Abstract
Organic anion transporter 3 (OAT3) is predominantly expressed in the kidney and plays a vital role in drug clearance. Consequently, co-ingestion of two OAT3 substrates may alter the pharmacokinetics of the substrate. This review summarizes drug–drug interactions (DDIs) and herbal–drug interactions (HDIs) mediated by OAT3, and inhibitors of OAT3 in natural active compounds in the past decade. This provides a valuable reference for the combined use of substrate drugs/herbs for OAT3 in clinical practice in the future and for the screening of OAT3 inhibitors to avoid harmful interactions.
1. Introduction
The two primary superfamilies, ATP binding cassette (ABC) and solute carrier (SLC), play a crucial role in the absorption, distribution, and elimination of various endogenous substances such as hormones and signal molecules, as well as exogenous substances and drugs [1,2]. Generally, SLC transporters mediate the influx of substances from the blood into the epithelium, while ABC transporters mediate the efflux of substances [3,4,5]. The SLC22 family comprises six primary subfamilies: organic anion transporter (OAT), OAT-like, OAT-related, organic cation transporter (OCT), organic cation and carnitine transporter (OCTN), and OCT/OCTN-related [6,7].
OAT3 is considered the primary transporter in OAT due to its broad substrate specificity. OAT3 (encoded by SLC22A8) is widely distributed in the kidney, liver, choroid plexus [8], olfactory mucosa, brain [9], retina, and placenta [10], but it is pre-dominantly expressed on the basolateral membrane of renal tubular cells [11,12]. OAT3 utilizes dicarboxylate (e.g., α-Ketoglutarate) to exchange organic anions, with the concentration gradient as the driving force [13]. It first transports organic anions (OAs) from the blood to the proximal tubules through the basolateral membrane and then discharges OAs into the urine through the apical membrane of tubular epithelial cells, reabsorbing specific compounds in the glomerular filtrate back into the internal circulation [14,15].
OAT3 plays a crucial role in the uptake, distribution, and excretion of various endogenous/exogenous substances, but current research has primarily focused on the interactions between OAT3 and clinical drugs [16,17,18]. When two or more drugs are taken simultaneously, OAT3 becomes a target of drug competition [19,20], potentially altering the toxicity [21], pharmacokinetics [22], and function of these drugs and resulting in potential drug–drug interactions (DDIs) [23,24]. However, when herbal medicines and OAT3 substrate drugs are taken together, they may lead to severe herbal drug interactions (HDIs), such as liver injury [25], increased clotting time, headache, insomnia, and even coma and death [26,27]. Research has shown that not all of the interactions mentioned above are negative. For example, enalaprilat and quinaprilat are two types of antihypertensive drugs. When used in combination with some synthetic drugs or flavonoids, their transport through OAT3 is inhibited, leading to synergistic blood pressure reduction [28,29]. OAT3 has a significant role in DDIs and HDIs [13], with effects that can be either beneficial or adverse. In this review, we summarize the DDIs and HDIs mediated by OAT3, as well as OAT3 inhibitors in natural products over the past decade. Through this review, we can predict and avoid DDIs/HDIs mediated by OAT3.
2. OAT3 and Synthetic Drug–Drug Interactions
The kidney is primarily responsible for drug clearance after administration [22]. Kidney drug transporters play a crucial role in drug transport between the blood and lumen [30], including antibiotics, diuretics, proton pump inhibitors, non-steroidal anti-inflammatory drugs, antiviral drugs, and anticancer drugs [31,32]. Simultaneous administration of multiple drugs can result in changes in drug levels compared to a single administration [33]. DDIs can cause higher or lower levels of drugs, thereby altering their toxicity or efficacy (Table 1) [5,34]. Table 1 summarizes the DDIs mediated by OAT3 reported in the last decade.

Table 1.
OAT3 and synthetic drug–drug interactions (DDIs).
Imipenem is an antibiotic with antibacterial effects, but when administered alone, it is rapidly degraded by dehydropeptidase-1 or renal dipeptidase (also termed DPEP1) [44]. Cilastatin and imipenem are both substrates of OAT3. When administered together intravenously, the plasma concentration–time curve (AUC) for imipenem is significantly increased by 1.65-fold, while the plasma/serum concentration half-time (t1/2β) of imipenem increased by 2.35-fold. Cilastatin also inhibits the uptake of imipenem mediated by OAT3 in the kidney, thereby reducing the hydrolysis of and acting as a complement to imipenem [35,45,46].
In addition to its role in DDIs, OAT3 inhibition has been shown to have a renoprotective effect against nephrotoxic drugs [47]. For example, diclofenac [48], a commonly used non-steroidal anti-inflammatory drug (NSAID), can cause kidney damage [49]. However, when diclofenac is co-administered with cilastatin, an OAT3 inhibitor, the AUC0–12h of diclofenac increases by 35.4% and its plasma clearance (CLp) significantly decreases. Co-administration of cilastatin with diclofenac acyl glucuronide, a metabolite of diclofenac, leads to an increase in the concentration of diclofenac acyl glucuronide in the plasma by 46.7%, but a slight decrease in AUC0–12h of diclofenac acyl glucuronide in the kidney by 18.0%. Moreover, diclofenac acyl glucuronide but not diclofenac exhibited OAT-dependent cytotoxicity and was identified as an OAT1/3 substrate. The accumulation of diclofenac acyl glucuronide in primary proximal tubule cells (RPTCs) is reduced, and the cytotoxicity caused by diclofenac acyl glucuronide is also reduced [36].
Methotrexate (MTX) is a drug used for the high-dose treatment of various malignant tumors or the low-dose treatment of rheumatoid arthritis and psoriasis [50,51]. Delayed elimination of MTX can lead to severe toxicity, such as bone marrow suppression, mucositis, and kidney damage [37]. Kidney clearance is the main pathway for MTX clearance (65–80%). Proton pump inhibitors (PPIs) inhibit the hOAT3-mediated uptake of MTX with IC50 values of 0.40–5.5 μM [37]. Rhein, the main metabolite of diacerein, markedly inhibited MTX accumulation in rat kidney slices and hOAT3-HEK293 cells, indicating that OAT3 is involved in DDIs in the kidney. When the two drugs were co-administered orally, the maximal plasma/serum concentration (Cmax) and AUC of MTX were increased by 2.5- and 4.4-fold, respectively, and the CL of MTX was decreased by 66.7%. When the two drugs were co-administered intravenously, the t1/2β of MTX was prolonged by 86.7%, the CLP was decreased by 57.6%, and the AUC was increased by 183.4%. Rhein alleviated MTX-induced renal toxicity in vivo mainly by inhibiting OAT3 to reduce the renal clearance of MTX [38]. Masahiro Iwaki et al. found that seven NSAIDs-Glu exhibit a concentration-dependent inhibitory effect on MTX uptake through OAT3, with diclofenac-Glu having the most effective inhibitory ability (IC50 = 3.17 μM) [39]. In addition to the above-mentioned drugs, tranilast, an anti-allergic drug, can inhibit MTX transport.
OAT3 renal sections and HEK293T-OAT3 cell uptake experiments have shown that tranilast can inhibit MTX uptake by OAT3. Pharmacokinetic studies in rats have shown that when MTX (5 mg/kg) is administered orally alongside tranilast (10 mg/kg), the Cmax and AUC0–24h of MTX increased to 2.14- and 1.46-fold, respectively, while CLz/f and Vz/f decreased by 24.90% and 39.23%, respectively [40].
Quinapril is an angiotensin-converting enzyme (ACE) inhibitor used to treat hypertension and congestive heart failure. In a pharmacokinetic study on rats, co-administration of quinapril (3 mg/kg) with gemcabene (30 mg/kg) resulted in a 40% decrease in the urinary excretion of quinaprilat (quinaprilat, the metabolite of the quinapril, is also the substrate of OAT3) and a 53% increase in the AUC0–24h of quinaprilat, leading to a reduction in blood pressure. Subsequent studies discovered that gemcabene inhibited quinaprilat uptake by hOAT3 and rOat3 at IC50 values of 35 and 48 μM. Moreover, gemcabene acylglucuronide, the major metabolite of gemcabene glucuronidation, also inhibited the hOAT3- and rOat3-mediated uptake of quinaprilat at IC50 values of 197 and 133 μM, respectively. This indicated the mechanism by which concomitant intake of gemcabene with quinapril can reduce blood pressure [28]. In another study, Ni et al. investigated the effects of several common drugs on the OAT3-mediated uptake of enalaprilat (another oral ACE). Benzbromarone was found to be the most effective inhibitor of OAT3 (IC50 = 0.14 μM), while diclofenac sodium was the weakest inhibitor (IC50 = 6.13 μM) [29].
One study showed that concurrent treatment with mizoribine and bezafibrate can result in rhabdomyolysis. It was suggested that both bezafibrate and mizoribine are substrates of OAT3, and when bezafibrate was co-administered orally with mizoribine, the t1/2β of bezafibrate increased to 1.39-fold, and the renal clearance (CLr) decreased to 0.81-fold. However, when bezafibrate was co-administrated with mizoribine intravenously, the AUC and t1/2β of bezafibrate increased to 1.29- and 1.25-fold, respectively. This study also suggested that mizoribine can competitively inhibit the uptake of bezafibrate by OAT3 [41].
Similarly, in a study in which benzylpenicillin and acyclovir were co-administered intravenously in rats, the cumulative urinary excretion of acyclovir decreased by 45% and CLR decreased by 44%, while the t1/2β of acyclovir increased by 1.9-fold. This indicates that benzylpenicillin can inhibit the renal excretion of acyclovir by inhibiting OAT3 [42].
Wen et al. found that when piperacillin and tazobactam were administered simultaneously, both CLp and CLR of tazobactam were decreased, and the AUC, t1/2β, and km of tazobactam were increased to 2.15-, 1.24-, and 1.56-fold, respectively. Moreover, piperacillin can competitively inhibit the uptake of tazobactam mediated by OAT3 [43].
3. OAT3 and Herb–Drug Interactions (HDIs)
Natural medicines containing flavonoids and other functional components, particularly polyphenols, are increasingly used in clinical therapy [52]. In the elderly population with chronic diseases such as hypertension, hyperlipidemia [53], and hyperuricemia, it is common to use prescription drugs, over-the-counter drugs, and natural products simultaneously [47,54]. A survey showed that 78% of elderly respondents take both prescription drugs and dietary supplements, and 32.6% of them experience potential adverse drug reactions when they were taking a combination of herbs and other drugs [55]. Some herbs may directly cause organ toxicity, alter the pharmacokinetics or efficacy of prescription drugs mediated by OAT3, or inhibit enzymes involved in drug metabolism [26].
Purarin (PUR) is an isoflavone component extracted from Pueraria lobata [56]. When PUR and MTX are orally administered in combination, the Cmax, AUC, and t1/2β of MTX are increased by 79%, 74%, and 70%, respectively. When PUR and MTX are co-administered intravenously, the AUC and t1/2β of MTX increase by 59% and 37%, respectively, and a 37% decrease in CLp was observed. PUR also simultaneously inhibits MTX uptake in rat kidney slices and HEK293-OAT3 cells. Therefore, the combined administration of PUR can inhibit OAT3 and enhance MTX exposure (Table 2) [57].

Table 2.
OAT3 and herb–drug interactions.
Steviol glucuronide is the major metabolite of steviol, and its uptake is primarily mediated by OAT3 (Wang et al.). Inhibition studies have shown that three drugs (with IC50 values between 2.92 and 9.97 μM) can inhibit steviol glucuronide uptake mediated by OAT3 in a concentration-dependent manner, and may also alter its renal clearance [58]. Steviol acyl glucuronide, the main circulating metabolite of steviol glycosides after oral administration, is also a substrate of OAT3 [63]. Therefore, if the activity of OAT3 is inhibited, it may alter the pharmacokinetic characteristics of steviol acyl glucuronide. Zhou et al. showed that probenecid and glimepiride inhibit hOAT3/rOat3-mediated steviol acyl glucuronide transport in a concentration-dependent manner (IC50 < 5 μM) (Table 2). When administered in combination with probenecid or glimepiride, the Cmax of steviol acyl glucuronide was increased to 10.8- or 9.7-fold, respectively. The AUC6–8h of steviol acyl glucuronide was increased to 2.9- or 2.5-fold, respectively. Therefore, people with impaired renal function should be cautious when taking steviol glycoside products [59].
The increased renal transport of imipenem mediated by OAT3 can lead to certain renal toxicity. Apigenin, a flavonoid compound widely distributed in traditional Chinese medicine, is a potent OAT3 inhibitor [64]. In cases with the combined administration of apigenin and imipenem, the survival rate of hOAT3-HEK93 cells significantly increased, and the IC50 value of imipenem within the cells increased to 8.1-fold. At the same time, the AUC0–6h of imipenem increased by 46%, while the CLp and CLr of imipenem decreased by 29% and 52%, respectively. The intracellular accumulation of imipenem decreased, thereby partially reducing the cytotoxicity of imipenem. These results indicate that the inhibition of apigenin on hOAT3 can protect the cytotoxicity induced by imipenem in vitro. Therefore, apigenin can be used as a potential clinical drug to alleviate adverse renal reactions caused by imipenem [60].
Natural drugs containing flavonoids are often taken concomitantly with prescribed or over-the-counter drugs, which may lead to potential interactions [65]. For instance, Ni et al. reported the inhibitory effects of various flavonoids with distinct structures on the activity of OAT3 transport drugs [66]. Among these flavonoids, galangin exhibited the strongest inhibitory effect on the activity of OAT3 with an IC50 value of 0.03 μM, while myricetin exhibited the weakest inhibitory effect, with an IC50 value of 22.58 μM (Table 2) [29].
Lu et al. [61] reported that dichloromethane extract from Juncus effusus can alter the pharmacokinetics of furosemide (FS), an OAT3 substrate drug, in rats. The AUC0–t of FS was increased by 80% and 55% after oral or intravenous administration of Juncus effusus (D), respectively. Therefore, caution is needed to avoid potential side effects resulting from the interaction between OAT3 substrate drugs and Juncus effusus (D). Ma et al. observed a 32% and 52% increase in the AUC0–t of FS following single or multiple dose co-administration of FS and rhubarb extract, respectively [62].
4. Natural Bioactive Inhibitors of OAT3
Many natural bioactive compounds, including flavonoids and their metabolites such as sulfates and glucuronides, have been identified as potent inhibitors and/or substrates of OAT3 [67,68]. The study of OAT3 inhibitors can help us predict and avoid potential OAT3-mediated DDIs/HDIs [69], and these inhibitors may also have potentially beneficial effects on renal diseases, such as AAI-induced kidney disease [70]. Table 3 summarizes the natural bioactive compounds identified as OAT3 inhibitors in the last decade. These compounds are classified into nine groups: phenolic acids, flavonoids, alkaloids, anthraquinones, phenols, phenylpropanoids, terpenoids, phenanthrenoids, and others.

Table 3.
Natural active compounds with OAT3 inhibitory activity.
4.1. Phenolic Acids
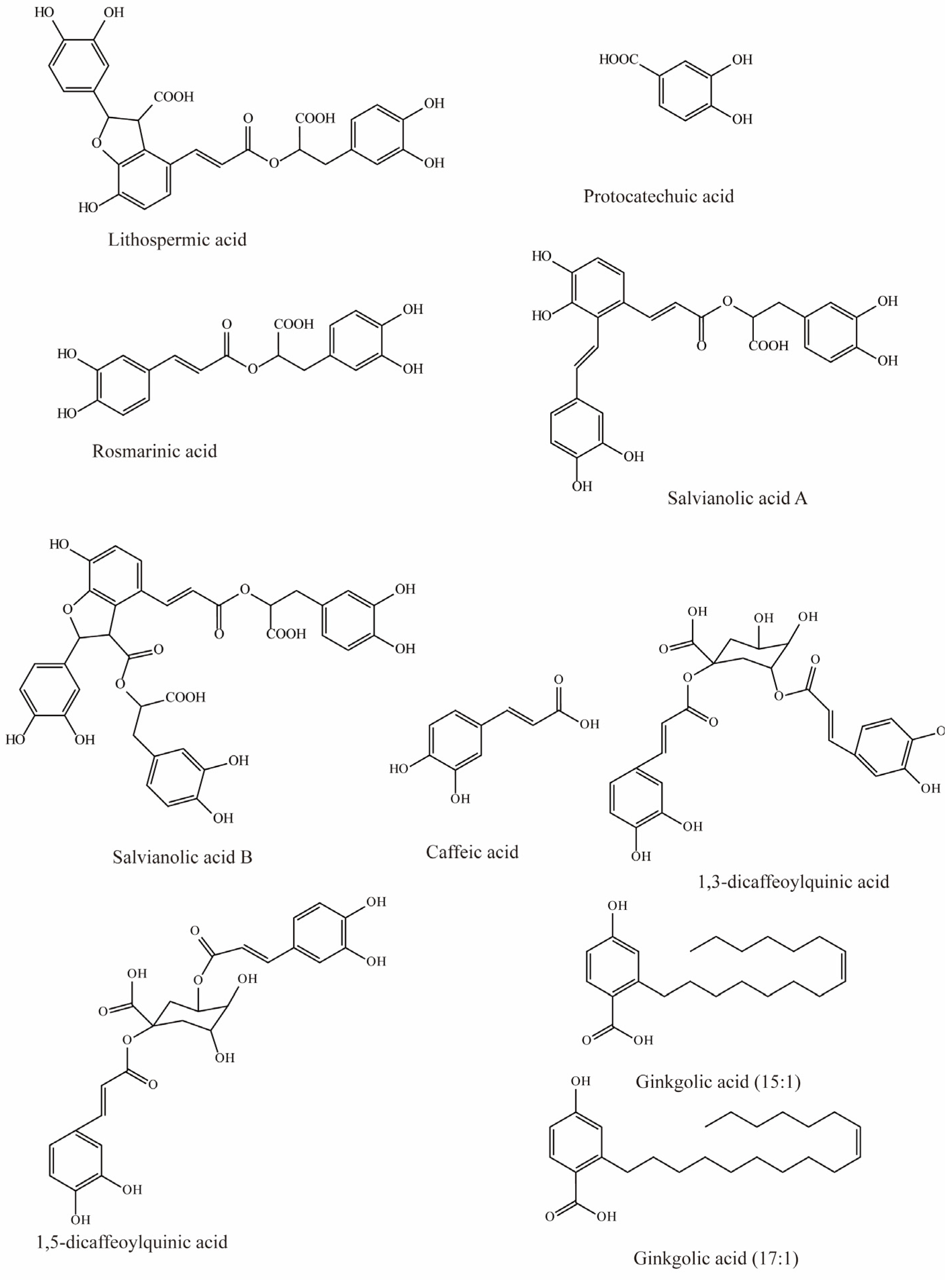
Salvia miltiorrhiza has been used to treat cardiovascular diseases, but its biochemical mechanisms are still unclear. However, studies by Wang et al. have shown that six hydrophilic components from Salvia miltiorrhiza, tanshinol, rosmarinic, lithospermic acid, salvianolic acid A, salvianolic acid B, and protocatechuic acid (Figure 1), have significant inhibitory effects on substrate uptake mediated by mOAT3. Among these, lithospermic acid (Ki = 31.3 μM), rosmarinic (Ki = 4.3 μM), and salvianolic acid A (Ki = 21.3 μM) produce virtually complete inhibition [79].
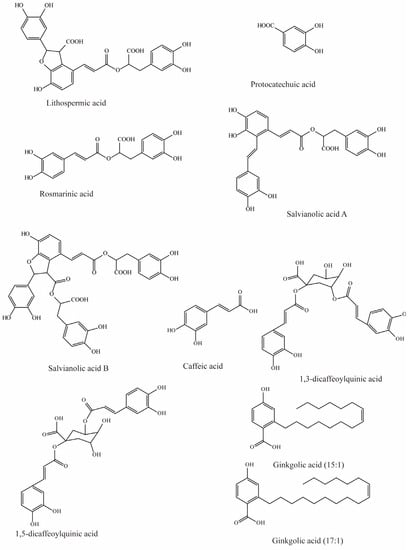
Figure 1.
Structural diagram showing OAT3 inhibitors of phenolic acids.
Previous studies have demonstrated that caffeic acid from coffee, fruits, and vegetables significantly inhibits OAT3 (IC50 = 5.4 μM) [71]. Yuichi et al. uncovered that caffeic acid inhibits substrate uptake by OAT3 in a concentration-dependent manner (IC50 < 10 μM) (Table 3) [80].
Wang et al. investigated the effects of nine compounds extracted from natural diets and herbs on hOAT3-mediated uptake. The results indicated that four of them significantly inhibited the transport of hOAT3, with 1,3-dicaffeoylquinic acid and ginkgolic acid (17:1) exhibiting 41% inhibition, while a 30–35% reduction in hOAT3 mediated uptake was observed in response to 1,5-dicaffeoylquinic acid and ginkgolic acid (15:1) [72].
4.2. Flavonoids
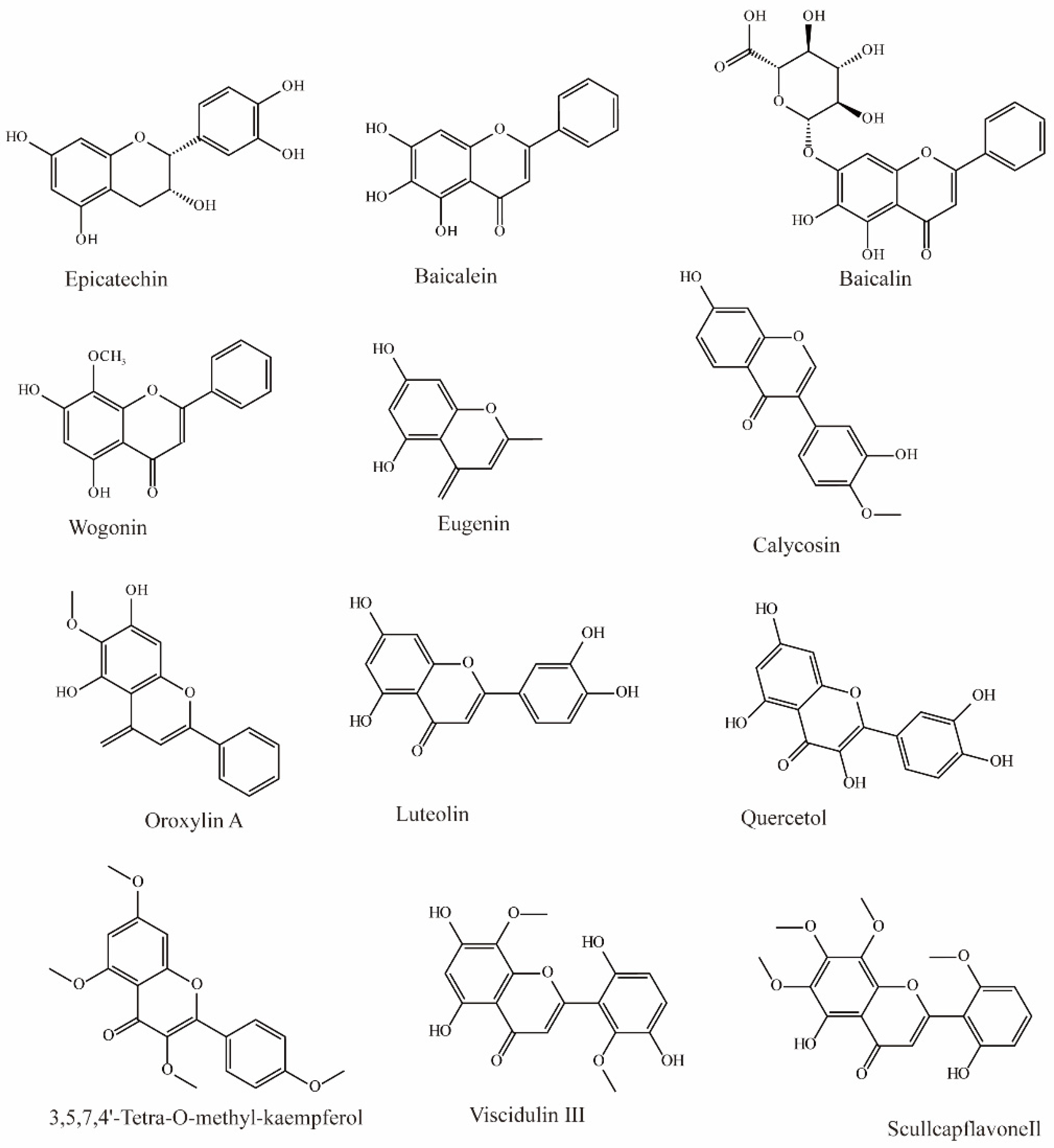
In Chinese herbal medicine, Scutellaria baicalensis is used to treat inflammation and hypertension. Research by Xu found that three main bioactive substances in Scutellaria baicalensis exhibit OAT3 inhibition. Baicalin can effectively inhibit the influx of OAT3 substrate (IC50 = 13.0 μM), and baicalein (IC50 = 2.4 μM) and wogonin (IC50 = 1.3 μM) can also inhibit OAT3 significantly (Figure 2) [70].
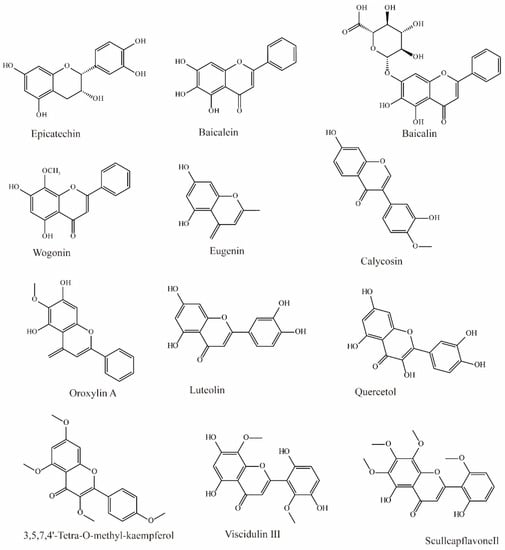
Figure 2.
Structural diagram showing OAT3 inhibitors of flavonoids.
Li et al. extracted 270 substances from Chinese herbal medicine to screen OAT3 inhibitors and found 10 flavonoids that can significantly inhibit OAT3 (IC50 between 1.51 and 14.77 μM). Among them, eugenin, calycosin, wogonin, luteolin, quercetol, and scullcapflavone II had noncompetitive inhibitory effects on OAT3. However, oroxylin A, viscidulin III, and 3,5,7,4′-Tetra-O-methyl-kaempferol competitively inhibit OAT3 [73].
Another study demonstrated that epicatechin may significantly inhibit substrate transport by hOAT3 (IC50 > 50 μM) [72].
4.3. Alkaloids
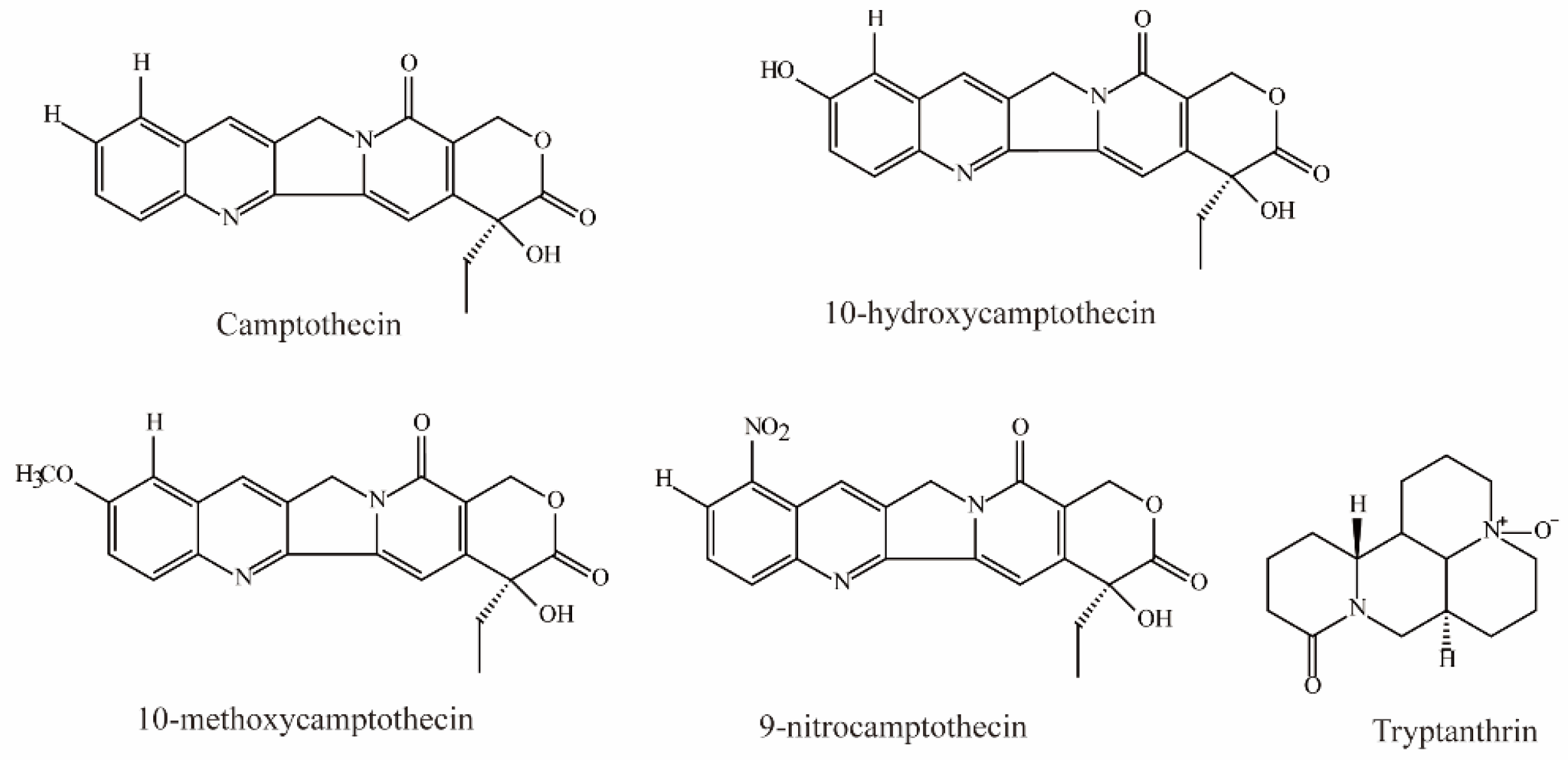
Zheng et al. demonstrated that camptothecin, 10-hydroxycamptothecin, 10-methoxycamptothecin (MCPT), and 9-nitrocamptothecin (Figure 3) could significantly inhibit OAT3-mediated substrate uptake (IC50 < 10 μM) [74].
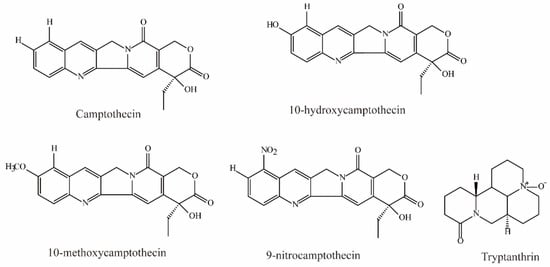
Figure 3.
Structural diagram showing OAT3 inhibitors of alkaloids.
Tryptanthrin, an alkaloid isolated from the medicinal indigo plant Strobilanthes cusia, was shown to be a potent OAT3 noncompetitive inhibitor (IC50 = 0.93 ± 0.22 μM, Ki = 0.43 μM) [81].
4.4. Anthraquinones
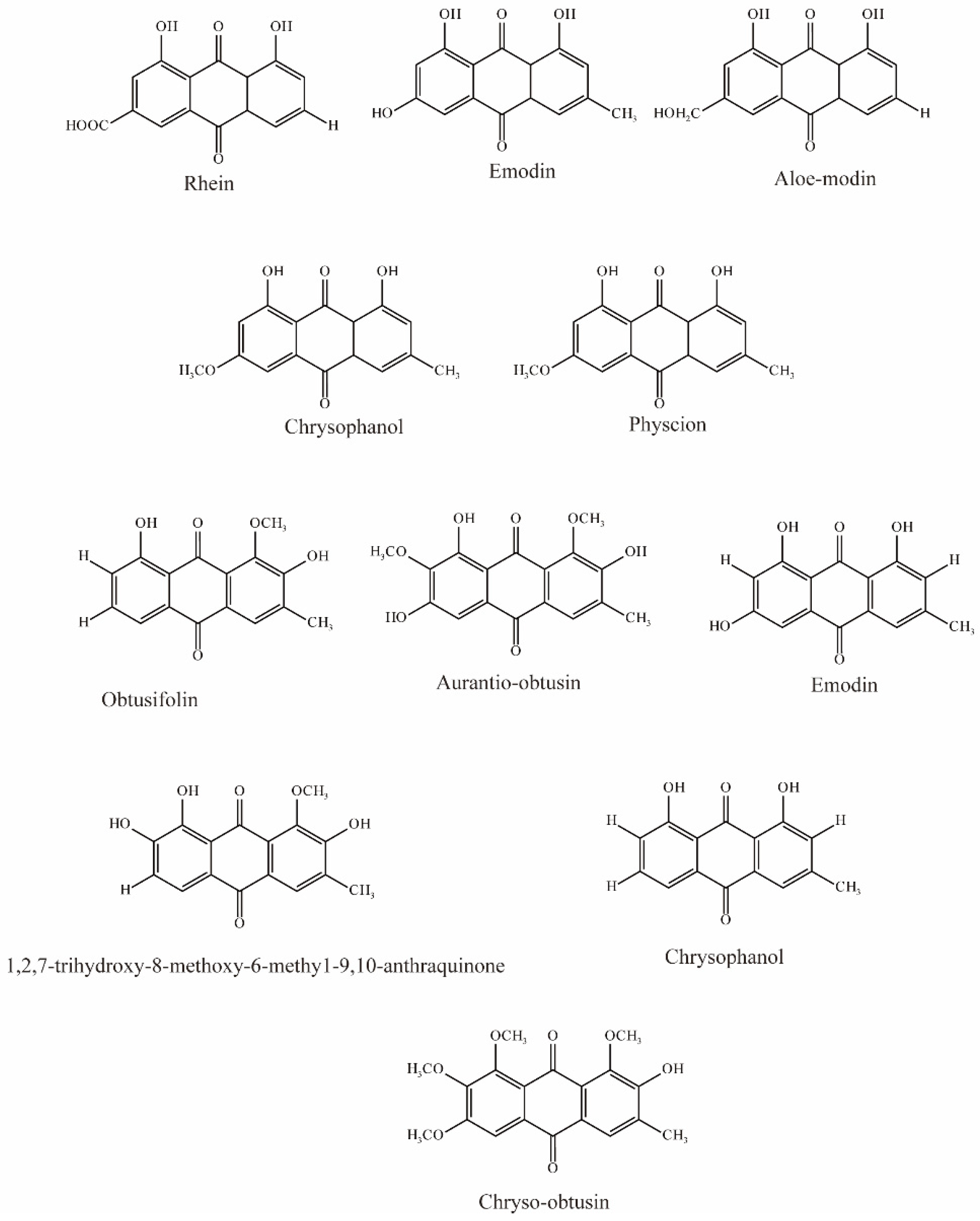
Ma et al. evaluated five anthraquinones isolated from rhubarb (Figure 4); among them, rhein, emodin, and aloeemodin significantly inhibited the uptake of 6-carboxyl fluorescein (6-CF, substrate of OAT3) via hOAT3, while chrysophanol and physcion showed slight inhibition [62].
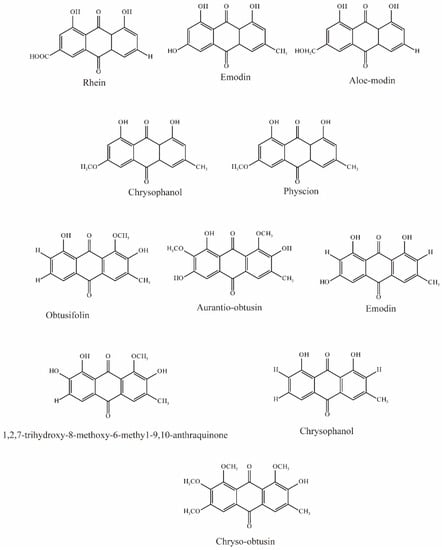
Figure 4.
Structural diagram showing OAT3 inhibitors of anthraquinones.
Wang et al. evaluated 22 substances isolated from Semen cassiae, and 6 anthraquinones significantly (IC50 < 10 μM) inhibited OAT3-mediated transport. In vivo experimental results demonstrated that Semen cassiae extract can nearly eliminate the alterations in renal histology induced by mercury chloride in rats. With docking and validation of OAT3 inhibitors, it may become a drug for treating Hg-induced renal injury [75].
4.5. Phenols
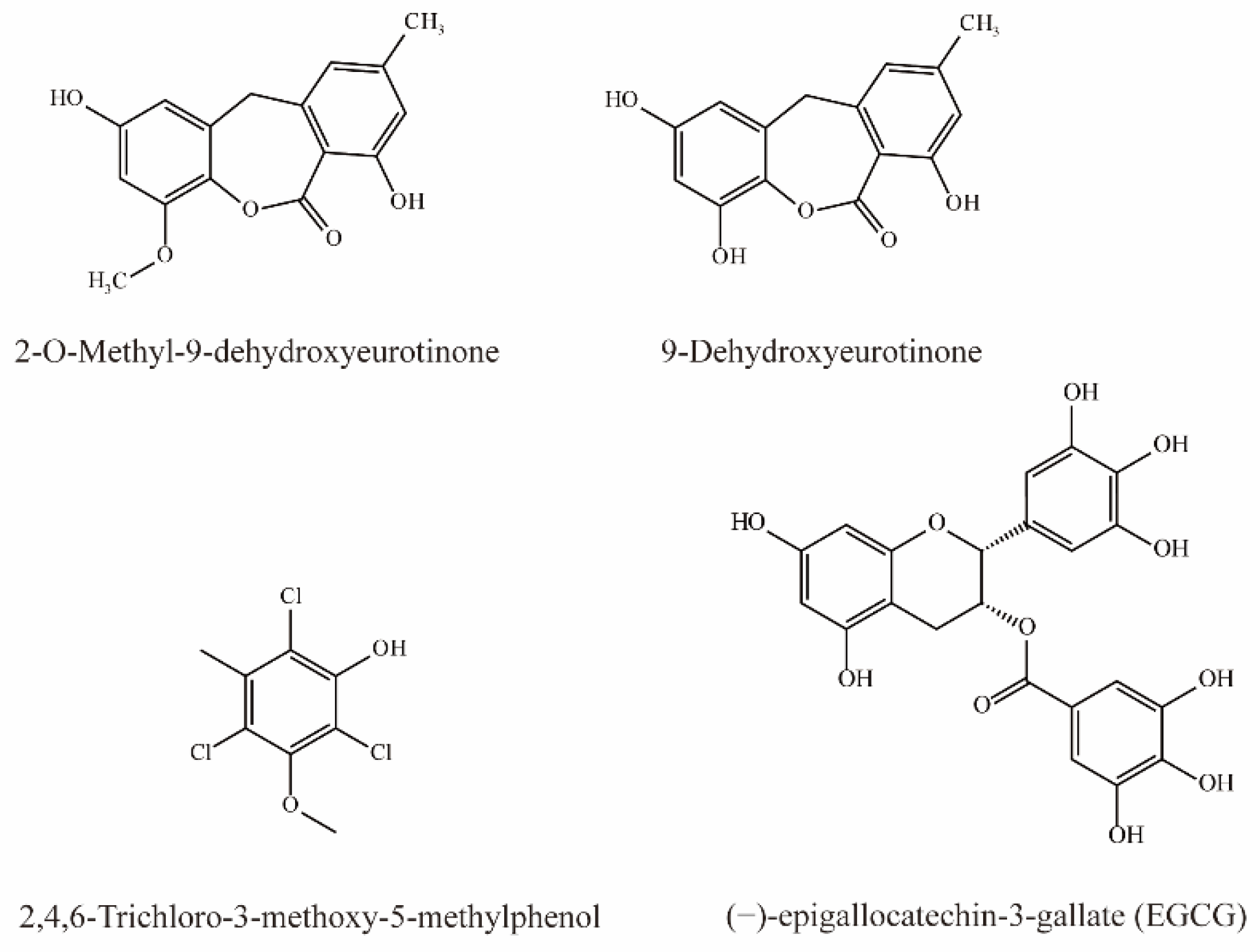
As shown in Figure 5, two phenolic compounds, 9-Dehydroxyeurotinone and 2-O-Methyl-9-dehydroxyeurotinone from extracts of Semen cassiae are potent inhibitors of OAT3 (IC50 < 10 μM). In addition, 9-dihydroxyurotinone is a noncompetitive inhibitor of OAT3 [75].
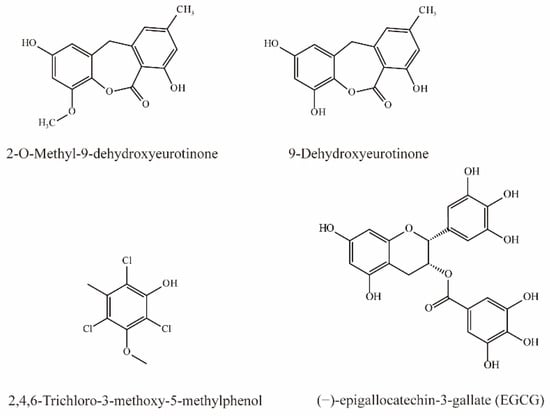
Figure 5.
Structural diagram showing OAT3 inhibitors of phenols.
Tatsuya et al. simultaneously injected 6-CF (1 mg/kg) and EGCG (60 mg/kg) intravenously in rats and detected the concentration of 6-CF in the blood and urine. Their results demonstrated that simultaneous injection elevated the plasma concentration of 6-CF by 8-fold after 1 h, while the AUC0–1h was significantly increased to 2.2-fold. In contrast, EGCG significantly reduced the CLr of 6-CF, indicating that EGCG can inhibit OAT3 [76].
Li et al. demonstrated that 2,4,6-Trichloro-3-methoxy-5-methylphenol extract from Lilium maximowiczii significantly competitively inhibited OAT3 (IC50 = 3.93 μM) [35].
4.6. Phenylpropanoids
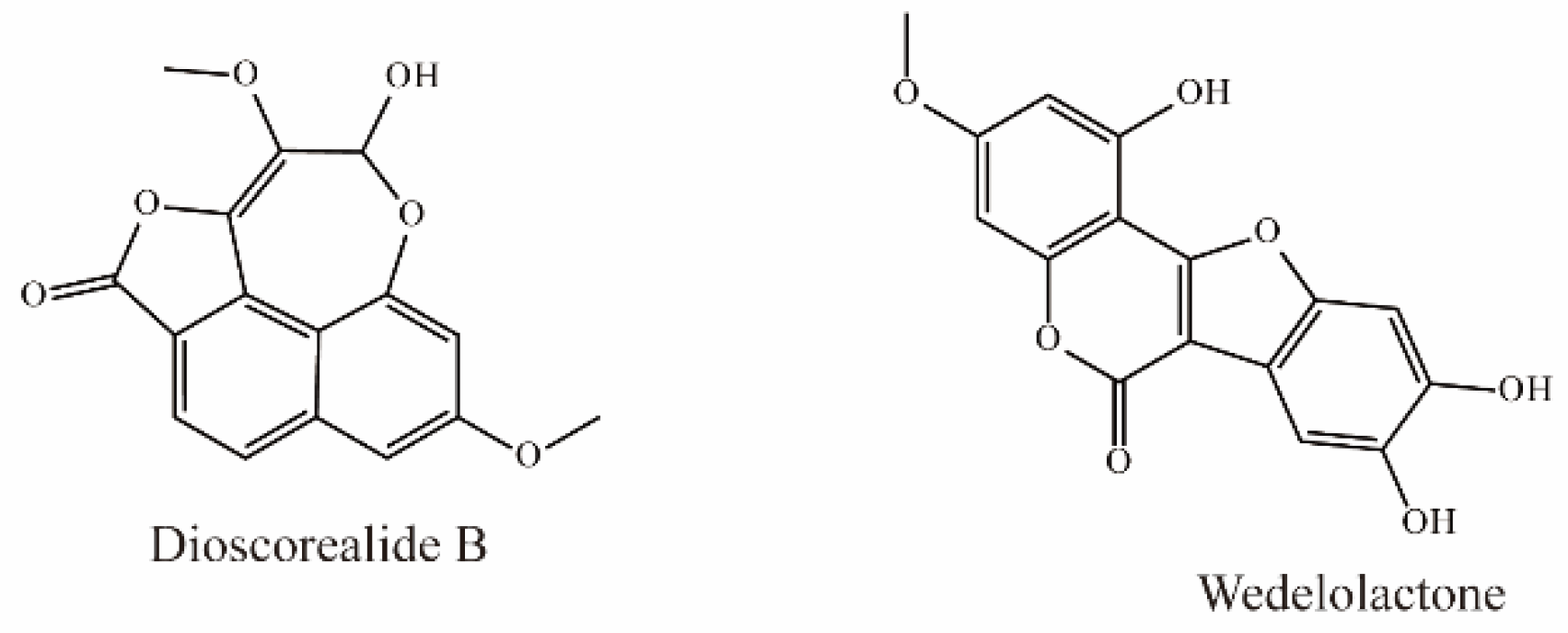
Dioscorealide B is a phenylpropanoid compound extracted from Dioscorea esculenta that has been shown to be an effective noncompetitive inhibitor of OAT3 (IC50 < 10 μM). Another phenylpropanoid compound, wedelolactone, extracted from Eclipta prostrata L., is also an effective inhibitor of OAT3 (IC50 < 10 μM). Moreover, wedelolactone significantly increased serum AAI concentrations and ameliorated renal injuries in AAI-treated mice (Figure 6) [35].
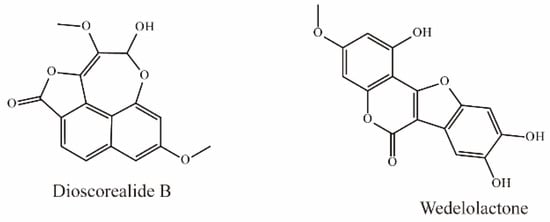
Figure 6.
Structural diagram showing OAT3 inhibitors of phenylpropanoids.
4.7. Terpenes
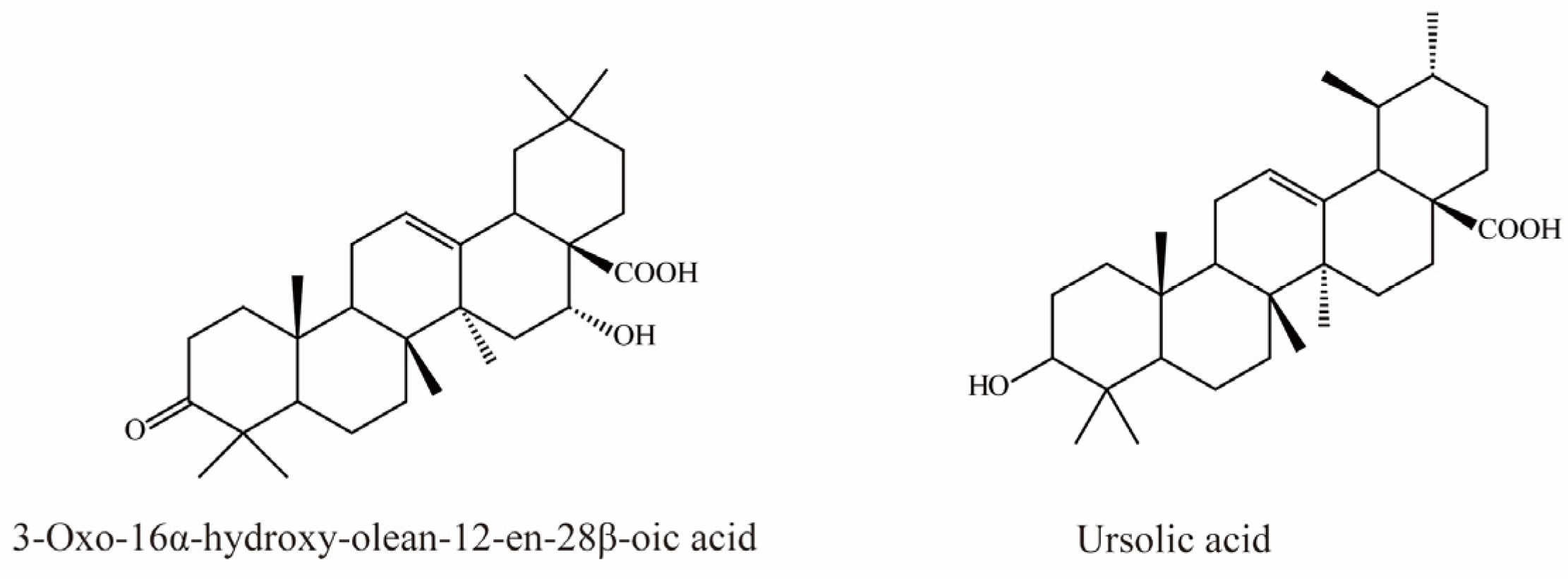
There is currently only one terpene compound, 3-Oxo-16α- hydroxy-olean-12-en-28β-oic acid, which was isolated from Eclipta prostrata L. It was identified as an OAT3 inhibitor with an IC50 value of 8.05 μM, inhibiting OAT3 in a competitive manner (Figure 7) [35].
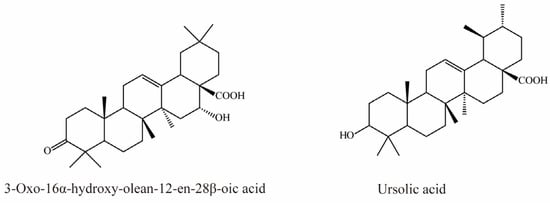
Figure 7.
Structural diagram showing OAT3 inhibitor of terpenes.
Li et al. demonstrated that ursolic acid, the major bioactive component in pomegranate, significantly inhibits transport mediated by OAT3 (IC50 = 18.9 μM) [77].
4.8. Phenanthrenoids
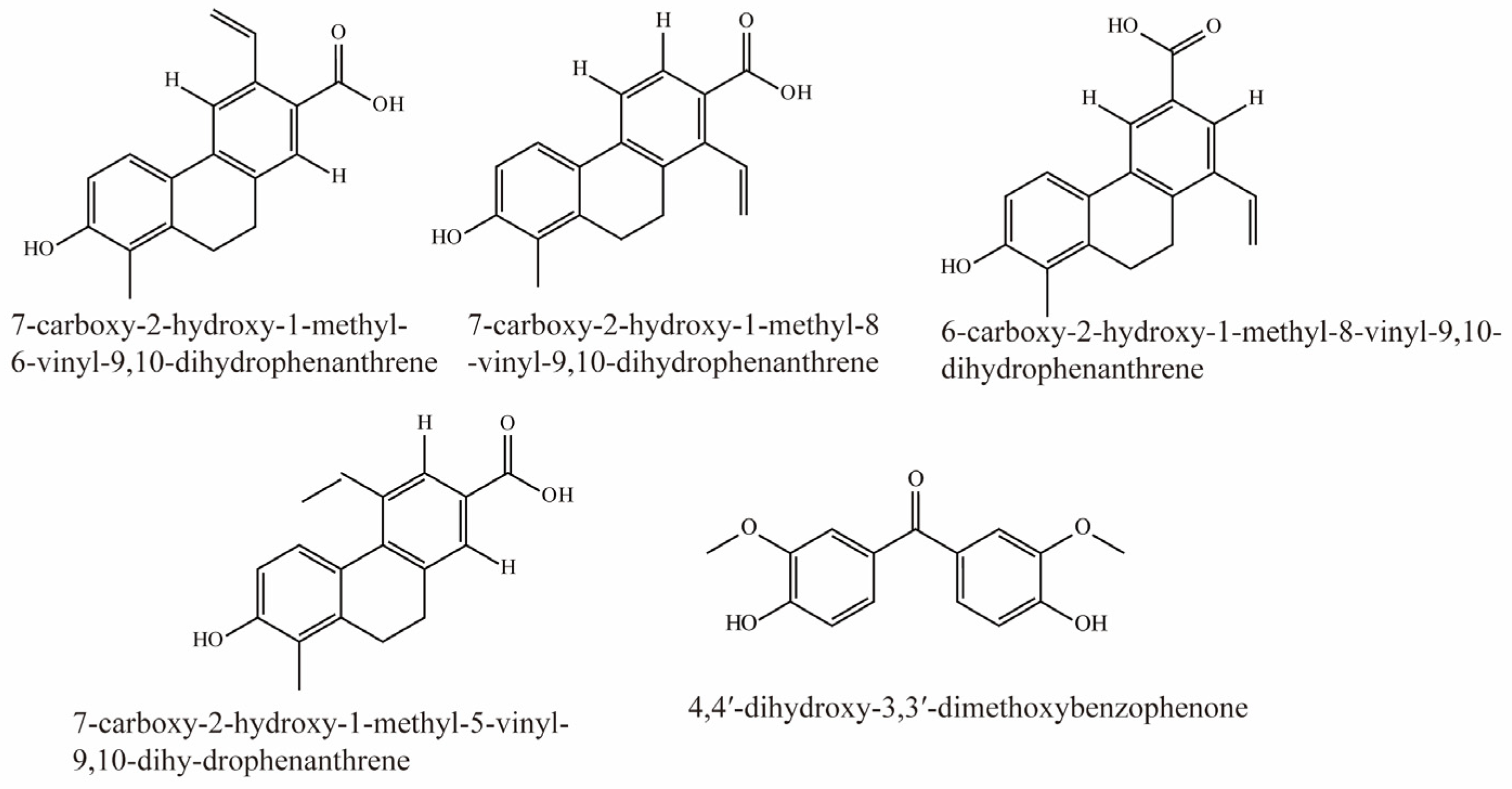
Li et al. identified 16 compounds from Juncus effusus. Among them, 7-carboxy-2-hydroxy-1-methyl-6-vinyl-9,10-dihydrophenanthrene, 7-carboxy-2-hydroxy-1-methyl-8-vinyl-9,10-dihydrophenanthrene, 6-carboxy-2-hydroxy-1-methyl-8-vinyl-9,10-dihydrophenanthrene, 7-carboxy-2-hydroxy-1-methyl-5-vinyl-9,10-dihy-drophenanthrene, and 4,4′-dihydroxy-3,3′-dimethoxybenzophenone were potent inhibitors of OAT3 (IC50 < 5 μM). Their structural formulas are shown in Figure 8 [78].
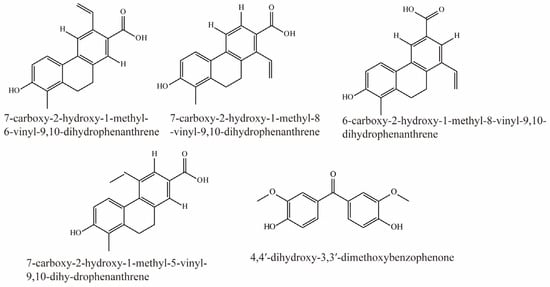
Figure 8.
Structural diagram showing OAT3 inhibitors of phenanthrenoids.
4.9. Others
Lu et al. [61] evaluated the effects on OAT3 of 172 extracts and showed that 14 were strong inhibitors of OAT3 (IC50 between 0.3 and 4.8 µg/mL). Generally speaking, the n-Butanol extract from plants is more effective. For example, the n-Butanol extract IC50 value for Anchusa azurea, Symphytum asperum, and Echium russicum are 0.343, 0.406, 0.460, respectively.
With regards to the effects of natural bioactive compounds, most studies used the IC50 value as an indicator for OAT3 inhibition. However, depending on the potential differences in protein binding and tissue-specific concentrations, IC50 alone may not be sufficient to indicate that exposure to the active compound can cause effective effects. Therefore, further in vivo studies are needed to verify the efficacy of these natural bioactive compounds.
In conclusion, when these substances are administered alongside other drugs or herbs, they may change the pharmacokinetics of the drug or herbal elimination and have certain clinical consequences, including reduced therapeutic efficacy or specific side effects caused by interactions. Therefore, caution should be exercised when administering multiple drugs involving the above substances.
5. Conclusions
OAT3-mediated DDIs/HDIs have both potential benefits and risks. On the one hand, they can enhance drug efficacy by prolonging its half-life, but on the other hand, they may lead to nephrotoxicity by increasing drug accumulation in the kidneys. Therefore, it is important to pay attention to the potential DDIs/HDIs mediated by OAT3 during drug/herb development and in clinical practice. In this regard, the identification of natural active OAT3 inhibitors, which are summarized into nine categories, can help predict and avoid potential interactions. A better understanding of the regulatory pathways involved can also lead to the development of new methods to alleviate or avoid DDIs/HDIs mediated by OAT3. While taking advantage of OAT3-mediated interactions can be beneficial in clinical treatment, it is also important to remain vigilant with respect to potential interactions with unreported drugs. The key role played by OAT3 in DDIs/HDIs has been confirmed, and this related review may also provide a reference for future clinical application of drugs as OAT3 substrates to avoid adverse effects caused by DDIs/HDIs. In addition, there is limited research on natural OAT3 inhibitors, with most studies limited to the in vitro level. The effects of and mechanisms of action for specific natural compounds on OTA3 inhibition in vivo require further research.
Author Contributions
Writing—original draft preparation, Y.C. and H.L.; writing—review and editing, Y.C., K.W. and Y.W.; funding acquisition, Y.W. and Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (grant number 31972127; 31471626) and the Natural Science Foundation of Rizhao (grant number 202117).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are included in this article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Muller, F.; Fromm, M.F. Transporter-mediated drug-drug interactions. Pharmacogenomics 2011, 12, 1017–1037. [Google Scholar] [CrossRef]
- Burckhardt, G. Drug transport by Organic Anion Transporters (OATs). Pharmacol. Ther. 2012, 136, 106–130. [Google Scholar] [CrossRef] [PubMed]
- Ivanyuk, A.; Livio, F.; Biollaz, J.; Buclin, T. Renal Drug Transporters and Drug Interactions. Clin. Pharm. 2017, 56, 825–892. [Google Scholar] [CrossRef]
- Nigam, S.K. What do drug transporters really do? Nat. Rev. Drug Discov. 2015, 14, 29–44. [Google Scholar] [CrossRef]
- International Transporter, C.; Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [CrossRef]
- Nigam, S.K. The SLC22 Transporter Family: A Paradigm for the Impact of Drug Transporters on Metabolic Pathways, Signaling, and Disease. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 663–687. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Matsson, P.; Stecula, A.; Ngo, H.X.; Zur, A.A.; Giacomini, K.M. Drug Metabolites Potently Inhibit Renal Organic Anion Transporters, OAT1 and OAT3. J. Pharm. Sci. 2021, 110, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.H.; Miller, D.S.; Pritchard, J.B.; Fujiwara, Y.; Beier, D.R.; Nigam, S.K. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J. Biol. Chem. 2002, 277, 26934–26943. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, K.; Tachikawa, M. Roles of organic anion/cation transporters at the blood-brain and blood-cerebrospinal fluid barriers involving uremic toxins. Clin. Exp. Nephrol. 2011, 15, 478–485. [Google Scholar] [CrossRef]
- Nigam, S.K.; Bush, K.T.; Martovetsky, G.; Ahn, S.Y.; Liu, H.C.; Richard, E.; Bhatnagar, V.; Wu, W. The organic anion transporter (OAT) family: A systems biology perspective. Physiol. Rev. 2015, 95, 83–123. [Google Scholar] [CrossRef]
- Bush, K.T.; Wu, W.; Lun, C.; Nigam, S.K. The drug transporter OAT3 (SLC22A8) and endogenous metabolite communication via the gut-liver-kidney axis. J. Biol. Chem. 2017, 292, 15789–15803. [Google Scholar] [CrossRef]
- He, L.; Vasiliou, K.; Nebert, D.W. Analysis and update of the human solute carrier (SLC) gene superfamily. Hum. Genom. 2009, 3, 195–206. [Google Scholar] [CrossRef]
- Burckhardt, G.; Burckhardt, B.C. In vitro and in vivo evidence of the importance of organic anion transporters (OATs) in drug therapy. In Drug Transporters; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 29–104. [Google Scholar] [CrossRef]
- Otani, N.; Ouchi, M.; Hayashi, K.; Jutabha, P.; Anzai, N. Roles of organic anion transporters (OATs) in renal proximal tubules and their localization. Anat. Sci. Int. 2017, 92, 200–206. [Google Scholar] [CrossRef]
- Hosoya, K.; Makihara, A.; Tsujikawa, Y.; Yoneyama, D.; Mori, S.; Terasaki, T.; Akanuma, S.; Tomi, M.; Tachikawa, M. Roles of inner blood-retinal barrier organic anion transporter 3 in the vitreous/retina-to-blood efflux transport of p-aminohippuric acid, benzylpenicillin, and 6-mercaptopurine. J. Pharmacol. Exp. Ther. 2009, 329, 87–93. [Google Scholar] [CrossRef]
- Mor, A.L.; Kaminski, T.W.; Karbowska, M.; Pawlak, D. New insight into organic anion transporters from the perspective of potentially important interactions and drugs toxicity. J. Physiol. Pharmacol. 2018, 69, 307–324. [Google Scholar] [CrossRef]
- Astorga, B.; Wunz, T.M.; Morales, M.; Wright, S.H.; Pelis, R.M. Differences in the substrate binding regions of renal organic anion transporters 1 (OAT1) and 3 (OAT3). Am. J. Physiol. Renal Physiol. 2011, 301, F378–F386. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bischoff, A.; Bucher, M.; Gekle, M.; Sauvant, C. PAH clearance after renal ischemia and reperfusion is a function of impaired expression of basolateral Oat1 and Oat3. Physiol. Rep. 2014, 2, e00243. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.; Waldenberger, M.; Krumsiek, J.; Evans, A.M.; Jeratsch, U.; Breier, M.; Adamski, J.; Koenig, W.; Zeilinger, S.; Fuchs, C.; et al. Metabolite profiling reveals new insights into the regulation of serum urate in humans. Metabolomics 2014, 10, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, B.; Ma, L.; Fu, P. Traditional Chinese herbs and natural products in hyperuricemia-induced chronic kidney disease. Front. Pharmacol. 2022, 13, 971032. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Fan, Y.; Yu, Z.; You, G. Regulation of organic anion transporters: Role in physiology, pathophysiology, and drug elimination. Pharmacol. Ther. 2021, 217, 107647. [Google Scholar] [CrossRef]
- Saito, H. Pathophysiological regulation of renal SLC22A organic ion transporters in acute kidney injury: Pharmacological and toxicological implications. Pharmacol. Ther. 2010, 125, 79–91. [Google Scholar] [CrossRef]
- Chung, S.; Kim, G.H. Urate Transporters in the Kidney: What Clinicians Need to Know. Electrolyte Blood Press. 2021, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sweet, D.H. Renal organic anion transporters (SLC22 family): Expression, regulation, roles in toxicity, and impact on injury and disease. AAPS J. 2013, 15, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Voigt, N.; Ort, K.; Sossalla, S. Drug-drug interactions you should know! Dtsch. Med. Wochenschr. 2019, 144, 264–275. [Google Scholar] [CrossRef]
- Parvez, M.K.; Rishi, V. Herb-Drug Interactions and Hepatotoxicity. Curr. Drug Metab. 2019, 20, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Liu, K. Renal organic anion transporters in drug-drug interactions and diseases. Eur. J. Pharm. Sci. 2018, 112, 8–19. [Google Scholar] [CrossRef]
- Yuan, H.; Feng, B.; Yu, Y.; Chupka, J.; Zheng, J.Y.; Heath, T.G.; Bond, B.R. Renal organic anion transporter-mediated drug-drug interaction between gemcabene and quinapril. J. Pharmacol. Exp. Ther. 2009, 330, 191–197. [Google Scholar] [CrossRef]
- Ni, Y.; Duan, Z.; Zhou, D.; Liu, S.; Wan, H.; Gui, C.; Zhang, H. Identification of Structural Features for the Inhibition of OAT3-Mediated Uptake of Enalaprilat by Selected Drugs and Flavonoids. Front. Pharmacol. 2020, 11, 802. [Google Scholar] [CrossRef]
- Nigam, S.K.; Wu, W.; Bush, K.T.; Hoenig, M.P.; Blantz, R.C.; Bhatnagar, V. Handling of Drugs, Metabolites, and Uremic Toxins by Kidney Proximal Tubule Drug Transporters. Clin. J. Am. Soc. Nephrol. 2015, 10, 2039–2049. [Google Scholar] [CrossRef]
- Wu, W.; Jamshidi, N.; Eraly, S.A.; Liu, H.C.; Bush, K.T.; Palsson, B.O.; Nigam, S.K. Multispecific drug transporter Slc22a8 (Oat3) regulates multiple metabolic and signaling pathways. Drug Metab. Dispos. 2013, 41, 1825–1834. [Google Scholar] [CrossRef]
- Zamek-Gliszczynski, M.J.; Chu, X.; Polli, J.W.; Paine, M.F.; Galetin, A. Understanding the transport properties of metabolites: Case studies and considerations for drug development. Drug Metab. Dispos. 2014, 42, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Matsson, P.; Fenu, L.A.; Lundquist, P.; Wisniewski, J.R.; Kansy, M.; Artursson, P. Quantifying the impact of transporters on cellular drug permeability. Trends Pharmacol. Sci. 2015, 36, 255–262. [Google Scholar] [CrossRef]
- Hagos, Y.; Wolff, N.A. Assessment of the role of renal organic anion transporters in drug-induced nephrotoxicity. Toxins 2010, 2, 2055–2082. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huo, X.; Wang, C.; Meng, Q.; Liu, Z.; Sun, H.; Tan, A.; Ma, X.; Peng, J.; Liu, K. Organic anion transporters also mediate the drug-drug interaction between imipenem and cilastatin. Asian J. Pharm. Sci. 2020, 15, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Meng, Q.; Wang, C.; Wu, J.; Wang, C.; Zhu, Y.; Ma, X.; Sun, H.; Liu, K. Protective effect of cilastatin against diclofenac-induced nephrotoxicity through interaction with diclofenac acyl glucuronide via organic anion transporters. Br. J. Pharmacol. 2020, 177, 1933–1948. [Google Scholar] [CrossRef] [PubMed]
- Narumi, K.; Sato, Y.; Kobayashi, M.; Furugen, A.; Kasashi, K.; Yamada, T.; Teshima, T.; Iseki, K. Effects of proton pump inhibitors and famotidine on elimination of plasma methotrexate: Evaluation of drug-drug interactions mediated by organic anion transporter 3. Biopharm. Drug Dispos. 2017, 38, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Jia, Y.M.; Wang, C.Y.; Meng, Q.; Huo, X.K.; Sun, H.J.; Sun, P.Y.; Yang, X.B.; Ma, X.D.; Peng, J.Y.; et al. Organic anion transporters 1 (OAT1) and OAT3 meditated the protective effect of rhein on methotrexate-induced nephrotoxicity. RSC Adv. 2017, 7, 25461–25468. [Google Scholar] [CrossRef]
- Iwaki, M.; Shimada, H.; Irino, Y.; Take, M.; Egashira, S. Inhibition of Methotrexate Uptake via Organic Anion Transporters OAT1 and OAT3 by Glucuronides of Nonsteroidal Anti-inflammatory Drugs. Biol. Pharm. Bull. 2017, 40, 926–931. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, W.; Shen, Q.; Wu, Q.; Jiang, Z.; Wu, W.; Zhang, L.; Huang, X. The key role of organic anion transporter 3 in the drug-drug interaction between tranilast and methotrexate. J. Biochem. Mol. Toxicol. 2022, 36, e22983. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, C.; Liu, Q.; Meng, Q.; Huo, X.; Liu, Z.; Sun, P.; Yang, X.; Sun, H.; Qin, J.; et al. Bezafibrate-mizoribine interaction: Involvement of organic anion transporters OAT1 and OAT3 in rats. Eur. J. Pharm. Sci. 2016, 81, 119–128. [Google Scholar] [CrossRef]
- Ye, J.; Liu, Q.; Wang, C.; Meng, Q.; Sun, H.; Peng, J.; Ma, X.; Liu, K. Benzylpenicillin inhibits the renal excretion of acyclovir by OAT1 and OAT3. Pharmacol. Rep. 2013, 65, 505–512. [Google Scholar] [CrossRef]
- Wen, S.; Wang, C.; Duan, Y.; Huo, X.; Meng, Q.; Liu, Z.; Yang, S.; Zhu, Y.; Sun, H.; Ma, X.; et al. OAT1 and OAT3 also mediate the drug-drug interaction between piperacillin and tazobactam. Int. J. Pharm. 2018, 537, 172–182. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Lob, S.H.; Young, K.; Motyl, M.R.; Sahm, D.F. Activity of imipenem-relebactam against multidrug-resistant Pseudomonas aeruginosa from the United States-SMART 2015–2017. Diagn. Microbiol. Infect. Dis. 2019, 95, 212–215. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, T.; Zhang, Y.; Wang, Y.; Liao, G.; Zhang, B.; Wang, C.; Tian, X.; Feng, L.; Fang, B.; et al. Beneficial herb-drug interaction of rhein in Jinhongtang and Imipenem/Cilastatin mediated by organic anion transporters. J. Ethnopharmacol. 2023, 312, 116449. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Meng, Q.; Wang, C.; Zhu, Y.; Liu, Z.; Ma, X.; Ma, X.; Peng, J.; Sun, H.; Liu, K. Cilastatin protects against imipenem-induced nephrotoxicity via inhibition of renal organic anion transporters (OATs). Acta Pharm. Sin. B 2019, 9, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Shi, B.; Zeng, T.; Zhang, Y.; Huang, B.; Ouyang, B.; Cai, Z.; Liu, M. Drug Transporters in the Kidney: Perspectives on Species Differences, Disease Status, and Molecular Docking. Front. Pharmacol. 2021, 12, 746208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, Y.H.; Putluru, S.P.; Matta, M.K.; Kole, P.; Mandlekar, S.; Furlong, M.T.; Liu, T.; Iyer, R.A.; Marathe, P.; et al. Diclofenac and Its Acyl Glucuronide: Determination of In Vivo Exposure in Human Subjects and Characterization as Human Drug Transporter Substrates In Vitro. Drug Metab. Dispos. 2016, 44, 320–328. [Google Scholar] [CrossRef]
- Brandoni, A.; Torres, A.M. Altered Renal Expression of Relevant Clinical Drug Transporters in Different Models of Acute Uremia in Rats. Role of Urea Levels. Cell Physiol. Biochem. 2015, 36, 907–916. [Google Scholar] [CrossRef]
- Uwai, Y.; Saito, H.; Inui, K. Interaction between methotrexate and nonsteroidal anti-inflammatory drugs in organic anion transporter. Eur. J. Pharmacol. 2000, 409, 31–36. [Google Scholar] [CrossRef]
- El-Sheikh, A.A.; Greupink, R.; Wortelboer, H.M.; van den Heuvel, J.J.; Schreurs, M.; Koenderink, J.B.; Masereeuw, R.; Russel, F.G. Interaction of immunosuppressive drugs with human organic anion transporter (OAT) 1 and OAT3, and multidrug resistance-associated protein (MRP) 2 and MRP4. Transl. Res. 2013, 162, 398–409. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Zhang, D.; Liu, J.; Wu, Q.; Chen, J.; Tan, P.; Xing, B.; Han, Y.; Zhang, P.; et al. Mechanism of drug-induced liver injury and hepatoprotective effects of natural drugs. Chin. Med. 2021, 16, 135. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xin, M.; Liang, S.; Xu, X.; Cai, T.; Dong, L.; Wang, C.; Wang, M.; Cui, Y.; Song, X.; et al. New insight into the management of renal excretion and hyperuricemia: Potential therapeutic strategies with natural bioactive compounds. Front. Pharmacol. 2022, 13, 1026246. [Google Scholar] [CrossRef]
- Endou, H. Recent advances in molecular mechanisms of nephrotoxicity. Toxicol. Lett. 1998, 102–103, 29–33. [Google Scholar] [CrossRef]
- Agbabiaka, T.B.; Spencer, N.H.; Khanom, S.; Goodman, C. Prevalence of drug-herb and drug-supplement interactions in older adults: A cross-sectional survey. Br. J. Gen. Pract. 2018, 68, e711–e717. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Huang, G. Preparation, Structural Analysis and Antioxidant Activity of Polysaccharides and Their Derivatives from Pueraria lobata. Chem. Biodivers. 2023, 20, e202201253. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Meng, Q.; Huo, X.; Sun, H.; Peng, J.; Ma, X.; Sun, P.; Liu, K. MDR1 and OAT1/OAT3 mediate the drug-drug interaction between puerarin and methotrexate. Pharm. Res. 2014, 31, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qi, H.; Li, J.; Xu, Y.; Zhang, H. Transmembrane transport of steviol glucuronide and its potential interaction with selected drugs and natural compounds. Food Chem. Toxicol. 2015, 86, 217–224. [Google Scholar] [CrossRef]
- Zhou, D.; Xu, Y.; Wang, Y.; Li, J.; Gui, C.; Zhang, H. Interaction of Organic Anion Transporter 3-Mediated Uptake of Steviol Acyl Glucuronide, a Major Metabolite of Rebaudioside A, with Selected Drugs. J. Agric. Food Chem. 2020, 68, 1579–1587. [Google Scholar] [CrossRef]
- Huo, X.; Meng, Q.; Wang, C.; Wu, J.; Zhu, Y.; Sun, P.; Ma, X.; Sun, H.; Liu, K. Targeting renal OATs to develop renal protective agent from traditional Chinese medicines: Protective effect of Apigenin against Imipenem-induced nephrotoxicity. Phytother. Res. 2020, 34, 2998–3010. [Google Scholar] [CrossRef]
- Lu, H.; Lu, Z.; Li, X.; Li, G.; Qiao, Y.; Borris, R.P.; Zhang, Y. Interactions of 172 plant extracts with human organic anion transporter 1 (SLC22A6) and 3 (SLC22A8): A study on herb-drug interactions. PeerJ 2017, 5, e3333. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, L.; Hu, H.; Qin, Y.; Bian, Y.; Jiang, H.; Zhou, H.; Yu, L.; Zeng, S. Interaction of five anthraquinones from rhubarb with human organic anion transporter 1 (SLC22A6) and 3 (SLC22A8) and drug-drug interaction in rats. J. Ethnopharmacol. 2014, 153, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, D.; Wang, Y.; Li, J.; Wang, M.; Lu, J.; Zhang, H. CYP2C8-mediated interaction between repaglinide and steviol acyl glucuronide: In vitro investigations using rat and human matrices and in vivo pharmacokinetic evaluation in rats. Food Chem. Toxicol. 2016, 94, 138–147. [Google Scholar] [CrossRef]
- Bao, Y.Y.; Zhou, S.H.; Fan, J.; Wang, Q.Y. Anticancer mechanism of apigenin and the implications of GLUT-1 expression in head and neck cancers. Future Oncol. 2013, 9, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- van Herwerden, E.F.; Sussmuth, R.D. Sources for Leads: Natural Products and Libraries. Handb. Exp. Pharmacol. 2016, 232, 91–123. [Google Scholar] [CrossRef] [PubMed]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef]
- Parvez, M.M.; Shin, H.J.; Jung, J.A.; Shin, J.G. Evaluation of para-Aminosalicylic Acid as a Substrate of Multiple Solute Carrier Uptake Transporters and Possible Drug Interactions with Nonsteroidal Anti-inflammatory Drugs In Vitro. Antimicrob. Agents Chemother. 2017, 61, e02392-16. [Google Scholar] [CrossRef]
- Xiao, S.Q.; Wang, W.; Liu, Y. Research Progress on Extraction and Separation of Active Components from Loquat Leaves. Separations 2023, 10, 126. [Google Scholar] [CrossRef]
- Hagos, F.T.; Daood, M.J.; Ocque, J.A.; Nolin, T.D.; Bayir, H.; Poloyac, S.M.; Kochanek, P.M.; Clark, R.S.; Empey, P.E. Probenecid, an organic anion transporter 1 and 3 inhibitor, increases plasma and brain exposure of N-acetylcysteine. Xenobiotica 2017, 47, 346–353. [Google Scholar] [CrossRef]
- Xu, F.; Li, Z.; Zheng, J.; Gee Cheung, F.S.; Chan, T.; Zhu, L.; Zhuge, H.; Zhou, F. The inhibitory effects of the bioactive components isolated from Scutellaria baicalensis on the cellular uptake mediated by the essential solute carrier transporters. J. Pharm. Sci. 2013, 102, 4205–4211. [Google Scholar] [CrossRef]
- Uwai, Y.; Ozeki, Y.; Isaka, T.; Honjo, H.; Iwamoto, K. Inhibitory effect of caffeic acid on human organic anion transporters hOAT1 and hOAT3: A novel candidate for food-drug interaction. Drug Metab. Pharm. 2011, 26, 486–493. [Google Scholar] [CrossRef]
- Wang, L.; Sweet, D.H. Interaction of Natural Dietary and Herbal Anionic Compounds and Flavonoids with Human Organic Anion Transporters 1 (SLC22A6), 3 (SLC22A8), and 4 (SLC22A11). Evid. Based Complement. Altern. Med. 2013, 2013, 612527. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Bi, Y.; Yu, H.; Wei, J.; Zhang, Y.; Han, L.; Zhang, Y. Potent Inhibitors of Organic Anion Transporters 1 and 3 From Natural Compounds and Their Protective Effect on Aristolochic Acid Nephropathy. Toxicol. Sci. 2020, 175, 279–291. [Google Scholar] [CrossRef]
- Zheng, J.; Chan, T.; Zhu, L.; Yan, X.; Cao, Z.; Wang, Y.; Zhou, F. The inhibitory effects of camptothecin (CPT) and its derivatives on the substrate uptakes mediated by human solute carrier transporters (SLCs). Xenobiotica 2016, 46, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, L.; Li, G.; Peng, W.; Gao, X.; Klaassen, C.D.; Fan, G.; Zhang, Y. From the Cover: Identification of Natural Products as Inhibitors of Human Organic Anion Transporters (OAT1 and OAT3) and Their Protective Effect on Mercury-Induced Toxicity. Toxicol. Sci. 2018, 161, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kondo, M.; Hiramatsu, R.; Nabekura, T. (-)-Epigallocatechin-3-gallate Inhibits Human and Rat Renal Organic Anion Transporters. ACS Omega 2021, 6, 4347–4354. [Google Scholar] [CrossRef]
- Li, Z.; Wang, K.; Zheng, J.; Cheung, F.S.; Chan, T.; Zhu, L.; Zhou, F. Interactions of the active components of Punica granatum (pomegranate) with the essential renal and hepatic human Solute Carrier transporters. Pharm. Biol. 2014, 52, 1510–1517. [Google Scholar] [CrossRef]
- Li, X.; Qiao, Y.; Wang, X.; Ma, R.; Li, T.; Zhang, Y.; Borris, R.P. Dihydrophenanthrenes from Juncus effusus as Inhibitors of OAT1 and OAT3. J. Nat. Prod. 2019, 82, 832–839. [Google Scholar] [CrossRef]
- Wang, L.; Sweet, D.H. Active Hydrophilic Components of the Medicinal Herb Salvia miltiorrhiza (Danshen) Potently Inhibit Organic Anion Transporters 1 (Slc22a6) and 3 (Slc22a8). Evid. Based Complement. Alternat. Med. 2012, 2012, 872458. [Google Scholar] [CrossRef]
- Uwai, Y.; Kawasaki, T.; Nabekura, T. Caffeic acid inhibits organic anion transporters OAT1 and OAT3 in rat kidney. Drug Metabol. Drug Interact. 2013, 28, 247–250. [Google Scholar] [CrossRef]
- Shams, T.; Lu, X.; Zhu, L.; Zhou, F. The inhibitory effects of five alkaloids on the substrate transport mediated through human organic anion and cation transporters. Xenobiotica 2018, 48, 197–205. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).