The Pathophysiological Changes and Clinical Effects of Tetramethylpyrazine in ICR Mice with Fluoride-Induced Hepatopathy
Abstract
1. Introduction
2. Results
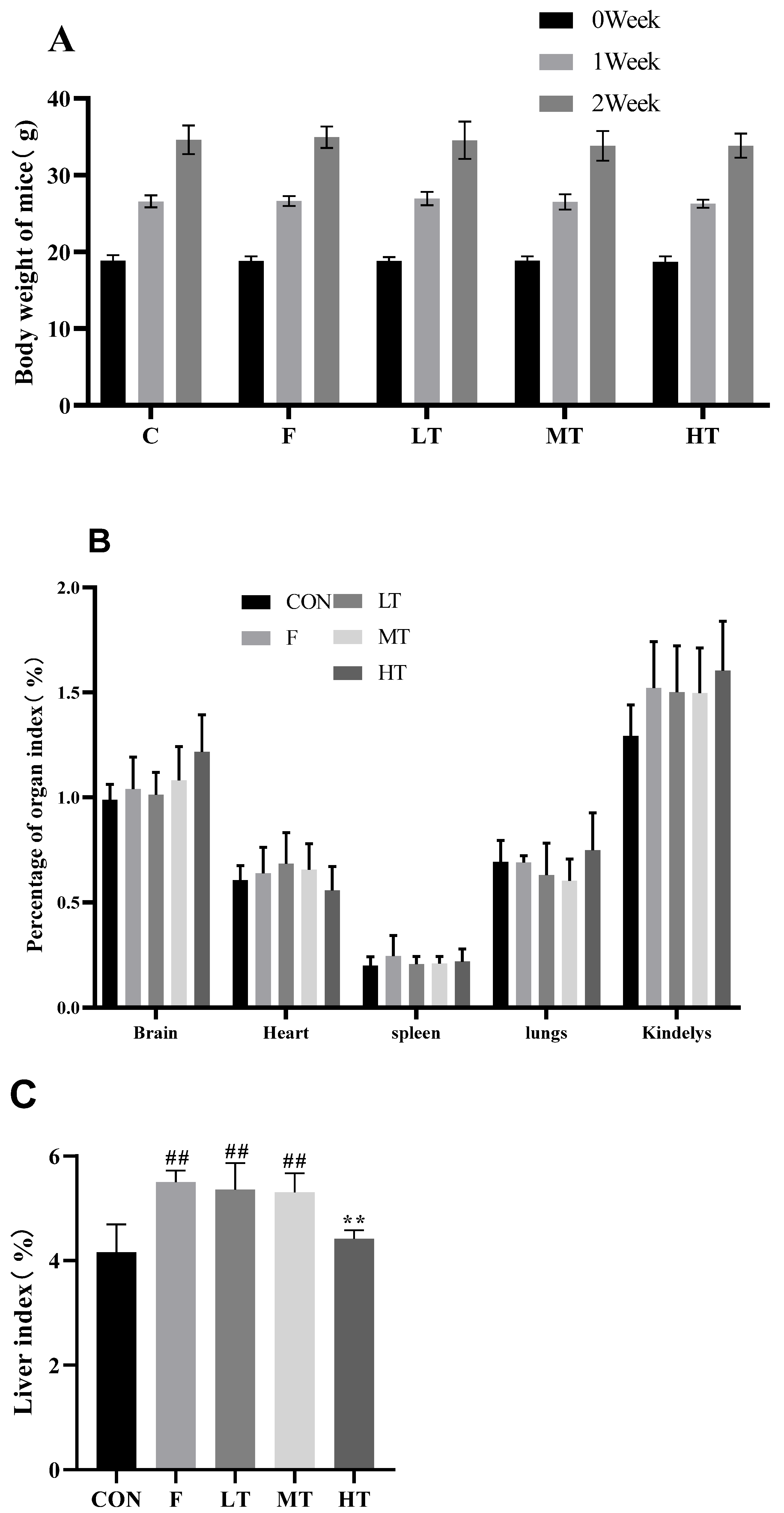
2.1. Body Weight and Liver Index
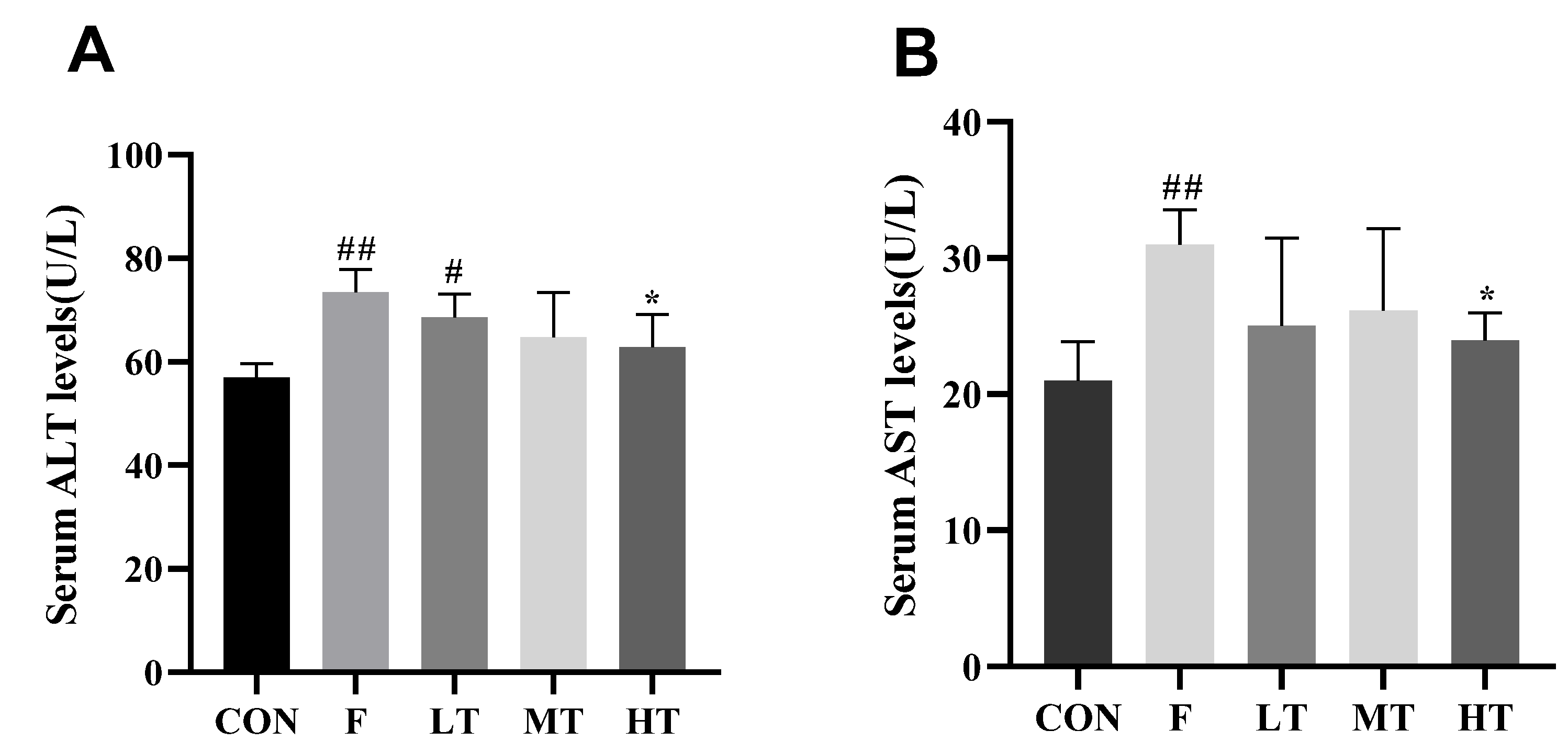
2.2. Serum Levels of ALT and AST
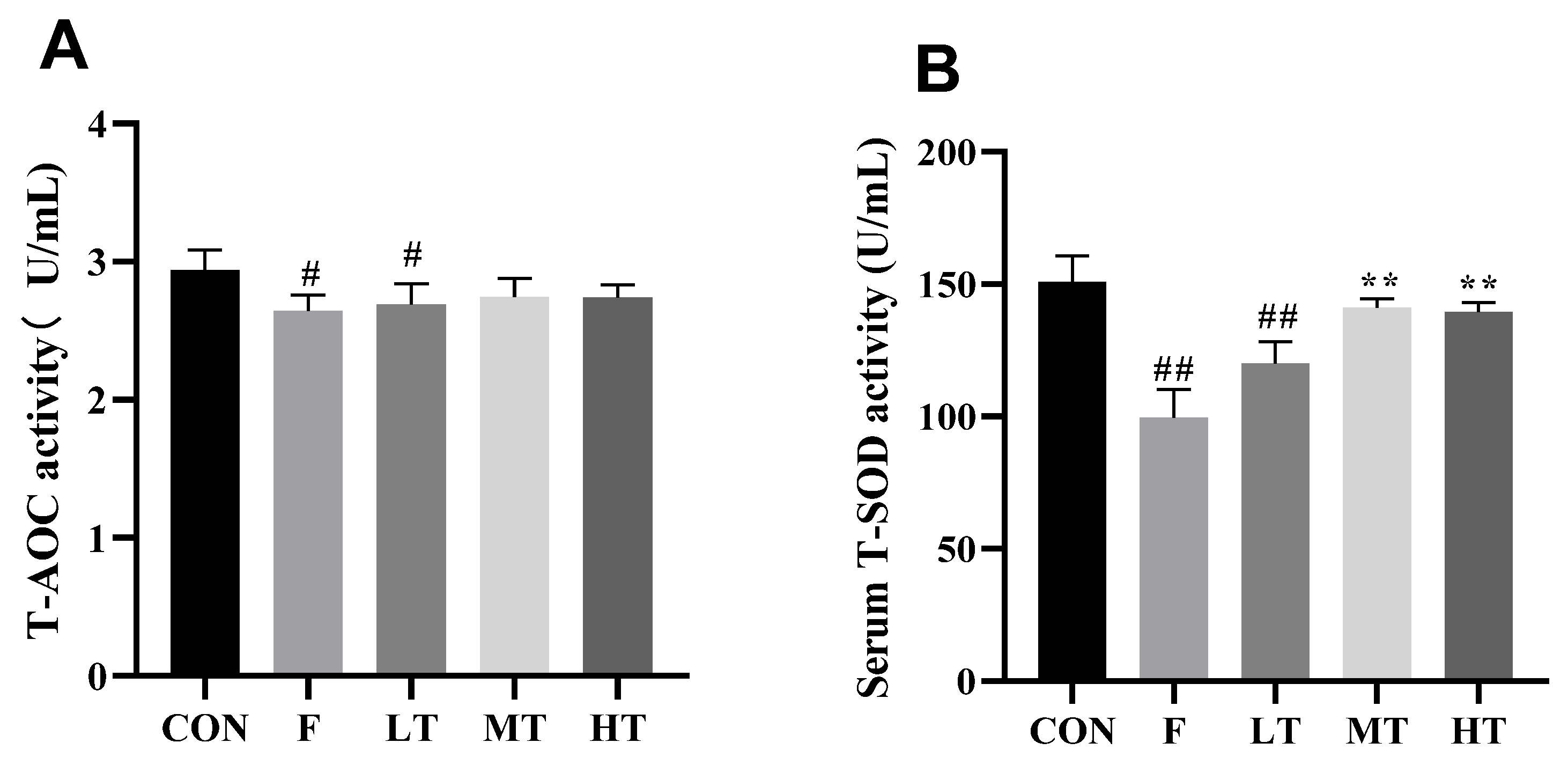
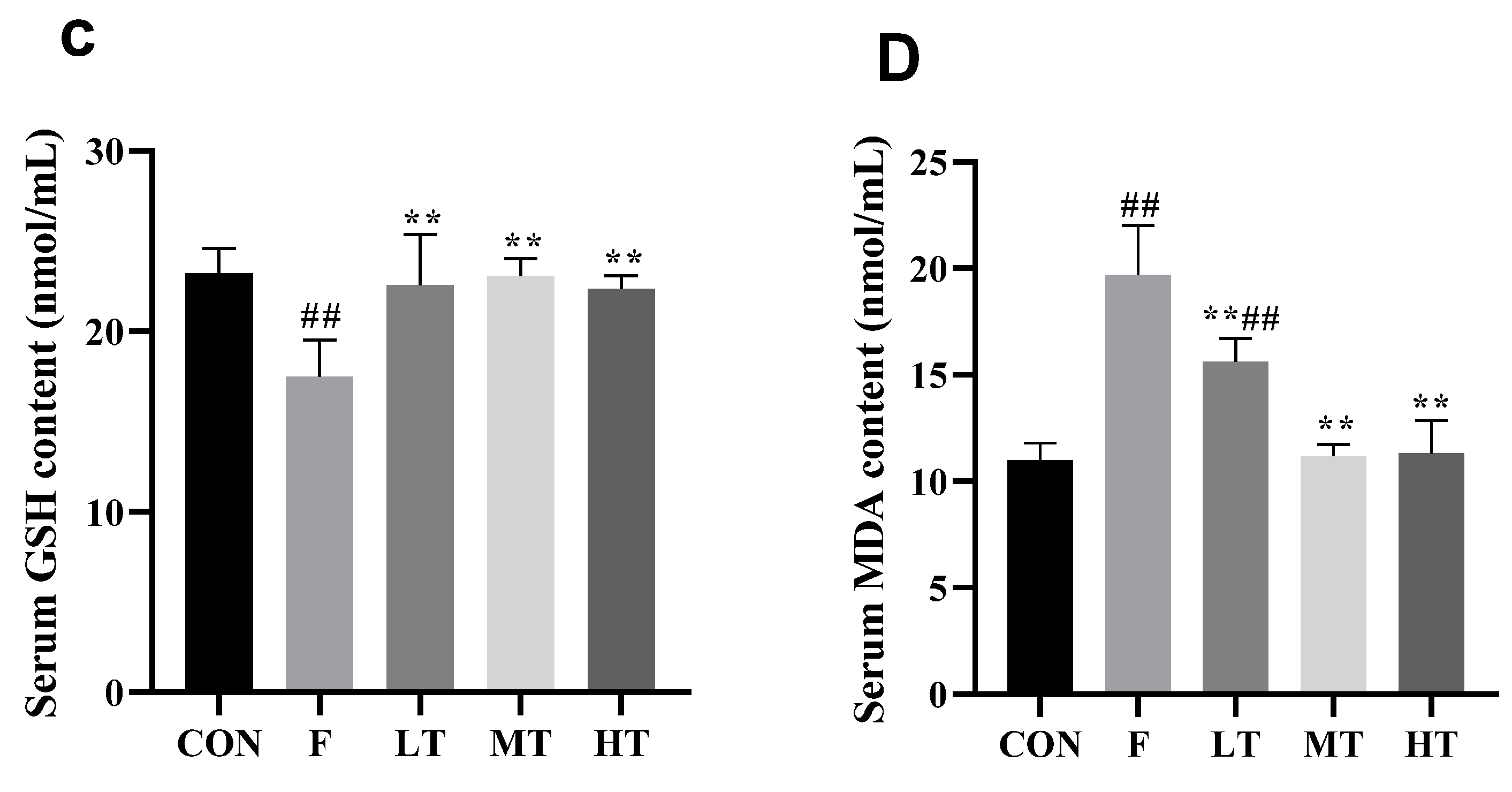
2.3. Serum Antioxidant Biomarkers
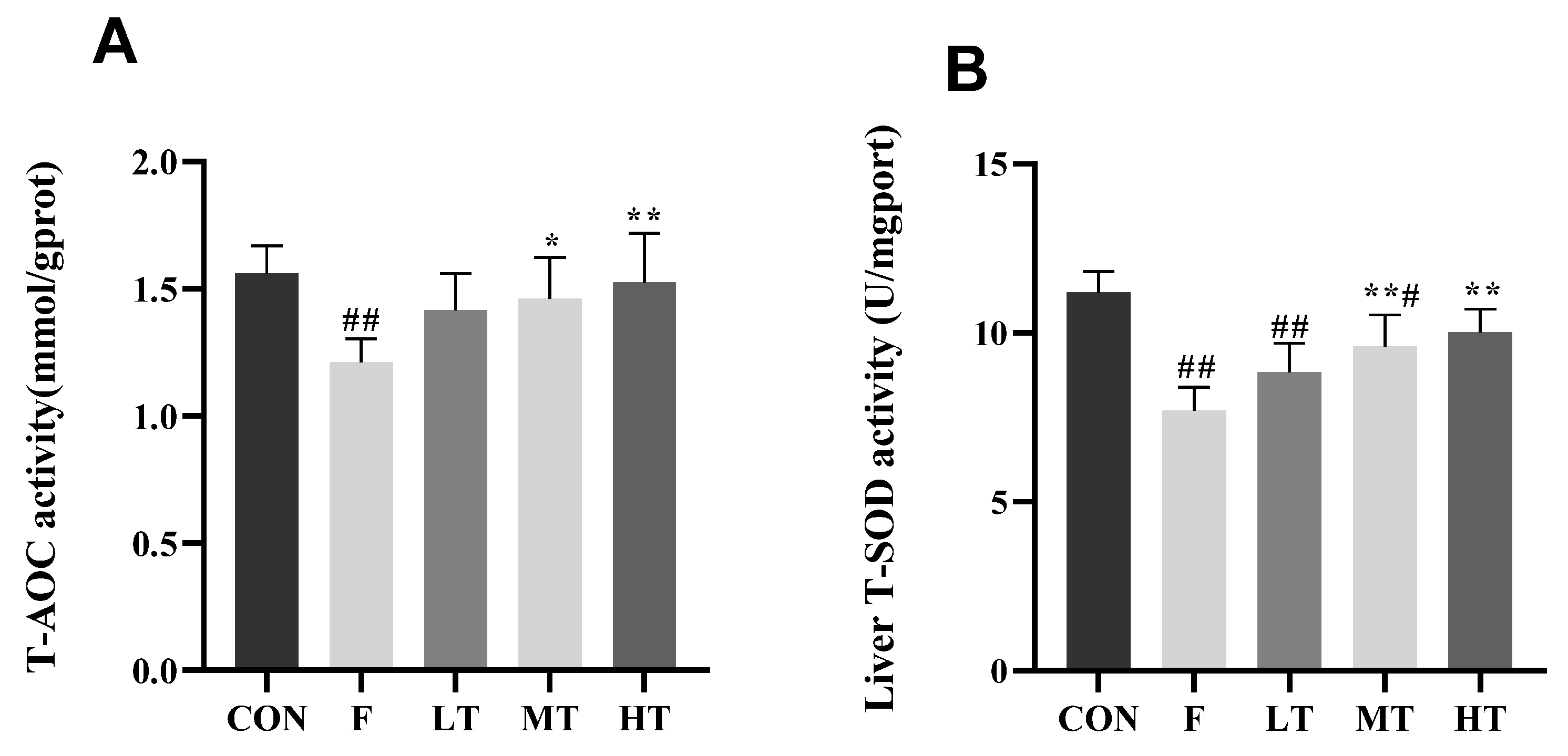
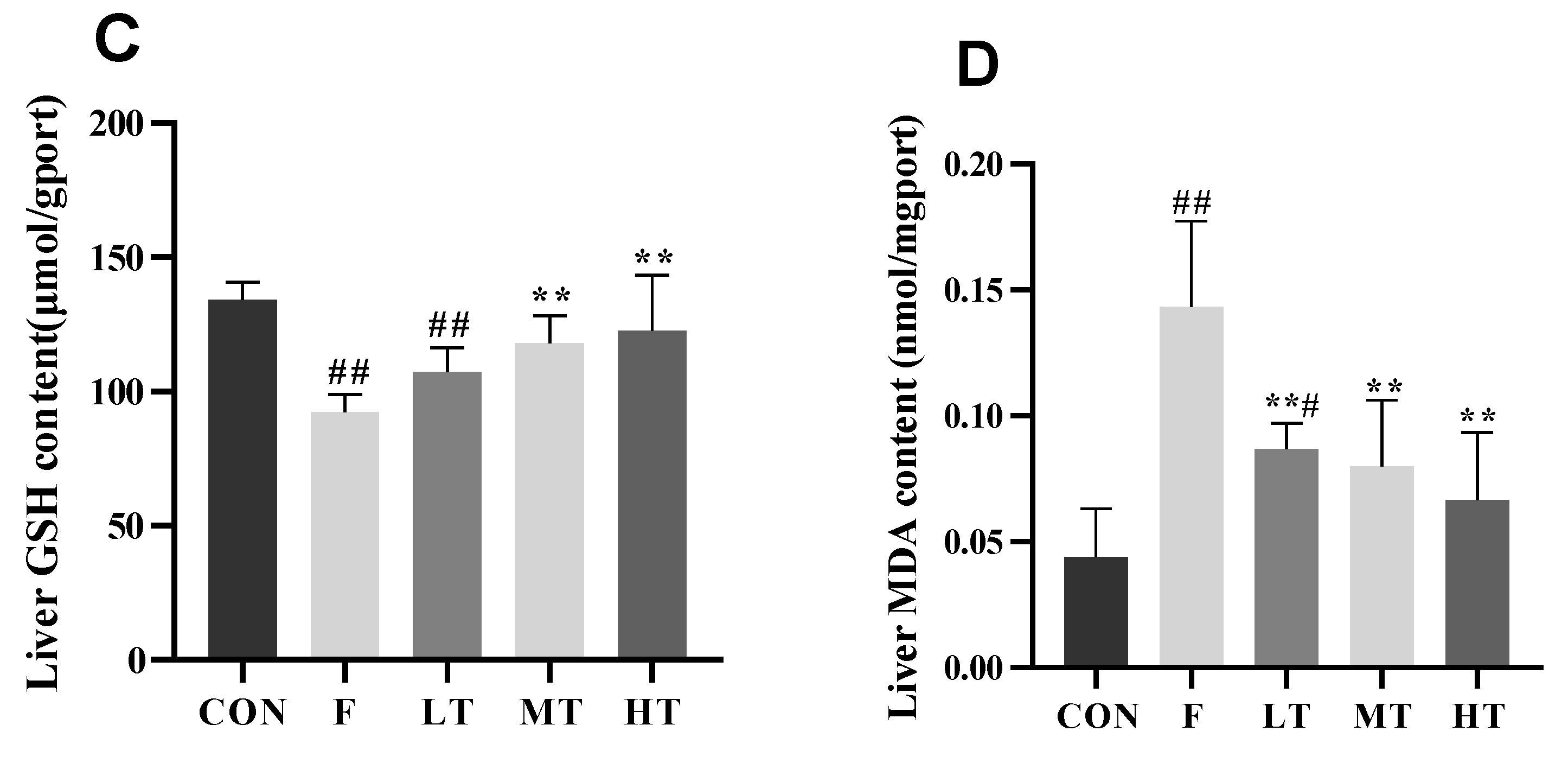
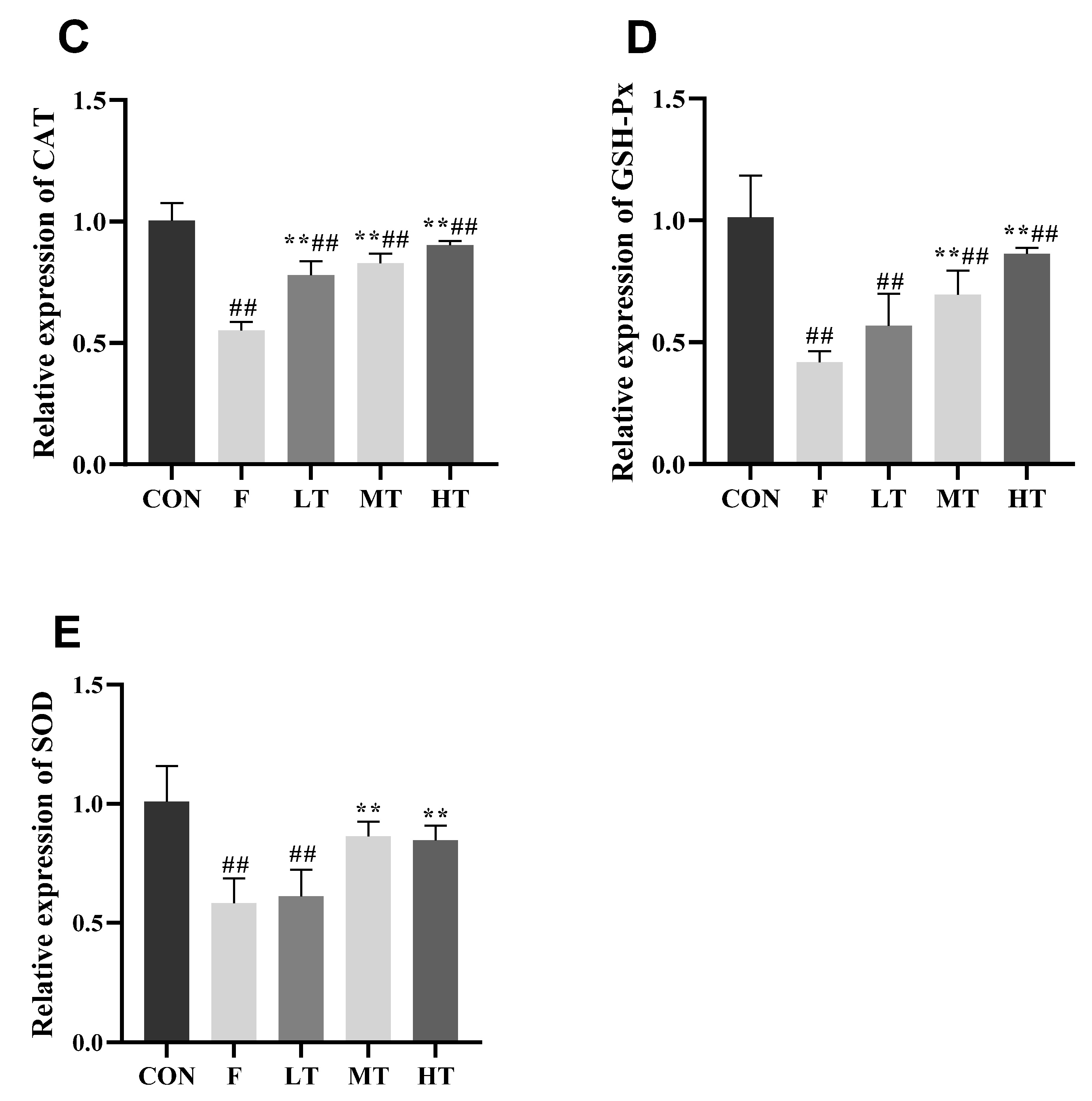
2.4. Hepatic Antioxidant Indicators
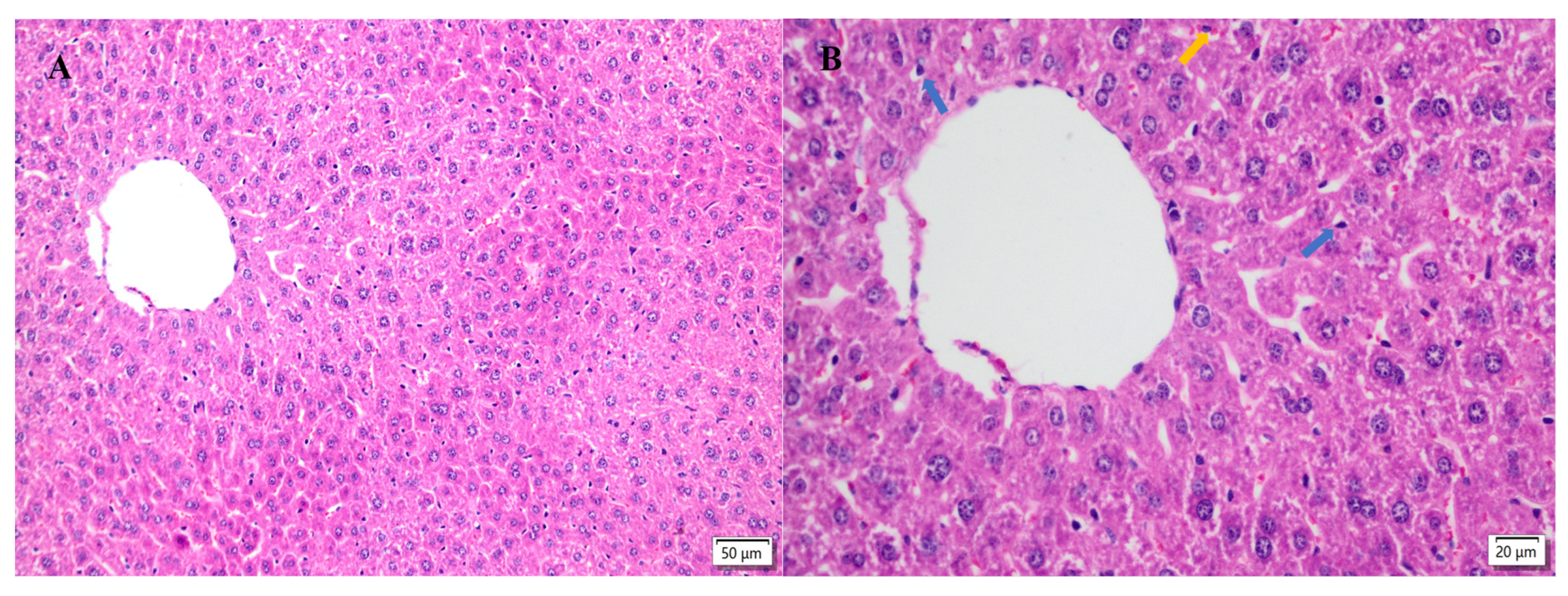
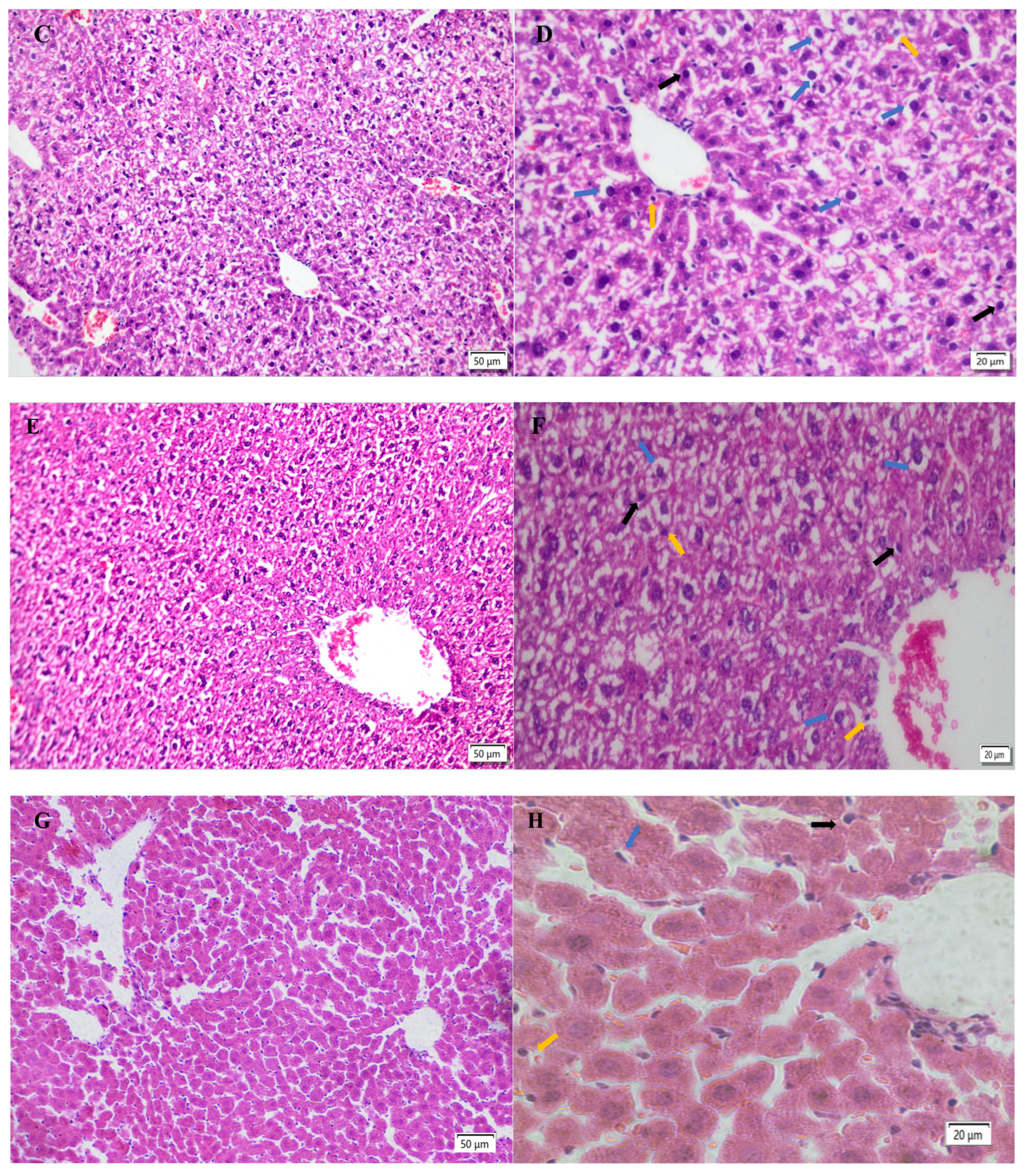
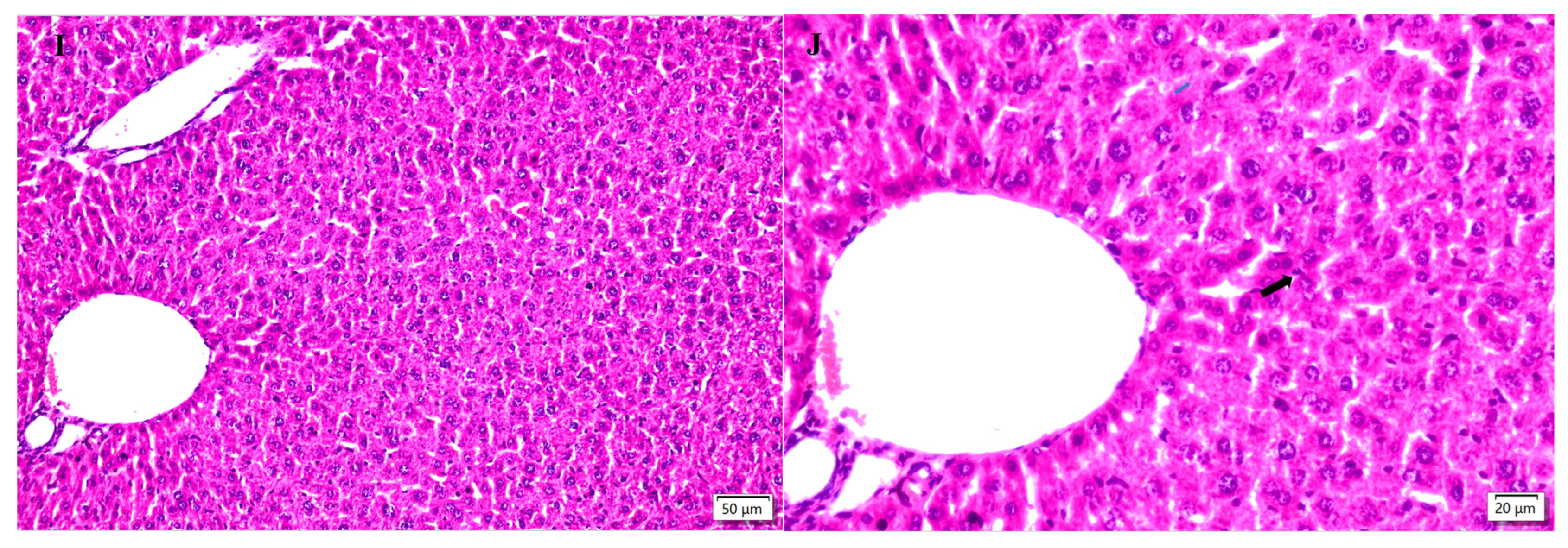
2.5. Hepatic Histopathological Changes
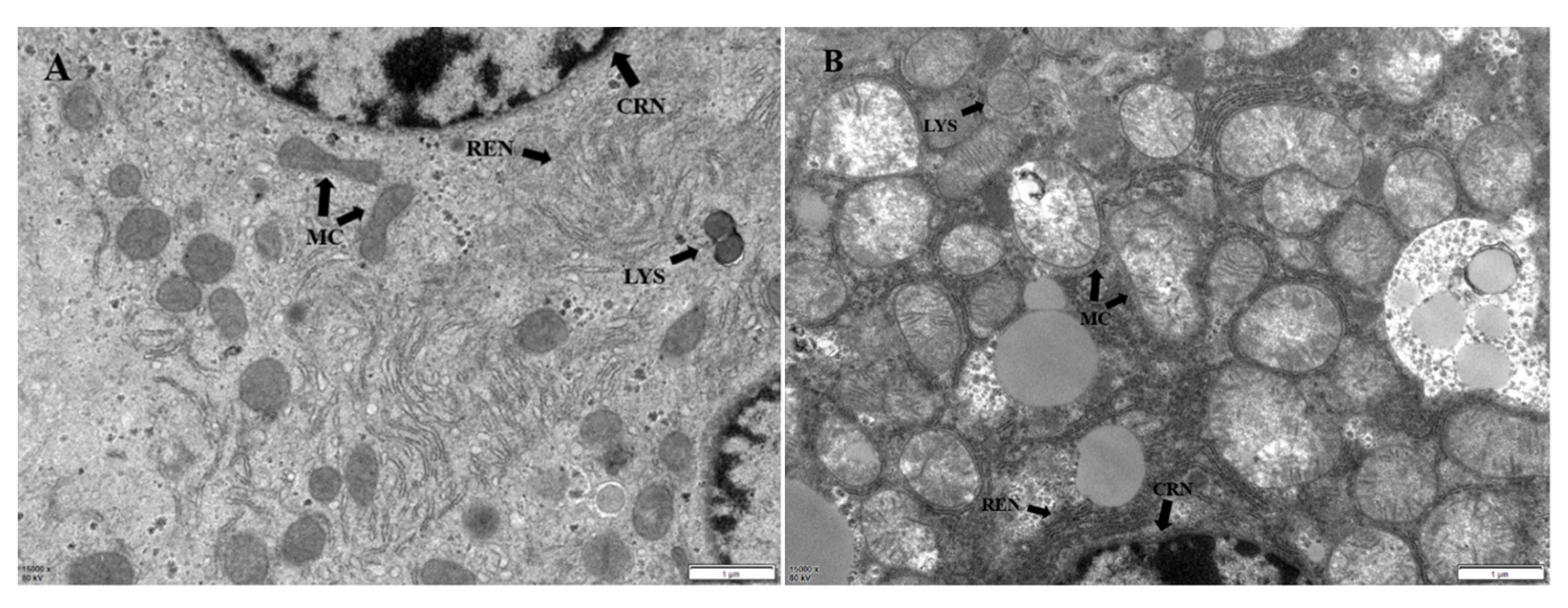
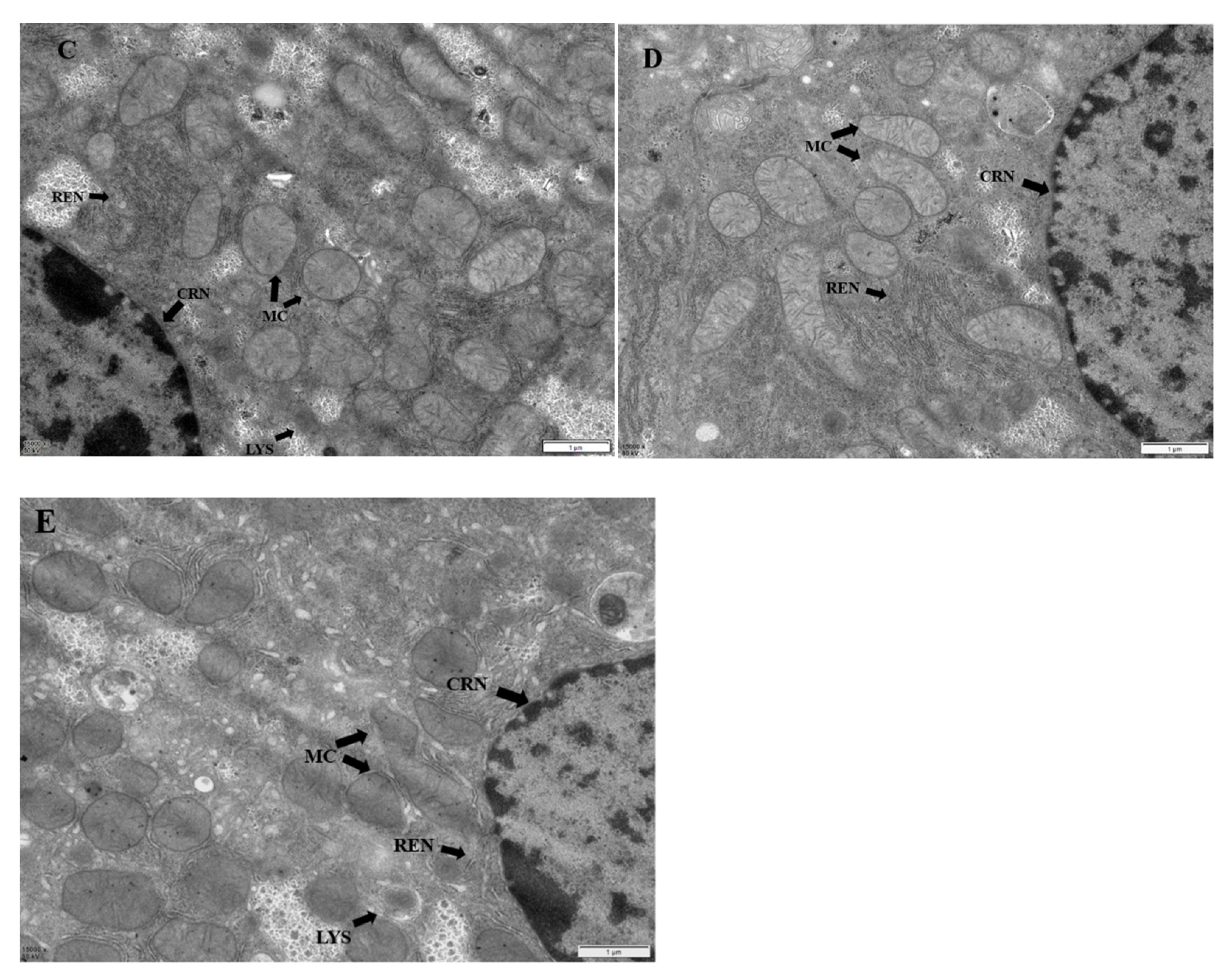
2.6. Hepatic Ultrastructural Changes
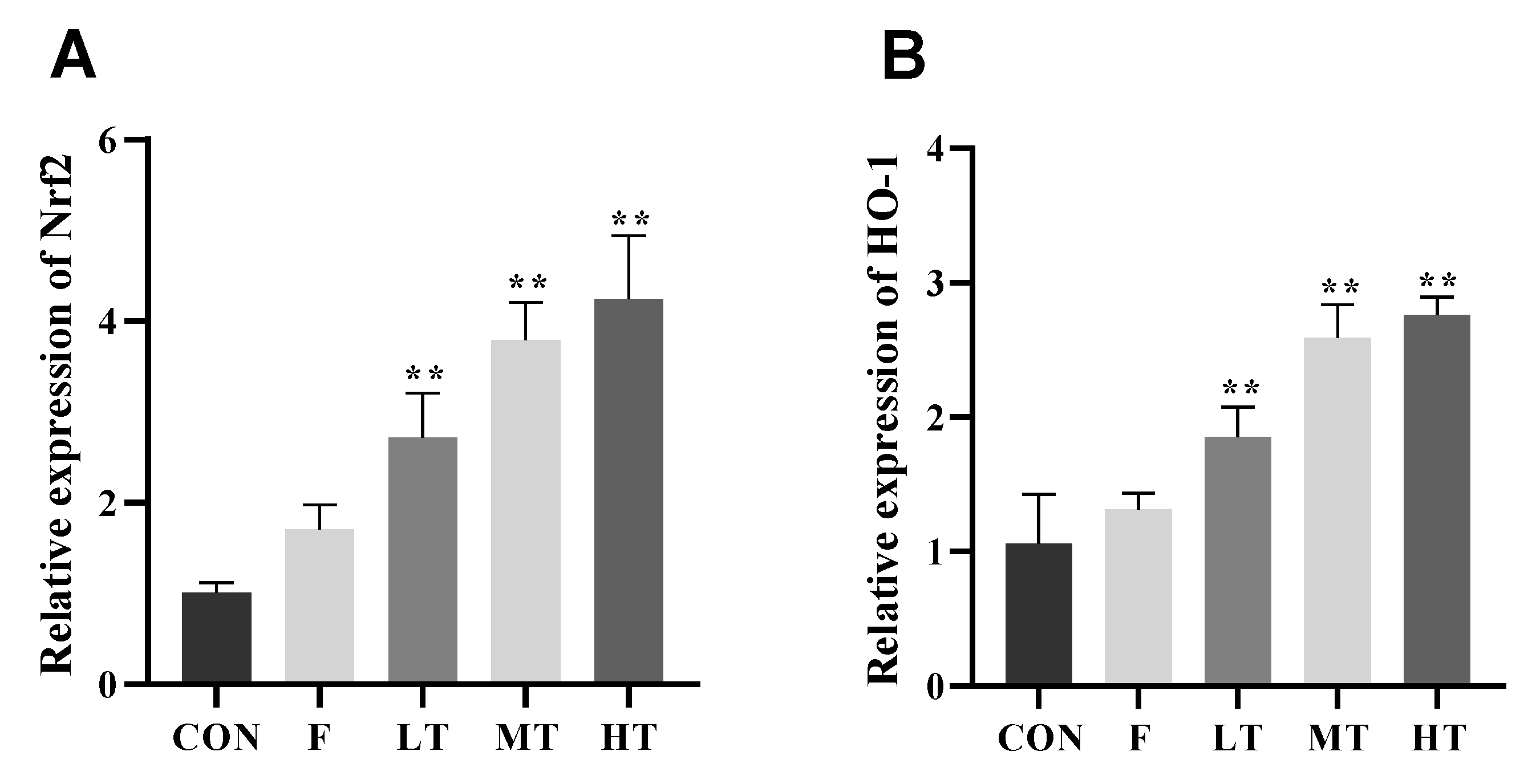
2.7. mRNA Expression
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Animals and Methods
3.3. Body Weight, Organ Index, and Liver Index
3.4. Detection of Serum Levels of ALT and AST
3.5. Detection of Serum Levels of Oxidative Stress Biomarkers
3.6. Detection of Hepatic Oxidative Stress Biomarkers
3.7. Hepatic Histopathological Changes
3.8. Ultrastructural Changes to Hepatocytes
3.9. RNA Extraction and qRT-PCR
3.10. Data Statistics and Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zou, J. Alteration of Liver Oxidative Stress Injury and Keap1-Nrf2 Signaling Pathway in Rats with Chronic Fluorosis and Screening of Antioxidant Activity of Botanical Extracts. Master’s Thesis, Guizhou Medical University, Guiyang, China, 2022. [Google Scholar]
- Kerdoun, M.A.; Mekhloufi, S.; Adjaine, O.; Bechki, Z.; Gana, M.; Belkhalfa, H. Fluoride concentrations in drinking water and health risk assessment in the south of Algeria. Regul. Toxicol. Pharmacol. 2022, 128, 105086. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Flora, S. Fluoride in Drinking Water and Skeletal Fluorosis: A Review of the Global Impact. Curr. Environ. Health Rep. 2020, 7, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.M.; Lakhkar, B.B.; Patil, S.S. Curse of Fluorosis. Indian J. Pediatr. 2018, 85, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Boone, J.S.; Vigo, C.; Boone, T.; Byrne, C.; Ferrario, J.; Benson, R.; Donohue, J.; Simmons, J.E.; Kolpin, D.W.; Furlong, T.E. Per- and polyfluoroalkyl substances in source and treated drinking waters of the United States. Sci. Total Environ. 2019, 653, 359–369. [Google Scholar] [CrossRef]
- Kabore, H.A.; Vo, D.S.; Munoz, G.; Méité, L.; Desrosiers, M.; Liu, J.X.; Sory, T.K.; Sauvé, S. Worldwide drinking water occurrence and levels of newly-identified perfluoroalkyl and polyfluoroalkyl substances. Sci. Total Environ. 2018, 616–617, 1089–1100. [Google Scholar] [CrossRef]
- Riddell, J.K.; Malin, A.J.; McCague, H.; Flora, D.B.; Till, C. Urinary Fluoride Levels among Canadians with and without Community Water Fluoridation. Int. J. Environ. Res. Public Health 2021, 18, 6203. [Google Scholar] [CrossRef]
- US Department of Health and Human Services Federal Panel on Community Water Fluoridation. U.S. Public Health Service Recommendation for Fluoride Concentration in Drinking Water for the Prevention of Dental Caries. Public Health Rep. 2015, 130, 318–331. [Google Scholar] [CrossRef]
- Ren, C.; Li, H.H.; Zhang, C.Y.; Song, X.C. Effects of chronic fluorosis on the brain. Ecotoxicol. Environ. Saf. 2022, 244, 114021. [Google Scholar] [CrossRef]
- Efe, U.; Dede, S.; Yuksek, V.; Çetin, S. Apoptotic and Oxidative Mechanisms in Liver and Kidney Tissues of Sheep with Fluorosis. Biol. Trace Elem. Res. 2021, 199, 136–141. [Google Scholar] [CrossRef]
- Perera, T.; Ranasinghe, S.; Alles, N.; Waduge, R. Effect of fluoride on major organs with the different time of exposure in rats. Environ. Health Prev. Med. 2018, 23, 17. [Google Scholar] [CrossRef]
- Kan, P.J.; Yao, X.W.; Wang, W. Overview of research on drugs for the treatment of endemic fluorosis. Endem. Dis. Control China 2022, 37, 385–386. [Google Scholar]
- Gong, Y.; Zheng, D.; Chen, F.P.; Yu, Y.N.; Guan, Z.Z.; Lou, D.D. Current status of endemic fluorosis and forensic identification. China Forensic Identif. 2022, 121, 35–40. [Google Scholar]
- Chen, Q.X.; Jiang, S.H.; Zhu, J. Current status of research on the therapeutic effects of Chinese medicine on endemic fluorosis. Endem. Dis. Control China 2021, 36, 215–216. [Google Scholar]
- Wang, L.D.; Wei, W.; Xiao, Q.F.; Yang, H.H.; Ci, X.X. Farrerol Ameliorates APAP-induced Hepatotoxicity via Activation of Nrf2 and Autophagy. Int. J. Biol. Sci. 2019, 15, 788–799. [Google Scholar] [CrossRef]
- Wang, D.; Yin, K.; Zhang, Y.; Lu, H.M.; Hou, L.L.; Zhao, H.J.; Xing, M.W. Novel pathways of fluoride-induced hepatotoxicity: P53-dependent ferroptosis induced by the SIRT1/FOXOs pathway and Nrf2/HO-1 pathway. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 264, 109526. [Google Scholar] [CrossRef]
- Yang, R.; Zhao, Y.Q.; Zhang, Q. Research progress of fluorosis on liver system damage. China Endem. Dis. Control 2022, 37, 198–199. [Google Scholar]
- Tian, X.L.; Feng, J.; Dong, N.S.; Lyu, Y.; Wei, C.L.; Li, B.; Ma, Y.Q.; Xie, J.X.; Qiu, Y.L.; Song, G.H. Subchronic exposure to fluoride and arsenite induces oxidative stress and activates the Nrf2 signaling pathway in the kidneys of rat offspring. Sci. Total Environ. 2019, 686, 1229–1237. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, Y.T.; Wei, N.; Guan, Z.Z. Expression of Nrf2 and HO1 protein in brain tissue of rats with chronic fluorosis. J. Guiyang Med. Coll. 2014, 39, 285–289. [Google Scholar]
- Feng, D.M. The Role of Nrf2/ARE Pathway in the Reproductive Damage of Male Rats Exposed to Fluoride and the Effect of NAC. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2016. [Google Scholar]
- Zhou, X.; Sun, X.Y.; Jia, L.Y.; Guo, M.; Jie, J.; Wang, Y.D.; Huang, L.; Li, Y.H.; He, Z.J.; Cao, H.L. Research progress on the application and mechanism of the Chinese medicine monomer Tetramethylpyrazine in the treatment of diseases. Shaanxi TCM 2022, 43, 541–544. [Google Scholar]
- AlKreathy, H.M.; Alghamdi, M.K.; Esmat, A. Tetramethylpyrazine ameliorates indomethacin-induced gastric ulcer in rats: Impact on oxidative, inflammatory, and angiogenic machineries. Saudi Pharm. J. 2020, 28, 916–926. [Google Scholar] [CrossRef]
- Xiao, L.; Mei, G.C.; Wu, L.H.; Xie, Y.X. Study on the protective effect of Danshen TMP injection on acute liver injury in young rats with sepsis. Chin. Tradit. Chin. Med. Emerg. 2019, 28, 1585–1589. [Google Scholar]
- Feng, L.; Zhao, J. Effect of Tetramethylpyrazine hydrochloride injection on hepatic ischemia-reperfusion injury through Nrf2/HO-1 pathway. Mod. Drugs Clin. 2023, 38, 22–28. [Google Scholar]
- Wang, W.Y.; Gui, C.Z.; Guan, Z.Z. Research progress of antagonists for fluorosis. Occup. Health 2021, 37, 2433–2435. [Google Scholar]
- Lu, C.F.; Xu, W.; Shao, J.; Zhang, F.; Chen, A.P.; Zheng, S.Z. Nrf2 Activation Is Required for Ligustrazine to Inhibit Hepatic Steatosis in Alcohol-Preferring Mice and Hepatocytes. Toxicol. Sci. Off. J. Soc. Toxicol. 2017, 155, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.J.; Li, J.S.; Sun, Y.M. Effects of acute fluorosis on nitric oxide synthase protein expression in mouse cerebral cortex. J. Environ. Health 2008, 25, 445–446. [Google Scholar]
- Li, X.; Yang, J.; Liang, C.; Yang, W.; Zhu, Q.L.; Luo, H.F.; Liu, X.Y.; Wang, J.D.; Zhang, J.H. Potential Protective Effect of Riboflavin against Pathological Changes in the Main Organs of Male Mice Induced by Fluoride Exposure. Biol. Trace Elem. Res. 2022, 200, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Li, J.; Yang, Y.; Wang, Y.; Jin, X.X.; Dong, Y.; Liu, Q.S.; Duan, X.X.; Yan, N. Sodium Butyrate Ameliorates Fluorosis-Induced Neurotoxicity by Regulating Hippocampal Glycolysis In Vivo. Biol. Trace Elem. Res. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Chen, L.; Huang, W.; Zhang, Z.L.; Wang, L.; Zhang, F.; Zheng, S.Z. Combined therapy with ligustrazine and paeonol mitigates hepatic fibrosis through destroying mitochondrial integrity of stellate cell. Am. J. Transl. Res. 2020, 12, 1255–1266. [Google Scholar] [PubMed]
- Zhao, Y.; Wang, J.; Zhang, J.; Sun, Z.L.; Niu, R.Y.; Manthari, R.K.; Ommati, M.M.; Wang, S.L.; Wang, J.D. Fluoride exposure induces mitochondrial damage and mitophagy via activation of the IL-17A pathway in hepatocytes. Sci. Total Environ. 2022, 804, 150184. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Q.Y.; Huang, M.; Jiang, W.; Ding, B.W.; Zhang, J.X.; Zhu, Q.X. The relationship between TNFR2 and immune liver injury in trichloroethylene sensitized mice. J. Anhui Med. Univ. 2021, 56, 602–607. [Google Scholar]
- Lehmann-Werman, R.; Magenheim, J.; Moss, J.; Neiman, J.; Abraham, O.; Piyanzin, S.; Zemmour, H.; Fox, I.; Dor, T.; Grompe, M. Monitoring liver damage using hepatocyte-specific methylation markers in cell-free circulating DNA. JCI Insight 2018, 3, e120687. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.W.; Zhang, X.L.; Wu, Q.H.; Jin, Y.B.; Ning, C.T.; Wang, R.; Mao, J.X.; Chen, M. The hepatoprotective effects of Sedum sarmentosum extract and its isolated major constituent through Nrf2 activation and NF-kappaB inhibition. Phytomedicine 2019, 53, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, D.; Andjelic, T.; Ninkovic, M.; Dejanovic, B.; Kotur-Stevuljevic, K. Superoxide dismutase (SOD), advanced oxidation protein products (AOPP), and disease-modifying treatment are related to better relapse recovery after corticosteroid treatment in multiple sclerosis. Neurol. Sci. 2021, 42, 3241–3247. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Effect of Different Doses of Calcium on Liver Injury in Fluorosis Rats. Master’s Thesis, Shanxi Agricultural University, Shanxi, China, 2019. [Google Scholar]
- Chen, W.J.; Wu, L.W.; Peng, J.Y.; Liang, C.N.; HE, T.G.; Wu, C.Z.; Tang, W.C. Protective effect of Xiao Chai Hu Tang on liver injury caused by chronic fluorosis in rats. China Hosp. Drug Eval. Anal. 2022, 22, 277–280. [Google Scholar]
- Zhang, Z.; Zhou, B.; Wang, H.; Wang, F.; Song, Y.L.; Liu, S.N.; Xi, S.H. Maize purple plant pigment protects against fluoride-induced oxidative damage of liver and kidney in rats. Int. J. Environ. Res. Public Health 2014, 11, 1020–1033. [Google Scholar] [CrossRef]
- Zhou, B.H.; Wei, S.S.; Jia, L.S.; Zhang, Y.; Miao, C.Y.; Wang, H.W. Drp1/Mff signaling pathway is involved in fluoride-induced abnormal fission of hepatocyte mitochondria in mice. Sci. Total Environ. 2020, 725, 138192. [Google Scholar] [CrossRef]
- Chan, B.; Elmasry, M.; Forootan, S.S.; Russomanno, G.; Bunday, T.M.; Zhang, F.; Brillant, N.; Lewis, P.S.; Aird, R.; Ricci, E. Pharmacological Activation of Nrf2 Enhances Functional Liver Regeneration. Hepatology 2021, 74, 973–986. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Yang, H.; Zhang, X.H. Study of Nrf2/HO-1 signaling pathway in chronic liver disease. Chin. J. Liver Dis. (Electron. Version) 2017, 9, 36–39. [Google Scholar]
- Jiang, L.; Rui, Y.J.; Dai, J.Q.; Ding, G.F. Effects of tanshinone IIA on hydrogen peroxide-induced apoptosis and oxidative stress in human lens epithelial cells. New Adv. Ophthalmol. 2021, 41, 838–842. [Google Scholar]
- Chen, J.Q.; Zhao, L.; Ding, X.H.; Wang, L.D.; Wen, Y.; Xie, H.; Pen, H. Ligustrazine inhibits high glucose-induced neutrophil extracellular capture network formation by regulating NRF2/HO-1 pathway. J. Army Med. Univ. 2022, 44, 2157–2164. [Google Scholar]
- Lu, C.; Xu, W.; Zhang, F.; Shao, J.J.; Zheng, S.Z. Nrf2 knockdown attenuates the ameliorative effects of ligustrazine on hepatic fibrosis by targeting hepatic stellate cell transdifferentiation. Toxicology 2016, 365, 35–47. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zheng, Y.; Du, H.; Zhang, W.; Li, H.; Ou, Y.; Xu, F.; Lin, J.; Fu, H.; Ni, X.; et al. The Pathophysiological Changes and Clinical Effects of Tetramethylpyrazine in ICR Mice with Fluoride-Induced Hepatopathy. Molecules 2023, 28, 4849. https://doi.org/10.3390/molecules28124849
Zhang S, Zheng Y, Du H, Zhang W, Li H, Ou Y, Xu F, Lin J, Fu H, Ni X, et al. The Pathophysiological Changes and Clinical Effects of Tetramethylpyrazine in ICR Mice with Fluoride-Induced Hepatopathy. Molecules. 2023; 28(12):4849. https://doi.org/10.3390/molecules28124849
Chicago/Turabian StyleZhang, Shuai, Yilei Zheng, Hong Du, Wei Zhang, Haohuan Li, Yangping Ou, Funeng Xu, Juchun Lin, Hualin Fu, Xueqing Ni, and et al. 2023. "The Pathophysiological Changes and Clinical Effects of Tetramethylpyrazine in ICR Mice with Fluoride-Induced Hepatopathy" Molecules 28, no. 12: 4849. https://doi.org/10.3390/molecules28124849
APA StyleZhang, S., Zheng, Y., Du, H., Zhang, W., Li, H., Ou, Y., Xu, F., Lin, J., Fu, H., Ni, X., Chang, L.-J., & Shu, G. (2023). The Pathophysiological Changes and Clinical Effects of Tetramethylpyrazine in ICR Mice with Fluoride-Induced Hepatopathy. Molecules, 28(12), 4849. https://doi.org/10.3390/molecules28124849