Hydrothermal Carbon Coating of an Activated Carbon—A New Adsorbent
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Characterisation of Adsorbents
2.1.1. Composition
2.1.2. Acidic and Basic Properties
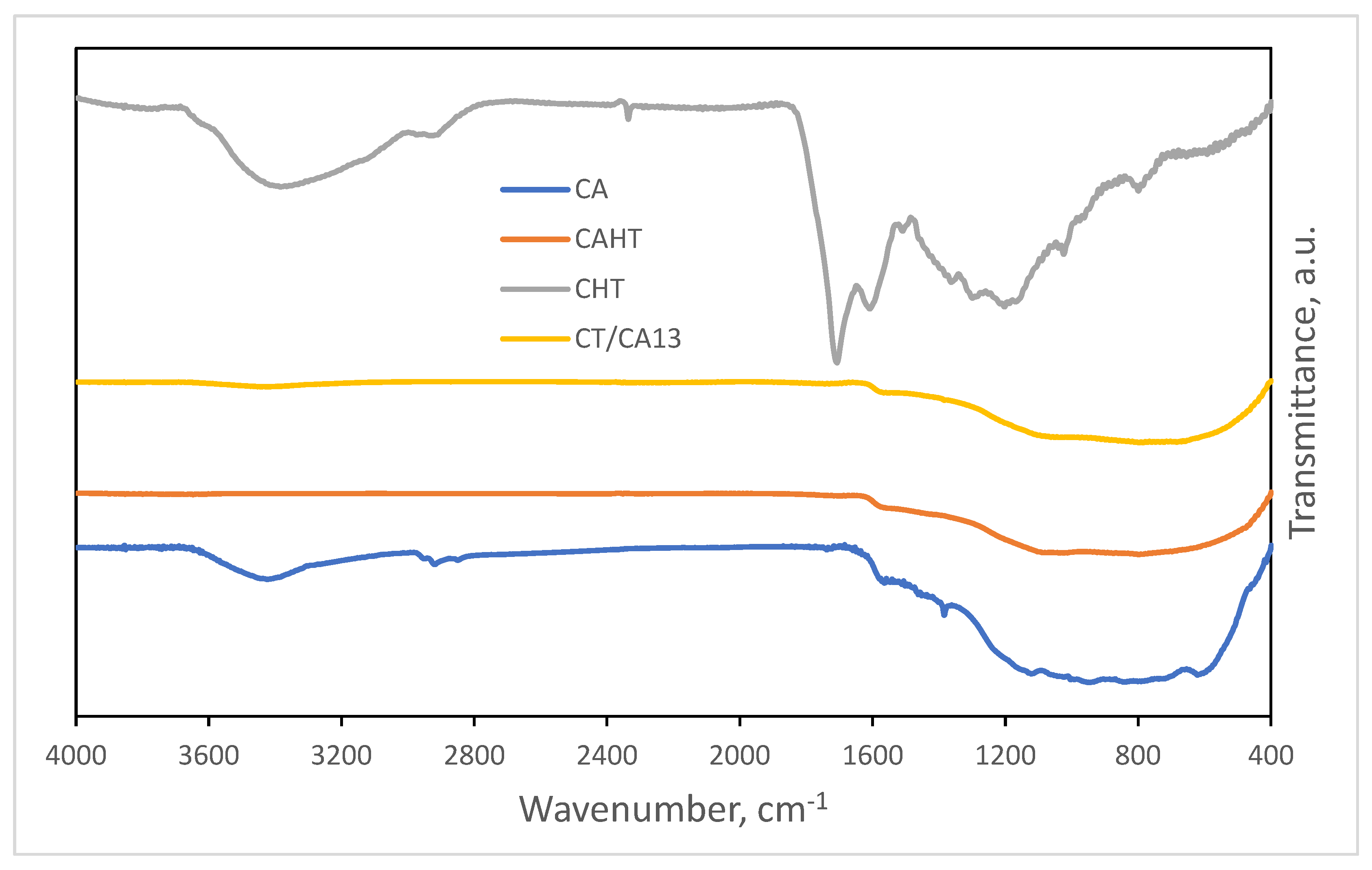
2.2. Structural Characterisation
2.2.1. N2 Adsorption
2.2.2. Scanning Electron Microscopy
2.3. Acetamiprid Adsorption
2.3.1. Adsorbent Selection
2.3.2. Adsorption Kinetics
2.3.3. Adsorption Isotherms
2.3.4. Influence of pH
2.3.5. Influence of Temperature
2.3.6. Mechanism
3. Materials and Methods
3.1. Raw Material
3.2. Synthesis of Coated Carbons
3.3. Coated Carbons Characterisation
3.4. Adsorption of Acetamiprid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Scaria, J.; Gopinath, A.; Ranjith, N.; Ravindran, V.; Ummar, S.; Nidheesh, P.; Kumar, M.S. Carbonaceous materials as effective adsorbents and catalysts for the removal of emerging contaminants from water. J. Clean. Prod. 2022, 350, 131319. [Google Scholar] [CrossRef]
- Rakowska, M.; Kupryianchyk, D.; Harmsen, J.; Grotenhuis, T.; Koelmans, A. In situ remediation of contaminated sediments using carbonaceous materials. Environ. Toxicol. Chem. 2012, 31, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Ngadi, N.; Inuwa, I.M.; Hassan, O. Recent advances in applications of activated carbon from biowaste for wastewater treatment: A short review. J. Clean. Prod. 2018, 175, 361–375. [Google Scholar] [CrossRef]
- Abioye, A.M.; Ani, F.N. Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: A review. Renew. Sustain. Energy Rev. 2015, 52, 1282–1293. [Google Scholar] [CrossRef]
- McNamara, J.D.; Franco, R.; Mimna, R.; Zappa, L. Comparison of Activated Carbons for Removal of Perfluorinated Compounds From Drinking Water. J.-Am. Water Work. Assoc. 2018, 110, E2–E14. [Google Scholar] [CrossRef]
- Diamadopoulos, E.; Samaras, P.; Sakellaropoulos, G.P. The Effect of Activated Carbon Properties on the Adsorption of Toxic Substances. Water Sci. Technol. 1992, 25, 153–160. [Google Scholar] [CrossRef]
- Jeguirim, M.; Belhachemi, M.; Limousy, L.; Bennici, S. Adsorption/reduction of nitrogen dioxide on activated carbons: Textural properties versus surface chemistry–A review. Chem. Eng. J. 2018, 347, 493–504. [Google Scholar] [CrossRef]
- Durán-Valle, C.J.; Madrigal-Martínez, M.; Martínez-Gallego, M.; Fonseca, I.M.F.L.; Matos, I.A.; Rego, A. Activated carbon as a catalyst for the synthesis of N-alkylimidazoles and imidazolium ionic liquids. Catal. Today 2012, 187, 108–114. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Rodrigues, D.L.C.; Machado, F.M.; Osório, A.G.; De Azevedo, C.F.; Lima, E.C.; Da Silva, R.S.; Lima, D.; Gonçalves, F.M. Adsorption of amoxicillin onto high surface area–activated carbons based on olive biomass: Kinetic and equilibrium studies. Environ. Sci. Pollut. Res. 2020, 27, 41394–41404. [Google Scholar] [CrossRef]
- Lima, D.R.; Lima, E.C.; Thue, P.S.; Dias, S.L.; Machado, F.M.; Seliem, M.K.; Sher, F.; dos Reis, G.S.; Saeb, M.R.; Rinklebe, J. Comparison of acidic leaching using a conventional and ultrasound-assisted method for preparation of magnetic-activated biochar. J. Environ. Chem. Eng. 2021, 9, 105865. [Google Scholar] [CrossRef]
- Zhao, L.; Baccile, N.; Gross, S.; Zhang, Y.; Wei, W.; Sun, Y.; Antonietti, M.; Titirici, M.-M. Sustainable nitrogen-doped carbonaceous materials from biomass derivatives. Carbon 2010, 48, 3778–3787. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Falco, C.; Sevilla, M. Black perspectives for a green future: Hydrothermal carbons for environment protection and energy storage. Energy Environ. Sci. 2012, 5, 6796–6822. [Google Scholar] [CrossRef]
- Durán-Valle, C.J.; Botet-Jiménez, A.B.; Omenat-Morán, D. Hydrothermal Carbonisation: An Eco-Friendly Method for the Production of Carbon Adsorbents. Adsorpt. Process. Water Treat. Purif. 2017, 77–108, 77–108. [Google Scholar] [CrossRef]
- Roldán, L.; Santos, I.; Armenise, S.; Fraile, J.M.; García-Bordejé, E. The formation of a hydrothermal carbon coating on graphite microfiber felts for using as structured acid catalyst. Carbon 2012, 50, 1363–1372. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.; Jin, Z.; Gui, J.; Tan, R.; Qiu, J. Insight into the impact of surface hydrothermal carbon layer on photocatalytic performance of ZnO nanowire. Appl. Catal. A Gen. 2019, 583, 117145. [Google Scholar] [CrossRef]
- Liu, C.; He, Y.; Wei, L.; Zhang, Y.; Zhao, Y.; Hong, J.; Chen, S.; Wang, L.; Li, J. Hydrothermal Carbon-Coated TiO2 as Support for Co-Based Catalyst in Fischer–Tropsch Synthesis. ACS Catal. 2018, 8, 1591–1600. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Q.; Liu, C.; Zhang, X.; Chung, D. Carbon-coated sepiolite clay fibers with acid pre-treatment as low-cost organic adsorbents. Carbon 2017, 123, 259–272. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.; Zhao, Z.; Li, B.; Gui, J.; Liu, D.; Qiu, J. One-step synthesis of flowerlike C/Fe2O3 nanosheet assembly with superior adsorption capacity and visible light photocatalytic performance for dye removal. Carbon 2017, 116, 59–67. [Google Scholar] [CrossRef]
- Fang, C.; Hu, P.; Dong, S.; Song, J.; Zhang, X. An efficient hydrothermal transformation approach for construction of controllable carbon coating on carbon fiber from renewable carbohydrate. Appl. Surf. Sci. 2019, 491, 478–487. [Google Scholar] [CrossRef]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Bernabeu, A.; Vercher, R.; Santos-Juanes, L.; Simón, P.; Lardín, C.; Martínez, M.; Vicente, J.; González, R.; Llosá, C.; Arques, A.; et al. Solar photocatalysis as a tertiary treatment to remove emerging pollutants from wastewater treatment plant effluents. Catal. Today 2011, 161, 235–240. [Google Scholar] [CrossRef]
- Jiménez, M.; Maldonado, M.I.; Rodríguez, E.M.; Hernández-Ramírez, A.; Saggioro, E.; Carra, I.; Pérez, J.A.S. Supported TiO2solar photocatalysis at semi-pilot scale: Degradation of pesticides found in citrus processing industry wastewater, reactivity and influence of photogenerated species. J. Chem. Technol. Biotechnol. 2015, 90, 149–157. [Google Scholar] [CrossRef]
- Guillossou, R.; Le Roux, J.; Mailler, R.; Vulliet, E.; Morlay, C.; Nauleau, F.; Gasperi, J.; Rocher, V. Organic micropollutants in a large wastewater treatment plant: What are the benefits of an advanced treatment by activated carbon adsorption in comparison to conventional treatment? Chemosphere 2019, 218, 1050–1060. [Google Scholar] [CrossRef]
- Mohammad, S.G.; Ahmed, S.M.; Amr, A.E.-G.E.; Kamel, A.H. Porous Activated Carbon from Lignocellulosic Agricultural Waste for the Removal of Acetampirid Pesticide from Aqueous Solutions. Molecules 2020, 25, 2339. [Google Scholar] [CrossRef]
- Sahraoui, N.; Tassalit, D.; Rekhila, G.; Chekir, N.; Trari, M. Laboratory Studies on the Adsorption of Acetamiprid to Activated Carbon from Pomegranate Waste. Water Air Soil Pollut. 2022, 233, 290. [Google Scholar] [CrossRef]
- Cruz-Alcalde, A.; Sans, C.; Esplugas, S. Priority pesticides abatement by advanced water technologies: The case of acetamiprid removal by ozonation. Sci. Total Environ. 2017, 599–600, 1454–1461. [Google Scholar] [CrossRef]
- Gorito, A.M.; Ribeiro, A.R.; Gomes, C.R.; Almeida, C.M.R.; Silva, A.M. Constructed wetland microcosms for the removal of organic micropollutants from freshwater aquaculture effluents. Sci. Total Environ. 2018, 644, 1171–1180. [Google Scholar] [CrossRef]
- Wang, J.; Hirai, H.; Kawagishi, H. Biotransformation of acetamiprid by the white-rot fungus Phanerochaete sordida YK-624. Appl. Microbiol. Biotechnol. 2012, 93, 831–835. [Google Scholar] [CrossRef]
- Araujo-Andrade, C.; Ruiz, F.; Martínez-Mendoza, J.; Terrones, H. Infrared and Raman spectra, conformational stability, ab initio calculations of structure, and vibrational assignment of α and β glucose. J. Mol. Struct. THEOCHEM 2005, 714, 143–146. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y. Colloidal Carbon Spheres and Their Core/Shell Structures with Noble-Metal Nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 597–601. [Google Scholar] [CrossRef]
- Sakaki, T.; Shibata, M.; Miki, T.; Hirosue, H.; Hayashi, N. Reaction model of cellulose decomposition in near-critical water and fermentation of products. Bioresour. Technol. 1996, 58, 197–202. [Google Scholar] [CrossRef]
- Ibarra, J.; Muñoz, E.; Moliner, R. FTIR study of the evolution of coal structure during the coalification process. Org. Geochem. 1996, 24, 725–735. [Google Scholar] [CrossRef]
- Lua, A.C.; Yang, T. Effect of activation temperature on the textural and chemical properties of potassium hydroxide activated carbon prepared from pistachio-nut shell. J. Colloid Interface Sci. 2004, 274, 594–601. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 111, 3973–3993. [Google Scholar] [CrossRef]
- Murano, H.; Suzuki, K.; Kayada, S.; Saito, M.; Yuge, N.; Arishiro, T.; Watanabe, A.; Isoi, T. Influence of humic substances and iron and aluminum ions on the sorption of acetamiprid to an arable soil. Sci. Total Environ. 2018, 615, 1478–1484. [Google Scholar] [CrossRef]
- Domínguez, J.R.; Durán-Valle, C.J.; Mateos-García, G. Synthesis and characterisation of acid/basic modified adsorbents. Application for chlorophenols removal. Environ. Res. 2022, 207, 112187. [Google Scholar] [CrossRef]
- Zhao, X.; Farajtabar, A.; Zhao, H.; Han, G. Solubility and Solvent Effect of Acetamiprid in Thirteen Pure Solvents and Aqueous Solutions of Ethanol. J. Chem. Eng. Data 2019, 64, 3505–3513. [Google Scholar] [CrossRef]
- Montero, M.R.; Valle, C.D.; Vargas, M.J.; Jiménez, A.B. Radioactive content of charcoal. Appl. Radiat. Isot. 2009, 67, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Bernalte García, Á.; Valenzuela, C. Un Método Termogravimétrico Rápido Para Análisis Inmediato de Carbones. Boletín Geológico Y Min. 1985, 96, 58–61. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Nguyen, C.; Do, D. The Dubinin–Radushkevich equation and the underlying microscopic adsorption description. Carbon 2001, 39, 1327–1336. [Google Scholar] [CrossRef]
- Valente Nabais, J.M.; M Carrott, P.J. Chemical Characterization of Activated Carbon Fibers and Activated Carbons. J. Chem. Educ. 2006, 83, 436–438. [Google Scholar] [CrossRef]
- Guzsvány, V.; Lazic, S.; Vidakovic, N.; Papp, Z. Derivative spectrophotometric determination of acetamiprid in the presence of 6-chloronicotinic acid. J. Serb. Chem. Soc. 2012, 77, 911–917. [Google Scholar] [CrossRef]
- Lagergren, S. About the Theory of So-Called Adsorption of Soluble Substances. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die Adsorption in Lösungen. Z. Phys. Chem. 1906, 57, 471. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Y.; Li, X.; Sun, B.; Li, Y.; Ji, H.; Qiu, J.; Wang, C. Surface Activated Hydrothermal Carbon-Coated Electrospun PAN Fiber Membrane with Enhanced Adsorption Properties for Herbicide. ACS Sustain. Chem. Eng. 2016, 4, 2584–2592. [Google Scholar] [CrossRef]








| Sample | %C | %H | %N | %S | %(O + Ash) |
|---|---|---|---|---|---|
| CA | 84.43 | 1.29 | 0.47 | 0.45 | 13.36 |
| CAHT | 89.69 | 1.63 | 1.21 | 0.39 | 7.08 |
| CT/CA11 | 90.97 | 1.20 | 1.61 | 0.56 | 5.66 |
| CT/CA12 | 90.52 | 0.93 | 1.45 | 0.50 | 6.60 |
| CT/CA13 | 89.21 | 1.05 | 1.32 | 0.30 | 8.12 |
| CT/CA14 | 91.21 | 0.80 | 1.34 | 0.59 | 6.06 |
| CT/CA15 | 91.28 | 0.49 | 1.78 | 0.34 | 6.11 |
| CT/CA16 | 91.04 | 0.77 | 1.64 | 0.57 | 5.98 |
| CT/CA17 | 89.48 | 0.80 | 1.30 | 0.49 | 7.93 |
| CT/CA18 | 89.82 | 0.70 | 1.39 | 0.52 | 7.57 |
| CT/CA19 | 90.45 | 0.68 | 1.31 | 0.31 | 7.25 |
| CHT | 64.30 | 6.66 | 0.00 | 0.01 | 29.03 |
| CA | CAHT | CHT | CT/CA13 | |
|---|---|---|---|---|
| Volatile | 2.46 | 3.79 | 50.95 | 3.93 |
| Fixed carbon | 92.36 | 91.97 | 49.05 | 94.11 |
| Ash | 5.19 | 4.23 | 0.00 | 1.96 |
| P.z.c. | Acidic Groups meq g−1 | Basic Groups meq g−1 | |
|---|---|---|---|
| CA | 9.09 | 0.32 | 0.56 |
| CAHT | 8.04 | 0.51 | 0.26 |
| CHT | 2.77 | 1.96 | --- |
| CT/CA13 | 8.71 | 0.68 | 0.46 |
| Sample | SBET, m2 g−1 | VDR, cm3 g−1 | Vme, cm3 g−1 | Rporo, nm | Vtotal, cm3 g−1 |
|---|---|---|---|---|---|
| CA | 1025.5 | 0.498 | 0.145 | 0.741 | 0.668 |
| CAHT | 1144.5 | 0.542 | 0.182 | 0.591 | 0.728 |
| CT/CA13 | 1051.9 | 0.516 | 0.109 | 0.591 | 0.628 |
| CHT | 27.1 1 | 0.001 | 0.025 | --- | 0.027 |
| CA | CHT | CT/CA13 | ||
|---|---|---|---|---|
| Pseudo-1st | k1 | 0.0958 | 0.0088 | 0.1971 |
| qe (calc) | 72.79 | 1.41 | 104.02 | |
| R2 | 0.5452 | 0.0023 | 0.903 | |
| Pseudo-2nd | k2 | 0.0080 | 0.1819 | 0.0104 |
| qe (calc) | 336.70 | 21.14 | 326.80 | |
| R2 | 0.9995 | 0.9981 | 0.9998 |
| Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|
| pH | Parameter | CA | CT/CA13 | CA | CT/CA13 | |
| 3 | Sm | 294.1 | 344.8 | KF | 31.59 | 35.20 |
| KL | 0.0622 | 0.0523 | n | 2.077 | 2.016 | |
| R2 | 0.9773 | 0.9956 | R2 | 0.9393 | 0.9347 | |
| Normal | Sm | 384.6 | 476.2 | KF | 24.05 | 20.00 |
| KL | 0.0430 | 0.0331 | n | 1.605 | 1.379 | |
| R2 | 0.9975 | 0.9573 | R2 | 0.9090 | 0.9437 | |
| 11 | Sm | 588.2 | 769.2 | KF | 16.89 | 14.94 |
| KL | 0.0239 | 0.0175 | n | 1.272 | 1.161 | |
| R2 | 0.9080 | 0.8607 | R2 | 0.9480 | 0.9754 | |
| Code | CA Mass (g) | Sucrose Concentration (g L−1) | Temperature (°C) | Isothermal Time (h) |
|---|---|---|---|---|
| CA | Carbon Norit RX-3 Extra without treatment | |||
| CAHT | 4 | - | 180 | 20 |
| CHT | - | 20 g dissolved in 50 mL of water (400 g L−1) | 180 | 20 |
| CT/CA11 | 4 | 0.01 | 160 | 10 |
| CT/CA12 | 4 | 0.05 | 180 | 15 |
| CT/CA13 | 4 | 0.10 | 200 | 20 |
| CT/CA14 | 8 | 0.05 | 160 | 20 |
| CT/CA15 | 8 | 0.10 | 180 | 10 |
| CT/CA16 | 8 | 0.01 | 200 | 15 |
| CT/CA17 | 12 | 0.10 | 160 | 15 |
| CT/CA18 | 12 | 0.01 | 180 | 20 |
| CT/CA19 | 12 | 0.05 | 200 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adame-Pereira, M.; Durán-Valle, C.J.; Fernández-González, C. Hydrothermal Carbon Coating of an Activated Carbon—A New Adsorbent. Molecules 2023, 28, 4769. https://doi.org/10.3390/molecules28124769
Adame-Pereira M, Durán-Valle CJ, Fernández-González C. Hydrothermal Carbon Coating of an Activated Carbon—A New Adsorbent. Molecules. 2023; 28(12):4769. https://doi.org/10.3390/molecules28124769
Chicago/Turabian StyleAdame-Pereira, Marta, Carlos J. Durán-Valle, and Carmen Fernández-González. 2023. "Hydrothermal Carbon Coating of an Activated Carbon—A New Adsorbent" Molecules 28, no. 12: 4769. https://doi.org/10.3390/molecules28124769
APA StyleAdame-Pereira, M., Durán-Valle, C. J., & Fernández-González, C. (2023). Hydrothermal Carbon Coating of an Activated Carbon—A New Adsorbent. Molecules, 28(12), 4769. https://doi.org/10.3390/molecules28124769









