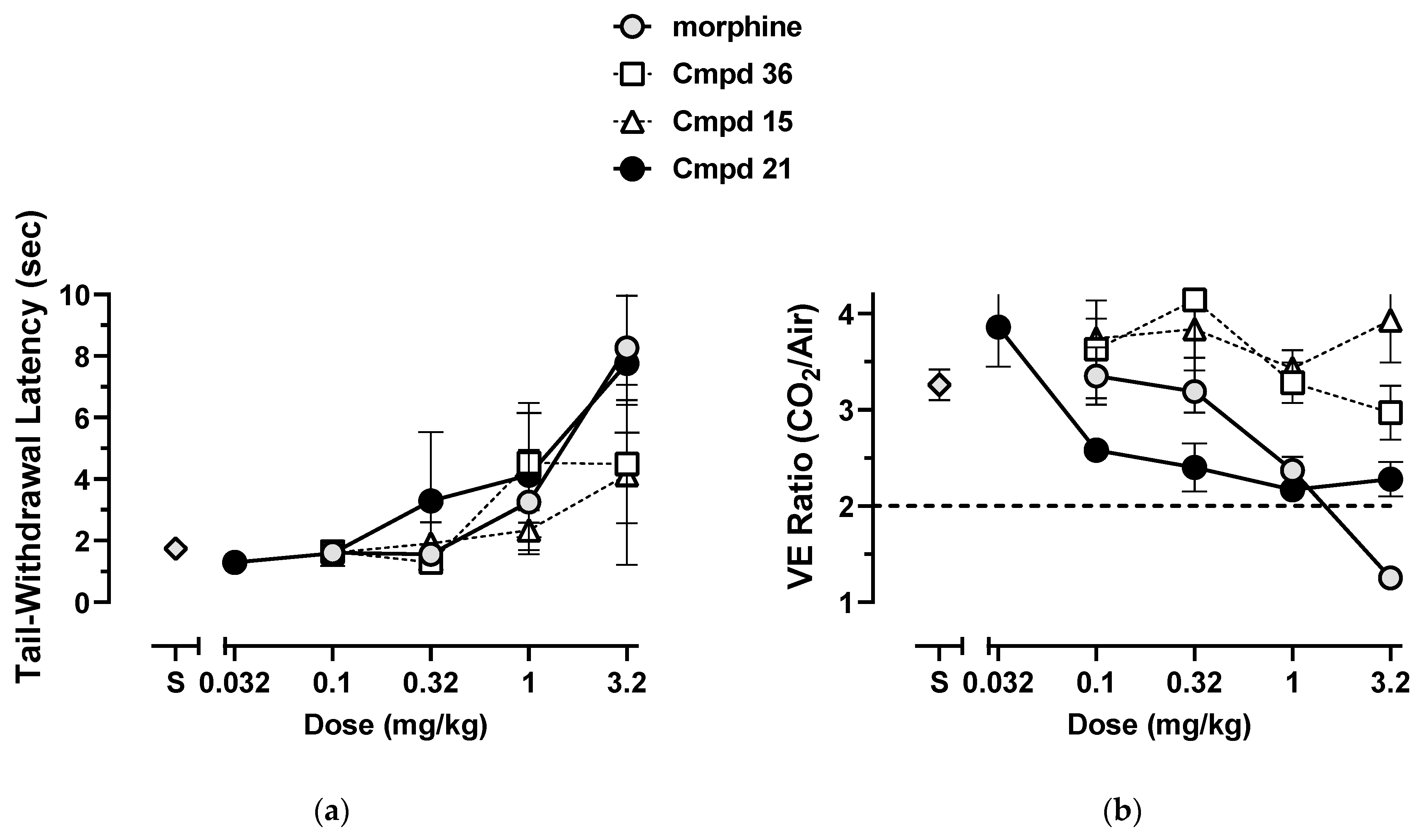
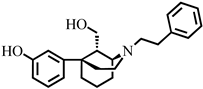
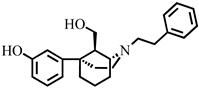
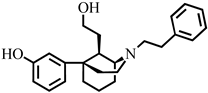
3.2. Synthesis
General crystallization method for C9-hydroxy(alkyl)-5-phenylmorphan salts. The free base was dissolved in isopropanol (2–4 mL/g) to make a saturated solution. A solution of 48% HBr (1 equiv.) was added dropwise. This mixture was stirred at room temperature for 1 h. In the case of crystals not forming after 1 h, diethyl ether was added dropwise until the solution remained briefly cloudy before becoming clear again then left to stir overnight. In the case of neither method working, the isopropanol–free base solution was placed in a sealed chamber of diethyl ether to allow vapor diffusion overnight. Obtained solid was recrystallized from 8% methanol in isopropanol (10–20 mL/g) at 80 °C. The solution was allowed to slowly cool to room temperature.
(1R,5R)-5-(3-Methoxyphenyl)-2-azabicyclo[3.3.1]nonan-9-one (
5). Tertiary amine
4 (408 mg, 1.573 mmol, 1.0 equiv) was dissolved in dry acetonitrile (10 mL) and to this solution was added K
2CO
3 (435 mg, 3.146 mmol, 2.0 equiv) followed by cyanogen bromide (472 µL of a 5.0 M solution in acetonitrile, 3.146 mmol, 2.0 equiv). The reaction was stirred at room temperature for 2 h before being brought to reflux for 1 h. Methanol (1.5 mL) was added and stirred for 10 min. Solvent was removed and the residue taken up in CHCl
3 (20 mL) and washed with water (15 mL). The organic layer was dried with MgSO
4. Chloroform was removed under vacuum and the residue was dissolved in 3N HCl (10 mL) and heated at reflux for 16 h. The reaction mixture was transferred to a separatory funnel and made basic (pH > 10.5) with 2 M KOH. The aqueous layer was extracted with CHCl
3 (20 mL × 2) and the organic layer was dried over MgSO
4 and concentrated. The crude residue was purified via flash chromatography eluting with 0–10% 50:45:5 CHCl
3:MeOH:NH
4OH in CHCl
3 to yield
9 as a brown oil (197 mg, 51%). Data for
5 were consistent with compound
4 in reference [
17].
(1R,5R)-5-(3-Methoxyphenyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-9-one (
6). Secondary amine
5 (404 mg, 1.647 mmol, 1.0 equiv) was dissolved in dry acetonitrile (10 mL) and to this solution was added K
2CO
3 (455 mg, 3.294 mmol, 2.0 equiv) followed by phenethyl bromide (457 mg, 337 µL, 2.47 mmol, 1.5 equiv). The solution was brought to reflux and left under N
2 for 16 h. The mixture was filtered through celite, concentrated, and purified by flash chromatography eluting with 0–10% 50:45:5 CHCl
3:MeOH:NH
4OH to yield
6 as a colorless foam (445 mg, 77%). Data for
6 were consistent with compound
5 in reference [
17].
(1R,5R, E&Z)-9-(Methoxymethylene)-5-(3-methoxyphenyl)-2-phenethyl-2-azabicyclo [3.3.1]nonane (
7). To as suspension of tertiary amine 6 (445 mg, 1.273 mmol, 1.0 equiv) and (methoxymethyl)triphenylphosphonium chloride (1.310 g, 3.820 mmol, 3.0 equiv) in dry tetrahydrofuran (6 mL) at 0 °C was added LiHMDS (3.31 mL of 1.0 M solution in THF, 2.6 equiv) dropwise over 10 min. After 30 min the ice bath was removed, and the solution was stirred under argon for 16 h. The mixture was cooled to 0 °C and methanol (4.5 mL) was added and stirred for 10 min. The solvents were removed under vacuum and the residue was taken up in CHCl
3 (50 mL) and washed with water which was made basic (pH > 10.5) with NH
4OH. The combined organic extracts were washed with brine, dried over MgSO
4, concentrated, and purified via flash chromatography eluting with 25–100% ethyl acetate in
n-hexane to give 7 as a yellow oil (413 mg, 86%). Data matched compound 12 in reference [
15].
((1R,5S,9R & 1R,5S,9S)-5-(3-Methoxyphenyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-9-yl)methanol (8 & 9). A mixture of the E and Z isomers of enol ether 7 (810 mg, 2.146 mmol, 1.0 equiv) in dry tetrahydrofuran (8 mL) was added dropwise to a stirred solution of 4 N HCl (8 mL) and this solution was stirred under argon for 16 h. Methanol (5 mL) was added dropwise, and the reaction was stirred for 15 min before removing volatile solvents under vacuum. The aqueous mixture was cooled to 0 °C and made basic (pH > 10.5) with NH4OH and extracted with 9:1 CHCl3:MeOH (20 mL × 2). The organic extracts were washed with brine, dried over MgSO4, and solvent removed in vacuo. The crude residue was taken directly to the next step. The crude material was dissolved in tetrahydrofuran (8 mL) and this solution was cooled to 0 °C. To the cooled solution was added NaCNBH3 (192 mg, 3.219 mmol, 1.5 equiv) and the solution was stirred at 0 °C for 10 min. The ice bath was removed and the solution stirred at room temperature for 1 h. The solution was again cooled to 0 °C and water (10 mL) added dropwise. The mixture was diluted with CHCl3 (25 mL) and the aqueous layer was made basic (pH > 10.5) with NH4OH and extracted with 9:1 CHCl3:MeOH (20 mL × 2). The organic extracts were washed with brine, dried over MgSO4, and solvent removed in vacuo. The crude residue was purified via flash chromatography eluting with 0.5–10% 50:45:5 CHCl3:MeOH:NH4OH to yield 8 as a teal oil and 9 as a yellow foam in a 3.6:1 diastereomeric ratio (369 mg of 9, 47% and 103 mg of 8, 13%).
For 1R,5S,9S-8: 1H-NMR (400 MHz; CDCl3): δ 7.31–7.18 (m, 6H), 7.04–6.97 (m, 2H), 6.73 (dd, J = 8.1, 2.1 Hz, 1H), 3.79 (s, 3H), 3.53 (t, J = 10.5 Hz, 1H), 3.41 (dd, J = 11.2, 4.5 Hz, 1H), 3.28 (s, 1H), 3.03 (dd, J = 10.3, 4.1 Hz, 2H), 2.91–2.80 (m, 4H), 2.50 (dt, J = 9.1, 4.2 Hz, 1H), 2.25 (dt, J = 13.6, 9.6 Hz, 1H), 1.98–1.93 (m, 4H), 1.82–1.72 (m, 2H), 1.57–1.49 (m, 1H); 13C-NMR (100 MHz; CDCl3): δ 159.8, 151.3, 140.9, 129.4, 128.9, 128.5, 126.1, 118.1, 112.3, 110.6, 61.3, 58.4, 55.3, 53.1, 49.9, 48.6, 41.7, 37.5, 34.9, 29.7, 21.9; HRMS-ESI (m/z): [M +H]+ calcd for C24H31NO2: 366.2433; found: 366.2433.
For 1R,5S,9R-9: 1H-NMR (400 MHz; CDCl3): δ 7.30–7.17 (m, 6H), 6.92–6.88 (m, 2H), 6.71 (dd, J = 8.1, 2.0 Hz, 1H), 3.78 (s, 3H), 3.64 (dd, J = 11.2, 4.0 Hz, 1H), 3.57 (dd, J = 10.8, 3.2 Hz, 1H) 3.30 (s, 1H), 3.09–3.15 (m, 2H), 2.88–2.71 (m, 5H), 2.32–2.27 (m, 1H), 2.05–1.83 (m, 4H), 1.75–1.68 (m, 2H), 1.56–1.49 (m, 1H); 13C-NMR (100 MHz; CDCl3): δ 159.6, 150.9, 140.2, 129.2, 128.7, 128.5, 126.2, 118.1, 112.4, 110.2, 64.4, 57.0, 56.9, 55.2, 48.9, 45.2, 43.0, 38.4, 34.5, 31.3, 25.3, 23.2; HRMS-ESI (m/z): [M +H]+ calcd for 366.2433 C24H31NO2, found: 366.2433.
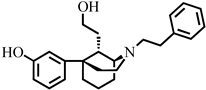
3-((1R,5S,9S)-9-(Hydroxymethyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (10). A solution of 8 (247 mg, 0.676 mmol, 1.0 equiv.) in dry dichloromethane (9 mL) was brought to −78 °C under an atmosphere of argon. To this cooled solution was added BBr3 (677 mg, 256 µL, 2.703 mmol, 4.0 equiv.) dropwise and the solution was stirred and allowed to warm to room temperature under argon for 16 h. The solution was cooled to 0 °C and methanol (3 mL) was added dropwise and stirred for 30 min. A solution of 1N HCl (4 mL) was added, and the mixture was brought to 100 °C. After 1 h, the solution was cooled to 0 °C, made basic (pH > 10.5) with NH4OH, and then extracted with 9:1 CHCl3 and MeOH (30 mL × 2). The organic extracts were washed with brine, dried over MgSO4, and solvent was removed in vacuo. The crude residue was purified via flash chromatography eluting with 20–100% EtOAc in n-hexane to give 10 as a colorless oil (167 mg, 70%) [α]D25 −27.3° (c 0.55, CHCl3). The free base was crystallized as the hydrobromide salt from isopropanol and diethyl ether by adding 48% HBr, mp 243–245 °C. 1H-NMR (400 MHz; CDCl3): δ 7.30–7.09 (m, 7H), 6.79 (d, J = 8.7 Hz, 2H), 6.58 (dd, J = 7.9, 1.4 Hz, 1H), 3.68 (dd, J = 11.1, 4.0 Hz, 1H), 3.55 (dd, J = 11.2, 2.6 Hz, 1H), 3.33 (s, 1H), 3.17–3.13 (m, 2H), 2.93–2.78 (m, 5H), 2.34–2.29 (m, 1H), 2.04–1.87 (m, 5H), 1.76–1.69 (m, 2H), 1.60–1.50 (m, 1H); 13C-NMR (100 MHz; CDCl3): δ 156.5, 150.6, 140.0, 129.4, 128.70, 128.67, 126.4, 117.4, 113.2, 112.8, 64.7, 57.5, 56.9, 48.9, 45.0, 42.7, 38.3, 34.4, 31.3, 25.3, 23.2; HRMS-ESI (m/z): [M +H]+ calcd for C23H29NO2 352.2277; found: 352.2274. HBr salt: mp: 243–245 °C; anal. calcd for C23H30BrNO2·0.5 H2O: C, 62.62%; H, 7.02%; N, 3.19%. Found C, 62.58%; H, 7.08%; N, 3.17%.
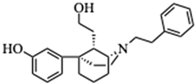
3-((1R,5S,9R)-9-(Hydroxymethyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (11). A solution of 9 (165 mg, 0.451 mmol, 1.0 equiv.) was dissolved in dry dichloromethane (7 mL) and brought to −78 °C under an atmosphere of argon. To this cooled solution was added BBr3 (452 mg, 171 µL, 1.806 mmol, 4.0 equiv.) dropwise and the solution was stirred and allowed to warm to room temperature under argon for 16 h. The solution was cooled to 0 °C and methanol (2 mL) was added dropwise and stirred for 30 min. A solution of 1N HCl (4 mL) was added and the mixture was brought to 100 °C in a distillation apparatus. After 1 h, the solution was cooled to 0 °C, made basic (pH >10.5) with NH4OH, and then extracted with 9:1 CHCl3:MeOH (30 mL × 2). The organic extracts were washed with brine, dried over MgSO4, and concentrated. The crude residue was purified via flash chromatography eluting with 20–100% EtOAc in n-hexane to give 11 as a colorless oil (116 mg, 73%) [α]D25 + 24.5° (c 0.27, CHCl3). The free base was crystallized as the hydrobromide salt from isopropanol and diethyl ether by adding 48% HBr, mp 257–259 °C. 1H-NMR (400 MHz; CDCl3): δ 7.28–7.07 (m, 7H), 6.82 (d, J = 7.7 Hz, 1H), 6.71 (d, J = 7.9 Hz, 1H), 3.57–3.50 (m, 3H), 3.09–3.02 (m, 2H), 2.91–2.77 (m, 4H), 2.29–2.21 (m, 1H), 2.01–1.85 (m, 4H), 1.75–1.64 (m, 2H), 1.38–1.15 (m, 2H). 13C-NMR (100 MHz; CDCl3): δ 156.9, 150.0, 139.3, 129.5, 128.7, 128.5, 126.3, 116.7, 114.1, 112.5, 59.8, 57.6, 52.9, 49.9, 46.7, 41.1, 36.8, 33.1, 29.0, 21.5, 17.5; HRMS-ESI (m/z): [M +H]+ calcd for C23H29NO2 352.2277; found: 352.2275. HBr salt: mp: 257–259 °C; anal. calcd for C23H30BrNO2·0.1 H2O: C, 63.46%; H, 6.90%; N, 3.20%. Found C, 63.57%; H, 7.01%; N, 3.22%.
(1S,5S,E&Z)-9-(Methoxymethylene)-5-(3-methoxyphenyl)-2-phenethyl-2-azabicyclo [3.3.1]nonane (12). See synthesis of compound 7. 1H NMR (400 MHz; CDCl3): δ 7.32–7.17 (m, 6H), 7.02 (d, J = 7.9 Hz, 1H), 6.97 (s, 1H), 6.76 (d, J = 8.3 Hz, 1H), 5.85 (s, 1H), 5.20 (s, 1H), 4.07 (bs, 1H), 3.82 (s, 3H), 3.41 (s, 3H), 3.08 (dt, J = 11.5, 5.9 Hz, 1H), 2.92–2.75 (m, 5H), 2.34 (dt, J = 13.5, 6.7 Hz, 1H), 2.20–1.92 (m, 5H), 1.75–1.68 (m, 1H), 1.54–1.42 (m, 1H), 13C NMR (101 MHz; CDCl3): δ 159.1, 149.5, 141.6, 140.8, 128.8, 128.6, 128.3, 125.9, 123.2, 119.9, 113.7, 110.6, 59.5, 58.8, 55.2, 52.1, 49.0, 40.6, 39.0, 38.2, 34.5, 29.7, 20.6. HRMS-ESI (m/z): [M +H]+ calcd for C25H32NO2 378.2433, found 378.2432.
((1S,5R,9R & 1S,5R,9S)-5-(3-Methoxyphenyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-9-yl)methanol (13 & 14). A 25 mL single-neck round-bottom flask was charged with 4N aq HCl (7.3 mL). A solution of enol ether 12 (0.276 g, 0.73 mmol) in THF (7.3 mL) was added dropwise to the flask and stirred under argon at room temperature for 18 h. TLC analysis revealed complete consumption of the enol ethers. The reaction was cooled to 0 °C in an ice bath and charged with NaCNBH3 (0.069 g, 0.1 mmol). TLC analysis revealed complete consumption of the intermediate aldehydes after 2 h. The reaction was quenched with MeOH (5 mL) and stirred for 10 min. The bulk of the solvent was removed in vacuo and the residue was taken up in CHCl3 (10 mL) and H2O (10 mL). The aqueous phase was made alkaline with concentrated aq NH4OH (1 mL) and extracted with CHCl3 (3 × 10 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated in vacuo. The resulting residue was purified via flash chromatography eluting with EtOAc/hexanes (0 to 100%) to afford 9-hydroxymethyl-5-phenylmorphans 13:14 as a 1:1.5 mixture of epimers (0.183g, 0.52 mmol, 69%).
For 1S,5R,9R-13: 1H NMR (400 MHz; CDCl3): δ 7.31–7.16 (m, 6H), 6.91 (d, J = 7.9 Hz, 1 H), 6.87 (s, 1H), 6.71 (d, J = 8.1 Hz, 1H), 3.79 (s, 3H), 3.64 (dd, J = 11.3, 3.4 Hz, 1H), 3.56 (dd, J = 11.5, 2.6 Hz, 1H), 3.31 (s, 1H), 3.13 (d, J = 10.1 Hz, 2H), 2.92–2.67 (m, 5H), 2.30 (dd, J = 14.6, 4.7 Hz, 1H), 2.06 (s, 1H), 2.00–1.81 (m, 2H), 1.77–1.65 (m, 2H), 1.59–1.47 (m, 1H); 13C NMR (101 MHz; CDCl3): δ 159.5, 150.8, 140.1, 129.1, 128.6, 128.4, 118.0, 112.4, 110.1, 64.4, 56.9, 55.2, 48.8, 45.0, 42.9, 38.3, 34.4, 31.2, 25.2, 23.1. HRMS-ESI (m/z): [M +H]+ calcd For C24H32NO2 366.2433, found 366.2437.
For 1S,5R,9S-14: 1H NMR (400 MHz; CDCl3): δ 7.33–7.18 (m, 6H), 7.04 (d, J = 8.0 Hz, 1H), 6.98 (s, 1H), 6.74 (d, J = 7.8 Hz, 1H), 3.81 (s, 3H), 3.53 (t, J = 10.5 Hz, 1H), 3.42 (dd, J = 11.1, 4.4 Hz, 1H), 3.31 (s, 1H), 3.05 (dd, J = 9.2, 2.8 Hz, 1H), 2.95–2.79 (m, 4H), 2.53 (bs, 1H), 2.32–2.21 (m, 1H), 2.01–1.93 (m, 4H), 1.84–1.71 (m, 2H), 1.59–1.47 (m, 1H). 13C NMR (101 MHz; CDCl3): δ 159.6, 151.0, 140.6, 129.3, 128.8, 128.4, 126.0, 118.0, 112.1, 110.5, 61.2, 58.2, 55.2, 52.9, 49.7, 48.4, 41.4, 37.3, 34.6, 29.5, 21.7, 18.7; HRMS-ESI (m/z): [M +H]+ calcd for C24H32NO2 366.2433, found 366.2434.
3-((1S,5R,9R)-9-(Hydroxymethyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (15). A 10 mL flame-dried round-bottom flask was charged with phenylmorphan 13 (0.099 g, 0.27 mmol) and DCM (2.7 mL). The flask was cooled to −78 °C and charged with BBr3 (0.077 mL, 0.81 mmol) dropwise over 5 min. The reaction was allowed to warm gradually to room temperature over the course of 4 h at which time all the starting material was consumed as determined by TLC. The reaction was cooled to 0 °C and quenched by the dropwise addition of MeOH (2 mL). The crude reaction mixture was transferred to a separatory funnel and portioned between water (10 mL) and CHCl3 (10 mL). The aqueous layer was made basic by addition of saturated aq NH4OH and extracted with 9:1 CHCl3:MeOH (3 × 10 mL). The combined organic extracts were dried over MgSO4, filtered, and concentrated in vacuo. The resulting residue was purified by column chromatography eluting with EtOAc/hexanes (0 to 100%) to afford 15 as a white foam (0.076 g, 0.22 mmol, 81%): 1H-NMR (400 MHz; CDCl3): δ 7.32–7.28 (m, 2H), 7.22–7.20 (m, 3H), 7.15 (t, J = 8.1 Hz, 1H), 6.82 (m, 2H), 6.65 (d, J = 8.0 Hz, 1H), 3.64 (dd, J = 11.3, 3.5 Hz, 1H), 3.55 (dd, J = 11.1, 2.4 Hz, 1H), 3.32 (bs, 1H), 3.17–3.13 (m, 2H), 2.91–2.78 (m, 4H), 2.71 (q, J = 11.6 Hz, 1H), 2.31 (d, J = 15.3 Hz, 1H), 2.05–1.88 (m, 5H), 1.78–1.69 (m, 3H); 13C NMR (101 MHz; CDCl3): δ 156.4, 150.5, 139.9, 129.3, 128.6, 128.5, 126.2, 117.2, 113.0, 112.7, 64.4, 57.1, 56.8, 48.8, 45.1, 42.4, 38.1, 34.3, 31.2, 25.1, 23.0; HRMS-ESI (m/z): [M +H]+ calcd for C23H30NO2 352.2277, found 352.2275. The free base was converted to its HBr salt for analysis and optical rotation, mp 237–240 °C, [α]D25 +37.8° (c 0.27, MeOH). Anal. calcd for C25H30BrNO2·0.3 H2O C, 63.10%; H, 7.04; N, 3.20%. Found C, 63.24%; H, 7.06%; N, 3.10%.
3-((1S,5R,9S)-9-(Hydroxymethyl)-2-phenethyl-2-azabicyclo [3.3.1]nonan-5-yl)phenol (16). See procedure for synthesis of 15, phenylmorphan 16 was isolated a white foam (0.045 g, 0.13 mmol, 71%). 1H-NMR (400 MHz; CDCl3): δ 7.23 (d, J = 7.3 Hz, 2H), 7.15 (m, 2H), 7.06 (d, J = 7.2 Hz, 2H), 7.00 (s, 1H), 6.82 (d, J = 7.8 Hz, 1H), 6.68 (d, J = 8.0 Hz, 1H), 3.56–3.48 (m, 3H), 3.04–2.95 (m, 2H), 2.89–2.66 (m, 5H), 2.15 (q, J = 11.5 Hz, 1H), 2.05–1.75 (m, 5H), 1.72–1.59 (m, 1H), 1.60–1.49 (m, 1H); 13C NMR (101 MHz; CDCl3): δ 157.0, 150.5, 139.9, 129.5, 128.7, 128.4, 126.1, 116.9, 114.5, 113.4, 60.1, 57.7, 52.1, 50.1, 47.4, 41.3, 37.0, 33.5, 29.1, 21.7, 17.6; HRMS-ESI (m/z): [M +H]+ calcd for C23H30NO2 352.2277, found 352.2276. The free base was converted to its HBr salt for analysis. Anal. calcd for C25H30BrNO2·0.2 H2O C, 63.36%; H, 7.03; N, 3.21%. Found C, 63.31%; H, 7.02%; N, 3.09%.
tert-Butyl (1S,5S)-5-(3-methoxyphenyl)-9-oxo-2-azabicyclo[3.3.1]nonane-2-carboxylate (17). To a solution of the 1S,5S-relative of 5, (1S,5S)-5-(3-methoxyphenyl)-2-azabicyclo[3.3.1]nonan-9-one (4.6 g, 18 mmol) in acetonitrile (27 mL) was added potassium carbonate (5.4 g, 2.2 equiv, 39 mmol). The flask was purged with argon and cyanogen bromide (3.8 g, 7.1 mL, 5.0 molar, 2.0 equiv, 35 mmol) was added dropwise. The mixture was stirred at room temperature for 1.5 h, then brought to reflux for 1.5 h. At this point TLC showed consumption of starting material. Methanol (3.0 mL) and 2N HCl (37 mL) were added, and the solution was brought to reflux. The solution was left to stir at reflux overnight. After 18 h the solution was cooled to 0 °C and 7 M NH4OH in MeOH was added until pH 11. The aqueous mixture was extracted with CHCl3 and concentrated in vacuo. The crude mixture was dissolved in dry dichloromethane (20 mL) at 0 °C and to this was added di-tert-butyl dicarbonate (3.9 g, 1.1 equiv, 19 mmol), N,N-dimethylpyridin-4-amine (430 mg, 0.2 equiv, 3.5 mmol), and triethylamine (1.8 g, 2.5 mL, 1.1 equiv, 18 mmol) dropwise. The solution was stirred under argon. After 1 h, TLC showed consumption of starting material. Saturated ammonium chloride was added, and the mixture was extracted with dichloromethane (30 mL × 2), washed with brine, and dried over sodium sulfate. The crude mixture was loaded onto silica and purified via flash chromatography eluting with 0–30% ethyl acetate in hexane to yield 17 as a yellow oil (3.39 g, 55%) [α]D25 −33.8° (c 1.4, CHCl3). 1H-NMR (400 MHz; CDCl3): δ 7.30–7.26 (m, 1H), 6.81–6.77 (m, 3H), 4.39–4.09 (m, 2H), 3.80 (s, 3H), 3.21–3.14 (m, 1H), 2.65–2.48 (m, 2H), 2.39–2.27 (m, 2H), 2.23–2.14 (m, 2H), 1.80–1.64 (m, 2H), 1.49 (s, 9H); 13C-NMR (100 MHz; CDCl3): δ 159.6, 155.0, 145.9, 129.4, 119.7, 113.9, 111.7, 80.7, 64.0, 55.5, 53.2, 41.3, 41.0, 38.5, 35.9, 28.7, 17.9; HRMS-ESI (m/z): [M +H]+ calcd for C20H27NO4Na 368.1838; found: 368.1833.
tert-Butyl (1S,5S,E)-9-(2-ethoxy-2-oxoethylidene)-5-(3-methoxyphenyl)-2-azabicyclo[3.3.1]nonane-2-carboxylate (18). To dry tetrahydrofuran (10 mL) was added sodium hydride (275 mg, 60% weight, 3.0 equiv, 6.87 mmol), followed by slow addition of ethyl 2-(diethoxyphosphoryl)acetate (1.540 g, 1.36 mL, 3.0 equiv., 6.87 mmol). After 15 min, tert-butyl 17 (791 mg, 1 equiv., 2.29 mmol) was added dropwise as a solution in tetrahydrofuran (10 mL). The mixture was brought to reflux under an argon atmosphere. After 16 h, the mixture was cooled to 0 °C and ethanol (3 mL) was added. After stirring for 15 min, silica (3 g) was added directly to the mixture and then concentrated in vacuo. The mixture was purified via flash chromatography eluting with 0–45% ethyl acetate in hexane to give 18 as a colorless foam (834 mg, 88%) 1H-NMR (400 MHz; CDCl3): δ 7.32–7.25 (m, 1H), 6.90–6.78 (m, 3H), 6.01–5.95 (m,1H), 5.11 (s, 1H), 4.12–4.07 (m, 4H), 3.81 (s, 3H), 3.22–3.14 (m, 1H), 2.34–2.26 (m, 3H), 2.07 (d, J = 11.8 Hz, 3H), 1.71–1.68 (m, 1H), 1.51 (s, 9H), 1.22 (t, J = 7.1 Hz, 3H); 13C-NMR (100 MHz; CDCl3): δ 166.0, 164.5, 159.4, 155.5, 149.00, 148.95, 129.2, 119.91, 119.90, 119.88, 116.1, 113.9, 111.0, 79.7, 60.0, 55.2, 51.3, 45.7, 40.32, 40.26, 38.5, 33.8, 28.5, 18.1, 14.2; HRMS-ESI (m/z): [M +H]+ calcd for C24H33NO5 416.2437; found: 416.2445.
Ethyl 2-((1S,5R,9R & 1S,5R,9S)-5-(3-methoxyphenyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-9-yl)acetate (19 and 20). Compound 18 (185 mg, 1 equiv, 443 µmol) was dissolved in isopropyl acetate (50 mL) and isopropanol (5 mL) in a 100 mL pear shaped flask. The vessel was attached to a Thales-Nano H-Cube Pro flow reactor. The solution was put through the reactor at a temperature of 80 °C, a pressure of 45 psi, and a flow rate of 0.4 mL/min. The reaction was monitored by 1H NMR to determine the consumption of starting material. The resulting solution was concentrated and redissolved in dichloromethane (5 mL) and brought to 0 °C. To this cooled solution was added 2,2,2-trifluoroacetic acid (505 mg, 339 µL, 10 equiv, 4.43 mmol) dropwise. After 15 min, the reaction was allowed to warm to room temperature. After 1 h, TLC showed consumption of starting material. Saturated NaHCO3 (15 mL) was added to quench the reaction and the solution was extracted with dichloromethane (15 mL × 3). The organic fractions were washed with brine, dried over MgSO4, and concentrated. The crude residue was dissolved in dry acetonitrile (20 mL) and K2CO3 (122 mg, 886 µmol, 2.0 equiv.) was added, followed by (2-bromoethyl)benzene (98.4 mg, 71.9 µL, 532 µmol, 1.2 equiv.). This mixture was brought to reflux and stirred for 16 h. The solution was then cooled to room temperature, filtered through celite, concentrated, and purified via flash chromatography eluting with 3–50% ethyl acetate in hexane to give the diastereomers 19 (96 mg, 51%) and 20 (21 mg, 11%).
For 19-9R: [α]D25 +5.64° (c 3.0, CHCl3). 1H-NMR (400 MHz; CDCl3): δ 7.27–7.16 (m, 6H), 6.93–6.88 (m, 2H), 6.72–6.70 (m, 1H), 4.02 (q, J = 7.1 Hz, 2H), 3.78 (s, 3H), 3.13–2.96 (m, 3H), 2.86–2.62 (m, 6H), 2.29–2.25 (m, 1H), 2.14–2.07 (m, 1H), 2.00 (dd, J = 13.4, 4.8 Hz, 1H), 1.92–1.82 (m, 3H), 1.75 (dd, J = 12.6, 5.0 Hz, 1H), 1.70–1.66 (m, 1H), 1.60–1.54 (m, 1H), 1.18 (t, J = 7.1 Hz, 3H); 13C-NMR (100 MHz; CDCl3): δ 174.3, 159.6, 151.6, 140.9, 129.2, 128.7, 128.2, 125.7, 117.9, 111.8, 110.5, 59.9, 56.7, 55.1, 54.5, 49.0, 42.6, 41.8, 38.7, 34.4, 32.7, 30.1, 25.7, 23.4, 14.2; HRMS-ESI (m/z): [M +H]+ calcd for C24H31NO2: 422.2695; found: 422.2698.
For 20-9S: [α]D20 +1.84° (c 2.4, CHCl3). 1H-NMR (400 MHz; CDCl3): δ 1H-NMR (400 MHz; CDCl3): δ 7.32–7.18 (m, 6H), 7.03–6.98 (m, 2H), 6.75–6.73 (m, 1H), 4.05 (t, J = 7.1 Hz, 2H), 3.80 (s, 3H), 3.05–3.01 (d, 3H), 2.90–2.80 (m, 5H), 2.29–2.23 (m, 2H), 2.09–1.94 (m, 5H), 1.81–1.78 (m, 2H), 1.58–1.56 (m, 1H), 1.19 (t, J = 7.1 Hz, 3H); 13C-NMR (100 MHz; CDCl3): δ13-C NMR (101 MHz; CDCl3): δ 13C-NMR (100 MHz; CDCl3): δ 173.0, 159.6, 150.9, 140.6, 129.2, 128.7, 128.3, 125.9, 118.1, 111.9, 110.9, 60.2, 58.0, 55.1, 54.4, 49.7, 41.9, 41.2, 38.2, 34.5, 33.4, 29.7, 28.9, 21.6, 18.6, 14.2; HRMS-ESI (m/z): [M +H]+ calcd for C24H31NO2: 422.2695; found: 422.2699.
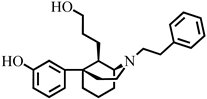
3-((1S,5R,9R)-9-(2-Hydroxyethyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (21). A flame-dried flask was charged with lithium aluminum hydride (55.7 mg, 95% weight, 3.0 equiv, 1.20 mmol) and brought to 0 °C under an argon atmosphere. To this flask was added dry tetrahydrofuran (1.5 mL). After 5 min, 19 (196 mg, 1 equiv, 401 µmol) was added dropwise as a solution in dry tetrahydrofuran (1.0 mL × 2). After 20 min, the ice bath was removed. Reaction was complete by TLC after 1 h. The mixture was cooled to 0 °C and water (400 µL) added to quench the reaction. After 10 min, sodium sulfate (500 mg) was added directly to the solution and the mixture was stirred for 10 min. The solution was filtered through celite and the filter was washed with dichloromethane (10 mL × 3). The filtrate was stripped of solvent in vacuo and used without purification in the next reaction. The crude reaction mixture was transferred in dry dichloromethane (3 mL) to a flame-dried round-bottom flask and the mixture was cooled to −78 °C. Tribromoborane (232 mg, 88 µL, 2 equiv, 0.93 mmol) was added dropwise and the reaction was stirred for 20 min. The cold bath was then removed, and the reaction continued to stir for 1.5 h at room temperature. At this point a small aliquot was removed and extracted with an ammonium hydroxide solution buffered to pH 9.5 with sodium bicarbonate. TLC of this mixture indicated complete consumption of starting material. The reaction mixture was cooled to 0 °C and quenched with 3 mL of methanol dropwise and stirred for 20 min. Then, 2N HCl (4 mL) was added, and a short-path distillation apparatus was fitted to the flask and distilled at 100 °C for 1 h. The resulting aqueous mixture was then cooled to 0 °C and made basic (~9.5) with NH4OH and extracted with 9:1 CHCl3:MeOH (15 mL × 3). The combined organic layers were washed with water and brine, dried with sodium sulfate, and concentrated. The crude mixture was purified with flash chromatography eluting with 5–45% ethyl acetate in hexanes to give 21 as a colorless foam (74 mg, 44% over two steps). [α]D20 +33.8° (c 1.1, CHCl3). The free base was crystallized as the hydrobromide salt from isopropanol by adding 48% HBr, mp 215–217 °C. HBr salt 1H NMR (400 MHz; CD3OD): δ 7.37–7.26 (m, 5H), 7.16 (t, J = 8.0 Hz, 1H), 6.83–6.77 (m, 2H), 6.65 (dd, J = 8.0, 2.0 Hz, 1H), 4.08 (s, 1H), 3.63 (tdd, J = 16.6, 9.2, 4.0 Hz, 4H), 3.53–3.39 (m, 2H), 3.14–3.07 (m, 2H), 2.61–2.40 (m, 3H), 2.24 (d, J = 14.7 Hz, 1H), 2.10–2.02 (m, 3H), 1.86–1.84 (m, 2H), 1.71–1.65 (m, 1H), 1.48–1.43 (m, 1H); 13C NMR (101 MHz; CD3OD): δ 158.8, 150.0, 137.7, 130.7, 130.0, 129.91, 129.87, 128.3, 117.4, 114.2, 113.4, 61.0, 57.7, 56.8, 51.3, 43.3, 42.2, 38.6, 31.6, 29.2, 28.8, 24.8, 21.9; HRMS-ESI (m/z): [M +H]+ calcd for C24H31NO2 366.2433; found: 366.2430. HBr salt: mp: 215–217 °C; anal. calcd for C24H32BrNO2·0.3 H2O: C, 63.74%; H, 6.97%; N, 2.81%. Found C, 63.93%; H, 7.26%; N, 3.11%.
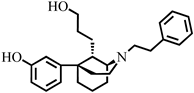
3-((1S,5R,9S)-9-(2-Hydroxyethyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (22). A flame-dried flask was charged with lithium aluminum hydride (82.4 mg, 95% weight, 2.06 mmol) and brought to 0 °C under an argon atmosphere. To this flask was added dry tetrahydrofuran (1.5 mL). After 5 min, 20 (290 mg, 1 equiv, 688 µmol) was added dropwise as a solution in dry tetrahydrofuran (1.2 mL × 2). After 20 min, the ice bath was removed. Reaction was complete by TLC after 1 h. The mixture was cooled to 0 °C and water (400 µL) added to quench the reaction. After 10 min, sodium sulfate (500 mg) was added directly to the solution and stirred for 10 min. The solution was filtered through celite and the filter was washed with dichloromethane (10 mL × 3). The filtrate was stripped of solvent in vacuo and used without purification in the next reaction. The crude reaction mixture was transferred in dry dichloromethane (3 mL) to a flame-dried round-bottom flask and the mixture was cooled to −78 °C. Tribromoborane (226 mg, 85.5 µL, 0.90 mmol) was added dropwise and the reaction was stirred for 20 min. The cold bath was then removed, and the reaction continued to stir for 1.5 h at room temperature. At this point a small aliquot was removed and extracted with an ammonium hydroxide solution buffered to pH 9.5 with sodium bicarbonate. TLC of this mixture indicated complete consumption of starting material. The reaction mixture was cooled to 0 °C and quenched with 3 mL of methanol dropwise and stirred for 20 min. Then, 2N HCl (4 mL) was added, and a short-path distillation apparatus was fitted to the flask and distilled at 100 °C for 1 h. The resulting aqueous mixture was then cooled to 0 °C and made basic (~9.5) with NH4OH and extracted with 9:1 CHCl3:MeOH (15 mL × 3). The combined organic layers were washed with water and brine, dried with sodium sulfate, and concentrated. The crude mixture was purified with flash chromatography eluting with 5–65% ethyl acetate in hexanes to give 22 as a colorless foam (140 mg, 64% over two steps). [α]D20 −35.1° (c 1.0, CHCl3). The free base was crystallized as the hydrobromide salt from isopropanol by adding a solution of 48% HBr in water, mp 257–259 °C. 1H-NMR (400 MHz; CDCl3): δ 7.30–7.14 (m, 7H), 6.95 (s, 1H), 6.88 (d, J = 7.7 Hz, 1H), 6.68 (dd, J = 8.0, 1.8 Hz, 1H), 3.51–3.38 (m, 2H), 3.12–2.97 (m, 3H), 2.86 (t, J = 10.6 Hz, 4H), 2.51 (d, J = 10.2 Hz, 1H), 2.32–2.23 (m, 1H), 2.01–1.89 (m, 4H), 1.81–1.76 (m, 2H), 1.60–1.41 (m, 2H), 1.35–1.29 (m, 1H); 13C NMR (101 MHz; CDCl3): δ 156.8, 151.4, 140.2, 129.2, 128.7, 128.4, 126.1, 117.3, 113.7, 113.4, 60.8, 58.5, 55.4, 49.6, 41.0, 40.7, 38.2, 34.1, 30.0, 28.9, 21.7, 18.3; HRMS-ESI (m/z): [M +H]+ calcd for C24H31NO2 366.2433; found: 366.2434. HBr salt: mp: 257–259 °C; anal. calcd for C24H32BrNO2: C, 64.42%; H, 6.92%; N, 2.85%. Found C, 64.57%; H, 7.22%; N, 3.14%.
tert-Butyl (1R,5R)-5-(4-methoxyphenyl)-9-oxo-2-azabicyclo[3.3.1]nonane-2-carboxylate (23). To a cooled (0 °C) solution of 5 (1.0 g, 4.08 mmol) in dry dichloromethane (50 mL) in a 100 mL round-bottom flask was added di-tert-butyl decarbonate (1.03 mL, 1.1 equiv., 4.49 mmol), N,N-dimethylpyridin-4-amine (10 mg, cat.), and triethylamine (0.63 mL, 1.1 equiv, 4.49 mmol) dropwise. The solution was stirred under argon. After 2 h, TLC showed consumption of starting material. Saturated ammonium chloride was added, and the mixture was extracted with dichloromethane (30 mL × 3), washed with brine, and dried over sodium sulfate. The crude product was purified via flash chromatography (EtOAc in hexanes, gradient 0–20%) to yield 23 as a yellow oil (1.10 g, 78%). Spectroscopic data matched enantiomer 17.
tert-Butyl (1R,5R,Z)-9-(2-ethoxy-2-oxoethylidene)-5-(4-methoxyphenyl)-2-azabicyclo[3.3.1]nonane-2-carboxylate (24). To dry tetrahydrofuran (10 mL) was added sodium hydride (348 mg, 60% weight, 3.0 equiv, 8.7 mmol), followed by slow addition of ethyl 2-(diethoxyphosphoryl)acetate (1.70 mL, 3.0 equiv, 8.7 mmol). After 15 min, 23 (1.0 g, 1 equiv, 2.9 mmol) was added dropwise as a solution in tetrahydrofuran (10 mL). The mixture was brought to reflux under an argon atmosphere. After 16 h, the mixture was cooled to 0 °C and ethanol (3 mL) was added. After stirring for 15 min, the mixture was concentrated and loaded onto silica (3 g). The mixture was purified via flash chromatography (EtOAc in hexanes, gradient 0–20%) to give 24 as a white solid (1.14 g, 95%). Spectroscopic data matched enantiomer 18.
Ethyl 2-((1R,5S,9S & 1R,5S,9R)-5-(4-methoxyphenyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-9-yl)acetate (25 and 26). (i) Compound 24 (1.0 g, 2.41 mmol) was dissolved in ethanol (20 mL) and transferred to a 250 mL pressure-tested reaction bottle. The vessel was charged with Escat 103 (5% Pd/C, 100 mg). The vessel was pressurized to 50 psi H2 in a Parr shaker at 60 °C for 1 h. The reaction mixture was filtered through celite and concentrated under vacuum to afford a yellow oil, used without further purification. (ii) The residue was dissolved in anhydrous dichloromethane (25 mL) and added to a 50 mL round-bottom flask, cooled to 0 °C, trifluoroacetic acid (1.83 mL, 24.0 mmol) was added slowly, and stirred at 0 °C for 1 h. The reaction was quenched with saturated aq NaHCO3, extracted with dichloromethane (3 × 25 mL), dried with Na2SO4, filtered, and concentrated in vacuo. The resultant yellow oil was used without further purification. (iii) The oil was dissolved in anhydrous acetonitrile (25 mL) and added to a 50 mL round-bottom flask. The flask was charged with K2CO3 (663 mg, 4.8 mmol) and 2-phenylethyl bromide (490 µL, 3.6 mmol) and heated to reflux for 18 h. The reaction was filtered through celite and concentrated in vacuo. The crude product was purified via flash chromatography (EtOAc in hexanes, gradient 0–50%), to afford 25 as a clear oil (600 mg, 60% yield) and 26 as a clear oil (200 mg, 20%).
For 25: [α]D25 −6.6° (c 1.4, CHCl3) 1H NMR (400 MHz; CDCl3) δ 7.27—7.14 (m, 6H), 6.92 (d, J = 7.9 Hz, 1H), 6.87 (t, J = 2.0 Hz, 1H), 6.70 (dd, J = 8.1, 2.4 Hz, 1H), 4.01 (q, J = 7.1 Hz, 2H), 3.81—3.76 (m, 3H), 3.08 (td, J = 12.0, 5.3 Hz, 1H), 3.00 (dd, J = 11.2, 7.9 Hz, 1H), 2.95 (d, J = 2.8 Hz, 1H), 2.62 (dd, J = 10.0, 1.5 Hz, 1H), 2.26 (dd, J = 14.8, 2.4 Hz, 1H), 2.16—2.07 (m, 1H), 2.00 (dd, J = 13.5, 5.3 Hz, 1H), 1.92—1.81 (m, 3H), 1.74 (dd, J = 13.0, 5.7 Hz, 1H), 1.68 (dd, J = 12.1, 4.9 Hz, 1H), 1.59—1.54 (m, 1H), 1.17 (t, J = 7.1 Hz, 3H). 13C-NMR (101 MHz, CDCl3): δ 174.3, 159.6, 151.5, 140.9, 129.2, 128.7, 128.1, 125.7, 117.9, 111.8, 110.5, 59.9, 56.7, 55.1, 54.4, 48.9, 42.6, 41.7, 38.7, 34.4, 32.7, 30.1, 25.7, 23.3, 14.2. HRMS-ESI (m/z): [M +H]+ calcd for C27H36NO3 422.2695; found: 422.2695.
For 26: [α]D25 −0.5° (c 1.4, CHCl3), 1H NMR (400 MHz; CDCl3) δ 7.30—7.26 (m, 2H), 7.24—7.19 (m, 5H), 7.02—7.00 (m, 1H), 6.96 (t, J = 2.1 Hz, 1H), 6.72 (dd, J = 8.1, 1.9 Hz, 1H), 4.03 (q, J = 7.1 Hz, 2H), 3.79 (s, 3H), 3.02 (dd, J = 9.0, 6.4 Hz, 3H), 2.86—2.80 (m, 5H), 2.24 (dd, J = 15.3, 11.3 Hz, 2H), 2.06 (dd, J = 15.4, 3.7 Hz, 1H), 2.02—1.92 (m, 4H), 1.78 (ddd, J = 14.0, 4.3, 2.7 Hz, 2H), 1.59—1.53 (m, 1H), 1.18 (t, J = 7.1 Hz, 3H). 13C-NMR (101 MHz, CDCl3): δ 173.0, 159.6, 150.9, 140.6, 129.2, 128.7, 128.3, 125.9, 118.1, 111.9, 110.9, 63.7, 60.2, 58.1, 55.2, 54.4, 49.7, 42.0, 41.2, 39.2, 38.2, 34.5, 33.4, 28.9, 21.6, 18.6, 14.2. HRMS-ESI (m/z): [M +H]+ calcd for C27H36NO3 422.2695; found: 422.2695.
Ethyl 2-((1R,5S,9S & 1R,5S,9R)-5-(4-hydroxyphenyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-9-yl)acetate (27 and 28). A solution of 25 and 26 (200 mg, 0.475 mmol, 1.0 equiv) in dry dichloromethane (95 mL) was brought to −78 °C under an atmosphere of argon. To this cooled solution was added BBr3 (224 µL, 2.38 mmol, 5.0 equiv) dropwise and the solution was stirred and allowed to warm to room temperature under argon for 16 h. The solution was cooled to 0 °C and ethanol (3 mL) was added dropwise and stirred for 30 min. The solution was made basic (pH > 10.5) with NH4OH, and then extracted with CHCl3 (30 mL × 3). The organic extracts were washed with brine, dried over MgSO4, and solvent was removed in vacuo. The crude residue was purified via flash chromatography (50:45:5 CHCl3:MeOH:NH4OH in CHCl3, gradient 0–10%) (190 mg, 98%). Both epimers were used without further characterization.
4-((1R,5S,9S)-9-(2-Hydroxyethyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (29). A solution of 27 (100 mg, 0.26 mmol) in dry tetrahydrofuran (5 mL) was cooled to 0 °C and lithium aluminum hydride (370 µL, 0.74 mmol, 3.0 equiv., 2.0M solution in THF) added dropwise. The reaction was warmed to room temperature and quenched with Na2SO4·10H2O, stirred for 15 min, and filtered through celite. The filtrate was concentrated in vacuo and purified via flash chromatography (50:45:5 CHCl3:MeOH:NH4OH, gradient 0–10%) to yield 29 as a white foam (62.4 mg, 66%). [α]D25 −36.1° (c 1.4, CHCl3), 1H NMR (400 MHz; CDCl3) δ 7.2–7.23 (m, 2H), 7.1–7.11 (m, 4H), 6.82 (d, J = 7.9 Hz, 1H), 6.79 (d, J = 2.0 Hz, 1H), 6.64 (dd, J = 7.9, 2.0 Hz, 1H), 3.5–3.44 (m, 2H), 3.18 (dd, J = 11.6, 8.2 Hz, 1H), 3.03 (dt, J = 12.5, 6.1 Hz, 2H), 2.8–2.72 (m, 4H), 2.45 (td, J = 12.5, 8.6 Hz, 1H), 2.2–2.21 (m, 2H), 1.96 (dd, J = 13.7, 5.3 Hz, 1H), 1.9–1.80 (m, 2H), 1.70 (ddt, J = 23.7, 13.9, 6.3 Hz, 3H), 1.5–1.46 (m, 1H), 1.4–1.35 (m, 1H). 13C-NMR (101 MHz, CDCl3): δ 156.3, 151.0, 140.1, 129.3, 128.8, 128.3, 126.0, 117.3, 112.8, 112.7, 60.6, 57.7, 55.2, 48.7, 43.6, 42.9, 38.6, 33.9, 31.2, 29.6, 25.9, 23.0. HRMS-ESI (m/z): [M +H]+ calcd for C24H32NO2 366.2433; found: 366.2429. Anal. calcd for C24H31NO2·0.15 H2O·0.2 CHCl3: C, 78.86%; H, 8.55%; N, 3.83%. Found C, 74.14%; H, 8.08%; N, 3.52%.
4-((1R,5S,9R)-9-(2-Hydroxyethyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (30). A solution of 28 (75 mg, 0.18 mmol) in dry tetrahydrofuran (5 mL) was cooled to 0 °C and lithium aluminum hydride (272 µL, 0.55 mmol, 2.0M solution in THF) added dropwise. The reaction was warmed to room temperature and quenched with Na2SO4·10H2O, stirred for 15 min, and filtered through celite. The filtrate was concentrated in vacuo and purified via flash chromatography (50:45:5 CHCl3:MeOH:NH4OH, gradient 0–10%) to yield 30 as a white foam (45 mg, 47%). [α]D25 +38.7° (c 1.4, CHCl3), 1H NMR (400 MHz; CDCl3) δ 7.26 (t, J = 7.4 Hz, 2H), 7.2–7.10 (m, 4H), 6.91 (s, 1H), 6.84 (d, J = 7.9 Hz, 1H), 6.67 (dd, J = 8.0, 1.7 Hz, 1H), 3.4–3.34 (m, 2H), 3.0–3.03 (m, 2H), 2.97 (td, J = 12.0, 4.9 Hz, 1H), 2.8–2.78 (m, 4H), 2.47 (d, J = 10.0 Hz, 1H), 2.24 (td, J = 12.9, 7.7 Hz, 1H), 1.9–1.86 (m, 4H), 1.7–1.70 (m, 2H), 1.55 (t, J = 9.6 Hz, 1H), 1.43 (td, J = 9.5, 5.1 Hz, 1H), 1.3–1.25 (m, 1H). 13C-NMR (101 MHz, CDCl3): δ 157.0, 151.2, 140.0, 129.2, 128.7, 128.4, 126.1, 117.1, 113.7, 113.7, 60.9, 58.3, 55.3, 49.6, 41.2, 40.7, 38.2, 33.9, 29.9, 28.8, 21.7, 18.0. HRMS-ESI (m/z): [M +H]+ calcd for C24H32NO2 366.2433; found: 366.2430. Anal. calcd for C24H31NO2·0.5 H2O·0.25 CH2Cl2: C, 78.87%; H, 8.55%; N, 3.83%. Found C, 73.70%; H, 8.43%; N, 3.47%.
Ethyl (E&Z)-3-((1R,5S,9R)-5-(3-methoxyphenyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-9-yl)acrylate (31 [9R] and 32 [9S]). In step 1, compound 7 (1.192 g, 3.157 mmol) was dissolved in THF (8 mL) and to this was added 6 N HCl (8 mL). After 4 h, TLC showed consumption of starting material. The solution was cooled to 0 °C and made basic (pH > 9.5) with 12 N NH4OH and extracted with dichloromethane (25 mL × 3) and the organic layers were washed with brine, filtered through sodium sulfate, and concentrated in a flame-dried flask. To another flame-dried flask was added NaH (328.4 mg, 60% weight, 2.6 equiv, 8.209 mmol) anhydrous THF (5 mL). To this solution was added ethyl 2-(diethoxyphosphoryl)acetate (2.189 g, 1.94 mL, 97% weight, 3.0 equiv, 9.472 mmol). The mixture was brought to 0 °C and the crude material from step 1 was added dropwise over 5 min in a solution in THF (2.5 mL × 2). After 30 min the ice bath was removed, and the reaction was left for 16 h. The solution was cooled to 0 °C and ethanol (5 mL) was added and stirred for 10 min to quench the reaction. Silica (20 g) was added directly to the mixture and solvents removed in vacuo. The material was loaded onto a column and purified via flash chromatography eluting with 5–65% ethyl acetate in hexanes. The C9R and C9S isomers were each separated as mixtures of E and Z (834 mg, 88%, 1.2:1 C9R:C9S).
For 31-9R: 1H NMR (400 MHz; CDCl3): δ 7.40–7.19 (m, J = 18.9, 9.4 Hz, 6H), 6.95–6.88 (m, 2H), 6.69 (dd, J = 8.1, 2.0 Hz, 1H), 5.83 (d, J = 15.7 Hz, 1H), 4.08 (q, J = 7.1 Hz, 2H), 3.74 (s, 3H), 3.25–3.23 (m, 1H), 3.06–3.01 (m, 3H), 2.82 (q, J = 7.4 Hz, 4H), 2.14 (t, J = 5.8 Hz, 2H), 1.95 (d, J = 11.6 Hz, 2H), 1.82 (dd, J = 13.2, 3.6 Hz, 2H), 1.52–1.45 (m, 1H), 1.26–1.18 (m, 4H); 13C NMR (101 MHz; CDCl3): δ 166.3, 159.5, 150.6, 148.9, 140.4, 129.18, 129.0, 128.7, 128.4, 126.1, 123.1, 118.2, 112.3, 110.7, 60.2, 58.3, 57.8, 55.1, 49.6, 48.5, 41.1, 38.2, 34.7, 29.7, 22.0, 19.9, 14.2; HRMS-ESI (m/z): [M +H]+ calcd for C28H36NO3 434.2695; found: 434.2691.
For 32-9S: 1H NMR (400 MHz; CDCl3): δ 7.27–7.10 (m, 7H), 6.87–6.81 (m, 2H), 6.68 (dd, J = 8.1, 2.1 Hz, 1H), 5.77–5.67 (m, 1H), 4.08 (qd, J = 7.1, 2.1 Hz, 2H), 3.76 (s, 3H), 3.14–3.07 (m, 3H), 2.86–2.72 (m, 5H), 2.44–2.36 (m, 1H), 2.30–2.27 (m, 1H), 2.09–2.05 (m, 1H), 1.99–1.79 (m, 3H), 1.67 (t, J = 5.0 Hz, 1H), 1.53–1.44 (m, 1H), 1.21 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz; CDCl3): δ 166.7, 159.4, 151.2, 150.8, 140.9, 129.1, 128.8, 128.2, 125.8, 121.9, 118.2, 112.4, 110.3, 59.9, 57.9, 57.1, 55.1, 49.1, 49.01, 42.1, 38.7, 34.3, 30.4, 25.7, 23.1, 14.3; HRMS-ESI (m/z): [M +H]+ calcd for C28H36NO3 434.2695; found: 434.2694.
3-((1R,5S,9R)-5-(3-Methoxyphenyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-9-yl)propan-1-ol (33). Compound 31 (9R) (570 mg, 1 equiv., 1.31 mmol) was dissolved in a 10:1 mixture of ethanol:ethyl acetate (20 mL) and transferred to a Parr shaker vessel. Escat Pd/c (5%) (0.1 equiv) was added and the vessel was attached to a Parr shaker, charged with hydrogen up to 45 psi, and shaken overnight. The mixture was filtered through celite and sodium sulfate into a flame-dried flask. This flask was brought to 0 °C under an argon atmosphere and anhydrous tetrahydrofuran (1.5 mL) was added. After 5 min, lithium aluminum hydride (155 mg, 2.05 mL, 2.0 molar, 3.0 equiv, 4.10 mmol) was added dropwise as a solution in tetrahydrofuran. After 20 min, the ice bath was removed. TLC taken after 1 h showed complete reaction. The mixture was cooled to 0 °C and water (600 µL) added to quench the reaction which was then stirred for 10 min. Sodium sulfate (500 mg) was added directly to the mixture, and it was filtered through a pad of celite. The celite was washed with 10% MeOH in dichloromethane and the crude material was purified via flash chromatography eluting with 0.5–20% 50:45:5 CHCl3:MeOH:NH4OH to give 33 as a yellow foam (480 g, 89%). 1H NMR (400 MHz; CDCl3): δ 7.31–7.18 (m, 6H), 7.00–6.94 (m, 2H), 6.84–6.70 (m, 2H), 3.78 (s, 3H), 3.54–3.40 (m, 2H), 3.08–3.01 (m, 2H), 2.88–2.80 (m, 4H), 2.45–2.15 (m, 4H), 2.10–1.86 (m, 5H), 1.84–1.65 (m, 3H), 1.60–1.48 (m, 2H), 1.42–1.25 (m, 3H), 1.06–0.99 (m, 1H); 13C NMR (101 MHz; CDCl3): δ 159.5, 151.7, 140.4, 129.1, 128.7, 128.4, 126.1, 118.1, 112.2, 110.4, 62.7, 58.2, 55.1, 54.2, 49.9, 44.9, 41.3, 38.8, 34.4, 30.7, 28.8, 23.2, 21.8, 18.1; HRMS-ESI (m/z): [M +H]+ calcd for C26H36NO2 394.2746; found: 394.2752.
3-((1R,5S,9R)-9-(3-Hydroxypropyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (34). A solution of 33 (185 mg, 0.470 mmol, 1.0 equiv.) was dissolved in dry dichloromethane (3 mL) and brought to −78 °C under an atmosphere of argon. To this cooled solution was added BBr3 (177 mg, 67 µL, 0.705 mmol, 1.5 equiv.) dropwise and the solution was stirred and allowed to warm to room temperature under argon for 16 h. The solution was cooled to 0 °C and methanol (2 mL) was added dropwise and stirred for 30 min. A solution of 2N HCl (4 mL) was added, and the mixture was brought to 100 °C in a distillation apparatus for removal of the dichloromethane. After 1 h, the solution was cooled to 0 °C, made basic (pH 9.5) with 14 N NH4OH and sodium bicarbonate, and then extracted with 9:1 CHCl3:MeOH (30 mL × 2). The organic extracts were washed with brine, dried over MgSO4, and concentrated in vacuo. The crude residue was purified via flash chromatography eluting with 20–100% ethyl acetate in n-hexane to give 34 as a colorless oil (65 mg, 36%) [α]D20 +42.7° (c 0.9, CHCl3). The free base was crystallized as the hydrobromide salt from isopropanol and diethyl ether by adding a solution of 48% HBr, mp 218–220 °C. 1H NMR (400 MHz; CDCl3): δ 7.31–7.13 (m, 6H), 6.92–6.86 (m, 2H), 6.67–6.64 (m, 1H), 3.52–3.43 (m, 2H), 3.08–2.99 (m, 3H), 2.89–2.84 (m, 3H), 2.33–2.22 (m, 2H), 2.06–1.74 (m, 6H), 1.58–1.49 (m, 2H), 1.38–1.15 (m, 3H); 13C NMR (101 MHz; CDCl3): δ 156.6, 151.6, 140.2, 129.2, 128.7, 128.4, 126.1, 117.3, 113.3, 113.2, 62.2, 58.3, 54.6, 49.8, 43.9, 41.0, 38.6, 34.0, 30.0, 28.8, 22.9, 21.7, 18.0; HRMS-ESI (m/z): [M +H]+ calcd for C25H34NO2 380.2590; found: 380.2589. C25H34BrNO2 0.3 H2O: C, 64.46%; H, 7.49%; N, 3.01%. Found C, 64.50%; H, 7.49%; N, 3.02%.
Ethyl 3-((1S,5R,9S)-5-(3-methoxyphenyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-9-yl)acrylate (37). See the procedure for the synthesis of compounds 31 and 32. 1H NMR (400 MHz; CDCl3): δ 7.32–7.19 (m, 6H), 6.95–6.88 (m, 3H), 6.70 (dd, J = 8.1, 2.4 Hz, 1H), 5.82 (dd, J = 15.8, 0.9 Hz, 1H), 4.10 (q, J = 7.2 Hz, 2H), 3.77 (s, 3H), 3.24 (dd, J = 8.2, 2.7 Hz, 1H), 3.12–2.99 (m, 3H), 2.83 (q, J = 7.5 Hz, 4H), 2.14 (t, J = 4.6 Hz, 3H), 2.01–1.94 (m, 2H), 1.86–1.79 (m, 2H), 1.53 (qt, J = 8.8, 4.3 Hz, 1H), 1.22 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz; CDCl3): δ 166.4, 159.5, 150.6, 148.9, 140.4, 129.1, 128.7, 128.4, 126.0, 123.1, 118.2, 112.2, 110.7, 60.2, 58.3, 57.7, 55.1, 49.6, 48.5, 41.1, 38.2, 34.7, 29.7, 22.0, 19.8, 14.2; HRMS-ESI (m/z): [M +H]+ calcd for C28H36NO3 434.2695; found: 434.2693.
Ethyl 3-((1S,5R,9S)-5-(3-hydroxyphenyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-9-yl)acrylate (38). A solution of 37 (548 mg, 1.26 mmol, 1.0 equiv.) was dissolved in dry dichloromethane (12 mL) and brought to −78 °C under an atmosphere of argon. To this cooled solution was added BBr3 (633 mg, 239 µL, 2.53 mmol, 2.0 equiv.) dropwise and the solution was stirred and allowed to warm to room temperature under argon for 16 h. The solution was cooled to 0 °C and methanol (2 mL) was added dropwise and stirred for 30 min. A solution of 2N HCl (4 mL) was added, and the mixture was brought to 100 °C in a distillation apparatus. After 1 h, the solution was cooled to 0 °C, made basic (pH 9.5) with 14 N NH4OH and sodium bicarbonate, and then extracted with CHCl3 (30 mL × 2). The organic extracts were washed with brine, dried over MgSO4, and concentrated. The crude residue was purified via flash chromatography eluting with 5–55% ethyl acetate in n-hexane to give 38 as a yellow oil (310 mg, 59%). 1H NMR (400 MHz; CDCl3): δ 7.31–7.18 (m, 5H), 7.17–7.14 (m, 1H), 6.95–6.88 (m, 2H), 6.81–6.76 (m, 1H), 6.63 (dd, J = 7.9, 2.1 Hz, 1H), 5.81 (d, J = 15.8 Hz, 1H), 4.12–4.05 (m, 2H), 3.24–3.22 (m, 1H), 3.09–3.01 (m, 3H), 2.89–2.80 (m, 4H), 2.19–2.05 (m, 3H), 2.03–1.92 (m, 2H), 1.84–1.78 (m, 2H), 1.59–1.50 (m, 1H), 1.21 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz; CDCl3): δ 166.6, 156.0, 150.6, 148.9, 140.2, 129.4, 128.7, 128.4, 126.1, 123.1, 117.7, 113.4, 113.2, 60.3, 58.0, 57.7, 49.6, 48.1, 40.7, 38.0, 34.3, 29.6, 21.9, 19.6, 14.2; HRMS-ESI (m/z): [M +H]+ calcd for C27H34NO3 420.2539; found: 420.2539.
3-((1S,5R,9S)-9-(3-Hydroxypropyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (39). Phenolic ester 38 (300 mg, 1 equiv, 0715 mmol) was dissolved in a 10:1 mixture of ethanol:ethyl acetate (20 mL) and transferred to a Parr shaker vessel. Escat Pd/C (5%) (0.1 equiv) was added and the vessel was attached to a Parr shaker, charged with hydrogen up to 45 psi, and shaken overnight. The mixture was filtered through celite and sodium sulfate into a flame-dried flask. This flask was brought to 0 °C under an argon atmosphere and anhydrous tetrahydrofuran (1.5 mL) was added. After 5 min, lithium aluminum hydride (84.5 mg, 1.11 mL, 2.0 molar, 3.0 equiv, 2.23 mmol) was added dropwise as a solution in tetrahydrofuran. After 20 min, the ice bath was removed. TLC taken after 1 h showed complete reaction. The mixture was cooled to 0 °C and water (600 µL) was added to quench the reaction which was then stirred for 10 min. Sodium sulfate (500 mg) was added directly to the mixture and the mixture was filtered through a pad of celite. The celite was washed with 10% MeOH in dichloromethane and the crude material was purified via flash chromatography eluting with 0.5–20% 50:45:5 CHCl3:MeOH:NH4OH in CHCl3 to give 39 as a yellow foam (203 mg, 72%) [α]D20 −42.7° (c 0.9, CHCl3). The free base was crystallized as the hydrobromide salt from isopropanol and diethyl ether by adding a solution of 48% HBr, mp 218–220 °C. 1H NMR (400 MHz; CDCl3): δ 7.30–7.12 (m, 6H), 6.88–6.86 (m, 2H), 6.66–6.64 (m, 1H), 3.51–3.40 (m, 2H), 3.10–3.01 (m, 3H), 2.85 (q, J = 9.7 Hz, 4H), 2.32–2.19 (m, 2H), 2.05–1.85 (m, 4H), 1.81–1.71 (m, 2H), 1.57–1.48 (m, 2H), 1.37–1.14 (m, 3H); 13C NMR (101 MHz; CDCl3):δ 156.6, 151.6, 140.2, 129.2, 128.7, 128.4, 126.1, 117.2, 113.4, 113.2, 62.2, 58.3, 54.6, 49.8, 43.9, 41.0, 38.6, 34.0, 30.0, 28.8, 22.9, 21.7, 18.0; HRMS-ESI (m/z): [M +H]+ calcd for C25H34NO2 380.2590; found: 380.2586. C25H34BrNO2: C, 65.21%; H, 7.44%; N, 3.04%. Found C, 65.36%; H, 7.58%; N, 3.14%.
3-((1R,5R,E)-9-(Methoxymethylene)-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (
40). Ketone
6 (2.28 g, 1 equiv, 6.52 mmol) was dissolved in dry dichloromethane (30 mL) in a flame-dried round-bottom flask and the mixture was cooled to −78 °C. Tribromoborane (8.17 g, 3.10 mL, 5 equiv, 32.6 mmol) was added dropwise and the reaction was stirred for 30 min. The cold bath was then removed, and the reaction continued to stir for 30 min at room temperature. At this point a small aliquot was removed and extracted with an ammonium hydroxide solution buffered to pH 9.5 with sodium bicarbonate. TLC of this mixture indicated complete consumption of starting material. The reaction mixture was cooled to 0 °C and quenched with 3 mL of methanol dropwise and stirred for 20 min. Then, 2N HCl (20 mL) was added, and a short-path distillation apparatus was fitted to the flask and distilled at 100 °C for 1 h. The resulting aqueous mixture was then cooled to 0 °C and made basic (~9.5) with NH
4OH and extracted with 9 CHCl
3 (50 mL × 3). The combined organic layers were washed with water and brine, dried with sodium sulfate, and purified via flash chromatography, eluting with 10–40% ethyl acetate in hexane to give free phenol as a colorless oil (1.634 g, 75%). The free phenolic tertiary amine (1.634 g, 1 equiv, 4.871 mmol) from the reaction was dissolved in dry tetrahydrofuran (40 mL) and (methoxymethyl)triphenylphosphonium chloride (5.009 g, 14.61 mmol, 3.0 equiv) was added and the solution brought to 0 °C. LiHMDS (2.119 g, 12.66 mL, 2.6 equiv, 1.0 M solution in THF, 12.66 mmol) was added dropwise over 10 min. After 30 min the ice bath was removed, and the solution was stirred under argon for 16 h. The mixture was cooled to 0 °C and methanol (20 mL) was added and stirred for 10 min. The solvents were removed in vacuo and the residue was taken up in CHCl
3 (50 mL) and washed with water which was made basic (pH 9–9.5) with NH
4OH. The combined organic extracts were washed with brine, dried over MgSO
4, concentrated in vacuo, and purified via flash chromatography eluting with 0.5–8% 50:45:5 CHCl
3:MeOH:NH
4OH to give
40 as a yellow oil (385 mg, 21.7%) [
15].
3-((1R,5S,9R)-9-(Methoxymethyl)-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (41). Enol ether 40 (240 mg, 1 equiv., 660 umol) was dissolved in ethanol (20 mL) and transferred to a Parr shaker vessel. Escat Pd/c (10%) (0.2 equiv.) was added and the vessel was attached to a Parr shaker, charged with hydrogen up to 45 psi, and shaken overnight. The mixture was filtered through celite and purified via flash chromatography 0.5–10% 50:45:5 CHCl3:MeOH:NH4OH to give 41 and 42 as a colorless foam (113 mg, 46.8% and 20 mg, 8.3%). Then, 41 was further purified by crystallization as the hydrobromide salt from isopropanol. [α]D20 +34.5° (c 1.0, CHCl3). mp 250–252 °C. 1H NMR (400 MHz; CDCl3): δ 7.30–7.26 (m, 3H), 7.21–7.16 (m, 4H), 6.95 (d, J = 7.9 Hz, 1H), 6.88 (t, J = 1.9 Hz, 1H), 6.69 (dd, J = 7.9, 2.1 Hz, 1H), 3.38 (t, J = 10.5 Hz, 1H), 3.30 (s, 1H), 3.18 (s, 3H), 3.08–2.98 (m, 3H), 2.90–2.78 (m, 4H), 2.68–2.64 (m, 1H), 2.23 (dt, J = 13.7, 9.8 Hz, 1H), 1.97–1.77 (m, 6H), 1.74–1.69 (m, 1H), 1.61–1.52 (m, 1H). 13C NMR (101 MHz; CDCl3): δ156.1, 151.3, 140.6, 129.3, 128.7, 128.3, 125.9, 117.5, 113.2, 113.1, 70.5, 58.6, 58.2, 52.6, 49.6, 44.6, 41.5, 36.9, 34.3, 29.5, 21.7, 18.4. HRMS-ESI (m/z): [M +H]+ calcd for C24H32NO2 366.2433; found: 366.2430. C24H32BrNO2. 0.2 H2O: C, 64.05%; H, 7.26%; N, 3.11%. Found C, 64.14%; H, 7.19%; N, 3.05%.