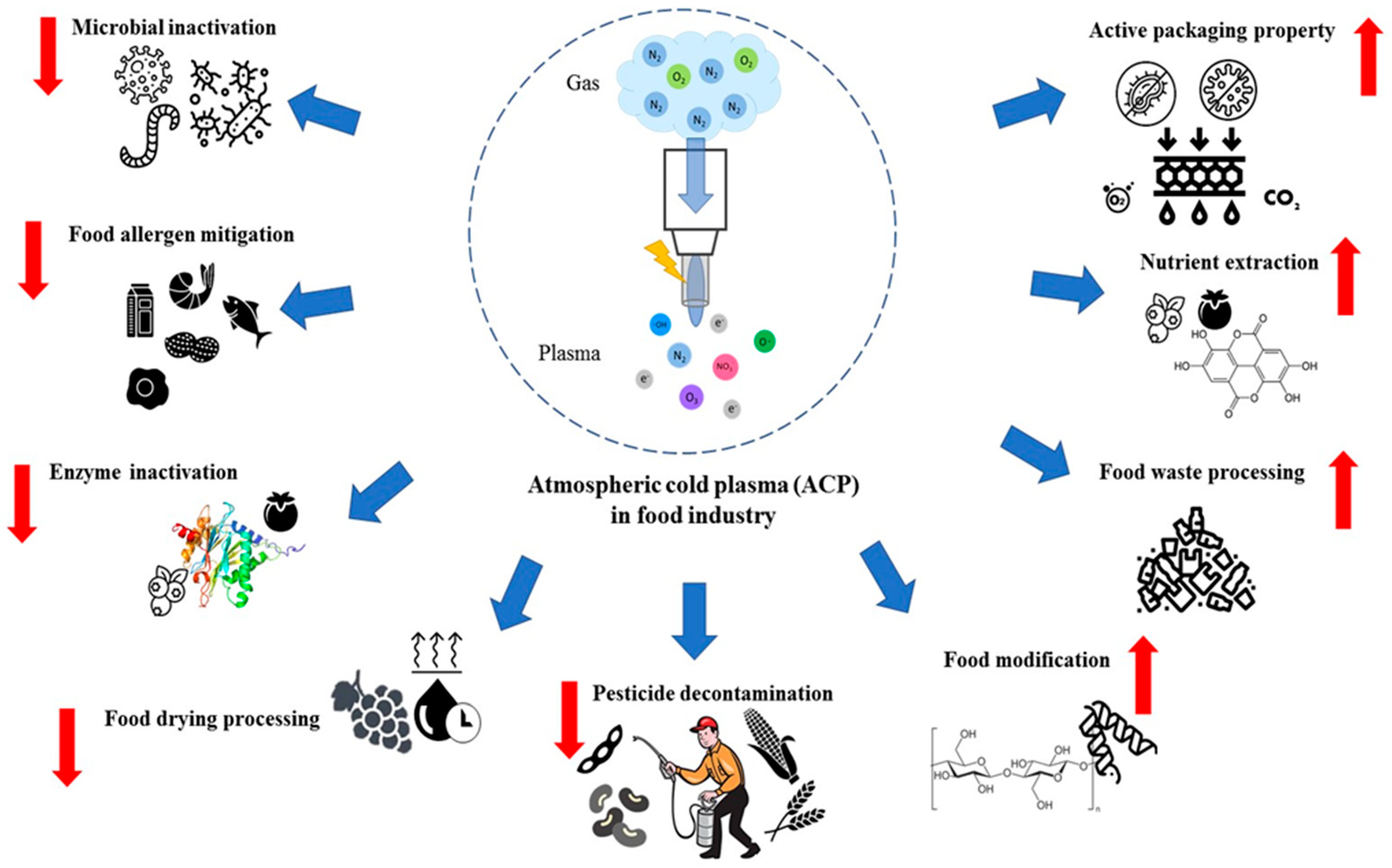
Current and Potential Applications of Atmospheric Cold Plasma in the Food Industry
Abstract
1. Introduction
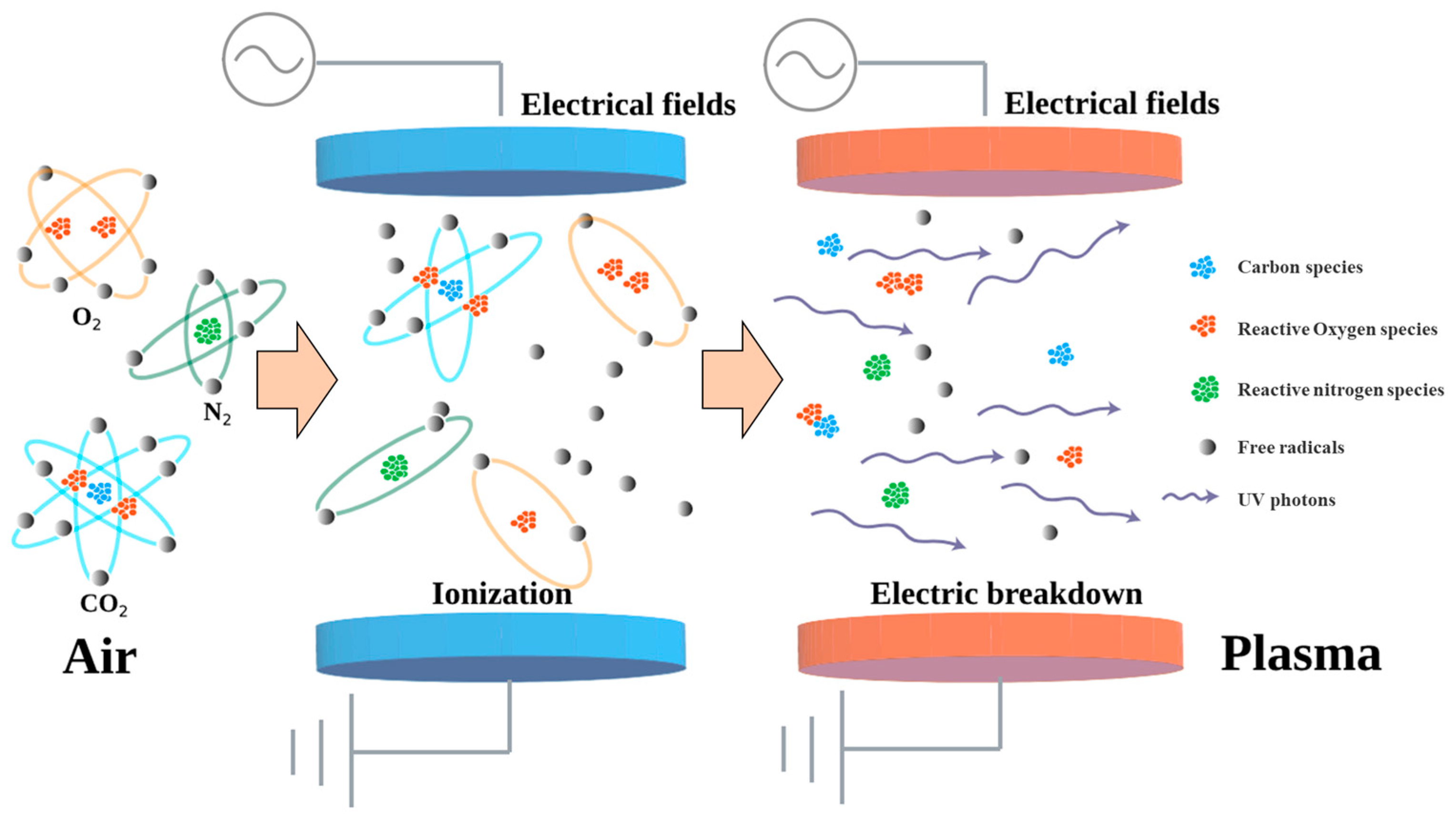
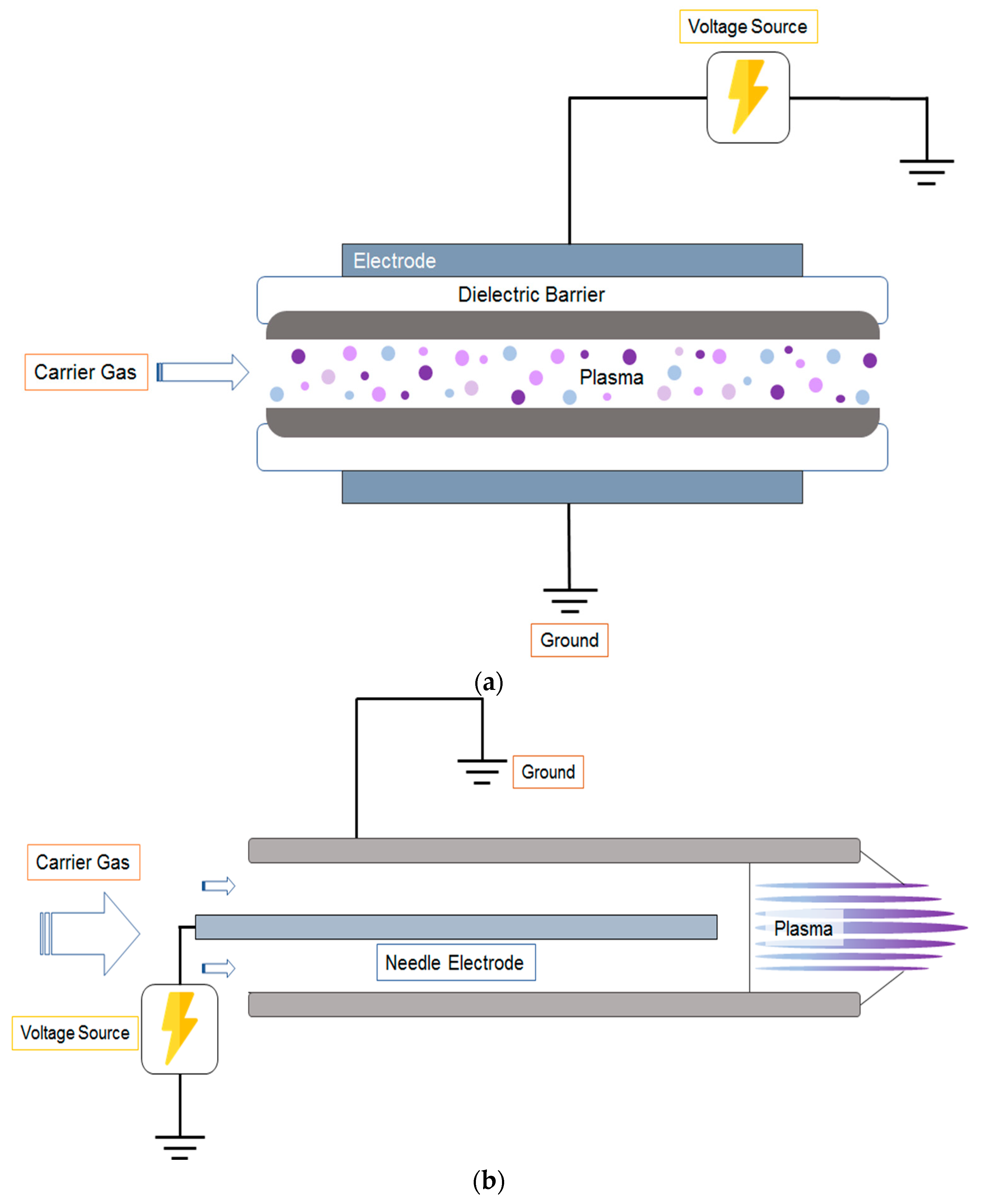
2. Mechanism of ACP
3. Atmospheric Cold Plasma (ACP) in Food Technology
3.1. Microbial Inactivation
3.2. Active Food Packaging
3.3. Food Allergen Mitigation
3.4. Enzyme Inactivation
| Enzyme Inactivation | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Food Product | Plasma Device | Enzymes | Reduction of Enzyme Activity | Parameters | Reference | ||||
| Exposure Time (s) | Exposure Distance (mm) | Input Power (W) | Voltage (kV) | Frequency | |||||
| White mushroom | DBD | β-1,3-glucanase, MDA, and PPO | 46.2%; 47.5%; 42.0% | 60 | 100 | 30 | - | 13.6 MHz | [7] |
| Mushroom (Agaricus bisporus) | DBD | PPO | 70.0% | 600 | 38 | - | 50 | - | [54] |
| Milk | DBD | ALP | 50.0% | 120 | 40 | - | 60 | 50.0 Hz | [55] |
| Wheatgerm | DBD | Lipase and lipoxygenase | 25.0%; 50.0% | 1500 | 20 | - | 24 | 50.0 Hz | [53] |
| Hen egg white | DBD | Lysozyme | 50.0% | 720 | 3 | - | 0.14 | 16.0 kHz | [56] |
| Hen egg white | Plasma jet | Lysozyme | 60.0% | 720 | 6 | - | 0.08 | 24.0 kHz | [56] |
| Fresh-cut melon | DBD | POD and PME | 17.0%; 7.0% | 900 | 5 | - | 15 | 12.5 kHz | [57] |
| Bananas | DBD | POD and PPO | 64.4%; 62.6% | 120 | 6 | - | 0.040 | 10.0 kHz | [58] |
3.5. Food Drying Pre-Treatment
3.6. Pesticide Decontamination
| Pesticide Decontamination | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Food Product | Plasma Device | Pesticide Active Ingredients | Degradation of Pesticides | Parameters | Reference | ||||
| Time (s) | Distance (mm) | Input Power (W) | Voltage (kV) | Frequency | |||||
| Strawberries | DBD | Azoxystrobin, cyprodinil, fludioxonil, and pyriproxyfen | 69%; 45%; 71%; 46% | 300 | 40 | - | 80 | 50.0 Hz | [75] |
| Blueberries | DBD | Boscalid and imidacloprid | 80.2%; 75.6% | 300 | 40 | - | 80 | 50.0 Hz | [76] |
| Corn | DBD | Chlorpyrifos and carbaryl | 86.2%; 66.6% | 60 | 6 | 20 | - | 12.0 kHz | [73] |
| Mango | Gliding arc | Chlorpyrifos and cypermethrin | 74%; 62.9% | 300 | 2.5 | 600 | 8 | - | [77] |
| Apple | DBD | Chlorpyrifos and diazinon | 87.0%; 87.4% | 600 | 7 | - | 13 | 13.0 kHz | [74] |
| Cucumber | DBD | Chlorpyrifos and diazinon | 33.7%; 82.2% | 600 | 7 | - | 13 | 13.0 kHz | [74] |
| Lettuce | DBD | Chlorpyrifos and malathion | 51.4%; 53.1% | 120 | 35 | - | 80 | 50.0 Hz | [78] |
3.7. Food Modification
| Food Modification | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Food Product | Plasma Device | Modification | Results | Parameters | Reference | ||||
| Time (s) | Distance (mm) | Input Power (W) | Voltage (kV) | Frequency | |||||
| Fenugreek | DBD | Galactomannand yield | 122% | 1800 | 40 | - | 80 | 60 Hz | [82] |
| Maize | DBD | Increase in crystallinity | 36.90% | 600 | 5 | - | 0.138 | 50 Hz | [83] |
| Wheat | DBD | Increase in viscosity | 17.60% | 1800 | 30 | - | 80 | 50 Hz | [81] |
| Whey protein isolate | DBD | Emulsification enhancement | 25.00% | 300 | 44 | - | 70 | - | [84] |
| Xanthan gum | SBD | Increase in viscosity | 40.00% | 1800 | 53 | 250 | 3.5 | 15 kHz | [85] |
| Pomegranate juice | Plasma jet | Increase in phenolic compounds | 33.00% | 300 | 22 | 6 | 2.5 | 25 kHz | [86] |
| White grapes | Plasma jet | Drying speed | 20.00% | 36,000 | 10 | 500 | - | 25 kHz | [61] |
| Chili pepper | Gliding arc | Drying speed | 16.70% | 30 | 60 | 750 | - | 20 kHz | [64] |
| Wolfberry | Gliding arc | Drying speed | 14.10% | 60 | 60 | 750 | - | 20 kHz | [68] |
3.8. Nutrient Extraction
3.9. Food Waste Processing
| Food Waste Processing | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Food Waste | Plasma Device | Products | Results | Parameters | Reference | |||||
| Gas Type | Time (s) | Distance (mm) | Input Power (W) | Voltage (V) | Frequency (Hz) | |||||
| Grape pomace | DBD | Phenolic compounds | 22.8% | Air | 900 | 52 | - | 120 | 60 | [96] |
| Pineapple peel | DBD | Bacterial cellulose | 3.82 g/L | Ar, Air | 900 | 10 | 600 | - | - | [111] |
| Sugarcane bagasse | Plasma jet | Bioethanol production | 38.5% | Ar | 1500 | 10 | 80–200 | - | - | [109] |
| Bacterial cellulose | 1.68 g/L | Ar | 1500 | 10 | 200 | - | - | [110] | ||
| Wheat straw | DBD | Methane | 45.0% | Air | 3600 | 20 | 230 | - | 10 kHz | [112] |
4. Challenges of ACP in Food Industry
5. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Loke, X.-J.; Chang, C.-K.; Hou, C.-Y.; Cheng, K.-C.; Hsieh, C.-W. Plasma-treated polyethylene coated with polysaccharide and protein containing cinnamaldehyde for active packaging films and applications on tilapia (Orechromis niloticus) fillet preservation. Food Control 2021, 125, 108016. [Google Scholar] [CrossRef]
- Moradi, E.; Moosavi, M.H.; Hosseini, S.M.; Mirmoghtadaie, L.; Moslehishad, M.; Khani, M.R.; Jannatyha, N.; Shojaee-Aliabadi, S. Prolonging shelf life of chicken breast fillets by using plasma-improved chitosan/low density polyethylene bilayer film containing summer savory essential oil. Int. J. Biol. Macromol. 2020, 156, 321–328. [Google Scholar] [CrossRef]
- Bermudez-Aguirre, D. Advances in Cold Plasma Applications for Food Safety and Preservation; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Contini, C.; Katsikogianni, M.G.; O’Neill, F.; O’Sullivan, M.; Boland, F.; Dowling, D.; Monahan, F. Storage stability of an antioxidant active packaging coated with citrus extract following a plasma jet pretreatment. Food Bioprocess Technol. 2014, 7, 2228–2240. [Google Scholar] [CrossRef]
- Kang, J.H.; Jeon, Y.J.; Min, S.C. Effects of packaging parameters on the microbial decontamination of Korean steamed rice cakes using in-package atmospheric cold plasma treatment. Food Sci. Biotechnol. 2021, 30, 1535–1542. [Google Scholar] [CrossRef]
- Oldham, C.J. Applications of Atmospheric Plasmas; North Carolina State University: Raleigh, NC, USA, 2009. [Google Scholar]
- Chang, C.-K.; Cheng, K.-C.; Hou, C.-Y.; Wu, Y.-S.; Hsieh, C.-W. Development of Active Packaging to Extend the Shelf Life of Agaricus bisporus by Using Plasma Technology. Polymers 2021, 13, 2120. [Google Scholar] [CrossRef] [PubMed]
- Dobslaw, C.; Glocker, B. Plasma Technology and Its Relevance in Waste Air and Waste Gas Treatment. Sustainability 2020, 12, 8981. [Google Scholar] [CrossRef]
- Misra, N.N.; Schlüter, O.; Cullen, P.J. Chapter 1—Plasma in Food and Agriculture. In Cold Plasma in Food and Agriculture; Academic Press: San Diego, CA, USA, 2016; pp. 1–16. [Google Scholar]
- Lin, S.-P.; Khumsupan, D.; Chou, Y.-J.; Hsieh, K.-C.; Hsu, H.-Y.; Ting, Y.; Cheng, K.-C. Applications of atmospheric cold plasma in agricultural, medical, and bioprocessing industries. Appl. Microbiol. Biotechnol. 2022, 106, 7737–7750. [Google Scholar] [CrossRef]
- Conrads, H.; Schmidt, M. Plasma generation and plasma sources. Plasma Sources Sci. Technol. 2000, 9, 441. [Google Scholar] [CrossRef]
- Mouele, E.S.M.; Tijani, J.O.; Badmus, K.O.; Pereao, O.; Babajide, O.; Fatoba, O.O.; Zhang, C.; Shao, T.; Sosnin, E.; Tarasenko, V. A critical review on ozone and co-species, generation and reaction mechanisms in plasma induced by dielectric barrier discharge technologies for wastewater remediation. J. Environ. Chem. Eng. 2021, 9, 105758. [Google Scholar] [CrossRef]
- Fröhling, A.; Durek, J.; Schnabel, U.; Ehlbeck, J.; Bolling, J.; Schlüter, O. Indirect plasma treatment of fresh pork: Decontamination efficiency and effects on quality attributes. Innov. Food Sci. Emerg. Technol. 2012, 16, 381–390. [Google Scholar] [CrossRef]
- Nasiru, M.M.; Frimpong, E.B.; Muhammad, U.; Qian, J.; Mustapha, A.T.; Yan, W.; Zhuang, H.; Zhang, J. Dielectric barrier discharge cold atmospheric plasma: Influence of processing parameters on microbial inactivation in meat and meat products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2626–2659. [Google Scholar] [CrossRef]
- Varilla, C.; Marcone, M.; Annor, G.A. Potential of cold plasma technology in ensuring the safety of foods and agricultural produce: A review. Foods 2020, 9, 1435. [Google Scholar] [CrossRef]
- Hensel, K.; Kučerová, K.; Tarabová, B.; Janda, M.; Machala, Z.; Sano, K.; Mihai, C.T.; Ciorpac, M.; Gorgan, L.D.; Jijie, R.; et al. Effects of air transient spark discharge and helium plasma jet on water, bacteria, cells, and biomolecules. Biointerphases 2015, 10, 029515. [Google Scholar] [CrossRef]
- Fridman, A.; Nester, S.; Kennedy, L.A.; Saveliev, A.; Mutaf-Yardimci, O. Gliding arc gas discharge. Prog. Energy Combust. Sci. 1999, 25, 211–231. [Google Scholar] [CrossRef]
- Ivanov, V.; Paunska, T.; Lazarova, S.; Bogaerts, A.; Kolev, S. Gliding arc/glow discharge for CO2 conversion: Comparing the performance of different discharge configurations. J. CO2 Util. 2023, 67, 102300. [Google Scholar] [CrossRef]
- Hertrich, S.M.; Boyd, G.; Sites, J.; Niemira, B.A. Cold Plasma Inactivation of Salmonella in Prepackaged, Mixed Salads Is Influenced by Cross-Contamination Sequence. J. Food Prot. 2017, 80, 2132–2136. [Google Scholar] [CrossRef] [PubMed]
- Kilonzo-Nthenge, A.; Liu, S.; Yannam, S.; Patras, A. Atmospheric Cold Plasma Inactivation of Salmonella and Escherichia coli on the Surface of Golden Delicious Apples. Front. Nutr. 2018, 5, 120. [Google Scholar] [CrossRef]
- Roh, S.H.; Oh, Y.J.; Lee, S.Y.; Kang, J.H.; Min, S.C. Inactivation of Escherichia coli O157:H7, Salmonella, Listeria monocytogenes, and Tulane virus in processed chicken breast via atmospheric in-package cold plasma treatment. LWT 2020, 127, 109429. [Google Scholar] [CrossRef]
- Mahnot, N.K.; Mahanta, C.L.; Farkas, B.E.; Keener, K.M.; Misra, N.N. Atmospheric cold plasma inactivation of Escherichia coli and Listeria monocytogenes in tender coconut water: Inoculation and accelerated shelf-life studies. Food Control 2019, 106, 106678. [Google Scholar] [CrossRef]
- Wang, J.; Fu, T.; Wang, Y.; Zhang, J. Effects of High-Voltage Atmospheric Cold Plasma Treatment on Microbiological and Quality Characters of Tilapia Fillets. Foods 2022, 11, 2398. [Google Scholar] [CrossRef]
- Lacombe, A.; Niemira, B.A.; Gurtler, J.B.; Sites, J.; Boyd, G.; Kingsley, D.H.; Li, X.; Chen, H. Nonthermal inactivation of norovirus surrogates on blueberries using atmospheric cold plasma. Food Microbiol. 2017, 63, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Abbaszadeh, S.; Salari, A. Optimization of decontamination conditions for Aspergillus flavus inoculated to military rations snack and physicochemical properties with atmospheric cold plasma. J. Food Saf. 2020, 40, e12850. [Google Scholar] [CrossRef]
- Devi, Y.; Thirumdas, R.; Sarangapani, C.; Deshmukh, R.; Annapure, U. Influence of cold plasma on fungal growth and aflatoxins production on groundnuts. Food Control 2017, 77, 187–191. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lin, C.-H.; Aljuffali, I.A.; Fang, J.-Y. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef]
- Eng, S.-K.; Pusparajah, P.; Ab Mutalib, N.-S.; Ser, H.-L.; Chan, K.-G.; Lee, L.-H. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States--major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Camargo, A.C.; Woodward, J.J.; Nero, L.A. The continuous challenge of characterizing the foodborne pathogen Listeria monocytogenes. Foodborne Pathog. Dis. 2016, 13, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Sarangapani, C.; Patange, A.; Bourke, P.; Keener, K.; Cullen, P.J. Recent Advances in the Application of Cold Plasma Technology in Foods. Annu. Rev. Food Sci. Technol. 2018, 9, 609–629. [Google Scholar] [CrossRef]
- Li, X.; Ye, M.; Neetoo, H.; Golovan, S.; Chen, H. Pressure inactivation of Tulane virus, a candidate surrogate for human norovirus and its potential application in food industry. Int. J. Food Microbiol. 2013, 162, 37–42. [Google Scholar] [CrossRef]
- Filipić, A.; Gutierrez-Aguirre, I.; Primc, G.; Mozetič, M.; Dobnik, D. Cold plasma, a new hope in the field of virus inactivation. Trends Biotechnol. 2020, 38, 1278–1291. [Google Scholar] [CrossRef]
- Ryu, Y.-H.; Kim, Y.-H.; Lee, J.-Y.; Shim, G.-B.; Uhm, H.-S.; Park, G.; Choi, E.H. Effects of Background Fluid on the Efficiency of Inactivating Yeast with Non-Thermal Atmospheric Pressure Plasma. PLoS ONE 2013, 8, e66231. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar-Mathur, P.; Sunkara, S.; Bhatnagar-Panwar, M.; Waliyar, F.; Sharma, K.K. Biotechnological advances for combating Aspergillus flavus and aflatoxin contamination in crops. Plant Sci. 2015, 234, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Brody, A.L.; Strupinsky, E.; Kline, L.R. Active Packaging for Food Applications; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Wong, L.-W.; Loke, X.-J.; Chang, C.-K.; Ko, W.-C.; Hou, C.-Y.; Hsieh, C.-W. Use of the plasma-treated and chitosan/gallic acid-coated polyethylene film for the preservation of tilapia (Orechromis niloticus) fillets. Food Chem. 2020, 329, 126989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Application of cold plasma in food packaging. In Applications of Cold Plasma in Food Safety; Springer: Berlin/Heidelberg, Germany, 2022; pp. 309–324. [Google Scholar]
- Guragain, R.P.; Baniya, H.B.; Dhungana, S.; Chhetri, G.K.; Gautam, S.; Pandey, B.P.; Joshi, U.M.; Subedi, D.P. Improvement of hydrophilicity of polypropylene film by dielectric barrier discharge generated in air at atmospheric pressure. Rev. Adhes. Adhes. 2021, 9, 153. [Google Scholar]
- Hosseini, S.; Kadivar, M.; Shekarchizadeh, H.; Abaee, M.S.; Alsharif, M.A.; Karevan, M. Cold plasma treatment to prepare active polylactic acid/ethyl cellulose film using wheat germ peptides and chitosan. Int. J. Biol. Macromol. 2022, 223, 1420–1431. [Google Scholar] [CrossRef]
- Wang, L.-J.; Mu, S.-C.; Lin, M.-I.; Sung, T.-C.; Chiang, B.-L.; Lin, C.-H. Clinical Manifestations of Pediatric Food Allergy: A Contemporary Review. Clin. Rev. Allergy Immunol. 2021, 62, 180–199. [Google Scholar] [CrossRef]
- Valenta, R.; Hochwallner, H.; Linhart, B.; Pahr, S. Food allergies: The basics. Gastroenterology 2015, 148, 1120–1131.e4. [Google Scholar] [CrossRef]
- Rahman, S.S.A.; Vaishnavi, T.; Vidyasri, G.S.; Sathya, K.; Priyanka, P.; Venkatachalam, P.; Karuppiah, S. Production of bacterial cellulose using Gluconacetobacter kombuchae immobilized on Luffa aegyptiaca support. Sci. Rep. 2021, 11, 2912. [Google Scholar] [CrossRef]
- Ng, S.W.; Lu, P.; Rulikowska, A.; Boehm, D.; O’Neill, G.; Bourke, P. The effect of atmospheric cold plasma treatment on the antigenic properties of bovine milk casein and whey proteins. Food Chem. 2021, 342, 128283. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Liu, L.-J.; Zhou, Y.-X.; Tan, Y.-C.; Cheng, J.-H.; Bekhit, A.E.-D.; Inam-Ur-Raheem, M.; Aadil, R.M. Dielectric-barrier discharge (DBD) plasma treatment reduces IgG binding capacity of β-lactoglobulin by inducing structural changes. Food Chem. 2021, 358, 129821. [Google Scholar] [CrossRef]
- Venkataratnam, H.; Cahill, O.; Sarangapani, C.; Cullen, P.; Barry-Ryan, C. Impact of cold plasma processing on major peanut allergens. Sci. Rep. 2020, 10, 17038. [Google Scholar] [CrossRef] [PubMed]
- Meinlschmidt, P.; Ueberham, E.; Lehmann, J.; Reineke, K.; Schlüter, O.; Schweiggert-Weisz, U.; Eisner, P. The effects of pulsed ultraviolet light, cold atmospheric pressure plasma, and gamma-irradiation on the immunoreactivity of soy protein isolate. Innov. Food Sci. Emerg. Technol. 2016, 38, 374–383. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Zhou, Y.-X.; Wang, F.; Tan, Y.-C.; Cheng, J.-H.; Bekhit, A.E.-D.; Aadil, R.M.; Liu, X.-B. Oxidation induced by dielectric barrier discharge (DBD) plasma treatment reduces IgG/IgE binding capacity and improves the functionality of glycinin. Food Chem. 2021, 363, 130300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, Z.; Zhang, J.; Nasiru, M.M.; Wang, Y.; Fu, L. Atmospheric cold plasma treatment of soybean protein isolate: Insights into the structural, physicochemical, and allergenic characteristics. J. Food Sci. 2021, 86, 68–77. [Google Scholar] [CrossRef]
- Sharma, S.; Prabhakar, H.; Singh, R.K. Atmospheric Cold Plasma-Induced Changes in Milk Proteins. Food Bioprocess Technol. 2022, 15, 2737–2748. [Google Scholar] [CrossRef]
- Liburdi, K.; Benucci, I.; Esti, M. Lysozyme in Wine: An Overview of Current and Future Applications. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1062–1073. [Google Scholar] [CrossRef]
- Takai, E.; Kitano, K.; Kuwabara, J.; Shiraki, K. Protein Inactivation by Low-temperature Atmospheric Pressure Plasma in Aqueous Solution. Plasma Process. Polym. 2012, 9, 77–82. [Google Scholar] [CrossRef]
- Tolouie, H.; Mohammadifar, M.A.; Ghomi, H.; Yaghoubi, A.S.; Hashemi, M. The impact of atmospheric cold plasma treatment on inactivation of lipase and lipoxygenase of wheat germs. Innov. Food Sci. Emerg. Technol. 2018, 47, 346–352. [Google Scholar] [CrossRef]
- Zhu, Y.; Elliot, M.; Zheng, Y.; Chen, J.; Chen, D.; Deng, S. Aggregation and conformational change of mushroom (Agaricus bisporus) polyphenol oxidase subjected to atmospheric cold plasma treatment. Food Chem. 2022, 386, 132707. [Google Scholar] [CrossRef]
- Segat, A.; Misra, N.N.; Cullen, P.J.; Innocente, N. Effect of atmospheric pressure cold plasma (ACP) on activity and structure of alkaline phosphatase. Food Bioprod. Process. 2016, 98, 181–188. [Google Scholar] [CrossRef]
- Choi, S.; Attri, P.; Lee, I.; Oh, J.; Yun, J.-H.; Park, J.H.; Choi, E.H.; Lee, W. Structural and functional analysis of lysozyme after treatment with dielectric barrier discharge plasma and atmospheric pressure plasma jet. Sci. Rep. 2017, 7, 1027. [Google Scholar] [CrossRef] [PubMed]
- Tappi, S.; Gozzi, G.; Vannini, L.; Berardinelli, A.; Romani, S.; Ragni, L.; Rocculi, P. Cold plasma treatment for fresh-cut melon stabilization. Innov. Food Sci. Emerg. Technol. 2016, 33, 225–233. [Google Scholar] [CrossRef]
- Gu, Y.; Shi, W.; Liu, R.; Xing, Y.; Yu, X.; Jiang, H. Cold plasma enzyme inactivation on dielectric properties and freshness quality in bananas. Innov. Food Sci. Emerg. Technol. 2021, 69, 102649. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Shen, J.; Li, X.; Ding, L.; Ma, J.; Lan, Y.; Xia, W.; Cheng, C.; Sun, Q.; et al. Effects and Mechanism of Atmospheric-Pressure Dielectric Barrier Discharge Cold Plasmaon Lactate Dehydrogenase (LDH) Enzyme. Sci. Rep. 2015, 5, 10031. [Google Scholar] [CrossRef]
- Bermúdez-Aguirre, D.; Guerrero-Beltrán, J.Á.; Barbosa-Cánovas, G.V.; Welti-Chanes, J. Study of the inactivation of Escherichia coli and pectin methylesterase in mango nectar under selected high hydrostatic pressure treatments. Food Sci. Technol. Int. 2011, 17, 541–547. [Google Scholar] [CrossRef]
- Huang, C.-C.; Wu, J.S.-B.; Wu, J.-S.; Ting, Y. Effect of novel atmospheric-pressure jet pretreatment on the drying kinetics and quality of white grapes. J. Sci. Food Agric. 2019, 99, 5102–5111. [Google Scholar] [CrossRef]
- Ashtiani, S.-H.M.; Rafiee, M.; Morad, M.M.; Khojastehpour, M.; Khani, M.R.; Rohani, A.; Shokri, B.; Martynenko, A. Impact of gliding arc plasma pretreatment on drying efficiency and physicochemical properties of grape. Innov. Food Sci. Emerg. Technol. 2020, 63, 102381. [Google Scholar] [CrossRef]
- Li, S.; Chen, S.; Liang, Q.; Ma, Z.; Han, F.; Xu, Y.; Jin, Y.; Wu, W. Low temperature plasma pretreatment enhances hot-air drying kinetics of corn kernels. J. Food Process. Eng. 2019, 42, e13195. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Zhong, C.-S.; Mujumdar, A.S.; Yang, X.-H.; Deng, L.-Z.; Wang, J.; Xiao, H.-W. Cold plasma pretreatment enhances drying kinetics and quality attributes of chili pepper (Capsicum annuum L.). J. Food Eng. 2019, 241, 51–57. [Google Scholar] [CrossRef]
- Karim, N.; Shishir, M.R.I.; Bao, T.; Chen, W. Effect of cold plasma pretreated hot-air drying on the physicochemical characteristics, nutritional values and antioxidant activity of shiitake mushroom. J. Sci. Food Agric. 2021, 101, 6271–6280. [Google Scholar] [CrossRef]
- Loureiro, A.d.C.; Souza, F.d.C.d.A.; Sanches, E.A.; Bezerra, J.d.A.; Lamarão, C.V.; Rodrigues, S.; Fernandes, F.A.; Campelo, P.H. Cold plasma technique as a pretreatment for drying fruits: Evaluation of the excitation frequency on drying process and bioactive compounds. Food Res. Int. 2021, 147, 110462. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Hao, X.; Shishir, M.R.I.; Karim, N.; Chen, W. Green alternative methods for pretreatment of whole jujube before the drying process. J. Sci. Food Agric. 2021, 102, 1030–1039. [Google Scholar] [CrossRef]
- Zhou, Y.-H.; Vidyarthi, S.K.; Zhong, C.-S.; Zheng, Z.-A.; An, Y.; Wang, J.; Wei, Q.; Xiao, H.-W. Cold plasma enhances drying and color, rehydration ratio and polyphenols of wolfberry via microstructure and ultrastructure alteration. LWT 2020, 134, 110173. [Google Scholar] [CrossRef]
- Tabibian, S.; Labbafi, M.; Askari, G.; Rezaeinezhad, A.; Ghomi, H. Effect of gliding arc discharge plasma pretreatment on drying kinetic, energy consumption and physico-chemical properties of saffron (Crocus sativus L.). J. Food Eng. 2020, 270, 109766. [Google Scholar] [CrossRef]
- Li, S.; Chen, S.; Han, F.; Xv, Y.; Sun, H.; Ma, Z.; Chen, J.; Wu, W. Development and optimization of cold plasma pretreatment for drying on corn kernels. J. Food Sci. 2019, 84, 2181–2189. [Google Scholar] [CrossRef]
- Gavahian, M.; Khaneghah, A.M. Cold plasma as a tool for the elimination of food contaminants: Recent advances and future trends. Crit. Rev. Food Sci. Nutr. 2020, 60, 1581–1592. [Google Scholar] [CrossRef]
- Watts, M. Chlorpyrifos as a Possible Global POP; Pesticide Action Network North America: Oakland, CA, USA, 2012. [Google Scholar]
- Liu, H.; Guo, D.; Feng, X. Plasma Degradation of Pesticides on the Surface of Corn and Evaluation of Its Quality Changes. Sustainability 2021, 13, 8830. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Imani, S.; Dorranian, D.; Larijani, K.; Shojaee, M. Effect of cold plasma on degradation of organophosphorus pesticides used on some agricultural products. J. Plant Prot. Res. 2017, 57, 26–35. [Google Scholar] [CrossRef]
- Misra, N.N.; Pankaj, S.K.; Walsh, T.; O’Regan, F.; Bourke, P.; Cullen, P.J. In-package nonthermal plasma degradation of pesticides on fresh produce. J. Hazard. Mater. 2014, 271, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sarangapani, C.; O’Toole, G.; Cullen, P.J.; Bourke, P. Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries. Innov. Food Sci. Emerg. Technol. 2017, 44, 235–241. [Google Scholar] [CrossRef]
- Phan, K.T.K.; Phan, H.T.; Boonyawan, D.; Intipunya, P.; Brennan, C.S.; Regenstein, J.M.; Phimolsiripol, Y. Non-thermal plasma for elimination of pesticide residues in mango. Innov. Food Sci. Emerg. Technol. 2018, 48, 164–171. [Google Scholar] [CrossRef]
- Cong, L.; Huang, M.; Zhang, J.; Yan, W. Effect of dielectric barrier discharge plasma on the degradation of malathion and chlorpyrifos on lettuce. J. Sci. Food Agric. 2021, 101, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Cullen, P.J.; Lalor, J.; Scally, L.; Boehm, D.; Milosavljević, V.; Bourke, P.; Keener, K. Translation of plasma technology from the lab to the food industry. Plasma Process. Polym. 2018, 15, 1700085. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Chang, C.-R.; Chang, T.-J.; Chang, Y.-J.; Liew, Y.; Chau, C.-F. Changes in physicochemical properties of corn starch upon modifications by atmospheric pressure plasma jet. Food Chem. 2019, 283, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Chaple, S.; Sarangapani, C.; Jones, J.; Carey, E.; Causeret, L.; Genson, A.; Duffy, B.; Bourke, P. Effect of atmospheric cold plasma on the functional properties of whole wheat (Triticum aestivum L.) grain and wheat flour. Innov. Food Sci. Emerg. Technol. 2020, 66, 102529. [Google Scholar] [CrossRef]
- Rashid, F.; Bao, Y.; Ahmed, Z.; Huang, J.-Y. Effect of high voltage atmospheric cold plasma on extraction of fenugreek galactomannan and its physicochemical properties. Food Res. Int. 2020, 138, 109776. [Google Scholar] [CrossRef]
- Trinh, K.S.; Nguyen, T.L. Structural, functional properties and in vitro digestability of maize starch under heat-moisture and atmospheric-cold plasma treatments. Vietnam J. Sci. Technol. 2018, 56, 751–760. [Google Scholar]
- Segat, A.; Misra, N.N.; Cullen, P.J.; Innocente, N. Atmospheric pressure cold plasma (ACP) treatment of whey protein isolate model solution. Innov. Food Sci. Emerg. Technol. 2015, 29, 247–254. [Google Scholar] [CrossRef]
- Misra, N.N.; Yong, H.I.; Phalak, R.; Jo, C. Atmospheric pressure cold plasma improves viscosifying and emulsion stabilizing properties of xanthan gum. Food Hydrocoll. 2018, 82, 29–33. [Google Scholar] [CrossRef]
- Herceg, Z.; Kovačević, D.B.; Kljusurić, J.G.; Jambrak, A.R.; Zorić, Z.; Dragović-Uzelac, V. Gas phase plasma impact on phenolic compounds in pomegranate juice. Food Chem. 2016, 190, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Bulbul, V.; Bhushette, P.R.; Zambare, R.S.; Deshmukh, R.; Annapure, U.S. Effect of cold plasma treatment on Xanthan gum properties. Polym. Test. 2019, 79, 106056. [Google Scholar] [CrossRef]
- Xu, L.; Hou, H.; Farkas, B.; Keener, K.M.; Garner, A.L.; Tao, B. High voltage atmospheric cold plasma modification of bovine serum albumin. LWT 2021, 149, 111995. [Google Scholar] [CrossRef]
- Chen, G.; Dong, S.; Zhao, S.; Li, S.; Chen, Y. Improving functional properties of zein film via compositing with chitosan and cold plasma treatment. Ind. Crops Prod. 2019, 129, 318–326. [Google Scholar] [CrossRef]
- Han, Y.; Cheng, J.-H.; Sun, D.-W. Activities and conformation changes of food enzymes induced by cold plasma: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 794–811. [Google Scholar] [CrossRef]
- Ji, H.; Dong, S.; Han, F.; Li, Y.; Chen, G.; Li, L.; Chen, Y. Effects of dielectric barrier discharge (DBD) cold plasma treatment on physicochemical and functional properties of peanut protein. Food Bioprocess Technol. 2018, 11, 344–354. [Google Scholar] [CrossRef]
- Pan, Y.-W.; Cheng, J.-H.; Sun, D.-W. Inhibition of fruit softening by cold plasma treatments: Affecting factors and applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 1935–1946. [Google Scholar] [CrossRef]
- Yu, J.-j.; Zhang, Y.-f.; Yan, J.; Li, S.-h.; Chen, Y. A novel glycoprotein emulsion using high-denatured peanut protein and sesbania gum via cold plasma for encapsulation of β-carotene. Innov. Food Sci. Emerg. Technol. 2021, 74, 102840. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, R.; Gan, Z.; Shao, T.; Zhang, X.; He, M.; Sun, A. Effect of cold plasma on blueberry juice quality. Food Chem. 2019, 290, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Reddivari, L.; Huang, J.-Y. Development of cold plasma pretreatment for improving phenolics extractability from tomato pomace. Innov. Food Sci. Emerg. Technol. 2020, 65, 102445. [Google Scholar] [CrossRef]
- Bao, Y.; Reddivari, L.; Huang, J.-Y. Enhancement of phenolic compounds extraction from grape pomace by high voltage atmospheric cold plasma. LWT 2020, 133, 109970. [Google Scholar] [CrossRef]
- Măgureanu, M.; Sîrbu, R.; Dobrin, D.; Gîdea, M. Stimulation of the Germination and Early Growth of Tomato Seeds by Non-thermal Plasma. Plasma Chem. Plasma Process. 2018, 38, 989–1001. [Google Scholar] [CrossRef]
- Sajib, S.A.; Billah, M.; Mahmud, S.; Miah, M.; Hossain, F.; Omar, F.B.; Roy, N.C.; Hoque, K.M.F.; Talukder, M.R.; Kabir, A.H. Plasma activated water: The next generation eco-friendly stimulant for enhancing plant seed germination, vigor and increased enzyme activity, a study on black gram (Vigna mungo L.). Plasma Chem. Plasma Process. 2020, 40, 119–143. [Google Scholar] [CrossRef]
- Rodríguez, Ó.; Gomes, W.F.; Rodrigues, S.; Fernandes, F.A. Effect of indirect cold plasma treatment on cashew apple juice (Anacardium occidentale L.). LWT 2017, 84, 457–463. [Google Scholar] [CrossRef]
- Kashfi, A.S.; Ramezan, Y.; Khani, M.R. Simultaneous study of the antioxidant activity, microbial decontamination and color of dried peppermint (Mentha piperita L.) using low pressure cold plasma. LWT 2020, 123, 109121. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Wan, Z.; Colonna, W.; Keener, K.M. Effect of high voltage atmospheric cold plasma on white grape juice quality. J. Sci. Food Agric. 2017, 97, 4016–4021. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.D.L.; Cavalcante, R.S.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Fernandes, F.A.; Rodrigues, S. Effects of atmospheric cold plasma and ozone on prebiotic orange juice. Innov. Food Sci. Emerg. Technol. 2015, 32, 127–135. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Jiang, A.; Guan, Q.; Sun, X.; Liu, S.; Hao, K.; Hu, W. The effects of cold plasma-activated water treatment on the microbial growth and antioxidant properties of fresh-cut pears. Food Bioprocess Technol. 2019, 12, 1842–1851. [Google Scholar] [CrossRef]
- Fernandes, F.A.; Santos, V.O.; Rodrigues, S. Effects of glow plasma technology on some bioactive compounds of acerola juice. Food Res. Int. 2019, 115, 16–22. [Google Scholar] [CrossRef]
- Silveira, M.R.; Coutinho, N.M.; Esmerino, E.A.; Moraes, J.; Fernandes, L.M.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.; Ranadheera, C.S. Guava-flavored whey beverage processed by cold plasma technology: Bioactive compounds, fatty acid profile and volatile compounds. Food Chem. 2019, 279, 120–127. [Google Scholar] [CrossRef]
- Poomanee, W.; Wattananapakasem, I.; Panjan, W.; Kiattisin, K. Optimizing anthocyanins extraction and the effect of cold plasma treatment on the anti-aging potential of purple glutinous rice (Oryza sativa L.) extract. Cereal Chem. 2021, 98, 571–582. [Google Scholar] [CrossRef]
- Faria, G.; Souza, M.; Oliveira, J.; Costa, C.; Collares, M.; Prentice, C. Effect of ultrasound-assisted cold plasma pretreatment to obtain sea asparagus extract and its application in Italian salami. Food Res. Int. 2020, 137, 109435. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, M.; Najafi, G.; Ahmadi Gavlighi, H.; Seyfi, P.; Ghomi, H. Enhancement of polyphenolic content extraction rate with maximal antioxidant activity from green tea leaves by cold plasma. J. Food Sci. 2020, 85, 3415–3422. [Google Scholar] [CrossRef]
- Lin, S.-P.; Kuo, T.-C.; Wang, H.-T.; Ting, Y.; Hsieh, C.-W.; Chen, Y.-K.; Hsu, H.-Y.; Cheng, K.-C. Enhanced bioethanol production using atmospheric cold plasma-assisted detoxification of sugarcane bagasse hydrolysate. Bioresour. Technol. 2020, 313, 123704. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-P.; Huang, S.-H.; Ting, Y.; Hsu, H.-Y.; Cheng, K.-C. Evaluation of detoxified sugarcane bagasse hydrolysate by atmospheric cold plasma for bacterial cellulose production. Int. J. Biol. Macromol. 2022, 204, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Santoso, S.P.; Lin, S.-P.; Wang, T.-Y.; Ting, Y.; Hsieh, C.-W.; Yu, R.-C.; Angkawijaya, A.E.; Soetaredjo, F.E.; Hsu, H.-Y.; Cheng, K.-C. Atmospheric cold plasma-assisted pineapple peel waste hydrolysate detoxification for the production of bacterial cellulose. Int. J. Biol. Macromol. 2021, 175, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Heiske, S.; Schultz-Jensen, N.; Leipold, F.; Schmidt, J.E. Improving Anaerobic Digestion of Wheat Straw by Plasma-Assisted Pretreatment. J. At. Mol. Phys. 2013, 2013, 791353. [Google Scholar] [CrossRef]
- Kodama, S.; Thawatchaipracha, B.; Sekiguchi, H. Enhancement of essential oil extraction for steam distillation by DBD surface treatment. Plasma Process. Polym. 2014, 11, 126–132. [Google Scholar] [CrossRef]
- Pragna, C.; Gracy, T.R.; Mahendran, R.; Anandharamakrishnan, C. Effects of microwave and cold plasma assisted Hydrodistillation on lemon Peel oil extraction. Int. J. Food Eng. 2019, 15, 20190093. [Google Scholar] [CrossRef]
| Food Product | Plasma Device | Microbial Strains | Reduction (log CFU) | Parameters | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Exposure Time (s) | Exposure Distance (mm) | Input Power (W) | Treatment Voltage (kV) | Frequency | |||||
| Prepackaged mixed salad | DBD | Salmonella | 0.8/g | 180 | 30 | - | 35 | 1.1 kHz | [19] |
| Golden Delicious apples | DBD | Salmonella and E. coli | 5.3/cm2; 5.5/cm2 | 240 | 35 | 200 | - | 50 Hz | [20] |
| Boiled chicken breast cubes | DBD | Salmonella, E. coli, L. monocytogenes, and Tulane virus | 3.7/cube; 3.9/cube; 3.5/cube; 2.2 PFU/cube | 210 | 12 | - | 39 | - | [21] |
| Tender coconut water | DBD | L. monocytogenes and E. coli | 2.0/mL; 2.2/mL | 120 | 10 | - | 90 | 60 Hz | [22] |
| Tilapia fillet | DBD | V. parahaemolyticus | 1.8/g | 60 | 100 | 30 | - | 13.6 MHz | [1] |
| S. enteritis, L. monocytogenes | 2.34 log CFU/g; 1.69 log CFU/g | 300 | 52 | 70 | 80 | 60 | [23] | ||
| Blueberries | Plasma jet | Tulane virus and murine norovirus | 3.5/g; 5.0/g | 120; 90 | 75 | 549 | - | 47 kHz | [24] |
| Military rations snack | Plasma jet | A. flavus, yeast-mold, and aflatoxin | 4.3/g; 4.6/g; 3.0/g | 360 | 30 | - | 9 | - | [25] |
| Groundnuts | DBD | A. flavus, A. parasiticus, and aflatoxin | 1.2/g; 1.2/g; 0.3/g | 720 | 30 | 60 | 2 | - | [26] |
| Food Matrix | Plasma Device | Film Materials | Treatment Conditions | Physicochemical Change (Optimisation Methods Were Chosen) | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Input Power (W) | Treatment Time (s) | Frequency (MHz) | Thickness (mm) | Tensile Strength (MPa) | Elastic Modulus (Mpa) | Elongation at Break | Water Vapour Permeability | ||||
| Tilapia fillets | RF- plasma | CNMA- CMC/LDPE | 30 | 60 | 13.56 | +29.97% | +13.58% | - | - | - | [1] |
| Chicken breast fillets | - | 1–3% SEO- CS/LDPE | 84 | 10 | - | +650% | −3% | - | −27% | −96.7% | [2] |
| Cooked turkey meat | Plasma Jet | Citrus/PET | - | - | 30 kHz | +150% | - | - | - | - | [4] |
| Korean steamed rice cakes | DBD- plasma | Nylon/PP Nylon/LDPE | 21 kW | 180 | - | - | Nylon/PP + 1.6% Nylon/PE − 0.5% | Nylon/PP + 1.2% Nylon/PE + 0.5% | Nylon/PP − 0.3% Nylon/PE + 0.9% | Nylon/PP − 6.25% Nylon/PE − 7.7% | [5] |
| Button mushroom (Agaricus bisporus) | RF- Plasma | CMC, COL/LDPE | 30 | 60 | 13.56 | - | +7.6% | +47.43% | - | +114% | [7] |
| Food Allergen Mitigation | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Allergens | Plasma Device | Sample Types | Antibody Binding Capacity | Parameters | Reference | ||||
| Exposure Time (min) | Exposure Distance (mm) | Input Power | Input Voltage (kV) | Frequency | |||||
| Casein | Plasma jet (spark discharge) | Allergenic protein solution | ↓ 49.9% | 30 | 2.5 | - | 8 | 25 kHz | [44] |
| α-lactalbumin | ↓ 49.5% | ||||||||
| β-lactoglobulin | ↑ 250% | 10 | |||||||
| Casein | Plasma jet (glow discharge) | ↓ 91.1% | 30 | 5 | - | 5 | 25 kHz | ||
| α-lactalbumin | ↓ 45.5% | ||||||||
| β-lactoglobulin | ↑ 300% | 10 | |||||||
| β-lactoglobulin | DBD | Allergenic protein solution | ↓ 58.21% | 4 | - | - | 40 | 12 kHz | [45] |
| Ara h 1 | Plasma jet (pin-to-plate) | Whole peanut | ↓ 39.32% | 60 | 70 | - | 32 | 52 kHz | [46] |
| Defatted peanut flour | ↓ 65% | ||||||||
| Ara h 2 | Whole peanut | ↓ 46% | |||||||
| Defatted peanut flour | ↓ 66% | ||||||||
| β-conglycinin (Gly m5) | Plasma jet | Soy protein isolate | ↓ 89% | 90 | - | 12 kW | - | 2.45 GHz | [47] |
| Glycinin | DBD | Allergenic protein solution | ↓ 91.64% | 5 | 50 | 40 | 20 kHz | [48] | |
| ↓ 81.49% * | |||||||||
| Soy allergens | DBD | Soy protein isolate | ↓ 75% * | 5 | 35 | - | 40 | 120 Hz | [49] |
| Food Drying Processing | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Food Product | Plasma Device | Drying Temperature (°C) | Reduction of Drying Time | Parameters | Reference | ||||
| Exposure Time | Exposure Distance (mm) | Input Power (W) | Input Voltage (kV) | Frequency | |||||
| Grape | Plasma jet | 70 | 20% | 3 times | 10 | 500 | - | 25 kHz | [61] |
| Plasma jet | 60 | 26.27% | 50 s | 35 | 300 | 27 | 50 Hz | [62] | |
| Corn kernels | DBD | 37.5 | 21.52% | 30 s | - | 500 | - | 40 kHz | [63] |
| Chili pepper | Plasma jet | 70 | ~16.6% | 30 s | 60 | 750 | - | 20 kHz | [64] |
| Shiitake mushroom | Plasma jet | 50, 60, 70 | The higher drying rate at 50 and 60 °C. | 60 s | 50 | 650 | - | - | [65] |
| Plasma-activated water | - | ||||||||
| Tucumã | DBD | 60 | 61.1% | 10 min | 15 | - | 20 | 200 Hz | [66] |
| Jujube | Plasma jet | 70 | 12.08% | 1 min | 50 | 650 | 5 | 40 kHz | [67] |
| Plasma-activated water | Non-effect | 10 min | - | ||||||
| Wolfberry | Plasma jet | 65 | 50% | 30 s | 60 | 750 | - | 20 kHz | [68] |
| Saffron | Plasma jet | 60 | 54.05% | 60 s | - | 1000 | 8 | 50 Hz | [69] |
| Nutrient Extraction | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Food Matrix | Plasma Device | Parameters | Results | Reference | |||||
| Input Power (W) | Voltage (kV) | Time (min) | Working Gas | Frequency (kHz) | Gas Flow Rate (L/min) | ||||
| White grapes | Jet plasma | 500 | - | 3–7 | Air | 25.00 | 40.00 | TPC and antioxidant capacity increased more than twofold | [61] |
| Blueberry juice | Jet plasma | - | 11 | - | Ar, O2 | 1.00 | 1.00 | TPC increased by 7.34%, and antioxidant capacity increased | [94] |
| Tomato pomace | DBD plasma | - | 60 | 15 | Ar, He, N2, air | - | - | TPC increased by 24.07%, and antioxidant capacity by 30% | [95] |
| Grape pomace | DBD plasma | - | 60 | 5–15 | He | - | - | Increased the yield of phenolic extracts; improved antioxidant capacity | [96] |
| Fenugreek | DBD plasma | - | 120 V | 30 | air | 0.06 | - | Increased the extraction yield of fenugreek galactomannan | [82] |
| Tomato | DBD-plasma | 0.55–1.43 | 13–17 | 5–45 | air | 0.05 | 15.00 | Increased the weight of tomato by 20–40% | [97] |
| Black gram | Jet plasma | - | 3–6 | 3–15 | O2 | 3.00–10.00 | 0.25 | Chlorophyll content increased by 23.80% and total soluble protein and sugar concentrations increased by 33.28% and 51.73%, respectively | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khumsupan, D.; Lin, S.-P.; Hsieh, C.-W.; Santoso, S.P.; Chou, Y.-J.; Hsieh, K.-C.; Lin, H.-W.; Ting, Y.; Cheng, K.-C. Current and Potential Applications of Atmospheric Cold Plasma in the Food Industry. Molecules 2023, 28, 4903. https://doi.org/10.3390/molecules28134903
Khumsupan D, Lin S-P, Hsieh C-W, Santoso SP, Chou Y-J, Hsieh K-C, Lin H-W, Ting Y, Cheng K-C. Current and Potential Applications of Atmospheric Cold Plasma in the Food Industry. Molecules. 2023; 28(13):4903. https://doi.org/10.3390/molecules28134903
Chicago/Turabian StyleKhumsupan, Darin, Shin-Ping Lin, Chang-Wei Hsieh, Shella Permatasari Santoso, Yu-Jou Chou, Kuan-Chen Hsieh, Hui-Wen Lin, Yuwen Ting, and Kuan-Chen Cheng. 2023. "Current and Potential Applications of Atmospheric Cold Plasma in the Food Industry" Molecules 28, no. 13: 4903. https://doi.org/10.3390/molecules28134903
APA StyleKhumsupan, D., Lin, S.-P., Hsieh, C.-W., Santoso, S. P., Chou, Y.-J., Hsieh, K.-C., Lin, H.-W., Ting, Y., & Cheng, K.-C. (2023). Current and Potential Applications of Atmospheric Cold Plasma in the Food Industry. Molecules, 28(13), 4903. https://doi.org/10.3390/molecules28134903









