In Vitro Anti-Microbial Activity and Anti-Cancer Potential of Novel Synthesized Carbamothioyl-Furan-2-Carboxamide Derivatives
Abstract
1. Introduction
2. Results and Discussion
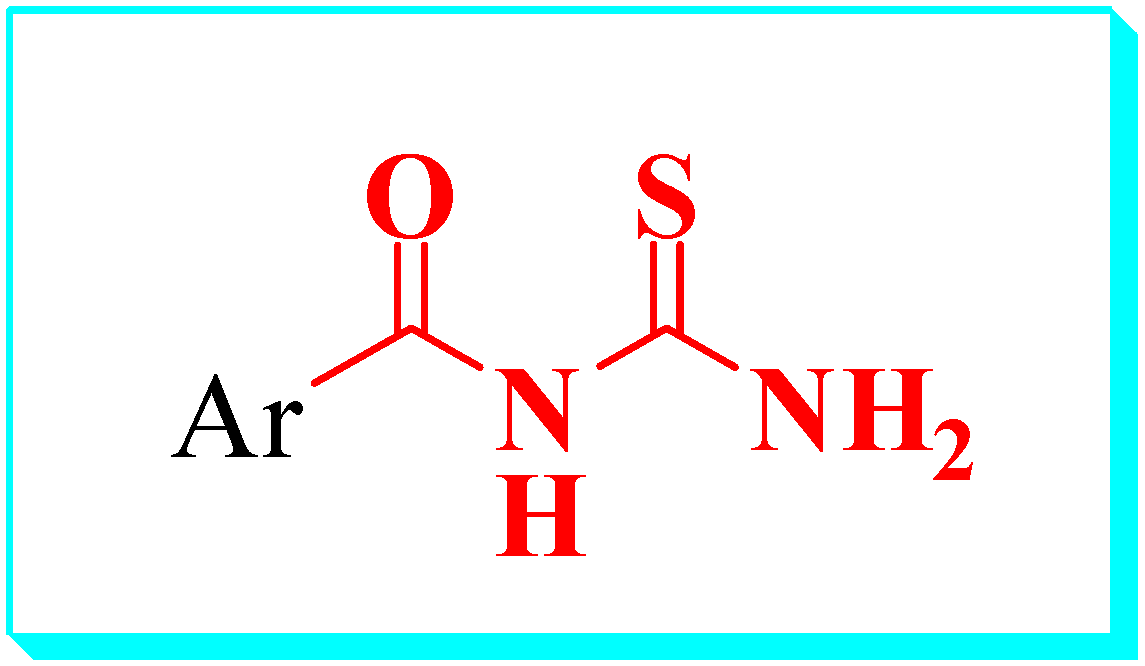
2.1. Synthesis
2.2. Pharmacology
2.2.1. Anti-Cancer Activity of Synthesized Carbamothioyl-Furan-2-Carboxamide Derivatives against HepG2, Huh-7, and MCF-7 Cancer Cell Line
2.2.2. Hemolytic Activity
2.2.3. Anti-Microbial Activity
3. Materials and Methods
3.1. General Method of Obtaining Caramothioyl-Furan-2-Carboxamide Derivatives 4a–f
3.2. Characterization Data
3.3. Biological Activities
3.3.1. Anti-Cancer Activity
Cell Culture and Treatment
Determination of Cell Viability
3.3.2. Anti-Microbial Activity by Well Diffusion strategy
Determination of MIC Values
3.3.3. Hemolytic Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koch, K.R. New chemistry with old ligands: N-alkyl-and N, N-dialkyl-N′-acyl (aroyl) thioureas in co-ordination, analytical and process chemistry of the platinum group metals. Coord. Chem. Rev. 2001, 216, 473–488. [Google Scholar] [CrossRef]
- Aly, A.A.; Ahmed, E.K.; El-Mokadem, K.M.; Hegazy, M.E.-A.F. Update survey on aroyl substituted thioureas and their applications. J. Sulfur. Chem. 2007, 28, 73–93. [Google Scholar] [CrossRef]
- Saeed, A.; Flörke, U.; Erben, M.F. A review on the chemistry, coordination, structure and biological properties of 1-(acyl/aroyl)-3-(substituted) thioureas. J. Sulfur. Chem. 2014, 35, 318–355. [Google Scholar] [CrossRef]
- Saeed, A.; Qamar, R.; Fattah, T.A.; Flörke, U.; Erben, M.F. Recent developments in chemistry, coordination, structure and biological aspects of 1-(acyl/aroyl)-3-(substituted) thioureas. Res. Chem. Intermed. 2017, 43, 3053–3093. [Google Scholar] [CrossRef]
- Singh, D.P.; Gangwar, M.; Kumar, D.; Nath, G.; Pratap, S. Synthesis, spectroscopic characterization, crystal structure, antimicrobial and in vitro hemolytic studies of some novel substituted thiourea derivatives. J. Chem. Crystallogr. 2013, 43, 610–621. [Google Scholar] [CrossRef]
- Saeed, S.; Rashid, N.; Jones, P.G.; Ali, M.; Hussain, R. Synthesis, characterization and biological evaluation of some thiourea derivatives bearing benzothiazole moiety as potential antimicrobial and anticancer agents. Eur. J. Med. Chem. 2010, 45, 1323–1331. [Google Scholar] [CrossRef]
- Zhang, K.E.; Naue, J.A.; Arison, B.; Vyas, K.P. Chemial Researdhin Тoкіo) oдy. Chem. Res. Toxicol. 1996, 9, 547–554. [Google Scholar] [CrossRef]
- Christian, M.C.; Wittes, R.E.; Leyland-Jones, B.; McLemore, T.L.; Smith, A.C.; Grieshaber, C.K.; Chabner, B.A.; Boyd, M.R. 4-Ipomeanol: A novel investigational new drug for lung cancer. JNCI J. Natl. Cancer Inst. 1989, 81, 1133–1143. [Google Scholar] [CrossRef]
- Tang, Y.; Varyambath, A.; Ding, Y.; Chen, B.; Huang, X.; Zhang, Y.; Yu, D.-G.; Kim, I.; Song, W. Porous organic polymers for drug delivery: Hierarchical pore structures, variable morphologies, and biological properties. Biomater. Sci. 2022. [Google Scholar] [CrossRef]
- Song, W.; Zhang, M.; Huang, X.; Chen, B.; Ding, Y.; Zhang, Y.; Yu, D.G.; Kim, I. Smart l-borneol-loaded hierarchical hollow polymer nanospheres with antipollution and antibacterial capabilities. Mater. Today Chem. 2022, 26, 101252. [Google Scholar] [CrossRef]
- Oppil, R.J.; Bishayee, A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 2011, 3, 228–249. [Google Scholar]
- Tan, X.W.; Xia, H.; Xu, J.H.; Cao, J.G. Induction of apoptosis in human liver carcinoma HepG2 cell line by 5-allyl-7-gendiuoromethylenechrysin. World J. Gastroenterol. 2009, 15, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Andreana, L.; Isgrò, G.; Marelli, L.; Davies, N.; Yu, D.; Navalkissoor, S.; Burroughs, A.K. Treatment of hepatocellular carcinoma (HCC) by intra-arterial infusion of radioemitter compounds: Trans-arterial radio-embolisation of HCC. Cancer Treat. Rev. 2012, 38, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Alisi, A.; Balsano, C. Enhancing the efficacy of hepatocellular carcinoma chemotherapeutics with natural anticancer agents. Nutr. Rev. 2007, 65, 550–553. [Google Scholar] [CrossRef]
- Khan, M.; Li, T.; Ahmad Khan, M.K.; Rasul, A.; Nawaz, F.; Sun, M.; Zheng, Y.; Ma, T. Alantolactone induces apoptosis in HepG2 cells through GSH depletion, inhibition of STAT3 activation, and mitochondrial dysfunction. BioMed Res. Int. 2013, 2013, 719858. [Google Scholar] [CrossRef]
- Aziz, H.; Saeed, A.; Khan, M.A.; Afridi, S.; Jabeen, F. Synthesis, characterization, antimicrobial, antioxidant and computational evaluation of N-acyl-morpholine-4-carbothioamides. Mol. Divers. 2021, 25, 763–776. [Google Scholar] [CrossRef]
- Aziz, H.; Saeed, A.; Khan, M.A.; Afridi, S.; Jabeen, F.; Hashim, M. Novel N-acyl-1H-imidazole-1-carbothioamides: Design, synthesis, biological and computational studies. Chem. Biodivers. 2020, 17, e1900509. [Google Scholar] [CrossRef]
- Soni, L.K.; Narsinghani, T.; Jain, R. Synthesis and antibacterial screening of some 1-Aroyl-3-aryl thiourea derivatives. Int. Sch. Res. Not. 2014, 2014, 393102. [Google Scholar] [CrossRef]
- Abdel Hamid, A.M. Addition–cyclization reactions of furan-2-carbonyl isothiocyanate with nitrogen nucleophiles as a synthetic route to novel azines and azoles of potential biological activity. J. Iran. Chem. Soc. 2019, 16, 1853–1861. [Google Scholar] [CrossRef]
- Jamil, M.; Zubair, M.; Rasool, N.; Altaf, A.A.; Rizwan, K.; Hafeez, S.; Bukhari, I.H.; Langer, P. Synthesis, Characterization, Antibacterial and Urease Inhibition Studies of Some Novel Symmetrical N3, N3′-bis-(disubstituted) isophthalyl-bis-(thioureas). Asian J. Chem. 2013, 25, 5328. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Wei, T.-B.; Gao, L.-M. Synthesis and biological activity of N-aroyl-N′-substituted thiourea derivatives. Synth. Commun. 2001, 31, 3099–3105. [Google Scholar] [CrossRef]
- Yusof, M.S.M.; Wong, S.T.; Yamin, B.M. 1-(Biphenyl-4-ylcarbonyl)-3-(4-nitrophenyl) thiourea. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o2483. [Google Scholar] [CrossRef] [PubMed]
- Dubbelboer, I.R.; Pavlovic, N.; Heindryckx, F.; Sjögren, E.; Lennernäs, H. Liver cancer cell lines treated with doxorubicin under normoxia and hypoxia: Cell viability and oncologic protein profile. Cancers 2019, 11, 1024. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, I.; Zahoor, A.F.; Rasul, A.; Rasool, N.; Raza, Z.; Faisal, S.; Parveen, B.; Kamal, S.; Zia-ur-Rehman, M.; Zahid, F.M. Synthesis, anticancer, and computational studies of 1, 3, 4-oxadiazole-purine derivatives. J. Heterocycl. Chem. 2020, 57, 2782–2794. [Google Scholar] [CrossRef]
- Koca, İ.; Özgür, A.; Coşkun, K.A.; Tutar, Y. Synthesis and anticancer activity of acyl thioureas bearing pyrazole moiety. Bioorg. Med. Chem. 2013, 21, 3859–3865. [Google Scholar] [CrossRef]
- Akhtar, R.; Zahoor, A.F.; Rasul, A.; Ahmad, M.; Anjum, M.N.; Ajmal, M.; Raza, Z. Design, synthesis, in-silico study and anticancer potential of novel n-4-piperazinyl-ciprofloxacin-aniline hybrids. Pak. J. Pharm. Sci. 2019, 32, 2215–2222. [Google Scholar]
- Rizwan, K.; Zubair, M.; Rasool, N.; Mahmood, T.; Ayub, K.; Alitheen, N.B.; Aziz, M.N.M.; Akhtar, M.N.; Nasim, F.-u.-H.; Bukhary, S.M. Palladium (0) catalyzed Suzuki cross-coupling reaction of 2, 5-dibromo-3-methylthiophene: Selectivity, characterization, DFT studies and their biological evaluations. Chem. Cent. J. 2018, 12, 1–12. [Google Scholar] [CrossRef]
- Khan, S.A.; Rizwan, K.; Shahid, S.; Noamaan, M.A.; Rasheed, T.; Amjad, H. Synthesis, DFT, computational exploration of chemical reactivity, molecular docking studies of novel formazan metal complexes and their biological applications. Appl. Organomet. Chem. 2020, 34, e5444. [Google Scholar] [CrossRef]
- Khan, S.A.; Shahid, S.; Kanwal, S.; Rizwan, K.; Mahmood, T.; Ayub, K. Synthesis of novel metal complexes of 2-((phenyl (2-(4-sulfophenyl) hydrazono) methyl) diazenyl) benzoic acid formazan dyes: Characterization, antimicrobial and optical properties studies on leather. J. Mol. Struct. 2019, 1175, 73–89. [Google Scholar] [CrossRef]
- Cock, I. Antibacterial activity of selected Australian native plant extracts. Internet J. Microbiol. 2008, 4, 1–8. [Google Scholar]
- Lebleu, N.; Roques, C.; Aimar, P.; Causserand, C. Role of the cell-wall structure in the retention of bacteria by microfiltration membranes. J. Membr. Sci. 2009, 326, 178–185. [Google Scholar] [CrossRef]
- Arslan, H.; Duran, N.; Borekci, G.; Koray Ozer, C.; Akbay, C. Antimicrobial activity of some thiourea derivatives and their nickel and copper complexes. Molecules 2009, 14, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Hoey, A.J.; Jackson, C.M.; Pegg, G.G.; Sillence, M.N.; Tropical Beef Centre: A joint venture between. Characteristics of cyanopindolol analogues active at the β3-adrenoceptor in rat ileum. Br. J. Pharmacol. 1996, 119, 564–568. [Google Scholar] [CrossRef]
- Ikokoh, P.P.; Onigbanjo, H.O.; Adedirin, O.; Akolade, J.O.; Amuzie, U.; Fagbohun, A. Synthesis and Antimicrobial Activities of Copper (I) Thiourea and Silver (I) Thiourea. Open J. Res. 2015, 2, 86–91. [Google Scholar]
- Buddelmeijer, N. The molecular mechanism of bacterial lipoprotein modification—How, when and why? FEMS Microbiol. Rev. 2015, 39, 246–261. [Google Scholar] [CrossRef]
- Chen, Z.; Rasul, A.; Zhao, C.; Millimouno, F.M.; Tsuji, I.; Yamamura, T.; Iqbal, R.; Malhi, M.; Li, X.; Li, J. Antiproliferative and apoptotic effects of pinocembrin in human prostate cancer cells. Bangladesh J. Pharmacol. 2013, 8, 255–262. [Google Scholar] [CrossRef]
- Dadpe, M.V.; Dhore, S.V.; Dahake, P.T.; Kale, Y.J.; Kendre, S.B.; Siddiqui, A.G. Evaluation of antimicrobial efficacy of Trachyspermum ammi (Ajwain) oil and chlorhexidine against oral bacteria: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2018, 36, 357. [Google Scholar]
- Weatherburn, M. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate based antibacterial assay incorporating resazurin as an Indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Riaz, M.; Rasool, N.; Bukhari, I.; Shahid, M.; Zubair, M.; Rizwan, K.; Rashid, U. In Vitro antimicrobial, antioxidant, cyto toxicity and GC-MS analysis of Mazus goodenifolius. Molecules 2012, 17, 14275–14287. [Google Scholar] [CrossRef]
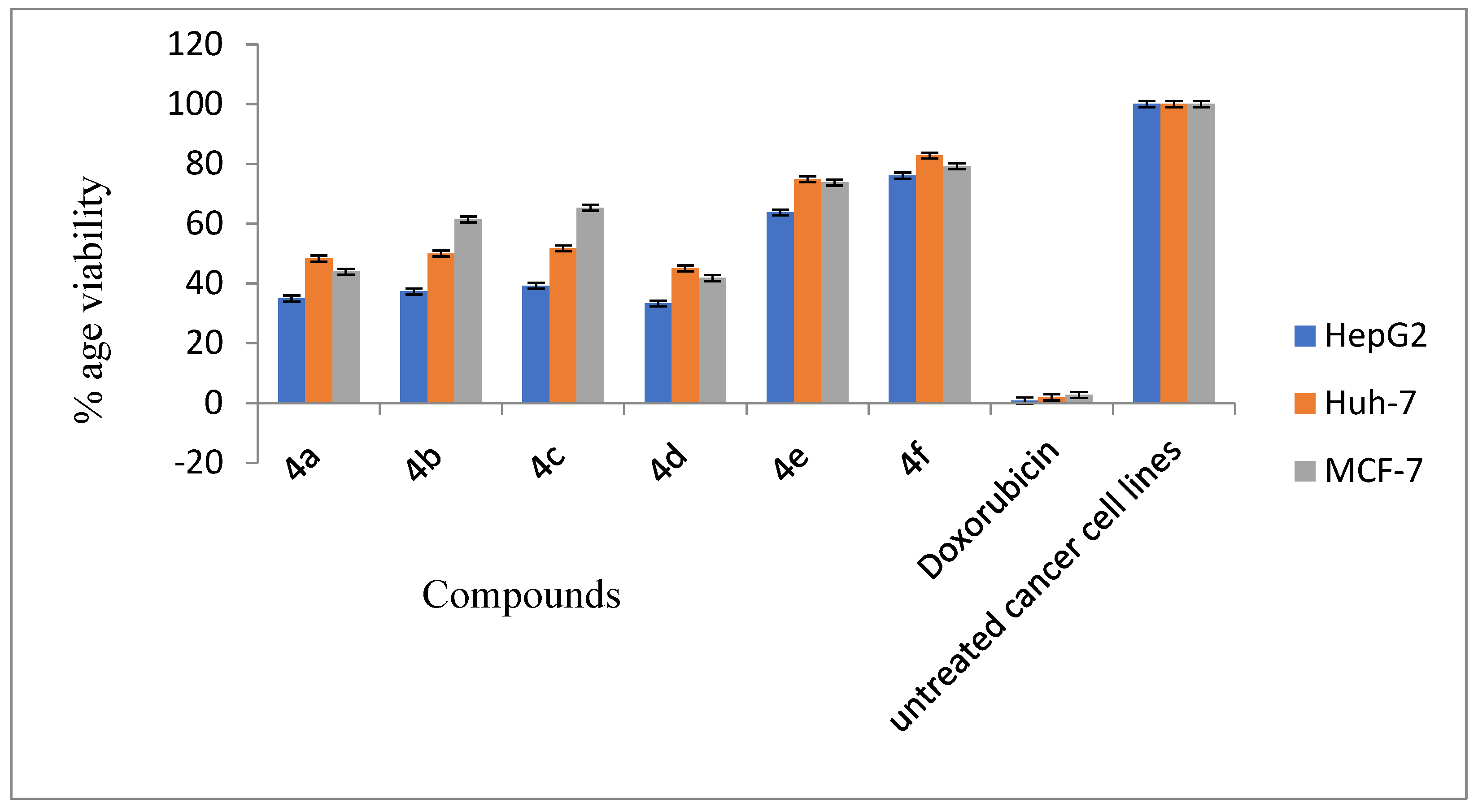

| Sr # | Entries (µM) | %Age Cell Viability (HepG2) | %Age Cell Viability (Huh-7) | %Age Cell Viability (MCF-7) | %Age Hemolysis |
|---|---|---|---|---|---|
| 1 | 4a (340) | 35.01 ± 1.55 | 48.32 ± 0.38 | 43.96 ± 1.42 | 2.60 ± 0.18 |
| 2 | 4b (340) | 37.31 ± 1.72 | 50.02 ± 1.54 | 61.42 ± 0.87 | 4.12 ± 0.22 |
| 3 | 4c (340) | 39.22 ± 4.04 | 51.76 ± 1.39 | 65.33 ± 0.17 | 4.84 ± 0.15 |
| 4 | 4d (380) | 33.29 ± 5.22 | 45.09 ± 0.23 | 41.81 ± 1.42 | 4.56 ± 0.34 |
| 5 | 4e (350) | 63.75 ± 0.30 | 74.91 ± 0.35 | 73.74 ± 0.41 | 8.32 ± 0.17 |
| 6 | 4f (280) | 76.07 ± 0.88 | 82.81 ± 1.61 | 79.26 ± 0.76 | 7.25 ± 0.29 |
| 7 | Doxorubicin (180) | 00.86 ± 0.56 | 01.89 ± 0.73 | 02.71 ± 0.21 | - |
| 8 | Untreated cancer cell linings | 100.0 ± 0.00 | 100.0 ± 0.00 | 100.0 ± 0.00 | - |
| 9 | DMSO | - | - | - | 0.01 ± 0.08 |
| 10 | ABTS | - | - | - | 95.9 ± 0.02 |
| Sr. | Entries Dose (10 mg/mL) | E. coli I.Z. (mm) | S. aureus I.Z. (mm) | B. cereus I.Z. (mm) | F. bracchygibossum I.Z. (mm) | A. niger I.Z. (mm) | A. flavus I.Z. (mm) |
|---|---|---|---|---|---|---|---|
| 1 | 4a | - | 13.00 ± 0.11 | 15.00 ± 0.13 | 16.00 ± 0.17 | 16.00 ± 0.13 | 14.00 ± 0.11 |
| 2 | 4b | 10.50 ± 0.10 | - | 14.00 ± 0.15 | 16.00 ± 0.13 | 16.00 ± 0.12 | 14.00 ± 0.13 |
| 3 | 4c | 10.00 ± 0.10 | - | 15.00 ± 0.16 | 17.00 ± 0.11 | 19.00 ± 0.14 | 15.00 ± 0.17 |
| 4 | 4d | - | 12.00 ± 0.12 | - | 11.00 ± 0.12 | 16.00 ± 0.13 | - |
| 5 | 4e | 09.00 ± 0.09 | - | - | 18.00 ± 0.11 | - | 12.00 ± 0.12 |
| 6 | 4f | 09.00 ± 0.11 | 10.00 ± 0.11 | 16.00 ± 0.13 | 13.00 ± 0.12 | 17.00 ± 0.12 | 12.00 ± 0.11 |
| 7 | Gentamicine (control) | 21.00 ± 0.11 | 21.00 ± 0.21 | 20.00 ± 0.18 | 14.00 ± 0.11 | 14.00 ± 0.14 | 16.00 ± 0.11 |
| Sr. | MIC (µg/mL) Entries | E. coli | S. aureus | B. cereus | F. bracchygibossum | A. niger | A. flavus |
|---|---|---|---|---|---|---|---|
| 1 | 4a | - | 265 | 240 | 150.2 | 145.2 | 155 |
| 2 | 4b | 280 | - | 250 | 150.0 | 145.0 | 155 |
| 3 | 4c | 280 | - | 240 | 137.5 | 120.7 | 140 |
| 4 | 4d | - | 277 | - | 270.1 | 154.0 | - |
| 5 | 4e | 300 | - | - | 122.1 | - | 186 |
| 6 | 4f | 295 | 280 | 230 | 150.7 | 157.4 | 190 |
| 7 | Gentamicine (control) | 120.35 | 122.50 | 120.47 | 160.25 | 163.31 | 164.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, M.S.; Zubair, M.; Rizwan, K.; Jamil, M. In Vitro Anti-Microbial Activity and Anti-Cancer Potential of Novel Synthesized Carbamothioyl-Furan-2-Carboxamide Derivatives. Molecules 2023, 28, 4583. https://doi.org/10.3390/molecules28124583
Javed MS, Zubair M, Rizwan K, Jamil M. In Vitro Anti-Microbial Activity and Anti-Cancer Potential of Novel Synthesized Carbamothioyl-Furan-2-Carboxamide Derivatives. Molecules. 2023; 28(12):4583. https://doi.org/10.3390/molecules28124583
Chicago/Turabian StyleJaved, Muhammad Salman, Muhammad Zubair, Komal Rizwan, and Muhammad Jamil. 2023. "In Vitro Anti-Microbial Activity and Anti-Cancer Potential of Novel Synthesized Carbamothioyl-Furan-2-Carboxamide Derivatives" Molecules 28, no. 12: 4583. https://doi.org/10.3390/molecules28124583
APA StyleJaved, M. S., Zubair, M., Rizwan, K., & Jamil, M. (2023). In Vitro Anti-Microbial Activity and Anti-Cancer Potential of Novel Synthesized Carbamothioyl-Furan-2-Carboxamide Derivatives. Molecules, 28(12), 4583. https://doi.org/10.3390/molecules28124583







