Photocatalytic Transformation of Biomass and Biomass Derived Compounds—Application to Organic Synthesis
Abstract
1. Introduction
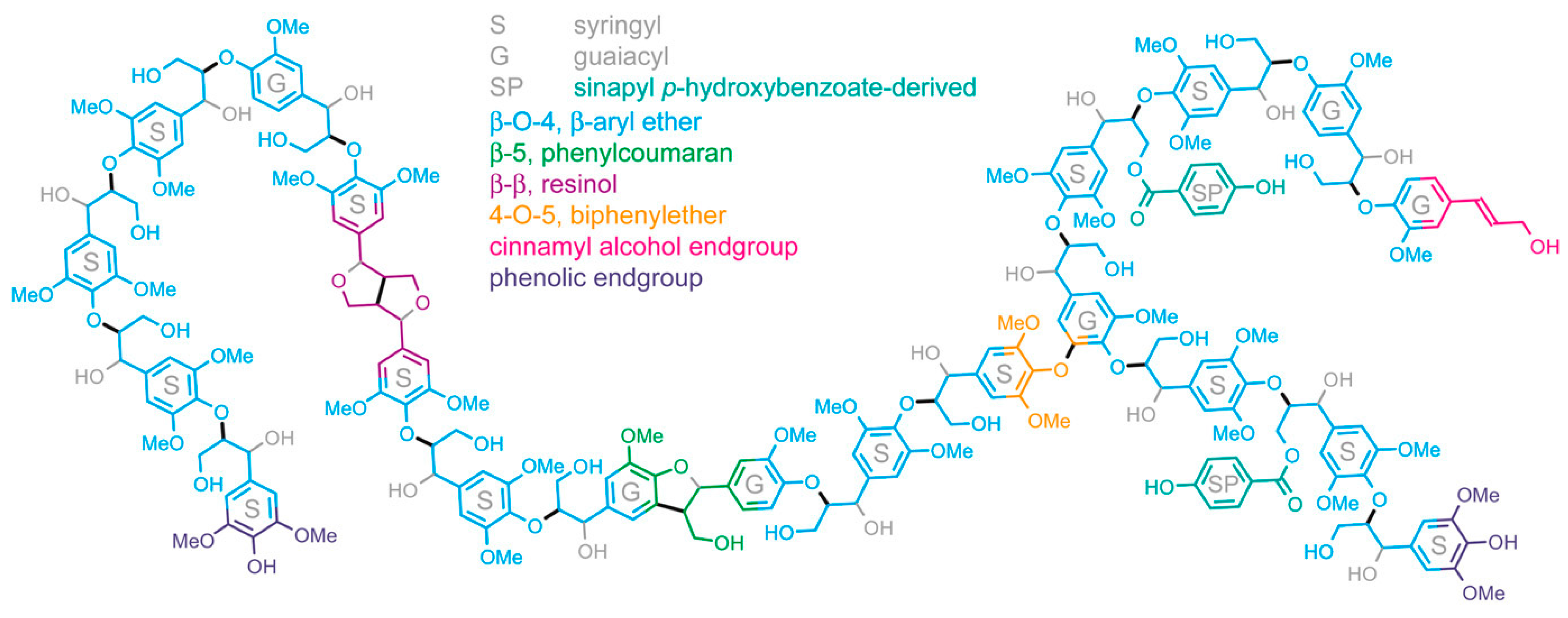
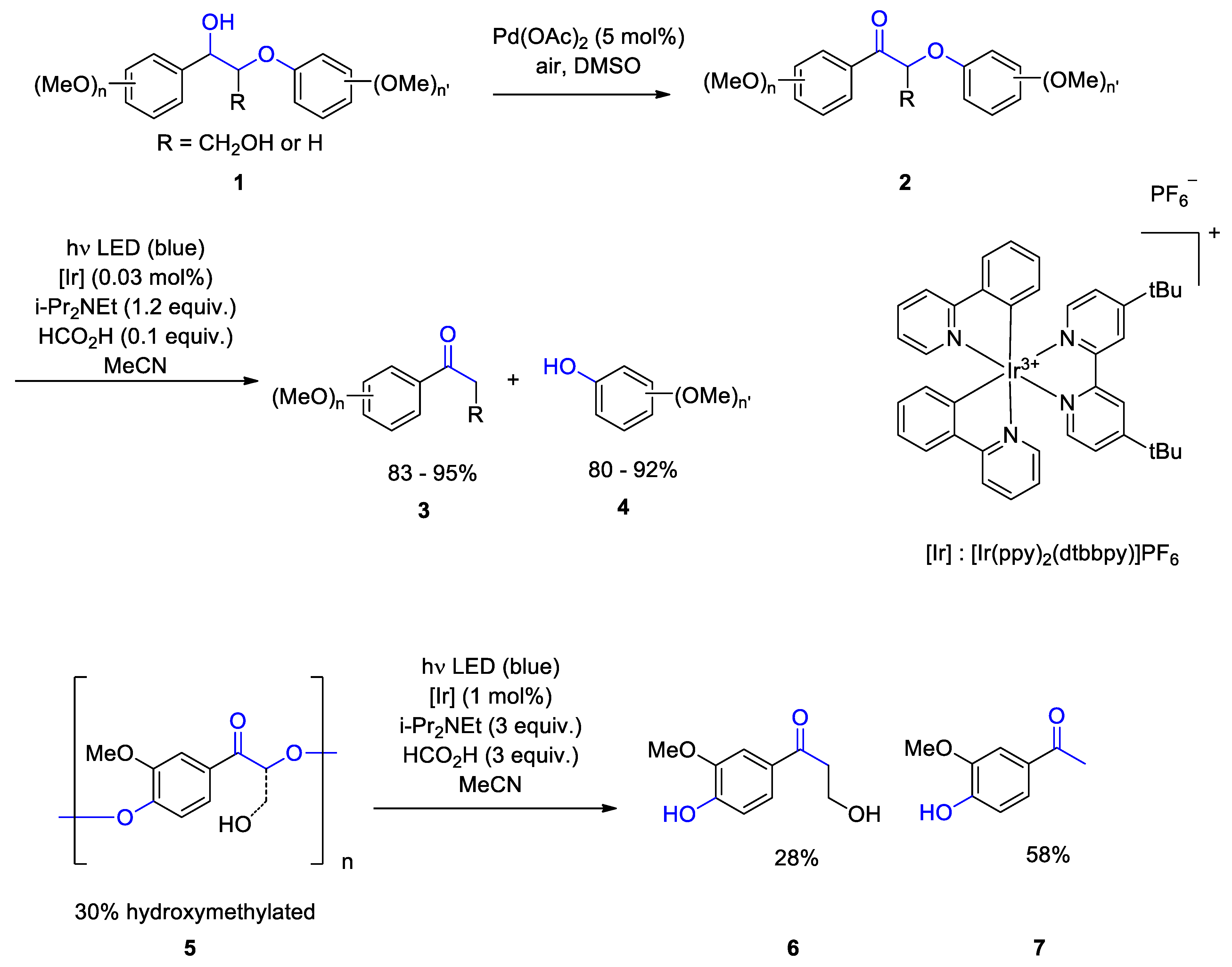
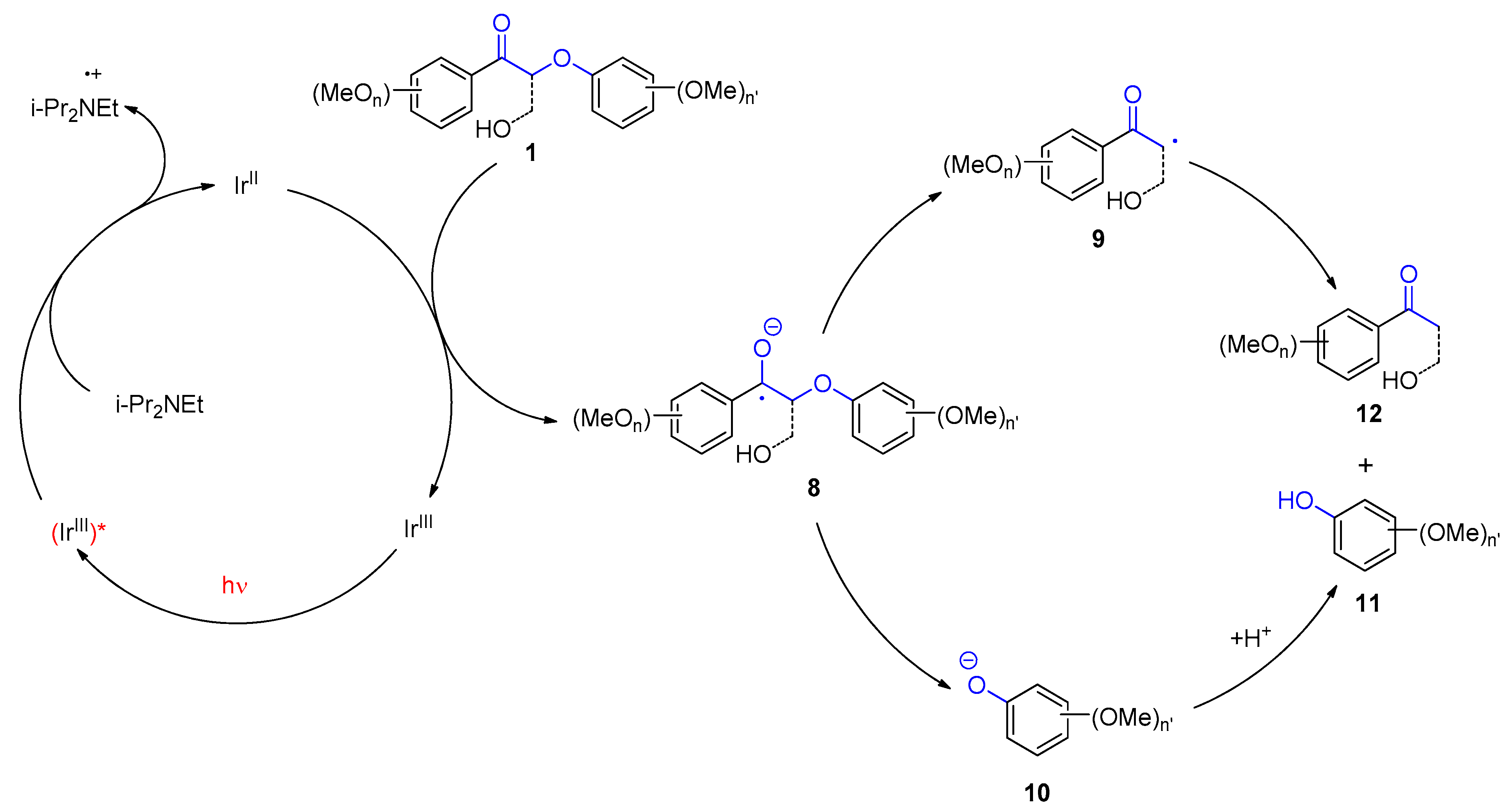
2. Photocatalytic Transformations of Biopolymers
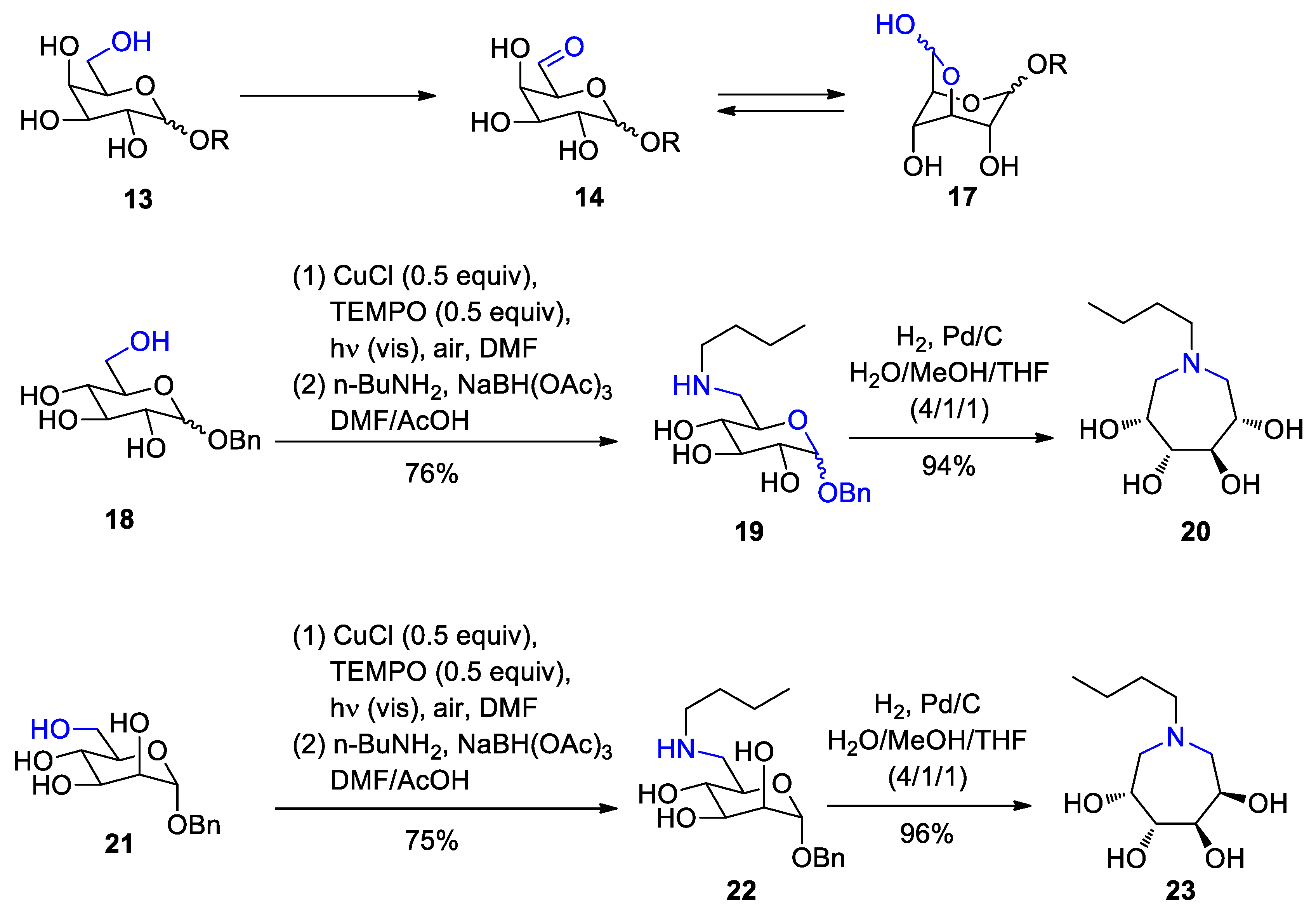
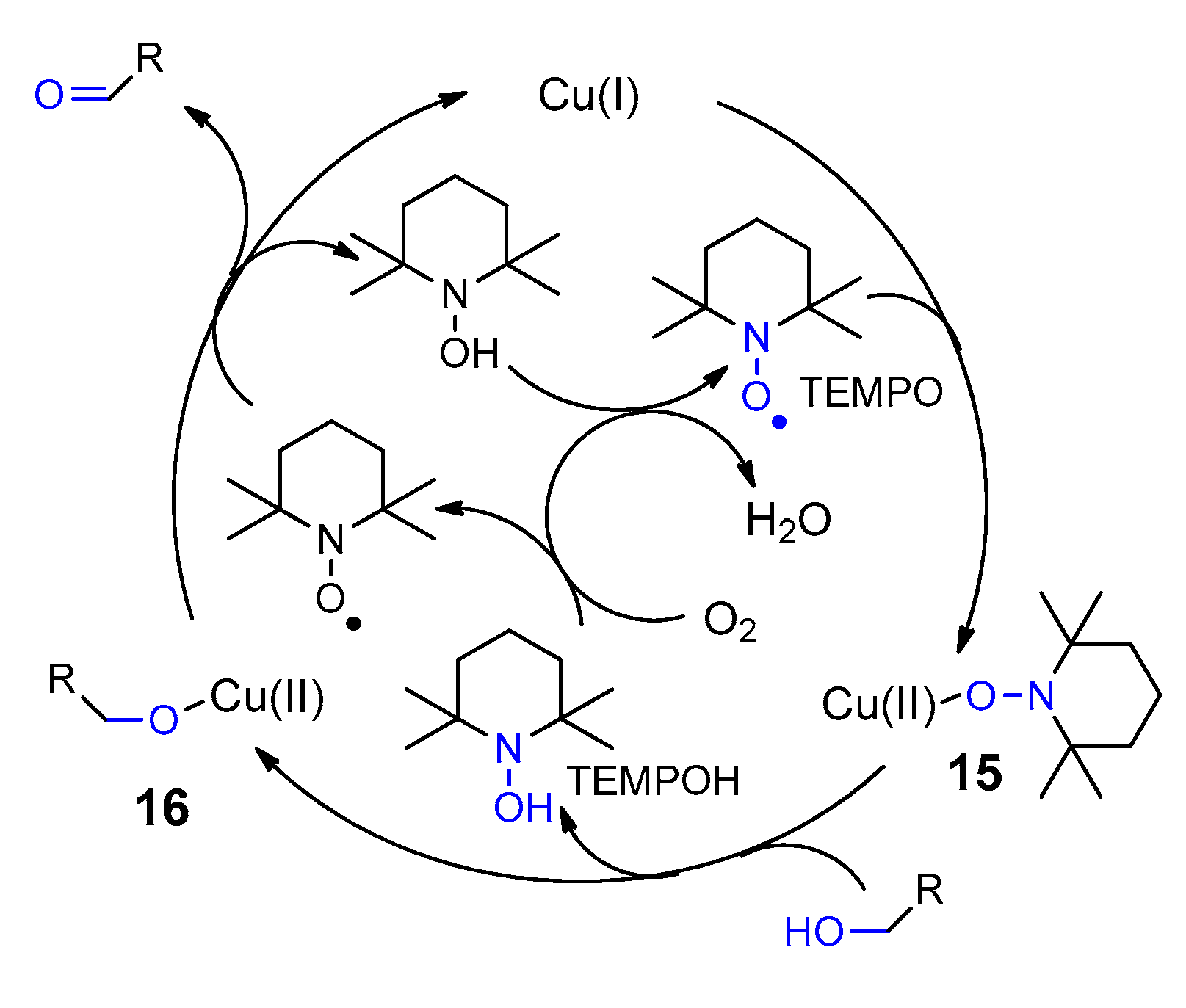
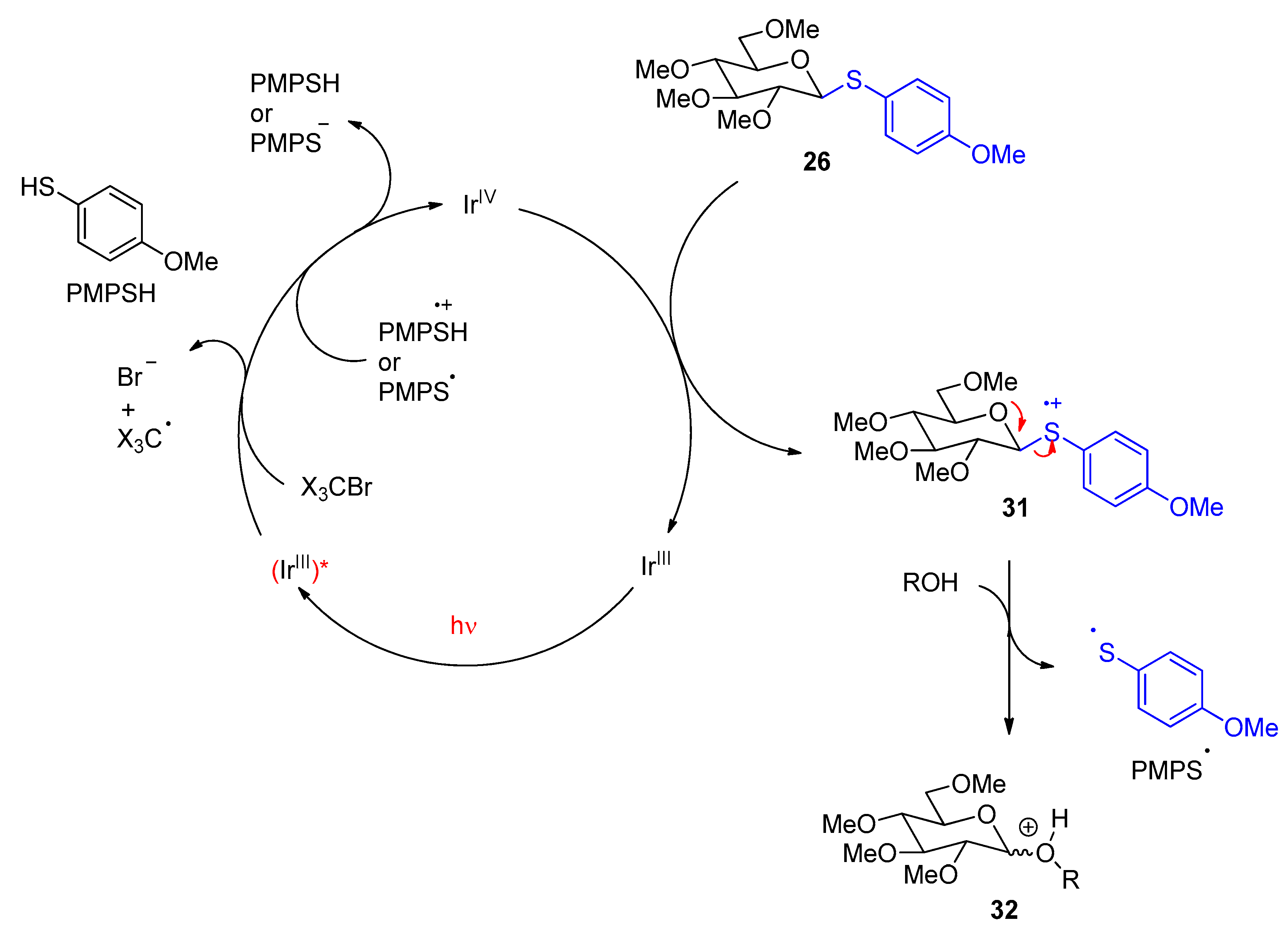
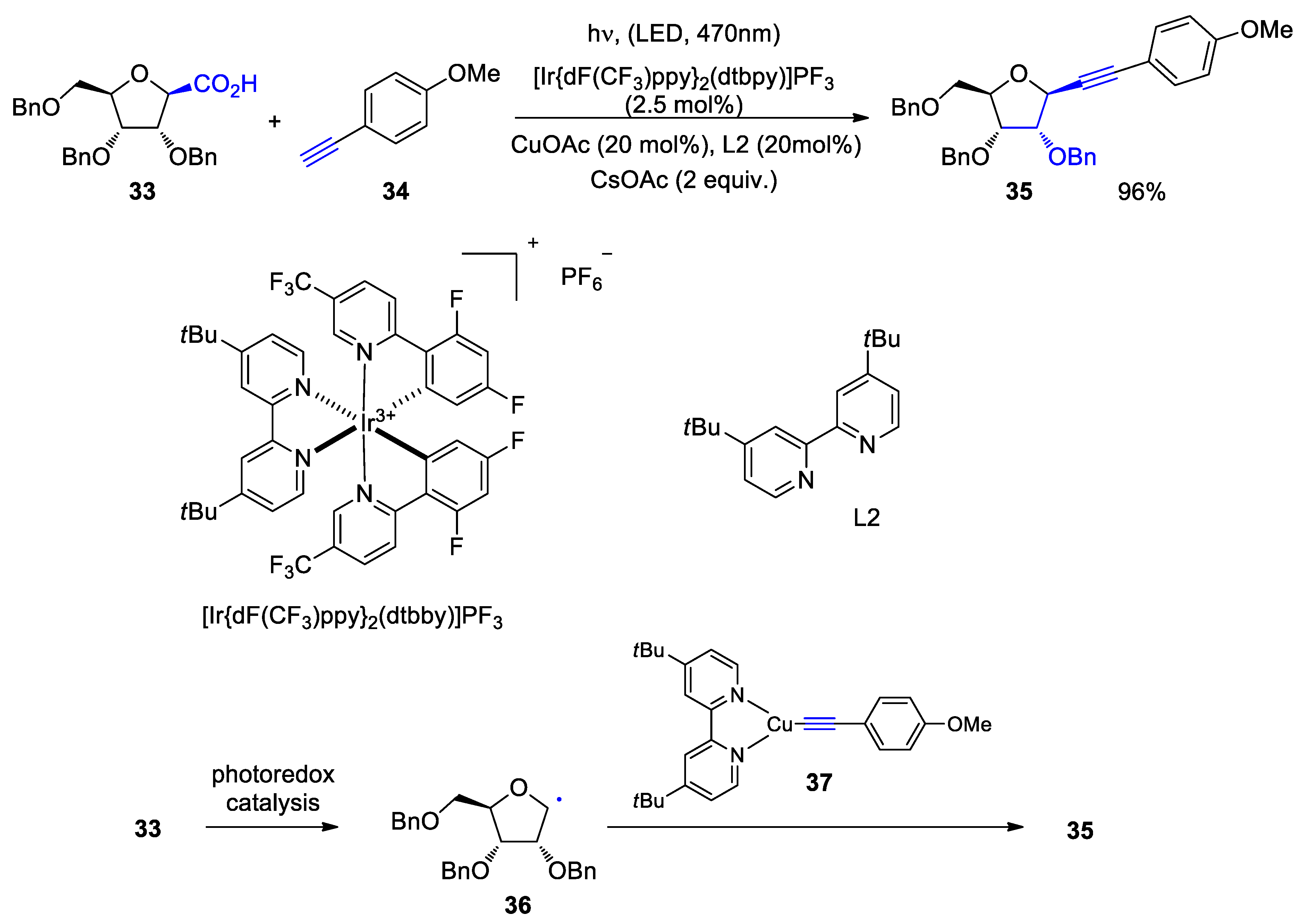
3. Carbohydrates
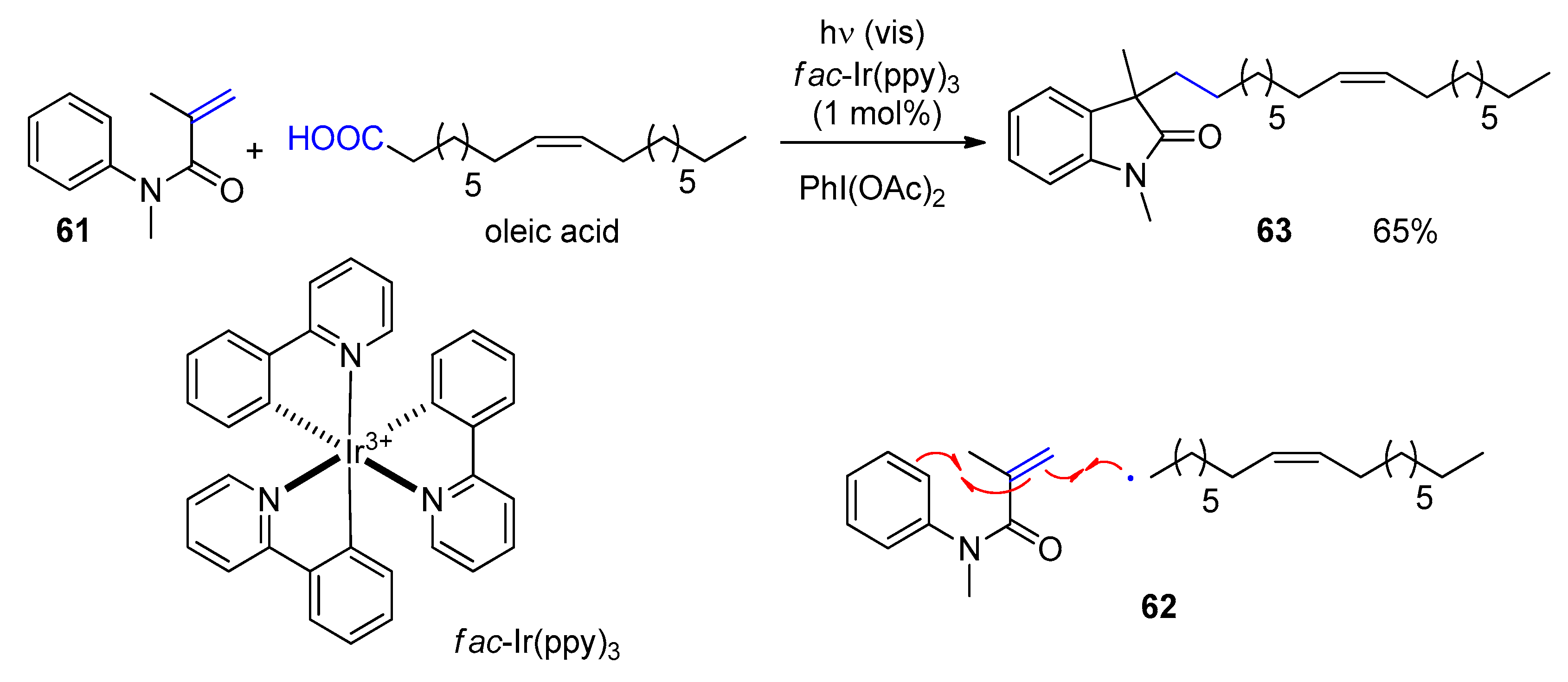
4. Fatty Acids
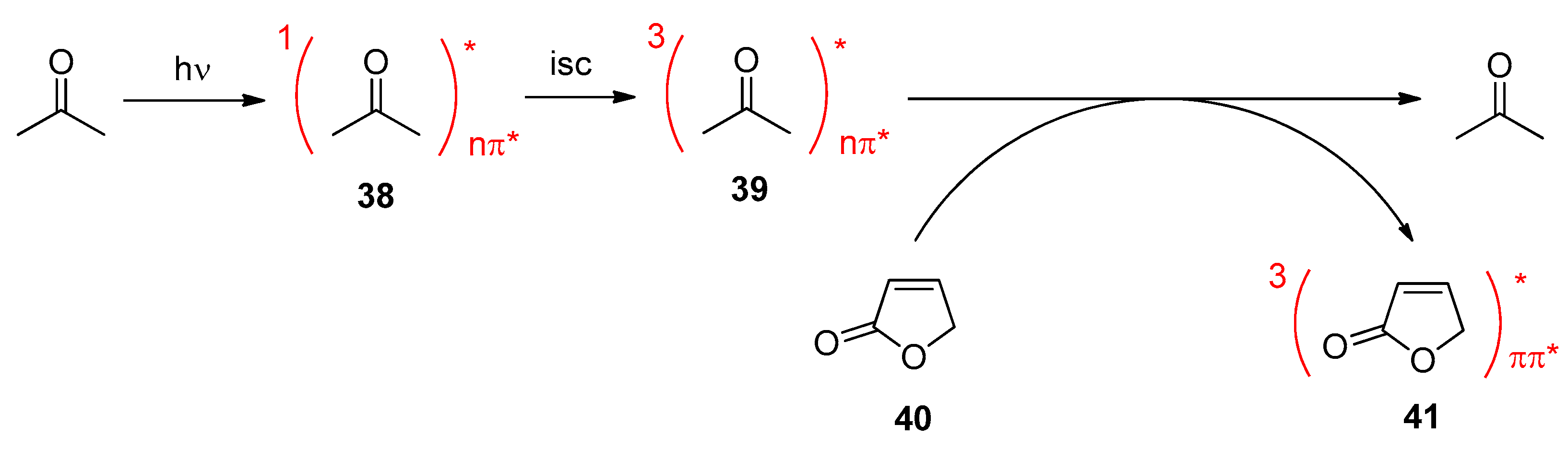
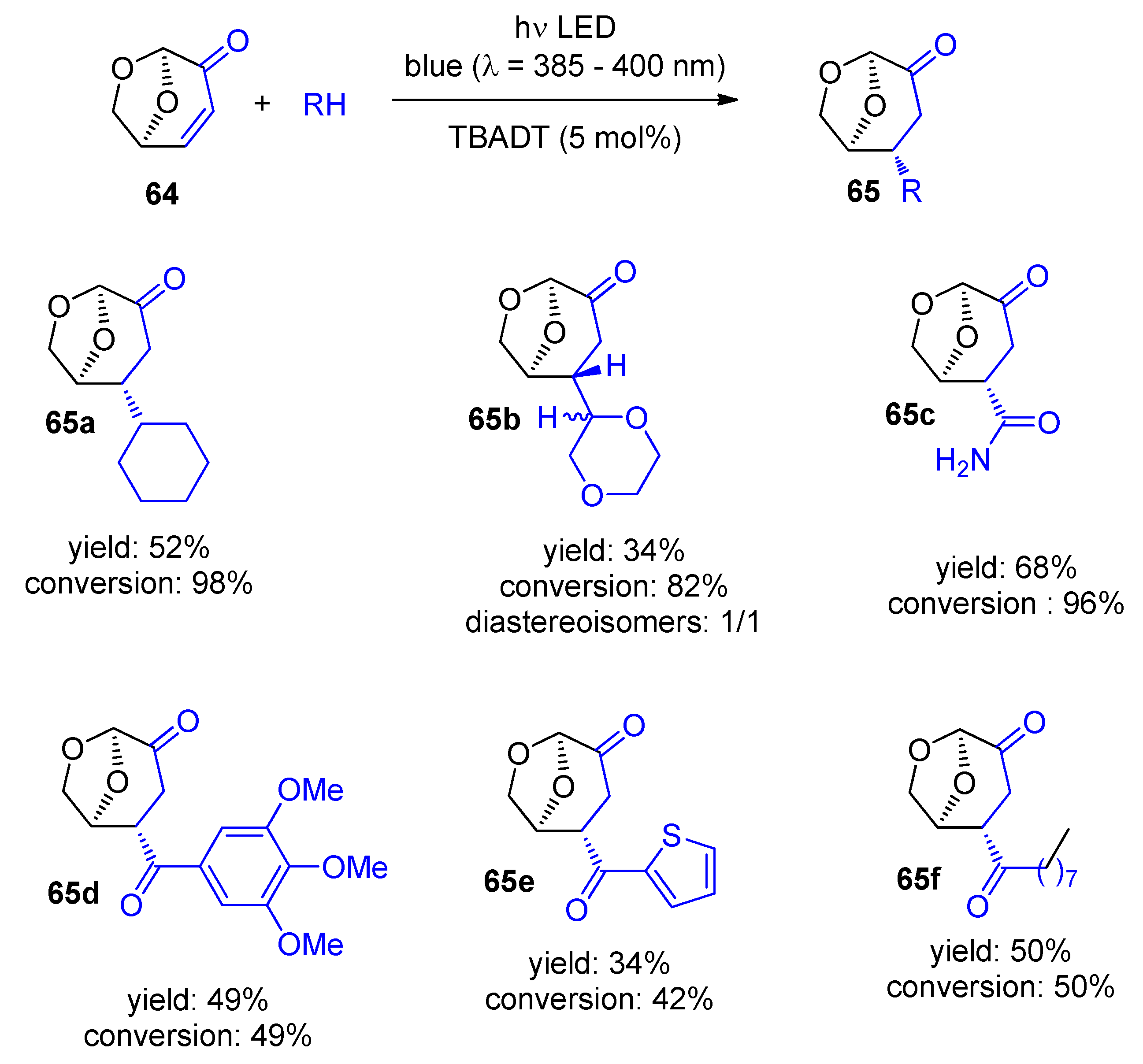
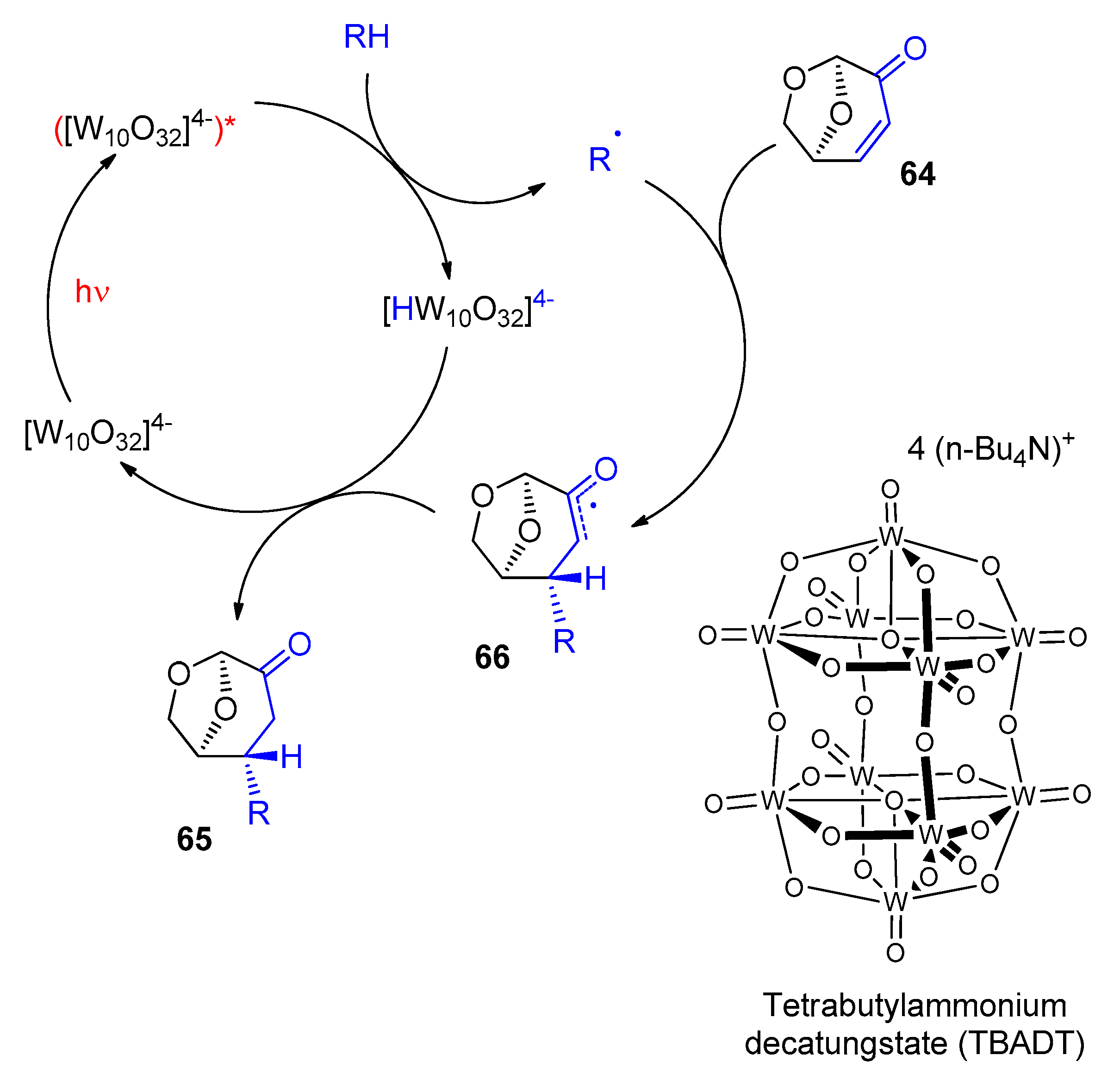
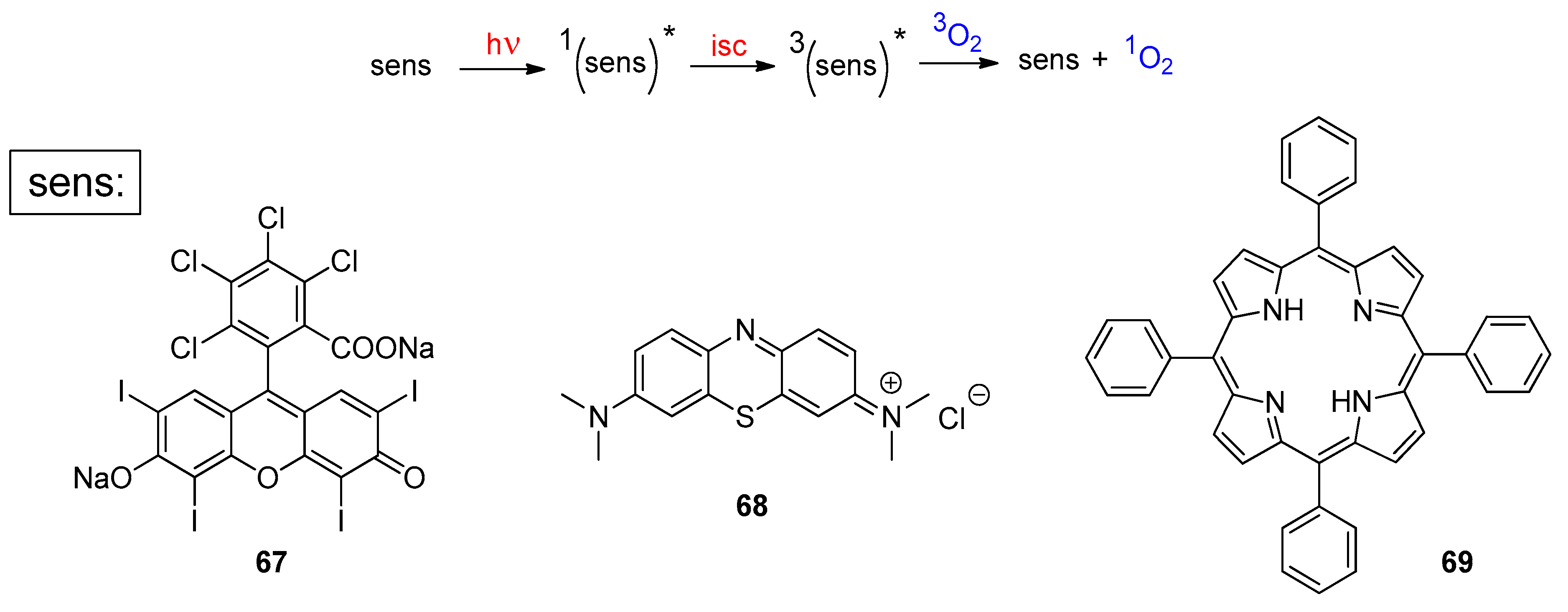
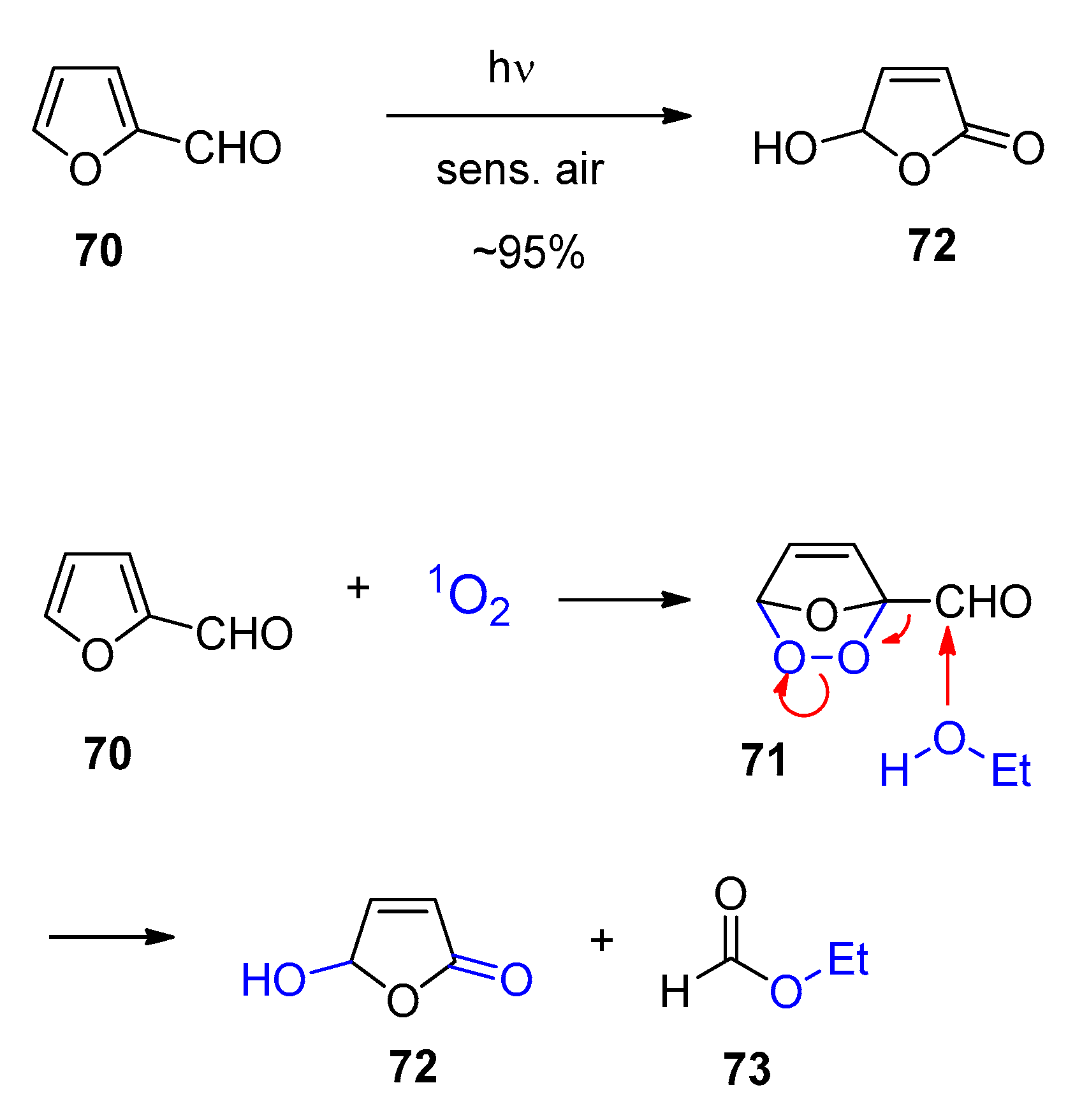
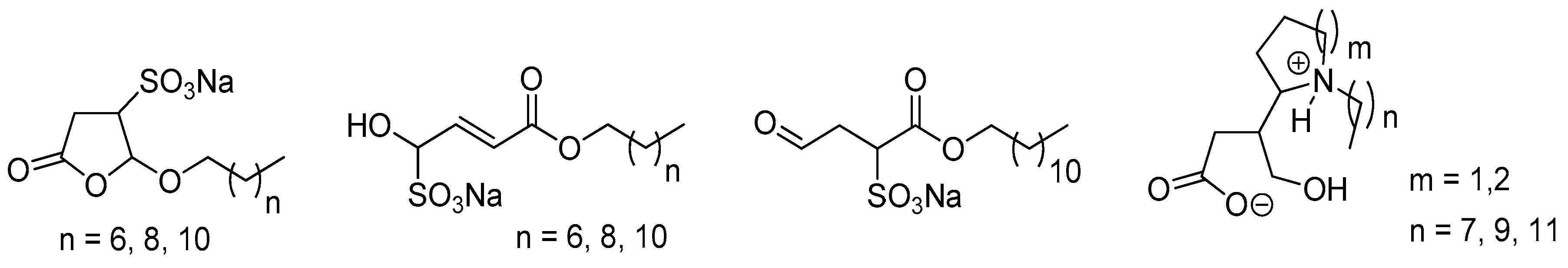
5. Biomass-Derived Platform Chemicals
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anastas, P.T.; Kirchhoff, M.M. Origins, Current Status, and Future Challenges of Green Chemistry. ACC Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Wertz, J.-L.; Bédué, O. Lignocellulosic Biorefineries; EPFL Press: Lausanne, Switzerland, 2013. [Google Scholar]
- Barrault, J.; Kervennal, J.; Isnard, P. La chimie durable: Pour l’environement, l’économie notre socciété (Numéro thématique). Actual. Chim. 2018, 15–116. [Google Scholar]
- Marion, P.; Bernela, B.; Piccirilli, A.; Estrine, B.; Patoullard, N.; Guilbot, J.; Jérôme, F. Sustainable chemistry: How to produce better and more from less? Green Chem. 2017, 19, 4973–4989. [Google Scholar] [CrossRef]
- Lichtenthaler, F.W.; Peters, S. Carbohydrates as green raw materials for the chemical industry. Comptes Rendus Chim. 2004, 7, 65–90. [Google Scholar] [CrossRef]
- Lichtenthaler, F.W. Carbohydrate-based Product Lines. In Biorefineries–Industrial Processes and Products; Kamm, B., Gruber, P.R., Kamm, M., Eds.; Wiley-VCH: Weinheim, Germany, 2006; Volume 1, pp. 2–59. [Google Scholar]
- Heitner, C.; Dimmel, D.R.; Schmidt, J.A. (Eds.) Lignin and Lignans; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Farmer, T.J.; Mascal, M. Platfom molecules. In Introduction to Chemicals from Biomass, 2nd ed.; Clark, J., Deswarte, F., Eds.; Wiley: Chichester, UK, 2015; pp. 89–152. [Google Scholar]
- Cramail, H.; Bizet, B.O.; Durand, P.-L.; Hibert, G.; Grau, E. Bio-Sourced Polymers: Recent Advances In Avanced Green Chemistry, Part 2; Horváth, I.T., Malacria, M., Eds.; World Scientific: Singapore, 2020; pp. 167–328. [Google Scholar]
- Esposito, D.; Antonietti, M. Redefining biorefinery: The search for unconventional building blocks for materials. Chem. Soc. Rev. 2015, 44, 5821–5835. [Google Scholar] [CrossRef]
- Monet, E. La forêt: Un gisement privilege et durable de molecules biosourcées. Actual. Chim. 2023, 14–23. [Google Scholar]
- de Rezende Locatel, W.; Guilhaume, N.; Schuurman, Y.; Laurenti, D. La pyrolyse du bois et la conversion catalytique de ses vapeurs. Actual. Chim. 2023, 71–77. [Google Scholar]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horvath, I.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Ravelli, D.; Samorì, C. (Eds.) Biomass Valorisation; Wiley-VCH: Weinheim, Germany, 2021. [Google Scholar]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Chatterjee, C.; Pong, F.; Sen, A. Chemical conversion pathways for carbohydrates. Green Chem. 2015, 17, 40–71. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef] [PubMed]
- Groß, J.; Kühlborn, J.; Opatz, T. Applications of xylochemistry from laboratory to industrial scale. Green Chem. 2020, 22, 4411–4425. [Google Scholar] [CrossRef]
- Kühlborn, J.; Groß, J.; Opatz, T. Making natural products from renewable feedstocks: Back to the roots? Nat. Prod. Rep. 2020, 37, 380–424. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Arends, I.; Hanefeld, U. (Eds.) Green Chemistry and Catalysis; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Centi, G.; van Santen, R.A. (Eds.) Catalysis for Renewables; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Imhof, P.; van der Waal, J.C. (Eds.) Catalytic Process Development for Renewable Materials; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Protti, S.; Manzini, S.; Fagnoni, M.; Albini, A. RSC Green Chemistry Book Series, Eco-Friendly Synthesis of Fine Chemicals; Ballini, R., Ed.; Royal Society of Chemistry: London, UK, 2009; pp. 80–111. [Google Scholar]
- Ciamician, G. The Photochemistry of the Future. Science 1912, 36, 385–394. [Google Scholar] [CrossRef]
- Ciamician, G. Sur les actions de la lumière. Bull. Soc. Chim. Fr. 1908, 3, i. [Google Scholar]
- Hoffmann, N. Photochemical reactions of aromatic compounds and the concept of the photon as a traceless reagent. Photochem. Photobiol. Sci. 2012, 11, 1613–1641. [Google Scholar] [CrossRef]
- Oelgemöller, M.; Jung, C.; Mattay, J. Green photochemistry: Production of fine chemicals with sunlight. Pure Appl. Chem. 2007, 79, 1939–1947. [Google Scholar] [CrossRef]
- Turro, N.J.; Schuster, G. Photochemical Reactions as a Tool in Organic Syntheses. Science 1975, 187, 303–312. [Google Scholar] [CrossRef]
- Hoffmann, N. Photochemical Key Steps in organic Synthesis. Chem. Rev. 2008, 108, 1052–1103. [Google Scholar] [CrossRef]
- Bach, T.; Hehn, J.P. Photochemical Reactions as Key Steps in Natural Product Synthesis. Angew. Chem. Int. Ed. 2011, 50, 1000–1052. [Google Scholar] [CrossRef] [PubMed]
- Kärkäs, M.D.; Porco, J.A.; Stephenson, C.R.J. Photochemical Approaches to Complex Chemotypes: Applications in Natural Product Synthesis. Chem. Rev. 2016, 116, 9683–9747. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, H.E.; Knauber, T.; Lévesque, F.; Moschetta, E.G.; Susanne, F.; Edwards, L.J. Photons as a 21st century reagent. Nat. Commun. 2020, 11, 804. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Fortier, L.; Hoffmann, N. Photochemical Rearrangements in Heterocyclic Chemistry. Eur. J. Org. Chem. 2020, 2020, 1393–1404. [Google Scholar] [CrossRef]
- Latrache, M.; Hoffmann, N. Photochemical radical cyclization reactions with imines, hydrazones, oximes and related compounds. Chem. Soc. Rev. 2021, 50, 7418–7435. [Google Scholar] [CrossRef]
- Hoffmann, N. Enantioselective synthesis of heterocyclic compounds using photochemical reactions. Photochem. Photobiol. Sci. 2021, 20, 1657–1674. [Google Scholar] [CrossRef]
- Michelin, C.; Hoffmann, N. Photocatalysis applied to organic synthesis—A green chemistry approach. Curr. Opin. Green Sustain. Chem. 2018, 10, 40–45. [Google Scholar] [CrossRef]
- Michelin, C.; Hoffmann, N. Photosensitization and Photocatalysis—Perspectives in Organic Synthesis. ACS Catal. 2018, 8, 12046–12055. [Google Scholar] [CrossRef]
- Tyburski, R.; Liu, T.; Glover, S.D.; Hammarström, L. Proton-Coupled Electron Transfer Guidelines, Fair and Square. J. Am. Chem. Soc. 2021, 143, 560–576. [Google Scholar] [CrossRef]
- Miller, D.C.; Tarantino, K.T.; Knowles, R.R. Proto-Coupled Electron Transfer in Organic Synthesis: Fundamentals, Applications, and Opportunities. Top. Curr. Chem. 2016, 374, 30. [Google Scholar] [CrossRef]
- Hoffmann, N. Proton-Coupled Electron Transfer in Photoredox Catalytic Reactions. Eur. J. Org. Chem. 2017, 2017, 1982–1992. [Google Scholar] [CrossRef]
- Stephenson, C.R.J.; Yoon, T.P.; MacMillan, D.W.C. (Eds.) Visible Light Photocatalysis in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- König, B. (Ed.) Chemical Photocatalysis, 2nd ed.; De Gruyter: Berlin, Germany, 2020. [Google Scholar]
- Marzo, L.; Pagire, S.K.; Reiser, O.; König, B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef] [PubMed]
- Barata-Vallejo, S.; Yerien, D.E.; Postigo, A. Bioinspired Photocatalyzed Organic Synthetic Transformations. The Use of Natural Pigments and Vitamins in Photocatalysis. ChemCatChem 2022, e202200623. [Google Scholar] [CrossRef]
- Mattay, J. Charge Transfer and Radical Ions in Photochemistry. Angew. Chem. Int. Ed. 1987, 26, 825–845. [Google Scholar] [CrossRef]
- Zeitler, K.; Neumann, M. Synergistic visible light photoredox catalysis. In Chemical Photocatalysis, 2nd ed.; König, B., Ed.; De Gruyter: Berlin, Germany, 2020; pp. 245–283. [Google Scholar]
- Skubi, K.L.; Blum, T.R.; Yoon, T.P. Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev. 2016, 116, 10035–10074. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Sahoo, B.; Li, J.-L.; Glorius, F. Dual Catalysis Sees the Light: Combining Photoredox with Organo-, Acid, and Transition-Metal Catalysis. Chem. Eur. J. 2014, 20, 3874–3886. [Google Scholar] [CrossRef]
- Ooi, T. Molecular Technology: Energy Innovation; Yamamoto, H., Kato, T., Eds.; Wiley-VCH: Weinheim, Germany, 2018; pp. 131–163. [Google Scholar]
- Mastandrea, M.M.; Pericàs, M. Photoredox Dual Catalysis: A Fertile Playground for the Discovery of New reactivities. Eur. J. Inorg. Chem. 2021, 2021, 3421–3431. [Google Scholar] [CrossRef]
- Lévêque, C.; Levernier, E.; Corcé, V.; Fensterbank, L.; Malacria, M.; Ollivier, C. Photoredox Catalysis, an Opportunity for Sustainable Radical Chemistry. In Avanced Green Chemistry, Part 2; Horváth, I.T., Malacria, M., Eds.; World Scientific: Singapore, 2020; pp. 49–121. [Google Scholar]
- Yang, N.; Tian, Y.; Zhang, M.; Peng, X.; Li, F.; Li, J.; Li, Y.; Fan, B.; Wang, F.; Song, H. Photocatalyst-enzyme hybrid systems for light-driven biotransformations. Biotechnol. Adv. 2022, 54, 107808. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, D.S.; Kuk, S.K.; Park, C.B. Photobiocatalysis: Activating Redox Enzymes by Direct or Indirect Transfer of Photoinduced Electrons. Angew. Chem. Int. Ed. 2018, 57, 7958–7985. [Google Scholar] [CrossRef]
- Rudorff, F.; Mihovilovic, M.D.; Gröger, H.; Snajdrova, R.; Iding, H.; Bornscheuer, U.T. Opportunities and challenges for combining chemo- and biocatalysis. Nat. Catal. 2018, 1, 12–22. [Google Scholar]
- Schmermund, L.; Jurkas, V.; Özgen, F.F.; Barone, G.D.; Büchsenschütz, H.C.; Winkler, C.K.; Schmidt, S.; Kourist, R.; Kroutil, W. Photo-Biocatalysis: Biotransformations in the Resence of Light. ACS Catal. 2019, 9, 4115–4144. [Google Scholar] [CrossRef]
- Zhang, W.; Hollmann, F. Noncoventional regeneration of redox enzymes—A practical approach for organic synthesis. Chem. Commun. 2018, 54, 7281–7289. [Google Scholar] [CrossRef] [PubMed]
- Nicewicz, D.A.; MacMillan, D.W.C. Merging Photoredox Catalysis with Organocatalysis: The Direct Asymmetric Alkylation of Aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef]
- Saha, D. Catalytic Enantioselective Radical Transformations Enabled by Visible Light. Chem. Asian J. 2020, 15, 2129–2152. [Google Scholar] [CrossRef] [PubMed]
- Brimioulle, R.; Lenhart, D.; Maturi, M.M.; Bach, T. Enantioselective Catalysis of Photochemical Reactions. Angew. Chem. Int. Ed. 2015, 54, 3872–3890. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Chen, W.; Zheng, W.-H.; Lu, H. Advances in asymmetric visible-light photocatalysis, 2015–2019. Org. Biomol. Chem. 2019, 17, 8673–8689. [Google Scholar] [CrossRef]
- Fidaly, K.; Ceballos, C.; Falguières, A.; Sylla-Iyarreta Veitia, M.; Guy, A.; Ferroud, C. Visible light photoredox organocatalysis: A fully transition metal-free direct asymmetric α-alkylation of aldehydes. Green Chem. 2012, 14, 1293–1297. [Google Scholar] [CrossRef]
- Cheung, K.P.S.; Sarkar, S.; Gevorgyan, V. Visible Light-Induced Transition Metal Catalysis. Chem. Rev. 2022, 122, 1543–1625. [Google Scholar] [CrossRef]
- Lipp, A.; Badir, S.O.; Molander, G.A. Stereoinduction in Metallaphotoredox Catalysis. Angew. Chem. Int. Ed. 2021, 60, 1714–1726. [Google Scholar] [CrossRef]
- Hoffmann, N. Combining Photoredox and Metal Catalysis. ChemCatChem 2015, 7, 393–394. [Google Scholar] [CrossRef]
- Fabry, D.C.; Rueping, M. Merging Visible Light Photoredox Catalysis with Metal Catalyzed C-H Activations: On the Role of Oxygen and Superoxide Ions as Oxidants. ACC Chem. Res. 2016, 49, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Sadek, O.; Abdellaoui, M.; Millanvois, A.; Ollivier, C.; Fensterbank, L. Organometallic catalysis under visible light activation: Benefits and preliminary rationals. Photochem. Photobiol. Sci. 2022, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Shen, X.; Wang, C.; Zhang, L.; Röse, P.; Chen, L.A.; Harms, K.; Marsch, M.; Hilt, G.; Meggers, E. Asymmetric photoredox transition-metal catalysis ativated by visible light. Nature 2014, 515, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Luo, N.; Xie, S.; Zhang, H.; Zhang, Q.; Wang, F.; Wang, Y. Photocatalytic transformations of lignocellulosic biomass into chemicals. Chem. Soc. Rev. 2020, 49, 6198–6223. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wan, K.; Zheng, F.; Zhang, Z.; Zhang, H.; Zhang, Y.; Long, D. Recent Advances in Photocatalytic Transformation on Carbohydrates into Valuable Platform Chemicals. Front. Chem. Eng. 2021, 3, 615309. [Google Scholar] [CrossRef]
- Rao, V.N.; Malu, T.J.; Cheralathan, K.K.; Sakar, M.; Pitchaimuthu, S.; Rodríguez-González, V.; Kumari, M.M.; Shankar, M.V. Light-driven transformation of biomass into chemicals using photocatalysts—Vistas and challenges. J. Environ. Manag. 2021, 284, 111983. [Google Scholar]
- Colmenares, J.C.; Luque, R. Heterogeneous photocatalytic nanomaterials: Prospects and challenges in selective transformations of biomass-derived compounds. Chem. Soc. Rev. 2014, 43, 765–778. [Google Scholar] [CrossRef]
- Granone, L.I.; Sieland, F.; Zheng, N.; Dillert, R.; Bahnemann, D.W. Photocatalytic conversion of biomass into valuable products: A meaningful approach? Green Chem. 2018, 20, 1169–1192. [Google Scholar] [CrossRef]
- Fan, H.; Li, G.; Yang, F.; Yang, L.; Zhang, S. Photodegradation of cellulose under UV light catalysed by TiO2. J. Chem. Technol. Biotechnol. 2011, 86, 1107–1112. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Zhang, L.; Xue, S.; Doherty, W.O.S.; O’Hara, I.M.; Ke, X. Sustainable conversion of cellulosic biomass to chemicals under visible-light irradiation. RSC Adv. 2015, 5, 85242–85247. [Google Scholar] [CrossRef]
- Laugel, C.; Estrine, B.; Le Bras, J.; Hoffmann, N.; Marinkovic, S.; Muzart, J. Visible Light-Accelerated Depolymerisation of Starch under Fenton Conditions and Preparation of Calcium Sequestering Compounds. Catal. Lett. 2014, 144, 1674–1680. [Google Scholar] [CrossRef]
- Li, S.-H.; Liu, S.; Colmenares, J.C.; Xu, Y.-J. A sustainable approach for lignin valorization by heterogeneous photocatalysis. Green Chem. 2016, 18, 594–607. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; Ulbrich, A.; Coon, J.J.; Stahl, S.S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 2014, 515, 249–252. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef]
- Nayak, J.; Basu, A.; Dey, P.; Kumar, R.; Upadhaya, A.; Ghosh, S.; Bishayee, B.; Mishra, S.R.; Tripathy, S.K.; Banerjee, S.; et al. Transformation of agro-biomass into vanillin through novel membrane integrated value-addition process: A state-of-the-art review. Biomass Conv. Bioref. 2022, 1–24. [Google Scholar] [CrossRef]
- Kärkäs, M.D.; Matsuura, B.S.; Monos, T.M.; Magallanes, G.; Stephenson, C.R.J. Transition-metal catalyzed valorization of lignin: The key to a sustainable carbon-neutral future. Org. Biomol. Chem. 2016, 14, 1853–1914. [Google Scholar] [CrossRef]
- Magallanes, G.; Kärkäs, M.D.; Bosque, I.; Lee, S.; Maldonado, S.; Sephenson, C.R.J. Selective C–O Bond Cleavage of Lignin Systems and Polymers Enabled by Sequential Palladium-Catalyzed Aerobic Oxidation and Visible-Light Photoredox Catalysis. ACS Catal. 2019, 9, 2252–2260. [Google Scholar] [CrossRef]
- Muzart, J. Palladium-catalysed oxidation of primary and secondary alcohols. Tetrahedron 2003, 59, 5789–5816. [Google Scholar] [CrossRef]
- Hoffmann, N. Electron and hydrogen transfer in organic photochemical reactions. J. Phys. Org. Chem. 2015, 28, 121–136. [Google Scholar] [CrossRef]
- Zhang, J. Conversion of Lignin Models by Photoredox Catalysis. ChemSusChem 2018, 11, 3071–3080. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; König, B. Transition metal- and photoredox-catalyzed valorisation of lignin subunits. Green Chem. 2018, 20, 4844–4852. [Google Scholar] [CrossRef]
- Shatskiy, A.; Kärkäs, M.D. Biomass Processing via Photochemical Means. In Biomass Valorisation; Ravelli, D., Samorì, C., Eds.; Wiley-VCH: Weinheim, Germany, 2021; pp. 265–288. [Google Scholar]
- Martín-Perales, A.I.; Rodríguez-Padron, D.; García Coleto, A.; Len, C.; de Miguel, G.; Muñoz-Batista, M.J.; Rafael Luque, R. Photocatalytic Production of Vanilline over CeOx and ZrO2 Modified Biomass-Templated Titania. Ind. Eng. Chem. Res. 2020, 59, 17085–17093. [Google Scholar] [CrossRef]
- Camera-Roda, G.; Augugliaro, V.; Cardillo, A.; Loddo, V.; Palmisano, G.; Palmisano, L. A pervaporation reactor for the green Synthesis of Vanillin. Chem. Eng. J. 2013, 224, 136–143. [Google Scholar] [CrossRef]
- Desvals, A.; Baudron, S.A.; Bulach, V.; Hoffmann, N. Photocycloadditions of Arenes Derived from Lignin. J. Org. Chem. 2021, 86, 13310–13321. [Google Scholar] [CrossRef]
- Desvals, A.; Hoffmann, N. Photocycloadditions of benzene derivatives and their systematic application to organic synthesis. Aus. J. Chem. 2023, 76, 117–129. [Google Scholar] [CrossRef]
- Remy, R.; Bochet, C.G. Arene-Alkene Cycloaddition. Chem. Rev. 2016, 116, 9816–9849. [Google Scholar] [CrossRef]
- Chapleur, Y.; Chrétien, F. Sugars as Chiral Starting Materials in Enantiospecific Synthesis. In The Organic Chemistry of Sugars; Levy, D.E., Fügedi, P., Eds.; CRC Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 489–573. [Google Scholar]
- Vogel, P. Total Asymmetric Synthesis of Monosaccharides and Analogs. In The Organic Chemistry of Sugars; Levy, D.E., Fügedi, P., Eds.; CRC Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 629–725. [Google Scholar]
- Lichtenthaler, F.W. Emil Fischer’s Proof of the Configuration of Sugars: A Centennial Tribute. Angew. Chem. Int. Ed. 1992, 31, 1541–1556. [Google Scholar] [CrossRef]
- Stick, R.V. Carbohydrates—The Sweet Molecules of Life; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Toshima, K. Synthesis of Carbohydrate Containing Complex Natural Compounds. In The Organic Chemistry of Sugars; Levy, D.E., Fügedi, P., Eds.; CRC Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 575–627. [Google Scholar]
- Kuszmann, J. Ingtoduction to Carbohydrates. In The Organic Chemistry of Sugars; Levy, D.E., Fügedi, P., Eds.; CRC Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 25–52. [Google Scholar]
- Shatskiy, A.; Stepanova, E.V.; Kärkäs, M.D. Exploiting photoredox catalysis for carbohydrate modification through C-H and C-C activation. Nat. Rev. Chem. 2022, 6, 782–805. [Google Scholar] [CrossRef]
- Gassama, A.; Hoffmann, N. Selective Light Supported Oxidation of Hexoses Using Air as Oxidant—Synthesis of Tetrahydroxyazepanes. Adv. Synth. Catal. 2008, 350, 35–39. [Google Scholar] [CrossRef]
- Ito, N.; Phillips, S.E.V.; Stevens, C.; Ogel, Z.B.; McPhersonn, M.J.; Keen, J.N.; Yadav, K.D.S.; Knowles, P.F. Novel thioether bond revealed by a 1.7 Å crystal structure of galactose oxidase. Nature 1991, 350, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F. Ten Years of a Biomimetic Approach to the Copper(II) Radical Site of Galactose Oxidase. Eur. J. Inorg. Chem. 2007, 2007, 2379–2404. [Google Scholar] [CrossRef]
- Pierre, J.-L. One electron at a time oxidations and enzymatic paradigms: From metallic to non-metallic redox centers. Chem. Soc. Rev. 2000, 29, 251–257. [Google Scholar] [CrossRef]
- Berkessel, A.; Dousset, M.; Bulat, S.; Glaubitz, K. Combinatorial approaches to functional models for galactose oxidase. Biol. Chem. 2005, 386, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, P.; Hess, M.; Hildenbrand, K.; Bill, E.; Weyhermüller, T.; Wieghardt, K. Aerobic Oxidation of Primary Alcohols (Including Methanol) by Copper(II)− and Zinc(II)−Phenoxyl Radical Catalysts. J. Am. Chem. Soc. 1999, 121, 9599–9610. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Schmid, C.R.; Cortés, D.A.; Chou, C.S. Oxidation of alcohols to aldehydes with oxygen and cupric ion, mediated by nitrosonium ion. J. Am. Chem. Soc. 1984, 106, 3374–3376. [Google Scholar] [CrossRef]
- Ryland, B.L.; Stahl, S.S. Practical Aerobic Oxidations of Alcohols and Amines with Homogeneous Copper/TEMPO and Related Catalyst Systems. Angew. Chem. Int. Ed. 2014, 53, 8824–8838. [Google Scholar] [CrossRef]
- Dijksman, A.; Arends, I.W.C.E.; Sheldon, R.A. Cu(ii)-nitroxyl radicals as catalytic galactose oxidase mimics. Org. Biomol. Chem. 2003, 1, 3232–3237. [Google Scholar] [CrossRef]
- Abderrazak, Y.; Bhattacharyya, A.; Reiser, O. Visible-Light-Induced Homolysis of Earth-Abundant Metal-Substrate Complexes: A Complementary Activation Strategy in Photoredox Catalysis. Angew. Chem. Int. Ed. 2021, 60, 21100–21115. [Google Scholar] [CrossRef]
- Compain, P.; Martin, O.R. (Eds.) Iminosugars; Wiley & Sons: Chichester, UK, 2007. [Google Scholar]
- Li, H.; Marcelo, F.; Bello, C.; Vogel, P.; Butters, T.D.; Rauter, A.P.; Zhang, Y.; Sollogoub, M.; Blériot, Y. Design and synthesis of acetamido tri- and tetra-hydroxyazepanes: Potent and selective β-N-acetylhexosaminidase inhibitors. Bioorg. Med. Chem. 2009, 17, 5598–5604. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.C.; Regeling, H.; Zwanenburg, B.; Chittenden, G.J.F. Syntheses of (3R,4R,5R,6R)-tetrahydroxyazepane (1,6-dideoxy-1,6-imino-d-mannitol) and (3S,4R,5R,6R)-tetrahydroxyazepane (1,6-dideoxy-1,6-imino-d-glucitol). Tetrahedron 2002, 58, 6907–6911. [Google Scholar] [CrossRef]
- Oelgemöller, M. Solar Photochemical Synthesis: From the Beginnings of Organic Photochemistry to the Solar Manufacturing of Commodity Chemicals. Chem. Rev. 2016, 116, 9664–9682. [Google Scholar] [CrossRef]
- Sangwan, R.; Mandal, P.K. Recent advances in photoinduced glycosylation: Oligosaccharides, glycoconjugates and their synthetic applications. RSC Adv. 2017, 7, 26256–26321. [Google Scholar] [CrossRef]
- Ling, J.; Bennett, C.S. Recent Developments in Stereoselective Chemical Glycosylation. Asian J. Org. Chem. 2019, 8, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Fügedi, P. Glycosylation Methods. In The Organic Chemistry of Sugars; Levy, D.E., Fügedi, P., Eds.; CRC Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 89–179. [Google Scholar]
- Collins, P.; Ferrier, R. Monosaccharides; Wiley: Chichester, UK, 1995. [Google Scholar]
- Hashimoto, S.; Kurimoto, I.; Fujii, Y.; Noyori, R. Novel nucleophilic substitution reaction by radical cation intermediates. Photosensitized transacetalization via SON1 mechanism. J. Am. Chem. Soc. 1985, 107, 1427–1429. [Google Scholar] [CrossRef]
- Wever, W.J.; Cinelli, M.A.; Bowers, A.A. Visible Light Mediated Activation and O-Glycosylation of Thioglycosides. Org. Lett. 2013, 15, 30–33. [Google Scholar] [CrossRef]
- Tolbert, L.M.; Solntsev, K.M. Excited-State Proton Transfer: From Constrained Systems to “Super” Photoacids to Superfast Proton Transfer. ACC Chem. Res. 2002, 35, 19–27. [Google Scholar] [CrossRef]
- Zivic, N.; Kuroishi, P.K.; Dumur, F.; Gigmes, D.; Dove, A.P.; Sardon, H. Recent Advances and Challenges in the Design of Organic Photoacid and Photobase Generators for Polymerizations. Angew. Chem. Int. Ed. 2019, 58, 10410–10422. [Google Scholar] [CrossRef]
- Saway, J.; Salem, Z.M.; Badillo, J.J. Recent Advances in Photoacid Catalysis for Organic Synthesis. Synthesis 2021, 53, 489–497. [Google Scholar]
- Iwata, R.; Uda, K.; Takahashi, D.; Toshima, K. Photo-induced glycosylation using reusable organophotoacids. Chem. Commun. 2014, 50, 10695–10698. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Eto, T.; Takahashi, D.; Toshima, K. Stereocontrolled Photoinduced Glycosylation Using an Aryl Thiourea as an Organo photoacid. Org. Lett. 2016, 18, 3190–3193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Li, J.; Wang, T. Visible-light-induced photoacid catalysis: Application in glycosylation with O-glycosyl trichloroacetimidates. Chem. Commun. 2021, 57, 12659–12662. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.E. Strategies towards C-Glycosides. In The Organic Chemistry of Sugars; Levy, D.E., Fügedi, P., Eds.; CRC Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 269–348. [Google Scholar]
- Zhang, M.; Kong, L.; Gong, R.; Iorio, M.; Donatio, S.; Deng, Z.; Sosio, M.; Chen, W. Biosynthesis of C-nucleoside antibiotics in actinobacteria: Recent advances and future developments. Microbiol. Cell Factories 2022, 21, 2. [Google Scholar] [CrossRef]
- Zhu, M.; Messoudi, S. Diastereoselective Decarboxylative Alkynylation of Anomeric Carboxylic Acids Using Cu/Photoredox Dual Catalysis. ACS Catal. 2021, 11, 6334–6342. [Google Scholar] [CrossRef]
- Schwarz, J.; König, B. Decarboxylative reactions with and without light—A comparison. Green Chem. 2018, 20, 323–361. [Google Scholar] [CrossRef]
- Martinez, R.D.; Buitrago, A.A.; Howell, N.W.; Hearn, C.H.; Joens, J.A. The near UV absorption spectra of several aliphatic aldehydes and ketones at 300 K. Atmos. Environ. 1992, 26A, 785–792. [Google Scholar] [CrossRef]
- Jahjah, R.; Gassama, A.; Bulach, V.; Suzuki, C.; Abe, M.; Hoffmann, N.; Martinez, A.; Nuzillard, J.-M. Stereoselective Triplet-Sensitised Radical Reactions of Furanone Derivatives. Chem. Eur. J. 2010, 16, 3341–3354. [Google Scholar] [CrossRef]
- Oelgemöller, M.; Hoffmann, N.; Shvydkiv, O. From ‘Lab & Light on a Chip’ to Parallel Microflow Photochemistry. Aust. J. Chem. 2014, 67, 337–342. [Google Scholar]
- Hoffmann, N. Photochemical Electron and Hydrogen Transfer in Organic Synthesis: The Control of Selectivity. Synthesis 2016, 48, 1782–1802. [Google Scholar] [CrossRef]
- Wessig, P.; Mühling, O. Abstraction of (γ ± n)-Hydrogen by Excited Carbonyls. In Synthetic Organic Photochemistry; Giesbeck, A.G., Mattay, J., Eds.; Marcel Dekker: New York, NY, USA, 2005; pp. 41–87. [Google Scholar]
- Martín, A.; Suárez, E. Carbohydrate Spiro-heterocycles via Radical Chemistry. Top. Heterocycl. Chem. 2019, 57, 51–104. [Google Scholar]
- Thiering, S.; Sund, C.; Thiem, J.; Giesler, A.; Kopf, J. Syntheses of imido-substituted glycosans and their photocyclisation towards highly functionalised heterotricycles. Carbohydr. Res. 2001, 336, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, L.; Ravelli, D. Hydrogen Atom Transfer (HAT): A Versatile Strategy for Substrate Activation in Photocatalyzed Organic Synthesis. Eur. J. Org. Chem. 2017, 2017, 2056–2071. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, D.; Protti, S.; Fagnoni, M. Carbon–Carbon Bond Forming Reactions via Photogenerated Intermediates. Chem. Rev. 2016, 116, 9850–9913. [Google Scholar] [CrossRef]
- Fréneau, M.; de Sainte-Claire, P.; Abe, M.; Hoffmann, N. The structure of electronically excited α,β-unsaturated lactones. J. Phys. Org. Chem. 2016, 29, 718–724. [Google Scholar] [CrossRef]
- Lejeune, G.; Font, J.; Parella, T.; Alibés, R.; Figueredo, M. Intramolecular Photoreactions of (5S)-5-Oxymethyl-2(5H)-furanones as a Tool for the Stereoselective Generation of Diverse Polycyclic Scaffolds. J. Org. Chem. 2015, 80, 9437–9445. [Google Scholar] [CrossRef]
- Tabanelli, T.; Cavani, F. Industrial Perspectives of Biomass Processing. In Biomass Valorisation; Ravelli, D., Samorì, C., Eds.; Wiley-VCH: Weinheim, Germany, 2021; pp. 369–410. [Google Scholar]
- Welter, R.A.; Santana, H.; de la Torre, L.G.; Robertson, M.; Taranto, O.P.; Oelgemöller, M. Methyl Oleate Synthesis by TiO2 Photocatalytic Esterification of Oleic Acid: Optimisation by Response Surface Quadratic Methodology, Reaction Kinetics and Thermodynamics. ChemPhotoChem 2022, 6, e202200007. [Google Scholar] [CrossRef]
- Huang, J.; Jian, Y.; Zhu, P.; Abdelaziz, O.; Li, H. Research Progress on the Photo-Driven Catalytic Production of Biodiesel. Front. Chem. 2022, 10, 904251. [Google Scholar] [CrossRef]
- Belousov, A.S.; Suleimanov, E.V. Application of metal–organic frameworks as an alternative to metal oxide-based photocatalysts for the production of industrially important organic chemicals. Green Chem. 2021, 23, 6172–6204. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, G.; Sun, P. Transition metal-free decarboxylative alkylation reactions. Org. Biomol. Chem. 2016, 14, 10763–10777. [Google Scholar] [CrossRef]
- Schwarz, J. Photocatalytic decarboxylations. Phys. Sci. Rev. 2018, 3, 20170186. [Google Scholar] [CrossRef]
- Tabandeh, M.; Cheng, C.K.; Centi, G.; Show, P.L.; Chen, W.-H.; Ling, T.C.; Ong, H.C.; Ng, E.-P.; Juan, J.C.; Lam, S.S. Recent advancement in deoxygenation of fatty acids via homogeneous catalysis for biofuel production. Mol. Catal. 2022, 523, 111207. [Google Scholar] [CrossRef]
- Patra, T.; Maiti, D. Decarboxylation as the Key Step in C−C Bond-Forming Reactions. Chem. Eur. J. 2017, 23, 7382–7401. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Kramer, W.; Oelgemöller, M. Photoinduced decarboxylation reactions. Radical chemistry in water. Green Chem. 1999, 1, 205–208. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Hoffmann, N.; Warzecha, K.-D. Photoinduced-Electron-Transfer Chemistry: From Studies on PET Processes to Applications in Natural Product Synthesis. ACC Chem. Res. 2007, 40, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Saito, H.; Osaka, K.; Nishikawa, K.; Sugie, M.; Morita, T.; Takahashi, I.; Yoshimi, Y. Direct modification of tripeptides using photoinduced decarboxylative radical reactions. Tetrahedron 2015, 71, 1117–1123. [Google Scholar] [CrossRef]
- Kicatt, D.M.; Nicolle, S.; Lee, A.-L. Direct decarboxylative Giese reactions. Chem. Soc. Rev. 2022, 51, 1415–1453. [Google Scholar] [CrossRef]
- Rodríguez, N.; Goossen, L.J. Decarboxylative coupling reactions: A modern strategy for C–C-bond formation. Chem. Soc. Rev. 2011, 40, 5030–5048. [Google Scholar] [CrossRef]
- McMurray, L.; McGuire, T.M.; Howells, R.L. Recent Advances in Photocatalytic Decarboxylative Coupling Reactions in Medicinal Chemistry. Synthesis 2020, 52, 1719–1737. [Google Scholar] [CrossRef]
- Yoshimi, Y.; Itou, T.; Hatanaka, M. Decarboxylative reduction of free aliphatic carboxylic acids by photogenerated cation radical. Chem. Comm. 2007, 5244–5246. [Google Scholar] [CrossRef]
- Griffin, J.D.; Zeller, M.A.; Nicewicz, D.A. Hydrodecarboxylation of Carboxylic and Malonic Acid Derivatives via Organic Photoredox Catalysis: Substrate Scope and Mechanistic Insight. J. Am. Chem. Soc. 2015, 137, 11340–11348. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhao, Z.; Zhang, C.; Liu, J.; Luo, N.; Zhang, J.; Wang, F. Enhanced photocatalytic alkane production from fatty acid decarboxylation via inhibition of radical oligomerization. Nat. Catal. 2020, 3, 170–178. [Google Scholar] [CrossRef]
- Sorigué, D.; Légeret, B.; Cuiné, S.; Blangy, S.; Moulin, S.; Billon, E.; Richaud, P.; Brugière, S.; Couté, Y.; Nurizzo, D.; et al. An algal photoenzyme converts fatty acids to hydrocarbons. Science 2017, 357, 903–907. [Google Scholar] [CrossRef]
- Sorigué, D.; Hadjidemetriou, K.; Blangy, S.; Gotthard, G.; Bonvalet, A.; Coquelle, N.; Samire, P.; Aleksandrov, A.; Antonucci, L.; Benachir, A.; et al. Mechanism and dynamics of fatty acid photodecarboxylase. Science 2021, 372, eabd5687. [Google Scholar] [CrossRef]
- Harrison, W.; Huang, X.; Zhao, H. Photobiocatalysis for Abiological Transformations. Acc. Chem. Res. 2022, 55, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.; Wojcik, E.Z.; Sun, C.; Hoeven, R.; Hughes, J.M.X.; Faulkner, M.; Yunus, I.S.; Tait, S.; Johannissen, L.O.; Hardman, S.J.O.; et al. Low carbon strategies for sustainable bio-alkane gas production and renewable energy. Energy Environ. Sci. 2020, 13, 1818–1831. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, M.; Huijbers, M.M.E.; Filonenko, G.A.; Pidko, E.A.; van Schie, M.; de Boer, S.; Burek, B.O.; Bloh, J.Z.; van Berkel, W.J.H.; et al. Hydrocarbon Synthesis via Photoenzymatic Decarboxylation of Carboxylic Acids. J. Am. Chem. Soc. 2019, 141, 3116–3120. [Google Scholar] [CrossRef]
- Duong, H.T.; Wu, Y.; Sutor, A.; Burek, B.O.; Hollmann, F.; Bloh, J.Z. Intensification of Photobiocatalytic Decarboxylation of Fatty Acids for the Production of Biodiesel. ChemSusChem 2021, 14, 1053–1056. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Kramer, W.; Oelgemöller, M. Synthetic Applications of Photoinduced Electron Transfer Decarboxylation Reactions. Synlett 1999, 1169–1178. [Google Scholar] [CrossRef]
- Xuan, J.; Zhang, Z.G.; Xiao, W.J. Visible-Light-Induced Decarboxylative Functionalization of Carboxylic Acids and Their Derivatives. Angew. Chem. Int. Ed. 2015, 54, 15632–15641. [Google Scholar] [CrossRef]
- Zheng, Y.; Shao, X.; Ramadoss, V.; Tian, L.; Wang, Y. Recent Developments in Photochemical and Electrochemical Decarboxylative C(sp3)–N Bond Formation. Synthesis 2020, 52, 1357–1368. [Google Scholar]
- Mumtaz, S.; Robertson, M.J.; Oelgemöller, M. Recent advances in photodecarboxylations involving phthalimides. Aust. J. Chem. 2018, 71, 634–648. [Google Scholar] [CrossRef]
- Jin, Y.; Fu, H. Visible-Light Photoredox Decarboxylative Couplings. Asian J. Org. Chem. 2017, 6, 368–385. [Google Scholar] [CrossRef]
- Schwarz, J.; König, B. Decarboxylative Alkynylation of Biomass-Derived Compounds by Metal-Free Visible Light Photocatalysis. ChemPhotoChem 2017, 1, 237–242. [Google Scholar] [CrossRef]
- Garza-Sanchez, R.A.; Tlahuext-Aca, A.; Tavakoli, G.; Glorius, F. Visible Light-Mediated Direct Decarboxylative C–H Functionalization of Heteroarenes. ACS Catal. 2017, 6, 4057–4061. [Google Scholar] [CrossRef]
- Hu, D.; Wang, L.; Li, P. Decarboxylative Borylation of Aliphatic Esters under Visible-Light Photoredox Conditions. Org. Lett. 2017, 19, 2770–2773. [Google Scholar] [CrossRef]
- Fawcett, A.; Pradeilles, J.; Wang, Y.; Mutsuga, T.; Myers, E.L.; Aggarwal, V.K. Photoinduced decarboxylative borylation of carboxylic acids. Science 2017, 357, 283–286. [Google Scholar] [CrossRef]
- Tlahuext-Aca, A.; Candish, L.; Garza-Sanchez, R.A.; Glorius, F. Decarboxylative Olefination of Activated Aliphatic Acids Enabled by Dual Organophotoredox/Copper Catalysis. ACS Catal. 2018, 8, 1715–1719. [Google Scholar] [CrossRef]
- Xie, J.; Xu, P.; Li, H.; Xue, Q.; Jin, H.; Cheng, Y.; Zhu, C. A room temperature decarboxylation/C–H functionalization cascade by visible-light photoredox catalysis. Chem. Commun. 2013, 49, 5672–5674. [Google Scholar] [CrossRef]
- De bruyn, M.; Fan, J.; Budarin, V.L.; Macquarrie, D.J.; Gomez, L.D.; Simister, R.; Farmer, T.J.; Raverty, W.D.; McQueen-Mason, S.J.; Clark, J.H. A new perspective in bio-refining: Levoglucosenone and cleaner lignin from waste biorefinery hydrolysis lignin by selective conversion of residual saccharides. Energy Environ. Sci. 2016, 9, 2571–2574. [Google Scholar] [CrossRef]
- Halpern, Y.; Ritter, R.; Broido, A. Levoglucosenone (1,6-anhydro-3,4-dideoxy-.DELTA.3-.beta.-D-pyranosen-2-one). Major product of the acid-catalyzed pyrolysis of cellulose and related carbohydrates. J. Org. Chem. 1973, 38, 204–209. [Google Scholar] [CrossRef]
- He, J.; Liu, M.; Huang, K.; Walker, T.W.; Maravelias, C.T.; Dumesic, J.A.; Huber, G.W. Production of levoglucosenone and 5-hydroxymethylfurfural from cellulose in polar aprotic solvent–water mixtures. Green Chem. 2017, 19, 3642–3653. [Google Scholar] [CrossRef]
- Sherwood, J.; De bruyn, M.; Constantinou, A.; Moity, L.; McElroy, C.R.; Farmer, T.J.; Duncan, T.; Raverty, W.; Hunt, A.J.; Clark, J.H. Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents. Chem. Commun. 2014, 50, 9650–9652. [Google Scholar] [CrossRef]
- Comba, M.B.; Tsai, Y.; Sarotti, A.M.; Mangione, M.I.; Suárez, A.G.; Spanevello, R.A. Levoglucosenone and Its New Applications: Valorization of Cellulose Residues. Eur. J. Org. Chem. 2018, 2018, 590–604. [Google Scholar] [CrossRef]
- Awad, L.; Demange, R.; Zhu, Y.-H.; Vogel, P. The use of levoglucosenone and isolevoglucosenone as templates for the construction of C-linked disaccharides. Carbohydr. Res. 2006, 341, 1235–1252. [Google Scholar] [CrossRef] [PubMed]
- Sarotti, A.M.; Zanardi, M.M.; Spanevello, R.A.; Suárez, A.G. Recent Applications of Levoglucosenone as Chiral Synthon. Curr. Org. Synth. 2012, 9, 439–459. [Google Scholar] [CrossRef]
- Tsai, Y.; Borini Etichetti, C.M.; Di Benedetto, C.; Girardini, J.E.; Terra Martins, F.; Spanevello, R.A.; Suárez, A.; Sarotti, A.M. Synthesis of Triazole Derivatives of Levoglucosenone as Promising Anticancer Agents: Effective Exploration of the Chemical Space through retro-aza-Michael//aza-Michael Isomerizations. J. Org. Chem. 2018, 83, 3516–3528. [Google Scholar] [CrossRef]
- Camp, J.E.; Greatrex, B.W. Levoglucosenone: Bio-Based Platform for Drug Discovery. Front. Chem. 2022, 10, 902239. [Google Scholar] [CrossRef]
- Diot-Néant, F.; Rastoder, E.; Miller, S.A.; Allais, F. Chemo-Enzymatic Synthesis and Free Radical Polymerization of Renewable Acrylate Monomers from Cellulose-Based Lactones. ACS Sustain. Chem. Eng. 2018, 6, 17284–17293. [Google Scholar] [CrossRef]
- Bonneau, G.; Peru, A.A.M.; Flourat, A.L.; Allais, F. Organic solvent- and catalyst-free Baeyer–Villiger oxidation of levoglucosenone and dihydrolevoglucosenone (Cyrene®): A sustainable route to (S)-γ-hydroxymethyl-α,β-butenolide and (S)-γ-hydroxymethyl-γ-butyrolactone. Green Chem. 2018, 20, 2455–2458. [Google Scholar] [CrossRef]
- Lefebvre, C.; Van Gysel, T.; Michelin, C.; Rousset, E.; Djiré, D.; Allais, F.; Hoffmann, N. Photocatalytic Radical Addition to Levoglucosenone. Eur. J. Org. Chem. 2022, 2022, e202101298. [Google Scholar] [CrossRef]
- Raviola, C.; Protti, S.; Ravelli, D.; Fagnoni, M. Photogenerated acyl/alkoxycarbonyl/carbamoyl radicals for sustainable synthesis. Green Chem. 2019, 21, 748–764. [Google Scholar] [CrossRef]
- Taniellan, C. Decatungstate photocatalysis. Coord. Chem. Rev. 1998, 178–180, 1165–1181. [Google Scholar] [CrossRef]
- Ravelli, D.; Protti, S.; Fagnoni, M. Decatungstate Anion for Photocatalyzed “Window Ledge” Reactions. Acc. Chem. Res. 2016, 49, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Fukuyama, T.; Fujii, S.; Ravelli, D.; Fagnoni, M.; Ryu, I. Cooperative Polar/Steric Strategy in Achieving Site-Selective Photocatalyzed C(sp3)−H Functionalization. Chem. Eur. J. 2017, 23, 8615–8618. [Google Scholar] [CrossRef]
- Ravelli, D.; Fagnoni, M.; Fukuyama, T.; Nishikawa, T.; Ryu, I. Site-Selective C–H Functionalization by Decatungstate Anion Photocatalysis: Synergistic Control by Polar and Steric Effects Expands the Reaction Scope. ACS Catal. 2018, 8, 701–713. [Google Scholar] [CrossRef]
- Mika, L.T.; Cséfalvay, E. Conversion of Carbohydrates to Chemicals. In Avanced Green Chemistry, Part 1; Horváth, I.T., Malacria, M., Eds.; World Scientific: Singapore, 2020; pp. 19–76. [Google Scholar]
- Zhu, J.; Yin, G. Catalytic Transformation of the Furfural Platform into Bifunctionalized Monomers for Polymer Synthesis. ACS Catal. 2021, 11, 10058–10083. [Google Scholar] [CrossRef]
- Martel, F.; Estrine, B.; Plantier-Royon, R.; Hoffmann, N.; Portella, C. Development of Agriculture Left-Overs: Fine Organic Chemicals from Wheat Hemicellulose-Derived Pentoses. Top. Curr. Chem. 2010, 294, 79–115. [Google Scholar]
- Bergman, J.A.; Kessler, M.R. Monomers and Resulting Polymers from Biomass. In Introduction to Chemicals from Biomass, 2nd ed.; Clark, J., Deswarte, F., Eds.; Wiley: Chichester, UK, 2015; pp. 157–204. [Google Scholar]
- Yue, X.; Queneau, Y. 5-Hydroxymethylfurfural Chemistry toward Biobased Surfactants. ChemSusChem 2022, 15, e202102660. [Google Scholar] [CrossRef]
- Velty, A.; Iborra, S.; Corma, A. Synthetic Routes for Designing Furanic and Non Furanic Biobased Surfactants from 5-Hydroxymethylfurfural. ChemSusChem 2022, 15, e202200181. [Google Scholar] [CrossRef]
- Girka, Q.; Hausser, N.; Estrine, B.; Hoffmann, N.; Le Bras, J.; Marinković, S.; Muzart, J. β-Amino acid derived gemini surfactants from diformylfuran (DFF) with particularly low critical micelle concentration (CMC). Green Chem. 2017, 19, 4074–4079. [Google Scholar] [CrossRef]
- Minaev, B.F. Electronic mechanisms of activation of molecular oxygen. Russ. Chem. Rev. 2007, 76, 988–1010. [Google Scholar] [CrossRef]
- Schweitzer, C.; Schmidt, R. Physical Mechanisms of Generation and Deactivation of Singlet Oxygen. Chem. Rev. 2003, 103, 1685–1757. [Google Scholar] [CrossRef] [PubMed]
- Espada, J. (Ed.) Reactive Oxygen Species; Springer Science + Business Media: Berlin, Germany, 2021. [Google Scholar]
- Krumova, K.; Cosa, G. Overview of Reactive Oxygen Species. In Singlet Oxygen, Applications in Biosciences and Nanosciences; Nonell, S., Flors, C., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2016; Volume 1, pp. 3–21. [Google Scholar]
- Boix-Garriga, E.; Rodríguez-Amigo, B.; Planas, O.; Nonell, S. Properties of singelet oxygen. In Singlet Oxygen, Applications in Biosciences and Nanosciences; Nonell, S., Flors, C., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2016; Volume 1, pp. 23–46. [Google Scholar]
- Gellé, A.; Price, G.D.; Voisard, F.; Brodusch, N.; Gauvin, R.; Amara, Z. Enhancing Singlet Oxygen Photocatalysis with Plasmonic Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 35606–35616. [Google Scholar] [CrossRef]
- Tambosco, B.; Segura, K.; Seyrig, C.; Cabrera, D.; Port, M.; Ferroud, C.; Amara, Z. Outer-sphere effects in visible-light photochemical oxidations with immobilized and recyclable ruthenium bipyridyl salts. ACS Catal. 2018, 8, 4383–4389. [Google Scholar] [CrossRef]
- Ghogare, A.A.; Greer, A. Using Singlet Oxygen to Synthesize Natural Products and Drugs. Chem. Rev. 2016, 116, 9994–10034. [Google Scholar] [CrossRef]
- Turconi, J.; Griolet, F.; Guevel, R.; Oddon, G.; Villa, R.; Geatti, A.; Hvala, M.; Rossen, K.; Göller, R.; Burgard, A. Semisynthetic Artemisinin, the Chemical Path to Industrial Production. Org. Process Res. Dev. 2014, 18, 417–422. [Google Scholar] [CrossRef]
- Turconi, J.; Mackievicz, P. Paludisme et hémisynthèse industrielle de l’artémisinine: Du rêve à la réalité! Actual. Chim. 2018, 39–47. [Google Scholar]
- Tu, Y. Artemisinin—A Gift from Traditional Chinese Medicine to the World. Angew. Chem. Int. Ed. 2016, 55, 10210–10226. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Höinck, L.-O.; Neudörfl, J.M. Synthesis of spiroannulated and 3-arylated 1,2,4-trioxanes from mesitylol and methyl 4-hydroxytiglate by photooxygenation and peroxyacetalization. Beilstein J. Org. Chem. 2010, 6, 61. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; El-Idreesy, T.T.; Fiege, M.; Brun, R. Synthesis of Antimalarial 1,2,4-Trioxanes via Photooxygenation of a Chiral Allylic Alcohol. Org. Lett. 2002, 4, 4193–4195. [Google Scholar] [CrossRef]
- Singh, C.; Varma, V.P.; Naikade, N.K.; Singh, A.S.; Hassam, M.; Pun, S.K. Novel Bis- and Tris-1,2,4-trioxanes: Synthesis and Antimalarial Activity against Multidrug-Resistant Plasmodium yoelii in Swiss Mice. J. Med. Chem. 2008, 51, 7581–7592. [Google Scholar] [CrossRef] [PubMed]
- Tsolakis, N.; Bam, W.; Srai, J.S.; Kumar, M. Renewable chemical feedstock supply network design: The case of terpenes. J. Cleaner Prod. 2019, 222, 802–822. [Google Scholar] [CrossRef]
- Behr, A.; Johnen, L. Myrcene as a Natural Base Chemical in Sustainable Chemistry: A Critical Review. ChemSusChem 2009, 2, 1072–1095. [Google Scholar] [CrossRef] [PubMed]
- Monica, F.D.; Kleij, A.W. From terpenes to sustainable and functional polymers. Polym. Chem. 2020, 11, 5109–5127. [Google Scholar] [CrossRef]
- Mewalal, R.; Rai, D.K.; Kainer, D.; Chen, F.; Külheim, C.; Peter, G.F.; Tuskan, G.A. Plant-Derived Terpenes: A Feedstock for Specialty Biofuels. Trends Biotechnol. 2017, 35, 227–240. [Google Scholar] [CrossRef]
- Rojahn, W.; Warnecke, H.U. Die photosensibilisierte Sauerstoffübertragung–eine Methode zur Herstellung hochwertiger Riechstoffe. Dragoco Report 1980, 27, 159–164. [Google Scholar]
- Surburg, H.; Panten, J. (Eds.) Common Fragrance and Flavor Materials, 6th ed.; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Park, C.Y.; Kim, Y.J.; Lim, H.J.; Park, J.H.; Kim, M.J.; Seo, S.W.; Park, C.P. Continuous flow photooxygenation of monoterpenes. RSC Adv. 2015, 5, 4233–4237. [Google Scholar] [CrossRef]
- Wau, J.S.; Robertson, M.J.; Oelgemöller, M. Solar Photooxygenations for the Manufacturing of Fine Chemicals—Technologies and Applications. Molecules 2021, 26, 1685. [Google Scholar] [CrossRef]
- Radjagobalou, R.; Blanco, J.-F.; Petrizza, L.; Le Bechec, M.; Dechy-Cabaret, O.; Lacombe, S.; Save, M.; Loubière, K. Efficient Photooxygenation Process of Biosourced α-Terpinene by Combining Controlled LED-Driven Flow Photochemistry and Rose Bengal-Anchored Polymer Colloids. ACS Sustain. Chem. Eng. 2020, 8, 18568–18576. [Google Scholar] [CrossRef]
- Lee, D.S.; Amara, Z.; Clark, C.A.; Xu, Z.; Kakimpa, B.; Morvan, H.P.; Pickering, S.J.; Poliakoff, M.; Gorge, M.W. Continuous Photo-Oxidation in a Vortex Reactor: Efficient Operations Using Air Drawn from the Laboratory. Org. Process Res. Dev. 2017, 21, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Radjagobalou, R.; Blanco, J.-F.; Dechy-Cabaret, O.; Oelgemöller, M.; Lobière, K. Photooxygenation in an advanced led-driven module: Experimental investigations and modelling. Chem. Eng. Process. Process Intensif. 2018, 130, 214–228. [Google Scholar] [CrossRef]
- Lévesque, F.; Seeberger, P.H. Continuous-Flow Synthesis of the Anti-Malaria Drug Artemisinin. Angew. Chem. Int. Ed. 2012, 51, 1706–1709. [Google Scholar] [CrossRef] [PubMed]
- Michelin, C.; Lefebvre, C.; Hoffmann, N. Les réactions photochimiques à l’échelle industrielle. Actual. Chim. 2019, 436, 19–26. [Google Scholar]
- Emmanuel, N.; Mendoza, C.; Winter, M.; Horn, C.R.; Vizza, A.; Dreesen, L.; Heinrichs, B.; Monbaliu, J.-C.M. Scalable Photocatalytic Oxidation of Methionine under Continious-Flow Conditions. Org. Process Res. Dev. 2017, 21, 1435–1438. [Google Scholar] [CrossRef]
- Montagnon, T.; Kalaitzakis, D.; Sofadis, M.; Vassilikogiannakis, G. Chemoselective photooxygenations of furans bearing unprotected amines: Their use in alkaloid synthesis. Org. Biomol. Chem. 2016, 14, 8636–8640. [Google Scholar] [CrossRef]
- Gollnick, K.; Griesbeck, A. Singlet oxygen photooxygenation of furans: Isolation and reactions of (4+2)-cycloaddition products (unsaturated sec.-ozonides). Tetrahedron 1985, 41, 2057–2068. [Google Scholar] [CrossRef]
- Schenck, G.O. Photochemische Reaktionen II. Über die unsensibilisierte und photosensibilisierte Autoxydation von Furanen. Justus Liebigs Ann. Chem. 1953, 584, 156–176. [Google Scholar] [CrossRef]
- Cottier, L.; Descotes, G.; Nigay, H.; Parron, J.-C.; Grégoire, V. Photo-oxygénation des dérivés de l’hydroxyméthyl-5 furfural-2. Bull. Soc. Chim. Fr. 1986, 5, 844–850. [Google Scholar]
- Chauhan, D.K.; Battula, V.R.; Giri, A.; Patra, A.; Kailasam, K. Photocatalytic valorization of furfural to value-added chemicals via mesoporous carbon nitride: A possibility through a metal-free pathway. Catal. Sci. Technol. 2022, 12, 144–153. [Google Scholar] [CrossRef]
- Desvals, A.; Fortino, M.; Lefebvre, C.; Rogier, J.; Michelin, C.; Alioui, S.; Rousset, E.; Pedone, A.; Lemercier, G.; Hoffmann, N. Synthesis and characterization of polymethine dyes carrying thiobarbituric and carboxylic acid moieties. New J. Chem. 2022, 46, 8971–8980. [Google Scholar] [CrossRef]
- Miles, W.H. Synthetic Applications of γ-Hydroxybutenolides. Curr. Org. Synth. 2014, 11, 244–267. [Google Scholar] [CrossRef]
- Lefebvre, C.; Hoffmann, N. Les colorants et la lumière pour transformer la matière. Actual. Chim. 2019, 444–445, 38–43. [Google Scholar]
- Martel, J.; Tessier, J.; Demoute, J.-P. (Roussel Uclaf), Procédé de préparation de la 6,6-diméthyl-4-hydroxy-3-oxabicyclo(3.1.0)hexan-2-one et de ses éthers sous toutes leurs formes stéréoisomères. Eur. Pat. 1981, 0023454. [Google Scholar]
- Moradei, O.M.; Paquette, L.A. (5S)-(d-Menthyloxy)-2(5H)-furanone. Org. Synth. 2003, 80, 66–73. [Google Scholar]
- Marinković, S.; Brulé, C.; Hoffmann, N.; Prost, E.; Nuzillard, J.-M.; Bulach, V. Origin of Chiral Induction in Radical Reactions with the Diastereoisomers (5R)- and (5S)-5-l-Menthyloxyfuran-2[5H]-one. J. Org. Chem. 2004, 69, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- Feringa, B.L.; de Jong, J.C. New Strategies in Asymmetric Synthesis Based On γ-Alkoxybutenolides. Bull. Soc. Chim. Belg. 1992, 101, 627–640. [Google Scholar] [CrossRef]
- Gassama, A.; Ernenwein, C.; Youssef, A.; Agach, M.; Riguet, E.; Marinković, S.; Hoffmann, N. Sulfonated surfactants obtained from furfural. Green Chem. 2013, 15, 1558–1566. [Google Scholar] [CrossRef]
- Gassama, A.; Ernenwein, C.; Hoffmann, N. Photochemical Key Steps in the Synthesis of Surfactants from Furfural-Derived Intermediates. ChemSusChem 2009, 2, 1130–1137. [Google Scholar] [CrossRef]
- Hoffmann, N. Efficient photochemical electron transfer sensitization of homogeneous organic reactions. J. Photochem. Photobiol. C 2008, 9, 43–60. [Google Scholar] [CrossRef]
- Tan, J.-N.; Ahmar, M.; Queneau, Y. Isomaltulose Oxidation and Dehydration Products as Starting Materials towards Fine Chemicals. Curr. Org. Chem. 2014, 18, 1768–1787. [Google Scholar] [CrossRef]
- Tan, J.-N.; Ahmar, M.; Queneau, Y. Glucosyloxymethylfurfural (GMF): A creative renewable scaffold towards bioinspired architectures. Pure Appl. Chem. 2015, 87, 827–839. [Google Scholar] [CrossRef]
- Lichtenthaler, F.W.; Martin, D.; Weber, T.; Schiweck, H. 5-(α-D-Glucosyloxymethyl)furfural: Preparation from Isomaltulose and Exploration of Its Ensuing Chemistry. Liebigs Ann. Chem. 1993, 1993, 967–974. [Google Scholar] [CrossRef]
- Tadiparthi, K.; Venkatesh, S. Synthetic approaches toward butenolide-containing natural products. Dihydro-5-(hydroxymethyl)-2(3H)-furanone. J. Heterocycl. Chem. 2022, 59, 1285–1307. [Google Scholar] [CrossRef]
- Flourat, A.L.; Haudrechy, A.; Allais, F.; Renault, J.-H. (S)-γ-Hydroxymethyl-α,β-butenolide, a Valuable Chiral Synthon: Syntheses, Reactivity, and Applications. Org. Process Res. Dev. 2020, 24, 615–636. [Google Scholar] [CrossRef]

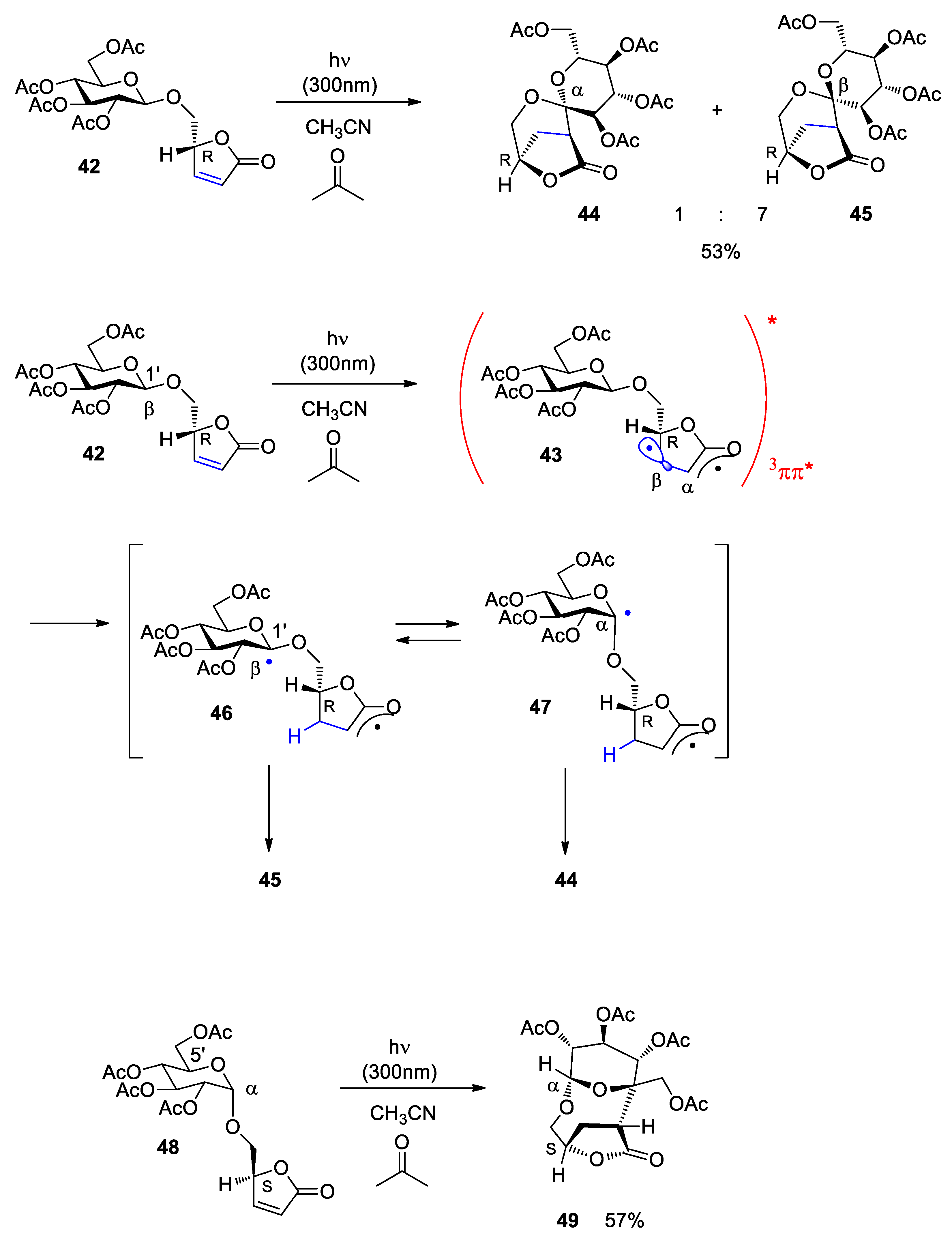

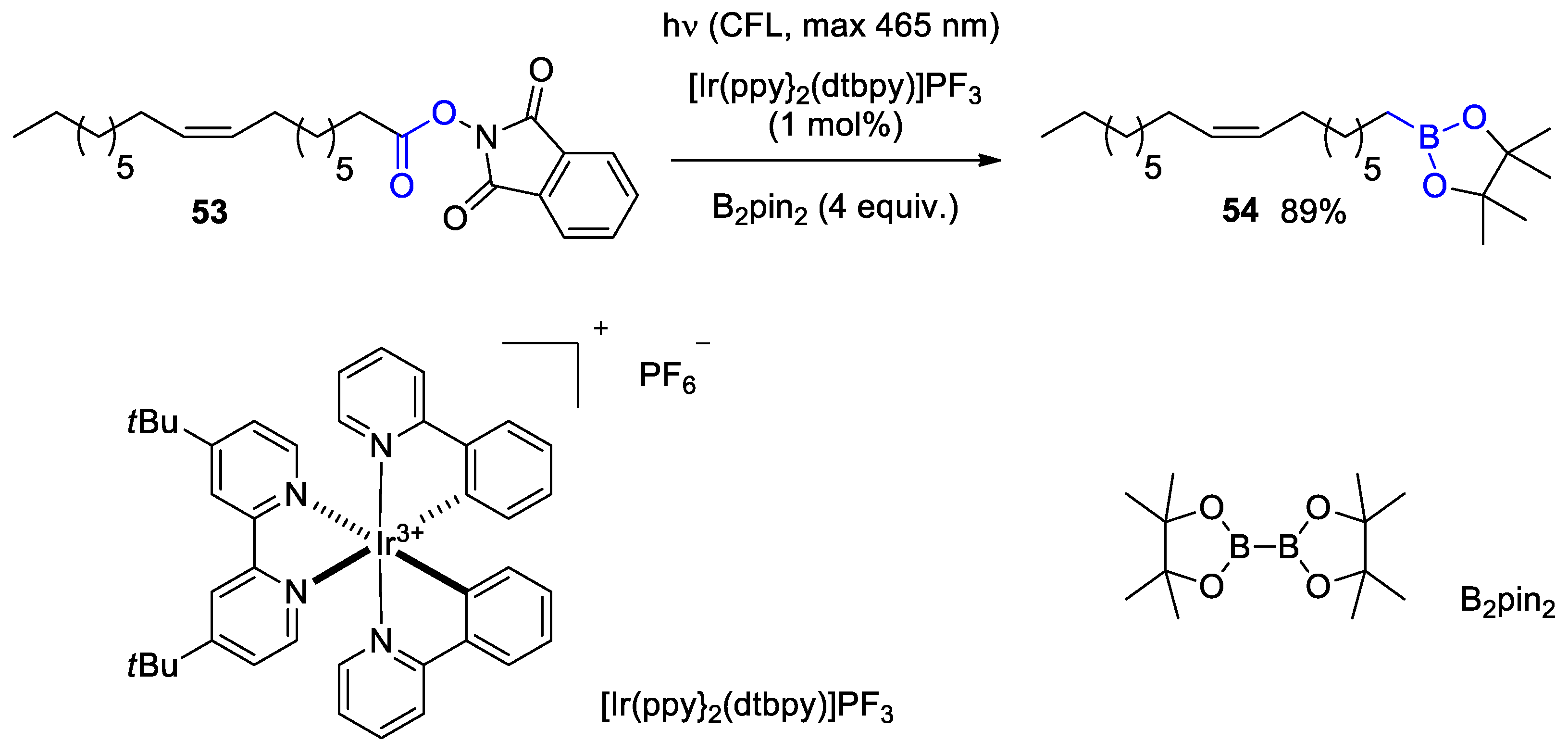
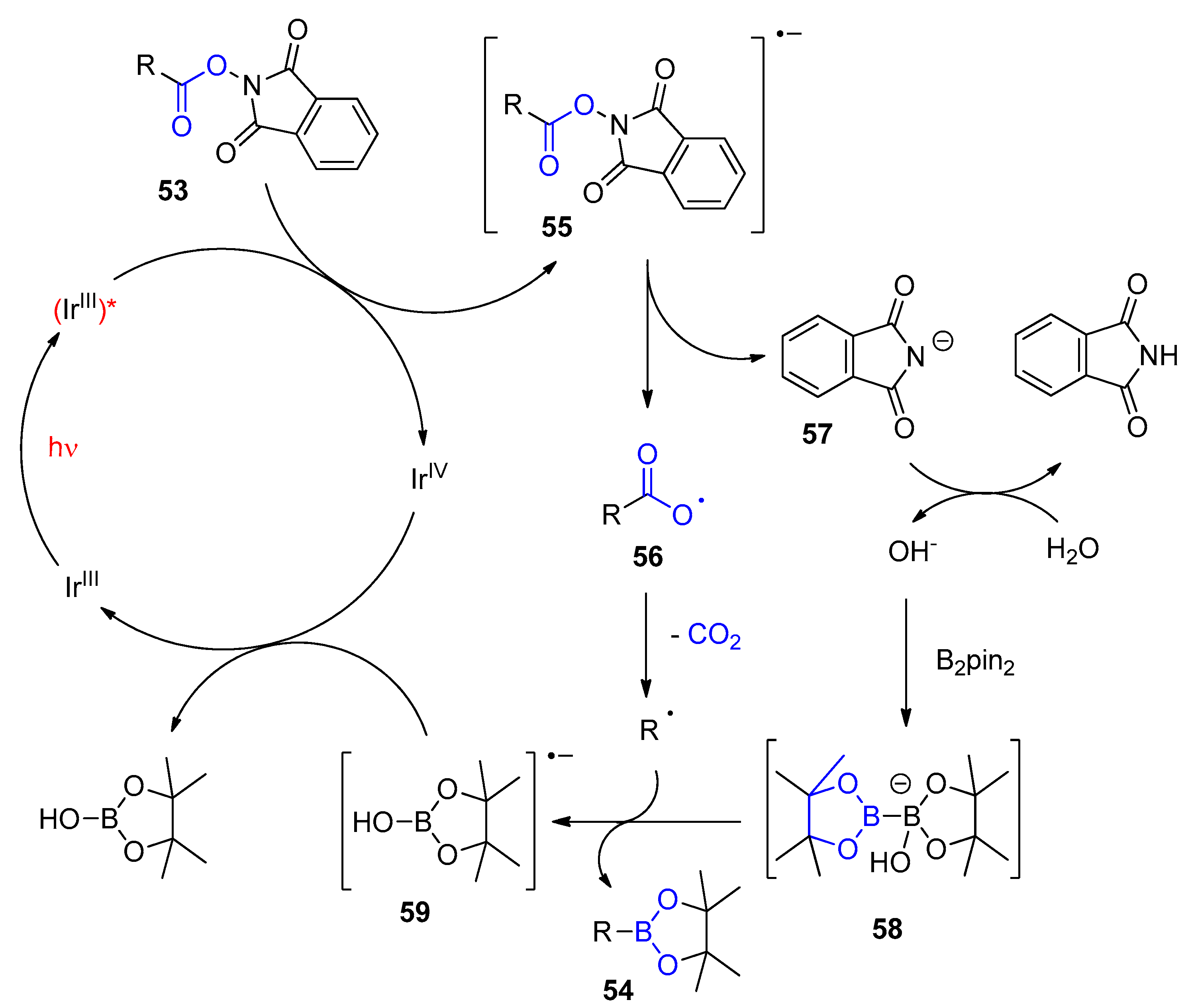
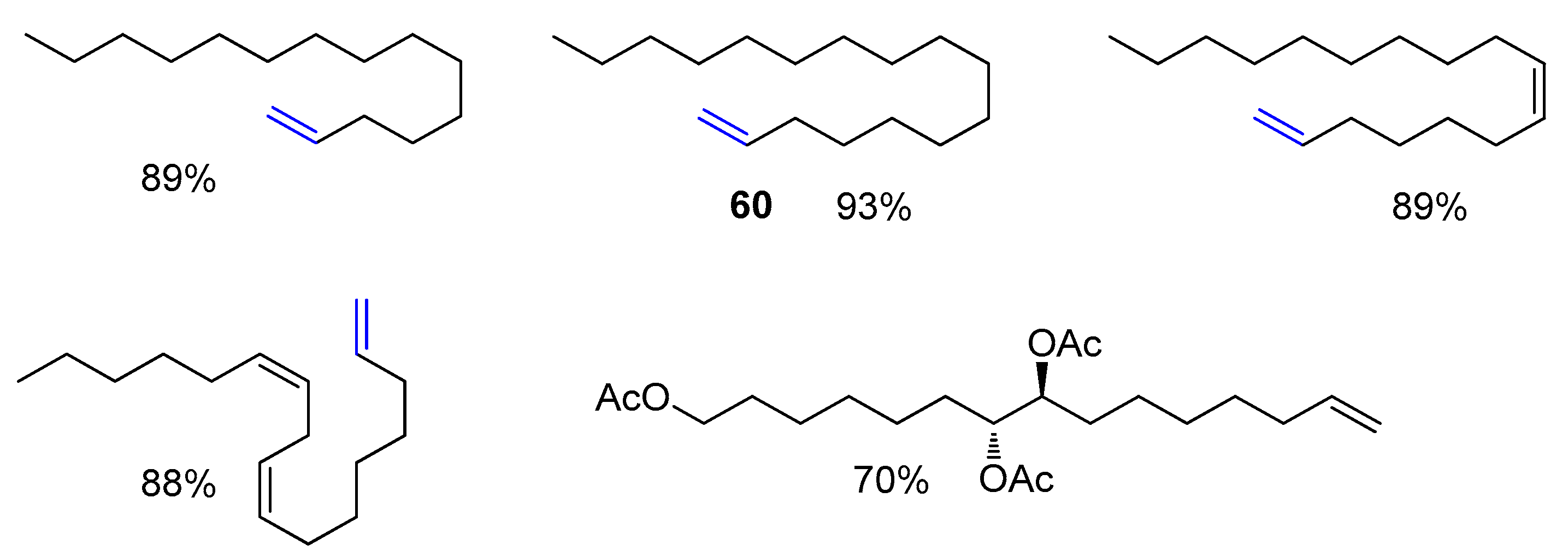

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez Fernández, M.A.; Hoffmann, N. Photocatalytic Transformation of Biomass and Biomass Derived Compounds—Application to Organic Synthesis. Molecules 2023, 28, 4746. https://doi.org/10.3390/molecules28124746
Gómez Fernández MA, Hoffmann N. Photocatalytic Transformation of Biomass and Biomass Derived Compounds—Application to Organic Synthesis. Molecules. 2023; 28(12):4746. https://doi.org/10.3390/molecules28124746
Chicago/Turabian StyleGómez Fernández, Mario Andrés, and Norbert Hoffmann. 2023. "Photocatalytic Transformation of Biomass and Biomass Derived Compounds—Application to Organic Synthesis" Molecules 28, no. 12: 4746. https://doi.org/10.3390/molecules28124746
APA StyleGómez Fernández, M. A., & Hoffmann, N. (2023). Photocatalytic Transformation of Biomass and Biomass Derived Compounds—Application to Organic Synthesis. Molecules, 28(12), 4746. https://doi.org/10.3390/molecules28124746







