 0.012 c | 0.0041 ± 0.0004 c |
| Malvidin 3-O-glucoside | M3G | 0.350 ± 0.041 a | 0.2131 ± 0.0049 b | 0.0179 ± 0.0017 c | 0.0391 ± 0.0033 c |
| Malvidin 3,5-O-diglucoside | MDG | 4.41 ± 0.17 a | 2.384 ± 0.083 b | 0.290 ± 0.025 c | 0.0170 ± 0.0033 c |
| Petunidin 3-O-glucoside | Pt3G | 0.1630 ± 0.0041 a | 0.0832 ±0.0041 b | 0.0022 ± 0.0005 c | 0.0044 ± 0.0005 c |
| ∑ Anthocyanins | 7.43 | 4.19 | 0.53 | 0.10 | |
| (+)-Catechin | C | 246 ± 35 a | 206 ± 16 a | 168 ± 15 a | 69.8 ± 3.3 b |
| (−)-Epicatechin | EC | 117 ± 15 a | 90.7 ± 3.4 a | 107.2 ± 8.4 a | 29.4 ± 2.5 b |
| ∑ Flavan-3-ols | 363.17 | 296.24 | 275.13 | 99.18 | |
| Procyanidin B1 | PCB1 | 51.9 ± 2.4 a | 40.6 ± 2.5 b | 4.32 ± 0.35 c | 13.4 ± 2.2 c |
| Procyanidin B2 | PCB2 | 124 ± 11 a | 104 ± 13 a,b | 35.8 ± 3.3 c | 69.3 ± 4.5 b,c |
| Procyanidin B3 | PCB3 | 46.3 ± 3.1 a | 34.0 ± 3.3 b | 5.73 ± 0.53 c | 9.42 ± 0.85 c |
| ∑ Procyanidins | 222.25 | 178.88 | 45.85 | 92.12 | |
| Resveratrol 3-O-glucoside | R3G | 2.72 ± 0.39 a | 1.87 ± 0.23 a | 0.437 ± 0.025 b | 0.652 ± 0.030 b |
| Resveratrol | RES | 3.25 ± 0.21 a | 1.559 ± 0.065 b | 0.566 ± 0.030 c | 1.08 ± 0.17 b,c |
| ∑ Stilbenoids | 6.97 | 3.43 | 1.00 | 1.72 | |
| Hydroxytyrosol | HYT | 0.560 ± 0.033 a | 0.378 ± 0.022 b | 0.144 ± 0.011 c | 0.0395 ± 0.0037 d |
| Verbascoside | VER | 0.219 ± 0.017 a | 0.1705 ± 0.0084 b | 0.0741 ± 0.0040 c | 0.0123 ± 0.0029 d |
| Oleuropein | OLE | 0.1089 ± 0.0074 a | 0.0504 ± 0.0029 b,c | 0.0638 ± 0.0036 b | 0.0297 ± 0.0041 c |
| Ligstroside | LIG | 0.0078 ± 0.0014 a | 0.0066 ± 0.0012 a | 0.0074 ± 0.0009 a | 0.0032 ± 0.0004 a |
| Phloridzin | PHZ | 2.72 ± 0.17 a | 2.425 ± 0.062 a | 2.77 ± 0.16 a | 0.198 ± 0.025 b |
| Phloretin | PHL | 0.0304 ± 0.0029 a | 0.0169 ± 0.0049 b | 0.0030 ± 0.0013 c | 0.0027 ± 0.0006 c |
| Luteolin | LUT | 0.0351 ± 0.0048 b | 0.0165 ± 0.0029 c | 0.0051 ± 0.0010 c | 0.0741 ± 0.0033 a |
| Eriodictyol | ERI | 0.0895 ± 0.0090 a | 0.101 ± 0.015 a | 0.0395 ± 0.0030 b | 0.0343 ± 0.0047 b |
| Naringenin | NAR | 0.105 ± 0.016 a | 0.118 ± 0.033 a | 0.111 ± 0.031 a | 0.1274 ± 0.0090 a |
| ∑ Others | 3.87 | 3.28 | 3.21 | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozzo, L.; Grande, T.; Raffaelli, A.; Longo, V.; Weidner, S.; Amarowicz, R.; Karamać, M. Characterization of Antioxidant and Antimicrobial Activity and Phenolic Compound Profile of Extracts from Seeds of Different Vitis Species. Molecules 2023, 28, 4924. https://doi.org/10.3390/molecules28134924
Pozzo L, Grande T, Raffaelli A, Longo V, Weidner S, Amarowicz R, Karamać M. Characterization of Antioxidant and Antimicrobial Activity and Phenolic Compound Profile of Extracts from Seeds of Different Vitis Species. Molecules. 2023; 28(13):4924. https://doi.org/10.3390/molecules28134924
Chicago/Turabian StylePozzo, Luisa, Teresa Grande, Andrea Raffaelli, Vincenzo Longo, Stanisław Weidner, Ryszard Amarowicz, and Magdalena Karamać. 2023. "Characterization of Antioxidant and Antimicrobial Activity and Phenolic Compound Profile of Extracts from Seeds of Different Vitis Species" Molecules 28, no. 13: 4924. https://doi.org/10.3390/molecules28134924
APA StylePozzo, L., Grande, T., Raffaelli, A., Longo, V., Weidner, S., Amarowicz, R., & Karamać, M. (2023). Characterization of Antioxidant and Antimicrobial Activity and Phenolic Compound Profile of Extracts from Seeds of Different Vitis Species. Molecules, 28(13), 4924. https://doi.org/10.3390/molecules28134924









