Carbonaceous Material Modified MoO2 Nanospheres with Oxygen Vacancies for Enhanced Visible-Light Photocatalytic Oxidative Coupling of Benzylamine
Abstract
:1. Introduction
2. Results and Discussion
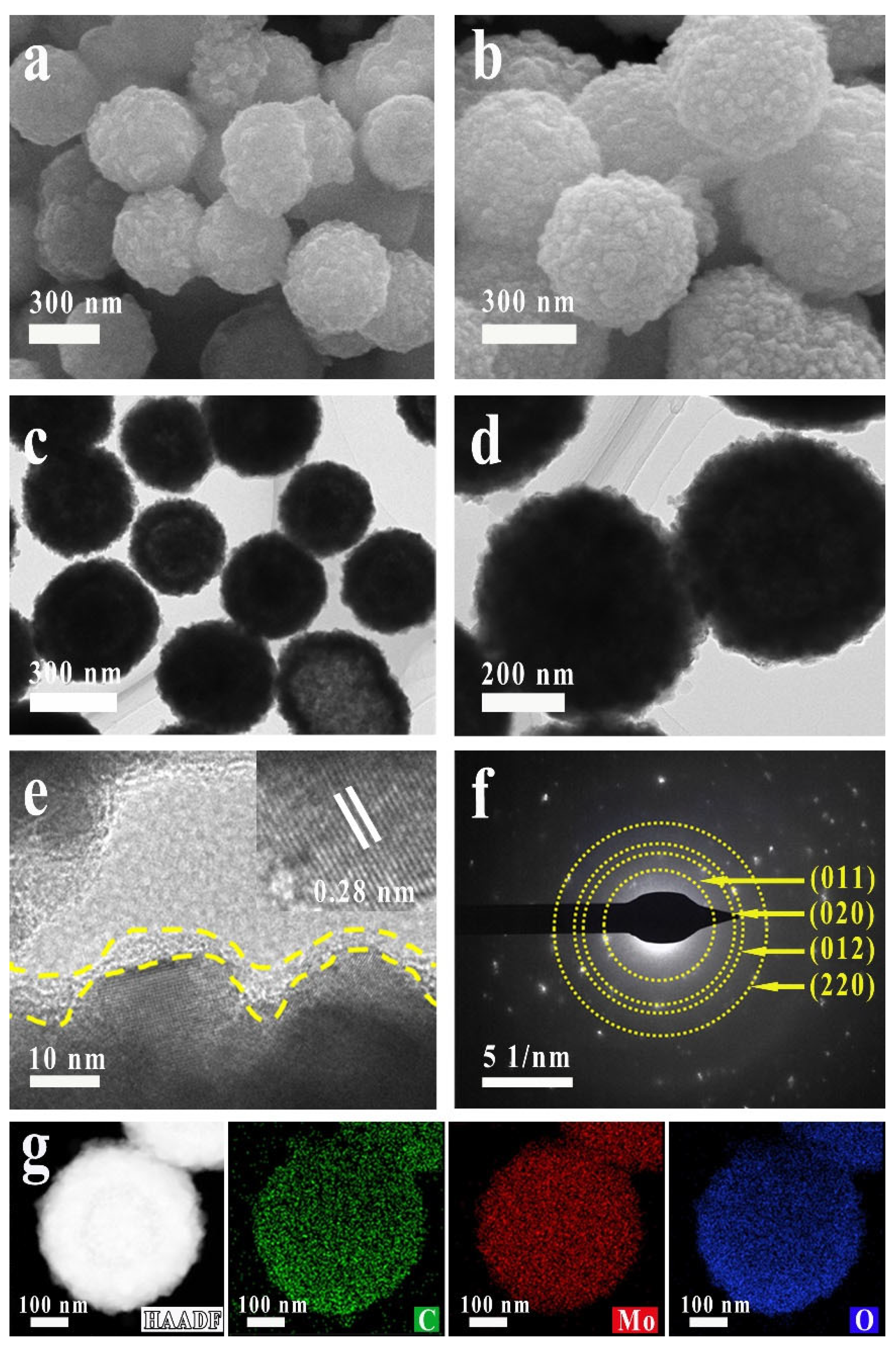
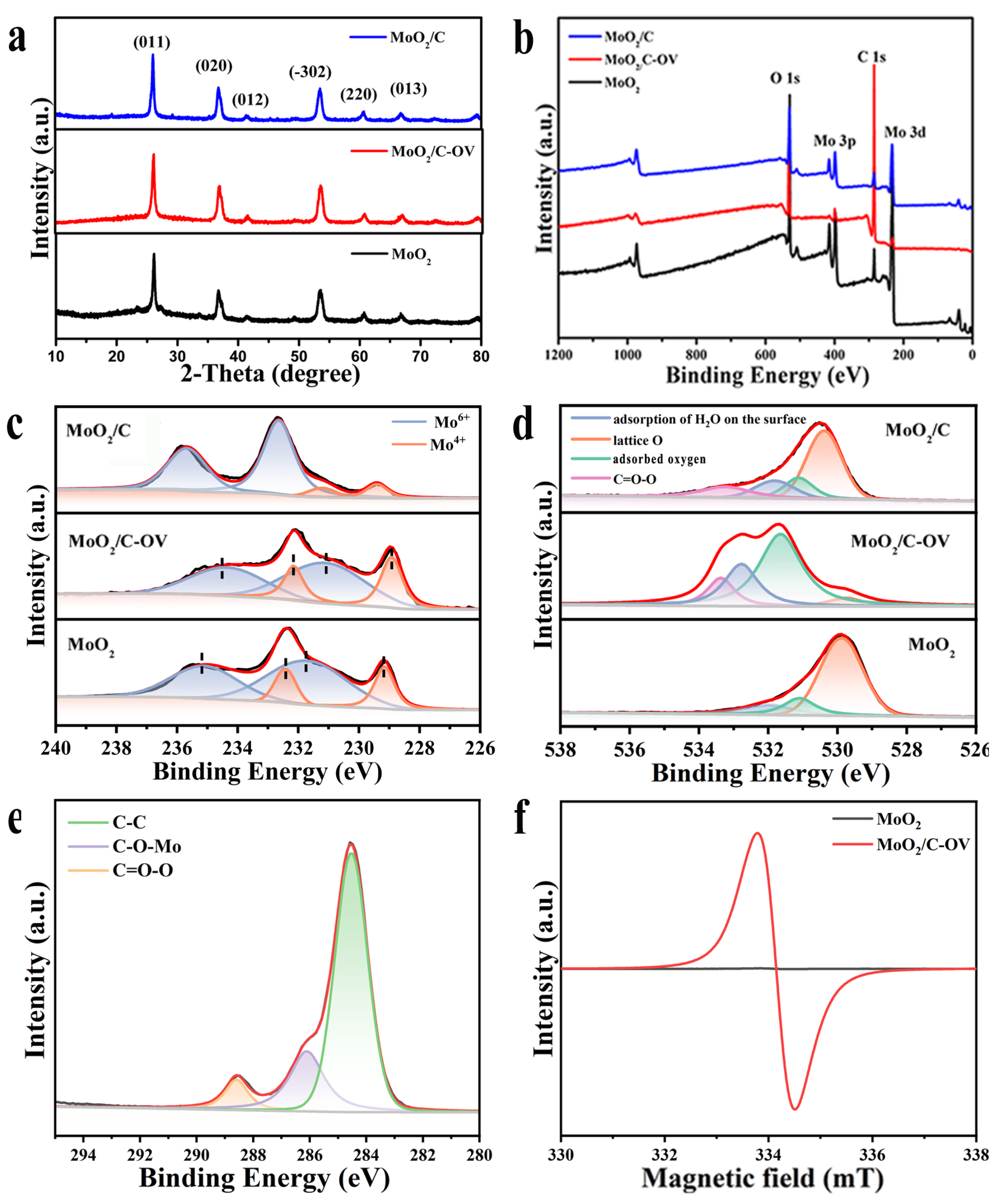
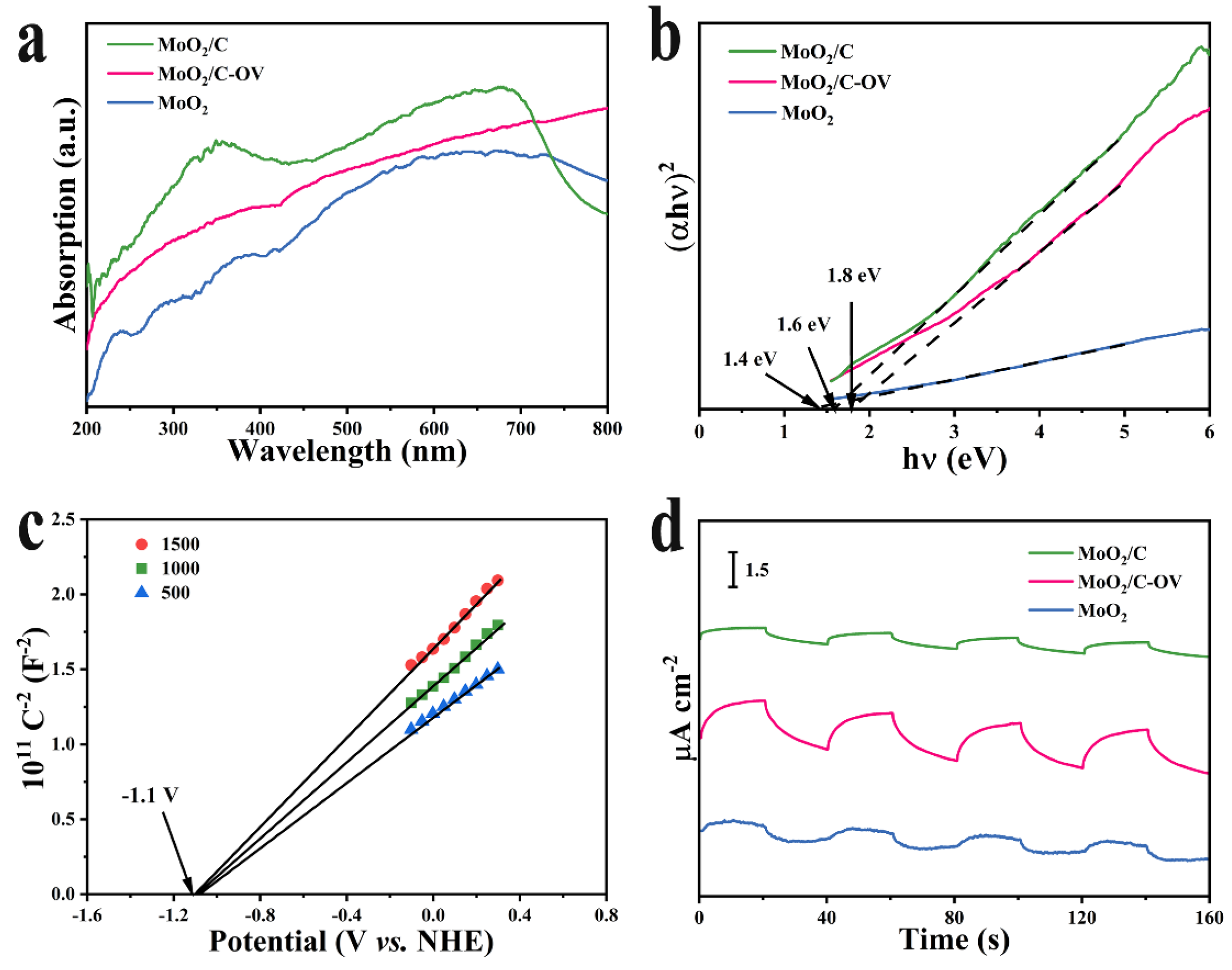
2.1. Synthesis and Characterization of the Samples
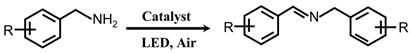
2.2. Photocatalytic Activity for Oxidation of Amines to Imines
2.3. Proposed Mechanism of Photocatalytic BA Coupling to N-benzylidenebenzylamine
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of MoO2 Nanospheres
3.3. Preparation of Defective MoO2/C Nanospheres
3.4. Electrochemistry Measurements
3.5. Photocatalytic Oxidative Coupling of Benzylamine
3.6. Detection of H2O2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Niu, Q.; Huang, Q.; Yu, T.Y.; Liu, J.; Shi, J.W.; Dong, L.Z.; Li, S.L.; Lan, Y.Q. Achieving high photo/thermocatalytic product selectivity and conversion via thorium clusters with switchable functional ligands. J. Am. Chem. Soc. 2022, 144, 18586–18594. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lang, Z.; Zhao, Y.; Zhao, X.; Qiu, T.; Hong, Q.; Wei, K.; Tan, H.; Kang, Z.; Li, Y. Cu-bridged tetrakis (4-ethynylphenyl) ethene aggregates with photo-regulated 1O2 and O2∙generation for selective photocatalytic aerobic oxidation. Angew. Chem. Int. Ed. 2022, 61, e202202914. [Google Scholar]
- Gao, K.; Li, H.; Meng, Q.; Wu, J.; Hou, H. Efficient and selective visible-light-driven oxidative coupling of amines to imines in air over CdS@Zr-MOFs. ACS Appl. Mater. Interfaces 2021, 13, 2779–2787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jia, J.; Zhi, Y.; Ma, S.; Liu, X. Porous organic polymers for light-driven organic transformations. Chem. Soc. Rev. 2022, 51, 2444–2490. [Google Scholar] [CrossRef]
- Sun, Z.X.; Sun, K.; Gao, M.L.; Metin, O.; Jiang, H.L. Optimizing Pt electronic states through formation of a schottky junction on non-reducible metal-organic frameworks for enhanced photocatalysis. Angew. Chem. Int. Ed. 2022, 61, e202206108. [Google Scholar]
- Liu, X.; Dai, D.; Cui, Z.; Zhang, Q.; Gong, X.; Wang, Z.; Liu, Y.; Zheng, Z.; Cheng, H.; Dai, Y.; et al. Optimizing the reaction pathway by active site regulation in the CdS/Fe2O3 Z-scheme heterojunction system for highly selective photocatalytic benzylamine oxidation integrated with H2 production. ACS Catal. 2022, 12, 12386–12397. [Google Scholar] [CrossRef]
- Martín-García, I.; Díaz-Reyes, G.; Sloan, G.; Moglie, Y.; Alonso, F. Sulfur-stabilised copper nanoparticles for the aerobic oxidation of amines to imines under ambient conditions. J. Mater. Chem. A 2021, 9, 11312–11322. [Google Scholar] [CrossRef]
- Chen, H.; Peng, L.; Bian, Y.; Shen, X.; Li, J.; Yao, H.C.; Zang, S.Q.; Li, Z. Exerting charge transfer to stabilize Au nanoclusters for enhanced photocatalytic performance toward selective oxidation of amines. Appl. Catal. B Environ. 2021, 284, 119704. [Google Scholar] [CrossRef]
- Ye, X.; Li, Y.; Luo, P.; He, B.; Cao, X.; Lu, T. Iron sites on defective BiOBr nanosheets: Tailoring the molecular oxygen activation for enhanced photocatalytic organic synthesis. Nano Res. 2021, 15, 1509–1516. [Google Scholar] [CrossRef]
- Chandra, M.; Guharoy, U.; Pradhan, D. Boosting the photocatalytic H2 evolution and benzylamine oxidation using 2D/1D g-C3N4/TiO2 Nanoheterojunction. ACS Appl. Mater. Interfaces 2022, 14, 22122–22137. [Google Scholar] [CrossRef]
- Zou, Y.; Xiao, K.; Qin, Q.; Shi, J.W.; Heil, T.; Markushyna, Y.; Jiang, L.; Antonietti, M.; Savateev, A. Enhanced organic photocatalysis in confined flow through a carbon nitride nanotube membrane with conversions in the millisecond regime. ACS Nano 2021, 15, 6551–6561. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Zhang, L.; Chen, Q.; Fan, H.; Zheng, J.; Fang, Y.; Duan, R.; Cao, X.; Hu, X. Self-assembly approach toward polymeric carbon nitrides with regulated heptazine structure and surface groups for improving the photocatalytic performance. Chem. Eng. J. 2021, 409, 127370. [Google Scholar] [CrossRef]
- Chu, C.; Qin, Y.; Ni, C.; Zou, J. Halogenated benzothiadiazole-based conjugated polymers as efficient photocatalysts for dye degradation and oxidative coupling of benzylamines. Chin. Chem. Lett. 2022, 33, 2736–2740. [Google Scholar] [CrossRef]
- Li, M.; Ma, L.; Luo, L.; Liu, Y.; Xu, M.; Zhou, H.; Wang, Y.; Li, Z.; Kong, X.; Duan, H. Efficient photocatalytic epoxidation of styrene over a quantum-sized SnO2 on carbon nitride as a heterostructured catalyst. Appl. Catal. B-Environ. 2022, 309, 121268. [Google Scholar] [CrossRef]
- He, B.; Wang, Z.; Xiao, P.; Chen, T.; Yu, J.; Zhang, L. Cooperative coupling of H2O2 production and organic synthesis over a floatable polystyrene-sphere-supported TiO2/Bi2O3 S-scheme photocatalyst. Adv. Mater. 2022, 34, 2203225. [Google Scholar] [CrossRef]
- Li, B.; Lai, C.; Lin, H.; Liu, S.; Qin, L.; Zhang, M.; Zhou, M.; Li, L.; Yi, H.; Chen, L. The promising NIR light-driven MO3-x (M=Mo, W) photocatalysts for energy conversion and environmental remediation. Chem. Eng. J. 2022, 431, 134044. [Google Scholar] [CrossRef]
- Liu, X.; Yang, L.; Huang, M.; Li, Q.; Zhao, L.; Sang, Y.; Zhang, X.; Zhao, Z.; Liu, H.; Zhou, W. Oxygen vacancy-regulated metallic semiconductor MoO2 nanobelt photoelectron and hot electron self-coupling for photocatalytic CO2 reduction in pure water. Appl. Catal. B-Environ. 2022, 319, 121887. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W.; Li, J.; Xiang, Q.; Liu, Z.; Liu, B. Identification of the active sites on metallic MoO2-x nano-sea-urchin for atmospheric CO2 photoreduction under UV, visible, and near-infrared light illumination. Angew. Chem. Int. Ed. 2022, 62, e202213124. [Google Scholar]
- Blackburn, T.J.; Tyler, S.M.; Pemberton, J.E. Optical spectroscopy of surfaces, interfaces, and thin films. Anal. Chem. 2022, 94, 515–558. [Google Scholar] [CrossRef]
- Song, X.; Yin, M.; Li, J.; Li, Y.; Yang, H.; Kong, Q.; Bai, H.; Xi, G.; Mao, L. Moving MoO2/C nanospheres with the functions of enrichment and sensing for online-high-throughput SERS detection. Anal. Chem. 2022, 94, 7029–7034. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, S.; Wei, C.; Fan, J.; Yao, H.; Dai, L.; Wang, G.; Li, H.; Su, B.; Guo, X. Synergistic effect between platinum single atoms and oxygen vacancy in MoO2 boosting pH-universal hydrogen evolution reaction at large current density. Chem. Eng. J. 2022, 427, 131309. [Google Scholar] [CrossRef]
- Zha, R.; Shi, T.; He, L.; Zhang, M. Synergetic excitonic and defective effects in confined SnO2/α-Fe2O3 nanoheterojunctions for efficient photocatalytic molecular oxygen activation. Chem. Eng. J. 2021, 421, 129883. [Google Scholar] [CrossRef]
- Li, M.; Qiu, J.; Xu, J.; Zhu, Y.; Yao, J. Self-induced oxygen vacancies on carboxyl-rich MIL-121 enable efficient activation and oxidation of benzyl alcohol under visible light. ACS Appl. Mater. Interfaces 2022, 14, 11509–11516. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shi, Y.; Li, H.; Mao, C.; Wang, X.; Liu, X.; Liu, X.; Zhang, L. Oxygen and chlorine dual vacancies enable photocatalytic O2 dissociation into monatomic reactive oxygen on BiOCl for refractory aromatic pollutant removal. Environ. Sci. Technol. 2022, 56, 3587–3595. [Google Scholar] [CrossRef]
- Hao, L.; Huang, H.; Zhang, Y.; Ma, T. Oxygen vacant semiconductor photocatalysts. Adv. Funct. Mater. 2021, 31, 2100919. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Z.; Fang, R.; Yan, Y.; Ran, J.; Zhang, L. A state-of-the-art review on action mechanism of photothermal catalytic reduction of CO2 in full solar spectrum. Chem. Eng. J. 2022, 429, 132322. [Google Scholar] [CrossRef]
- Han, X.; Zhang, T.; Cui, Y.; Wang, Z.; Dong, R.; Wu, Y.; Du, C.; Chen, R.; Yu, C.; Feng, J.; et al. A bifunctional BiOBr/ZIF-8/ZnO photocatalyst with rich oxygen vacancy for enhanced wastewater treatment and H2O2 generation. Molecules 2023, 28, 2422. [Google Scholar] [CrossRef]
- Ge, H.; Kuwahara, Y.; Kusu, K.; Yamashita, H. Plasmon-induced catalytic CO2 hydrogenation by a nano-sheet Pt/HxMoO3-y hybrid with abundant surface oxygen vacancies. J. Mater. Chem. A 2021, 9, 13898–13907. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, L.; Wang, Y.; Yue, Z.; Li, Y.; Ma, J.; Xiao, H.; Chen, W.; Zhao, M.; Zheng, Z.; et al. Defect engineering in Pd/NiCo2O4−x for selective hydrogenation of α,β-unsaturated carbonyl compounds under ambient conditions. ACS Sustain. Chem. Eng. 2020, 8, 7851–7859. [Google Scholar] [CrossRef]
- Duan, W.; Han, S.; Fang, Z.; Xiao, Z.; Lin, S. In situ filling of the oxygen vacancies with dual heteroatoms in Co3O4 for efficient overall water splitting. Molecules 2023, 28, 4134. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Huang, C.; Dong, G.; Chen, F.; Zhao, F.; Yu, Y.; Liu, X.; Li, Z.; Wang, Y. Plasmonic gold nanocrystals simulated efficient photocatalytic nitrogen fixation over Mo doped W18O49 nanowires. J. Mater. Chem. A 2021, 9, 14459–14465. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R.; Shi, M.; Zhou, P.; Han, K.; Li, C.; Li, R. Photo-induced carbon dioxide reduction on hexagonal tungsten oxide via an oxygen vacancies-involved process. Chin. Chem. Lett. 2023, 34, 107200. [Google Scholar] [CrossRef]
- Rej, S.; Hejazi, S.M.H.; Badura, Z.; Zoppellaro, G.; Kalytchuk, S.; Kment, Š.; Fornasiero, P.; Naldoni, A. Light-induced defect formation and Pt single atoms synergistically boost photocatalytic H2 production in 2D TiO2-bronze nanosheets. ACS Sustain. Chem. Eng. 2022, 10, 17286–17296. [Google Scholar] [CrossRef]
- Wei, S.; Zhong, H.; Wang, H.; Song, Y.; Jia, C.; Anpo, M.; Wu, L. Oxygen vacancy enhanced visible light photocatalytic selective oxidation of benzylamine over ultrathin Pd/BiOCl nanosheets. Appl. Catal. B-Environ. 2022, 305, 121032. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, H.; Wang, C.; Guan, R.; Duan, R.; Fang, Y.; Hu, X. Activation of molecular oxygen in selectively photocatalytic organic conversion upon defective TiO2 nanosheets with boosted separation of charge carriers. Appl. Catal. B-Environ. 2020, 262, 118258. [Google Scholar] [CrossRef]
- Chen, S.; Huang, D.; Cheng, M.; Lei, L.; Chen, Y.; Zhou, C.; Deng, R.; Li, B. Surface and interface engineering of two-dimensional bismuth-based photocatalysts for ambient molecule activation. J. Mater. Chem. A 2021, 9, 196–233. [Google Scholar] [CrossRef]
- Bui, H.T.; Weon, S.; Bae, J.W.; Kim, E.J.; Kim, B.; Ahn, Y.Y.; Kim, K.; Lee, H.; Kim, W. Oxygen vacancy engineering of cerium oxide for the selective photocatalytic oxidation of aromatic pollutants. J. Hazard. Mater. 2021, 404, 123976. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, M.; Ma, W.; Li, D.; Xu, Y. Sugar-disguised bullets for combating multidrug-resistant bacteria infections based on an oxygen vacancy-engineered glucose-functionalized MoO3-x photo-coordinated bienzyme. Chem. Eng. J. 2022, 431, 133943. [Google Scholar] [CrossRef]
- Du, Y.; He, Z.; Ma, F.; Jiang, Y.; Wan, J.; Wu, G.; Liu, Y. Anionic biopolymer assisted preparation of MoO2@C heterostructure nanoparticles with oxygen vacancies for ambient electrocatalytic ammonia synthesis. Inorg. Chem. 2021, 60, 4116–4123. [Google Scholar] [CrossRef]
- Wang, X.; Kim, S.Y.; Wallace, R.M. Interface chemistry and band alignment study of Ni and Ag contacts on MoS2. ACS Appl. Mater. Interfaces 2021, 13, 15802–15810. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.; Azcatl, A.; Addou, R.; Cheng, G.; Battaglia, C.; Chuang, S.; Cho, K.; Javey, A.; Wallace, R.M. Hole contacts on transition metal dichalcogenides: Interface chemistry and band alignments. ACS Nano 2014, 8, 6265–6272. [Google Scholar] [CrossRef]
- Wang, X.; Cormier, C.R.; Khosravi, A.; Smyth, C.M.; Shallenberger, J.R.; Addou, R.; Wallace, R.M. In situ exfoliated 2D molybdenum disulfide analyzed by XPS. Surf. Sci. Spectra 2020, 27, 014019. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, F.; Zhao, M.; Xu, J.; Zhou, J.; Wang, Y. Ultra-small yellow defective TiO2 nanoparticles for co-catalyst free photocatalytic hydrogen production. Nano Energy 2016, 24, 63–71. [Google Scholar] [CrossRef]
- Wei, W.; Tian, Q.; Sun, H.; Liu, P.; Zheng, Y.; Fan, M.; Zhuang, J. Efficient visible-light-driven photocatalytic H2 evolution over MoO2-C/CdS ternary heterojunction with unique interfacial microstructures. Appl. Catal. B-Environ. 2020, 260, 118153. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Lan, X.; Shi, Y.E.; Wang, Z. Modulating emission of nonconventional luminophores from nonemissive to fluorescence and room-temperature phosphorescence via dehydration-induced through-space conjugation. J. Phys. Chem. Lett. 2021, 12, 1413–1420. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, Y.E.; Liu, Y.; Xing, Y.; Yi, D.; Wang, Z.; Yan, D. Highly efficient and stable deep-blue room temperature phosphorescence via through-space conjugation. Chem. Eng. J. 2022, 442, 136179. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, X.; Liu, X.; Xu, L.; Liu, B.; Zhang, J.; Xu, H.; Han, Z.; Li, G. In-situ exfoliation and assembly of 2D/2D g-C3N4/TiO2(B) hierarchical microflower: Enhanced photo-oxidation of benzyl alcohol under visible light. Carbon 2022, 196, 401–409. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Yang, D.; Liu, J.; He, L.; Tang, M.; Feng, W.; Wu, X. Fabrication, characterization and high photocatalytic activity of Ag-ZnO heterojunctions under UV-visible light. RSC Adv. 2021, 11, 27257–27266. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhou, Q.; Xia, Y.; Wang, J.; Chen, H.; Xu, Q.; Liu, J.; Feng, W.; Chen, S. Preparation and characterization of Cu-doped TiO2 nanomaterials with anatase/rutile/brookite triphasic structure and their photocatalytic activity. J. Mater. Sci. Mater. Electron. 2021, 32, 21511–21524. [Google Scholar] [CrossRef]


| Entry | Substrate | Product | Conv. (%) | Sel. (%) |
|---|---|---|---|---|
| 1 | 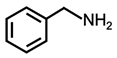 | 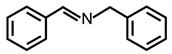 | 95 | >99 |
| 2 | 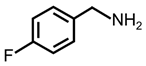 | 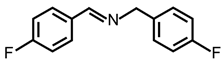 | 89 | >99 |
| 3 | 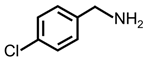 | 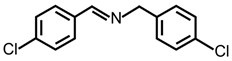 | 83 | 98 |
| 4 | 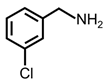 |  | 77 | >99 |
| 5 | 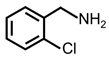 | 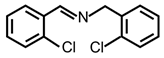 | 73 | >99 |
| 6 | 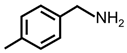 |  | 95 | >99 |
| 7 | 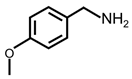 | 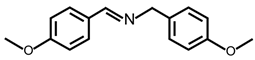 | 99 | >99 |
| 8 |  | 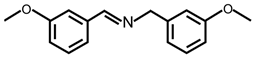 | 99 | >99 |
| 9 | 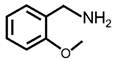 | 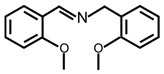 | 96 | 97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Zhang, Y.; Hu, T.; Chen, W.; Tang, T.; Luo, E.; Jia, J. Carbonaceous Material Modified MoO2 Nanospheres with Oxygen Vacancies for Enhanced Visible-Light Photocatalytic Oxidative Coupling of Benzylamine. Molecules 2023, 28, 4739. https://doi.org/10.3390/molecules28124739
Chang Y, Zhang Y, Hu T, Chen W, Tang T, Luo E, Jia J. Carbonaceous Material Modified MoO2 Nanospheres with Oxygen Vacancies for Enhanced Visible-Light Photocatalytic Oxidative Coupling of Benzylamine. Molecules. 2023; 28(12):4739. https://doi.org/10.3390/molecules28124739
Chicago/Turabian StyleChang, Yuhong, Yanxia Zhang, Tianjun Hu, Wenwen Chen, Tao Tang, Ergui Luo, and Jianfeng Jia. 2023. "Carbonaceous Material Modified MoO2 Nanospheres with Oxygen Vacancies for Enhanced Visible-Light Photocatalytic Oxidative Coupling of Benzylamine" Molecules 28, no. 12: 4739. https://doi.org/10.3390/molecules28124739
APA StyleChang, Y., Zhang, Y., Hu, T., Chen, W., Tang, T., Luo, E., & Jia, J. (2023). Carbonaceous Material Modified MoO2 Nanospheres with Oxygen Vacancies for Enhanced Visible-Light Photocatalytic Oxidative Coupling of Benzylamine. Molecules, 28(12), 4739. https://doi.org/10.3390/molecules28124739






