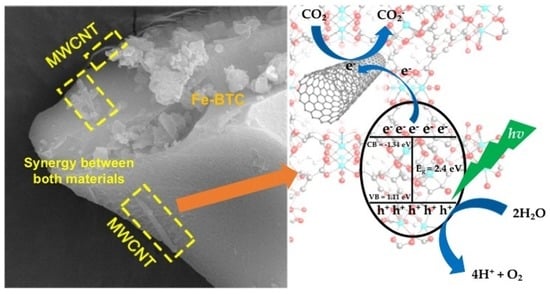
CNTs/Fe-BTC Composite Materials for the CO2-Photocatalytic Reduction to Clean Fuels: Batch and Continuous System
Abstract
1. Introduction
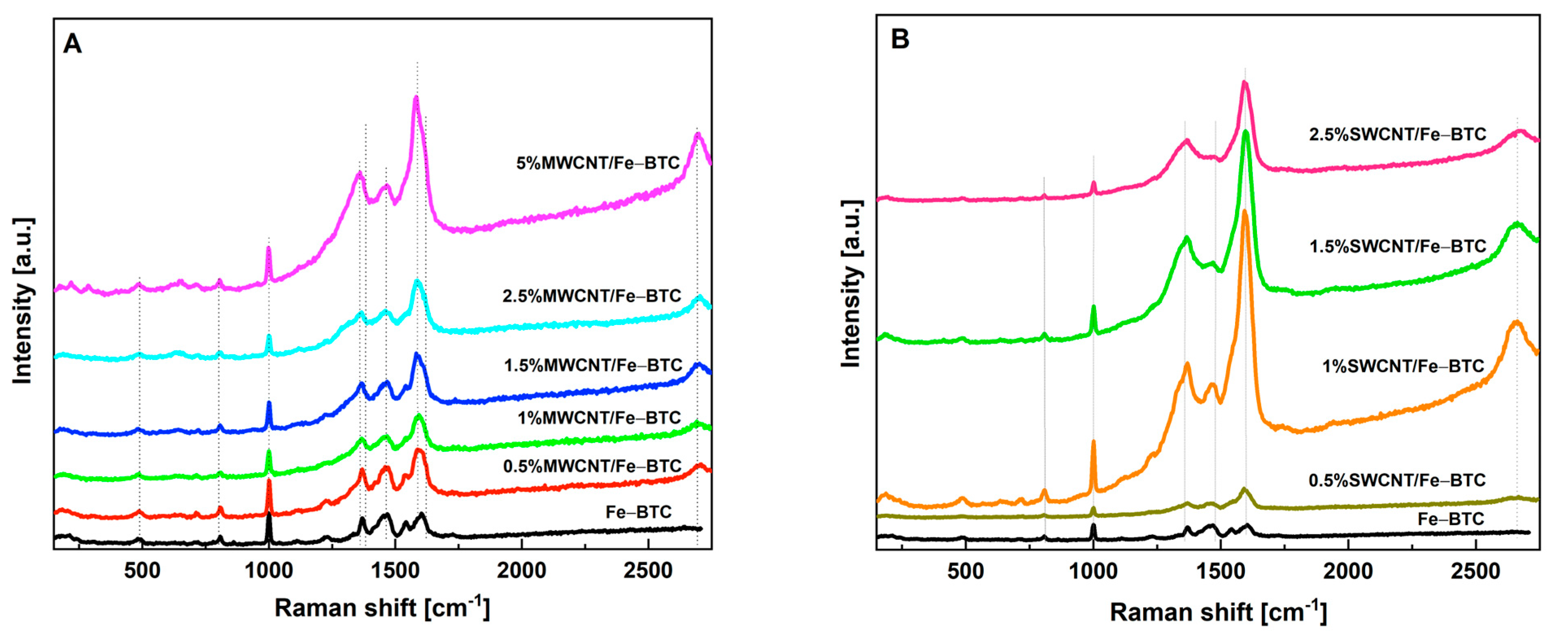
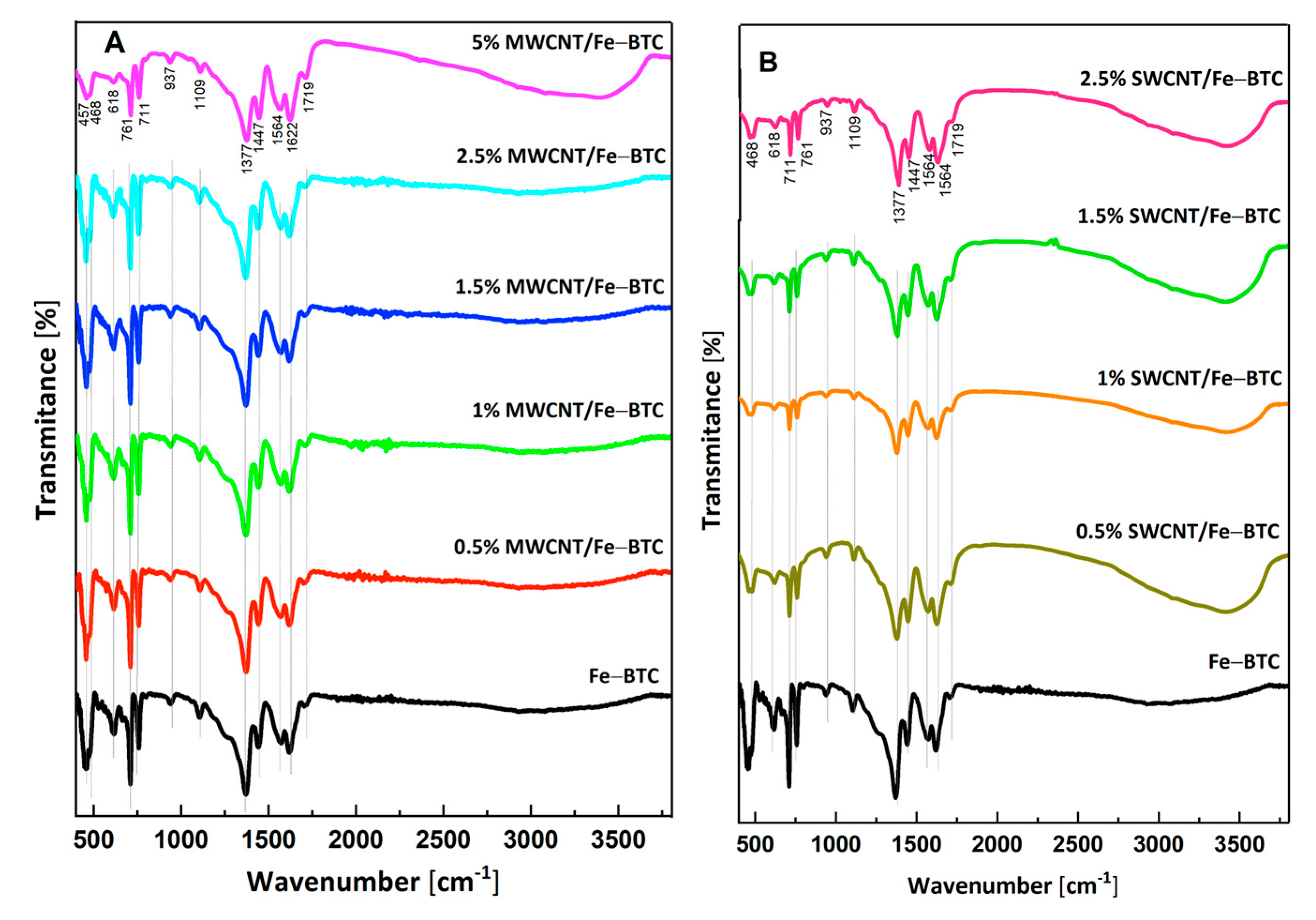
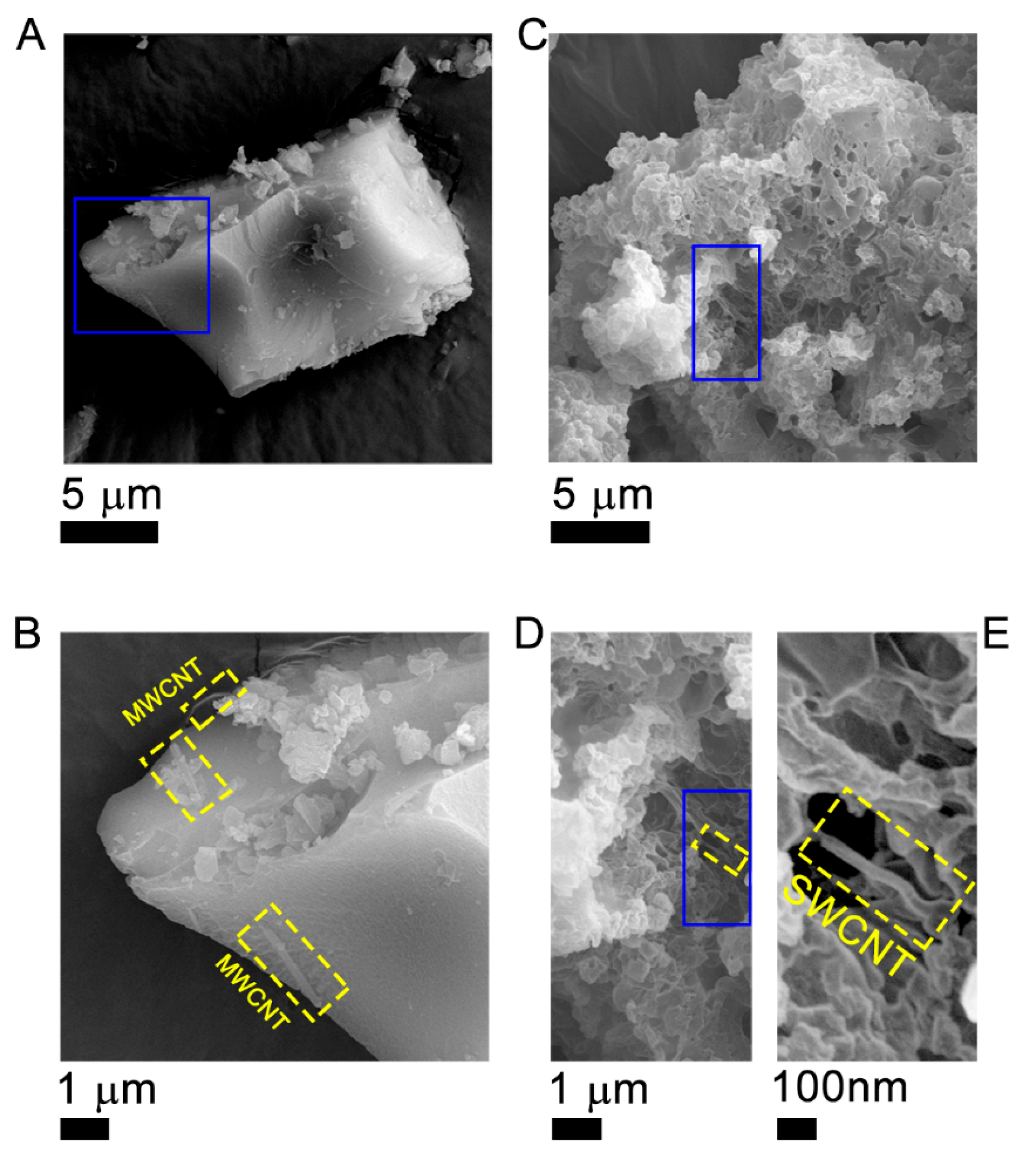
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Functionalization of the CNTs
3.3. Synthesis of Composite Materials
3.4. Characterization of Materials
3.5. Photocatalytic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tkemaladze, G.S.; Makhashvili, K.A. Climate changes and photosynthesis. Ann. Agr. Sci. 2016, 14, 119–126. [Google Scholar] [CrossRef]
- Tkemaladze, G.S. BioChemical Fundamentals of Protecting the World from Global Warming. In Proceedings of the International Scientific Conference Global Warming and Agrobiodiversity, Tbilisi, Georgia, 15–17 September 2011; pp. 32–41. (In Georgian). [Google Scholar]
- Randel, W.J.; Shine, K.P.; Austin, J.; Barnett, J.; Claud, C.; Gillett, N.P.; Keckhut, P.; Langematz, U.; Lin, R.; Long, C.; et al. An update of observed stratospheric temperature trends. J. Geophys. Res. 2009, 114. [Google Scholar] [CrossRef]
- Kim, S.; Shi, H.; Lee, J.Y. CO2 absorption mechanism in amine solvents and enhancement of CO2 capture capability in blended amine solvent. Int. J. Greenh. Gas Control 2016, 45, 181–188. [Google Scholar] [CrossRef]
- Bernhardsen, I.M.; Knuutila, H.K. A review of potential amine solvents for CO2 absorption process: Absorption capacity, cyclic capacity and pKa. Int. J. Greenh. Gas Control 2017, 61, 27–48. [Google Scholar] [CrossRef]
- Saidi, M.; Ho, P.H.; Yadav, P.; Salles, F.; Charnay, C.; Girard, L.; Boukli-Hacene, L.; Trens, P. Zirconium-Based Metal Organic Frameworks for the Capture of Carbon Dioxide and Ethanol Vapour. A Comparative Study. Molecules 2021, 26, 7620. [Google Scholar] [CrossRef]
- Wan, Y.; Miao, Y.; Qiu, T.; Kong, D.; Wu, Y.; Zhang, Q.; Shi, J.; Zhong, R.; Zou, R. Tailoring Amine-Functionalized Ti-MOFs via a Mixed Ligands Strategy for High-Efficiency CO2 Capture. Nanomaterials 2021, 11, 3348. [Google Scholar] [CrossRef] [PubMed]
- Renata, A.M.; Benoît, L.; Wanlin, G.; Qiang, W. CO2 adsorption mechanisms on MOFs: A case study of open metal sites, ultra-microporosity and flexible framework. React. Chem. Eng. 2021, 6, 1118–1133. [Google Scholar] [CrossRef]
- Lancheros, A.; Goswami, S.; Mian, M.R.; Zhang, X.; Zarate, X.; Schott, E.; Farha, O.K.; Hupp, J.T. Modulation of CO2 adsorption in novel pillar-layered MOFs based on carboxylate–pyrazole flexible linker. Dalton Trans. 2021, 50, 2880–2890. [Google Scholar] [CrossRef]
- Haopeng, J.; Mengyang, X.; Xiaoxue, Z.; Huiqin, W.; Pengwei, H. Fabricated local surface plasmon resonance Cu2O/Ni-MOF hierarchical heterostructure photocatalysts for enhanced photoreduction of CO2. J. Environ. Chem. Eng. 2023, 11, 109504. [Google Scholar] [CrossRef]
- Zhiliang, W.; Wei, L.; Linlin, H.; Qiuming, W.; Huixing, Y.; Yangyang, J.; Dingyuan, T. A novel Sunflower-like MOF@COF for improved photocatalytic CO2 reduction. Sep. Purif. Technol. 2023, 311, 123322. [Google Scholar] [CrossRef]
- Jie, L.; Shuang, W.; Yi, Z.; Kai, G.; Jiabo, W.; He, C.; Yang, Y.; Yongfang, Y. Photocatalytic reduction of CO2 by two-dimensional Zn-MOF-NH2/Cu heterojunctions. Catal. Commun. 2023, 175, 106613. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konish, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Fujita, S.; Arai, M.; Bhalchandra, M.B. Direct Transformation of Carbon Dioxide to Value-Added Products over Heterogeneous Catalysts. In Transformation and Utilization of Carbon Dioxide. Green Chemistry and Sustainable Technology; Bhalchandra, M.B., Arai, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 39–53. [Google Scholar] [CrossRef]
- Narayanan, H.; Viswanathan, B.; Suguna, Y. Photocatalytic Reduction of Carbon Dioxide: Issues and Prospects. Curr. Catal. 2016, 5, 79–107. [Google Scholar] [CrossRef]
- Wang, C.C.; Zhang, Y.Q.; Li, J.; Wang, P. Photocatalytic CO2 reduction in metal–organic frameworks: A mini review. J. Mol. Struct. 2015, 1083, 127–136. [Google Scholar] [CrossRef]
- Evans, J.R. Improving Photosynthesis. Plant Physiol. 2013, 162, 1780–1793. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Jiang, K.; Qiu, X.; Yu, J.; Liu, M. Product selectivity of photocatalytic CO2 reduction reactions. Mater. Today 2020, 32, 222–243. [Google Scholar] [CrossRef]
- Liu, Q.; Low, Z.X.; Li, L.; Razmjou, A.; Wang, K.; Yao, J.; Wang, H. ZIF-8/Zn2GeO4nanorods with an enhanced CO2 adsorption property in an aqueous medium for photocatalytic synthesis of liquid fuel. J. Mater. Chem. A 2013, 1, 11563–11569. [Google Scholar] [CrossRef]
- Yang, X.; Wen, Z.; Wu, Z.; Luo, X. Synthesis of ZnO/ZIF-8 hybrid photocatalysts derived from ZIF-8 with enhanced photocatalytic activity. Inorg. Chem. 2018, 5, 687–693. [Google Scholar] [CrossRef]
- Garay-Rodríguez, L.F.; Torres-Martínez, L.M. Extending the visible-light photocatalytic CO2 reduction activity of K2Ti6O13 with the MxOy (M = Co, Ni and Cu) incorporation. J. Mater. Sci. Mater. Electron. 2020, 31, 19248–19265. [Google Scholar] [CrossRef]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Ting, W.; Xiaojuan, L.; Yang, L.; Min, C.; Zhifeng, L.; Guangming, Z.; Binbin, S.; Qinghua, L.; Wei, Z.; Qingyun, H.; et al. Application of QD-MOF composites for photocatalysis: Energy production and environmental remediation, Coordin. Chem. Rev. 2020, 403, 213097. [Google Scholar] [CrossRef]
- Rasheed, T.; Hassan, A.A.; Bilal, M.; Hussain, T.; Rizwan, K. Metal-organic frameworks based adsorbents: A review from removal perspective of various environmental contaminants from wastewater. Chemosphere 2020, 259, 127369. [Google Scholar] [CrossRef]
- Ali, N.; Bilal, M.; Khan, A.; Ali, F.; Yang, Y.; Malik, S.; Ud Din, S.; Iqbal, H.M.N. Deployment of metal-organic frameworks as robust materials for sustainable catalysis and remediation of pollutants in environmental settings. Chemosphere 2021, 272, 129605. [Google Scholar] [CrossRef]
- Daglar, H.; Altintas, C.; Erucar, I.; Heidari, G.; Zare, E.N.; Moradi, O.; Srivastava, V.; Ifte-har, S.; Keskin, S.; Sillanpää, M. Metal-organic framework-based materials for the abatement of air pollution and decontamination of wastewater. Chemosphere 2022, 303, 135082. [Google Scholar] [CrossRef]
- Meng, J.; Chen, Q.; Lu, J.; Liu, H. Z-Scheme Photocatalytic CO2 Reduction on a Heterostructure of Oxygen-Defective ZnO/Reduced Graphene Oxide/UiO-66-NH2 under Visible Light. ACS Appl. Mater. Interfaces 2019, 11, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, N.; Sharifnia, S.; Do, T.-O. Enhanced CO2 photoreduction by a graphene–porphyrin metal–organic framework under visible light irradiation. J. Mater. Chem. A 2018, 6, 18031–18035. [Google Scholar] [CrossRef]
- Gomes Silva, C.; Corma, A.; García, H. Metal–organic frameworks as semiconductors. J. Mater. Chem. 2010, 20, 3141–3156. [Google Scholar] [CrossRef]
- Shen, L.; Liang, R.; Wu, L. Strategies for engineering metal-organic frameworks as efficient photocatalysts. Chin. J. Catal. 2015, 36, 2071–2088. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, Y.; Wu, G.; Mu, M.; Yin, X. 2D Cu-FeTCPP MOF assembled on ZnTi-LDH to construct 2D/2D direct Z-scheme heterojunction for enhanced photocatalytic CO2 reduction. Sol. Energy 2023, 253, 480–490. [Google Scholar] [CrossRef]
- Wu, K.; Liu, C.; Chen, Y.; Jiang, H.; Peng, Q.; Chen, Y.; Fang, D.; Shen, B.; Wu, Q.; Zhan, L.; et al. Constructing asymmetric unsaturated copper coordination in Zinc(II)/Copper(I, II)-based metal-organic framework toward productive CO2-to-methanol photocatalytic conversion from CO2-capturing solution. Appl. Catal. A 2023, 650, 118970. [Google Scholar] [CrossRef]
- Gulati, S.; Vijayan, S.; Mansi, R.; Sanjay, K.; Harikumar, B.; Trivedi, M.; Varma, R.S. Recent advances in the application of metal-organic frameworks (MOFs)-based nanocatalysts for direct conversion of carbon dioxide (CO2) to value-added chemicals. Coord. Chem. Rev. 2023, 474, 214853. [Google Scholar] [CrossRef]
- Sciortino, L.; Alessi, A.; Messina, F.; Buscarino, G.; Gelardi, F.M. Structure of the Fe-BTC Metal–Organic Framework: A Model Based on the Local Environment Study. J. Phys. Chem. C 2015, 119, 7826–7830. [Google Scholar] [CrossRef]
- Rojas García, E.; López Medina, R.; May Lozano, M.; Hernández Pérez, I.; Valero, M.J.; Maubert Franco, A.M. Adsorption of Azo-Dye Orange II from Aqueous Solutions Using a Metal-Organic Framework Material: Iron-Benzenetricarboxylate. Materials 2014, 7, 8037–8057. [Google Scholar] [CrossRef] [PubMed]
- Zukal, A.; Opanasenko, M.; Rubeš, M.; Nachtigall, P.; Jagiello, J. Adsorption of pentane isomers on metal-organic frameworks Cu-BTC and Fe-BTC. Catal. Today 2015, 243, 69–75. [Google Scholar] [CrossRef]
- Milakin, K.A.; Gavrilov, N.; Pašti, I.A.; Morávková, Z.; Acharya, U.; Unterweger, C.; Breitenbach, S.; Zhigunov, A.; Bober, P. Polyaniline-metal organic framework (Fe-BTC) composite for electrochemical applications. Polymer 2020, 208, 122945. [Google Scholar] [CrossRef]
- Martínez, F.; Leo, P.; Orcajo, G.; Díaz-García, M.; Sanchez-Sanchez, M.; Calleja, G. Sustainable Fe-BTC catalyst for efficient removal of mehylene blue by advanced fenton oxidation. Catal. Today 2018, 313, 6–11. [Google Scholar] [CrossRef]
- Nguyen, M.B.; Sy, D.T.; Thoa, V.T.K.; Hong, N.T.; Doan, H.V. Bimetallic Co-Fe-BTC/CN nanocomposite synthesised via a microwave-assisted hydrothermal method for highly efficient Reactive Yellow 145 dye photodegradation. J. Taiwan Inst. Chem. Eng. 2022, 140, 104543. [Google Scholar] [CrossRef]
- Yurduşen, A.; Yürüm, A.; Yürüm, Y. A remarkable increase in the adsorbed H2 amount: Influence of pore size distribution on the H2 adsorption capacity of Fe-BTC. Int. J. Hydrogen Energy 2020, 45, 12394–12407. [Google Scholar] [CrossRef]
- Castañeda-Ramírez, A.A.; Rojas-García, E.; López-Medina, R.; García-Martínez, D.C.; Nicolás- Antúnez, J.; Maubert-Franco, A.M. Magnetite nanoparticles into Fe-BTC MOF as adsorbent material for the remediation of metal (Cu(II), Pb(II, As(III) and Hg(II)) ions-contaminated water. Catal. Today 2022, 394–396, 94–102. [Google Scholar] [CrossRef]
- Castañeda Ramírez, A.A.; Rojas García, E.; López Medina, R.; Larios, J.L.C.; Suárez Parra, R.; Maubert Franco, A.M. Selective Adsorption of Aqueous Diclofenac Sodium, Naproxen Sodium, and Ibuprofen Using a Stable Fe3O4-FeBTC. Materials 2021, 14, 2293. [Google Scholar] [CrossRef]
- Singh, A.K.; Vishwakarma, P.K.; Pandey, S.K.; Pratap, R.; Giri, R.; Srivastava, A. A comparative study of band gap engineered in-situ and ex-situ MWCNTs/TiO2 heterostructures for their enhanced photocatalytic activity under visible light. Inorg. Chem. Commun. 2023, 150, 110540. [Google Scholar] [CrossRef]
- Valadez-Renteria, E.; Perez-Gonzalez, R.; Gomez-Solis, C.; Diaz-Torres, L.A.; Encinas, A.; Oliva, J.; Rodriguez-Gonzalez, V. A novel and stretchable carbon-nanotube/Ni@TiO2:W photocatalytic composite for the complete removal of diclofenac drug from the drinking water. J. Environ. Sci. 2023, 126, 575–589. [Google Scholar] [CrossRef]
- Alelyani, S.S.; Kavil, Y.N.; Al-Farawati, R.K. Superior photocatalytic aptitude of MWCNT/TiO2 for the removal of Cr (VI) from polluted water. Res. Chem. Intermed. 2023, 49, 1819–1842. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Yang, H.; Hsu, F.-K. Zr-Metal Organic Framework and Derivatives for Adsorptive and Photocatalytic Removal of Acid Dyes. Water Environ. Res. 2018, 90, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.; Ibrahim, M.G.; Fujii, M.; Diab, K.E.; ElKady, M.; Alalm, M.G. CNTs/MOF-808 painted plates for extended treatment of pharmaceutical and agrochemical wastewaters in a novel photocatalytic reactor. Chem. Eng. J. 2021, 406, 127152. [Google Scholar] [CrossRef]
- Bellusci, M.; Guglielmi, P.; Masi, A.; Padella, F.; Singh, G.; Yaacoub, N.; Peddis, D.; Secci, D. Magnetic Metal–Organic Framework Composite by Fast and Facile Mechanochemical Process. Inorg. Chem. 2018, 57, 1806–1814. [Google Scholar] [CrossRef]
- Lian, X.; Yan, B. A postsynthetically modified MOF hybrid as a ratiometric flourescent sensor for anion recognition anda detection. Dalton Trans. 2016, 45, 18668–18675. [Google Scholar] [CrossRef]
- Zhou, S.B.; Wang, X.F.; Du, C.C.; Wang, D.Z.; Jia, D. A series of new mixed-ligand complexes based on 3,6-bis(imidazole-1-yl)pyridazine: Syntheses, structures, and catalytic activities. CrystEngComm 2017, 19, 3124–3137. [Google Scholar] [CrossRef]

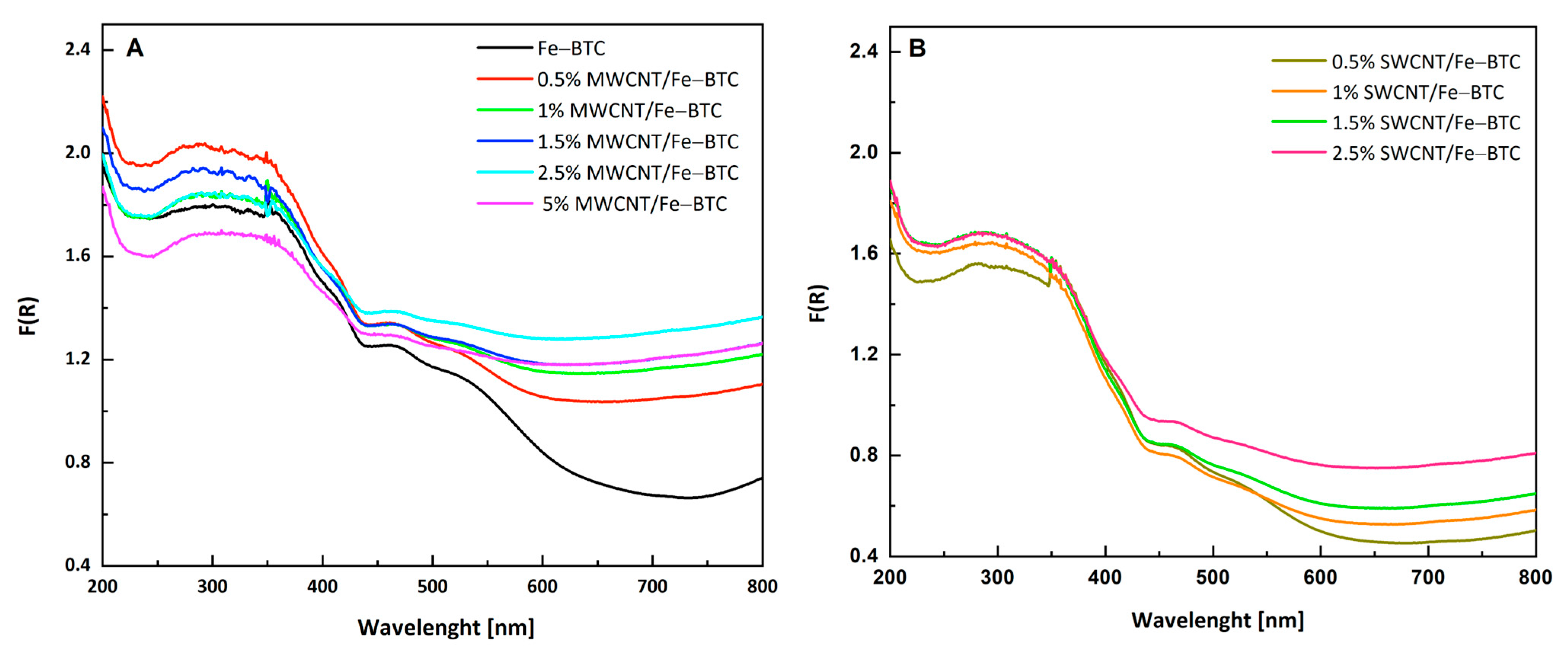
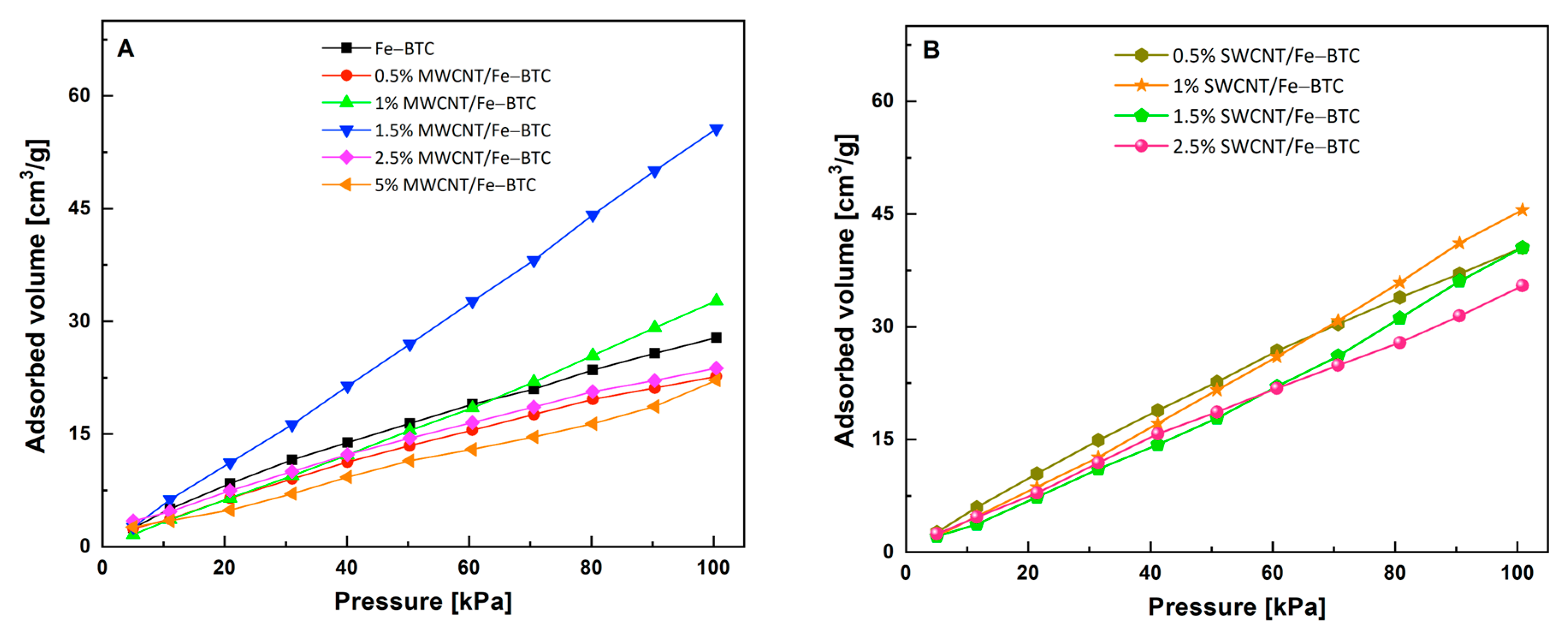
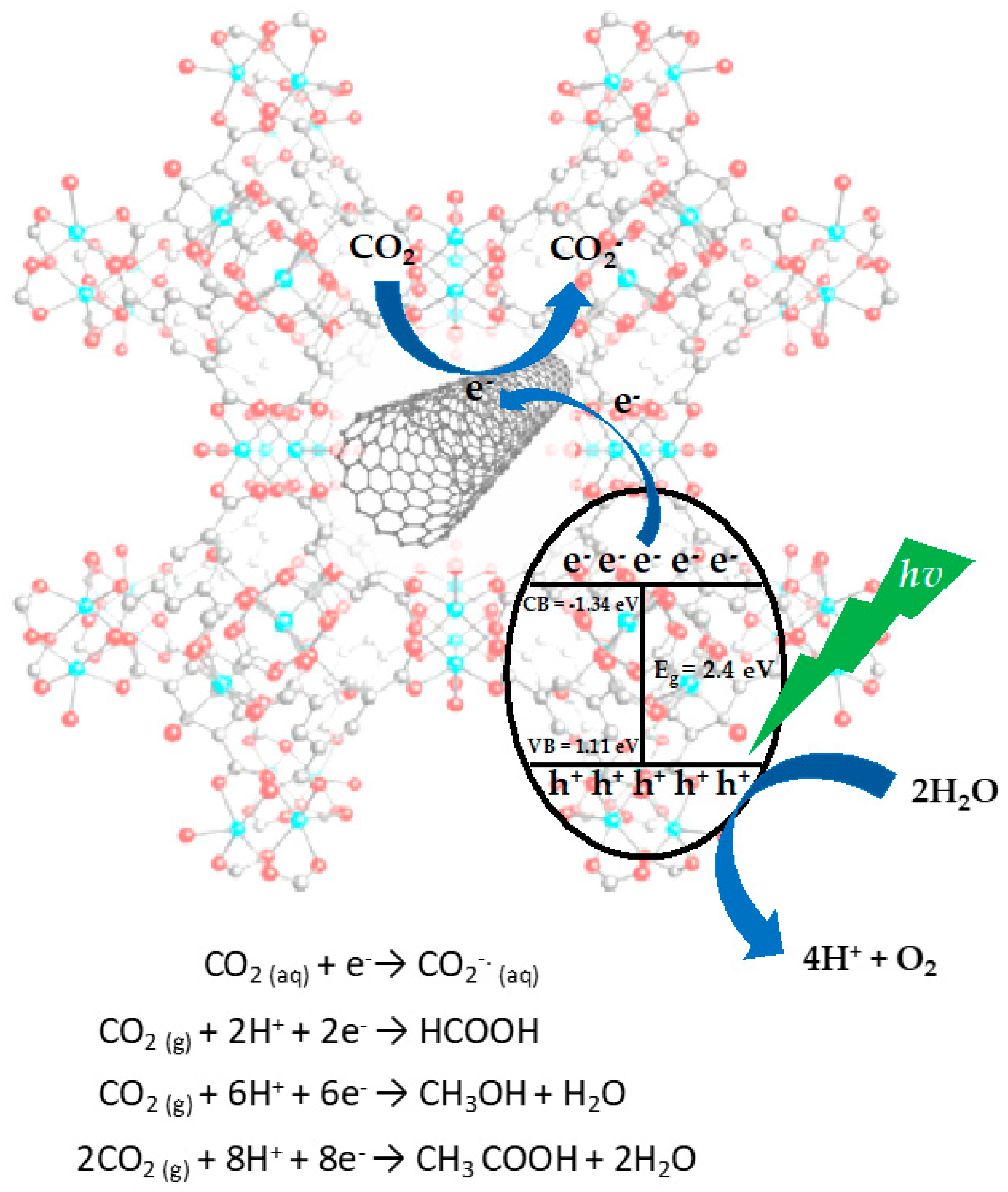

| Samples | Band Gap (eV) | CO2 Adsorption Capacity (cm3/g) |
|---|---|---|
| Fe-BTC | 2.49 | 27.8 |
| 0.5% MWCNT/Fe-BTC | 2.41 | 22.6 |
| 1% MWCNT/Fe-BTC | 2.61 | 32.7 |
| 1.5% MWCNT/Fe-BTC | 2.31 | 55.6 |
| 2.5% MWCNT/Fe-BTC | 2.13 | 23.7 |
| 5% MWCNT/Fe-BTC | 2.10 | 22.1 |
| 0.5% SWCNT/Fe-BTC | 2.41 | 40.5 |
| 1% SWCNT/Fe-BTC | 2.52 | 45.5 |
| 1.5% SWCNT/Fe-BTC | 2.50 | 40.5 |
| 2.5% SWCNT/Fe-BTC | 2.41 | 35.4 |
| Sample | Production Rate (μmol/g*h) | Selectivity (%) | ||||
|---|---|---|---|---|---|---|
| Methanol | Ethanol | Formic Acid | Methanol | Ethanol | Formic Acid | |
| Batch system—visible light | ||||||
| Fe-BTC | 88.5 | 292.9 | 0 | 23.2 | 76.8 | 0.0 |
| 0.5% MWCNT/Fe-BTC | 1566 | 671.2 | 25 | 69.2 | 29.7 | 1.1 |
| 1% MWCNT/Fe-BTC | 273 | 1569.3 | 0 | 14.8 | 85.2 | 0.0 |
| 1.5% MWCNT/Fe-BTC | 1443.3 | 650.8 | 600.5 | 53.6 | 24.2 | 22.3 |
| 2.5% MWCNT/Fe-BTC | 459.4 | 205.8 | 15 | 67.5 | 30.3 | 2.2 |
| 5% MWCNT/Fe-BTC | 883.6 | 282.2 | 0 | 75.8 | 24.2 | 0.0 |
| 0.5% SWCNT/Fe-BTC | 530.8 | 241 | 0 | 68.8 | 31.2 | 0.0 |
| 1% SWCNT/Fe-BTC | 192.8 | 602 | 0 | 24.3 | 75.7 | 0.0 |
| 1.5% SWCNT/Fe-BTC | 2631.8 | 700.8 | 0 | 79.0 | 21.0 | 0.0 |
| 2.5% SWCNT/Fe-BTC | 188.4 | 95.3 | 0 | 66.4 | 33.6 | 0.0 |
| Batch system—UV light | ||||||
| Fe-BTC | 178.3 | 144 | 0 | 55.3 | 44.7 | 0.0 |
| 1% MWCNT/Fe-BTC | 3163 | 1104 | 0 | 74.1 | 25.9 | 0.0 |
| 1.5% MWCNT/Fe-BTC | 163.9 | 132 | 0 | 55.4 | 44.6 | 0.0 |
| 1% SWCNT/Fe-BTC | 425.6 | 126.8 | 136.6 | 61.8 | 18.4 | 19.8 |
| 1.5% SWCNT/Fe-BTC | 194.7 | 144 | 0 | 57.5 | 42.5 | 0.0 |
| Continuous system—visible light | ||||||
| Fe-BTC | 476.9 | 159.1 | 137.4 | 61.7 | 20.6 | 17.8 |
| 1% MWCNT/Fe-BTC | 195.6 | 140.9 | 186.7 | 37.4 | 26.9 | 35.7 |
| 1.5% MWCNT/Fe-BTC | 163.9 | 96.4 | 0 | 63.0 | 37.0 | 0.0 |
| 1% SWCNT/Fe-BTC | 983 | 180.4 | 121.5 | 76.5 | 14.0 | 9.5 |
| 1.5% SWCNT/Fe-BTC | 451 | 205.2 | 76.8 | 61.5 | 28.0 | 10.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas García, E.; Pérez-Soreque, G.; López Medina, R.; Rubio-Marcos, F.; Maubert-Franco, A.M. CNTs/Fe-BTC Composite Materials for the CO2-Photocatalytic Reduction to Clean Fuels: Batch and Continuous System. Molecules 2023, 28, 4738. https://doi.org/10.3390/molecules28124738
Rojas García E, Pérez-Soreque G, López Medina R, Rubio-Marcos F, Maubert-Franco AM. CNTs/Fe-BTC Composite Materials for the CO2-Photocatalytic Reduction to Clean Fuels: Batch and Continuous System. Molecules. 2023; 28(12):4738. https://doi.org/10.3390/molecules28124738
Chicago/Turabian StyleRojas García, Elizabeth, Gloria Pérez-Soreque, Ricardo López Medina, Fernando Rubio-Marcos, and Ana M. Maubert-Franco. 2023. "CNTs/Fe-BTC Composite Materials for the CO2-Photocatalytic Reduction to Clean Fuels: Batch and Continuous System" Molecules 28, no. 12: 4738. https://doi.org/10.3390/molecules28124738
APA StyleRojas García, E., Pérez-Soreque, G., López Medina, R., Rubio-Marcos, F., & Maubert-Franco, A. M. (2023). CNTs/Fe-BTC Composite Materials for the CO2-Photocatalytic Reduction to Clean Fuels: Batch and Continuous System. Molecules, 28(12), 4738. https://doi.org/10.3390/molecules28124738