Abstract
Zaitra, Thymus satureioides, is an aromatic plant with a long history of use in traditional medicine. In this study, we assessed the mineral composition, nutritional value, phytocontents, and dermatological properties of the aerial parts of T. satureioides. The plant contained high contents of calcium and iron, moderate levels of magnesium, manganese, and zinc, and low contents of total nitrogen, total phosphorus, total potassium, and copper. It is rich in several amino acids, including asparagine, 4-hydroxyproline, isoleucine, and leucine, and the essential amino acids account for 60.8%. The extract contains considerable amounts of polyphenols and flavonoids (TPC = 118.17 mg GAE/g extract and TFC = 32.32 mg quercetin/g extract). It also comprises 46 secondary metabolites, identified through LC-MS/MS analysis, belonging to phenolic acids, chalcones, and flavonoids. The extract elicited pronounced antioxidant activities, inhibited the growth of P. aeruginosa (MIC = 50 mg/mL), and reduced biofilm formation by up to 35.13% using the ¼ sub-MIC of 12.5 mg/mL. Moreover, bacterial extracellular proteins and exopolysaccharides were diminished by 46.15% and 69.04%, respectively. Likewise, the swimming of the bacterium was impaired (56.94% decrease) in the presence of the extract. In silico, skin permeability and sensitization effects revealed that out of the 46 identified compounds, 33 were predicted to be exempt from any skin sensitivity risk (Human Sensitizer Score ≤ 0.5), while extensive skin permeabilities were observed (Log Kp = −3.35–−11.98 cm/s). This study provides scientific evidence about the pronounced activities of T. satureioides, supports its traditional uses, and promotes its utilization in the development of new drugs, food supplements, and dermatological agents.
1. Introduction
Thymus satureioides Coss., commonly known as savory thyme, is a medicinal plant from the Lamiaceae family [1,2]. It is a perennial shrub that grows between 10 and 60 cm in height and is found exclusively in semiarid regions of the Moroccan High Atlas and Anti-Atlas. Locally, it is referred to as “Zaitra” or “Azkuni” [3,4,5]. Traditional uses of this plant include the treatment of various conditions such as diabetes, hypertension, fever, skin and blood circulation disorders, bronchitis, pain perception, immune system issues, pharyngitis, and influenza [6]. Furthermore, several studies have investigated the pharmacological properties of T. satureioides extracts and essential oils, revealing their potential as antidiabetic [2], anticancer [7], anti-inflammatory [8], antimicrobial [9,10,11], insecticidal [12,13], and hypolipidemic [14] agents. However, the underlying mechanisms responsible for most of these pharmacological effects have received limited research attention [6].
Like others, T. satureioides has gained attention for its dermatological applications due to its high content of antioxidants, antimicrobials, and anti-inflammatory compounds. Plant-based dermatological agents were shown to improve the overall health and appearance of the skin by providing nutrients, hydration, and protection from environmental stressors. T. satureioides contains a range of bioactive compounds such as thymol, carvacrol, and rosmarinic acid, which have been shown to have anti-aging, anti-inflammatory, and antimicrobial properties [6]. Many of these phytoconstituents have been incorporated into various dermacosmeceutical products such as creams, lotions, and serums. These products have been shown to improve skin hydration, elasticity, and firmness while reducing the appearance of fine lines and wrinkles [15]. Additionally, T. satureioides has been found to have a protective effect against UV radiation, which is a major contributor to skin damage and aging [6].
Pseudomonas aeruginosa is a Gram-negative, aerobic, rod-shaped bacterium that is commonly found in soil, water, and moist environments. This bacterium is known to cause a variety of infections, including skin and wound infections [16]. P. aeruginosa is one of the most common bacteria responsible for wound infections, particularly in immunocompromised individuals or those with chronic wounds. The skin and wound manifestations of P. aeruginosa infections can vary widely depending on the severity of the infection and the location of the wound. Common symptoms of P. aeruginosa infections include redness, swelling, pain, and pus formation. In severe cases, the infection may progress to tissue destruction, necrosis, and sepsis, which can be life-threatening [17]. Effective treatment of P. aeruginosa infections requires a combination of antimicrobial therapy and wound management strategies [18]. In this context, the search for natural products that could counteract the skin and wound manifestations of P. aeruginosa infections is an attractive strategy for healthcare professionals.
Besides their flavor and medicinal properties, Thymus species are also known for their mineral and nutritional minerals, such as calcium, magnesium, potassium, and iron, which are important for various functions including bone health, nerve transmission, and muscle contraction [19]. They also contain various vitamins, such as vitamins A, C, and K, which have antioxidant properties and are important for maintaining healthy skin, vision, and blood clotting [19].
The review of the existing literature has revealed that there have been no studies so far on the potential nutritional value of T. satureioides. Therefore, the current study aimed to examine the chemical components, nutraceutical value, and mineral contents of the plant and explore its antioxidant properties. Additionally, the extract was also evaluated against P. aeruginosa growth and virulence factors, including biofilm formation, protein and exopolysaccharide production, and swarming and swimming mobilities. We also performed an in silico study and discussed the potential mechanisms of action of the plant’s bioactive compounds, their potential applications in the dermatological industry, and their safety profile.
2. Results
2.1. Mineral Composition
T. satureioides contains high contents of calcium and iron, moderate levels of magnesium, manganese, and zinc, and low contents of total nitrogen, total phosphorus, total potassium, and copper (Table 1).

Table 1.
Mineral composition of T. satureioides aqueous extract.
2.2. Amino Acid Contents
Amino acid analysis showed that asparagine, 4-hydroxyproline, isoleucine, and leucine are present in relatively high concentrations compared to the other amino acids (Table 2). Moreover, the essential amino acids represent 60.8% of the total amino acids, which is a relatively high value and indicates good protein quality.

Table 2.
Amino acid composition of T. satureioides.
2.3. LC-MS Profiling
The aqueous extract of T. satureioides aerial parts was analyzed by the HPLC-PDA-MS/MS. The phytoconstituents were tentatively identified using their MS, MS2 fragments and retention times. In total, 46 secondary metabolites belonging to phenolic acids, chalcones, and flavonoids were identified (Table 3 and Figure 1).

Table 3.
Polyphenolics from T. satureioides aerial parts extract.
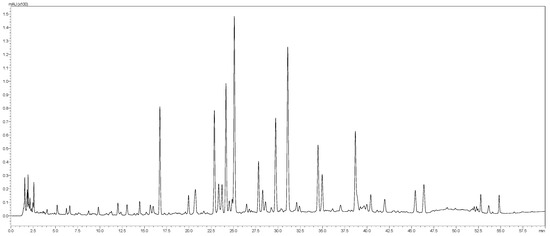
Figure 1.
LC-MS profile of T. satureioides aerial parts extract.
2.4. Phytocontents and In Vitro Antioxidant Assays
The extract contains substantial amounts of polyphenols and flavonoids (Table 4). Accordingly, the extract displayed noticeable antioxidant activity (Table 4). Comparatively, the standard antioxidant, quercetin, showed a significantly higher antioxidant effect, as evidenced by a lower IC50 in both assays.

Table 4.
Phytocontents and in vitro antioxidant activities of the aqueous extract of T. satureioides aerial parts.
2.5. Antibacterial Activities
In this study, T. satureioides aqueous extract was able to completely inhibit the growth of P. aeruginosa at a MIC of 50 mg/mL (Figure 2a). Noteworthy, the extract exhibited an inhibitory effect in a dose-dependent manner. Moreover, as targeting bacterial virulence factors is being explored as a novel approach to weakening microbial pathogenicity [20], we evaluated the effect of the extract on the ability of P. aeruginosa to form biofilm communities as well as their swimming and swarming mobilities. These two parameters are among the most important virulence determinants, allowing bacteria to form protected bacterial communities and move over biological surfaces [21,22]. Noticeably, T. satureioides extract at the 1/4 sub-MIC (12.5 mg/mL) substantially reduced the biofilm produced with an inhibition percentage of up to 35.13% (Figure 2b).
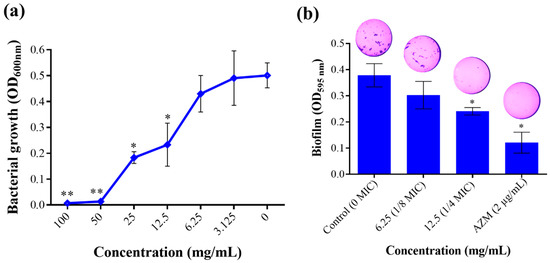
Figure 2.
Effect of T. satureioides aqueous extract on (a) bacterial growth rate and (b) biofilm production by P. aeruginosa cultured in the absence and presence of the extract at sub-inhibitory concentrations. Asterisks (*) indicate a significant difference compared to the control media at p < 0.05. Asterisks (**) indicate a significant difference compared to the control media at p < 0.01.
Antimicrobials are also known to interfere with many virulence factors secreted by pathogenic bacteria, including toxins, exo-proteases, adhesins, and exopolysaccharides. In this study, we demonstrated that T. satureioides aqueous extract at ¼ MIC (12.5 mg/mL) induced a significant reduction in the amount of extracellular proteins and sugar contents (Figure 3a). The extract showed high performance in inhibiting proteins’ secretion compared to the positive control, azithromycin (2 µg/mL), with up to 46.15% inhibition. Likewise, exopolysaccharides were significantly decreased by up to 69.04% as compared to untreated media (Figure 3a).
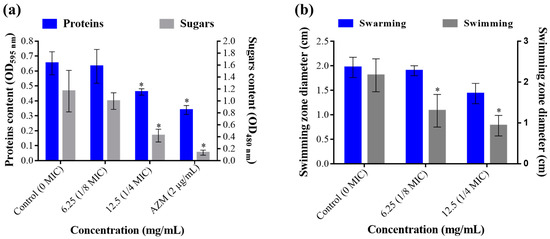
Figure 3.
Effect of T. satureioides aqueous extract on (a) total extracellular proteins and sugar contents and (b) swarming and swimming mobilities of P. aeruginosa in the absence and presence of the extract at sub-inhibitory concentrations. Asterisks (*) indicate a significant difference compared to the control media (p < 0.05).
In addition to virulence-related molecules, bacteria are endowed with different types of mobilities, allowing them to find a host, evade host immune responses, colonize tissues, and disperse and spread [21,23]. Here, we monitored the effect of the sub-inhibitory concentrations of T. satureioides aqueous extract on the swimming and swarming mobility of P. aeruginosa on plates. This showed that the extract significantly reduced the swimming (up to 56.94% decrease) mobility of the bacterium in a dose-dependent manner (Figure 3b). In addition, even though the extract also reduced the swarming zone by up to 27.04%, ANOVA analysis showed no significant differences compared to the negative control plates (0 mg/mL) (Figure 3b).
2.6. Skin Permeability and Sensitization Effects of Identified Compounds
In order to computationally evaluate the skin sensitization of the identified compounds, we used the SkinSensDB database, which is based on adverse outcome pathway (AOP)-based computational prediction models. Out of the 46 identified compounds, 33 were predicted to be exempt from any skin sensitivity risk (Human Sensitizer Score ≤ 0.5) (Table 1).
The prediction of the permeability coefficient (log Kp) for the transport of compounds through the mammalian epidermis is based on the linear model developed by Potts R.O. and Guy R.H. (1992) [24]. The more negative the log Kp value, the less skin-permeant the molecule. Here, the log Kp values were computed using the SwissADME online tool. The 46 compounds showed extensive skin permeability abilities, with log Kp values ranging from −3.35 cm/s (Phloretic acid caffeate) to −10.90 cm/s (Eriodictyol rutinoside) (Table 1). For instance, dimethyl quercetin and apigenin were shown to have higher skin permeability (−5.99 and −5.80 cm/s) than quercetin pentoside and galloyl glucose (−10.54 and −10.66 cm/s, respectively).
3. Discussion
T. satureioides is a species of thyme that is widely distributed in the Mediterranean region. This herb has been traditionally used as a medicinal plant, and recent studies have demonstrated its potential for a wide range of health benefits. In this study, we showed that the aqueous extract of its aerial parts contains a variety of phytochemicals. We also demonstrated that it is endowed with strong antioxidant, antibacterial, antibiofilm, and anti-quorum sensing activities in vitro.
After reviewing subsequent and more recent literature, we noticed that the nutritional value of T. satureioides has never been examined so far. Therefore, we analyzed the mineral content of T. satureioides. Although the normal mineral contents in medicinal plants can vary greatly depending on the specific plant species, its growing conditions, and the part of the plant being studied, general ranges showed that T. satureioides contains high contents of calcium and iron, moderate levels of magnesium, manganese, and zinc, and low contents of total nitrogen, total phosphorus, total potassium, and copper. This suggests that the plant may be a good source of calcium and iron, as well as other important minerals. Comparatively to closely related thymus species such as T. vulgaris, T. satureioides contains higher levels of iron (591.25 versus 174.5 mg/Kg DW) and manganese (48.6 versus 17.19 mg/Kg DW) but lower contents of zinc (14.66 versus 18.1 mg/Kg DW) [19]. Noteworthy, T. vulgaris was also shown to be rich in some important vitamins such as folic acid, B complex, β-carotene, and vitamins A, K, E, and C [19]. Hence, determining the vitamin content of T. satureioides would be of interest to extend the health benefits of this species.
In addition to the mineral content analysis, we also profiled the phytoconstituents of the extract using LC/MS-MS. We detected 46 compounds belonging mainly to phenolic acids, chalcones, and flavonoids. These include apigenin, quercetin, luteolin, and rosmarinic and salvianolic acids. Previous studies corroborate this phytochemical profile, where some similar compounds were identified from T. satureioides, mainly apigenin, luteolin, chlorogenic acid, feruloyl caffeic acid, and rosmarinic acid, among others [6]. The richness of this species in phenolic compounds and flavonoids is most likely responsible for its strong antioxidant properties. These compounds act as free radical scavengers and prevent cellular damage caused by oxidative stress, thus maintaining overall health and preventing chronic diseases such as cancer, diabetes, cardiovascular diseases, and age-related damage [25]. Here, we showed that the extract contains a high content of total phenolics and flavonoids and exhibits a strong antioxidant potential using both DPPH and ABTS assays. Similar observations were reported showing that T. satureioides extracts were the richest in total phenolics (up to 70.2 mg GAE/g) and flavonoids (up to 52.7 mg QuE/g) compared to the other extracts from Petroselinum crispum and the microalgae Spirulina platensis [26]. Elsewhere, the aqueous extract of T. satureioides was also shown to elicit ferric-reducing antioxidant power (FRAP) at up to 7.03 ± 0.29 mmol trolox/g [27]. Interestingly, salvianolic acid A, a compound detected in the tested extract, was previously shown to protect retinal pigment epithelial cells against oxidative stress via activating Nrf2/HO-1 signaling pathways [28]. In addition to these compounds, T. satureioides is also known to contain high amounts of essential oils, which contribute to its aromatic and flavor properties [29].
Although there is substantial data in the literature justifying the traditional use of T. satureioides in skin diseases [6,30,31], there is no biological and/or pharmacological experimental evidence regarding these claims. In this regard, the dermacosmeceutical potential of T. satureioides is one of the main original aspects addressed in this study. It was investigated by assessing the extract’s effect against P. aeruginosa, a main pathogenic bacterium with common skin-related symptoms. These can include redness, inflammation, and the formation of pus-filled lesions known as pyoderma. P. aeruginosa infections can also cause a condition called hot tub folliculitis, which is characterized by a rash of small, red, itchy bumps that appear after exposure to contaminated water sources, such as hot tubs. In severe cases, P. aeruginosa infections can lead to tissue necrosis, which can cause extensive tissue damage. The skin manifestation of P. aeruginosa infections can be particularly problematic for immunoexpressed subjects [18,32,33]. The antibacterial effect of T. satureioides was largely investigated using its essential oil against a large spectrum of species, including Enterobacter cloacae, Staphylococcus aureus, Acinetobacter baumannii, Escherichia coli, and Bacillus cereus [34]. In this work, we tested the aqueous extract of T. satureioides aerial parts and showed that it has a strong antibacterial effect against P. aeruginosa. In addition, lower concentrations, sub-MICs (6.25 and 12.5 mg/mL), were potent and significantly reduced the biofilm formation abilities of the bacterium. Biofilm formation by P. aeruginosa is a critical aspect of its pathogenicity, as it allows the bacterium to colonize and persist in a wide range of environments, including medical devices and human tissues. They also make the bacterium more resistant to many antibiotics [35].
Compared to other thymus species, Noumi et al. (2023) showed that the methanolic extract of Thymus musilii Velen. was able to completely inhibit the growth of P. aeruginosa at MIC = 12.5 mg/mL and induced high inhibitory effects on its ability to swarm (up to 39.73 ± 1.50%) and swim (up to 25.18 ± 1.00%). Noticeably, T. musilii extract did not significantly inhibit the biofilm formation at >12.5 mg/mL for P. aeruginosa, while it impaired its formation by Salmonella typhimurium (53.96 ± 4.21%) and Listeria monocytogenes (49.54 ± 4.5%) [36]. Therefore, T. satureioides and its phytoconstituents could be promising in counteracting the ability of P. aeruginosa to form biofilms during infections by inhibiting the formation of a protective matrix of bacterial communities and thus their adherence to biological surfaces. In addition to biofilm formation, P. aeruginosa produces a variety of virulence factors, including proteases, that contribute to its ability to cause infections in humans. The combination of biofilm formation and virulence factor production makes P. aeruginosa a serious pathogen that poses a significant threat to public health. In this study, total extracellular protein quantification revealed that T. satureioides extract, at the sub-MIC of 12.5 mg/mL, was able to significantly reduce protein secretion by the bacterium by up to 46.15%. Similarly, secreted exopolysaccharides were significantly decreased by up to 69.04% when culture media contained 12.5 mg/mL of the extract. These anti-quorum sensing effects were also investigated using Mangifera indica L. extract at 800 µg/mL, which was demonstrated to be potent in reducing total protease (56%), elastase (76%), chitinase (55%), exopolysaccharide secretion (58%), and swarming motility (74%), in P. aeruginosa PAO1 [37]. Our findings demonstrated that T. satureioides phytocompounds could target bacterial genes involved in proteases (e.g., lasR, lasI, rhlI, rhlR, exoU, lasB) and biofilm (e.g., pslA, pelA, ppyR) synthesis [38]. In fact, quercetin, one of the major compounds identified in our plant extract, was previously shown to be effective in reducing the expression of several quorum sensing genes, lasR, lasI, rhlI, and rhlR, by 68, 34, 57, and 50%, respectively [39]. Indeed, many studies have corroborated the potency of plant-based extracts and their phytoconstituents to impair bacterial virulence factors. For instance, the aqueous extracts from Conocarpus erectus, Callistemon viminalis, and Bucida buceras were investigated for their impact on P. aeruginosa’s virulence factors and quorum sensing. The findings showed that all three plants significantly suppressed LasA protease, LasB elastase, pyoverdin production, and biofilm formation. Moreover, each plant had a different effect on the Las and Rhl genes and their corresponding signaling molecules, highlighting that different mechanisms were involved in their effectiveness [40]. Outstandingly, the effect of T. satureioides, especially its polar extracts, has not been examined for their anti-quorum sensing activities. Considering these preliminary data, the utility of the anti-quorum sensing properties of T. satureioides extract in combating P. aeruginosa infections by preventing the formation of biofilms, reducing the production of toxins, and impairing the ability of bacteria to colonize and infect host tissues seems attractive. This approach may have several advantages over traditional antibiotics, such as reducing the risk of antibiotic resistance and promoting wound healing [41].
Besides phytochemicals, minerals, and vitamins, amino acids are very important in the dermacosmeceutical industry as they contribute to triggering different signaling pathways within human skin cells, which activate particular genes associated with aging and safeguard the cells from stress factors [42,43]. Interestingly, it was suggested that hydrophobic amino acids significantly contribute to the antioxidant ability of natural products [44]. In the present study, we noticed that T. satureioides is rich in asparagine, 4-hydroxyproline, isoleucine, and leucine compared to the other amino acids characterized, indicating that they may be important for protein function and structure in the plant. The presence of 4-hydroxyproline is noteworthy as it is a non-standard amino acid that is usually found in collagen, the main structural protein in animals, and it is less common in plant proteins. In addition, 4-hydroxyproline is a critical element in stabilizing the triple helical conformation of collagen [45], a protein that conforms the supportive and connecting tissues in the skin [46]. Moreover, the ratio of essential amino acids (isoleucine, leucine, phenylalanine, threonine, valine, histidine, and lysine) to non-essential amino acids (alanine, aspartic acid, asparagine, glutamic acid, serine, and tyrosine) can reflect the protein quality of the plant. Noticeably, the essential amino acids represented 60.8% of the total amino acids, which is a relatively high value and indicates good protein quality. The amino acid composition of T. satureioides suggests that it may have a nutritionally valuable protein content and could be integrated as an active ingredient in formulating dermacosmeceutical agents [44].
An important advancement in the field of toxicology in recent times has been the utilization of alternative methods to animal testing. These methods, including those for skin sensitization, have gradually been integrated into regulatory practices [47]. Hence, as topical dermacosmeceuticals are a popular category of skincare products that are formulated with active ingredients, ensuring the safety of consumers is mandatory and constitutes a critical concern in their manufacturing. Indeed, skin sensitization is a crucial aspect of the process of developing drugs and making regulatory decisions. Chemical sensitizers, including plants, act by binding to proteins, causing immune responses that may lead to allergic contact dermatitis [48]. Sensitization is a process where the body’s immune system reacts to a substance that it recognizes as foreign, even though the substance may not be harmful [49]. Here, out of the 46 identified compounds, 33 were predicted to be exempt from any skin sensitivity risk (Human Sensitizer Score ≤ 0.5). In addition, the compounds showed diverse skin permeabilities, with log Kp values ranging from −3.35 cm/s (Phloretic acid caffeate) to −10.90 cm/s (Eriodictyol rutinoside). This shows that most of the plant phytochemicals are potentially safe agents for dermatological applications. Nevertheless, dermatotoxicity assays in vivo involving skin irritation (e.g., occluded dermal irritation test method), skin sensitization (e.g., Mouse Ear Swelling Test (MEST)), acute dermal toxicity, and repeated toxicity tests would be mandatory to bridge the knowledge gap on the cosmeceutical and dermatological applications of T. satureioides.
4. Material and Methods
4.1. Plant Material and Extract Preparation
Flowering aerial parts of T. satureioides Coss. were purchased from a local herbalist in Ben Guerir, Morocco. The dried plant material was subjected to hydro-distillation (150 g/L, w/v) utilizing a Clevenger apparatus until no more essential oil (EO) was found. The residual water from the hydro-distillation process was collected, filtered, evaporated under reduced pressure (BUCHI, Flawil, Switzerland), and lyophilized to obtain the crude extract (24.7 g).
4.2. Mineral Analysis
Nitrogen content was analyzed using the Kjeldahl method [50], while inductively coupled plasma optical emission spectrometry (ICP-OES) was utilized to estimate the levels of total phosphorus, potassium, calcium, magnesium, sodium, iron, manganese, zinc, and copper.
4.3. LC-MS Analysis
The phytochemical characterization of the extract was performed using the HPLC-PDA-MS/MS system consisting of a Shimadzu Japan system (Tokyo, Japan) coupled to an MS 8050 mass spectrometer with an electrospray ionization (ESI) source, as previously described [51].
4.4. Analysis of Amino Acids
Amino acid characterization and quantification were analyzed using high-performance liquid chromatography (HPLC) tandem mass spectrometry (Shimadzu 8050, Tokyo, Japan) [51]. Forty mg of the plant extract was hydrolyzed in 10 mL of 6 M HCl for 22 h at 110 °C. The hydrolyzed sample was cooled down to 4 °C to stop the hydrolyzation process and then diluted in 50 mL of distilled water. The hydrolysate pH was adjusted to 4.5 and filtered using a 0.22-micrometer PTFE membrane to remove suspended particles. Liquid chromatography was performed at 40 °C using a Shim-pack GIST PFPP Kyoto, Japan (2.1 mm I.D. × 150 mm, 3.0 mm) column and a gradient system with the mobile phase consisting of solvent water and acetonitrile with 0.1% of formic acid in each solvent, at a flow rate of 0.25 mL/min, and an injection volume of 3 μL. The gradient program used 0–2 min 100% of A, 5 min 75% of A, 11 min 65% of A, 16 min 50%, 19 min 5%, 30 min 100% of B for 2 min, and hold for 4 min. The conditions of mass spectroscopy were in ESI positive and negative modes.
4.5. Phytocontents
The total polyphenolic content (TPC) in a 96-well microplate was determined by utilizing the Folin-Ciocalteu method [52]. Firstly, 20 μL of the extract with a concentration of 1 mg/mL was added to the microplate, followed by the addition of 100 μL of Folin-Ciocalteu reagent (10% v/v). The mixture was then incubated for 5 min in the dark at room temperature, after which 80 μL of 7.5% Na2CO3 (w/v) was added and mixed thoroughly. The resulting mixture was then incubated for an additional 30 min in the dark at room temperature, and its absorbance was measured at 765 nm using a UV-vis spectrophotometer (BMG LABTECH, Bath, UK). The TPC was expressed as mg GAE/g DW (micrograms of gallic acid equivalents per milligram of dry weight extract), and a calibration curve was established using gallic acid as the standard. The experiment was carried out in triplicate for all samples.
The total flavonoid content (TFC) in the extract was detected using the aluminum chloride (AlCl3) method [53]. To prepare the reaction mixture, 100 μL of the extract with a concentration of 1 mg/mL was combined with 0.5 μL of AlCl3 (1.2%) and 0.5 μL of potassium acetate (120 mM). The mixture was allowed to stand for 30 min at room temperature, and the absorbance of the reaction mixture was measured at 415 nm. The TFC was expressed as μg of quercetin equivalent per mg of dry weight extract (μg QE/mg DW), and all samples were tested in triplicate.
4.6. Antioxidant Activity In Vitro
4.6.1. DPPH Radical Scavenging Assay
The extract’s ability to scavenge DPPH was assessed using a modified spectrophotometric method [54]. Briefly, 100 μL of the extract and 100 μL of a 0.2 mM DPPH solution were combined in each well of a 96-well plate. The plate was then kept in the dark at room temperature for 30 min, after which the absorbance was measured at 517 nm. Quercetin was employed as a standard (1 to 1000 μg/mL), and all samples were tested in triplicate. The scavenging capacity was determined using the following equation:
4.6.2. ABTS Radical Scavenging Assay
The ABTS radical cation method was modified to evaluate the free radical-scavenging effect of the extract [55]. First, ABTS+ was generated by oxidizing ABTS with potassium persulfate. The resulting mixture was then kept in the dark at room temperature for 16 h. Afterward, the mixture was diluted with ethanol to an absorbance of 0.7 ± 0.02. Next, 180 μL of the ABTS reagent was mixed with 20 μL of the extract in a 96-well microplate, and the mixture was incubated at room temperature for 6 min. After the incubation period, the absorbance was measured at 734 nm. All determinations were carried out in triplicate. The ABTS scavenging effect was calculated using the following formula:
The IC50 ABTS values were obtained through extrapolation from the regression analysis.
4.7. Antibacterial Activity
4.7.1. Minimal Inhibitory Concentration (MIC) Determination
The antibacterial activity of T. satureioides extract was tested using the microdilution assay in a sterile 96-well microplate [56,57]. The extract was solubilized in Mueller Hinton (MH) broth, filtered using 0.22 μm sterile syringe filters, two-fold serially diluted (100, 50, 25, 12.5, and 6.25 mg/mL), and then transferred into the microplate’s wells in triplicates (200 μL per well). Afterward, 5 μL of a fresh overnight culture of P. aeruginosa adjusted to an OD600nm of 0.6 were inoculated into each well and incubated at 37 °C under 150 rpm shaking for 18 h. The minimum inhibitory concentration (MIC) corresponds to the lowest concentration that inhibits visible microbial growth. The bacterial viability was checked at OD600nm. The extract-free media as well as the uninoculated MH media were used as controls.
4.7.2. Bacterial Biofilm Inhibition Assay
The anti-biofilm effect of the extract was evaluated using the crystal violet colorimetric assay [58,59,60]. Firstly, the plant extract at 6.25 mg/mL (1/8 MIC) and 12.5 mg/mL (1/4 MIC) was filtered using 0.22 μm sterile syringe filters, inoculated, and incubated as described above. Media without bacterial inoculation were used as negative controls. After 18 h of incubation, the culture suspensions were discarded, and the wells were washed four times with a PSB solution to eliminate planktonic bacteria. Next, each well was filled with 1% crystal violet and kept for 15 min at room temperature. Afterward, a vigorous washing with distilled water was carried out to remove any excess dye. The biofilm was solubilized by 95% ethanol and quantified at OD595nm using a multimode plate reader. Azithromycin at 2 µg/mL was used as the standard antibiotic [37].
4.7.3. Swimming and Swarming Inhibition Assays
T. satureioides extract was also evaluated for its potential effect on the swimming and swarming motilities of P. aeruginosa. The concentrations of 6.25 mg/mL (1/8 MIC) and 12.5 mg/mL (1/4 MIC) of the extract were aseptically added to the swimming (1% tryptone, 0.5% sodium chloride, and 0.3% agar) and swarming (semisolid LB medium, 0.6%) media [61]. Next, 10 μL of a fresh overnight culture of P. aeruginosa (OD600nm = 1) was deposited at the center of each plate and incubated at 37 °C for 18 h. The mobilities’ zone diameters were measured in cm [56,58]. Extract-free media were used as the negative control.
4.7.4. Outer Membrane Protein Quantification
To evaluate the effect of the extract on the amount of virulence factors produced by the bacterium, the outer membrane proteins were quantified using the Bradford assay in a sterile 96-well microplate [62,63]. Briefly, P. aeruginosa was cultivated in the presence (1/8 and 1/4 MIC) and absence of the extract for 24 h. The cultures were centrifuged (10,000× g; 12 min), and the supernatants were filtered using 0.45 μm syringe filters. The filtered supernatants were used to quantify the total protein content at OD 595 nm. Azithromycin at 2 µg/mL was used as the standard molecule, and uninoculated media were used as the blank controls [37].
4.7.5. Exopolysaccharide Estimation
In addition to the total protein content, the supernatants were used to extract the EPS by the addition of chilled ethanol (95%) to completely precipitate the EPS and left overnight to precipitate the EPS at 4 °C. The concentrations of sugar content were determined using the protocol of [37,64]. Azithromycin at 2 µg/mL was used as the standard molecule, and media without inoculum were used as blanks [37].
4.8. In Silico Suitability for Skin Application
The rate at which a phytocompound can penetrate the stratum corneum, known as skin permeability (log Kp), is a commonly used parameter to quantify the transport of molecules across the outermost layer of the epidermis and indicate the extent of skin absorption. We checked all identified compounds for log Kp using the SwissADME database [65]. Furthermore, we assessed the skin sensitization potential of identified compounds using the human skin sensitizer score from the SkinSens database (available at: https://cwtung.kmu.edu.tw/skinsensdb/, accessed on 1 June 2023). The score is based on a scale from 0 to 1, with phytochemicals that score 0 being the least skin-sensitizing and those that score 1 being the most skin-sensitizing. Compounds scoring above a certain threshold (≥0.5) are considered to be skin sensitizers.
4.9. Statistical Analysis
Data analysis was performed in IBM SPSS Statistics 20 software using one-way analysis of variance (ANOVA) followed by many-to-one comparisons according to the post-hoc analysis with Dunnett’s test. The data represent the mean and standard deviation of three independent experiments. Each treatment was compared to a single control. Significant differences were set at p < 0.05.
5. Conclusions
In the present study, we demonstrated that T. satureioides is endowed with a wide range of nutritional, dermatological, and anti-quorum sensing properties. The plant contained high levels of calcium and iron, moderate amounts of magnesium, manganese, and zinc, and low levels of nitrogen, phosphorus, potassium, and copper. It is rich in amino acids, with essential amino acids comprising a substantial portion. The extract exhibited potent antioxidant activity, inhibited the growth of P. aeruginosa, reduced biofilm formation, and impacted bacterial swimming and extracellular protein and exopolysaccharide production. The plant’s compounds were also examined for their suitability for dermatological applications via in silico toxicity assessment of the skin sensitivity score and permeability index. This revealed a low skin sensitivity risk for most identified compounds and highlighted varying degrees of skin permeability. This makes the plant and its phytochemicals promising candidates for use in the development of new drugs and food supplements. Nevertheless, further research is needed to explore the full potential of T. satureioides, understand its mechanisms of action and potential dermatotoxicities, and develop effective formulations for various skin disorders.
Author Contributions
I.M. performed the biological activities and the in silico study and wrote the manuscript. N.F. and H.A. performed the in vitro assays. B.D. performed the characterization and quantification assays. M.B. and M.F.M. revised the manuscript. M.S. revised the manuscript and designed the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study is contained within the article.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationship that could have appeared to influence the work reported in this paper.
Sample Availability
Not applicable.
References
- El-Fassi Fihri, A. La Pharmacopée Marocaine Traditionnelle, Jamal Bellakhdar. Horiz. Maghrébins Le Droit À La Mémoire 1998, 35, 319–321. [Google Scholar]
- Kabbaoui, M.; Chda, A.; Mejrhit, N.; Azdad, O.; Farah, A.; Aarab, L.; Bencheikh, R.; Tazi, A. Antidiabetic Effect of Thymus Satureioides Aqueous Extract in Streptozotocin-Induced Diabetic Rats. Int. J. Pharm. Pharm. Sci. 2016, 8, 140–145. [Google Scholar] [CrossRef]
- Bellakhdar, J.; Claisse, R.; Fleurentin, J.; Younos, C. Repertory of Standard Herbal Drugs in the Moroccan Pharmacopoea. J. Ethnopharmacol. 1991, 35, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Benabid, A. Flore et Écosystèmes Du Maroc: Evaluation et Préservation de La Biodiversité; Ibis Press: Paris, France, 2000. [Google Scholar]
- Drioiche, A.; Zahra Radi, F.; Ailli, A.; Bouzoubaa, A.; Boutakiout, A.; Mekdad, S.; AL Kamaly, O.; Saleh, A.; Maouloua, M.; Bousta, D.; et al. Correlation between the Chemical Composition and the Antimicrobial Properties of Seven Samples of Essential Oils of Endemic Thymes in Morocco against Multi-Resistant Bacteria and Pathogenic Fungi. Saudi Pharm. J. 2022, 30, 1200–1214. [Google Scholar] [CrossRef]
- El Hachlafi, N.; Chebat, A.; Fikri-Benbrahim, K. Ethnopharmacology, Phytochemistry, and Pharmacological Properties of Thymus Satureioides Coss. Evid.-Based Complement. Altern. Med. 2021, 2021, 6673838. [Google Scholar] [CrossRef]
- Jaafari, A.; Mouse, H.A.; Rakib, E.M.; M’barek, L.A.; Tilaoui, M.; Benbakhta, C.; Boulli, A.; Abbad, A.; Zyad, A. Chemical Composition and Antitumor Activity of Different Wild Varieties of Moroccan Thyme. Rev. Bras. De Farmacogn. 2007, 17, 477–491. [Google Scholar] [CrossRef]
- Khouya, T.; Ramchoun, M.; Hmidani, A.; Amrani, S.; Harnafi, H.; Benlyas, M.; Filali Zegzouti, Y.; Alem, C. Anti-Inflammatory, Anticoagulant and Antioxidant Effects of Aqueous Extracts from Moroccan Thyme Varieties. Asian Pac. J. Trop. Biomed. 2015, 5, 636–644. [Google Scholar] [CrossRef]
- El Hattabi, L.; Talbaoui, A.; Amzazi, S.; Bakri, Y.; Harhar, H.; Costa, J.; Tabyaoui, M. Chemical Composition and Antibacterial Activity of Three Essential Oils from South of Morocco (Thymus Satureoides, Thymus Vulgaris and Chamaelum Nobilis). J. Mater. Environ. Sci. 2016, 7, 3110–3117. [Google Scholar]
- Amrouche, T.; Djenane, D.; Dziri, F.; Danoun, K.; Djerbal, M.; Rabinal, P.R. Growth Inhibition of Staphylococcus Aureus, Bacillus Cereus, and Escherichia Coli Assessed in Vitro and in Food System Using Thyme and Mentha Essential Oils. Syst. Agraires Et Environ. 2018, 2, 01–10. [Google Scholar]
- Asdadi, A.; Alilou, H.; Akssira, M.; Mina, L.; Hassani, I.; Chebli, B. Chemical Composition and Anticandidal Effect of Three Thymus Species Essential Oils from Southwest of Morocco against the Emerging Nosocomial Fluconazole-Resistant Strains. J. Biol. Agric. Healthc. 2014, 4, 16–26. [Google Scholar]
- Santana, O.; Andrés, M.F.; Sanz, J.; Errahmani, N.; Abdeslam, L.; González-Coloma, A. Valorization of Essential Oils from Moroccan Aromatic Plants. Nat. Prod. Commun. 2014, 9, 1934578X1400900812. [Google Scholar] [CrossRef]
- Avato, P.; Laquale, S.; Argentieri, M.P.; Lamiri, A.; Radicci, V.; D’Addabbo, T. Nematicidal Activity of Essential Oils from Aromatic Plants of Morocco. J. Pest Sci. 2017, 90, 711–722. [Google Scholar] [CrossRef]
- Ramchoun, M.; Harnafi, H.; Alem, C.; Büchele, B.; Simmet, T.; Rouis, M.; Atmani, F.; Amrani, S. Hypolipidemic and Antioxidant Effect of Polyphenol-Rich Extracts from Moroccan Thyme Varieties. e-SPEN J. 2012, 7, e119–e124. [Google Scholar] [CrossRef]
- Binic, I.; Lazarevic, V.; Ljubenovic, M.; Mojsa, J.; Sokolovic, D. Skin Ageing: Natural Weapons and Strategies. Evid.-Based Complement. Altern. Med. 2013, 2013, 827248. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Jones, V. Wound Pathogens. In Essential Microbiology for Wound Care; Oxford University Press: Oxford, UK, 2016; p. 67. [Google Scholar]
- Swaraj, M.; Bighneswar, B.; Suraja, K.N. Antimicrobial Resistance in Pseudomonas Aeruginosa: A Concise Review. In Antimicrobial Resistance; Mare, M., Lim, S.H.E., Lai, K.-S., Cristina, R.-T., Eds.; IntechOpen: Rijeka, Croatia, 2020; p. Ch. 3. ISBN 978-1-83962-433-9. [Google Scholar]
- Spernovasilis, N.; Psichogiou, M.; Poulakou, G. Skin Manifestations of Pseudomonas Aeruginosa Infections. Curr. Opin. Infect. Dis. 2021, 34, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Dauqan, E.M.; Abdullah, A. Medicinal and Functional Values of Thyme (Thymus vulgaris L.) Herb. J. Appl. Biol. Biotechnol. 2017, 5, 017–022. [Google Scholar] [CrossRef]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant Natural Products Targeting Bacterial Virulence Factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef]
- Kearns, D.B. A Field Guide to Bacterial Swarming Motility. Nat. Rev. Microbiol. 2010, 8, 634–644. [Google Scholar] [CrossRef]
- Rajkumari, J.; Borkotoky, S.; Murali, A.; Busi, S. Anti-Quorum Sensing Activity of Syzygium jambos (L.) Alston against Pseudomonas aeruginosa PAO1 and Identification of Its Bioactive Components. S. Afr. J. Bot. 2018, 118, 151–157. [Google Scholar] [CrossRef]
- Josenhans, C.; Suerbaum, S. The Role of Motility as a Virulence Factor in Bacteria. Int. J. Med. Microbiol. 2002, 291, 605–614. [Google Scholar] [CrossRef]
- Potts, R.O.; Guy, R.H. Predicting Skin Permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Fusco, D.; Colloca, G.; Monaco, M.R.L.; Cesari, M. Effects of Antioxidant Supplementation on the Aging Process. Clin. Interv. Aging 2007, 2, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Kouar, J.; Lamsaddek, A.; Benchekroun, R.; El Amrani, A.; Cherif, A.; Ould Bellahcen, T.; Kamil, N. Comparison between Electrocoagulation and Solvent Extraction Method in the Process of the Dechlorophyllation of Alcoholic Extracts from Moroccan Medicinal Plants Petroselinum Crispum, Thymus Satureioides and Microalgae Spirulina Platensis. SN Appl. Sci. 2018, 1, 132. [Google Scholar] [CrossRef]
- Ramchoun, M.; Sellam, K.; Harnafi, H.; Alem, C.; Benlyas, M.; Khallouki, F.; Amrani, S. Investigation of Antioxidant and Antihemolytic Properties of Thymus Satureioides Collected from Tafilalet Region, South-East of Morocco. Asian Pac. J. Trop. Biomed. 2015, 5, 93–100. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Jiang, Q.; Li, K.; Zhao, Y.; Cao, C.; Yao, J. Salvianolic Acid A Protects RPE Cells against Oxidative Stress through Activation of Nrf2/HO-1 Signaling. Free Radic. Biol. Med. 2014, 69, 219–228. [Google Scholar] [CrossRef]
- Jamali, C.A.; El Bouzidi, L.; Bekkouche, K.; Lahcen, H.; Markouk, M.; Wohlmuth, H.; Leach, D.; Abbad, A. Chemical Composition and Antioxidant and Anticandidal Activities of Essential Oils from Different Wild Moroccan Thymus Species. Chem. Biodivers. 2012, 9, 1188–1197. [Google Scholar] [CrossRef]
- Ouhaddou, H.; Boubaker, H.; Msanda, F.; El Mousadik, A. An Ethnobotanical Study of Medicinal Plants of the Agadir Ida Ou Tanane Province (Southwest Morocco). J. Appl. Biosci. 2014, 84, 7707–7722. [Google Scholar] [CrossRef]
- Fatiha, B.A.; Souad, S.; Ouafae, B.; Jamila, D.; Allal, D.; Lahcen, Z. Ethnobotanical Study of Medicinal Plants Used in the Region of Middle Oum Rbia (Morocco). Plant Arch. 2019, 19, 2005–2017. [Google Scholar]
- Wu, D.C.; Chan, W.W.; Metelitsa, A.I.; Fiorillo, L.; Lin, A.N. Pseudomonas Skin Infection. Am. J. Clin. Dermatol. 2011, 12, 157–169. [Google Scholar] [CrossRef]
- Baro, M.; Marín, M.A.; Ruiz-Contreras, J.; Fernandez de Miguel, S.; Sánchez-Díaz, I. Pseudomonas Aeruginosa Sepsis and Ecthyma Gangrenosum as Initial Manifestations of Primary Immunodeficiency. Eur. J. Pediatr. 2004, 163, 173–174. [Google Scholar] [CrossRef]
- Lakhrissi, B.; Rhaiem, N.; Ouhssine, M. Evaluation of the Bacteriostatic and Bactericidal Activity of Essential Oil of Thymus Satureioides. Dis. Manag. 2016, 2, 3. [Google Scholar]
- Chen, L.; Wen, Y. The Role of Bacterial Biofilm in Persistent Infections and Control Strategies. Int. J. Oral Sci. 2011, 3, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Noumi, E.; Ahmad, I.; Bouali, N.; Patel, H.; Ghannay, S.; ALrashidi, A.A.; Abdulhakeem, M.A.; Patel, M.; Ceylan, O.; Badraoui, R.; et al. Thymus Musilii Velen. Methanolic Extract: In Vitro and In Silico Screening of Its Antimicrobial, Antioxidant, Anti-Quorum Sensing, Antibiofilm, and Anticancer Activities. Life 2023, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.M.; Ahmad, I.; Al-thubiani, A.S.; Abulreesh, H.H.; AlHazza, I.M.; Aqil, F. Leaf Extracts of Mangifera indica L. Inhibit Quorum Sensing—Regulated Production of Virulence Factors and Biofilm in Test Bacteria. Front. Microbiol. 2017, 8, 727. [Google Scholar] [CrossRef]
- Pournajaf, A.; Razavi, S.; Irajian, G.; Ardebili, A.; Erfani, Y.; Solgi, S.; Yaghoubi, S.; Rasaeian, A.; Yahyapour, Y.; Kafshgari, R. Integron Types, Antimicrobial Resistance Genes, Virulence Gene Profile, Alginate Production and Biofilm Formation in Iranian Cystic Fibrosis Pseudomonas Aeruginosa Isolates. Infez. Med. 2018, 26, 226–236. [Google Scholar]
- Ouyang, J.; Sun, F.; Feng, W.; Sun, Y.; Qiu, X.; Xiong, L.; Liu, Y.; Chen, Y. Quercetin Is an Effective Inhibitor of Quorum Sensing, Biofilm Formation and Virulence Factors in Pseudomonas Aeruginosa. J. Appl. Microbiol. 2016, 120, 966–974. [Google Scholar] [CrossRef]
- Rowan-Nash Aislinn, D.; Korry Benjamin, J.; Mylonakis, E.; Belenky, P. Cross-Domain and Viral Interactions in the Microbiome. Microbiol. Mol. Biol. Rev. 2019, 83, e00044-18. [Google Scholar] [CrossRef]
- Boyen, F.; Eeckhaut, V.; Van Immerseel, F.; Pasmans, F.; Ducatelle, R.; Haesebrouck, F. Quorum Sensing in Veterinary Pathogens: Mechanisms, Clinical Importance and Future Perspectives. Vet. Microbiol. 2009, 135, 187–195. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Varriale, S.; Topakas, E.; Rova, U.; Christakopoulos, P.; Faraco, V. Enzymatic Synthesis of Bioactive Compounds with High Potential for Cosmeceutical Application. Appl. Microbiol. Biotechnol. 2016, 100, 6519–6543. [Google Scholar] [CrossRef]
- Saewan, N.; Vichit, W.; Prinyarux, T. Anti-Aging Efficacy of Thai Red Rice Callus Cosmetic Product. J. Appl. Sci. 2018, 17, 63–72. [Google Scholar]
- Pangestuti, R.; Kim, S.-K. Chapter 6—Seaweed Proteins, Peptides, and Amino Acids. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 125–140. ISBN 978-0-12-418697-2. [Google Scholar]
- Prockop, D.J.; Berg, R.A.; Kivirikko, K.I.; Uitto, J. Intracellular Steps in the Biosynthesis of Collagen. In Biochemistry of Collagen; Ramachandran, G.N., Reddi, A.H., Eds.; Springer: Boston, MA, USA, 1976; pp. 163–273. ISBN 978-1-4757-4602-0. [Google Scholar]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A Review on Its Sources and Potential Cosmetic Applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Kolle, S.N.; Flach, M.; Kleber, M.; Basketter, D.A.; Wareing, B.; Mehling, A.; Hareng, L.; Watzek, N.; Bade, S.; Funk-Weyer, D.; et al. Plant Extracts, Polymers and New Approach Methods: Practical Experience with Skin Sensitization Assessment. Regul. Toxicol. Pharmacol. 2023, 138, 105330. [Google Scholar] [CrossRef] [PubMed]
- Benezra, C.; Ducombs, G. Molecular Aspects of Allergic Contact Dermatitis to Plants. Recent Progress in Phytodermatochemistry. Derm. Beruf Umwelt. 1987, 35, 4–11. [Google Scholar] [PubMed]
- Vukmanović, S.; Sadrieh, N. Skin Sensitizers in Cosmetics and beyond: Potential Multiple Mechanisms of Action and Importance of T-Cell Assays for in Vitro Screening. Crit. Rev. Toxicol. 2017, 47, 422–439. [Google Scholar] [CrossRef] [PubMed]
- Adamu, H.M.A.; Ushie, O.; Gwangwala, A.H.; Yadav, R.P.; Singh, A.V.; Bhardwaj, A.K.; Lone, P.A.; Dar, M.M.; Parray, J.A.; Shah, K.W.; et al. Estimation of Total Flavonoids and Tannins in the Stem Bark and Leaves of Anogeisus Leiocarpus Plant Plant. Int. J. Tradit. Nat. Med. 2013, 2, 141–148. [Google Scholar]
- Tawfeek, N.; Fikry, E.; Mahdi, I.; Ochieng, M.A.; Bakrim, W.B.; Taarji, N.; Mahmoud, M.F.; Sobeh, M. Cupressus Arizonica Greene: Phytochemical Profile and Cosmeceutical and Dermatological Properties of Its Leaf Extracts. Molecules 2023, 28, 1036. [Google Scholar] [CrossRef] [PubMed]
- Lamuela-Raventós, R.M. Folin–Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity. In Measurement of Antioxidant Activity & Capacity; Wiley Online Library: Hoboken, NJ, USA, 2018; pp. 107–115. ISBN 978-1-119-13538-8. [Google Scholar]
- Matić, P.; Sabljić, M.; Jakobek, L. Validation of Spectrophotometric Methods for the Determination of Total Polyphenol and Total Flavonoid Content. J. AOAC Int. 2017, 100, 1795–1803. [Google Scholar] [CrossRef]
- Lee, K.J.; Oh, Y.C.; Cho, W.K.; Ma, J.Y. Antioxidant and Anti-Inflammatory Activity Determination of One Hundred Kinds of Pure Chemical Compounds Using Offline and Online Screening HPLC Assay. Evid.-Based Complement. Altern. Med. 2015, 2015, e165457. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Spectrophotometric Determination of Antioxidant Activity. Redox Rep. 1996, 2, 161–171. [Google Scholar] [CrossRef]
- Bakrim, W.B.; Nurcahyanti, A.D.R.; Dmirieh, M.; Mahdi, I.; Elgamal, A.M.; El Raey, M.A.; Wink, M.; Sobeh, M. Phytochemical Profiling of the Leaf Extract of Ximenia Americana Var. Caffra and Its Antioxidant, Antibacterial, and Antiaging Activities In Vitro and in Caenorhabditis Elegans: A Cosmeceutical and Dermatological Approach. Oxidative Med. Cell Longev. 2022, 2022, 3486257. [Google Scholar] [CrossRef]
- Abbas, H.A.; Elsherbini, A.M.; Shaldam, M.A. Repurposing Metformin as a Quorum Sensing Inhibitor in Pseudomonas Aeruginosa. Afr. Health Sci. 2017, 17, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, I.; Abbas, H.A.; Ashour, M.L.; Yasri, A.; El-Shazly, A.M.; Wink, M.; Sobeh, M. Polyphenols from Salix Tetrasperma Impair Virulence and Inhibit Quorum Sensing of Pseudomonas Aeruginosa. Molecules 2020, 25, 1341. [Google Scholar] [CrossRef] [PubMed]
- Musthafa, K.S.; Ravi, A.V.; Annapoorani, A.; Packiavathy, I.S.V.; Pandian, S.K. Evaluation of Anti-Quorum-Sensing Activity of Edible Plants and Fruits through Inhibition of the N-Acyl-Homoserine Lactone System in Chromobacterium Violaceum and Pseudomonas Aeruginosa. Chemotherapy 2010, 56, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Benjelloun, S.; Allaoui, A.; Biskri, L. Rhizospheric Phosphate Solubilizing Bacillus Atrophaeus GQJK17 S8 Increases Quinoa Seedling, Withstands Heavy Metals, and Mitigates Salt Stress. Sustainability 2021, 13, 3307. [Google Scholar] [CrossRef]
- Yeung, A.T.; Torfs, E.C.; Jamshidi, F.; Bains, M.; Wiegand, I.; Hancock, R.E.; Overhage, J. Swarming of Pseudomonas Aeruginosa Is Controlled by a Broad Spectrum of Transcriptional Regulators, Including MetR. J. Bacteriol. 2009, 191, 5592–5602. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Adonizio, A.; Kong, K.-F.; Mathee, K. Inhibition of Quorum Sensing-Controlled Virulence Factor Production in Pseudomonas Aeruginosa by South Florida Plant Extracts. Antimicrob. Agents Chemother. 2008, 52, 198–203. [Google Scholar] [CrossRef]
- Dubois, M. Calorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 351–356. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).