Ab Initio Calculations on the Ground and Excited Electronic States of Thorium–Ammonia, Thorium–Aza-Crown, and Thorium–Crown Ether Complexes
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ariyarathna, I.R.; Khan, S.N.; Pawłowski, F.; Ortiz, J.V.; Miliordos, E. Aufbau Rules for Solvated Electron Precursors: Be(NH3)40,± Complexes and Beyond. J. Phys. Chem. Lett. 2018, 9, 84–88. [Google Scholar] [CrossRef]
- Ariyarathna, I.R.; Pawłowski, F.; Ortiz, J.V.; Miliordos, E. Molecules mimicking atoms: Monomers and dimers of alkali metal solvated electron precursors. Phys. Chem. Chem. Phys. 2018, 20, 24186–24191. [Google Scholar] [CrossRef]
- Almeida, N.M.S.; Pawłowski, F.; Ortiz, J.V.; Miliordos, E. Transition-metal solvated-electron precursors: Diffuse and 3d electrons in V(NH3)0,±6. Phys. Chem. Chem. Phys. 2019, 21, 7090–7097. [Google Scholar] [CrossRef]
- Ariyarathna, I.R.; Almeida, N.M.S.; Miliordos, E. Stability and Electronic Features of Calcium Hexa-, Hepta-, and Octa-Coordinated Ammonia Complexes: A First-Principles Study. J. Phys. Chem. A 2019, 123, 6744–6750. [Google Scholar] [CrossRef]
- Jordan, Z.; Khan, S.N.; Jackson, B.A.; Miliordos, E. Can boron form coordination complexes with diffuse electrons? Evidence for linked solvated electron precursors. Electron. Struct. 2022, 4, 015001. [Google Scholar] [CrossRef]
- Almeida, N.M.S.; Miliordos, E. Electronic and structural features of octa-coordinated yttrium–ammonia complexes: The first neutral solvated electron precursor with eight ligands and three outer electrons. Phys. Chem. Chem. Phys. 2019, 21, 7098–7104. [Google Scholar] [CrossRef]
- Jackson, B.A.; Miliordos, E. Electronic and geometric structure of cationic and neutral chromium and molybdenum ammonia complexes. J. Chem. Phys. 2021, 155, 014303. [Google Scholar] [CrossRef]
- Khan, S.N.; Miliordos, E. Scandium in Neutral and Positively Charged Ammonia Complexes: Balancing between Sc2+ and Sc3+. J. Phys. Chem. A 2020, 124, 4400–4412. [Google Scholar] [CrossRef]
- Takasu, R.; Misaizu, F.; Hashimoto, K.; Fuke, K. Microscopic Solvation Process of Alkali Atoms in Finite Clusters: Photoelectron and Photoionization Studies of M(NH3)n and M(H2O)n (M = Li, Li-, Na-). J. Phys. Chem. A 1997, 101, 3078–3087. [Google Scholar] [CrossRef]
- Brockhaus, P.; Hertel, I.V.; Schulz, C.P. Electronically excited states in size-selected solvated alkali metal atoms. III. Depletion spectroscopy of Na(NH3)n-clusters. J. Chem. Phys. 1999, 110, 393–402. [Google Scholar] [CrossRef]
- Lee, J.I.; Sperry, D.C.; Farrar, J.M. Spectroscopy and reactivity of size-selected Mg+-ammonia clusters. J. Chem. Phys. 2004, 121, 8375–8384. [Google Scholar] [CrossRef]
- Mune, Y.; Ohashi, K.; Iino, T.; Inokuchi, Y.; Judai, K.; Nishi, N.; Sekiya, H. Infrared photodissociation spectroscopy of [Al(NH3)n]+ (n = 1–5): Solvation structures and insertion reactions of Al+ into NH3. Chem. Phys. Lett. 2006, 419, 201–206. [Google Scholar] [CrossRef]
- Inoue, K.; Ohashi, K.; Iino, T.; Judai, K.; Nishi, N.; Sekiya, H. Coordination and solvation of copper ion: Infrared photodissociation spectroscopy of Cu+(NH3)n (n = 3–8). Phys. Chem. Chem. Phys. 2007, 9, 4793–4802. [Google Scholar] [CrossRef]
- Salter, T.E.; Ellis, A.M. Structures of Small Li(NH3)n and Li(NH3)n+ Clusters (n = 1−5): Evidence from Combined Photoionization Efficiency Measurements and ab Initio Calculations. J. Phys. Chem. A 2007, 111, 4922–4926. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Ohashi, K.; Iino, T.; Sasaki, J.; Judai, K.; Nishi, N.; Sekiya, H. Coordination structures of the silver ion: Infrared photodissociation spectroscopy of Ag+(NH3)n (n = 3–8). Phys. Chem. Chem. Phys. 2008, 10, 3052–3062. [Google Scholar] [CrossRef]
- Ohashi, K.; Inoue, K.; Iino, T.; Sasaki, J.; Judai, K.; Nishi, N.; Sekiya, H. A molecular picture of metal ion solvation: Infrared spectroscopy of Cu+(NH3)n and Ag+(NH3)n in the gas phase. J. Mol. Liq. 2009, 147, 71–76. [Google Scholar] [CrossRef]
- Imamura, T.; Ohashi, K.; Sasaki, J.; Inoue, K.; Furukawa, K.; Judai, K.; Nishi, N.; Sekiya, H. Infrared photodissociation spectroscopy of Co+(NH3)n and Ni+(NH3)n: Preference for tetrahedral or square-planar coordination. Phys. Chem. Chem. Phys. 2010, 12, 11647–11656. [Google Scholar] [CrossRef]
- Koga, N.; Ohashi, K.; Furukawa, K.; Imamura, T.; Judai, K.; Nishi, N.; Sekiya, H. Coordination and Solvation of V+ with Ammonia Molecules: Infrared Photodissociation Spectroscopy of V+(NH3)n (n=4–8). Chem. Phys. Lett. 2012, 539–540, 1–6. [Google Scholar] [CrossRef]
- Albaqami, M.D.; Ellis, A.M. Infrared spectroscopy of Ca(NH3)n complexes. Chem. Phys. Lett. 2018, 706, 736–740. [Google Scholar] [CrossRef]
- Kozubal, J.; Heck, T.R.; Metz, R.B. Vibrational Spectroscopy of Cr+(NH3)n (n = 1–6) Reveals Coordination and Hydrogen-Bonding Motifs. J. Phys. Chem. A 2019, 123, 4929–4936. [Google Scholar] [CrossRef]
- Seel, A.G.; Swan, H.; Bowron, D.T.; Wasse, J.C.; Weller, T.; Edwards, P.P.; Howard, C.A.; Skipper, N.T. Electron Solvation and the Unique Liquid Structure of a Mixed-Amine Expanded Metal: The Saturated Li–NH3–MeNH2 System. Angew. Chem. Int. Ed. 2017, 56, 1561–1565. [Google Scholar] [CrossRef]
- Seel, A.G.; Zurek, E.; Ramirez-Cuesta, A.J.; Ryan, K.R.; Lodge, M.T.J.; Edwards, P.P. Low energy structural dynamics and constrained libration of Li(NH3)4, the lowest melting point metal. Chem. Comm. 2014, 50, 10778–10781. [Google Scholar] [CrossRef]
- Glaunsinger, W.S.; Von Dreele, R.B.; Marzke, R.F.; Hanson, R.C.; Chieux, P.; Damay, P.; Catterall, R. Structures and properties of metal-ammonia compounds on the trail of a new ammonia geometry. J. Phys. Chem. 1984, 88, 3860–3877. [Google Scholar] [CrossRef]
- Jackson, B.A.; Miliordos, E. Simultaneous CO2 capture and functionalization: Solvated electron precursors as novel catalysts. Chem. Comm. 2022, 58, 1310–1313. [Google Scholar] [CrossRef]
- Jackson, B.A.; Dale, S.G.; Camarasa-Gómez, M.; Miliordos, E. Introducing Novel Materials with Diffuse Electrons for Applications in Redox Catalysis and Quantum Computing via Theoretical Calculations. J. Phys. Chem. C 2023, 127, 9295–9308. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 93rd ed.; Taylor & Francis: Boca Raton, FL, USA, 2012. [Google Scholar]
- Tutson, C.D.; Gorden, A.E.V. Thorium coordination: A comprehensive review based on coordination number. Coord. Chem. Rev. 2017, 333, 27–43. [Google Scholar] [CrossRef]
- Blanchard, F.; Rivenet, M.; Vigier, N.; Hablot, I.; Grandjean, S.; Abraham, F. Solid State Chemistry of Ten-Fold Coordinate Thorium(IV) Complexes with Oxalates in the Presence of Ammonium and Hydrazinium Ions. Cryst. Growth Des. 2018, 18, 4593–4601. [Google Scholar] [CrossRef]
- Ariyarathna, I.R. Superatomic Chelates: The Cases of Metal Aza-Crown Ethers and Cryptands. Inorg. Chem. 2022, 61, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. IV. Calculation of static electrical response properties. J. Chem. Phys. 1994, 100, 2975–2988. [Google Scholar] [CrossRef]
- Peterson, K.A. Correlation consistent basis sets for actinides. I. The Th and U atoms. J. Chem. Phys. 2015, 142, 074105. [Google Scholar] [CrossRef] [PubMed]
- Weigand, A.; Cao, X.; Hangele, T.; Dolg, M. Relativistic Small-Core Pseudopotentials for Actinium, Thorium, and Protactinium. J. Phys. Chem. A 2014, 118, 2519–2530. [Google Scholar] [CrossRef] [PubMed]
- Ariyarathna, I.R.; Pawłowski, F.; Ortiz, J.V.; Miliordos, E. Aufbau Principle for Diffuse Electrons of Double-Shell Metal Ammonia Complexes: The Case of M(NH3)4@12NH3, M = Li, Be+, B2+. J. Phys. Chem. A 2020, 124, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Schlegel, G.W.T.H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; Li, X.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Ghigo, G.; Roos, B.O.; Malmqvist, P.A. A Modified Definition of the Zeroth-Order Hamiltonian in Multiconfigurational Perturbation Theory (CASPT2). Chem. Phys. Lett. 2004, 396, 142–149. [Google Scholar] [CrossRef]
- Azizi, Z.; Roos, B.O.; Veryazov, V. How accurate is the CASPT2 method? Phys. Chem. Chem. Phys. 2006, 8, 2727–2732. [Google Scholar] [CrossRef]
- Ariyarathna, I.R.; Miliordos, E. Geometric and electronic structure analysis of calcium water complexes with one and two solvation shells. Phys. Chem. Chem. Phys. 2020, 22, 22426–22435. [Google Scholar] [CrossRef]
- Jackson, B.A.; Miliordos, E. The nature of supermolecular bonds: Investigating hydrocarbon linked beryllium solvated electron precursors. J. Chem. Phys. 2022, 156, 194302. [Google Scholar] [CrossRef]
- Werner, H.-J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schütz, M.; Celani, P.; Györffy, W.; Kats, D.; Korona, T.; Lindh, R.; et al. MOLPRO, Version 2015.1, a Package of ab Initio Programs. 2015. Available online: https://www.molpro.net/ (accessed on 5 June 2023).
- Celani, P.; Werner, H.-J. Multireference perturbation theory for large restricted and selected active space reference wave functions. J. Chem. Phys. 2000, 112, 5546–5557. [Google Scholar] [CrossRef]
- Roos, B.O.; Andersson, K. Multiconfigurational Perturbation-Theory with Level Shift—The Cr2 Potential Revisited. Chem. Phys. Lett. 1995, 245, 215–223. [Google Scholar] [CrossRef]
- Miliordos, E.; Ruedenberg, K.; Xantheas, S.S. Unusual Inorganic Biradicals: A Theoretical Analysis. Angew. Chem. Int. Ed. 2013, 52, 5736–5739. [Google Scholar] [CrossRef] [PubMed]
- Ariyarathna, I.R.; Miliordos, E. Ground and excited states analysis of alkali metal ethylenediamine and crown ether complexes. Phys. Chem. Chem. Phys. 2021, 23, 20298–20306. [Google Scholar] [CrossRef] [PubMed]
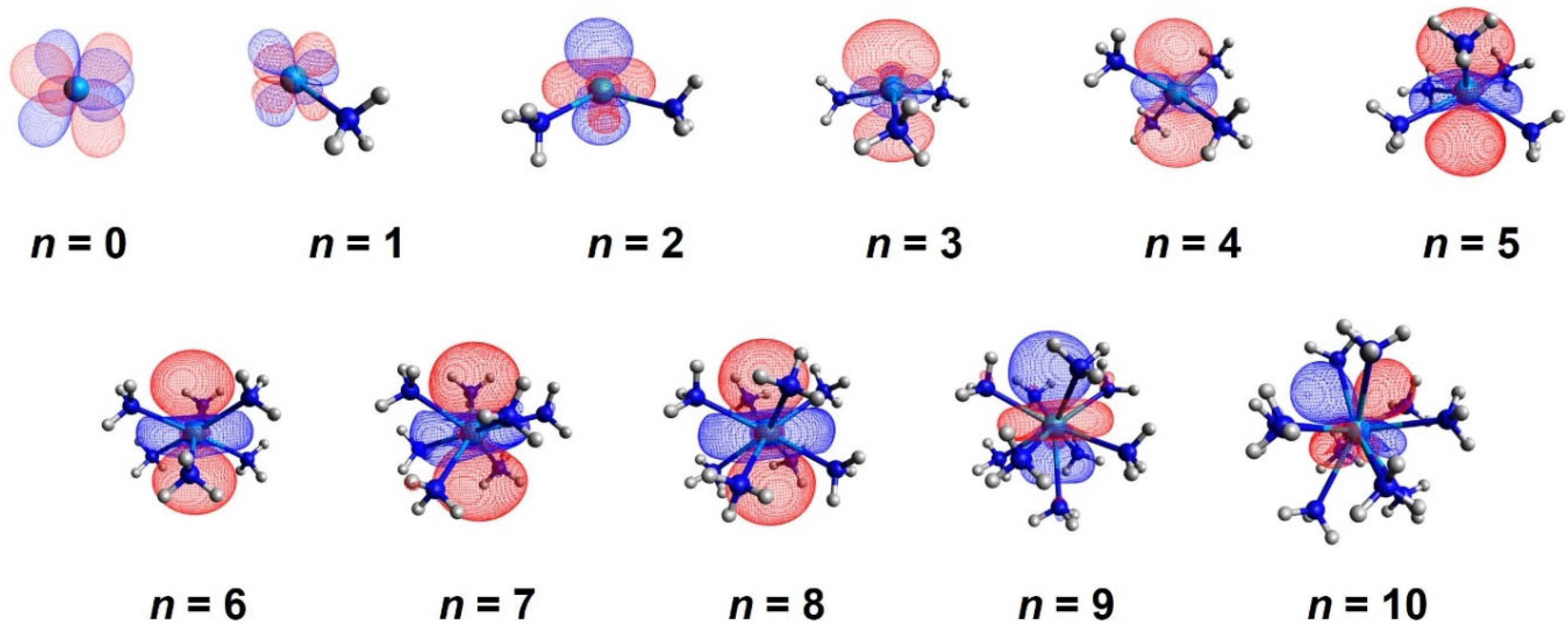
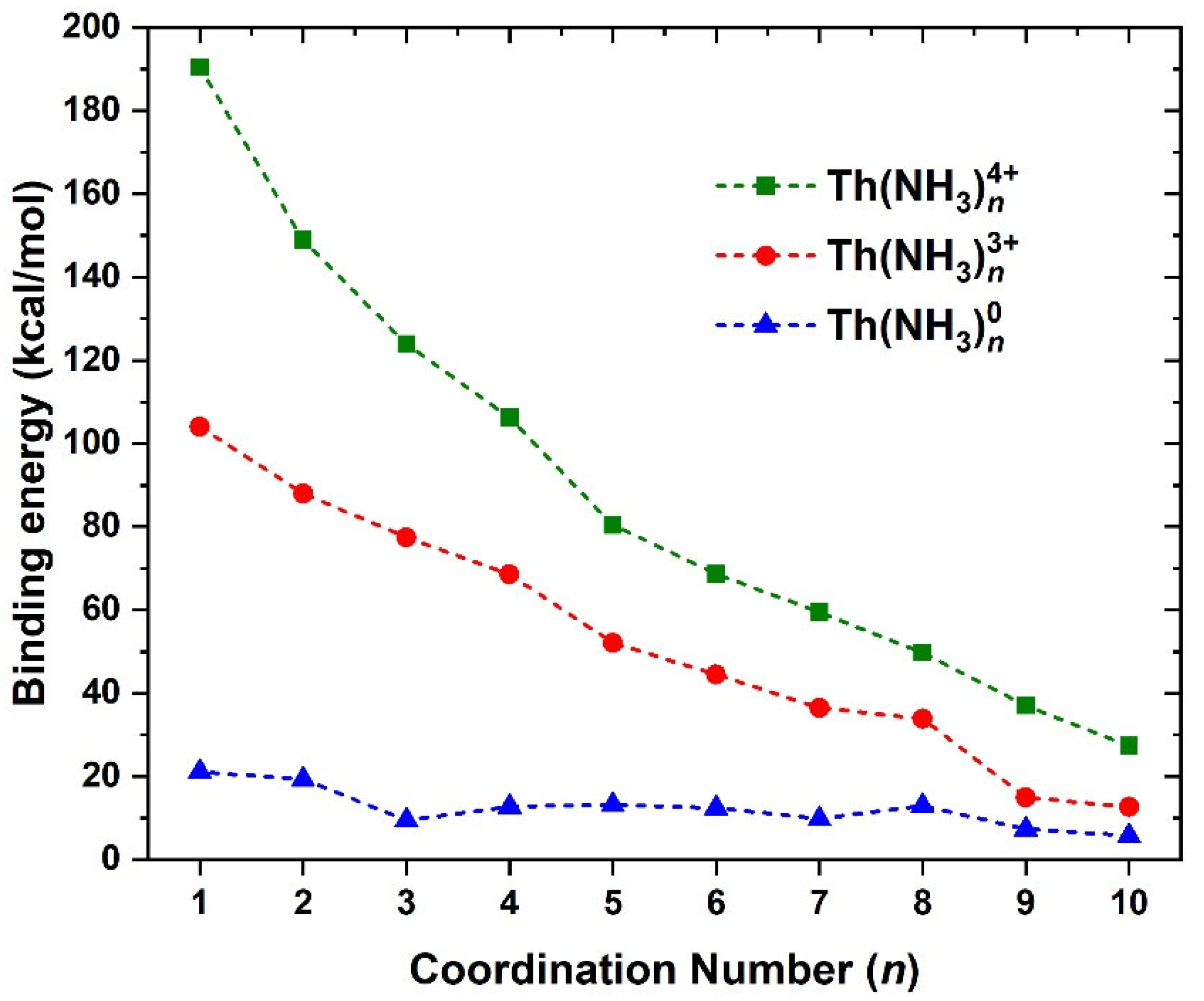

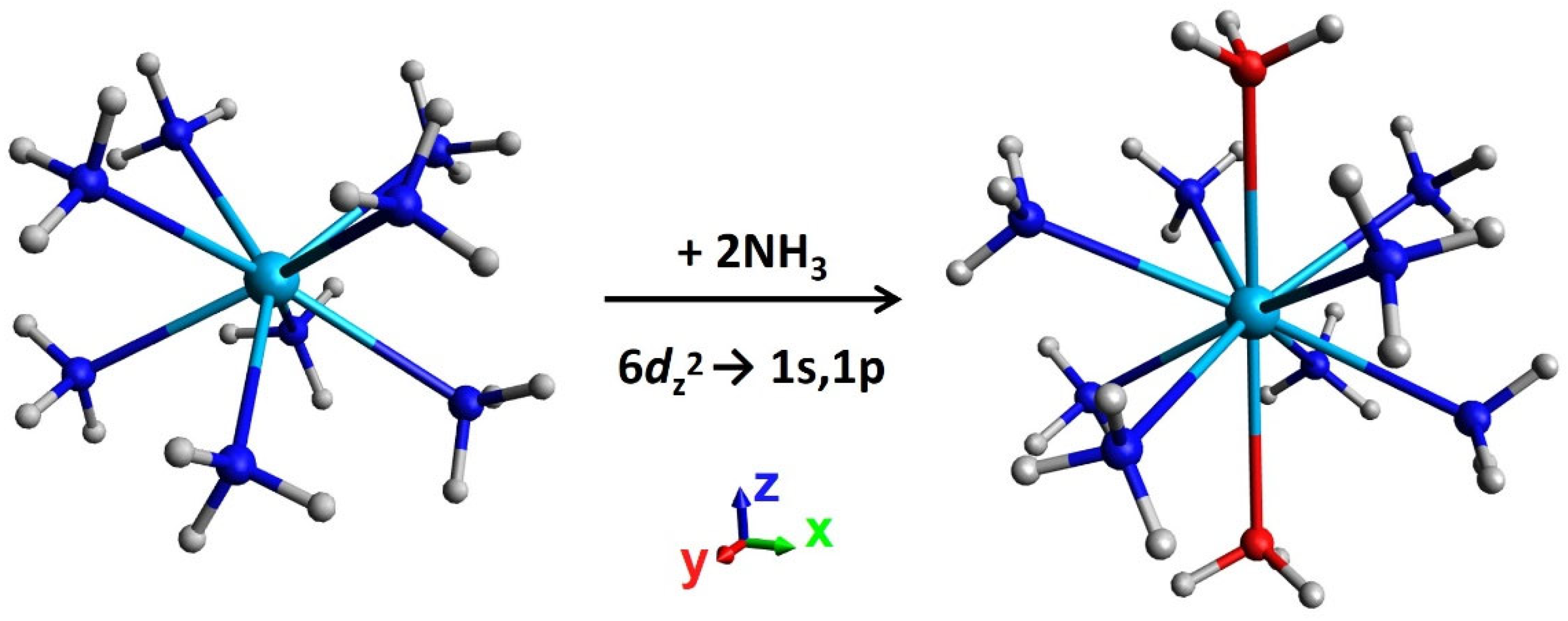
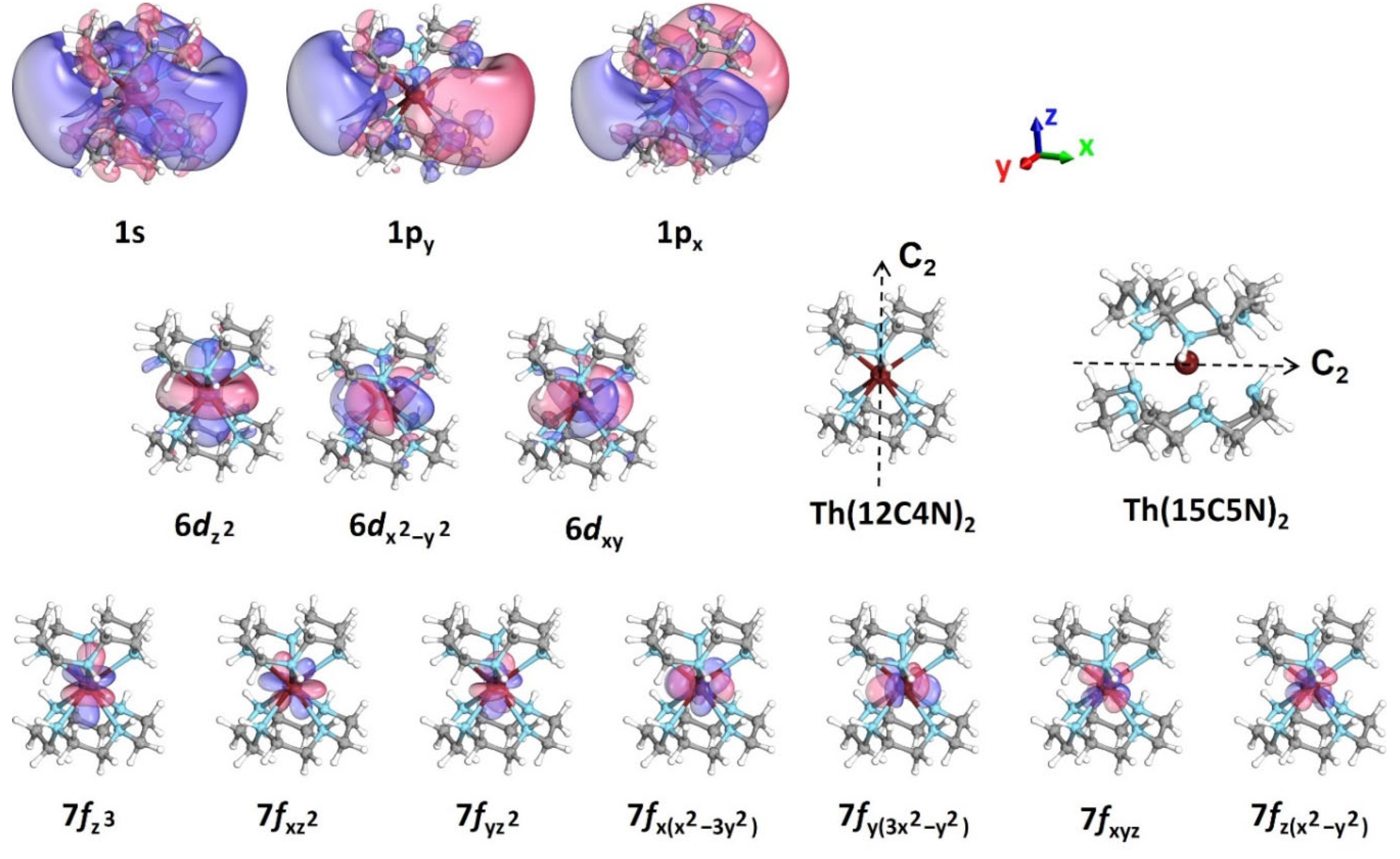
| State | EC | CASSCF | CASPT2 | CASSCF | CASPT2 | CASSCF | CASPT2 |
|---|---|---|---|---|---|---|---|
| ΔΕ[Th(NH3)83+] | ΔΕ[Th(12C4N)23+] a | ΔΕ[Th(12C4O)23+] a | |||||
| 2A1 | (6dz2)1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 12E2 | (6dxy)1/(6dx2−y2)1 | 1.35 | 1.28 | 1.03/1.05 | 1.04/1.02 | 1.61/1.29 | 1.57/1.27 |
| 12E3 | (6dxz)1/(6dyz)1 | 1.92 | 1.79 | 4.36/4.00 | 4.28/3.88 | ||
| 12B2 | (7fz3)1 | 2.08 | 1.94 | 2.03 | 2.03 | N/C b | N/C b |
| 12E1 | (7fxz2)1/(7fyz2)1 | 2.18 | 2.01 | 2.13/2.11 | 2.11/2.11 | N/C b | N/C b |
| 22A1 | (1s)1 | 2.15 | 2.05 | 3.03 | 2.76 | 3.15 | 3.06 |
| 22B2 | (1pz)1 | 3.19 | 3.10 | 3.73 | 3.60 | ||
| 22E1 | (1px)1/(1py)1 | 3.24 | 3.15 | 3.50/3.36 | 3.23/3.10 | 3.42/3.71 | 3.36/3.64 |
| 22E2 | (7fxyz)1/(7fz(x2−y2))1 | 3.30 | 3.18 | 3.24/3.26 | 3.22/3.25 | N/C b | N/C b |
| 22E3 | (7fx(x2−3y2))1/(7fy(3x2−y2))1 | 3.59 | 3.51 | 1.89/1.83 | 1.88/1.84 | N/C b | N/C b |
| 32A1 | (1dz2)1 | 4.24 | 4.20 | 3.95 | 3.82 | ||
| 32E2 | (1dx2−y2)1/(1dxy)1 | 4.46 | 4.40 | 4.29/4.26 | |||
| 32E3 | (1dxz)1/(1dyz)1 | 4.89 | 4.86 | ||||
| State | Electron Configuration | ΔE (CASSCF) | ΔE (CASPT2) |
|---|---|---|---|
| Th(NH3)81+ | |||
| 2A1a | (6dz2)11s2/(6dz2)21s1 | 0.00 | 0.00 |
| 12E1 a | (6dz2)11s11px,y1/1s21px,y1/(6dz2)21px,y1 | 0.47 | 0.50 |
| 14B2 | (6dz2)11s11pz1 | 0.48 | 0.51 |
| 14E1 | (6dz2)11s11px,y1 | 0.49 | 0.53 |
| 12B2a | 1s21pz1/(6dz2)21pz1/(6dz2)11s11pz1 | 0.53 | 0.56 |
| Th(NH3)80 | |||
| 3E1 | (6dz2)11s21px,y1 | 0.00 | 0.00 |
| 1E1 | (6dz2)11s21px,y1 | 0.01 | 0.00 |
| 13B2 | (6dz2)11s21pz1 | 0.04 | 0.04 |
| 11B2 | (6dz2)11s21pz1 | 0.11 | 0.09 |
| 13A2 | (6dz2)11s11px11py1 | 0.28 | 0.30 |
| 15A2 | (6dz2)11s11px11py1 | 0.27 | 0.31 |
| 15E3 | (6dz2)11s11pz11px,y1 | 0.32 | 0.36 |
| 13E3 | (6dz2)11s11pz11px,y1 | 0.38 | 0.39 |
| 11E2a,b | (6dz2)11s11p2/(6dz2)11s2(1dxy,x2−y2)1 | 0.56 | 0.54 |
| 13E2a,b | (6dz2)11s11p2/(6dz2)11s2(1dxy,x2-y2)1 | 0.55 | 0.56 |
| 11E3a,b | (6dz2)11s11p2/(6dz2)11s2(1dxz,yz)1 | 0.63 | 0.60 |
| 23E3a,b | (6dz2)11s11p2/(6dz2)11s2(1dxz,yz)1 | 0.63 | 0.62 |
| 13A1a,b | (6dz2)11s11p2/(6dz2)11s2(1dz2)1 | 0.63 | 0.63 |
| 11A1a,b | (6dz2)11s11p2/(6dz2)11s2(1dz2)1 | 0.65 | 0.63 |
| q | Th(NH3)8q | Th(12C4N)2q | Th(12C4O)2q | Th(NH3)10q | Th(15C5N)2q | Th(15C5O)2q |
|---|---|---|---|---|---|---|
| +4 | 103.5 | 108.9 | 108.0 | 89.2 | 93.0 | 92.9 |
| +3 | 63.1 | 63.7 | 64.1 | 53.2 | 55.0 | 54.9 |
| +2 | N/A | 38.8 | 38.6 | 34.1 | 36.8 | 36.9 |
| +1 | N/A | 19.3 | 18.7 | 19.1 | 15.4 | 14.2 |
| 0 | 13.6 | 8.6 | 6.1 | 12.2 | 7.1 | 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Jackson, B.A.; Miliordos, E. Ab Initio Calculations on the Ground and Excited Electronic States of Thorium–Ammonia, Thorium–Aza-Crown, and Thorium–Crown Ether Complexes. Molecules 2023, 28, 4712. https://doi.org/10.3390/molecules28124712
Lu Z, Jackson BA, Miliordos E. Ab Initio Calculations on the Ground and Excited Electronic States of Thorium–Ammonia, Thorium–Aza-Crown, and Thorium–Crown Ether Complexes. Molecules. 2023; 28(12):4712. https://doi.org/10.3390/molecules28124712
Chicago/Turabian StyleLu, Zhongyuan, Benjamin A. Jackson, and Evangelos Miliordos. 2023. "Ab Initio Calculations on the Ground and Excited Electronic States of Thorium–Ammonia, Thorium–Aza-Crown, and Thorium–Crown Ether Complexes" Molecules 28, no. 12: 4712. https://doi.org/10.3390/molecules28124712
APA StyleLu, Z., Jackson, B. A., & Miliordos, E. (2023). Ab Initio Calculations on the Ground and Excited Electronic States of Thorium–Ammonia, Thorium–Aza-Crown, and Thorium–Crown Ether Complexes. Molecules, 28(12), 4712. https://doi.org/10.3390/molecules28124712






