Novel Imine-Tethering Cationic Surfactants: Synthesis, Surface Activity, and Investigation of the Corrosion Mitigation Impact on Carbon Steel in Acidic Chloride Medium via Various Techniques
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Surface Activity Measurements
2.3. Weight Loss (WL) Studies
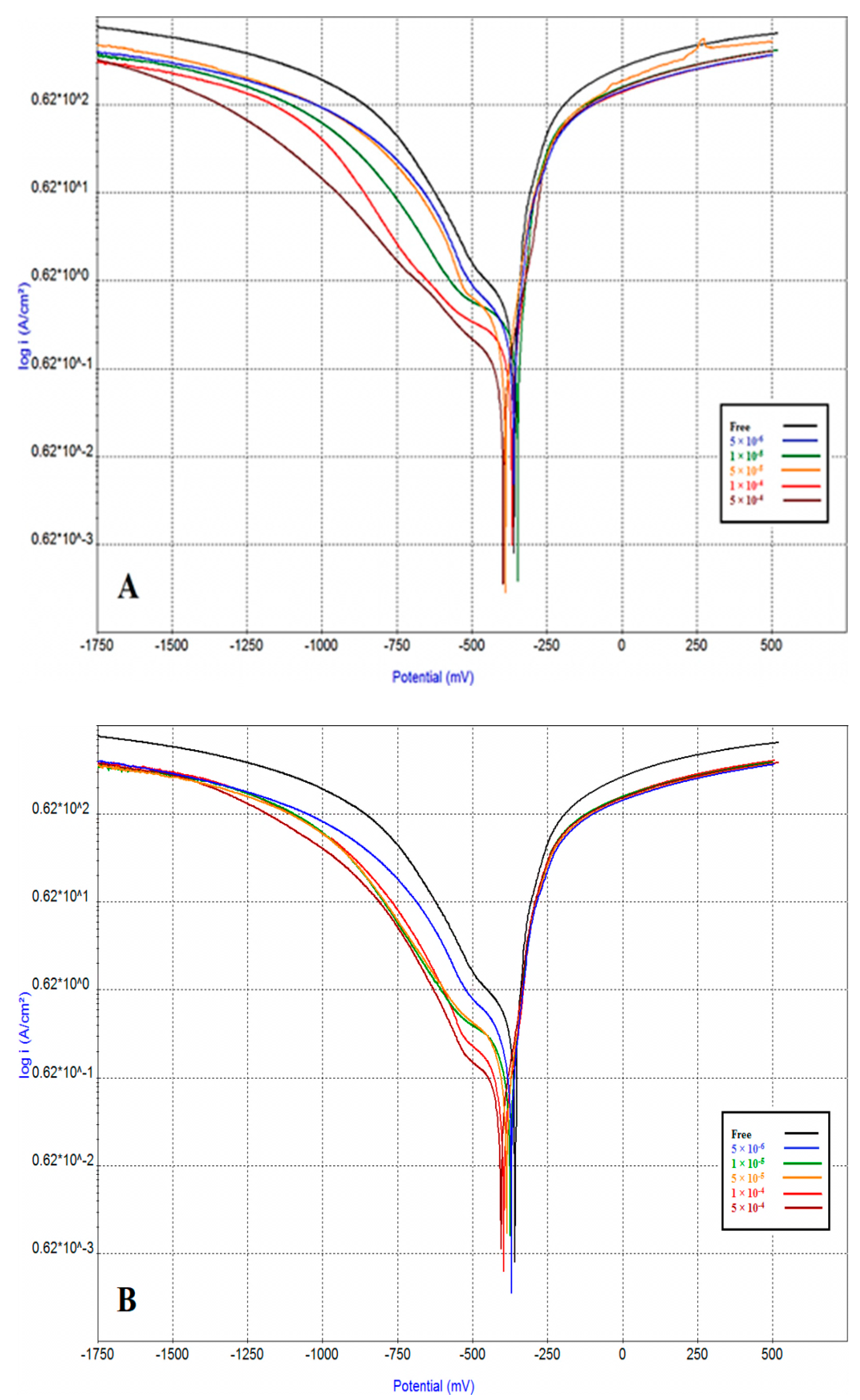
2.4. Potentiodynamic Polarization (PDP)
2.5. Morphology Studies
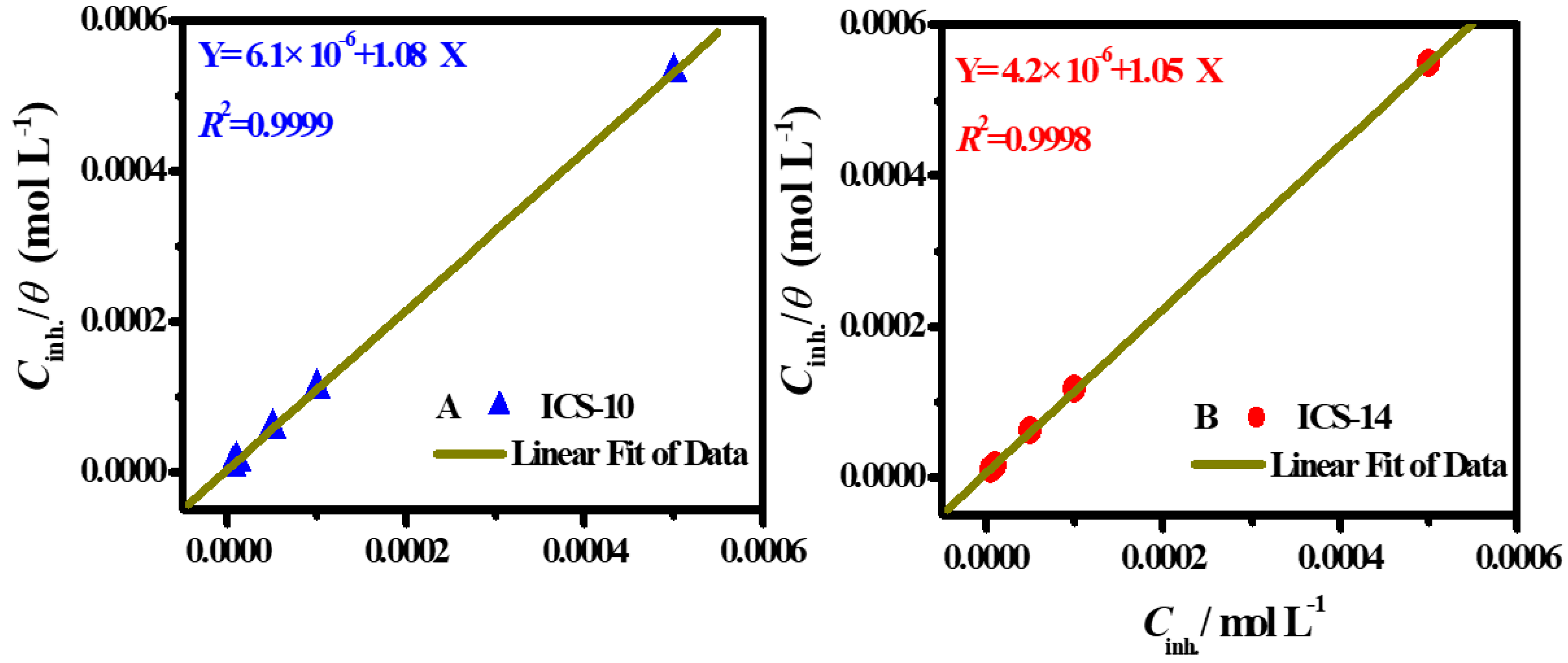
2.6. Adsorption Isotherm Considerations
2.7. Thermodynamic/Adsorption Calculations
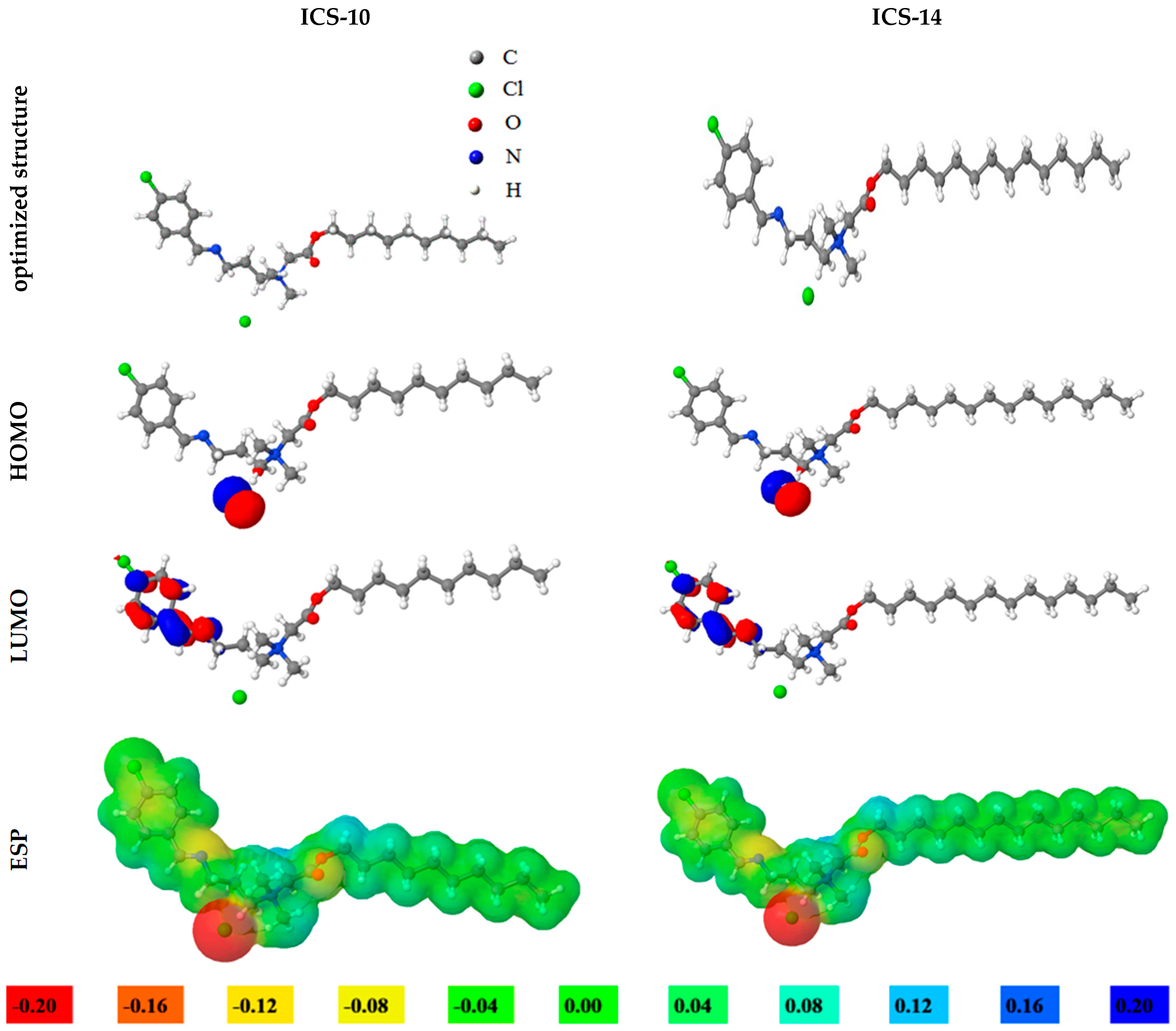
2.8. DFT Calculations
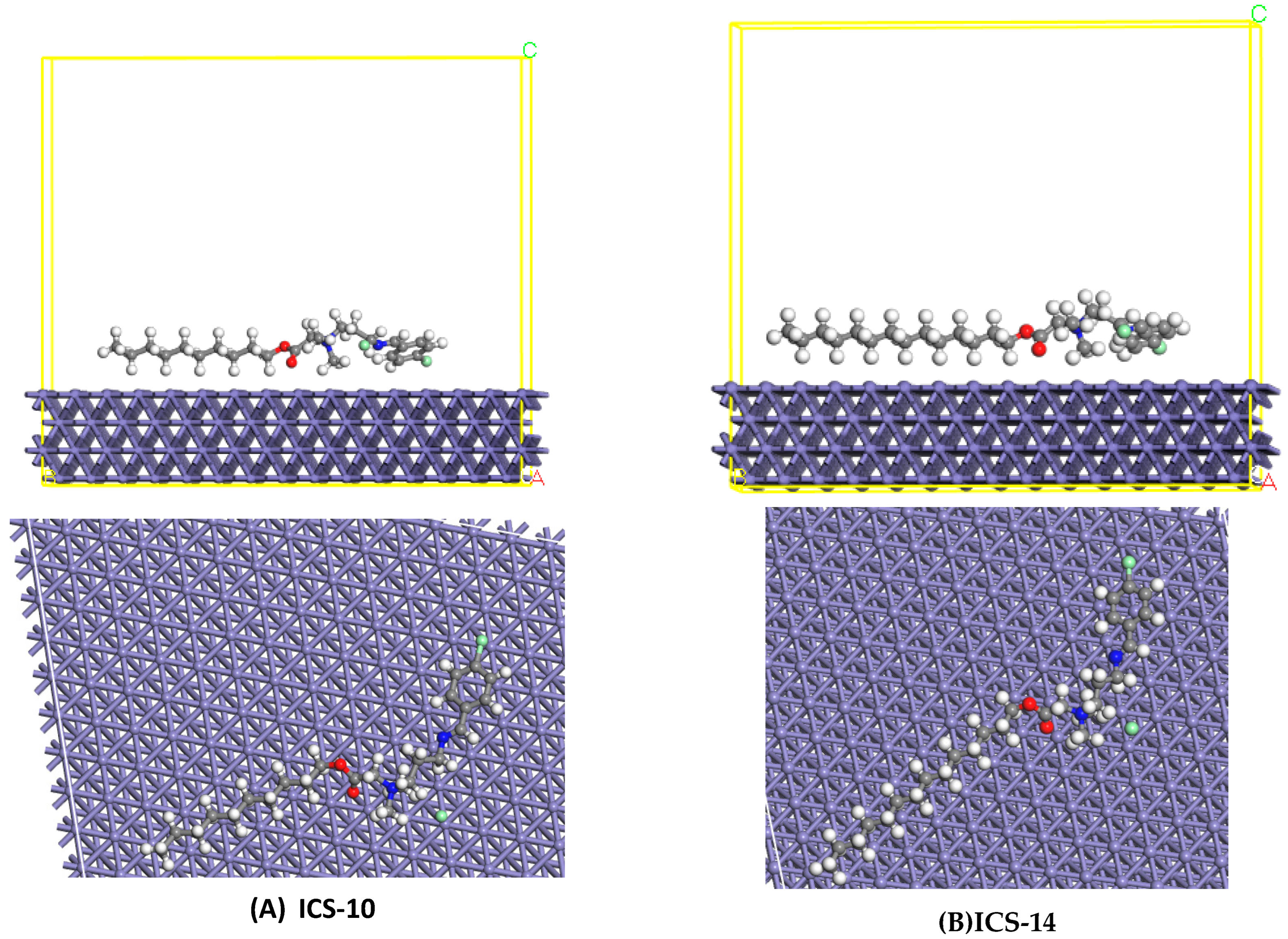
2.9. MC Simulation and the Mechanism of Inhibition
3. Experimental
3.1. Materials
3.2. Equipment and Instrumentation
3.3. Synthesis of the As-Prepared Cationic Surfactant
3.4. Weight Loss (WL) Measurements
3.5. Electrochemical Measurements
3.6. SEM Observations
3.7. Computational Details
3.8. MC Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Adway, A.I.; Abbas, M.A.; Zakaria, K. New Schiff base cationic surfactants as corrosion inhibitors for carbon steel in acidic medium: Weight loss, electrochemical and SEM characterization techniques. Res. Chem. Intermed. 2016, 42, 3385. [Google Scholar] [CrossRef]
- Yan, C.; Jia, J.; Liao, C.; Wu, S.; Xu, G. Rare earth separation in China. Tsinghua Sci. Technol. 2006, 11, 241–247. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Marikkannu, C.; Palaniswamy, N. Corrosion inhibition influence of tetramines for mild steel in 1M HCl. Appl. Surf. Sci 2005, 241, 477–484. [Google Scholar] [CrossRef]
- Trabanelli, G. 1991 Whitney Award Lecture: Inhibitors-An Old Remedy for a New Challenge. Corrosion 1991, 47, 410–419. [Google Scholar] [CrossRef]
- Lagrenee, M.; Mernari, B.; Bouanis, M.; Traisnel, M.; Bentiss, F. Study of the mechanism and inhibiting efficiency of 3,5-bis (4-methylthiophenyl)-4H-1, 2, 4-triazole on mild steel corrosion in acidic media. Corros. Sci. 2002, 44, 573–588. [Google Scholar] [CrossRef]
- Kosari, A.; Moayed, M.H.; Davoodi, A.; Parvizi, R.; Momeni, M.; Eshghi, H.; Moradi, H. Electrochemical and quantum chemical assessment of two organic compounds from pyridine derivatives as corrosion inhibitors for mild steel in HCl solution under stagnant condition and hydrodynamic flow. Corros. Sci. 2014, 78, 138–150. [Google Scholar] [CrossRef]
- Tawfik, S.M. Corrosion inhibition efficiency and adsorption behavior of N,N-dimethyl-4-(((1-methyl-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)-N-alkylbenzenaminium bromide surfactant at carbon steel/hydrochloric acid interface. J. Mol. Liq. 2015, 207, 185. [Google Scholar] [CrossRef]
- Thomas, J.G.N. Some New Fundamental Aspects in Corrosion Inhibition; Corrosion Inhibitors: Ferrara, Italy, 1980; pp. 453–470. [Google Scholar]
- Attia, M.M.; Soliman, K.A.; Eid, S.; Mabrouk, E.M. Experimental and theoretical study on some azo chromotropic acid dyes compounds as inhibitor for carbon steel corrosion in sulfuric acid. J. Iran. Chem. Soc. 2021, 19, 655–664. [Google Scholar] [CrossRef]
- Eid, S. Expired Desloratidine Drug as Inhibitor for Corrosion of Carbon Steel Pipeline in Hydrochloric acid Solution. Int. J. Electrochem. Sci. 2021, 16, 150852. [Google Scholar] [CrossRef]
- Moussa, M.N.H.; El-Far, A.A.; El-Shafei, A.A. The use of water-soluble hydrazones as inhibitors for the corrosion of C-steel in acidic medium. Mater. Chem. Phys. 2007, 105, 105–113. [Google Scholar] [CrossRef]
- Wagdy, E.D.; Eid, S.; Zaher, A.A.; El-Etre, A.Y. Inhibition of carbon steel corrosion in aqueous solutions using some fatty amido-cationic surfactant. J. Basic Environ. Sci. 2016, 3, 55–64. [Google Scholar]
- Ruso, J.M.; Pérez, A.G.; Prieto, G.; Sarmiento, F. Study of the interactions between lysozyme and a fully-fluorinated surfactant in aqueous solution at different surfactant–protein ratios. Int. J. Biol. 2003, 33, 67–73. [Google Scholar] [CrossRef]
- Betih, M.A.; El-Henawy, S.B.; Al-Sabagh, A.M.; Negm, N.A.; Mahmoud, T. Experimental evaluation of cationic-Schiff base surfactants based on 5-chloromethyl salicylaldehyde for improving crude oil recovery and bactericide. J. Mol. Liq. 2020, 316, 113862. [Google Scholar] [CrossRef]
- Rajput, S.M.; Mondal, K.; Kuddushi, M.; Aswal, D.R.; Malek, N.I. Formation of hydrotropic drug/gemini surfactant based catanionic vesicles as efficient nano drug delivery vehicles. Colloids Interface Sci. Commun. 2020, 37, 100273. [Google Scholar] [CrossRef]
- Pakiet, M.; Kowalczyk, I.; Leiva Garcia, R.; Akid, R.; Brycki, B. Cationic clevelable surfactants as highly efficient corrosion inhibitors of stainless steel AISI 304: Electrochemical study. J. Mol. Liq. 2020, 315, 113675. [Google Scholar] [CrossRef]
- Shalabi, K.; Helmy, A.M.; El-Askalany, A.H.; Shahba, M.M. New pyridinium bromide mono-cationic surfactant as corrosion inhibitor for carbon steel during chemical cleaning: Experimental and theoretical studies. J. Mol. Liq. 2019, 293, 111480. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Shalabi, K.; Tantawy, A.H. Corrosion inhibition and adsorption features of novel bioactive cationic surfactants bearing benzenesulphonamide on C1018-steel under sweet conditions: Combined modeling and experimental approaches. J. Mol. Liq. 2020, 320, 114564. [Google Scholar] [CrossRef]
- Godec, R.F.; Dolecek, V. Effect of sodium dodecylsulfate on the corrosion of copper in sulphuric acid media. Colloids Surf. A 2004, 244, 73–76. [Google Scholar] [CrossRef]
- Alfakeer, M.; Abdallah, M.; Abdel Hameed, R.S. Propoxylated Fatty Esters as Safe Inhibitors for Corrosion of Zinc in Hydrochloric Acid. Prot. Met. Phys. Chem. Surf. 2020, 56, 225–232. [Google Scholar] [CrossRef]
- Tang, F.; Wang, X.; Xu, X.; Li, L. Phytic acid doped nanoparticles for green anticorrosion coatings. Colloids Surf. A 2010, 369, 101–105. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Song, C.X.; Ying Kan, Y.; Fan, X.S.; Zhang, Y. Inhibition Corrosion Effect of Extract from Basella rubra on Carbon Steel in HCl Solution. Int. J. Electrochem. Sci. 2020, 15, 4032–4055. [Google Scholar] [CrossRef]
- Fergachi, O.; Benhiba, F.; Rbaa, M.; Ouakki, M.; Galai, M.; Touir, R.; Lakhrissi, B.; Oudda, H.; Touhami, M.E. Corrosion Inhibition of Ordinary Steel in 5.0 M HCl Medium by Benzimidazole Derivatives: Electrochemical, UV–Visible Spectrometry, and DFT Calculations. J. Bio- Tribo-Corros. 2019, 5, 21. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; El-Beltagi, H.S.; Mohamed, M.E.M.; Kandeel, M.; Bakir, E.; Toghan, A.; Shalabi, K.; Tantawy, A.H.; Khalaf, M.M. Novel Natural Surfactant-Based Fatty Acids and Their Corrosion-Inhibitive Characteristics for Carbon Steel-Induced Sweet Corrosion: Detailed Practical and Computational Explorations. Front. Mater. 2022, 9, 843438. [Google Scholar] [CrossRef]
- Cerchiaro, G.; Aquilano, K.; Filomeni, G.; Rotilio, G.; Ciriolo, M.R.; Ferreira, A.M.D.C. Isatin-Schiff base copper(II) complexes and their influence on cellular viability. J. Inorg. Biochem. 2005, 99, 1433–1440. [Google Scholar] [CrossRef]
- Vancoa, J.; Svajlenova, O.; Racanskac, E.; Muselıka, J.; Valentova, J. Antiradical activity of different copper(II) Schiff base complexes and their effect on alloxan-induced diabetes. J. Trace Elem. Med. Biol. 2004, 18, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Tebbji, K.; Hammouti, B.; Oudda, H.; Ramdani, A.; Benkadour, M. The inhibitive effect of bipyrazolic derivatives on the corrosion of steel in hydrochloric acid solution. Appl. Surf. Sci. 2005, 252, 1378–1385. [Google Scholar] [CrossRef]
- Prakash, M.; Moon, A.P.; Mondal, K.; Shekhar, S. Effect of machining configurations on the electrochemical response of mild steel in 3.5% NaCl solution. J. Mater. Eng. Perform. 2015, 24, 3643–3650. [Google Scholar] [CrossRef]
- Sorkhabia, H.A.; Shaabanib, B.; Seifzadeha, D. Corrosion inhibition of mild steel by some Schiff base compounds in hydrochloric acid. Appl. Surf. Sci. 2005, 239, 154–164. [Google Scholar] [CrossRef]
- Behpour, M.; Ghoreishi, S.M.; Salavati-Niasari, M.; Ebrahimi, B. Evaluating two new synthesized S–N Schiff bases on the corrosion of copper in 15% hydrochloric acid. Mater. Chem. Phys. 2008, 107, 153–157. [Google Scholar] [CrossRef]
- El Kacimi, Y.; Touir, R.; Galai, M.; Belakhmima, R.A.; Zarrouk, A.; Alaoui, K.; Harcharras, M.; El Kafssaoui, H.; Ebn Touhami, M. Effect of silicon and phosphorus contents in steel on its corrosion inhibition in 5 M HCl solution in the presence of Cetyltrimethylammonium/KI. J. Mater. Environ. Sci. 2016, 7, 371–381. Available online: https://www.jmaterenvironsci.com/Document/vol7/vol7_N1/40-JMES-2155-2015-El%20Kacimi.pdf (accessed on 15 January 2016).
- Dahmani, K.; Galai, M.; Ouakki, M.; Cherkaoui, M.; Touir, R.; Erkan, S.; Kaya, S.; El Ibrahimi, B. Quantum chemical and molecular dynamic simulation studies for the identification of the extracted cinnamon essential oil constituent responsible for copper corrosion inhibition in acidified 3.0 wt% NaCl medium. Inorg. Chem. Commun. 2021, 124, 108409. [Google Scholar] [CrossRef]
- Rbaa, M.; Galai, M.; Abousalem, A.S.; Lakhrissi, B.; Ebn Touhami, M.; Warad, I.; Zarrouk, A. Synthetic, spectroscopic characterization, empirical and theoretical investigations on the corrosion inhibition characteristics of mild steel in molar hydrochloric acid by three novel 8-hydroxyquinoline derivatives. Ionics 2020, 26, 503–522. [Google Scholar] [CrossRef]
- Negm, N.A.; Zaki, M.F.; Salem, M.A.I. Synthesis and Evaluation of 4-Diethyl Amino Benzaldehyde Schiff Base Cationic Amphiphiles as Corrosion Inhibitors for Carbon Steel in Different Acidic Media. J. Surfact. Deterg. 2009, 12, 321–329. [Google Scholar] [CrossRef]
- Kamboj, R.; Singh, S.; Bhadani, A.; Kataria, H.; Kaur, G. Gemini Imidazolium Surfactants: Synthesis and Their Biophysiochemical Study. Langmuir 2012, 28, 11969–11978. [Google Scholar] [CrossRef] [PubMed]
- Quagliotto, P.; Viscardi, G.; Barolo, C.; Barni, E.; Bellinvia, S.; Fisicaro, E.; Compari, C. Gemini Pyridinium Surfactants: Synthesis and Conductometric Study of a Novel Class of Amphiphiles. J. Org. Chem. 2003, 68, 7651. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Guo, J.; Feng, L.; Xu, X.; Zhu, D. Cationic Gemini surfactants based on adamantane: Synthesis, surface activity and aggregation properties. Colloids Surf. A 2014, 441, 572–580. [Google Scholar] [CrossRef]
- Mousli, R.; Tazerouti, A. Synthesis and Some Surface Properties of Glycine-Based Surfactants. J. Surfactants Deterg. 2011, 14, 65–72. [Google Scholar] [CrossRef]
- Tantawy, A.H.; Soliman, K.A.; Abd El-Lateef, H.M. Novel synthesized cationic surfactants based on natural piper nigrum as sustainable-green inhibitors for steel pipeline corrosion in CO2-3.5% NaCl: DFT, Monte Carlo simulations and experimental approaches. J. Clean. Prod. 2020, 250, 119510. [Google Scholar] [CrossRef]
- de Queiroz Baddini, A.L.; Cardoso, S.P.; Hollauer, E.; Gomes, J.A.D.C.P. Statistical analysis of a corrosion inhibitor family on three steel surfaces (duplex, super-13 and carbon) in hydrochloric acid solutions. Electrochim. Acta 2007, 53, 434. [Google Scholar] [CrossRef]
- Negm, N.A.; Kandile, N.G.; Aiad, I.A.; Mohammad, M.A. New eco-friendly cationic surfactants: Synthesis, characterization and applicability as corrosion inhibitors for carbon steel in 1 N HCl. Colloids Surf. A 2011, 391, 224–233. [Google Scholar] [CrossRef]
- Abboud, Y.; Abourriche, A.; Saffaj, T.; Berrada, M.; Charrouf, M.; Bennamara, A.; Al Himidi, N.; Hannache, H. 2, 3-Quinoxalinedione as a novel corrosion inhibitor for mild steel in 1 M HCl. Mater. Chem. Phys. 2007, 105, 1–5. [Google Scholar] [CrossRef]
- Fouda, A.S.; Elewady, Y.A.; Abd El-Aziz, H.K.; Ahmed, A.M. Corrosion Inhibition of Carbon Steel in 0.5 M HCl Solution Using Cationic Surfactants. Int. J. Electrochem. Sci. 2012, 7, 10456–10475. [Google Scholar]
- Abd El-Lateef, H.M.; Tantawy, A.H. Synthesis and evaluation of novel series of Schiff base cationic surfactants as corrosion inhibitors for carbon steel in acidic/chloride media: Experimental and theoretical investigations. RSC Adv. 2016, 6, 8681–8700. [Google Scholar] [CrossRef]
- Hamitouche, H.; Khelifa, A.; Kouache, A.; Moulay, S. Petroleum quaternary ammonium surfactants mixture synthesized from light naphtha as corrosion inhibitors for carbon steel in 1 m HCl. Corros. Rev. 2013, 31, 61. [Google Scholar] [CrossRef]
- Abiola, O.K.; Otaigbe, J. Effect of common water contaminants on the corrosion of aluminium alloys in ethylene glycol–water solution. Corros. Sci. 2008, 50, 242–247. [Google Scholar] [CrossRef]
- Abdallah, M.; Kamar, E.M.; El-Etre, A.Y.; Eid, S. Gelatin as Corrosion Inhibitor for Aluminum and Aluminum Silicon Alloys in Sodium Hydroxide Solutions. Prot. Met. Phys. Chem. 2016, 52, 140–148. [Google Scholar] [CrossRef]
- Al Otaibi, N.; Hammud, H.H. Corrosion Inhibition Using Harmal Leaf Extract as an Eco-Friendly Corrosion Inhibitor. Molecules 2021, 26, 7024. [Google Scholar] [CrossRef]
- Seyam, D.F.; Tantawy, A.H.; Eid, S.; El-Etre, A.Y. Study of the inhibition effect of two novel synthesized amidoamine- based cationic surfactants on aluminum corrosion in 0.5 M HCl solution. J. Surfact. Deterg. 2021, 25, 133–143. [Google Scholar] [CrossRef]
- Frumkin, A. Surface tension curves of higher fatty acids and the equation of condition of the surface layer. Z. Phys. Chem. 1925, 116, 466–484. [Google Scholar] [CrossRef]
- Eddy, N.O.; Awe, F.; Ebenso, E.E. Adsorption and Inhibitive Properties of Ethanol Extracts of Leaves of Solanum Melongena for the Corrosion of Mild Steel in 0.1 M HCl. Int. J. Electrochem. Sci. 2010, 5, 1996–2011. Available online: http://www.electrochemsci.org/papers/vol5/5121996.pdf (accessed on 7 May 2023).
- Eid, S.; Hassan, W.M.I. Chemical and theoretical studies for corrosion inhibition of magnesium in hydrochloric acid by tween 80 surfactant. Int. J. Electrochem. Sci. 2015, 10, 8017–8027. Available online: http://www.electrochemsci.org/papers/vol10/101008017.pdf (accessed on 7 May 2023).
- Eddy, N.O.; Ita, B.I. Theoretical and experimental studies on the inhibition potentials of aromatic oxaldehydes for the corrosion of mild steel in 0.1 M HCl. J. Mol. Model. 2011, 17, 633–647. [Google Scholar] [CrossRef]
- Ateya, B.G.; El-Anadauli, B.E.; El-Nizamy, F.M. The adsorption of thiourea on mild steel. Corros. Sci. 1984, 24, 509–515. [Google Scholar] [CrossRef]
- Emranuzzaman, T.K.; Vishwanatham, S.; Udayabhanu, G. Synergistic effects of formaldehyde and alcoholic extract of plant leaves for protection of N80 steel in 15%HCl. Corros. Eng. Sci. Technol. 2004, 39, 327. [Google Scholar] [CrossRef]
- Haque, J.; Srivastava, V.; Quraishi, M.A.; Chauhan, D.S.; Lgaz, H.; Chung, I.M. Polar group substituted imidazolium zwitterions as eco-friendly corrosion inhibitors for mild steel in acid solution. Corros. Sci. 2020, 172, 108665. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, Y.; Chen, L.J.; Wang, L.; Wu, Y.-C.; Li, H.-J. Aloe polysaccharide as an eco-friendly corrosion inhibitor for mild steel in simulated acidic oilfield water: Experimental and theoretical approaches. J. Mol. Liq. 2020, 307, 112950. [Google Scholar] [CrossRef]
- Dagdag, O.; Hsissou, R.; Berisha, A.; Erramli, H.; Hamed, O.; Jodeh, S.; El Harfi, A. Polymeric-Based Epoxy Cured with a Polyaminoamide as an Anticorrosive Coating for Aluminum 2024-T3 Surface: Experimental Studies Supported by Computational Modeling. J. Bio- Tribo-Corros. 2019, 5, 58. [Google Scholar] [CrossRef]
- Hsissou, R.; Abbout, S.; Berisha, A.; Berradi, M.; Assouag, M.; Hajjaji, N.; Elharfi, A. Experimental, DFT and molecular dynamics simulation on the inhibition performance of the DGDCBA epoxy polymer against the corrosion of the E24 carbon steel in 1.0 M HCl solution. J. Mol. Struct. 2019, 1182, 340. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Shalabi, K.; Sayed, A.R.; Gomha, S.M.; Bakir, E.M. The novel polythiadiazole polymer and its composite with α-Al(OH)3 as inhibitors for steel alloy corrosion in molar H2SO4:Experimental and computational evaluations. J. Ind. Eng. Chem. 2022, 105, 238–250. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Abdallah, Z.A.; Ahmed, M.S.M. Solvent-free synthesis and corrosion inhibition performance of Ethyl 2-(1,2,3,6-tetrahydro-6-oxo-2-thioxopyrimidin-4-yl)ethanoate on carbon steel in pickling acids: Experimental, quantum chemical and Monte Carlo simulation studies. J. Mol. Liq. 2019, 296, 111800. [Google Scholar] [CrossRef]
- Alnajjar, A.O.; Abd El-Lateef, H.M.; Khalaf, M.M.; Mohamed, I.M.A. Steel protection in acidified 3.5% NaCl by novel hybrid composite of CoCrO3/polyaniline: Chemical fabrication, physicochemical properties, and corrosion inhibition performance. Constr. Build. Mater. 2022, 317, 125918. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Shalabi, K.; Abdelhamid, A.A. One-pot synthesis of novel triphenyl hexyl imidazole derivatives catalyzed by ionic liquid for acid corrosion inhibition of C1018 steel: Experimental and computational perspectives. J. Mol. Liq. 2021, 334, 116081. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Shalabi, K.; Tantawy, A.H. Corrosion inhibition of carbon steel in hydrochloric acid solution using newly synthesized urea-based cationic fluorosurfactants: Experimental and computational investigations. N. J. Chem. 2020, 44, 17791. [Google Scholar] [CrossRef]
- Dao, V.-D.; Vu, N.H.; Choi, H.-S. All day Limnobium laevigatum inspired nano generator self-driven via water evaporation. J. Power Sources 2020, 448, 227388. [Google Scholar] [CrossRef]
- Dao, V.-D. An experimental exploration of generating electricity from nature-inspired hierarchical evaporator: The role of electrode materials. Sci. Total Environ. 2021, 759, 143490. [Google Scholar] [CrossRef] [PubMed]
- El-Etre, A.Y. Inhibition of acid corrosion of carbon steel using aqueous extract of olive leaves. J. Colloid Interface Sci. 2007, 314, 578–583. [Google Scholar] [CrossRef]
- El-Etre, A.; El-Karim, I.G.; Ibrahim, S.; El-Kattan, F. Preparation of Amino nictino nitril and its application as corrosion inhibitor for carbon steel. J. Basic Environ. Sci. 2017, 4, 226–235. [Google Scholar]
- Sobhi, M.; Eid, S. Chemical, electrochemical and morphology studies on Methyl hydroxyethyl cellulose as green inhibitor for corrosion of copper in hydrochloric acid solutions. Prot. Met. Phys. Chem. Surf. 2018, 54, 893–898. [Google Scholar] [CrossRef]
- Abdallah, M.; El-Etre, A.Y.; Kamar, E.M.; Eid, S. Licorice root extract as corrosion inhibitor for tin in sodium chloride solutions. J. Environ. Sci. 2020, 7, 191–194. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. Available online: http://www.lct.jussieu.fr/pagesperso/savin/papers/lyp/MieSavStoPre-89.pdf (accessed on 7 May 2023). [CrossRef]
- Perri, M.J.; Weber, S.H. Web-Based Job Submission Interface for the GAMESS Computational Chemistry Program. J. Chem. Educ. 2014, 91, 2206–2208. [Google Scholar] [CrossRef]
- Barca, G.M.J.; Bertoni, C.; Carrington, L.; Datta, D.; De Silva, N.; Deustua, J.E.; Włoch, M.; Xu, P.; Zahariev, F.; Gordon, M.S. Recent developments in the general atomic and molecular electronic structure system. J. Chem. Phys. 2020, 152, 154102. [Google Scholar] [CrossRef]
- Abdallah, M.; Soliman, K.A.; Alfattani, R.; Al-Gorair, A.S.; Fawzy, A.; Ibrahim, M.A. Insight of corrosion mitigation performance of SABIC iron in 0.5 M HCl solution by tryptophan and histidine: Experimental and computational approaches. Int. J. Hydrog. Energy 2022, 47, 12782–12797. [Google Scholar] [CrossRef]

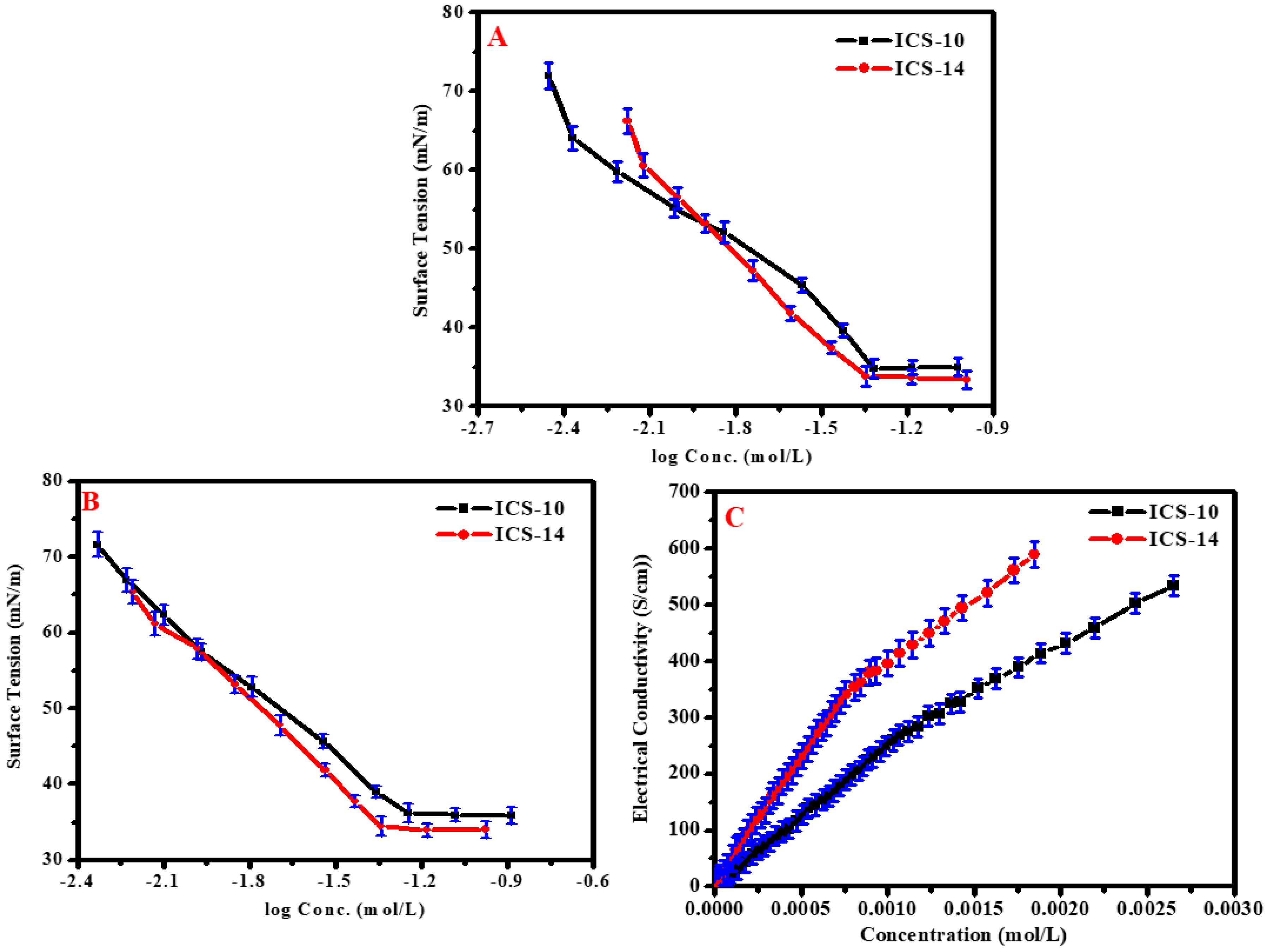



| Comp. | Surface Tension Measurements | Conductivity Measurements | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CMC a (mM L−1) | CMC b (mM L−1) | γCMC (mN/m) | ΠCMC (mN/m) | Γmax × 1011 (mol cm−2) | Amin (nm2) | CMC (mM L−1) | α | β | (KJ mol−1) | (KJ mol−1) | |
| ICS-10 | 1.34 | 0.95 | 36.17 | 35.83 | 7.31 | 1.88 | 1.17 | 0.44 | 0.56 | −26.66 | −30.72 |
| ICS-14 | 0.83 | 0.79 | 34.46 | 37.54 | 6.11 | 2.68 | 0.86 | 0.49 | 0.51 | −26.53 | −32.29 |
| Inhibitor Code | Inh. Conc. (M) | βa mV·Decade−1 | −βc mV·Decade−1 | Ecor mV (SCE) | icor µA/cm2 | I.E.% | θ |
|---|---|---|---|---|---|---|---|
| - | 0 | 63 | 214 | −379 | 720 | - | - |
| ICS-10 | 5 × 10−6 | 62 | 221 | −373 | 367 | 49.02 | 0.4902 |
| 1 × 10−5 | 56 | 237 | −372 | 346 | 51.94 | 0.5194 | |
| 5 × 10−5 | 50 | 208 | −374 | 170 | 76.39 | 0.7639 | |
| 1× 10−4 | 46 | 242 | −371 | 102 | 85.83 | 0.8583 | |
| 5 × 10−4 | 55 | 262 | −380 | 61 | 91.53 | 0.9153 | |
| ICS-14 | 5 × 10−6 | 51 | 220 | −364 | 203 | 71.81 | 0.7181 |
| 1 × 10−5 | 50 | 212 | −384 | 126 | 82.50 | 0.8250 | |
| 5 × 10−5 | 45 | 212 | −377 | 84 | 88.33 | 0.8833 | |
| 1× 10−4 | 39 | 188 | −386 | 47 | 93.47 | 0.9347 | |
| 5 × 10−4 | 43 | 188 | −397 | 39 | 94.58 | 0.9458 |
| Solution | CR1 293 K | CR2 333 K | θ1 293 K | θ2 333 K | Ea kJ·mol−1 | Qads kJ·mol−1 |
|---|---|---|---|---|---|---|
| Blank | 1.671 × 10−4 | 4.018 × 10−3 | - | - | 64.498 | - |
| ICS-10 | 1.504 × 10−5 | 3.221 × 10−3 | 0.910 | 0.198 | 108.858 | −75.276 |
| ICS-14 | 9.758 × 10−6 | 2.87 × 10−3 | 0.941 | 0.285 | 115.290 | −74.980 |
| Parameters | ICS-10 | ICS-14 |
|---|---|---|
| EHOMO (eV) | −4.71 | −4.69 |
| ELUMO (eV) | −1.33 | −1.34 |
| ΔE (eV) | 3.38 | 3.35 |
| (Debye) | 13.23 | 13.31 |
| 1.69 | 1.68 | |
| 0.59 | 0.60 | |
| ΔN (e) | 0.53 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Lateef, H.M.; Tantawy, A.H.; Soliman, K.A.; Eid, S.; Abo-Riya, M.A. Novel Imine-Tethering Cationic Surfactants: Synthesis, Surface Activity, and Investigation of the Corrosion Mitigation Impact on Carbon Steel in Acidic Chloride Medium via Various Techniques. Molecules 2023, 28, 4540. https://doi.org/10.3390/molecules28114540
Abd El-Lateef HM, Tantawy AH, Soliman KA, Eid S, Abo-Riya MA. Novel Imine-Tethering Cationic Surfactants: Synthesis, Surface Activity, and Investigation of the Corrosion Mitigation Impact on Carbon Steel in Acidic Chloride Medium via Various Techniques. Molecules. 2023; 28(11):4540. https://doi.org/10.3390/molecules28114540
Chicago/Turabian StyleAbd El-Lateef, Hany M., Ahmed H. Tantawy, Kamal A. Soliman, Salah Eid, and Mohamed A. Abo-Riya. 2023. "Novel Imine-Tethering Cationic Surfactants: Synthesis, Surface Activity, and Investigation of the Corrosion Mitigation Impact on Carbon Steel in Acidic Chloride Medium via Various Techniques" Molecules 28, no. 11: 4540. https://doi.org/10.3390/molecules28114540
APA StyleAbd El-Lateef, H. M., Tantawy, A. H., Soliman, K. A., Eid, S., & Abo-Riya, M. A. (2023). Novel Imine-Tethering Cationic Surfactants: Synthesis, Surface Activity, and Investigation of the Corrosion Mitigation Impact on Carbon Steel in Acidic Chloride Medium via Various Techniques. Molecules, 28(11), 4540. https://doi.org/10.3390/molecules28114540







