Pentagalloyl Glucose: A Review of Anticancer Properties, Molecular Targets, Mechanisms of Action, Pharmacokinetics, and Safety Profile
Abstract
1. Introduction
2. Sources of PGG
2.1. Synthesis
2.2. Natural Sources
3. Anticancer Activity of PGG
3.1. Breast Cancer
3.2. Prostate Cancer
3.3. Lung Cancer
3.4. Liver Cancer
3.5. Pancreatic Cancer
3.6. Head and Neck Cancer
3.7. Colorectal Cancer
3.8. Glioma Cancer
3.9. Cervical Cancer
3.10. Leukemia
| Cancer Type and Cell Lines | Plant Source of PGG | IC50 (Exposure Time) | Effect | References |
|---|---|---|---|---|
| Breast | ||||
| MDA-MB-231 | 47.25 ± 2.03 µg/mL (24 h) | Inhibit tumor cell proliferation and induce cell apoptosis | [38] | |
| <11.76 µg/mL (72 h) | Induced cell S-phase arrest | [39] | ||
| 23.52 µg/mL (24 h) | Inhibit tumor cell growth | [41] | ||
| Gallnut of Rhus chinensis Mill | 1.13 µg/mL (72 h) | Attenuate cell proliferation | [42] | |
| Bouea macrophylla seeds | 26.46 ± 6.53 µg/mL (72 h) | Induce cell apoptosis | [43] | |
| MDA-MB-468 | 33.60 ± 0.70 µg/mL (24 h) | Inhibit tumor cell proliferation and induce cell apoptosis | [38] | |
| MCF-7 | <11.76 µg/mL (72 h) | Induced cell S-phase arrest | [39] | |
| Bouea macrophylla seeds | >100 µg/mL (72 h) | Induce apoptosis | [43] | |
| Lung | ||||
| MRC5-SV2 | Anacardium occidentale L. | 52.24 µg/mL (48 h) | Induce cell oxidative stress, cytotoxicity | [69] |
| LLC | Gallnut of Rhus chinensis Mill | >70.55 µg/mL(48 h) | Induce cell apoptosis | [48] |
| Liver | ||||
| Huh7 | Paeonia lactiflora | 30 µg/mL (72 h) | Induce cell apoptosis, reduced the colony formation | [52] |
| Hep G2 | Paeonia lactiflora | 160 µg/mL (72 h) | Inhibit tumor cell proliferation | [52] |
| Hep3B | Paeonia lactiflora | 70 µg/mL (72 h) | Inhibit tumor cell proliferation | [52] |
| SK-HEP-1 | Paeonia lactiflora | 100 µg/mL (72 h) | Inhibit tumor cell proliferation | [52] |
| HepG2 | 27.94 µg/mL (48 h) | inhibit the proliferation, migration and invasion, induce G1 arrest and apoptosis | [70] | |
| Prostate | ||||
| LNCaP | Gallnut of Rhus chinensis Mill | ≤23.52 µg/mL (96 h) | Induce G1-cell cycle arrests | [47] |
| DU145 | ≤23.52 µg/mL (96 h) | Induce S-cell cycle arrests | ||
| Head and Neck | ||||
| CAL27 | Bouea macrophylla seed | 16.68 ± 1.20 µg/mL (48 h) | suppress the tumer cells stemness trait | [12] |
| FaDu | Bouea macrophylla seed | 26.50 ± 1.46 µg/mL (48 h) | suppress the tumer cells stemness trait | [12] |
| Colorectal | ||||
| HCT116 | 0.65 ± 0.34 µg/mL (48 h) | Induce cell apoptosis | [60] | |
| HT29 | 4.19 ± 1.09 µg/mL (48 h) | Induce cell apoptosis | [60] | |
| Glioma cancer | ||||
| U87 | 23.52 µg/mL | Inhibit tumor cell growth | [41] |
| Cancer Type and Cell Lines | Plant Source of PGG | Model Treatment Dose | (Administration Route) | Eeffect | References |
|---|---|---|---|---|---|
| Breast | |||||
| MDA-MB-231 | MDA-MB-231 injected subcutaneously into the right flank of each 6-week-old female athymic nude mouse | 20 mg/kg (O.G.) | Inhibition of breast cancer cell growth | [39] | |
| Gallnut of Rhus chinensis Mill | MDA-MB-231 injected subcutaneously into the right flank of a 6-week-old female BALB/c athymic nude mice | 10 mg/kg (O.G.) | Inhibition of MDA-MB-231 xenograft growth and lung metastasis | [40] | |
| Lung | |||||
| LLC | Gallnut of Rhus chinensis Mill | tumor inoculation in C57BL/6 mice | 4 or 20 mg/kg (I.P.) alternate days for 17 days from day 3 | Decrease in tumor volume over time, suppression of the weight of the tumor, and inhibite tumor angiogenesis | [48] |
| Liver | |||||
| Huh7 | Paeonia lactiflora | Huh7 cells subcutaneously implanted subcutaneously into Balb/c nude mice | 300 mg/kg (O.G.) | Suppression of the tumor growth by inhibiting the expression of MYC | [52,53] |
| Prostate | |||||
| PC-3 | Intratibial injection of PC-3 in nude mice | 25 mg/kg (I.P.) | Suppression of Tumorigenesis in nude mice | [44] | |
| Pancreatic | |||||
| MiaPaCa2 | tumor cells were transplanted subcutaneously in male athymic Balb/c mice | 20 mg/kg (O.G.) | Alleviates cancer cachexia | [54] | |
| A piece of tumor tissue made of MiaPaCa2 cells was embedded orthotopically in athymic mice | 20 mg/kg (O.G.) | Intra-pancreatic insulin normally combated the pharmacologic effects of PGG | [71] |
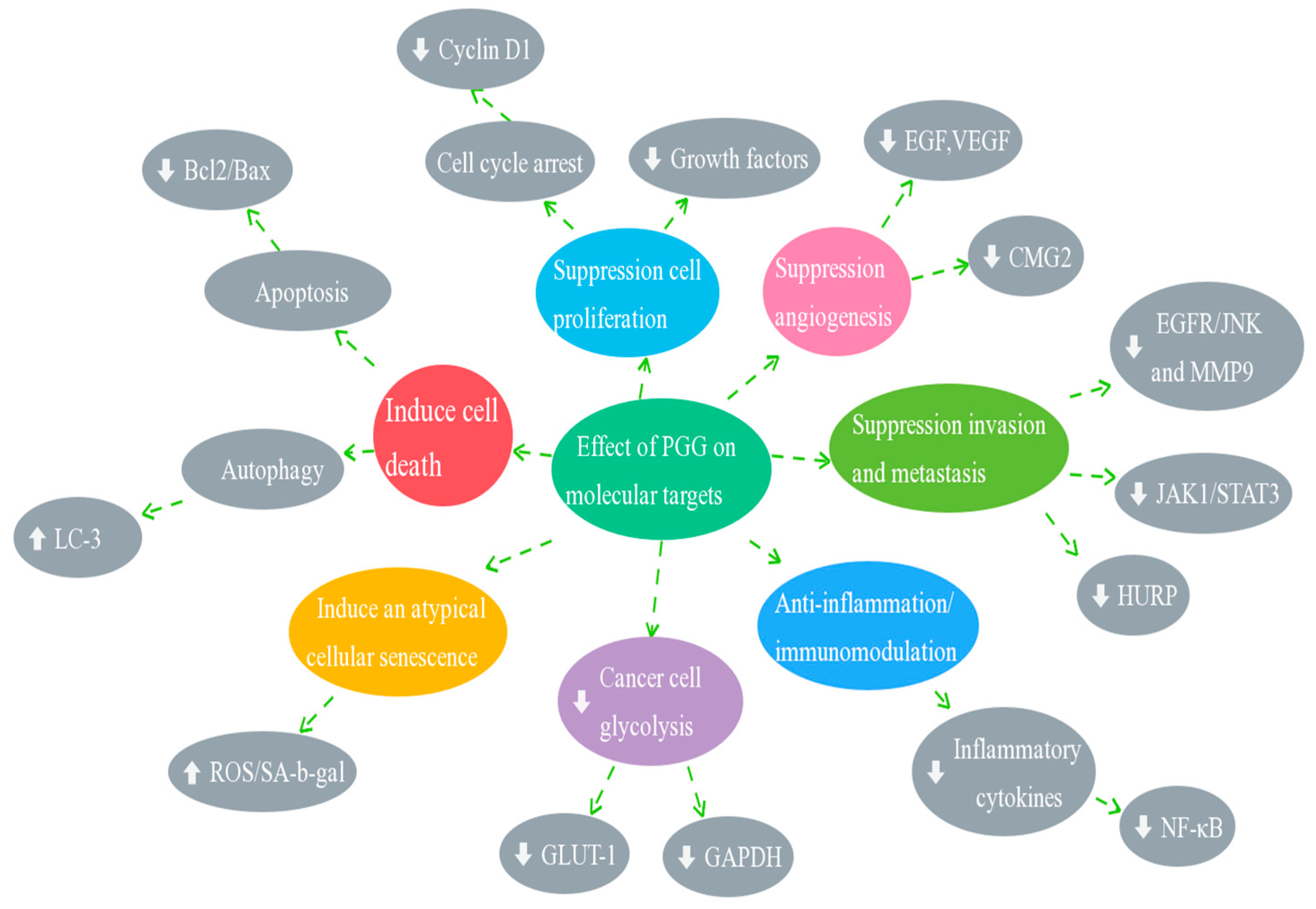
4. Molecular Mechanisms of Anticancer Effects of PGG
4.1. Effect of PGG on Cell Cycle Arrest
4.2. Effect of PGG on Inducing Cell Death (Apoptosis and Autophagy)
4.3. Effect of PGG on the Inhibition of Angiogenesis and Metastasis
4.4. Effect of PGG on STAT3 Transcription Factors
5. Synergistic Effect of PGG on Cancer Chemotherapy and Radiotherapy
5.1. Chemotherapy
5.2. Radiotherapy
6. Safety Profile and Pharmacokinetics of PGG
7. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Phillips, M.C.; Mousa, S.A. Clinical application of nano-targeting for enhancing chemotherapeutic efficacy and safety in cancer management. Nanomedicine 2022, 17, 405–421. [Google Scholar] [CrossRef]
- Nasir, A.; Bullo, M.M.H.; Ahmed, Z.; Imtiaz, A.; Yaqoob, E.; Jadoon, M.; Ahmed, H.; Afreen, A.; Yaqoob, S. Nutrigenomics: Epigenetics and cancer prevention: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1375–1387. [Google Scholar] [CrossRef]
- Li, G.; Lin, P.; Wang, K.; Gu, C.-C.; Kusari, S. Artificial intelligence-guided discovery of anticancer lead compounds from plants and associated microorganisms. Trends Cancer 2022, 8, 65–80. [Google Scholar] [CrossRef]
- Kimura, I.; Kagawa, S.; Tsuneki, H.; Tanaka, K.; Nagashima, F. Multitasking bamboo leaf-derived compounds in prevention of infectious, inflammatory, atherosclerotic, metabolic, and neuropsychiatric diseases. Pharmacol. Ther. 2022, 235, 108159. [Google Scholar] [CrossRef]
- Bailly, C. The traditional and modern uses of Selaginella tamariscina (P.Beauv.) Spring, in medicine and cosmetic: Applications and bioactive ingredients. J. Ethnopharmacol. 2021, 280, 114444. [Google Scholar] [CrossRef]
- Long, J.; Guan, P.; Hu, X.; Yang, L.; He, L.; Lin, Q.; Luo, F.; Li, J.; He, X.; Du, Z.; et al. Natural Polyphenols as Targeted Modulators in Colon Cancer: Molecular Mechanisms and Applications. Front. Immunol. 2021, 12, 635484. [Google Scholar] [CrossRef]
- Khan, H.; Reale, M.; Ullah, H.; Sureda, A.; Tejada, S.; Wang, Y.; Zhang, Z.-J.; Xiao, J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: Updates and future directions. Biotechnol. Adv. 2020, 38, 107385. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef]
- Zeng, J.; Han, J.; Liu, Z.; Yu, M.; Li, H.; Yu, J. Pentagalloylglucose disrupts the PALB2-BRCA2 interaction and potentiates tumor sensitivity to PARP inhibitor and radiotherapy. Cancer Lett. 2022, 546, 215851. [Google Scholar] [CrossRef]
- Park, K.-Y.; Lee, H.-J.; Jeong, S.-J.; Kim, H.-S.; Kim, S.-H.; Lim, S.; Kim, H.-C.; Lü, J.; Kim, S.-H. 1,2,3,4,6-Penta-O-galloly-beta-D-glucose Suppresses Hypoxia-Induced Accumulation of Hypoxia-Inducible Factor-1α and Signaling in LNCaP Prostate Cancer Cells. Biol. Pharm. Bull. 2010, 33, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Kantapan, J.; Dechsupa, N.; Tippanya, D.; Nobnop, W.; Chitapanarux, I. Gallotannin from Bouea macrophylla Seed Extract Suppresses Cancer Stem-like Cells and Radiosensitizes Head and Neck Cancer. Int. J. Mol. Sci. 2021, 22, 9253. [Google Scholar] [CrossRef]
- Meng, J.; Li, Q.; Cao, Z.; Gu, D.; Wang, Y.; Zhang, Y.; Wang, Y.; Yang, Y.; He, F. Rapid screening and separation of active compounds against α-amylase from Toona sinensis by ligand fishing and high-speed counter-current chromatography. Int. J. Biol. Macromol. 2021, 174, 270–277. [Google Scholar] [CrossRef]
- Chen, X.; Daniels, N.A.; Cottrill, D.; Cao, Y.; Wang, X.; Li, Y.; Shriwas, P.; Qian, Y.; Archer, M.W.; Whitticar, N.B.; et al. Natural Compound α-PGG and Its Synthetic Derivative 6Cl-TGQ Alter Insulin Secretion: Evidence for Diminishing Glucose Uptake as a Mechanism. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 759–772. [Google Scholar] [CrossRef]
- Sylla, T.; Pouységu, L.; Da Costa, G.; Deffieux, D.; Monti, J.-P.; Quideau, S. Gallotannins and Tannic Acid: First Chemical Syntheses and In Vitro Inhibitory Activity on Alzheimer’s Amyloid β-Peptide Aggregation. Angew. Chem. Int. Ed. 2015, 54, 8217–8221. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Yu, R.; Zheng, T.; Sun, D.; Zhou, Y.; Tang, J.; Zhu, H.; Li, G.; Niu, L.; Cui, L.; et al. A Novel Strategy for Unveiling Spatial Distribution Pattern of Gallotannins in Paeonia rockii and Paeonia ostii Based on LC–QTRAP–MS. Metabolites 2022, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Soares, S.; Sousa, C.F.; Dias, R.; Gameiro, P.; Soares, S.; de Freitas, V. Interaction of polyphenols with model membranes: Putative implications to mouthfeel perception. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, L.; Kim, S.-H.; Hagerman, A.E.; Lü, J. Anti-Cancer, Anti-Diabetic and Other Pharmacologic and Biological Activities of Penta-Galloyl-Glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef]
- Sekowski, S.; Veiko, A.; Olchowik-Grabarek, E.; Dubis, A.; Wilczewska, A.Z.; Markiewicz, K.H.; Zavodnik, I.B.; Lapshina, E.; Dobrzynska, I.; Abdulladjanova, N.; et al. Hydrolysable tannins change physicochemical parameters of lipid nano-vesicles and reduce DPPH radical—Experimental studies and quantum chemical analysis. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183778. [Google Scholar] [CrossRef]
- Chen, R.H.; Yang, L.J.; Hamdoun, S.; Chung, S.K.; Lam, C.W.-K.; Zhang, K.X.; Guo, X.; Xia, C.; Law, B.Y.K.; Wong, V.K.W. 1,2,3,4,6-Pentagalloyl Glucose, a RBD-ACE2 Binding Inhibitor to Prevent SARS-CoV-2 Infection. Front. Pharmacol. 2021, 12, 634176. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Thanigaimani, S.; Phie, J. A Systematic Review and Meta-Analysis of the Effect of Pentagalloyl Glucose Administration on Aortic Expansion in Animal Models. Biomedicines 2021, 9, 1442. [Google Scholar] [CrossRef] [PubMed]
- Torres-León, C.; Ventura-Sobrevilla, J.; Serna-Cock, L.; Ascacio-Valdés, J.A.; Contreras-Esquivel, J.; Aguilar, C.N. Pentagalloylglucose (PGG): A valuable phenolic compound with functional properties. J. Funct. Foods 2017, 37, 176–189. [Google Scholar] [CrossRef]
- Niemetz, R.; Gross, G.G. Enzymology of gallotannin and ellagitannin biosynthesis. Phytochemistry 2005, 66, 2001–2011. [Google Scholar] [CrossRef]
- Kim, B.-H.; Choi, M.S.; Lee, H.G.; Lee, S.-H.; Noh, K.H.; Kwon, S.; Jeong, A.J.; Lee, H.; Yi, E.H.; Park, J.Y.; et al. Photoprotective Potential of Penta-O-Galloyl-β-DGlucose by Targeting NF-κB and MAPK Signaling in UVB Radiation-Induced Human Dermal Fibroblasts and Mouse Skin. Mol. Cells 2015, 38, 982–990. [Google Scholar] [CrossRef]
- Wang, W.-C.; Wang, C.; Song, X.-Y.; Zhao, W.-H.; Wang, Q. Determination of 1, 2, 3, 4, 6-penta-O-galloyl-D-glucose in forty four kinds of Chinese traditional medicines by HPLC. China J. Chin. Mater. Med. 2008, 33, 656–659. [Google Scholar]
- Barreto, J.C.; Trevisan, M.T.S.; Hull, W.E.; Erben, G.; de Brito, E.S.; Pfundstein, B.; Würtele, G.; Spiegelhalder, B.; Owen, R.W. Characterization and Quantitation of Polyphenolic Compounds in Bark, Kernel, Leaves, and Peel of Mango (Mangifera indica L.). J. Agric. Food Chem. 2008, 56, 5599–5610. [Google Scholar] [CrossRef]
- Zhishuang, Y.; Wenhua, Z.; Xueying, S.; Lingjiang, K.; Qiao, W. Determination of 1, 2, 3, 4, 6-penta-O-galloyl-D-glucose in Radix Paeoniae Alba and Radix Paeoniae Rubra with HPLC. J. Cap. Med. Univ. 2008, 29, 601–604. [Google Scholar]
- Pellati, F.; Bruni, R.; Righi, D.; Grandini, A.; Tognolini, M.; Prencipe, F.P.; Poli, F.; Benvenuti, S.; Del Rio, D.; Rossi, D. Metabolite profiling of polyphenols in a Terminalia chebula Retzius ayurvedic decoction and evaluation of its chemopreventive activity. J. Ethnopharmacol. 2013, 147, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Juang, L.-J.; Sheu, S.-J.; Lin, T.-C. Determination of hydrolyzable tannins in the fruit of Terminalia chebula Retz. by high-performance liquid chromatography and capillary electrophoresis. J. Sep. Sci. 2004, 27, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.-H.; Lee, Y.-G.; Lim, Y.; Jeon, H.; Kim, E.H.; Choi, J.; Hong, W.; Jeon, H.; Ahrweiler, M.; Kim, H.; et al. Potent antiviral activity of the extract of Elaeocarpus sylvestris against influenza A virus in vitro and in vivo. Phytomedicine 2022, 97, 153892. [Google Scholar] [CrossRef] [PubMed]
- Jaszewska, E.; Kosmider, A.; Kiss, A.; Naruszewicz, M. Oenothera paradoxa defatted seeds extract containing pentagalloylglucose and procyanidins potentiates the cytotoxicity of vincristine. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2010, 61, 637–643. [Google Scholar]
- Aitani, M.; Kimura, H.; Abiru, Y.; Soyama, H.; Murakami, H.; Zhang, H.L.; Sugishita, T.; Konishi, Y. Effect of an Extract from Evening-Primrose Seeds on Postprandial Blood Glucose Level and Its Active Components. Nippon. Shokuhin Kagaku Kaishi 2003, 50, 180–187. [Google Scholar] [CrossRef]
- Fujimaki, T.; Sato, C.; Yamamoto, R.; Watanabe, S.; Fujita, H.; Kikuno, H.; Sue, M.; Matsushima, Y. Isolation of phenolic acids and tannin acids from Mangifera indica L. kernels as inhibitors of lipid accumulation in 3T3-L1 cells. Biosci. Biotechnol. Biochem. 2022, 86, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yu, X.; Wu, Y.; Shiraishi, F.; Kawahara, N.; Komatsu, K. Genetic and chemical characterization of white and red peony root derived from Paeonia lactiflora. J. Nat. Med. 2015, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Erşan, S.; Üstündağ, G.; Carle, R.; Schweiggert, R.M. Subcritical water extraction of phenolic and antioxidant constituents from pistachio (Pistacia vera L.) hulls. Food Chem. 2018, 253, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayed, E.; Singab, A.-N.; Ayoub, N.; Martiskainen, O.; Sinkkonen, J.; Pihlaja, K. HPLC–PDA–ESI–MS/MS profiling and chemopreventive potential of Eucalyptus gomphocephala DC. Food Chem. 2012, 133, 1017–1024. [Google Scholar] [CrossRef]
- Si, C.-L.; Qin, P.-P.; Hu, H.-Y.; Jiang, J.-Z.; Ni, Y.-H. Low Molecular Weight Extractives from Green Husks of Juglans sigillata and Their Antioxidant Activities. J. Biobased Mater. Bioenergy 2011, 5, 287–292. [Google Scholar] [CrossRef]
- Mendonca, P.; Alghamdi, S.; Messeha, S.; Soliman, K.F.A. Pentagalloyl glucose inhibits TNF-α-activated CXCL1/GRO-α expression and induces apoptosis-related genes in triple-negative breast cancer cells. Sci. Rep. 2021, 11, 5649. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Lee, H.-J.; Shaik, A.A.; Nkhata, K.; Xing, C.; Zhang, J.; Jeong, S.-J.; Kim, S.-H.; Lü, J. Penta-O-galloyl-β-D-glucose induces G1arrest and DNA replicative S-phase arrest independently of P21 cyclin-dependent kinase inhibitor 1A, P27 cyclin-dependent kinase inhibitor 1B and P53 in human breast cancer cells and is orally active against triple-negative xenograft growth. Breast Cancer Res. 2010, 12, R67. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Seo, N.-J.; Jeong, S.-J.; Park, Y.; Jung, D.-B.; Koh, W.; Lee, E.-O.; Ahn, K.S.; Lü, J.; Kim, S.-H. Oral administration of penta-O-galloyl-D-glucose suppresses triple-negative breast cancer xenograft growth and metastasis in strong association with JAK1-STAT3 inhibition. Carcinogenesis 2011, 32, 804–811. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Hao, W.; Zhao, M.; Peng, S. In vitro inhibition of fatty acid synthase by 1,2,3,4,6-penta-O-galloyl-β-d-glucose plays a vital role in anti-tumour activity. Biochem. Biophys. Res. Commun. 2014, 445, 346–351. [Google Scholar] [CrossRef]
- Deiab, S.; Mazzio, E.; Eyunni, S.; McTier, O.; Mateeva, N.; Elshami, F.; Soliman, K.F.A. 1,2,3,4,6-Penta-O-galloylglucose within Galla Chinensis Inhibits Human LDH-A and Attenuates Cell Proliferation in MDA-MB-231 Breast Cancer Cells. Evid.-Based Complement. Altern. Med. 2015, 2015, 276946. [Google Scholar] [CrossRef]
- Kantapan, J.; Paksee, S.; Chawapun, P.; Sangthong, P.; Dechsupa, N. Pentagalloyl Glucose- and Ethyl Gallate-Rich Extract from Maprang Seeds Induce Apoptosis in MCF-7 Breast Cancer Cells through Mitochondria-Mediated Pathway. Evid.-Based Complement. Altern. Med. 2020, 2020, 5686029. [Google Scholar] [CrossRef]
- Kuo, P.-T.; Lin, T.-P.; Liu, L.-C.; Huang, C.-H.; Lin, J.-K.; Kao, J.-Y.; Way, T.-D. Penta-O-galloyl-β-d-glucose Suppresses Prostate Cancer Bone Metastasis by Transcriptionally Repressing EGF-Induced MMP-9 Expression. J. Agric. Food Chem. 2009, 57, 3331–3339. [Google Scholar] [CrossRef]
- Hu, H.; Lee, H.-J.; Jiang, C.; Zhang, J.; Wang, L.; Zhao, Y.; Xiang, Q.; Lee, E.-O.; Kim, S.-H.; Lü, J. Penta-1,2,3,4,6-O-galloyl-β-d-glucose induces p53 and inhibits STAT3 in prostate cancer cells in vitro and suppresses prostate xenograft tumor growth in vivo. Mol. Cancer Ther. 2008, 7, 2681–2691. [Google Scholar] [CrossRef]
- Hu, H.; Chai, Y.; Wang, L.; Zhang, J.; Lee, H.J.; Kim, S.-H.; Lü, J. Pentagalloylglucose induces autophagy and caspase-independent programmed deaths in human PC-3 and mouse TRAMP-C2 prostate cancer cells. Mol. Cancer Ther. 2009, 8, 2833–2843. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, J.; Lee, H.J.; Kim, S.-H.; Lü, J. Penta-O-galloyl-beta-D-glucose induces S- and G1-cell cycle arrests in prostate cancer cells targeting DNA replication and cyclin D1. Carcinogenesis 2009, 30, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.-E.; Lee, E.-O.; Kim, M.-S.; Kang, K.-S.; Kim, C.-H.; Cha, B.-C.; Surh, Y.-J.; Kim, S.-H. Penta-O-galloyl-beta-d-glucose suppresses tumor growth via inhibition of angiogenesis and stimulation of apoptosis: Roles of cyclooxygenase-2 and mitogen-activated protein kinase pathways. Carcinogenesis 2005, 26, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.-S.; Pae, H.-O.; Oh, H.; Hong, S.-G.; Kim, I.-K.; Chai, K.-Y.; Yun, Y.-G.; Kwon, T.-O.; Chung, H.-T. In vitro anti-proliferative effect of 1,2,3,4,6-penta-O-galloyl-beta-d-glucose on human hepatocellular carcinoma cell line, SK-HEP-1 cells. Cancer Lett. 2001, 174, 17–24. [Google Scholar] [CrossRef]
- Yin, S.; Dong, Y.; Li, J.; Lü, J.; Hu, H. Penta-1,2,3,4,6-O-galloyl-beta-D-glucose induces senescence-like terminal S-phase arrest in human hepatoma and breast cancer cells. Mol. Carcinog. 2011, 50, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yin, S.; Jiang, C.; Luo, X.; Guo, X.; Zhao, C.; Fan, L.; Meng, Y.; Lu, J.; Song, X.; et al. Involvement of autophagy induction in penta-1,2,3,4,6-O-galloyl-β-D-glucose-induced senescence-like growth arrest in human cancer cells. Autophagy 2014, 10, 296–310. [Google Scholar] [CrossRef]
- Kant, R.; Yen, C.-H.; Lu, C.-K.; Lin, Y.-C.; Li, J.-H.; Chen, Y.-M.A. Identification of 1,2,3,4,6-Penta-O-galloyl-β-d-glucopyranoside as a Glycine N-Methyltransferase Enhancer by High-Throughput Screening of Natural Products Inhibits Hepatocellular Carcinoma. Int. J. Mol. Sci. 2016, 17, 669. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.; Yen, C.-H.; Hung, J.-H.; Lu, C.-K.; Tung, C.-Y.; Chang, P.-C.; Chen, Y.-H.; Tyan, Y.-C.; Chen, Y.-M.A. Induction of GNMT by 1,2,3,4,6-penta-O-galloyl-beta-D-glucopyranoside through proteasome-independent MYC downregulation in hepatocellular carcinoma. Sci. Rep. 2019, 9, 1968. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, F.; Chen, X.; Qiu, S.; Cui, L.; Hu, L. β-Pentagalloyl-Glucose Sabotages Pancreatic Cancer Cells and Ameliorates Ca-chexia in Tumor-Bearing Mice. Am. J. Chin. Med. 2019, 47, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Palamaris, K.; Felekouras, E.; Sakellariou, S. Epithelial to Mesenchymal Transition: Key Regulator of Pancreatic Ductal Adenocarcinoma Progression and Chemoresistance. Cancers 2021, 13, 5532. [Google Scholar] [CrossRef]
- Patil, K.; Khan, F.B.; Akhtar, S.; Ahmad, A.; Uddin, S. The plasticity of pancreatic cancer stem cells: Implications in therapeutic resistance. Cancer Metastasis Rev. 2021, 40, 691–720. [Google Scholar] [CrossRef] [PubMed]
- Gzil, A.; Zarębska, I.; Bursiewicz, W.; Antosik, P.; Grzanka, D.; Szylberg, Ł. Markers of pancreatic cancer stem cells and their clinical and therapeutic implications. Mol. Biol. Rep. 2019, 46, 6629–6645. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Lee, S.-U.; Kim, Y.H. 1,2,3,4,6-Penta-O-galloyl-β-D-glucose Inhibits CD44v3, a cancer stem cell marker, by regulating its transcription factor, in human pancreatic cancer cell line. Anim. Cells Syst. 2022, 26, 328–337. [Google Scholar] [CrossRef]
- Fan, C.-W.; Tang, J.; Jiang, J.-C.; Zhou, M.-M.; Li, M.-S.; Wang, H.-S. Pentagalloylglucose suppresses the growth and migration of human nasopharyngeal cancer cells via the GSK3β/β-catenin pathway in vitro and in vivo. Phytomedicine 2022, 102, 154192. [Google Scholar] [CrossRef]
- Kawk, S.H.; Kang, Y.R.; Kim, Y.H. 1,2,3,4,6-Penta-O-galloyl-β-d-glucose suppresses colon cancer through induction of tumor suppressor. Bioorg. Med. Chem. Lett. 2018, 28, 2117–2123. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Yeh, C.-L.; Chou, H.-C.; Yang, C.-H.; Fu, Y.-N.; Chen, Y.-T.; Cheng, H.-W.; Huang, C.-Y.F.; Liu, H.-P.; Huang, S.-F.; et al. Vaccinia H1-related Phosphatase is a Phosphatase of ErbB Receptors and is Down-regulated in Non-small Cell Lung Cancer. J. Biol. Chem. 2011, 286, 10177–10184. [Google Scholar] [CrossRef]
- Wu, S.; Vossius, S.; Rahmouni, S.; Miletic, A.V.; Vang, T.; Vazquez-Rodriguez, J.; Cerignoli, F.; Arimura, Y.; Williams, S.; Hayes, T.; et al. Multidentate Small-Molecule Inhibitors of Vaccinia H1-Related (VHR) Phosphatase Decrease Proliferation of Cervix Cancer Cells. J. Med. Chem. 2009, 52, 6716–6723. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, D.; Roh, K.M.; Ahn, D.; Kang, H.J.; Chung, S.J. Identification of Vaccinia-H1 Related Phosphatase as an Anticancer Target for 1,2,3,4,6-O-Pentagalloylglucose. Chem. Biodivers. 2020, 17, e1900414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gu, Y.; Chen, B. Mechanisms of drug resistance in acute myeloid leukemia. OncoTargets Ther. 2019, 12, 1937–1945. [Google Scholar] [CrossRef]
- Gay, F.; Palumbo, A. Management of disease- and treatment-related complications in patients with multiple myeloma. Med. Oncol. 2010, 27, 43–52. [Google Scholar] [CrossRef]
- Pan, M.-H.; Lin, J.-H.; Lin-Shiau, S.-Y.; Lin, J.-K. Induction of apoptosis by penta-O-galloyl-β-d-glucose through activation of caspase-3 in human leukemia HL-60 cells. Eur. J. Pharmacol. 1999, 381, 171–183. [Google Scholar] [CrossRef]
- Kwon, T.-R.; Jeong, S.-J.; Lee, H.-J.; Sohn, E.J.; Jung, J.H.; Kim, J.-H.; Jung, D.-B.; Lu, J.; Kim, S.-H. Reactive oxygen species-mediated activation of JNK and down-regulation of DAXX are critically involved in penta-O-galloyl-beta-d-glucose-induced apoptosis in chronic myeloid leukemia K562 cells. Biochem. Biophys. Res. Commun. 2012, 424, 530–537. [Google Scholar] [CrossRef]
- Tseeleesuren, D.; Kant, R.; Yen, C.-H.; Hsiao, H.-H.; Chen, Y.-M.A. 1,2,3,4,6-Penta-O-Galloyl-Beta-D-Glucopyranoside Inhibits Proliferation of Multiple Myeloma Cells Accompanied with Suppression of MYC Expression. Front. Pharmacol. 2018, 9, 2066–2080. [Google Scholar] [CrossRef]
- Taiwo, B.J.; Popoola, T.D.; Van Heerden, F.R.; Fatokun, A.A. Pentagalloylglucose, isolated from the leaf extract of Anacardium occidentale L., could elicit rapid and selective cytotoxicity in cancer cells. BMC Complement. Med. Ther. 2020, 20, 287. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.-Q.; Zhao, S.; Wang, J.-Y.; Zheng, H.-C.; Ma, C.-M. Inhibitory effects and molecular mechanisms of pentagalloyl glucose in combination with 5-FU on aggressive phenotypes of HepG2 cells. Nat. Prod. Res. 2021, 35, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chen, X.; Qiu, S.; Yang, J.; Liu, H.; Zhang, J.; Zhang, D.; Wang, F. Intra-Pancreatic Insulin Nourishes Cancer Cells: Do Insulin-Receptor Antagonists such as PGG and EGCG Play a Role? Am. J. Chin. Med. 2020, 48, 1005–1019. [Google Scholar] [CrossRef]
- Chen, T.-F.; Hsu, J.-T.; Wu, K.-C.; Hsiao, C.-F.; Lin, J.-A.; Cheng, Y.-H.; Liu, Y.-H.; Lee, D.-Y.; Chang, H.-H.; Cho, D.-Y.; et al. A systematic identification of anti-inflammatory active components derived from Mu Dan Pi and their applications in inflammatory bowel disease. Sci. Rep. 2020, 10, 17238. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, P.; Taka, E.; Bauer, D.; Reams, R.R.; Soliman, K.F. The attenuating effects of 1,2,3,4,6 penta-O-galloyl-β-d-glucose on pro-inflammatory responses of LPS/IFNγ-activated BV-2 microglial cells through NFƙB and MAPK signaling pathways. J. Neuroimmunol. 2018, 324, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.M.; Song, H.Y.; Lee, S.J.; Seo, W.Y.; Sin, D.H.; Goh, A.R.; Kang, Y.-H.; Kang, I.-J.; Won, M.-H.; Yi, J.-S.; et al. Suppression of thymus- and activation-regulated chemokine (TARC/CCL17) production by 1,2,3,4,6-penta-O-galloyl-β-d-glucose via blockade of NF-κB and STAT1 activation in the HaCaT cells. Biochem. Biophys. Res. Commun. 2009, 387, 115–120. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.M.; Ji, S.-T.; Mar, W.; Gho, Y.S. 1,2,3,4,6-Penta-O-galloyl-beta-d-glucose blocks endothelial cell growth and tube formation through inhibition of VEGF binding to VEGF receptor. Cancer Lett. 2004, 208, 89–94. [Google Scholar] [CrossRef]
- Lee, J.-H.; Yehl, M.; Ahn, K.S.; Kim, S.-H.; Lieske, J.C. 1,2,3,4,6-penta-O-galloyl-beta-d-glucose attenuates renal cell migration, hyaluronan expression, and crystal adhesion. Eur. J. Pharmacol. 2009, 606, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.F.; Nabil, M.; Hasan, R.A.; El-Shazly, A.M.; El-Ansari, M.A.; Sobeh, M. Pentagalloyl Glucose, a Major Compound in Mango Seed Kernel, Exhibits Distinct Gastroprotective Effects in Indomethacin-Induced Gastropathy in Rats via Modulating the NO/eNOS/iNOS Signaling Pathway. Front. Pharmacol. 2022, 13, 800986. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Feng, K.; Xiao, J.; Du, J.; Cao, Y.; Chen, Y. Immunomodulatory effect of pentagalloyl glucose in LPS-stimulated RAW264.7 macrophages and PAO1-induced Caenorhabditis elegans. Exp. Gerontol. 2021, 150, 111388. [Google Scholar] [CrossRef]
- Kang, D.G.; Moon, M.K.; Choi, D.H.; Lee, J.K.; Kwon, T.O.; Lee, H.S. Vasodilatory and anti-inflammatory effects of the 1,2,3,4,6-penta-O-galloyl-β-d-glucose (PGG) via a nitric oxide–cGMP pathway. Eur. J. Pharmacol. 2005, 524, 111–119. [Google Scholar] [CrossRef]
- Peng, J.; Li, K.; Zhu, W.; Nie, R.; Wang, R.; Li, C. Penta-O-galloyl-β-d-glucose, a hydrolysable tannin from Radix Paeoniae Alba, inhibits adipogenesis and TNF-α-mediated inflammation in 3T3-L1 cells. Chem. Interact. 2019, 302, 156–163. [Google Scholar] [CrossRef]
- Tong, J.; Fang, J.; Zhu, T.; Xiang, P.; Shang, J.; Chen, L.; Zhao, J.; Wang, Y.; Tong, L.; Sun, M. Pentagalloylglucose reduces AGE-induced inflammation by activating Nrf2/HO-1 and inhibiting the JAK2/STAT3 pathway in mesangial cells. J. Pharmacol. Sci. 2021, 147, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-E.; Hyam, S.R.; Jeong, J.-J.; Han, M.J.; Kim, D.-H. Penta-O-galloyl-β-D-glucose ameliorates inflammation by inhibiting MyD88/NF-κB and MyD88/MAPK signalling pathways. Br. J. Pharmacol. 2013, 170, 1078–1091. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yang, X.; Yamashita, S.; Kumazoe, M.; Huang, Y.; Nakahara, K.; Won, Y.S.; Murata, M.; Lin, I.-C.; Tachibana, H. 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose increases a population of T regulatory cells and inhibits IgE production in ovalbumin-sensitized mice. Int. Immunopharmacol. 2015, 26, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, T.P.; Nosalski, R.; Skiba, D.S.; Koziol, J.; Mazur, M.; Justo-Junior, A.S.; Kowalczyk, P.; Kusmierczyk, Z.; Schramm-Luc, A.; Luc, K.; et al. 1,2,3,4,6-Penta-O-galloyl-β-d-glucose modulates perivascular inflammation and prevents vascular dysfunction in angiotensin II-induced hypertension. Br. J. Pharmacol. 2019, 176, 1951–1965. [Google Scholar] [CrossRef]
- Dhital, S.; Rice, C.D.; Vyavahare, N.R. Reversal of elastase-induced abdominal aortic aneurysm following the delivery of nanoparticle-based pentagalloyl glucose (PGG) is associated with reduced inflammatory and immune markers. Eur. J. Pharmacol. 2021, 910, 174487. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Xu, M.; Zhang, J.; Feng, Y.; Hao, Z.; Tu, C.; Liu, Y. Pentagalloylglucose Inhibits the Replication of Rabies Virus via Mediation of the miR-455/SOCS3/STAT3/IL-6 Pathway. J. Virol. 2019, 93, e00539-19. [Google Scholar] [CrossRef]
- Park, J.H.; Kho, M.C.; Oh, H.C.; Kim, Y.C.; Yoon, J.J.; Lee, Y.J.; Kang, D.G.; Lee, H.S. 1,2,3,4,6-Penta-O-Galloyl-β-D-Glucose from Galla rhois Ameliorates Renal Tubular Injury and Microvascular Inflammation in Acute Kidney Injury Rats. Am. J. Chin. Med. 2018, 46, 785–800. [Google Scholar] [CrossRef]
- Ryu, H.-G.; Jeong, S.-J.; Kwon, H.-Y.; Lee, H.-J.; Lee, E.-O.; Lee, M.-H.; Choi, S.H.; Ahn, K.S.; Kim, S.-H. Penta-O-galloyl-β-d-glucose attenuates cisplatin-induced nephrotoxicity via reactive oxygen species reduction in renal epithelial cells and enhances antitumor activity in Caki-2 renal cancer cells. Toxicol. Vitr. 2012, 26, 206–214. [Google Scholar] [CrossRef]
- Xiang, Q.; Tang, J.; Luo, Q.; Xue, J.; Tao, Y.; Jiang, H.; Tian, J.; Fan, C. In vitro study of anti-ER positive breast cancer effect and mechanism of 1,2,3,4-6-pentyl-O-galloyl-beta-d-glucose (PGG). Biomed. Pharmacother. 2019, 111, 813–820. [Google Scholar] [CrossRef]
- Mendonca, P.; Taka, E.; Soliman, K.F.A. Proteomic analysis of the effect of the polyphenol pentagalloyl glucose on proteins involved in neurodegenerative diseases in activated BV-2 microglial cells. Mol. Med. Rep. 2019, 20, 1736–1746. [Google Scholar] [CrossRef]
- Kim, J.-A.; Lee, J.-E.; Kim, J.H.; Lee, H.-J.; Kang, N.J. Penta-1,2,3,4,6-O-Galloyl-β-d-Glucose Inhibits UVB-Induced Photoaging by Targeting PAK1 and JNK1. Antioxidants 2019, 8, 561. [Google Scholar] [CrossRef]
- Pae, H.O.; Oh, G.S.; Jeong, S.O.; Jeong, G.S.; Lee, B.S.; Choi, B.M.; Lee, H.S.; Chung, H.T. 1,2,3,4,6-penta-O-galloyl-beta-D-glucose up-regulates heme oxygenase-1 expression by stim-ulating Nrf2 nuclear translocation in an extracellular signal-regulated kinase-dependent manner in HepG2 cells. World J. Gastroenterol. 2006, 12, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, H.; Cao, F.; Zhen, L.; Bai, J.; Yuan, S.; Mei, Y. 1,2,3,4,6-penta-O-galloyl-β-D-glucose protects PC12 Cells from MPP(+)-mediated cell death by inducing heme oxygenase-1 in an ERK- and Akt-dependent manner. J. Huazhong Univ. Sci. Technol. 2012, 32, 737–745. [Google Scholar] [CrossRef]
- Bae, S.; Kim, S.Y.; Do, M.H.; Lee, C.H.; Song, Y.-J. 1,2,3,4,6-Penta-O-galloyl-ß-D-glucose, a bioactive compound in Elaeocarpus sylvestris extract, inhibits varicella-zoster virus replication. Antivir. Res. 2017, 144, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.C.-H.; Kuo, P.-T.; Lin, Y.-C.; Chen, Y.; Hseu, Y.-C.; Yang, H.-L.; Kao, J.-Y.; Ho, C.-T.; Way, T.-D. Penta-O-galloyl-β-d-glucose Suppresses EGF-Induced eIF3i Expression through Inhibition of the PI3K/AKT/mTOR Pathway in Prostate Cancer Cells. J. Agric. Food Chem. 2014, 62, 8990–8996. [Google Scholar] [CrossRef]
- Kim, J.-H.; Im, E.; Lee, J.; Lee, H.-J.; Sim, D.Y.; Park, J.E.; Ahn, C.-H.; Kwon, H.H.; Shim, B.S.; Kim, B.; et al. Apoptotic and DNA Damage Effect of 1,2,3,4,6-Penta-O-galloyl-beta-D-glucose in Cisplatin-Resistant Non-Small Lung Cancer Cells via Phosphorylation of H2AX, CHK2 and p53. Cells 2022, 11, 1343. [Google Scholar] [CrossRef]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef]
- Rahman, A.; Hannan, A.; Dash, R.; Rahman, H.; Islam, R.; Uddin, J.; Sohag, A.A.M.; Rahman, H.; Rhim, H. Phytochemicals as a Complement to Cancer Chemotherapy: Pharmacological Modulation of the Autophagy-Apoptosis Pathway. Front. Pharmacol. 2021, 12, 639628. [Google Scholar] [CrossRef]
- Chen, W.-J.; Chang, C.-Y.; Lin, J.-K. Induction of G1 phase arrest in MCF human breast cancer cells by pentagalloylglucose through the down-regulation of CDK4 and CDK2 activities and up-regulation of the CDK inhibitors p27(Kip) and p21(Cip). Biochem. Pharmacol. 2003, 65, 1777–1785. [Google Scholar] [CrossRef]
- Montalto, F.I.; De Amicis, F. Cyclin D1 in Cancer: A Molecular Connection for Cell Cycle Control, Adhesion and Invasion in Tumor and Stroma. Cells 2020, 9, 2648. [Google Scholar] [CrossRef]
- Alao, J.P. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol. Cancer 2007, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Gillett, C.; Smith, P.; Gregory, W.; Richards, M.; Millis, M.; Peters, G.; Barnes, D. Cyclin D1 and prognosis in human breast cancer. Int. J. Cancer 1996, 69, 92–99. [Google Scholar] [CrossRef]
- Sicinski, P.; Donaher, J.L.; Parker, S.B.; Li, T.; Fazeli, A.; Gardner, H.; Haslam, S.Z.; Bronson, R.T.; Elledge, S.J.; Weinberg, R.A. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 1995, 82, 621–630. [Google Scholar] [CrossRef]
- Gautschi, O.; Ratschiller, D.; Gugger, M.; Betticher, D.C.; Heighway, J. Cyclin D1 in non-small cell lung cancer: A key driver of malignant transformation. Lung Cancer 2007, 55, 1–14. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- O’Brien, M.A.; Kirby, R. Apoptosis: A review of pro-apoptotic and anti-apoptotic pathways and dysregulation in disease. J. Vet. Emerg. Crit. Care 2008, 18, 572–585. [Google Scholar] [CrossRef]
- Van Laethem, A.; Nys, K.; Van Kelst, S.; Claerhout, S.; Ichijo, H.; Vandenheede, J.R.; Garmyn, M.; Agostinis, P. Apoptosis signal regulating kinase-1 connects reactive oxygen species to p38 MAPK-induced mitochondrial apoptosis in UVB-irradiated human keratinocytes. Free. Radic. Biol. Med. 2006, 41, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Majidpoor, J.; Mortezaee, K. Angiogenesis as a hallmark of solid tumors—Clinical perspectives. Cell. Oncol. 2021, 44, 715–737. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Marti, H.J.; Bernaudin, M.; Bellail, A.; Schoch, H.; Euler, M.; Petit, E.; Risau, W. Hypoxia-Induced Vascular Endothelial Growth Factor Expression Precedes Neovascularization after Cerebral Ischemia. Am. J. Pathol. 2000, 156, 965–976. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Chen, S.-H.; Murphy, D.; Lassoued, W.; Thurston, G.; Feldman, M.D.; Lee, W.M. Activated STAT3 is a mediator and biomarker of VEGF endothelial activation. Cancer Biol. Ther. 2008, 7, 1994–2003. [Google Scholar] [CrossRef] [PubMed]
- Cryan, L.M.; Bazinet, L.; Habeshian, K.A.; Cao, S.; Clardy, J.; Christensen, K.A.; Rogers, M.S. 1,2,3,4,6-Penta-O-galloyl-β-d-glucopyranose Inhibits Angiogenesis via Inhibition of Capillary Morphogenesis Gene 2. J. Med. Chem. 2013, 56, 1940–1945. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Carlos-Reyes, A.; Lopez-Camarillo, C.; de la Cruz, O.N.H.; Lopez-Gonzalez, J.S. Contribution of Angiogenesis to Inflammation and Cancer. Front. Oncol. 2019, 9, 1399. [Google Scholar] [CrossRef]
- Ho, L.-L.; Chen, W.-J.; Lin-Shiau, S.-Y.; Lin, J.-K. Penta-O-galloyl-β-d-glucose inhibits the invasion of mouse melanoma by suppressing metalloproteinase-9 through down-regulation of activator protein-1. Eur. J. Pharmacol. 2002, 453, 149–158. [Google Scholar] [CrossRef]
- Coleman, R.E. Clinical Features of Metastatic Bone Disease and Risk of Skeletal Morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef]
- Lu, X.; Kang, Y. Epidermal growth factor signalling and bone metastasis. Br. J. Cancer 2010, 102, 457–461. [Google Scholar] [CrossRef]
- Yang, H.; Yue, G.G.-L.; Leung, P.-C.; Wong, C.-K.; Zhang, Y.-J.; Lau, C.B.-S. Anti-metastatic effects of 1,2,3,4,6-Penta-O-galloyl-β-D-glucose in colorectal cancer: Regulation of cathepsin B-mediated extracellular matrix dynamics and epithelial-to-mesenchymal transition. Pharmacol. Res. 2022, 184, 106457. [Google Scholar] [CrossRef]
- Tan, G.-J.; Peng, Z.-K.; Lu, J.-P.; Tang, F.-Q. Cathepsins mediate tumor metastasis. World J. Biol. Chem. 2013, 4, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, K.; Viswakarma, N.; Rana, A.; Rana, B. Transcription Factors in Cancer Development and Therapy. Cancers 2020, 12, 2296. [Google Scholar] [CrossRef]
- Loh, C.-Y.; Arya, A.; Naema, A.F.; Wong, W.F.; Sethi, G.; Looi, C.Y. Signal Transducer and Activator of Transcription (STATs) Proteins in Cancer and Inflammation: Functions and Therapeutic Implication. Front. Oncol. 2019, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Chai, E.Z.P.; Shanmugam, M.K.; Arfuso, F.; Dharmarajan, A.; Wang, C.; Kumar, A.P.; Samy, R.P.; Lim, L.H.; Wang, L.; Goh, B.C.; et al. Targeting transcription factor STAT3 for cancer prevention and therapy. Pharmacol. Ther. 2016, 162, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Kantapan, J.; Intachai, N.; Khamto, N.; Meepowpan, P.; Sangthong, P.; Wantanajittikul, K.; Dechsupa, N.; Chitapanarux, I. Pentagalloyl Glucose and Cisplatin Combination Treatment Exhibits a Synergistic Anticancer Effect in 2D and 3D Models of Head and Neck Carcinoma. Pharmaceuticals 2022, 15, 830. [Google Scholar] [CrossRef]
- Li, W.; Liao, L.-P.; Song, N.; Liu, Y.-J.; Ding, Y.-L.; Zhang, Y.-Y.; Zhou, X.-R.; Sun, Z.-Y.; Xiao, S.-H.; Wang, H.-B.; et al. Natural product 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose is a reversible inhibitor of glyceraldehyde 3-phosphate dehydrogenase. Acta Pharmacol. Sin. 2022, 43, 470–482. [Google Scholar] [CrossRef]
- Chen, W.-J.; Lin, J.-K. Induction of G1 Arrest and Apoptosis in Human Jurkat T Cells by Pentagalloylglucose through Inhibiting Proteasome Activity and Elevating p27Kip1, p21Cip1/WAF1, and Bax Proteins. J. Biol. Chem. 2004, 279, 13496–13505. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Chen, L.; Guo, X.; Cheng, C.; Luo, Y.; Wang, J.; Wang, J.; Liu, Y.; Cao, Y.; Li, P.; et al. Combating multidrug resistance and metastasis of breast cancer by endoplasmic reticulum stress and cell-nucleus penetration enhanced immunochemotherapy. Theranostics 2022, 12, 2987–3006. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Yang, Y.; Cai, C.-Y.; Teng, Q.-X.; Cui, Q.; Lin, J.; Assaraf, Y.G.; Chen, Z.-S. Multidrug resistance proteins (MRPs): Structure, function and the overcoming of cancer multidrug resistance. Drug Resist. Updates 2021, 54, 100743. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.-H.; Seow, K.-M.; Chen, K.-H. The Molecular Mechanisms of Actions, Effects, and Clinical Implications of PARP Inhibitors in Epithelial Ovarian Cancers: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 8125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Li, X.; Wang, D. Lysosomes contribute to radioresistance in cancer. Cancer Lett. 2018, 439, 39–46. [Google Scholar] [CrossRef]
- Ni, J.; Bucci, J.; Malouf, D.; Knox, M.; Graham, P.; Li, Y. Exosomes in Cancer Radioresistance. Front. Oncol. 2019, 9, 869. [Google Scholar] [CrossRef]
- Kwon, T.-R.; Lee, M.H.; Jeong, S.J.; Sohn, E.J.; Shin, E.A.; Jung, J.H.; Kim, J.-H. Penta-O-galloyl-beta-D-glucose enhances antitumor activity of imatinib and suppresses the growth of K562 cells in mice. Afr. J. Pharm. Pharmacol. 2013, 7, 552–559. [Google Scholar] [CrossRef]
- Lin, M.-H.; Chang, F.-R.; Hua, M.-Y.; Wu, Y.-C.; Liu, S.-T. Inhibitory Effects of 1,2,3,4,6-Penta-O-Galloyl-β-d-Glucopyranose on Biofilm Formation by Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Feldman, K.S.; Sahasrabudhe, K.; Lawlor, M.D.; Wilson, S.L.; Lang, C.H.; Scheuchenzuber, W.J. In vitro and In vivo inhibition of LPS-stimulated tumor necrosis factor-alpha secretion by the gallotannin beta-D-pentagalloylglucose. Bioorg. Med. Chem. Lett. 2001, 11, 1813–1815. [Google Scholar] [CrossRef]
- Jiamboonsri, P.; Pithayanukul, P.; Bavovada, R.; Gao, S.; Hu, M. A validated liquid chromatography–tandem mass spectrometry method for the determination of methyl gallate and pentagalloyl glucopyranose: Application to pharmacokinetic studies. J. Chromatogr. B 2015, 986–987, 12–17. [Google Scholar] [CrossRef]
- Jiamboonsri, P.; Pithayanukul, P.; Bavovada, R.; Leanpolchareanchai, J.; Yin, T.; Gao, S.; Hu, M. Factors Influencing Oral Bioavailability of Thai Mango Seed Kernel Extract and Its Key Phenolic Principles. Molecules 2015, 20, 21254–21273. [Google Scholar] [CrossRef]
- Li, L.; Shaik, A.A.; Zhang, J.; Nhkata, K.; Wang, L.; Zhang, Y.; Xing, C.; Kim, S.-H.; Lü, J. Preparation of penta-O-galloyl-β-d-glucose from tannic acid and plasma pharmacokinetic analyses by liquid–liquid extraction and reverse-phase HPLC. J. Pharm. Biomed. Anal. 2011, 54, 545–550. [Google Scholar] [CrossRef] [PubMed]
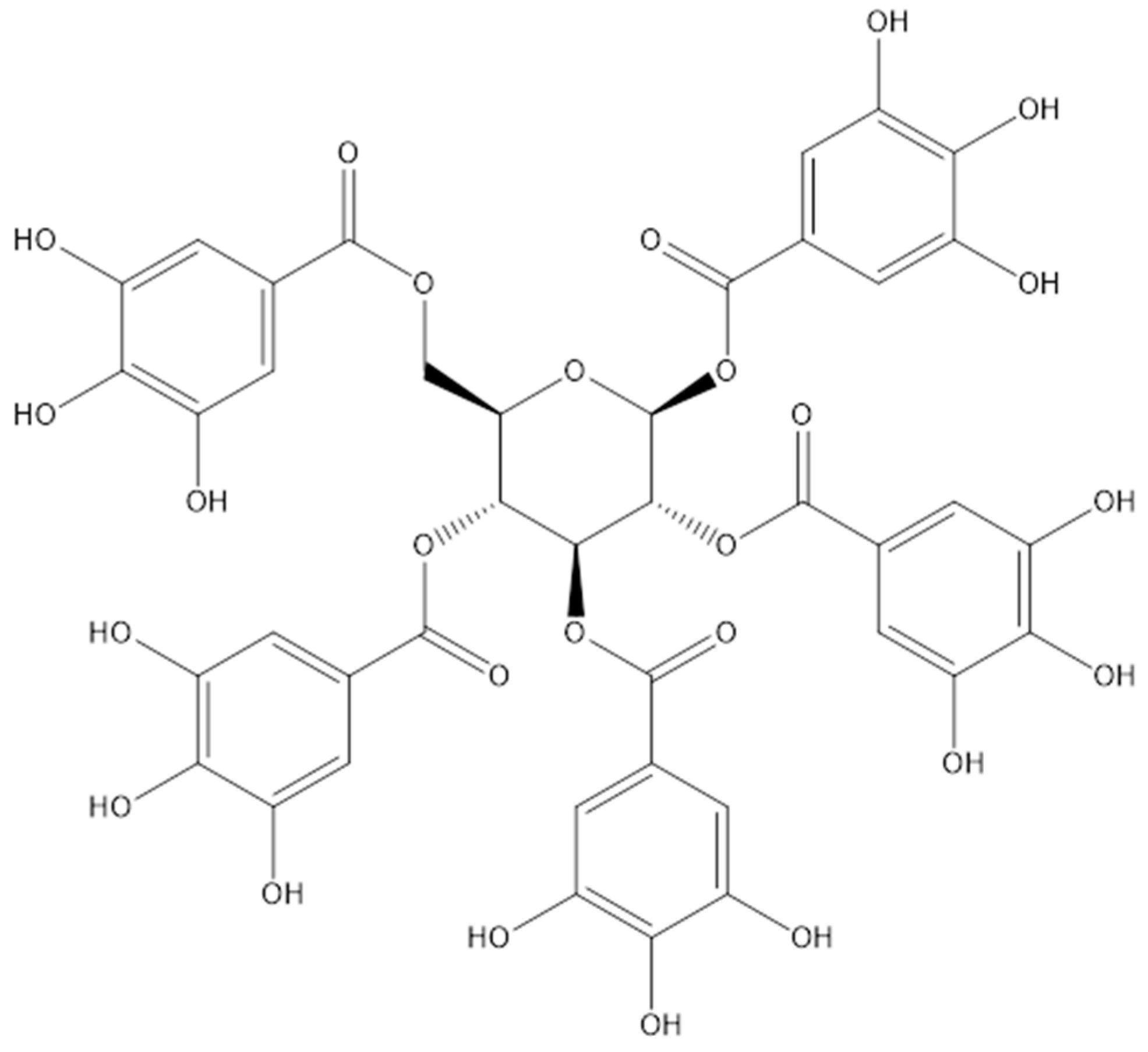
| Scientific Name | Common Name | Photo | Plant Part Used | Extraction/Purification Method | Yield | References | |
|---|---|---|---|---|---|---|---|
| Crude Extract | PGG | ||||||
| Bouea macrophylla | Marian plum, plum mango, Maprang, Gandara, Kundang |  | Seed | Ethanol maceration/Cold precipitation followed by centrifugation and HPLC | 400 g/kg (MPSE) | 52.88 g/kg | [12] |
| Mangifera indica | Mango |  | Young leaf | Methanol extraction/HPLC | 23.26 g/kg | [26,33] | |
| Old leaf | 1.82 g/kg | ||||||
| Peel | 17.71 g/kg | ||||||
| Kernel | 50.03 g/kg | ||||||
| Bark | 0.7 g/kg | ||||||
| Paeonia lactiflora | Chinese peony, Chinese herbaceous peony, common garden peony |  | Root | Methanol extraction/HPLC | 38.32 g/kg | [34] | |
| Aloe vera | Chinese Aloe, Indian Aloe, True Aloe, Barbados Aloe, Burn Aloe, First-aid plant |  | Leaf | Methanol extraction/HPLC | 1.520 g/kg | [25] | |
| Gallnuts:Galla Chinensis, Rhus chinensis, Rhus potaninii, Rhus punjabensis, Quercus infectoria, Quercus lusitanica | Gallnut, nutgall, gall oak, Galla rhois |  | Gall | Methanol extraction/HPLC | 58.4 g/kg | [25] | |
| Scutellaria baicalensis | Baikal skullcap, Chinese skullcap |  | Root | Methanol extraction/HPLC | 1.87 g/kg | [25] | |
| Cassia obtusifolia, Cassia tora | Cassia seed, Semen Cassia |  | Seed | Methanol extraction/HPLC | 1.00 g/kg | [25] | |
| Radix Paeoniae Alba | White peony root |  | Root | Ethanol extraction/HPLC | 2.48 g/kg | [27] | |
| Radix Paeoniae Rubra | Red peony root |  | Root | Ethanol extraction/HPLC | 2.24 g/kg | [27] | |
| Terminalia chebula | Chebulic myrobalan, Black myrobalan, Haritaki |  | Fruit | Ethanol extraction/HPLC | 13.53 g/kg | [28] | |
| Ethanol extraction/HPLC | 14.54 g/kg | [29] | |||||
| Ethanol extraction/Capillary electrophoresis | 20.7 g/kg | [29] | |||||
| Pistacia vera | Pistachio |  | Hull | Methanol extraction/HPLC | 9.77 g/kg | [35] | |
| Elaeocarpus sylvestris | Woodland elaeocarpus |  | No, specify | Ethanol extraction/Column chromatography/HPLC | 200 g/kg | 2.4 g/kg | [30] |
| Oenothera paradoxa | Evening primrose |  | Seed | Ethanolic extraction | 745.5 g/kg | 16.75 g/kg | [31] |
| Ethanolic extraction/HPLC | 27 g/kg | [32] | |||||
| Cancer Type | Mechanism of Action of Pentagalloyl Glucose | References | |
|---|---|---|---|
| Target Pathway/Molecule | Type of Effect | ||
| Lung cancer | ERK1/2, pJNK, p38 | ↓ | [48] |
| VEGF | ↓ | ||
| COX-2 | ↓ | ||
| p-H2AX | ↑ | [96] | |
| CHK2 | ↑ | ||
| P53 | ↑ | ||
| Breast cancer | MAPK | ↓ | [38] |
| IκBKE | ↓ | ||
| GRO-α/CXCL1 | ↓ | ||
| JAK1 | ↓ | [40] | |
| STAT3 | ↓ | ||
| HURP | ↓ | [89] | |
| Cyclin D1 | ↓ | ||
| Bcl-2 | ↓ | ||
| P53Ser15 | ↑ | [39] | |
| Cyclin D1 | ↓ | ||
| ROS | ↑ | [50] | |
| SA-β-gal | ↑ | ||
| LDH-A | ↓ | [42] | |
| Liver cancer | MYC | ↓ | [53] |
| GNMT | ↑ | ||
| P27 | ↑ | ||
| P21 | ↑ | ||
| Cyclin D1 | ↓ | ||
| GNMT | ↑ | [52] | |
| NF-κB | ↓ | [50] | |
| Cyclin D1 | ↓ | ||
| PI3K | ↓ | [70] | |
| Akt | ↓ | ||
| Bax | ↑ | ||
| Bcl-XL | ↓ | ||
| Bcl-2 | ↓ | ||
| ROS | ↓ | [50] | |
| SA-β-gal | ↓ | ||
| Brain cancer | FAS | ↓ | [41] |
| Caspase-3 | ↑ | [41] | |
| Head and neck cancer | GSK3β/β-catenin | ↑ | [59] |
| STAT3 | ↓ | [128] | |
| Bcl-2 | ↓ | ||
| VEGF | ↓ | ||
| Pancreatic cancer | HIF-1 | ↓ | [54,71] |
| Caveolin-1 | ↓ | [54] | |
| Akt | ↓ | [54,71] | |
| ERK | ↓ | [54,71] | |
| HK-II | ↓ | [54,71] | |
| PFK | ↓ | [54,71] | |
| GLUT-1 | ↓ | [54] | |
| IR/IGF1R | ↓ | [54,71] | |
| p-MEK | ↓ | [71] | |
| VEGF | ↓ | [71] | |
| Colorectal cancer | GAPDH | ↓ | [129] |
| P53 | ↑ | [60] | |
| P21 | ↑ | ||
| Bcl-2 | ↓ | ||
| c-Caspase-3 | ↑ | ||
| Prostate Cancer | P53 | ↑ | [45] |
| STAT3 | ↓ | ||
| pS6K | ↓ | [46] | |
| LC3-I/LC3-II | ↑ | ||
| PI3K/AKT/mTOR | ↓ | [95] | |
| EGF | ↓ | ||
| eIF3i | ↓ | ||
| PI3K/AKT/mTOR | ↓ | [11] | |
| VEGF | ↓ | ||
| HIF-1α | ¯ | ||
| EGFR/JNK | ↓ | [44] | |
| MMP-9 | ↓ | ||
| Leukemia | |||
| -CML | JNK | ↑ | [67] |
| Mcl-1, Bcl-2, Survivin | ↓ | ||
| DAXX | ↓ | ||
| -AML | Bax | ↑ | [130] |
| -MM | DEPTOR | ↓ | [68] |
| MYC | ↓ | ||
| Cervical cancer | PARP | ↑ | [63] |
| Cyclin D1 | ↓ | ||
| Bcl-2 | ↓ | ||
| STAT3 | ↓¯ | ||
| Pharmacokinetic Parameter | PGG Dose in the Animal Model | ||
|---|---|---|---|
| Mouse | Rat | ||
| 20 mg/kg (I.P.) | 20 mg/kg (I.P.) | 20 mg/kg (P.O.) | |
| Cmax (μM) | 3–4 | 6.39 ± 4.25 | ND |
| Tmax (h) | ~2 | 0.85 ± 0.70 | ND |
| t1/2(a) (h) | ND | ND | |
| t1/2(el) (h) | ND | 38.66 ± 22.89 | ND |
| Ke (h−1) | ND | 0.023 ± 0.012 | ND |
| AUC (0–24 h) (h* µM) | ND | 38.78 ± 22.89 | ND |
| Vd (L/kg) | ND | 7838.89 ± 3474.72 | ND |
| Cl (L/h/kg) | ND | 30.98 ± 21.73 | ND |
| MRT last (h) | ND | 12.47 ± 2.77 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, C.; Dechsupa, N.; Yu, Z.; Zhang, X.; Liang, S.; Lei, X.; Xu, T.; Gao, X.; Hu, Q.; Innuan, P.; et al. Pentagalloyl Glucose: A Review of Anticancer Properties, Molecular Targets, Mechanisms of Action, Pharmacokinetics, and Safety Profile. Molecules 2023, 28, 4856. https://doi.org/10.3390/molecules28124856
Wen C, Dechsupa N, Yu Z, Zhang X, Liang S, Lei X, Xu T, Gao X, Hu Q, Innuan P, et al. Pentagalloyl Glucose: A Review of Anticancer Properties, Molecular Targets, Mechanisms of Action, Pharmacokinetics, and Safety Profile. Molecules. 2023; 28(12):4856. https://doi.org/10.3390/molecules28124856
Chicago/Turabian StyleWen, Chengli, Nathupakorn Dechsupa, Zehui Yu, Xu Zhang, Sicheng Liang, Xianying Lei, Tao Xu, Xiaolan Gao, Qinxue Hu, Phattarawadee Innuan, and et al. 2023. "Pentagalloyl Glucose: A Review of Anticancer Properties, Molecular Targets, Mechanisms of Action, Pharmacokinetics, and Safety Profile" Molecules 28, no. 12: 4856. https://doi.org/10.3390/molecules28124856
APA StyleWen, C., Dechsupa, N., Yu, Z., Zhang, X., Liang, S., Lei, X., Xu, T., Gao, X., Hu, Q., Innuan, P., Kantapan, J., & Lü, M. (2023). Pentagalloyl Glucose: A Review of Anticancer Properties, Molecular Targets, Mechanisms of Action, Pharmacokinetics, and Safety Profile. Molecules, 28(12), 4856. https://doi.org/10.3390/molecules28124856







