Detachment of Dodecane from Silica Surfaces with Variable Surface Chemistry Studied Using Molecular Dynamics Simulation
Abstract
1. Introduction
2. Results
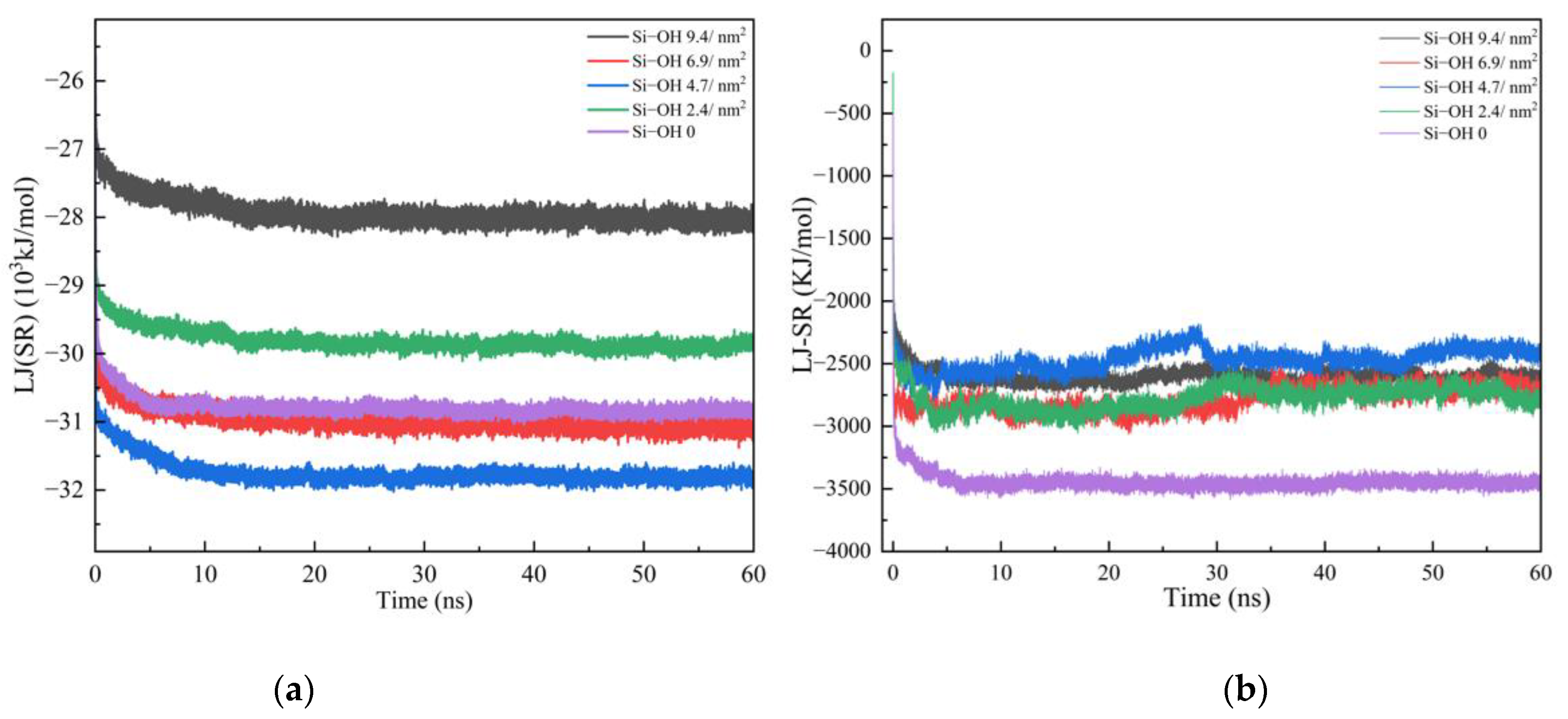
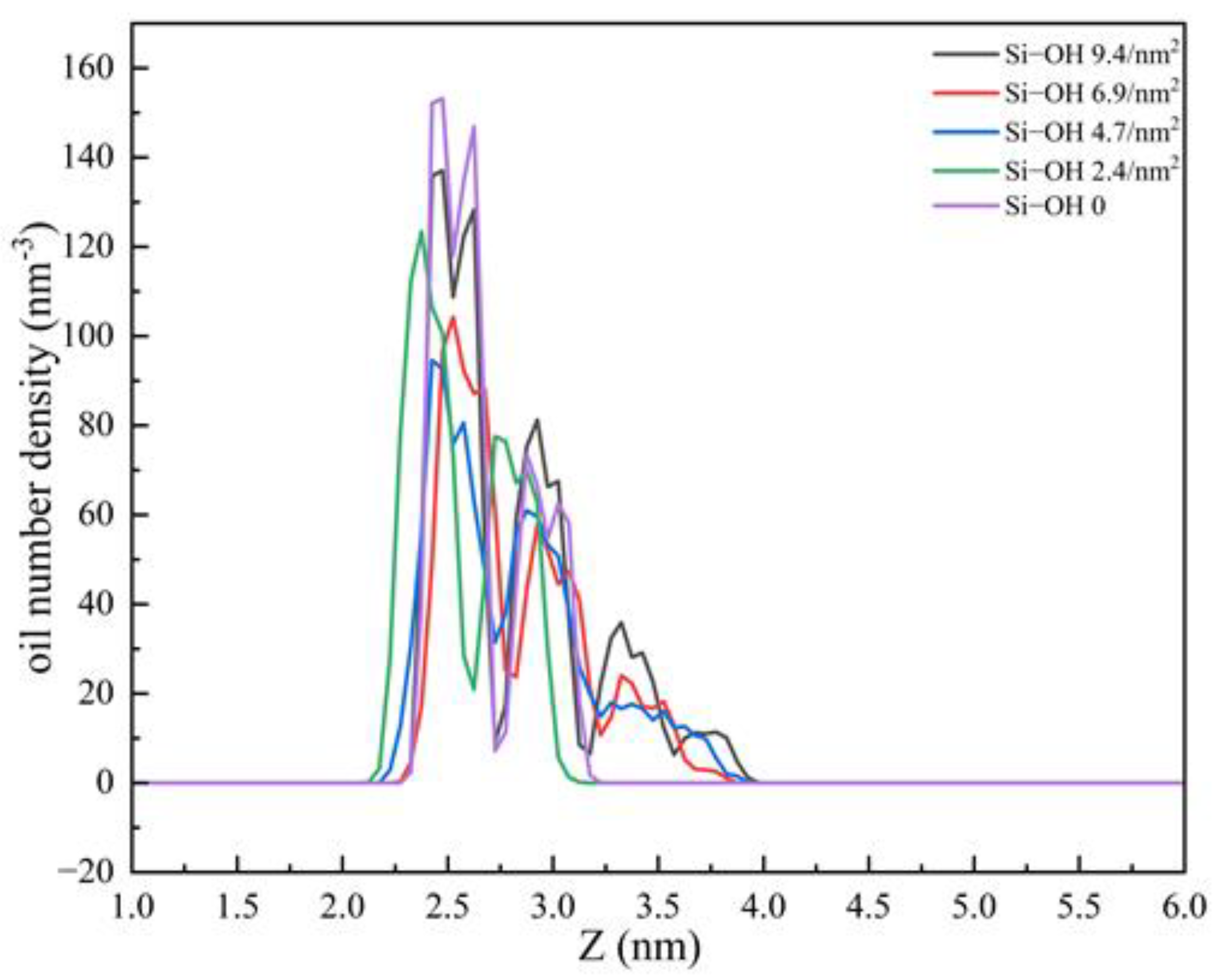
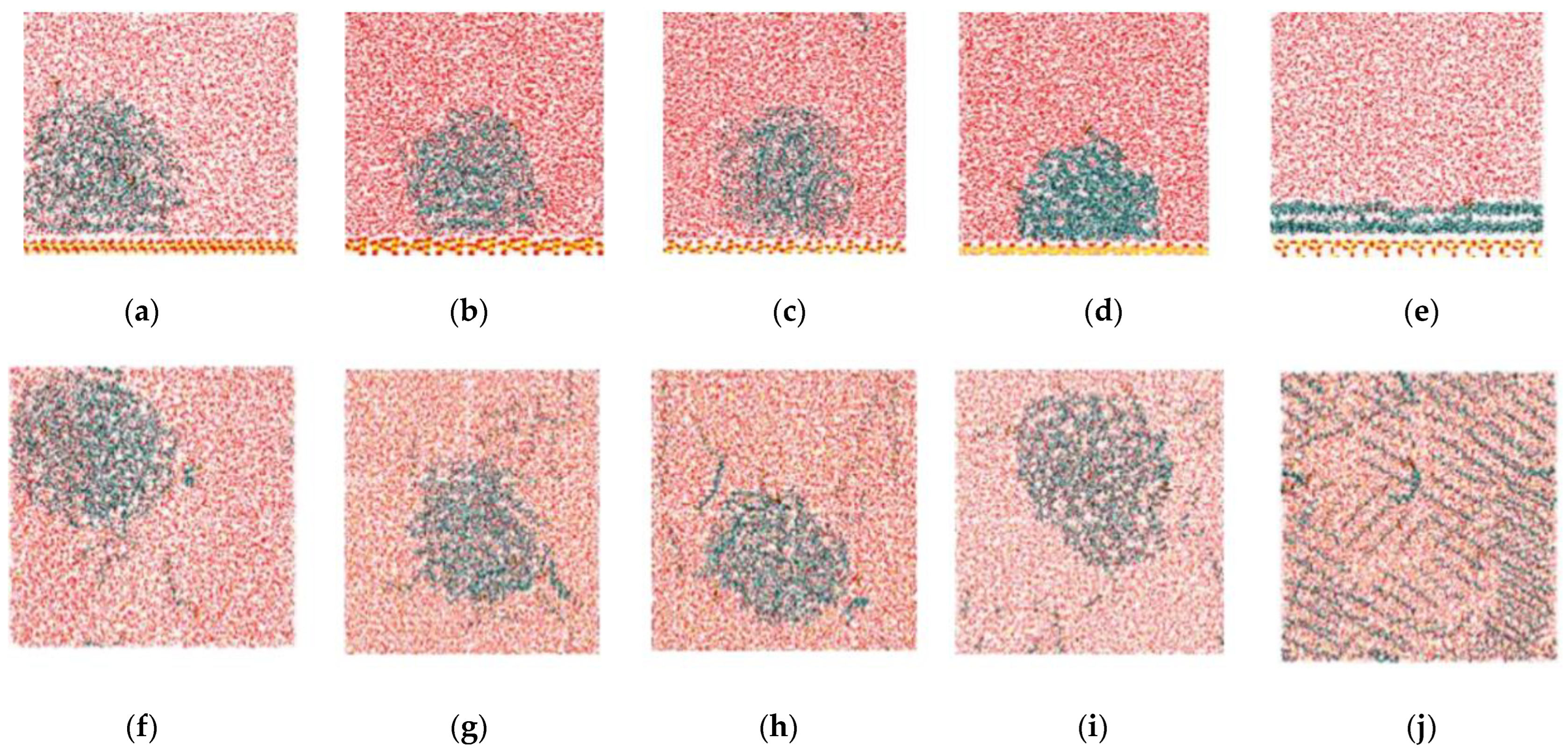
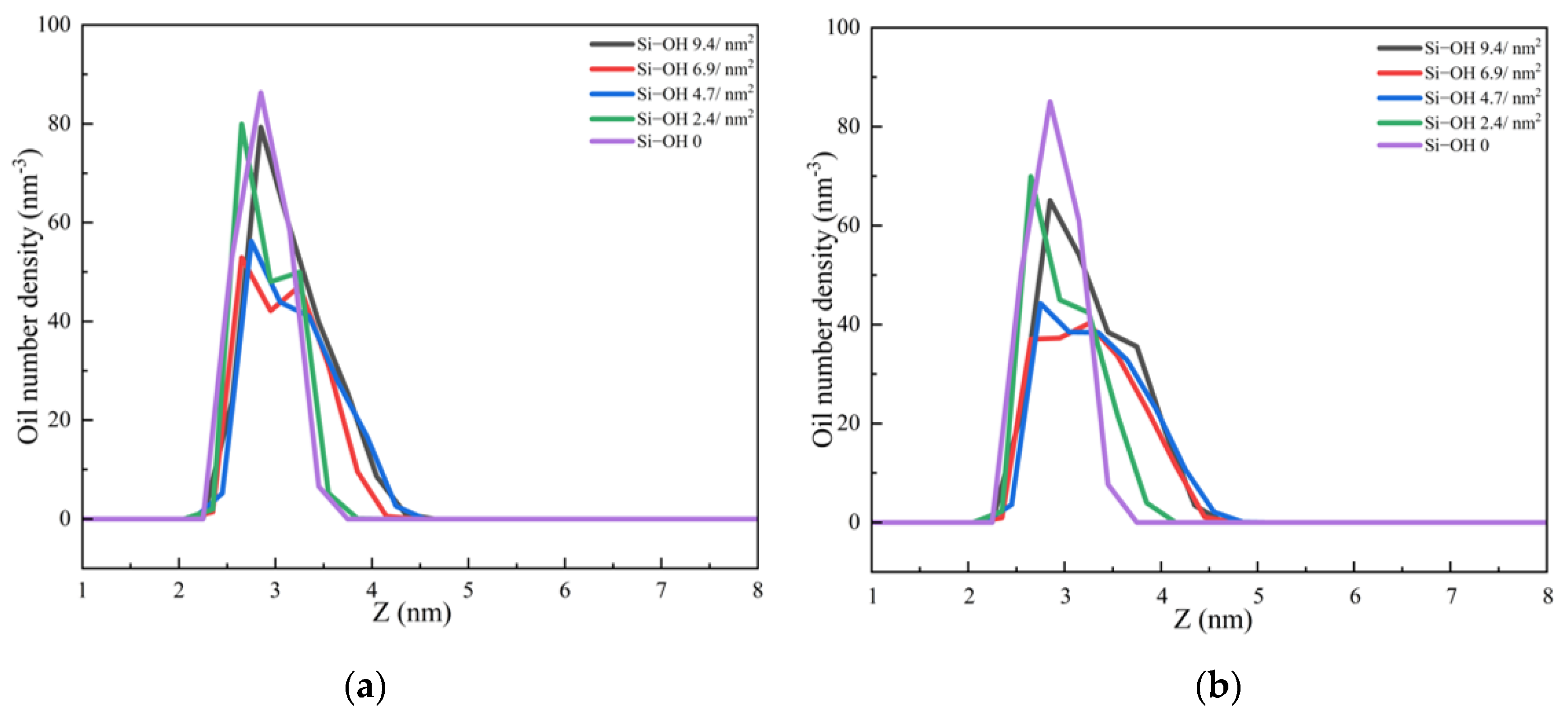
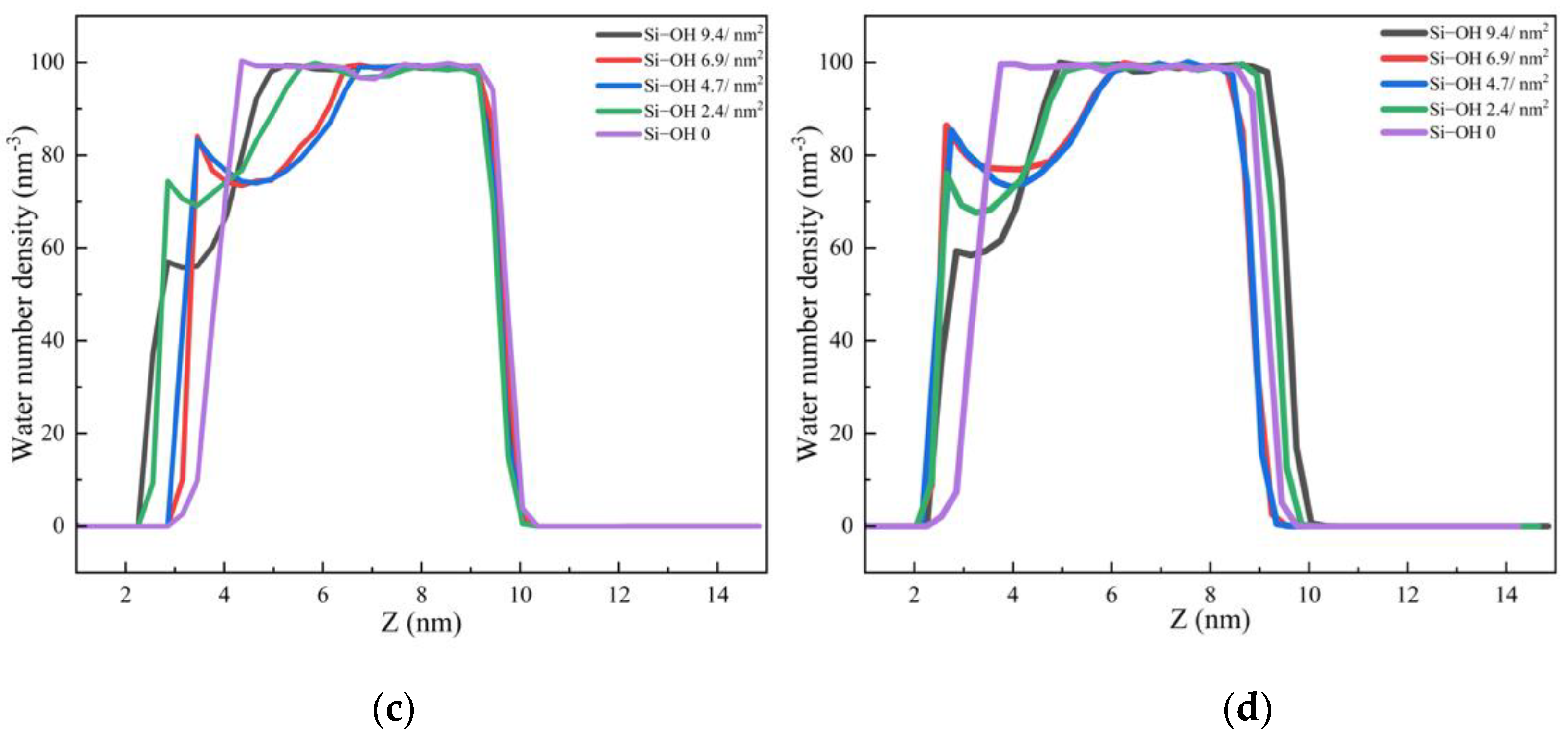
2.1. Adsorption of C12H26 on Different Silica Surfaces
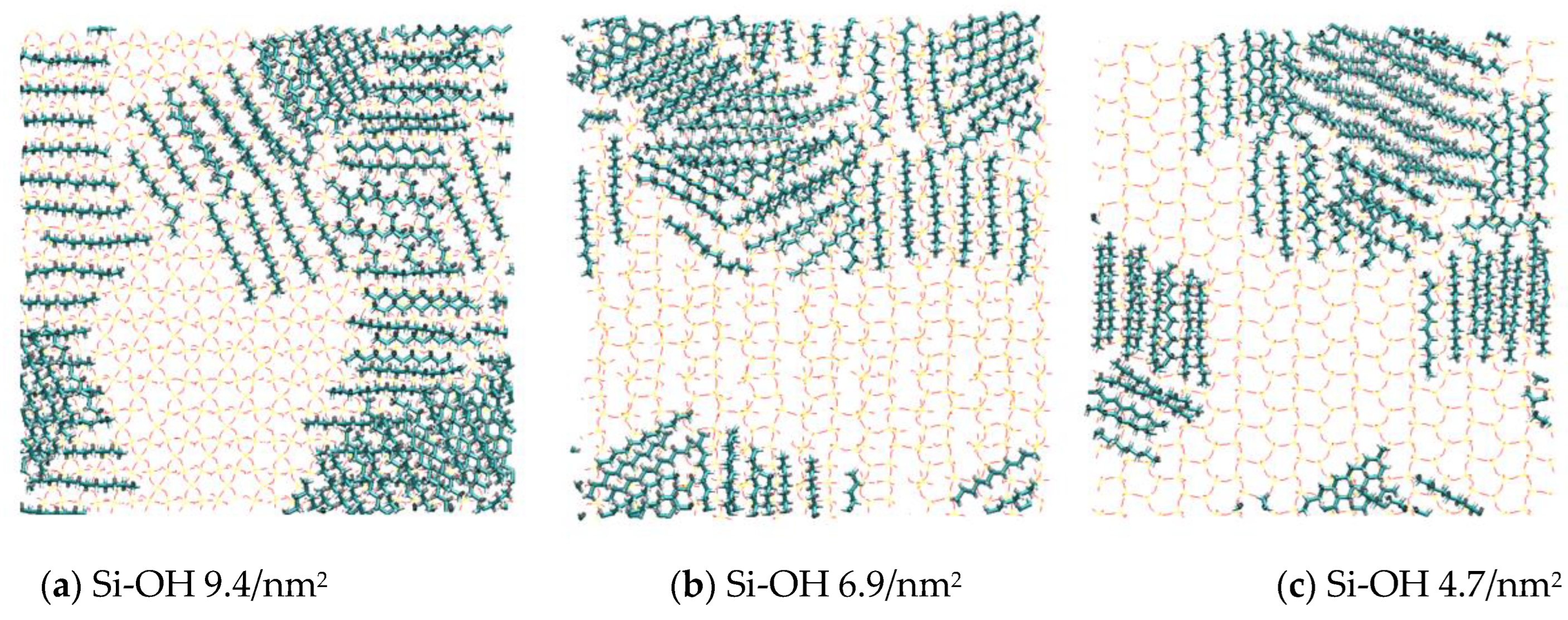
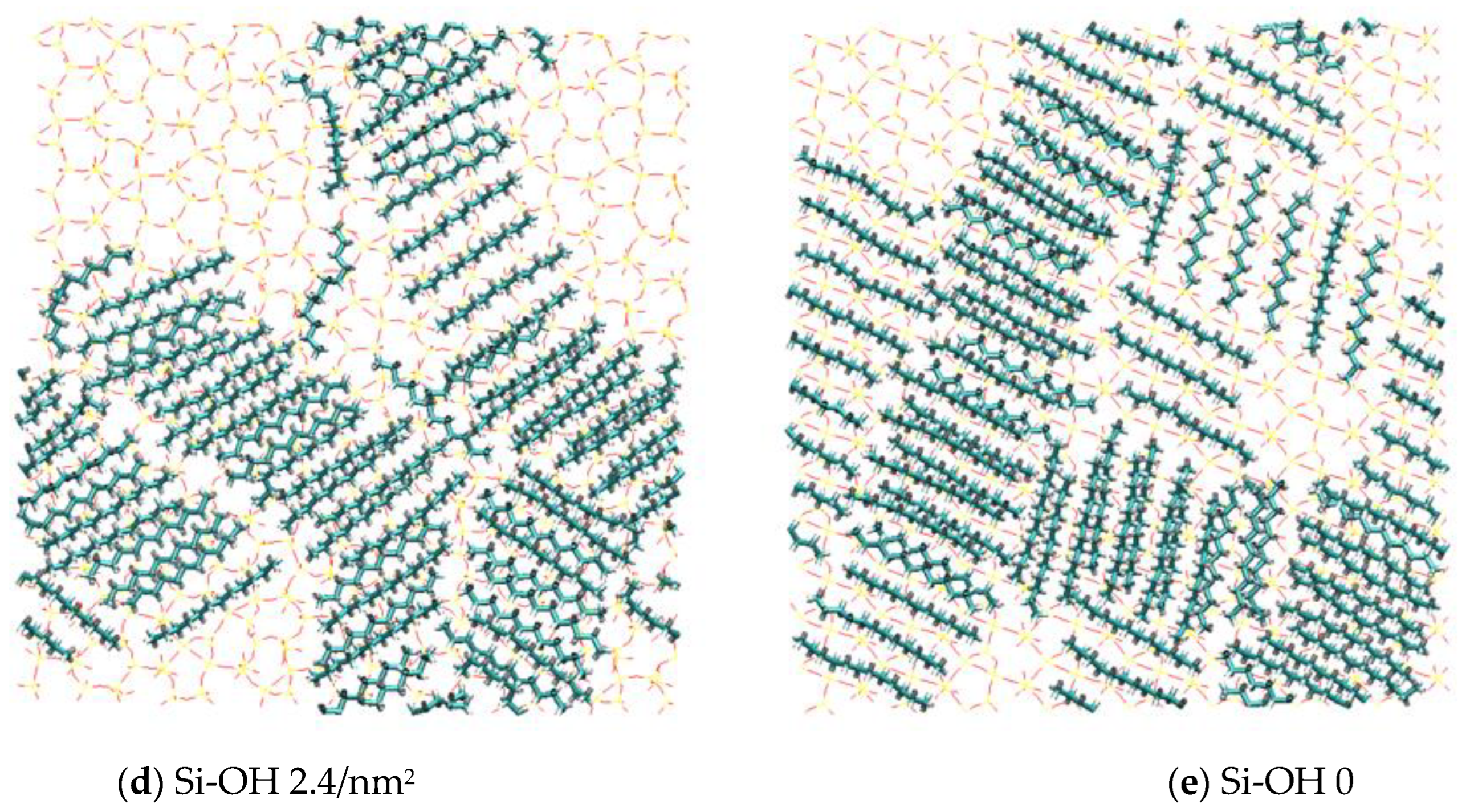
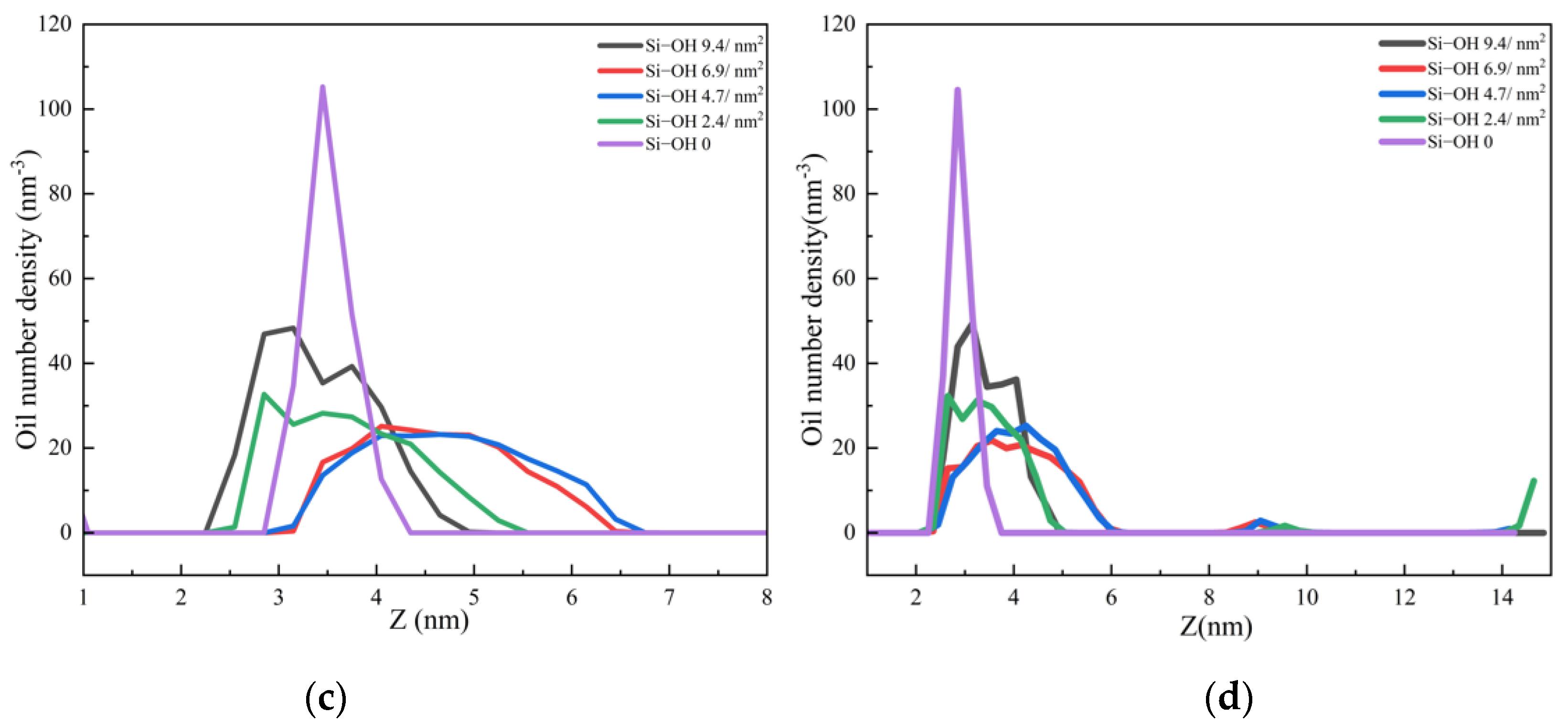
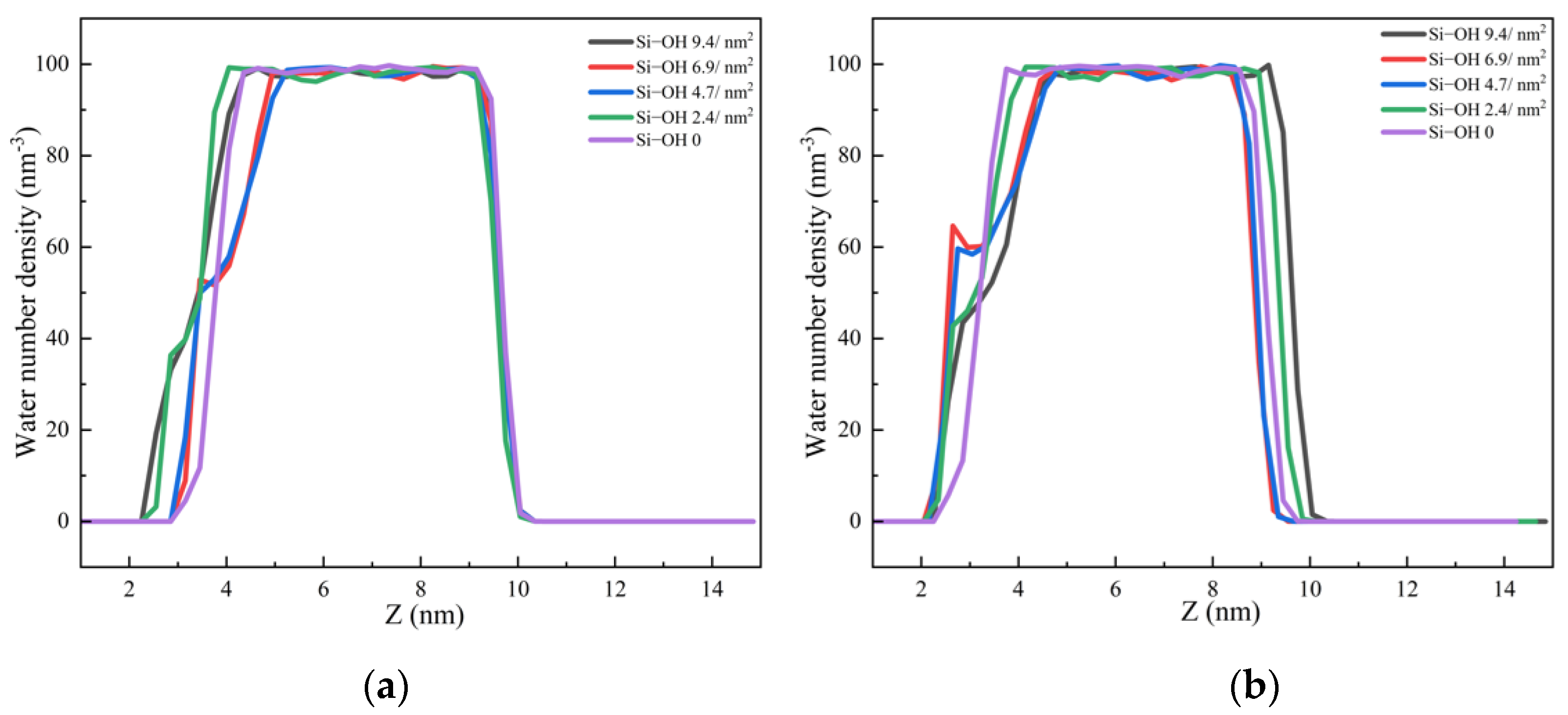
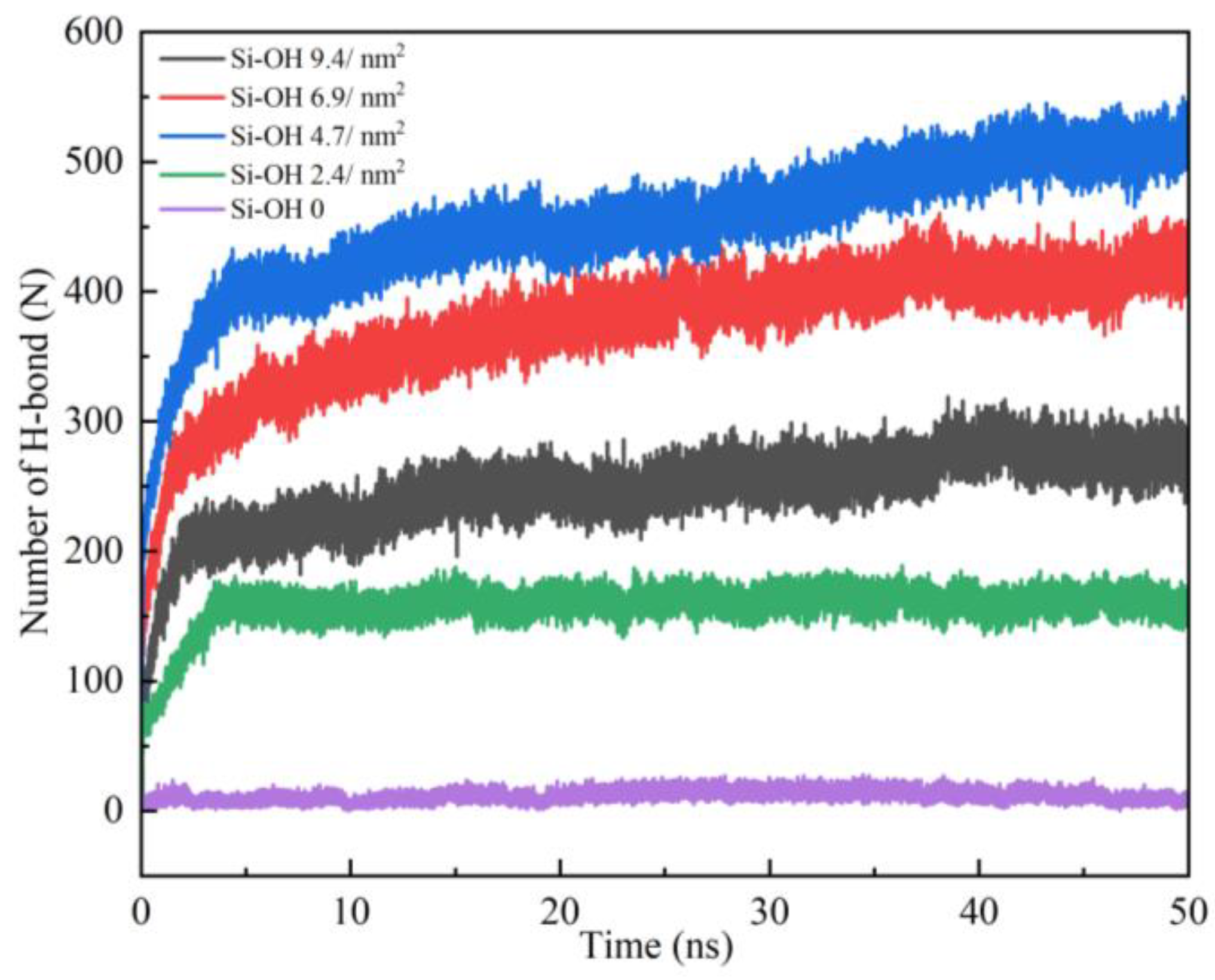
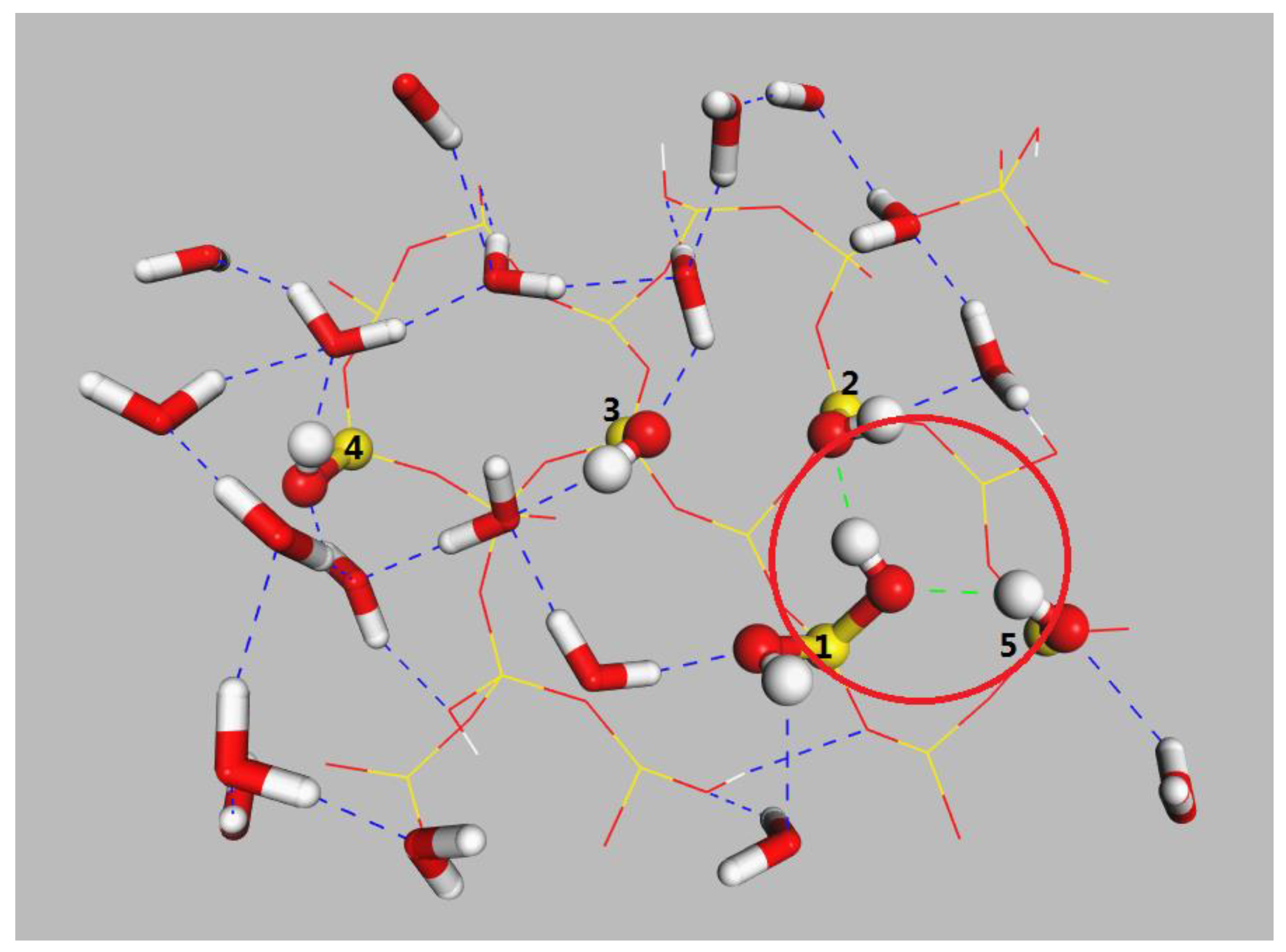
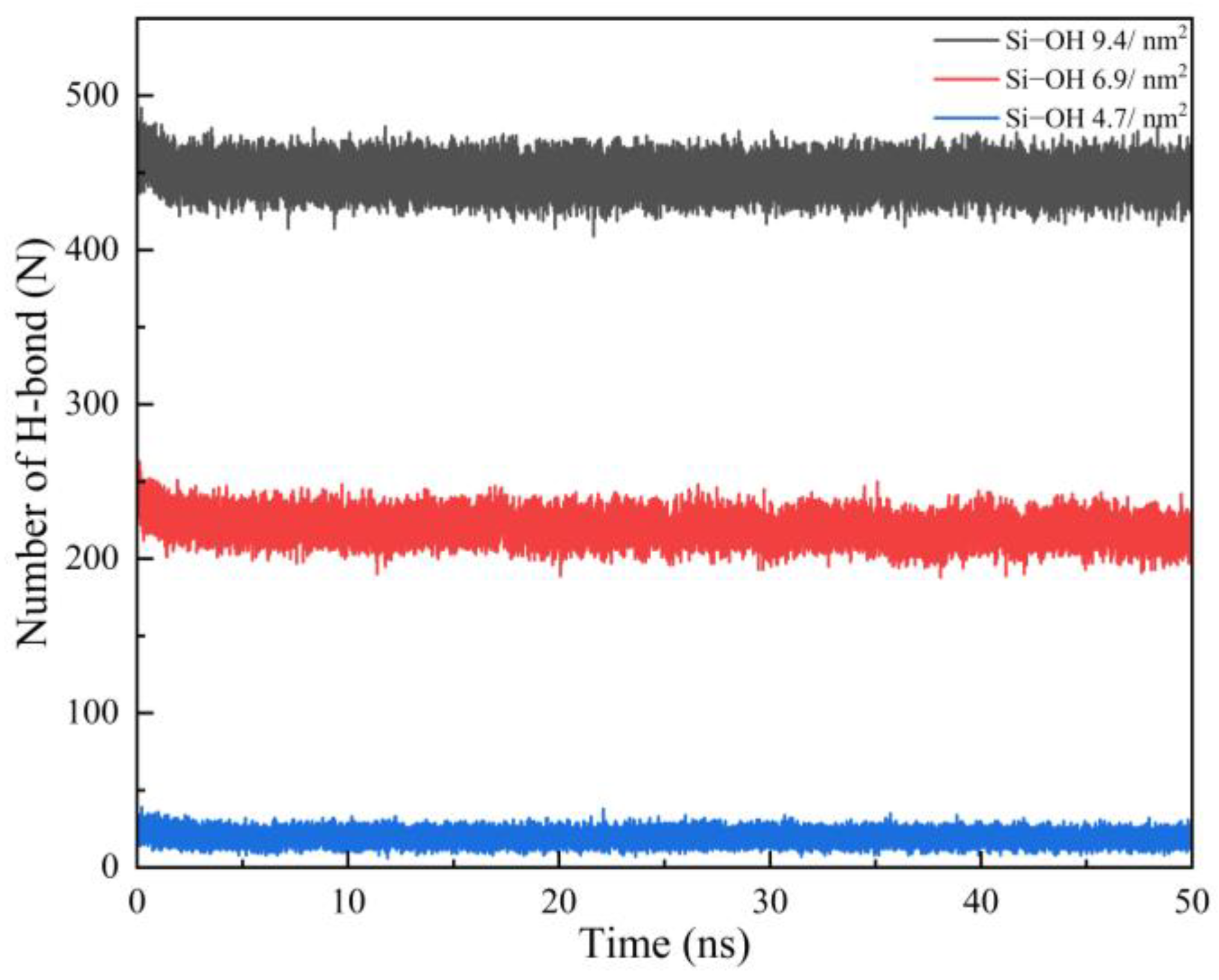
2.2. Detachment of C12H26 on Different Silica Surfaces
3. Discussion
4. Materials and Methods
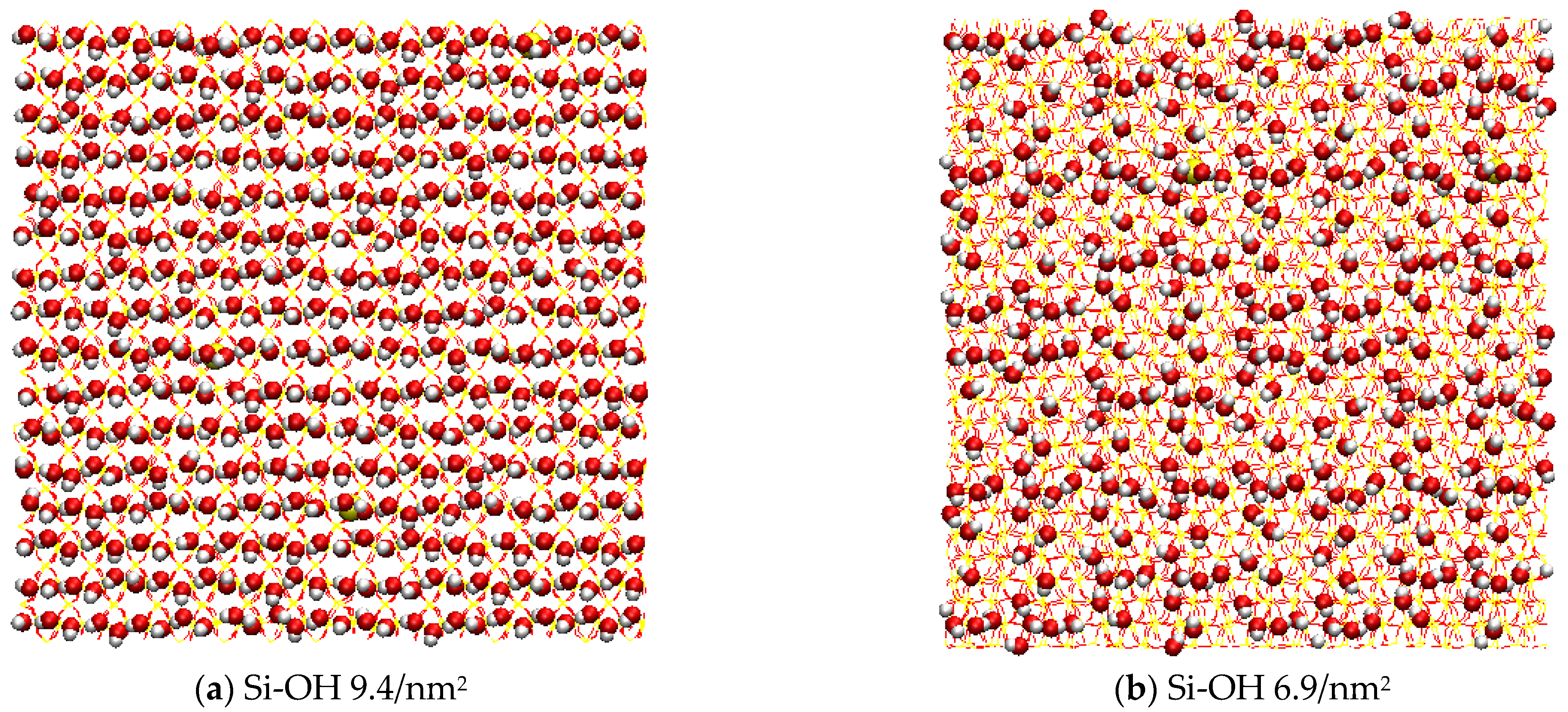
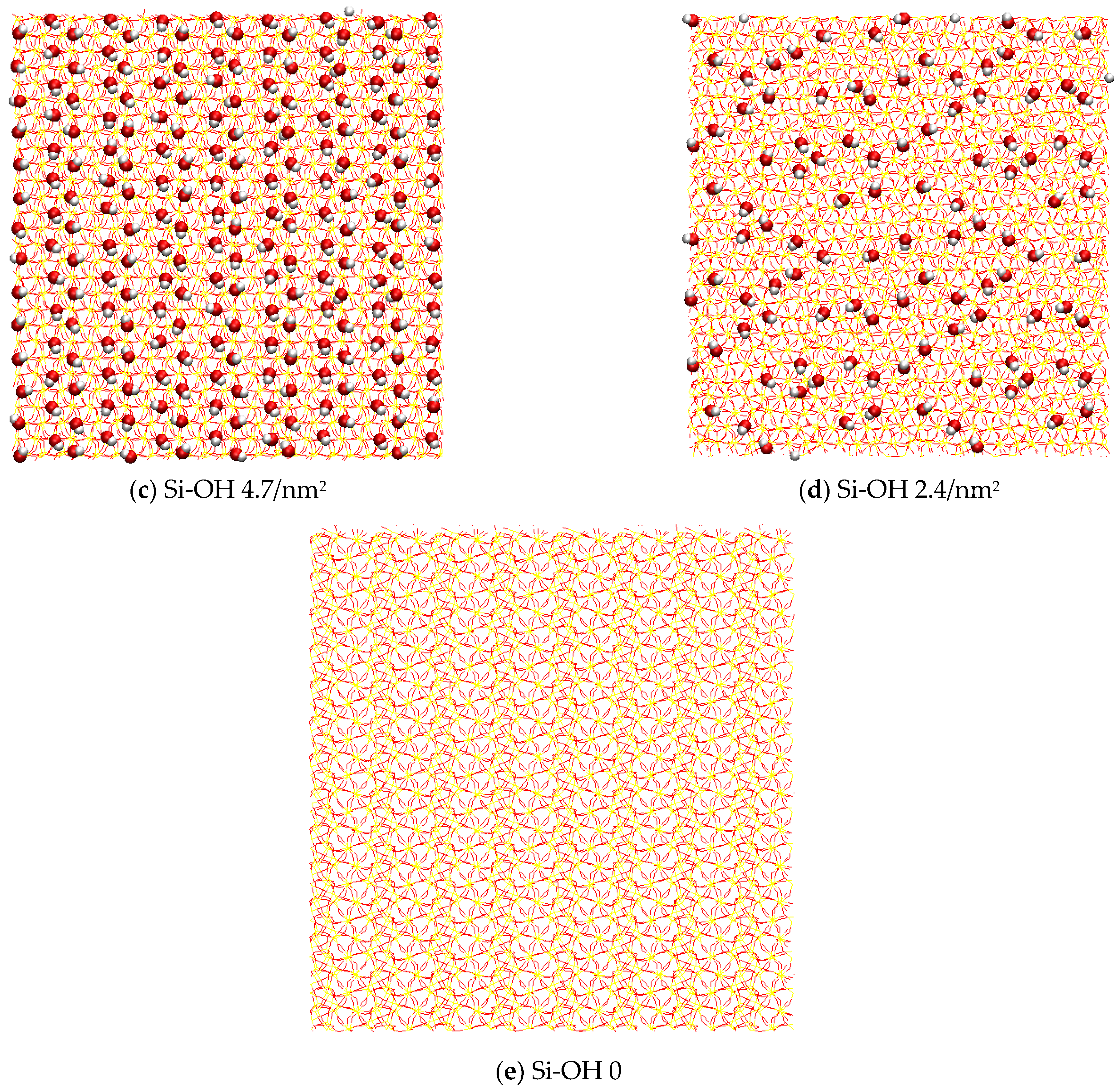
4.1. Model Systems
4.2. Computational Details
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dudášová, D.; Simon, S.; Hemmingsen, P.V.; Sjöblom, J. Study of asphaltenes adsorption onto different minerals and clays: Part 1. Experimental adsorption with UV depletion detection. Colloid Surf. A 2008, 317, 1–9. [Google Scholar] [CrossRef]
- He, L.; Lin, F.; Li, X.; Sui, H.; Xu, Z. Interfacial sciences in unconventional petroleum production: From fundamentals to applications. Chem. Soc. Rev. 2015, 44, 5446–5494. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Ma, S.; Liu, Q. Enhanced heavy oil recovery through interfacial instability: A study of chemical flooding for Brintnell heavy oil. Fuel 2009, 88, 1049–1056. [Google Scholar] [CrossRef]
- Alvarado, V.; Manrique, E. Enhanced Oil Recovery: An Update Review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Li, X.; Sun, W.; Wu, G.; He, L.; Li, H.; Sui, H. Ionic Liquid Enhanced Solvent Extraction for Bitumen Recovery from Oil Sands. Energy Fuels 2011, 25, 5224–5231. [Google Scholar] [CrossRef]
- Emami, F.S.; Puddu, V.; Berry, R.J.; Varshney, V.; Patwardhan, S.V.; Perry, C.C.; Heinz, H. Force Field and a Surface Model Database for Silica to Simulate Interfacial Properties in Atomic Resolution. Chem. Mater. 2014, 26, 2647–2658, Correction in Chem. Mater. 2016, 28, 406–407. [Google Scholar] [CrossRef]
- Johannessen, A.M.; Spildo, K. Enhanced Oil Recovery (EOR) by Combining Surfactant with Low Salinity Injection. Energy Fuels 2013, 27, 5738–5749. [Google Scholar] [CrossRef]
- Adams, J.J. Asphaltene Adsorption, a Literature Review. Energy Fuels 2014, 28, 2831–2856. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Z.; Masliyah, J. Processability of Oil Sand Ores in Alberta. Energy Fuels 2005, 19, 2056–2063. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Z.; Masliyah, J. Studies on Bitumen−Silica Interaction in Aqueous Solutions by Atomic Force Microscopy. Langmuir 2003, 19, 3911–3920. [Google Scholar] [CrossRef]
- Ahmadi, M.; Hou, Q.; Wang, Y.; Chen, Z. Interfacial and molecular interactions between fractions of heavy oil and surfactants in porous media: Comprehensive review. Adv. Colloid Interface Sci. 2020, 283, 102242. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Y.; Ren, S. Molecular Dynamics Simulation of Self-Aggregation of Asphaltenes at an Oil/Water Interface: Formation and Destruction of the Asphaltene Protective Film. Energy Fuels 2015, 29, 1233–1242. [Google Scholar] [CrossRef]
- Liu, Q.; Yuan, S.; Yan, H.; Zhao, X. Mechanism of Oil Detachment from a Silica Surface in Aqueous Surfactant Solutions: Molecular Dynamics Simulations. J. Phys. Chem. B 2012, 116, 2867–2875. [Google Scholar] [CrossRef]
- Moncayo-Riascos, I.; Cortes, F.B.; Hoyos, B.A. Chemical Alteration of Wettability of Sandstones with Polysorbate 80. Experimental and Molecular Dynamics Study. Energy Fuels 2017, 31, 11918–11924. [Google Scholar] [CrossRef]
- Moncayo-Riascos, I.; Hoyos, B.A. Fluorocarbon versus hydrocarbon organosilicon surfactants for wettability alteration: A molecular dynamics approach. J. Ind. Eng. Chem. 2020, 88, 224–232. [Google Scholar] [CrossRef]
- Moncayo-Riascos, I.; de León, J.; Hoyos, B.A. Molecular Dynamics Methodology for the Evaluation of the Chemical Alteration of Wettability with Organosilanes. Energy Fuels 2016, 30, 3605–3614. [Google Scholar] [CrossRef]
- Zhang, L.; Li, B.; Xia, Y.; Liu, S. Wettability modification of Wender lignite by adsorption of dodecyl poly ethoxylated surfactants with different degree of ethoxylation: A molecular dynamics simulation study. J. Mol. Graph. Model. 2017, 76, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, Y.; Liu, Y.; Jiang, Y.; Zhang, Q.; Sun, Z.; Di, C. A Mechanistic Study of Wettability Alteration of Calcite as an Example of Carbonate Reservoirs Using Molecular Dynamics Simulation. J. Energy Resour. Technol. 2022, 144, 103006. [Google Scholar] [CrossRef]
- Ahmadi, M.; Chen, Z. Molecular Dynamics Investigation of Wettability Alteration of Quartz Surface under Thermal Recovery Processes. Molecules 2023, 28, 1162. [Google Scholar] [CrossRef]
- Mohammed, S.; Gadikota, G. Dynamic Wettability Alteration of Calcite, Silica and Illite Surfaces in Subsurface Environments: A Case Study of Asphaltene Self-Assembly at Solid Interfaces. Appl. Surf. Sci. 2020, 505, 144516. [Google Scholar] [CrossRef]
- Li, X.; Xue, Q.; Zhu, L.; Jin, Y.; Wu, T.; Guo, Q.; Zheng, H.; Lu, S. How to select an optimal surfactant molecule to speed up the oil-detachment from solid surface: A computational simulation. Chem. Eng. Sci. 2016, 147, 47–53. [Google Scholar] [CrossRef]
- Yang, Z.; Hu, R.; Chen, Y.-F. Molecular Origin of Wetting Characteristics on Mineral Surfaces. Langmuir 2023, 39, 2932–2942. [Google Scholar]
- Zhu, X.; Chen, D.; Zhang, Y.; Wu, G. Insights into the Oil Adsorption and Cyclodextrin Extraction process on Rough Silica Surface by Molecular Dynamics Simulation. J. Phys. Chem. C 2018, 122, 2997–3005. [Google Scholar] [CrossRef]
- Peter, A.K.; Krassimir, D.D.; Vesselin, L.K.; Theodor, D.G.; Mila, I.T.; Gunter, B. Detachment of Oil Drops from Solid Surfaces in Surfactant Solutions: Molecular Mechanisms at a Moving Contact Line. Ind. Eng. Chem. Res. 2005, 44, 1309–1321. [Google Scholar]
- Koretsky, C.M.; Sverjensky, D.A.; Sahai, N. A model of surface site types on oxide and silicate minerals based on crystal chemistry; implications for site types and densities, multi-site adsorption, surface infrared spectroscopy, and dissolution kinetics. Am. J. Sci. 1998, 298, 349–438. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, X.; Liu, X.; Yang, K.; Zhou, H. Surface Wettability of Basal Surfaces of Clay Minerals: Insights from Molecular Dynamics Simulation. Energy Fuels 2016, 30, 149–160. [Google Scholar] [CrossRef]
- Toukan, K.; Rahman, A. Molecular-dynamics study of atomic motions in water. Phys. Rev. B 1985, 31, 2643–2648. [Google Scholar] [CrossRef] [PubMed]

| Atom | Atom Type | Charge (e) |
|---|---|---|
| Si | SC4 | 1.1 |
| Si-O-Si | OC23 | −0.55 |
| Si-O-H | OC24 | −0.675 |
| H | HOY | 0.4 |
| C12H26/N | SOL/N | SDS/N | |
|---|---|---|---|
| Si-OH 9.4/nm2 | 90 | 9964 | 5 |
| Si-OH 6.9/nm2 | 90 | 8941 | 5 |
| Si-OH 4.7/nm2 | 90 | 8890 | 5 |
| Si-OH 2.4/nm2 | 90 | 9650 | 5 |
| Si-OH 0 | 90 | 9096 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.; Hou, H.; Liu, Q.; Wang, H.; Li, Y.; Yang, B.; Su, C.; Wu, M. Detachment of Dodecane from Silica Surfaces with Variable Surface Chemistry Studied Using Molecular Dynamics Simulation. Molecules 2023, 28, 4765. https://doi.org/10.3390/molecules28124765
Jiang B, Hou H, Liu Q, Wang H, Li Y, Yang B, Su C, Wu M. Detachment of Dodecane from Silica Surfaces with Variable Surface Chemistry Studied Using Molecular Dynamics Simulation. Molecules. 2023; 28(12):4765. https://doi.org/10.3390/molecules28124765
Chicago/Turabian StyleJiang, Binbin, Huan Hou, Qian Liu, Hongyuan Wang, Yang Li, Boyu Yang, Chen Su, and Min Wu. 2023. "Detachment of Dodecane from Silica Surfaces with Variable Surface Chemistry Studied Using Molecular Dynamics Simulation" Molecules 28, no. 12: 4765. https://doi.org/10.3390/molecules28124765
APA StyleJiang, B., Hou, H., Liu, Q., Wang, H., Li, Y., Yang, B., Su, C., & Wu, M. (2023). Detachment of Dodecane from Silica Surfaces with Variable Surface Chemistry Studied Using Molecular Dynamics Simulation. Molecules, 28(12), 4765. https://doi.org/10.3390/molecules28124765






