Synthesis and Characterization of a New Series of Bis(allylic-α-aminophosphonates) under Mild Reaction Conditions
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure and Spectral Data for the Multicomponent Kabachnik–Fields Reaction for the Preparation of Bis(α-aminophosphonates) 4
- Tetraethyl [(ethane-1,2-diylbis(azanediyl))bis(phenylmethylene)]bis(phosphonate) (4a), (1.41 g, 55%) was obtained as a white solid following the general procedure (method A). The title compound 4a (1.54 g, 60%) was obtained as white solid as described in the general procedure (method B). Data for 4a: mp 97–98 °C. 1H NMR (400 MHz, CDCl3) δ 7.36–7.19 (m, 8H, Ar-CH), 4.09–3.69 (m, 10H, H2C-O and P-CH), 2.84–2.53 (m, 6H, CH2 and NH), 1.20 (t, 6H, 3JHH = 7.0 Hz, CH3), 1.06 (t, 6H, 3JHH = 7.1 Hz, CH3) ppm. 31P NMR (162 MHz, CDCl3) δ 23.3 ppm.
- Tetraethyl [(ethane-1,2-diylbis(azanediyl))bis(p-tolylmethylene)]bis(phosphonate) (4b), (1.92 g, 71%) was obtained as a viscous oil following the general procedure (method A). The title compound 4b (1.54 g, 57%) was obtained as viscous oil as described in the general procedure (method B). Data for 4b: 1H NMR (400 MHz, CDCl3) δ 7.26–7.22 (m, 4H, Ar-CH), 7.14–7.09 (m, 4H, Ar-CH), 4.13–3.78 (m, 10H, H2C-O and P-CH), 2.63–2.46 (m, 4H, CH2), 2.32 (s, 6H, CH3), 2.17 (bs, 2H, NH), 1.29–1.12 (m, 12H, CH3) ppm. 13C {1H} NMR (101 MHz, CDCl3) δ 137.5 (Ar-C), 132.8 (Ar-C), 129.1 (Ar-CH), 128.4 (Ar-CH), 62.9 (P-OCH2), 60.4 (d, 1JPC = 154.7 Hz, P-CH), 47.0 (CH2), 37.4 (CH2), 21.2 (CH3), 16.3 (CH3) ppm. 31P NMR (162 MHz, CDCl3) δ 23.7 ppm.
- Tetraethyl [(propane-1,3-diylbis(azanediyl))bis(phenylmethylene]bis(phosphonate) (4c), (1.82 g, 69%) was isolated as a viscous clear oil using the general procedure (method A). The title compound 4c (1.95 g, 74%) was produced as a viscous clear oil as described in the general procedure (method B). Data for 4c: 1H NMR (400 MHz, CDCl3) δ 7.42–7.13 (m, 8H, Ar-CH), 4.12–3.62 (m, 10H, H2C-O and P-CH), 2.63–2.33 (m, 4H, CH2), 2.20 (bs, 2H, NH), 1.69–1.46 (m, 2H, CH2), 1.33–0.95 (m, 12H, CH3) ppm. 31P NMR (162 MHz, CDCl3) δ 23.4 ppm.
- Tetraethyl [(propane-1,3-diylbis(azanediyl))bis(p-tolylmethylene]bis(phosphonate) (4d), (2.41 g, 87%) was isolated as a viscous oil using the general procedure (method A). The title compound 4d (2.19 g, 79%) was produced as a viscous oil as described in the general procedure (method B). Data for 4d: 1H NMR (400 MHz, CDCl3) δ 7.18 (d, 3JHH = 7.2 Hz, 4H, Ar-CH), 7.06 (d, 3JHH = 7.6 Hz, 4H, Ar-CH), 4.02–3.69 (m, 10H, H2C-O and P-CH), 2.55–2.37 (m, 4H, CH2), 2.26 (s, 6H, CH3), 2.09 (bs, 2H, NH), 1.57–1.45 (m, 2H, CH2), 1.18 (t, 6H, 3JHH = 7.1 Hz, CH3), 1.06 (t, 6H, 3JHH = 7.1 Hz, CH3) ppm. 13C {1H} NMR (101 MHz, CDCl3) δ 137.1 (Ar-C), 131.7 (Ar-C), 128.6 (Ar-CH), 128.0 (Ar-CH), 62.3 (P-OCH2), 59.9 (d, 1JPC = 153.1 Hz, P-CH), 45.5 (d, 3JPC = 15.2 Hz, CH2), 28.5 (CH2), 20.6 (CH3), 15.8 (d, 3JPC = 17.2 Hz, CH3) ppm. 31P NMR (162 MHz, CDCl3) δ 23.6 ppm.
- Tetraethyl [(propane-1,3-diylbis(azanediyl))bis((4-methoxyphenyl)methylene)]bis(phosphonate) (4e), (1.93 g, 66%) was isolated as a viscous clear oil as described in the general procedure (method A). The title compound 4e (1.85 g, 63%) was obtained as a viscous clear oil as described in the general procedure (method B). Data for 4e: 1H NMR (400 MHz, CDCl3) δ 7.25 (d, 3JHH = 7.9 Hz, 4H, Ar-CH), 6.83 (d, 3JHH = 8.5 Hz, 4H, Ar-CH), 4.35 (bs, 2H, NH), 4.03–3.88 (m, 10H, H2C-O and P-CH), 3.75 (s, 6H, CH3), 2.54–2.39 (m, 4H, CH2), 1.60–1.52 (m, 2H, CH2), 1.22 (t, 6H, 3JHH = 7.0 Hz, CH3), 1.09 (t, 6H, 3JHH = 7.0 Hz, CH3) ppm 31P NMR (162 MHz, CDCl3) δ 23.7 ppm.
- Tetraethyl [(propane-1,3-diylbis(azanediyl))bis((4-chlorophenyl)methylene)]bis(phosphonate) (4f), (2.02 g, 68%) was isolated as a viscous oil as described in the general procedure (method A). The title compound 4f (2.11 g, 71%) was obtained as viscous oil as described in the general procedure (method B). Data for 4f: 1H NMR (400 MHz, CDCl3) δ 7.22–7.07 (m, 8H, Ar-CH), 3.93–3.64 (m, 10H, H2C-O and P-CH), 2.45–2.24 (m, 4H, CH2), 1.76 (bs, 2H, NH), 1.46–1.37 (m, 2H, CH2), 1.09–0.95 (m, 12H, CH3) ppm. 13C {1H} NMR (101 MHz, CDCl3) δ 134.3 (Ar-C), 132.8 (Ar-C), 129.2 (d, 3JPC = 6.0 Hz,Ar-CH), 127.9 (Ar-CH), 62.3 (P-OCH2), 60.0 (d, 1JPC = 152.8 Hz, P-C), 45.4 (d, 3JPC = 16.6 Hz,CH2), 29.3 (CH2), 15.7 (CH3) ppm. 31P NMR (162 MHz, CDCl3) δ 22.6 ppm.
- Tetraethyl [(propane-1,3-diylbis(azanediyl))bis((4-fluorophenyl)methylene)]bis(phosphonate) (4g), (1.69 g, 60%) was obtained as a viscous oil following the general procedure (method A). The title compound 4g (1.63 g, 58%) was obtained as a viscous oil using the general procedure (method B). Data for 4g: 1H NMR (400 MHz, CDCl3) δ 7.28–6.94 (m, 4H, Ar-CH), 6.71–6.50 (m, 4H, Ar-CH), 3.93–3.44 (m, 10H, H2C-O and P-CH), 2.33–2.05 (m, 4H, CH2), 1.71–1.63 (m, 2H, NH), 1.37–1.23 (m, 2H, CH2), 1.03–0.70 (m, 12H, CH3) ppm. 13C {1H} NMR (101 MHz, CDCl3) δ 161.5 (d, 1JCF = 245.8 Hz, C-F), 131.3 (Ar-C), 129.2 (Ar-CH), 114.3 (d, 3JPC = 21.6 Hz, Ar-CH), 61.8 (P-OCH2), 59.6 (d, 1JPC = 153.3 Hz, P-CH), 45.1 (d, 3JPC = 17.3 Hz, CH2), 29.0 (CH2), 15.4 (d, 3JPC = 15.8 Hz, CH3) ppm. 31P NMR (162 MHz, CDCl3) δ 22.9 ppm. 19F NMR (376 MHz, CDCl3) δ –115.0 ppm.
- Tetraethyl [(propane-1,3-diylbis(azanediyl))bis(ethane-1,1-diyl)]bis(phosphonate) (4h), (1.59 g, 79%) was isolated as a viscous oil as described in the general procedure (method A). The title compound 4h (1.73 g, 86%) was obtained as a viscous oil as described in the general procedure (method B). Data for 4h: 1H NMR (400 MHz, CDCl3) δ 4.19–4.08 (m, 10H, H2C-O and P-CH), 3.00–2.91 (m, 2H, CH2), 2.84–2.67 (m, 4H, CH2), 1.48 (bs, 2H, NH), 1.34–1.25 (m, 18H, CH3). 13C {1H} NMR (101 MHz, CDCl3) 62.0 (P-OCH2), 50.5 (d, 1JPC = 153.9 Hz, P-CH), 46.1 (d, 3JPC = 11.0 Hz, CH2), 30.6 (CH2), 16.5 (d, 3JPC = 5.53 Hz,CH3), 15.2 (CH3) ppm. 31P NMR (162 MHz, CDCl3) δ 28.6 ppm.
3.1.2. General Procedure and Spectral Data for the Nucleophilic Substitution of Bis(α-aminophosphonates) 4 with Ethyl (2-Bromomethyl)acrylate 5 for the Synthesis of Bis(allylic-α-aminophosphonates) 6
- Diethyl 2,2′-[(ethane-1,2-diylbis(((diethoxyphosphoryl)(phenyl)methyl)azanediyl))bis(methylene)]diacrylate (6a), (0.36 g, 69%) was isolated as a white solid as described in the general procedure. Data for 6a: mp 150–151 °C. 1H NMR (300 MHz, CDCl3) δ 7.56–7.33 (m, 10H, Ar-CH), 6.24 (s, 2H, H2C=), 5.86 (s, 2H, H2C=), 4.21–4.08 (m, m, 10H, H2CO-P and HC-P), 3.92–3.86 (m, 2H, H2CO-C), 3.76–3.66 (m, 2H, H2CO-C and H2C-N), 3.35–3.30 (m, 2H, H2C-N), 3.21–3.17 (m, 2H, H2C-N), 2.50–2.37 (m, 2H, CH2), 1.28–1.22 (m, 12H, CH3), 0.98 (t, 3JHH = 7.2 Hz, 6H, CH3) ppm. 13C {1H} NMR (101 MHz, CDCl3) δ 166.7 (C=O), 138.6 (C=CH2), 132.4 (d, 2JPC = 5.8 Hz Ar-C), 130.8 (d, 3JPC = 8.7 Hz Ar-CH), 128.1 (Ar-CH), 127.9 (Ar-CH), 125.9 (H2C=), 62.4 (P-OCH2), 61.4 (d, 1JPC = 162.8 Hz, P-CH), 60.4 (C-OCH2), 52.7 (d, 3JPC = 10.3 Hz, N-CH2), 50.5 (d, 3JPC = 5.8 Hz, N-CH2), 31.6 (CH2), 29.0 (CH2), 16.4 (d, 3JPC = 5.8 Hz, CH3), 14.1 (CH3) ppm. 31P NMR (162 MHz, CDCl3) δ 23.1 ppm. ESI-HRMS (CI) m/z calcd for C36H55N2O10P2 ([M + H]+) 737.3332, found 737.3334.
- Diethyl 2,2′-[(ethane-1,2-diylbis(((diethoxyphosphoryl)(p-tolyl)methyl)azanediyl))bis(methylene)]diacrylate (6b), (0.46 g, 85%) was isolated as a viscous oil as described in the general procedure. Data for 6b: 1H NMR (400 MHz, CDCl3) δ 7.32 (d, 3JHH = 7.7 Hz, 4H, Ar-CH), 7.13 (d, 3JHH = 8.0 Hz, 4H, Ar-CH), 6.28 (s, 2H, H2C=), 5.93 (s, 2H, H2C=), 4.22–4.01 (m, 10H, H2CO-P and HC-P), 3.97–3.87 (m, 2H, H2CO-C), 3.80–3.66 (m, 4H, H2CO-C and H2C-N), 3.27–3.24 (m, 2H, H2C-N), 3.19–3.14 (m, 2H, CH2), 2.55–2.49 (m, 2H, CH2), 2.35 (s, 6H, CH3), 1.33 (t, 6H, 3JHH = 7.2 Hz, CH3), 1.26 (t, 6H, 3JHH = 7.2 Hz, CH3), 1.05 (t, 3JHH = 7.1 Hz, 6H, CH3) ppm. 13C {1H} NMR (101 MHz, CDCl3) δ 166.9 (C=O), 138.5 (C=CH2), 137.5 (Ar-C), 130.5 (Ar-CH), 130.4 (Ar-CH), 128.9 (Ar-C), 126.0 (H2C=), 62.5 (P-OCH2), 61.7 (d, 1JPC = 151.6 Hz, P-CH), 60.5 (C-OCH2), 52.5 (N-CH2), 50.6 (N-CH2), 21.1 (CH3), 16.6 (d, 3JPC = 5.6 Hz, CH3), 16.2 (d, 3JPC = 5.8 Hz, CH3), 14.2 (CH3) ppm. 31P NMR (162 MHz, CDCl3) δ 23.7 ppm. ESI-HRMS (CI) m/z calcd for C38H59N2O10P2 ([M + H]+) 765.3645, found 765.3638.
- Diethyl 2,2′-[(propane-1,3-diylbis(((diethoxyphosphoryl)(phenyl)methyl)azanediyl))bis(methylene)]diacrylate (6c), (0.39 g, 74%) obtained as a viscous clear oil as described in the general procedure. Data for 6c: 1H NMR (300 MHz, CDCl3) δ 7.39–7.18 (m, 10H, Ar-CH), 6.11 (s, 2H, H2C=), 5.68 (s, 2H, H2C=), 4.20–3.67 (m, 14H, H2CO-P, H2CO-C, HC-P), 3.16 (s, 4H, H2C-N), 2.50–2.32 (m, 4H, H2C-N), 1.61–1.52 (m, 2H, CH2), 1.27–1.14 (m, 12H, CH3), 1.06 (t, 3JHH = 7.3 Hz, 6H, CH3) ppm. 13C {1H} NMR (75 MHz, CDCl3) δ 165.8 (C=O), 137.3 (C=CH2), 135.0 (Ar-C), 127.4 (Ar-CH), 127.3 (Ar-CH), 126.7 (Ar-CH), 124.6 (C=CH2), 61.8 (P-OCH2), 60.1 (d, 1JPC = 153.1 Hz, CH-P), 59.5 (C-OCH2), 53.5 (N-CH2), 51.1 (CH2), 45.0 (CH2), 28.6 (CH2), 15.2 (CH3), 13.1 (CH3) ppm. 31P NMR (121 MHz, CDCl3) δ 23.6 ppm. ESI-HRMS (CI) m/z calcd for C37H56N2O10P2 ([M + H]+) 751.3488, found 751.3494.
- Diethyl 2,2′-[(propane-1,3-diylbis(((diethoxyphosphoryl)(p-tolyl)methyl)azanediyl))bis(methylene)]diacrylate (6d), (0.39 g, 71%) was isolated as a viscous clear oil as described in the general procedure. Data for 6d: 1H NMR (300 MHz, CDCl3) δ 7.37–7.00 (m, 8H, Ar-CH), 6.21 (s, 2H, H2C=), 5.89 (s, 2H, H2C=), 4.19–3.56 (m, 12H, H2CO-P, HC-P and H2CO-C), 3.72–3.56 (m, 4H, H2CO-C and H2C-N), 3.20–3.03 (m, 2H, H2C-N), 2.98–2.87 (m, 2H, CH2), 2.26 (s, 6H, CH3), 1.74–1.51 (m, 2H, CH2), 1.29–1.14 (m, 12H, CH3), 0.98–0.92 (m, 6H, CH3) ppm. 13C {1H} NMR (101 MHz, CDCl3) δ 166.7 (C=O), 138.3 (C=CH2), 137.3 (Ar-C), 130.3 (d, 2JPC = 8.6 Hz, Ar-C), 129.4 (d, 3JPC = 6.0 Hz, Ar-CH), 128.6 (Ar-CH), 125.7 (C=CH2), 62.0 (d, 2JPC = 7.1 Hz, P-OCH2), 61.9 (d, 2JPC = 7.0 Hz, P-OCH2), 61.2 (d, 1JPC = 138.7 Hz, P-CH), 60.2 (C-OCH2), 51.7 (N-CH2), 49.6 (N-CH2), 26.8 (CH2), 20.9 (CH3), 16.3 (d, 3JPC = 5.9 Hz, CH3), 16.0 (d, 3JPC = 5.6 Hz, CH3), 14.0 (CH3) ppm. 31P NMR (121 MHz, CDCl3) δ 23.7 ppm. ESI-HRMS (CI) m/z calcd for C39H61N2O10P2 ([M + H]+) 779.3801, found 779.3810.
- Diethyl 2,2′-[(propane-1,3-diylbis(((diethoxyphosphoryl)(4-methoxyphenyl)methyl)azanediyl))bis(methylene)]diacrylate (6e), (0.42 g, 74%) was isolated as a viscous clear oil as described in the general procedure. Data for 6e: 1H NMR (400 MHz, CDCl3) δ 7.39–7.36 (m, 4H, Ar-CH), 6.88–6.85 (m, 4H, Ar-CH), 6.29 (s, 2H, H2C=), 5.95 (s, 2H, H2C=), 4.20–3.86 (m, 12H, H2CO-P, HC-P and H2CO-C), 3.81 (s, 6H, OCH3), 3.75–3.67 (m, 4H, H2CO-C and H2C-N), 3.20–3.13 (m, 2H, H2C-N), 3.04–2.94 (m, 2H, CH2), 2.42–2.27 (m, 2H, CH2), 1.71–1.62 (m, 2H, CH2), 1.31–1.25 (m, 12H, CH3), 0.96 (t, 3JHH = 7.1, 3H, CH3), 0.94 (t, 3JHH = 7.1, 3H, CH3) ppm. 13C {1H} NMR (101 MHz, CDCl3) δ 166.9 (C=O), 159.2 (Ar-C), 138.5 (C=CH2), 131.9 (Ar-C), 125.9 (=CH2), 124.7 (d, 3JPC = 5.9 Hz, Ar-CH), 124.5 (d, 3JPC = 5.9 Hz, Ar-CH), 113.5 (Ar-CH), 62.2 (d, 2JPC = 7.3Hz, P-OCH2), 62.1 (d, 2JPC = 7.3 Hz, P-OCH2), 61.1 (d, 1JPC = 161.2 Hz, P-CH), 60.5 (C-OCH2), 55.1 (N-CH2), 51.7 (N-CH2), 49.8 (CH2), 27.0 (CH3), 16.5 (d, 3JPC = 5.8 Hz, CH3), 16.2 (d, 3JPC = 5.8 Hz, CH3), 14.2 (CH3) ppm. 31P NMR (162 MHz, CDCl3) δ 23.7 ppm. ESI-HRMS (CI) m/z calcd for C39H61N2O12P2 ([M + H]+) 811.3700, found 811.3697.
- Diethyl 2,2′-[(propane-1,3-diylbis(((4-chlorophenyl)(diethoxyphosphoryl)methyl)azanediyl))bis(methylene)]diacrylate (6f), (0.50 g, 87%) obtained as a viscous clear oil as described in the general procedure. Data for 6f: 1H NMR (300 MHz, CDCl3) δ 7.39–7.11 (m, 8H, Ar-CH), 6.21 (s, 2H, H2C=), 5.79 (s, 2H, H2C=), 4.25–3.51 (m, 16H, H2CO-P, HC-P, H2CO-C, and H2C-N), 3.24–2.70 (m, 4H, H2C-N), 2.34–2.14 (m, 2H, CH2), 1.76–1.44 (m, 2H, CH2), 1.23–0.76 (m, 18H, CH3) ppm. 13C {1H} NMR (75 MHz, CDCl3) δ 166.6 (C=O), 138.4 (C=CH2), 133.8 (Ar-C), 131.7 (Ar-CH), 128.2 (Ar-CH), 125.9 (=CH2), 124.6 (Ar-C), 62.2 (P-OCH2), 60.8 (d, 1JPC = 143.7 Hz, P-CH), 60.4 (C-OCH2), 51.9 (N-CH2), 49.7 (N-CH2), 26.9 (CH2), 16.3 (CH3), 14.0 (CH3) ppm. 31P NMR (121 MHz, CDCl3) δ 23.00 ppm. ESI-HRMS (CI) m/z calcd for C37H55Cl2N2O10P2 ([M + H]+) 819.2709, found 819.2714.
- Diethyl 2,2′-[(propane-1,3-diylbis(((diethoxyphosphoryl)(4-fluorophenyl)methyl)azanediyl))bis(methylene)]diacrylate (6g), (0.41 g, 75%) was isolated as a viscous clear oil as described in the general procedure. Data for 6g: 1H NMR (400 MHz, CDCl3) δ 7.45–7.41 (m, 4H, Ar-CH), 7.06–7.00 (m, 4H, Ar-CH), 6.28 (s, 2H, H2C=), 5.93 (s, 2H, H2C=), 4.22–4.07 (m, 10H, H2CO-P and HC-P), 3.97–3.87 (m, 2H, H2CO-C), 3.81–3.68 (m, 4H, H2CO-C and H2C-N), 3.23–3.18 (m, 2H, H2C-N), 3.05–2.98 (m, 2H, CH2), 2.35–2.29 (m, 2H, CH2), 1.68–1.60 (m, 2H, CH2), 1.33–1.26 (m, 12H, CH3), 1.06 (t, 6H, 3JHH = 7.3 Hz, CH3) ppm. 13C {1H} NMR (101 MHz, CDCl3) δ 166.7 (C=O), 162.4 (d, 1JCF = 245.9 Hz, C-F), 138.4 (C=CH2), 132.2 (Ar-CH), 128.9 (Ar-C), 126.0 (C=CH2), 115.2 (d, 3JPC = 21.1 Hz, Ar-CH), 62.2 (d, 2JPC = 20.9 Hz, P-OCH2), 61.0 (d, 1JPC = 159.4 Hz, P-C), 60.5 (C-OCH2), 51.8 (N-CH2), 49.7 (N-CH2), 27.0 (CH2), 16.5 (CH3), 16.2 (CH3) ppm. 31P NMR (162 MHz, CDCl3) δ 23.4 ppm. 19F NMR (376 MHz, CDCl3) δ −114.2 ppm. ESI-HRMS (CI) m/z calcd for C37H55F2N2O10P2 ([M + H]+) 787.3300, found 787.3295.
- Diethyl 2,2′-[(propane-1,3-diylbis((1-(diethoxyphosphoryl)ethyl)azanediyl))bis(methylene)]diacrylate (6h), (0.30 g, 69%) was isolated as a viscous clear oil as described in the general procedure. Data for 6h: 1H NMR (400 MHz, CDCl3) δ 6.13 (s, 2H, H2C=), 5.77 (s, 2H, H2C=), 4.14–3.95 (m, 12H, H2CO-P, HC-P and H2CO-C), 3.50–3.26 (m, 4H, H2CO-C and H2C-N), 3.07–2.99 (m, 2H, H2C-N), 2.52–2.41 (m, 2H, CH2), 1.49–1.45 (m, 2H, CH2), 1.25–1.13 (m, 24H, CH3) ppm. 13C {1H} NMR (101 MHz, CDCl3) δ 166.7 (C=O), 139.0 (C=CH2), 125.3 (=CH2), 61.0 (d, 2JPC = 7.2 Hz, P-OCH2), 60.3 (C-OCH2), 52.0 (N-CH2), 51.4 (d, 1JPC = 141.6 Hz, P-CH), 49.2 (N-CH2), 28.1 (CH2), 16.4 (CH3), 14.0 (CH3), 11.2 (d, 3JCP = 4.7 Hz, CH3), 10.7 (d, 3JCP = 4.8 Hz, CH3) ppm. 31P NMR (162 MHz, CDCl3) δ 28. 5 ppm. ESI-HRMS (CI) m/z calcd for C27H53N4O10P2 ([M + H]+) 627.3175, found, 627.3175.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tal, N.; Rudnick-Glick, S.; Margel, S. Novel bisphosphonates near infrared fluorescent and non-fluorescent nanoparticles of narrow size distribution for bone targeting. Polymer 2017, 132, 188–192. [Google Scholar] [CrossRef]
- Paredes, L.; Gonçalves, L.S.; Menezes Aguiar Miranda, A.M.; de Noronha Santos Netto, J.; da Cruz Perez, D.E.; Ramôa Pires, F. Knowledge of dental professionals and dental students on bisphosphonates and bisphosphonate-associated osteonecrosis of the jaws. Res. Soc. Dev. 2022, 11, e16211931553. [Google Scholar] [CrossRef]
- Fuggle, N.; Al-Daghri, N.; Bock, O.; Branco, J.; Bruyere, O.; Casado, E.; Cavalier, E.; Cortet, B.; de Wit, M.; Giusti, A.; et al. Novel formulations of oral bisphosphonates in the treatment of osteoporosis. Aging Clin. Exp. Res. 2022, 34, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Yu, X.; Jiang, J.; Yang, J.; Chen, L.; Yang, Z.; Yu, C. Small molecule-assisted assembly of multifunctional ceria nanozymes for synergistic treatment of atherosclerosis. Nat. Commun. 2022, 13, 6528. [Google Scholar] [CrossRef] [PubMed]
- Fields, S.C. Synthesis of natural products containing a C–P bond. Tetrahedron 1999, 55, 12237–12273. [Google Scholar] [CrossRef]
- Mucha, A.; Kafarski, P.; Berlicki, L. Remarkable potential of the α-aminophosphonate/phosphinate structural motif in Medicinal Chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef]
- Hiratake, J.; Oda, J. Aminophosphonic and aminoboronic acids as key elements of a transition state analogue inhibitor of enzymes. Biosci. Biotech. Biochem. 1997, 61, 211–218. [Google Scholar] [CrossRef]
- Lavielle, G.; Hautefaye, P.; Schaeffer, C.; Boutin, J.A.; Cudennec, C.A.; Pierre, A. New alpha-amino phosphonic acid derivatives of vinblastine: Chemistry and antitumor activity. J. Med. Chem. 1991, 34, 1998–2003. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Raut, D.S.; Rana, K.C.; Polanki, I.K.; Khan, M.S.; Iram, S. Diversity-oriented synthesis of α-aminophosphonates: A new class of potential anticancer agents. Eur. J. Med. Chem. 2013, 66, 146–152. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Rana, K.C.; Pannecouque, C.; De Clercq, E. An efficient synthesis of a hydroxyethylamine (HEA) isostere and its α-aminophosphonate and phosphoramidate derivatives as potential anti-HIV agents. ChemMedChem 2012, 7, 1601–1611. [Google Scholar] [CrossRef]
- Lejczak, B.; Kafarski, P.; Sztajer, H.; Mastalerz, P. Antibacterial activity of phosphono dipeptides related to alafosfalin. J. Med. Chem. 1986, 29, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.; Diel, P.J. Organic phosphorus compounds 94. Preparation, physical and biological properties of amino-arylmethylphosphonic- and –phosphonous acids. Phosphorus Sulfur Silicon Relat. Elem. 1991, 57, 57–64. [Google Scholar] [CrossRef]
- Kafarski, P.; Lejczak, B. Biological activity of aminophosphonic acids. Phosphorus Sulfur Silicon Relat. Elem. 1991, 63, 193–215. [Google Scholar] [CrossRef]
- Panwar, P.; Singh, S.; Kumar, N.; Rawat, H.; Mishra, A.K. Synthesis, characterization, and in vivo skeletal localization of a new 99mTc-based multidentate phosphonate chelate: 5-amino-1,3-bis(ethylamine-(N,N-dimethyl diphosphonic acid) acetamido) benzene. Bioorg. Med. Chem. 2007, 15, 1138–1145. [Google Scholar] [CrossRef]
- Cherkasov, R.A.; Garifzyanov, A.R.; Zakharov, S.V.; Vinokurov, A.V.; Galkin, V.I. Liquid extraction of noble metal ions with bis(α-aminophosphonates). Russ. J. Gen. Chem. 2006, 76, 417–420. [Google Scholar] [CrossRef]
- Garifzyanov, A.R.; Zakharoov, S.V.; Kryukov, S.V.; Galkin, V.I.; Cherkasov, R.A. Liquid extraction of noble metal ions with an α-amino phosphonate. Russ. J. Gen. Chem. 2005, 75, 1208–1211. [Google Scholar] [CrossRef]
- Jagodic, V.; Herak, M.J. Synthesis and physical properties of a novel aminophosphonic acid as an extracting agent for metals. J. Inorg. Nucl. Chem. 1970, 32, 1323–1332. [Google Scholar] [CrossRef]
- Bilgici, Z.S.; Ordu, O.D.; Isik, M.; Avci, D. Synthesis and polymerizations of six aminophosphonate-containing methacrylates. J. Polym. Sci. A Polym. Chem. 2011, 49, 5042–5048. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Yang, B.; Zhu, C.; Wei, Y.; Tao, L. ‘One pot’ synthesis of well-defined poly(aminophosphonate)s: Time for the Kabachnik-Fields reaction on the stage of polymer chemistry. Polym. Chem. 2014, 5, 1857–1862. [Google Scholar] [CrossRef]
- Rezaei, Z.; Firouzabadi, H.; Iranpoor, N.; Ghaderi, A.; Jafari, M.R.; Jafari, A.A.; Zare, H.R. Design and one-pot synthesis of α-aminophosphonates and bis(α-aminophosphonates) by iron(III) chloride and cytotoxic activity. Eur. J. Med. Chem. 2009, 44, 4266–4275. [Google Scholar] [CrossRef]
- Nag, S.; Batra, S. Applications of allylamines for the syntheses of aza-heterocycles. Tetrahedron 2011, 67, 8959–9061. [Google Scholar] [CrossRef]
- William, A.D.; Lee, A.C.-H.; Goh, K.C.; Blanchard, S.; Poulsen, A.; Teo, E.L.; Nagaraj, H.; Lee, C.P.; Wang, H.; Williams, M.; et al. Discovery of kinase spectrum selective macrocycle (16E)-14-methyl-20-oxa-5,7,14,26-tetraazatetracyclo[19.3.1.1(2,6).1(8,12)]heptacosa-1(25),2(26),3,5,8(27),9,11,16,21,23-decaene (SB1317/TG02), a potent inhibitor of cyclin dependent kinases (CDKs), Janus kinase 2 (JAK2), and Fms-like tyrosine kinase-3 (FLT3) for the treatment of cancer. J. Med. Chem. 2012, 55, 169–196. [Google Scholar] [CrossRef]
- Skoda, E.M.; Davis, G.C.; Wipf, P. Allylic amines as key building blocks in the synthesis of (E)-alkene peptide isosteres. Org. Process Res. Dev. 2012, 16, 26–34. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Q.; Wang, M.-X. (Eds.) Multicomponent Reactions in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2015; ISBN 978-3-527-67820-4. [Google Scholar]
- Wang, Y.; Cobo, A.A.; Franz, A.K. Recent advances in organocatalytic asymmetric multicomponent cascade reactions for enantioselective synthesis of spirooxindoles. Org. Chem. Front. 2021, 8, 4315–4348. [Google Scholar] [CrossRef]
- Lemos, B.C.; Venturini Filho, E.; Fiorot, R.G.; Medici, F.; Greco, S.J.; Benaglia, M. Enantioselective Povarov reactions: An update of a powerful catalytic synthetic methodology. Eur. J. Org. Chem. 2022, 2022, e202101171. [Google Scholar] [CrossRef]
- Fouad, M.A.; Abdel-Hamid, H.; Ayoup, M.S. Two decades of recent advances of Ugi reactions: Synthetic and pharmaceutical applications. RSC Adv. 2020, 10, 42644–42681. [Google Scholar] [CrossRef] [PubMed]
- Knapp, J.M.; Kurth, M.J.; Shaw, J.T.; Younai, A. Strategic Applications of Multicomponent Reactions in Diversity-Oriented Synthesis; Trabocchi, A., Ed.; Willey: New York, NY, USA, 2013; pp. 29–57. [Google Scholar] [CrossRef]
- Schreiber, S.L. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science 2000, 287, 1964–1969. [Google Scholar] [CrossRef] [PubMed]
- Varga, P.R.; Keglevich, G. Synthesis of α-aminophosphonates and related derivatives; the last decade of the Kabachnik-Fields reaction. Molecules 2021, 26, 2511. [Google Scholar] [CrossRef] [PubMed]
- Keglevich, G.; Balint, E. The Kabachnik–Fields reaction: Mechanism and synthetic use. Molecules 2012, 17, 12821–12835. [Google Scholar] [CrossRef]
- Mulla, S.A.R.; Pathan, M.Y.; Chavan, S.S.; Gample, S.P.; Sarkar, D. Highly efficient one-pot multi-component synthesis of α-aminophosphonates and bis-α-aminophosphonates catalyzed by heterogeneous reusable silica supported dodecatungstophosphoric acid (DTP/SiO2) at ambient temperature and their antitubercular evaluation against Mycobactrium Tuberculosis. RSC Adv. 2014, 4, 7666–7672. [Google Scholar] [CrossRef]
- Aissa, R.; Guezane-Lekoud, S.; Toffano, M.; Gali, L.; Aribi-Zouioueche, L. Fiaud’s acid, a novel organocatalyst for diastereoselective bis α-aminophosphonates synthesis with in-vitro biological evaluation of antifungal, antioxidant and enzymes inhibition potential. Bioorg. Med. Chem. Lett. 2021, 41, 128000. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, C.; Jmaï, M.; Jismy, B.; Abarbri, M.; M’rabet, H. An easy and regioselective synthesis of new functionalized isoxazoline derivatives via a 1,3-dipolar cycloaddition reaction. Synth. Commun. 2022, 52, 2291–2300. [Google Scholar] [CrossRef]
- Saroka, M.; Kaliniak, M.; Lecion, P.; Kwiecien, M.; Rosol, M.; Szczepaniec, A.; Buszkiewicz, P.; Banka, L. Method of producing new esters of α,ω-alkene diamino-N,N′-bis(1-aryl methyl phosponium) acids. Poland PL195533 B1, 28 September 2007. [Google Scholar]
- Liu, H.; Xu, K.; Ai, H.; Zhang, L.; Chen, M. Preparation and characterization of phosphorus-containing Mannich-type bases as curing agents for epoxy resin. Polym. Adv. Technol. 2009, 20, 753–758. [Google Scholar] [CrossRef]
- Villieras, J.; Rambaud, M. Ethyl α-(Hydroxymethyl)acrylate [2-propenoic acid, 2-(hydroxymethyl)-,ethyl ester]. Org. Synth. Coll. 1988, 66, 220. [Google Scholar] [CrossRef]
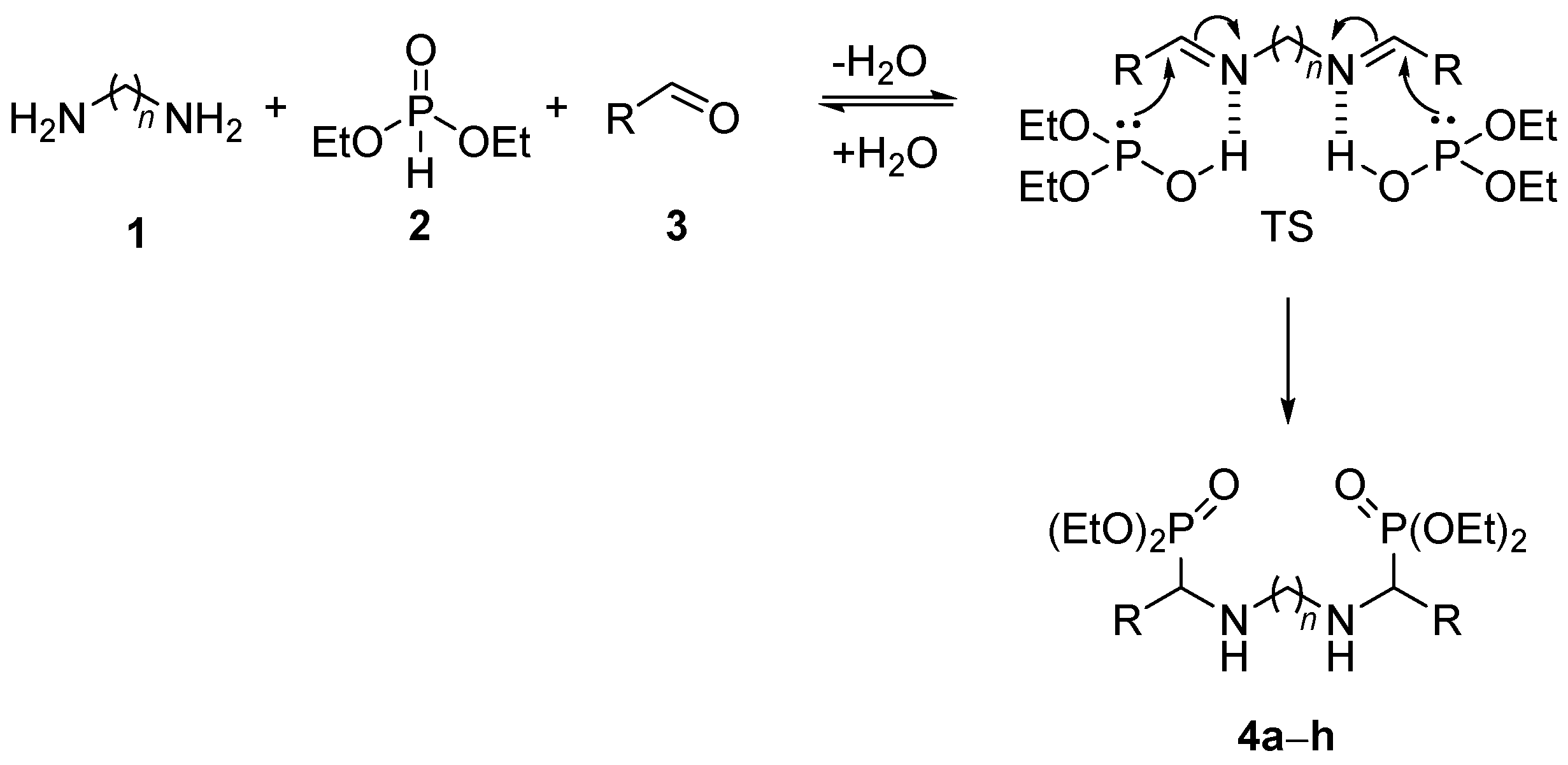
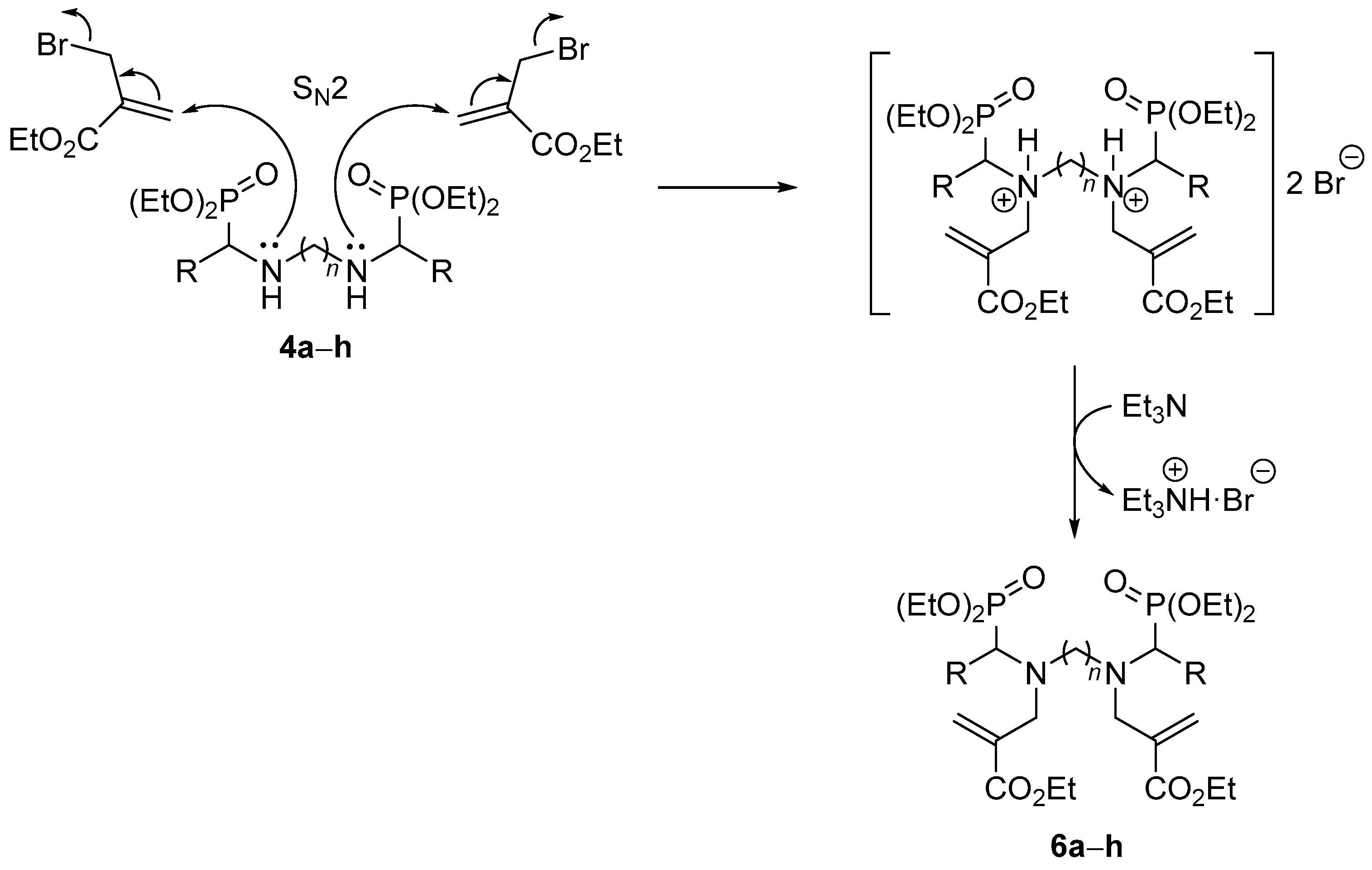
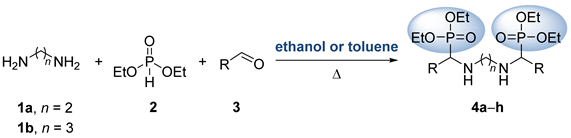
| Entry | Compound | R | n | Yield (%) [b] | |
|---|---|---|---|---|---|
| Method A | Method B | ||||
| 1 | 4a | C6H5 | 2 | 55 | 60 |
| 2 | 4b | p-MeC6H4 | 2 | 71 | 57 |
| 3 | 4c | C6H5 | 3 | 69 | 74 |
| 4 | 4d | p-MeC6H4 | 3 | 87 | 79 |
| 5 | 4e | p-MeOC6H4 | 3 | 66 | 63 |
| 6 | 4f | p-ClC6H4 | 3 | 68 | 71 |
| 7 | 4g | p-FC6H4 | 3 | 60 | 58 |
| 8 | 4h | Me | 3 | 79 | 86 |
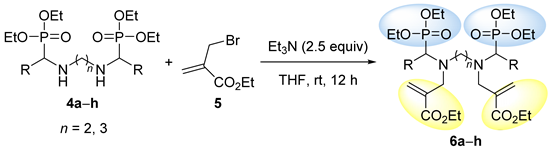
| Entry | Compound | R | n | Yield (%) [a] |
|---|---|---|---|---|
| 1 | 6a | C6H5 | 2 | 69 |
| 2 | 6b | p-MeC6H4 | 2 | 85 |
| 3 | 6c | C6H5 | 3 | 74 |
| 4 | 6d | p-MeC6H4 | 3 | 71 |
| 5 | 6e | p-MeOC6H4 | 3 | 74 |
| 6 | 6f | p-ClC6H4 | 3 | 87 |
| 7 | 6g | p-FC6H4 | 3 | 75 |
| 8 | 6h | Me | 3 | 69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souii, I.; Sanhoury, M.A.; Vicario, J.; Jiménez-Aberásturi, X.; Efrit, M.L.; M’rabet, H.; de los Santos, J.M. Synthesis and Characterization of a New Series of Bis(allylic-α-aminophosphonates) under Mild Reaction Conditions. Molecules 2023, 28, 4678. https://doi.org/10.3390/molecules28124678
Souii I, Sanhoury MA, Vicario J, Jiménez-Aberásturi X, Efrit ML, M’rabet H, de los Santos JM. Synthesis and Characterization of a New Series of Bis(allylic-α-aminophosphonates) under Mild Reaction Conditions. Molecules. 2023; 28(12):4678. https://doi.org/10.3390/molecules28124678
Chicago/Turabian StyleSouii, Ichrak, Med Abderrahmane Sanhoury, Javier Vicario, Xabier Jiménez-Aberásturi, Mohamed L. Efrit, Hedi M’rabet, and Jesús M. de los Santos. 2023. "Synthesis and Characterization of a New Series of Bis(allylic-α-aminophosphonates) under Mild Reaction Conditions" Molecules 28, no. 12: 4678. https://doi.org/10.3390/molecules28124678
APA StyleSouii, I., Sanhoury, M. A., Vicario, J., Jiménez-Aberásturi, X., Efrit, M. L., M’rabet, H., & de los Santos, J. M. (2023). Synthesis and Characterization of a New Series of Bis(allylic-α-aminophosphonates) under Mild Reaction Conditions. Molecules, 28(12), 4678. https://doi.org/10.3390/molecules28124678










