Analytics, Properties and Applications of Biologically Active Stilbene Derivatives
Abstract
1. Introduction
2. Biological Activity of Stilbene Derivatives
| Compound | Biological Activity | References |
|---|---|---|
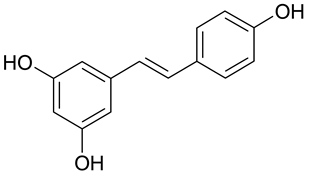 | P. oryzae, B. cinerea, P. capsici, P. viticola, C. cucumerinum, S. sapinea, P. colocasiae, C. gloeosporioides | [26,27,28] |
 | C. albicans, F. Oxysporum, P. aphanidermatum, R. solani, E. turcicum | [29,30] |
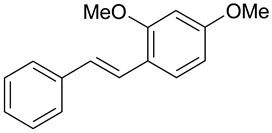 | B. cinerea, A. niger, B. subtilis, P. syringae, C. herbarum | [27] |
 | P. viticola, B. cinerea | [31] |
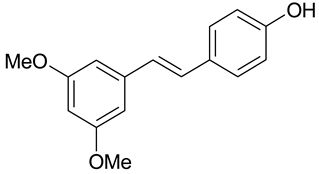 | C. albican, C. guilliermondii, C. famata, B. cinerea, P. viticola | [32] |
 | A. niger, B. cinerea, C. herbarum | [27] |
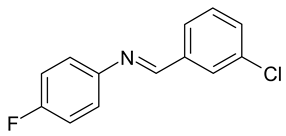 | A. niger, P. chrysogenum | [33] |
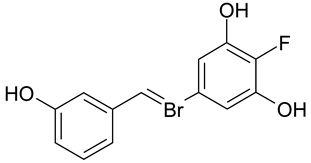 | B. brevis, B. subilis, M. luteus, M. miehei | [34] |
 | B. brevis, B. subilis, M. luteus | [34] |
 | E. coli, S. aureus, S. faecalis, B. subtilis, A. fumigatus | [35] |
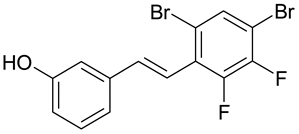 | R. miehei, P. notatum, C. graminicola, B. Cinerea | [34] |
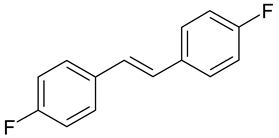 | A. niger, P. chrysogenum | [36] |
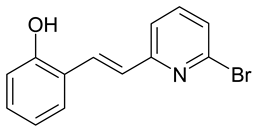 | M. indicus | [37] |
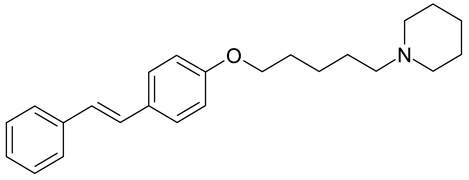 | S. aureus, S. faecalis, B. subtilis, A. fumigatus | [38] |
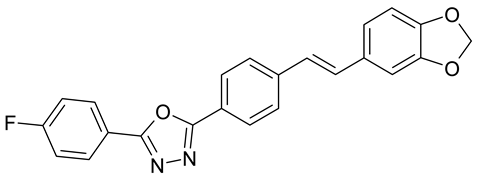 | B. cinerea | [39] |
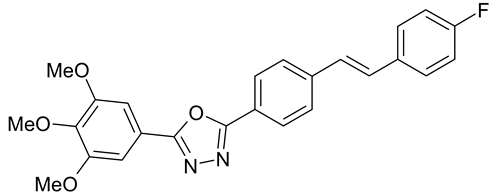 | C. lagenarium | [40] |
3. Estrogenic Effects
4. Preservatives in Drugs and Problems in Their Selection
| Name of Preservative | Concentration [%] | Form of Drug |
|---|---|---|
| Thiomersal | 0.005–0.01% [78] 0.0005–0.01% up to 0.007% [79] | vaccines, test solutions, topical preparations (creams), ear drops, eye drops |
| Parabens | 0.065–0.15% up to 0.5% | eye medications ointments, oral medications |
| Benzalkonium chloride | up to 0.02% [80] | drugs and eye preparations, |
| Chlorhexidine | up to 0.05% [81] | eye medications |
5. Stability and Analytics of Stilbene Derivatives
5.1. Solid Phase Extraction
5.2. Short- and Long-Term Stability
5.3. Isotachophoretic Separation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pecyna, P.; Wargula, J.; Murias, M.; Kucinska, M. More than Resveratrol: New Insights into Stilbene-Based Compounds. Biomolecules 2020, 10, 1111. [Google Scholar] [CrossRef]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of Environmental Factors on Stilbene Biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef]
- Olas, B. Antioxidants present in diet as anti atherosclerosis factors. Kosmos Probl. Nauk Biol. 2003, 52, 249–258. [Google Scholar]
- Kumar, S.N.; Siji, J.V.; Nambisan, B.; Mohandas, C. Activity and synergistic interactions of stilbenes and antibiotic combinations against bacteria in vitro. World J. Microbiol. Biotechnol. 2012, 28, 3143–3150. [Google Scholar] [CrossRef]
- Mahady, G.B.; Pendland, S.L. Resveratrol Inhibits The Growth of Helicobacter Pylori in Vitro. Am. J. Gastroenterol. 2000, 95, 1849. [Google Scholar] [CrossRef]
- Chan, M.M.-Y. Antimicrobial effect of resveratrol on dermatophytes and bacterial pathogens of the skin. Biochem. Pharmacol. 2002, 63, 99–104. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. Antibacterial properties of Polygonum cuspidatum roots and their major bioactive constituents. Food Chem. 2008, 109, 530–537. [Google Scholar] [CrossRef]
- Wang, W.-B.; Lai, H.-C.; Hsueh, P.-R.; Chiou, R.Y.-Y.; Lin, S.-B.; Liaw, S.-J. Inhibition of swarming and virulence factor expression in Proteus mirabilis by resveratrol. J. Med. Microbiol. 2006, 55, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Docherty, J.J.; Fu, M.M.; Tsai, M. Resveratrol selectively inhibits Neisseria gonorrhoeae and Neisseria meningitidis. J. Antimicrob. Chemother. 2001, 47, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Paulo, L.; Ferreira, S.; Gallardo, E.; Queiroz, J.; Domingues, F. Antimicrobial activity and effects of resveratrol on human pathogenic bacteria. World J. Microbiol. Biotechnol. 2010, 26, 1533–1538. [Google Scholar] [CrossRef]
- Almstrup, K.; Fernández, M.F.; Petersen, J.H.; Olea, N.; Skakkebaek, N.E.; Leffers, H. Dual effects of phytoestrogens result in u-shaped dose-response curves. Environ. Health Perspect. 2002, 110, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Gehm, B.D.; McAndrews, J.M.; Chien, P.-Y.; Jameson, J.L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 14138–14143. [Google Scholar] [CrossRef]
- Albert, S.; Horbach, R.; Deising, H.B.; Siewert, B.; Csuk, R. Synthesis and antimicrobial activity of (E) stilbene derivatives. Bioorganic Med. Chem. 2011, 19, 5155–5166. [Google Scholar] [CrossRef]
- Jeandet, P.; Douillet-Breuil, A.-C.; Bessis, R.; Debord, S.; Sbaghi, M.; Adrian, M. Phytoalexins from the Vitaceae: Biosynthesis, Phytoalexin Gene Expression in Transgenic Plants, Antifungal Activity, and Metabolism. J. Agric. Food Chem. 2002, 50, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.N.; Stevenson, P.C.; Kokubun, T.; Hall, D.R. Antibacterial and antifungal activity of cicerfuran and related 2-arylbenzofurans and stilbenes. Microbiol. Res. 2009, 164, 191–195. [Google Scholar] [CrossRef]
- Weber, K.; Schulz, B.; Ruhnke, M. Resveratrol and its antifungal activity against Candida species. Mycoses 2011, 54, 30–33. [Google Scholar] [CrossRef]
- Houillé, B.; Papon, N.; Boudesocque, L.; Bourdeaud, E.; Besseau, S.; Courdavault, V.; Enguehard-Gueiffier, C.; Delanoue, G.; Guérin, L.; Bouchara, J.-P.; et al. Antifungal Activity of Resveratrol Derivatives against Candida Species. J. Nat. Prod. 2014, 77, 1658–1662. [Google Scholar] [CrossRef]
- Kato, E.; Tokunaga, Y.; Sakan, F. Stilbenoids Isolated from the Seeds of Melinjo (Gnetum gnemon L.) and Their Biological Activity. J. Agric. Food Chem. 2009, 57, 2544–2549. [Google Scholar] [CrossRef]
- Shina, N.H.; Ryu, S.Y.; Choi, E.J.; Kangc, S.H.; Changd, I.L.M.; Min, K.R.; Kim, Y. Oxyresveratrol as the Potent Inhibitor on Dopa Oxidase Activity of Mushroom Tyrosinase. Biochem. Biophys. Res. Commun. 1998, 243, 801–803. [Google Scholar] [CrossRef]
- Sanoh, S.; Kitamura, S.; Sugihara, K.; Fujimoto, N.; Ohta, S. Estrogenic Activity of Stilbene Derivatives. J. Health Sci. 2003, 49, 359–367. [Google Scholar] [CrossRef]
- Singh, D.; Chauhan, N.; Koli, M.; Nayak, S.K.; Subramanian, M. Dimer stilbene, a resveratrol analogue exhibits synergy with antibiotics that target protein synthesis in eradicating Staphylococcus aureus infection. Biochimie 2022, 201, 128–138. [Google Scholar] [CrossRef]
- Cebrián, R.; Li, Q.; Peñalver, P.; Belmonte-Reche, E.; Andrés-Bilbao, M.; Lucas, R.; de Paz, M.V.; Kuipers, O.P.; Morales, J.C. Chemically Tuning Resveratrol for the Effective Killing of Gram-Positive Pathogens. J. Nat. Prod. 2022, 85, 1459–1473. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Adwan, G.; Mhanna, M. Synergistic Effects of plant extracts and antibiotics on Staphylococcus aureus strains isolated from clinical specimens. Middle-East J. Sci. Res. 2008, 3, 134–139. [Google Scholar]
- Adrian, M.; Jeandet, P. Effects of resveratrol on the ultrastructure of Botrytis cinerea conidia and biological significance in plant/pathogen interactions. Fitoterapia 2012, 83, 1345–1350. [Google Scholar] [CrossRef]
- Bostanghadiri, N.; Pormohammad, A.; Chirani, A.S.; Pouriran, R.; Erfanimanesh, S.; Hashemi, A. Comprehensive review on the antimicrobial potency of the plant polyphenol Resveratrol. Biomed. Pharmacother. 2017, 95, 1588–1595. [Google Scholar] [CrossRef]
- Kumar, S.N.; Nambisan, B. Antifungal Activity of Diketopiperazines and Stilbenes Against Plant Pathogenic Fungi In Vitro. Appl. Biochem. Biotechnol. 2014, 172, 741–754. [Google Scholar] [CrossRef]
- Park, H.B.; Crawford, J.M. Lumiquinone A, an α-Aminomalonate-Derived Aminobenzoquinone from Photorhabdus luminescens. J. Nat. Prod. 2015, 78, 1437–1441. [Google Scholar] [CrossRef]
- Shi, D.; An, R.; Zhang, W.; Zhang, G.; Yu, Z. Stilbene Derivatives from Photorhabdus temperata SN259 and Their Antifungal Activities against Phytopathogenic Fungi. J. Agric. Food Chem. 2017, 65, 60–65. [Google Scholar] [CrossRef]
- Chalal, M.; Klinguer, A.; Echairi, A.; Meunier, P.; Vervandier-Fasseur, D.; Adrian, M. Antimicrobial Activity of Resveratrol Analogues. Molecules 2014, 19, 7679–7688. [Google Scholar] [CrossRef]
- Li, D.-D.; Zhao, L.-X.; Mylonakis, E.; Hu, G.-H.; Zou, Y.; Huang, T.-K.; Yan, L.; Wang, Y.; Jiang, Y.-Y. In Vitro and In Vivo Activities of Pterostilbene against Candida albicans Biofilms. Antimicrob. Agents Chemother. 2014, 58, 2344–2355. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.S.; Butle, S.R.; Shaikh, R.M.; Zubaidha, P.K.; Pedgaonkar, G.S.; Shendarkar, G.S.; Rajput, C.G. Synthesis and biological evaluation of some novel substituted N-benzylideneaniline derivatives. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 707–717. [Google Scholar]
- Bavaresco, L.; Mattivi, F.; De Rosso, M.; Flamini, R. Effects of elicitors, viticultural factors, and enological practices on resveratrol and stilbenes in grapevine and wine. Mini-Rev. Med. Chem. 2012, 12, 1366–1381. [Google Scholar]
- Wyrzykiewicz, E.; Błaszczak, A.; Kędzia, B. Synthesis and antimicrobial activity of (E)-acetoxystilbenes and a,a′-dibromoacetoxybibenzyls. Il Farm. 2000, 55, 151–157. [Google Scholar] [CrossRef]
- Karki, S.S.; Bhutle, S.R.; Pedgaonkar, G.S.; Zubaidha, P.K.; Shaikh, R.M.; Rajput, C.G.; Shendarkar, G.S. Synthesis and biological evaluation of some stilbene-based analogues. Med. Chem. Res. 2011, 20, 1158–1163. [Google Scholar] [CrossRef]
- Chandrasekara Reddy, G.; Shiva Prakash, S.; Diwakar, L. Stilbene heterocycles: Synthesis, antimicrobial, antioxidant and anti-cancer activities. Pharm. Innov. 2015, 3, 24–30. [Google Scholar]
- Wyrzykiewicz, E.; Wendzonka, M.; Kędzia, B. Synthesis and antimicrobial activity of new (E)-4-[piperidino (4′-methylpiperidino-, morpholino-) N-alkoxy]stilbenes. Eur. J. Med. Chem. 2006, 41, 519–525. [Google Scholar] [CrossRef]
- Jian, W.; He, D.; Song, S. Synthesis, Biological Evaluation and Molecular Modeling Studies of New Oxadiazole-Stilbene Hybrids against Phytopathogenic Fungi. Sci. Rep. 2016, 6, 31045. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; He, D.; Xi, P.; Li, X. Synthesis and Biological Evaluation of Novel Fluorine-Containing Stilbene Derivatives as Fungicidal Agents against Phytopathogenic Fungi. J. Agric. Food Chem. 2015, 63, 9963–9969. [Google Scholar] [CrossRef] [PubMed]
- Piver, B.; Berthou, F.; Dreano, Y.; Lucas, D. Inhibition of CYP3A, CYP1A and CYP2E1 activities by resveratrol and other non volatile red wine components. Toxicol. Lett. 2001, 125, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Wierzchowski, M.; Dutkiewicz, Z.; Gielara-Korzańska, A.; Korzański, A.; Teubert, A.; Teżyk, A.; Stefański, T.; Baer-Dubowska, W.; Mikstacka, R. Synthesis, biological evaluation and docking studies of trans -stilbene methylthio derivatives as cytochromes P450 family 1 inhibitors. Chem. Biol. Drug Des. 2017, 90, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Kobylka, P.; Kucinska, M.; Kujawski, J.; Lazewski, D.; Wierzchowski, M.; Murias, M. Resveratrol Analogues as Selective Estrogen Signaling Pathway Modulators: Structure–Activity Relationship. Molecules 2022, 27, 6973. [Google Scholar] [CrossRef]
- Lephart, E.D. Phytoestrogens (Resveratrol and Equol) for Estrogen-Deficient Skin—Controversies/Misinformation versus Anti-Aging In Vitro and Clinical Evidence via Nutraceutical-Cosmetics. Int. J. Mol. Sci. 2021, 22, 11218. [Google Scholar] [CrossRef]
- Shah, A.A.; Shah, A.; Kumar, A.; Lakra, A.; Singh, D.; Nayak, Y. Phytoestrogenic Potential of Resveratrol by Selective Activation of Estrogen Receptor-α in Osteoblast Cells. Rev. Bras. Farm. 2022, 32, 248–256. [Google Scholar] [CrossRef]
- Rimando, A.M.; Suh, N. Biological/Chemopreventive Activity of Stilbenes and their Effect on Colon Cancer. Planta Med. 2008, 74, 1635–1643. [Google Scholar] [CrossRef]
- Kuršvietienė, L.; Stanevičienė, I.; Mongirdienė, A.; Bernatonienė, J. Multiplicity of effects and health benefits of resveratrol. Medicina 2016, 52, 148–155. [Google Scholar] [CrossRef]
- Fang, H.; Tong, W.; Shi, L.M.; Blair, R.; Perkins, R.; Branham, W.; Hass, B.S.; Xie, Q.; Dial, S.L.; Moland, C.L.; et al. Structure−Activity Relationships for a Large Diverse Set of Natural, Synthetic, and Environmental Estrogens. Chem. Res. Toxicol. 2001, 14, 280–294. [Google Scholar] [CrossRef]
- Elder, D.P. Effective formulation development strategies for poorly soluble active pharmaceutical ingredients (APIs). Am. Pharm. Rev. 2012, 13, 28–34. [Google Scholar]
- Stanojevic, D.; Comic, L.J.; Stefanovic, O.; Solujic-Sukdolak, S. Antimicrobial effects of sodium benzoate, sodium nitrite and potassium sorbate and their synergistic action in vitro. Bulg. J. Aqric. Sci. 2009, 15, 307–311. [Google Scholar]
- Bojarowicz, H.; Fronczak, P.; Krysiński, J. Can cosmetics be preservative-free? Hygeia Public Health 2018, 53, 124–131. [Google Scholar]
- Elder, D.P.; Crowley, P.J. Antimicrobial Preservatives Part Three: Chalenges Facing Preservative Systems. 2012. Available online: www.americanpharmaceutical-review.com (accessed on 12 March 2023).
- Strilets, O.P.; Petrovska, L.S.; Baranova, I.I.; Bespala, Y.O. A study of antimicrobial activity of foam-washing agent specimens at acidic pH values. Ann. Mechnikov’s Institude 2017, 2, 23–26. [Google Scholar] [CrossRef]
- Hall, M.J.; Middleton, R.F.; Westmacott, D. The fractional inhibitory concentration (FIC) index as a measure of synergy. J. Anti-microb. Chem. 1983, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Regulation of the Minister of Health on Preservatives, Sweeteners, Colors and Antioxidants That May Be Included in the Com-Position of Medicinal Products. Dz.U. 2003 nr 19, poz. 169. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20030190169 (accessed on 12 January 2023). (In Polish)
- Pharmacopeia Poland, 12th ed.; Polish Pharmaceutical Society: Warsaw, Poland, 2022. (In Polish)
- Penha, F.M.; Rodrigues, E.B.; Maia, M.; Furlani, B.A.; Regatieri, C.; Melo, G.B.; Magalhães, J.O.; Manzano, R.; Farah, M.E. Retinal and Ocular Toxicity in Ocular Application of Drugs and Chemicals—Part II: Retinal Toxicity of Current and New Drugs. Ophthalmic Res. 2010, 44, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Walsh, F. Muslim Fear Hamper Drive to Eradicate Polio. The Observer, 29 May 2005. Available online: www.theguardian.com/world/2005/may/29/theobserver1 (accessed on 23 February 2023).
- United States Pharmacopeia, General Chapter Antimicrobial Effectiveness Testing; USP 34–NF29; United States Pharmacopeial Convention: Rockville, MD, USA, 1 January 2015. Available online: www.amazon.com/United-States-Pharmacopeia-National-Formulary/dp/1936424320 (accessed on 12 January 2023).
- European Directorate for Quality of Medicines. European Pharmacopeia 5.1.3; Efficacy of Antimicrobial Preservation; FarmEuropa: Strasbourg, France, 2020; Available online: https://www.edqm.eu/en/web/edqm/european-pharmacopoeia-ph.-eur (accessed on 27 March 2023).
- Kireche, M.; Peiffer, J.-L.; Antonios, D.; Fabre, I.; Giménez-Arnau, E.; Pallardy, M.; Lepoittevin, J.-P.; Ourlin, J.-C. Evidence for Chemical and Cellular Reactivities of the Formaldehyde Releaser Bronopol, Independent of Formaldehyde Release. Chem. Res. Toxicol. 2011, 24, 2115–2128. [Google Scholar] [CrossRef]
- Anon. Benzyl alcohol may be toxic to newborns. FDA Drug. Bull. 1982, 12, 10. [Google Scholar]
- Cahill, E. Benzyl Alcohol Monograph. In Handbook of Pharmaceutical Excipients, 5th ed.; Rowe, R.C., Sheskey, P.J., Weller, P.J., Eds.; Pharmaceutical Press: London, UK, 2006; pp. 9–71. [Google Scholar]
- Weller, P.J. Benzoic Acid Monigraph. In Handbook of Pharmaceutical Excipients, 6th ed.; Rowe, R.C., Sheskey, P.J., Weller, P.J., Eds.; Pharmaceutical Press: London, UK, 2007; pp. 66–68. [Google Scholar]
- Downard, C.D.; Roberts, L.J.; Morrow, J.D. Topical benzoic acid induces the increased biosynthesis of prostaglandin D2 in human skin in vivo*. Clin. Pharmacol. Ther. 1995, 57, 441–445. [Google Scholar] [CrossRef]
- Abraham, J.; Dowling, K.; Florentine, S. Can copper products and surfaces reduce the spread of infectious microorganisms and hospital-acquired infections? Materials 2021, 14, 3444. [Google Scholar] [CrossRef]
- Różańska, A.; Chmielarczyk, A.; Romaniszyn, D.; Sroka-Oleksiak, A.; Bulanda, M.; Walkowicz, M.; Osuch, P.; Knych, T. Antimicrobial Properties of Selected Copper Alloys on Staphylococcus aureus and Escherichia coli in Different Simulations of Environmental Conditions: With vs. without Organic Contamination. Int. J. Environ. Res. Public Health 2017, 14, 813. [Google Scholar] [CrossRef]
- Barregard, L.; Rekić, D.; Horvat, M.; Elmberg, L.; Lundh, T.; Zachrisson, O. Toxicokinetics of Mercury after Long-Term Repeated Exposure to Thimerosal-Containing Vaccine. Toxicol. Sci. 2011, 120, 499–506. [Google Scholar] [CrossRef]
- Aronson, J.K. Meyler’s Side Effects of Drugs; Elsevier Science: Amsterdam, The Netherlands, 2015; p. 7674. [Google Scholar]
- Esposito, S.; Principi, N.; Cornaglia, G. Barriers to the vaccination of children and adolescents and possible solutions. Clin. Microbiol. Infect. 2014, 20, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; Geier, M.R. An assessment of downward trends in neurodevelopmental disorders in the United States following removal of Thimerosal from childhood vaccines. Experiment 2006, 12, CR231–CR239. [Google Scholar]
- Tan, M.; Parkin, J. Route of decomposition of thiomersal (thimerosal). Int. J. Pharm. 2000, 208, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Trümpler, S.; Meermann, B.; Nowak, S.; Buscher, W.; Karst, U.; Sperling, M. In vitro study of thimerosal reactions in human whole blood and plasma surrogate samples. J. Trace Elem. Med. Biol. 2014, 28, 125–130. [Google Scholar] [CrossRef]
- Geier, D.A.; Kern, J.K.; Geier, M.R. Premature Puberty and Thimerosal-Containing Hepatitis B Vaccination: A Case-Control Study in the Vaccine Safety Datalink. Toxics 2018, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Vena, G.A.; Foti, C.; Grandolfo, M.; Angelini, G. Mercury exanthem. Contact Dermat. 1994, 31, 214–216. [Google Scholar] [CrossRef]
- Lohiya, G. Asthma and urticaria after hepatitis B vaccination. West. J. Med. 1987, 147, 341. [Google Scholar]
- Aberer, W. Vaccination despite thimerosal sensitivity. Contact Dermat. 1991, 24, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.K.; Haley, B.E.; Geier, D.A.; Sykes, L.K.; King, P.G.; Geier, M.R. Thimerosal Exposure and the Role of Sulfation Chemistry and Thiol Availability in Autism. Int. J. Environ. Res. Public Health 2013, 10, 3771–3800. [Google Scholar] [CrossRef]
- Podgórska, A.; Puścion-Jakubik, A.; Grodzka, A.; Naliwajko, S.K.; Markiewicz-Zukowska, R.; Socha, K. Natural and conven-tional cosmetics-mercury exposure assessment. Molecules 2021, 26, 4088. [Google Scholar] [CrossRef]
- Liebert, M.A. Final report on the safety assessment of benzethonium chloride and methylbenzethonium chloride. J. Am. Coll. Toxicol. 1985, 4, 65–106. [Google Scholar]
- Rove, R.C.; Sheskey, P.J.; Owen, S.C. Handbook of Pharmaceutical Excipients, 5th ed.; Pharmaceutical Press: London, UK; American Pharmacists Association: Washington, DC, USA, 2006. [Google Scholar]
- Prukala, W.; Wyrzykiewicz, E.; Kedzia, B. Synthesis and antimicrobial properties of N-substituted halides of (E)-azastilbenols. Il Farm. 1995, 50, 779–782. [Google Scholar]
- Prukała, D.; Prukała, W.; Małkiewicz, K.; Szymalska, M.; Witkowska-Krajewska, E.; Kluska, M. New methodology of separation and determination of biologically active isomers of nitrobenzyl azastilbene derivatives. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 761–769. [Google Scholar] [CrossRef]
- Al-Suod, H.; Ratiu, I.-A.; Gadzała-Kopciuch, R.; Górecki, R.; Buszewski, B. Identification and quantification of cyclitols and sugars isolated from different morphological parts of Raphanus sativus L. Nat. Prod. Res. 2023, 37, 107–112. [Google Scholar] [CrossRef]
- Mencin, M.; Mikulic-Petkovsek, M.; Veberic, R.; Terpinc, P. Development and optimisation of solid-phase extraction of ex-tractable and bound phenolic acids in spelt (Triticum spelta L.) seeds. Antioxidants 2021, 10, 1085. [Google Scholar] [CrossRef]
- Nawała, J.; Szala, M.; Dziedzic, D.; Gordon, D.; Dawidziuk, B.; Fabisiak, J.; Popiel, S. Analysis of samples of explosives excavated from the Baltic Sea floor. Sci. Total Environ. 2020, 708, 135198. [Google Scholar] [CrossRef]
- Wianowska, D. Combination of sea sand disruption method and ion-pair solid-phase extraction for effective isolation and purification of chlorogenic acid from plants prior to the HPLC determination. Molecules 2022, 27, 5601. [Google Scholar] [CrossRef] [PubMed]
- Cigalski, P.; Kosobucki, P. Recent Materials Developed for Dispersive Solid Phase Extraction. Molecules 2020, 25, 4869. [Google Scholar] [CrossRef] [PubMed]
- Awang, M.A.; Chua, L.S.; Abdullah, L.C. Solid-Phase Extraction and Characterization of Quercetrin-Rich Fraction from Melastoma malabathricum Leaves. Separations 2022, 9, 373. [Google Scholar] [CrossRef]
- Kluska, M.; Krajewska, E.; Jabłońska, J.; Prukała, W. New Applications and Analysis of (E)-Azastilbenes in Environmental Samples. Crit. Rev. Anal. Chem. 2019, 49, 395–402. [Google Scholar] [CrossRef]
- Buszewski, B.; Szultka-Młyńska, M. Past, Present, and Future of Solid Phase Extraction: A Review. Crit. Rev. Anal. Chem. 2012, 42, 198–213. [Google Scholar] [CrossRef]
- Jabłońska, J.; Kluska, M.; Erchak, N. Development of a procedure for the isolation of electrostatically stabilized silanates from wheat samples. Przem. Chem. 2020, 99, 605–608. [Google Scholar] [CrossRef]
- Kluska, M.; Liepinsh, E.; Pypowski, K.; Erchak, N. Separation of Azepinio-Methyl Derivatives of ES-Silanates by the Use of Aryl Stationary Phases in HPLC. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 675–682. [Google Scholar] [CrossRef]
- Kluska, M.; Liepinsh, E.; Pypowski, K.; Chrząścik, I.; Michel, M.; Erchak, N. Separation of Selected Derivatives of Hoszczawa-Silanates, Taking Advantage of π–π Interactions. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 2812–2820. [Google Scholar] [CrossRef]
- Prukała, W.; Prukała, D.; Pypowski, K.; Chrząścik, I.; Kluska, M. Chromatography of Biologically Active Chlorides of (E)-N-o-(m- or p-)chlorobenzyl-γ-azastilbenols-2′(3′ or 4′). J. Liq. Chromatogr. Relat. Technol. 2008, 31, 2612–2620. [Google Scholar] [CrossRef]
- Prukała, W.; Pypowski, K.; Chrząścik, I.; Kluska, M. Separation of Biologically Active Isomers of (E)-N-Meta- and Para-Nitroazastilbenes by the HPLC Technique. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 578–585. [Google Scholar] [CrossRef]
- Gadzała-Kopciuch, R.; Kluska, M.; Wełniak, M.; Buszewski, B. Silicon dioxide surfaces with aryl interaction sites for chromatographic applications. Mater. Chem. Phys. 2005, 89, 228–237. [Google Scholar] [CrossRef]
- Kluska, M.; Jabłońska, J.; Erchak, N. Analytics and Application of Biologically Active Pentacoordinate Electrostatically Stabilized Silanates. Crit. Rev. Anal. Chem. 2021, 51, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, J.; Kluska, M.; Erchak, N.; Popiel, S. Development of an extraction procedure and analysis of electrostatically stabilized silanates from aqueous solutions. Oceanol. Hydrobiol. Stud. 2020, 49, 247–254. [Google Scholar] [CrossRef]
- Witkowska-Krajewska, E.; Kluska, M.; Prukała, W.; Mikulewicz, M.; Chojnacka, K.; Małkiewicz, K. Study on stability of (E)-azastilbenes as disinfectants and preservatives as well as their recovery from aqueous solutions. Przem. Chem. 2018, 97, 1320–1324. [Google Scholar] [CrossRef]
- Gopchak, I.; Kalko, A.; Basiuk, T.; Pinchuk, O.; Gerasimov, I.; Yaromenko, O.; Shkirynets, V. Assessment of surface water pol-lution in Western Bug River within the cross-border section of Ukraine. J. Water Land Dev. 2020, 46, 97–104. [Google Scholar] [CrossRef]
- Gopchak, I.; Basiuk, T.; Bialyk, I.; Pinchuk, O.; Gerasimov, I. Dynamics of changes in surface water quality indicators of the Western Bug River basin within Ukraine using GIS technologies. J. Water Land Dev. 2019, 42, 67–75. [Google Scholar] [CrossRef]
- Hagemann, N.; Blumensaat, F.; Tavares, F.; Trumper, J.; Burmeister, C.; Moynihan, R.; Scheifhackenf, N. The long road to improving the water quality of the Western Bug River (Ukraine)—A multi-scale analysis. J. Hydrol. 2014, 519, 2436–2447. [Google Scholar] [CrossRef]
- Odnorih, Z.; Manko, R.; Malovanyy, M.; Soloviy, C. Results of Surface Water Quality Monitoring of the Western Bug River Basin in Lviv Region. J. Ecol. Eng. 2020, 21, 18–26. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Konarzewska, J.; Piątek, M.; Mikulski, M. Environmental Protection Program for the Liw Commune for 2016–2019; Public Information Bulletin: Węgrów, Poland, 2015; p. 139. [Google Scholar]
- Kluska, M.; Jabłońska, J.; Prukała, W.; Popiel, S. Research on the Stability of Biologically Active (E)-azastilbene Derivatives in Polish Rivers. Pol. J. Environ. Stud. 2021, 30, 1647–1653. [Google Scholar] [CrossRef]
- Jabłońska, J.; Kluska, M. Determination of Mercury Content in Surface Waters Using an Environmentally Non-Toxic Terminating Electrolyte. Bull. Environ. Contam. Toxicol. 2020, 105, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, J.; Kluska, M. Dynamics of mercury content changes in snow in the heating season on the example of the city of Siedlce. Ochr. Srodowiska I Zasobow Nat. 2019, 30, 19–24. [Google Scholar] [CrossRef]
- Malá, Z.; Gebauer, P.; Boček, P. Analytical capillary isotachophoresis after 50 years of development: Recent progress 2014–2016. Electrophoresis 2017, 38, 9–19. [Google Scholar] [CrossRef]
- Jabłońska, J.; Kluska, M.; Erchak, N. The challenge of separating and determining biologically active electrostatically stabilized silanates using the high-performance liquid chromatography technique. J. Sep. Sci. 2020, 43, 3399–3407. [Google Scholar] [CrossRef]
- Jabłońska, J.; Kluska, M.; Erchak, N. Analytics of biologically active derivatives of electrostatically stabilized silanates by isotachophoresis. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 1098–1103. [Google Scholar] [CrossRef]
- Kluska, M.; Marciniuk-Kluska, A.; Prukala, D.; Prukala, W. Analytics of Quinine and its Derivatives. Crit. Rev. Anal. Chem. 2016, 46, 139–145. [Google Scholar] [CrossRef]
- Kluska, M.; Pypowski, K.; Chrząścik, I.; Erchak, N. Isotachophoresis of Chosen Heptacoordinated Goshchava Silanates. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 2396–2406. [Google Scholar] [CrossRef]
- Prukała, D.; Chrząścik, I.; Prukała, W.; Pypowski, K.; Szymalska, M.; Kluska, M. Isotachophoresis of Chosen Biologically Active (E)-Azastilbenes. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 2193–2202. [Google Scholar] [CrossRef]
- Kluska, M.; Pypowski, K.; Chrząścik, I.; Koval, T.; Erchak, N. Separation and Determination of Chosen λ5-Silanates by an Isotachophoresis Technique. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 896–907. [Google Scholar] [CrossRef]
- Kluska, M. Analytical Techniques in Determination of Biologically Active Organosilicons of the ES-Silanate Group. Crit. Rev. Anal. Chem. 2008, 38, 216–226. [Google Scholar] [CrossRef]
- Kosobucki, P.; Buszewski, B. Selected Imidazolium Ionic Liquids as a Terminating Electrolyte in Isotachophoresis. Anal. Lett. 2010, 43, 2631–2639. [Google Scholar] [CrossRef]
- Kosobucki, P.; Buszewski, B. Determination of tetrafluoroborate and chloride anions by capillary isotachophoresis and sup-pressed ion chromatography. Chem. Anal. 2008, 53, 895–903. [Google Scholar]
- Malá, Z.; Gebauer, P. Capillary moving-boundary isotachophoresis with electrospray ionization mass-spectrometric detection and hydrogen ion used as essential terminator: Methodology for sensitive analysis of hydroxyderivatives of s -triazine herbicides in waters. J. Chromatogr. A 2017, 1518, 97–103. [Google Scholar] [CrossRef]
- Malá, Z.; Gebauer, P. Recent progress in analytical capillary isotachophoresis. Electrophoresis 2019, 40, 55–64. [Google Scholar] [CrossRef]
- Datinská, V.; Voráčová, I.; Schlecht, U.; Berka, J.; Foret, F. Recent progress in nucleic acids isotachophoresis. J. Sep. Sci. 2018, 41, 236–247. [Google Scholar] [CrossRef]
- Guo, S.; Jacroux, T.; Ivory, C.F.; Li, L.; Dong, W.-J. Immunobinding-induced alteration in the electrophoretic mobility of proteins: An approach to studying the preconcentration of an acidic protein under cationic isotachophoresis. Electrophoresis 2019, 40, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Hradski, J.; Duriš, M.; Szucs, R.; Moravský, L.; Matejcík, Š.; Masár, M. Development of microchip isotachophoresis coupled with ion mobility spectrometry and evaluation of its potential for the analysis of food, biological and pharmaceutical samples. Molecules 2021, 26, 6094. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolska-Iwanek, J.; Jamka-Kasprzyk, M.; Rusin, M.; Paśko, P.; Grekh, S.; Jurczak, A. Developed and validated capillary isotachophoresis method for the rapid determining organic acids in children’s saliva. Molecules 2023, 28, 1092. [Google Scholar] [CrossRef]
- Jabłońska, J.; Kluska, M.; Erchak, N.; Prukała, W. A non-toxic for environmental electrolyte terminating for the analysis of stilbene derivatives by the isotachophoresis technique. Int. J. Environ. Anal. Chem. 2022, 102, 483–490. [Google Scholar] [CrossRef]

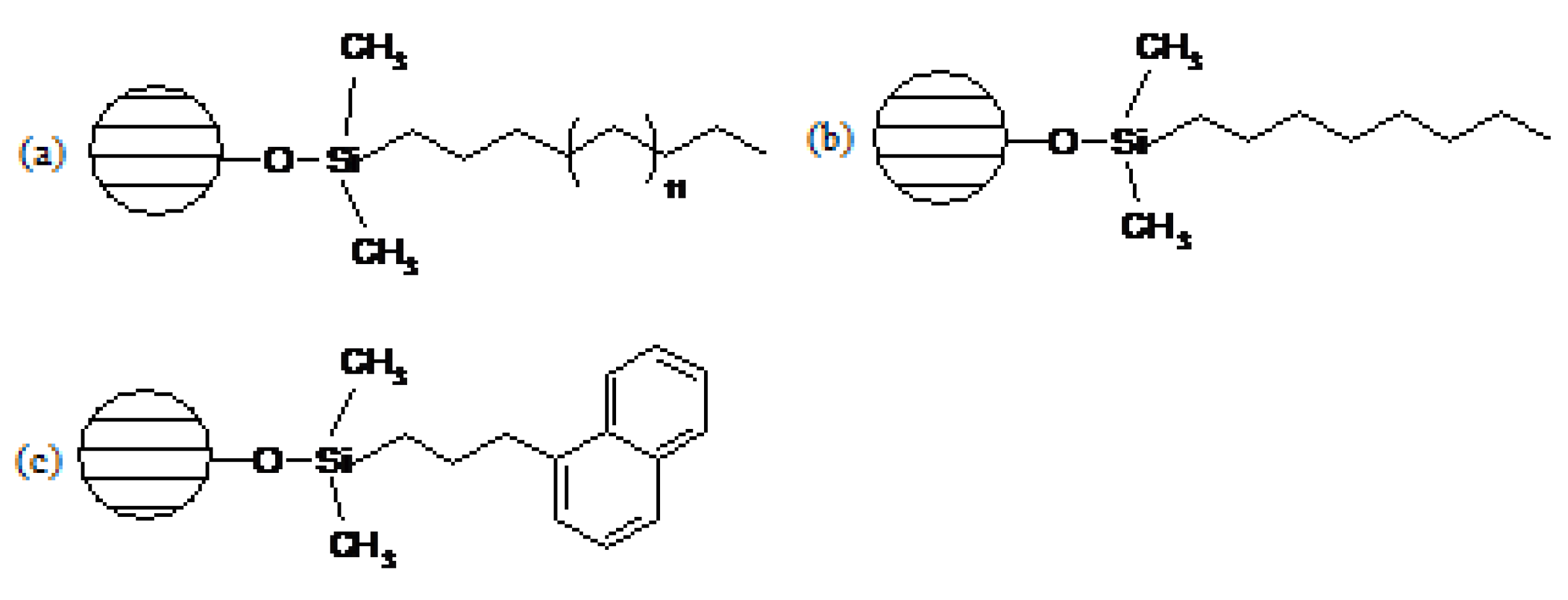

| Microorganisms | Criteria | Log Reduction | ||||
|---|---|---|---|---|---|---|
| 6 h | 24 h | 7 Days | 14 Days | 28 Days | ||
| Bacteria | A | 2 | 3 | - | - | BW |
| B | - | 1 | 3 | - | BN | |
| Fungi | A | - | - | 2 | - | BN |
| B | - | - | - | 1 | BN | |
| Compound | Minimum Inhibitory Concentration, μg/mL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| (A1) | 100 | 500 | 500 | 100 | 1000 | 1000 | >500 | >500 | >500 |
| (A2) | 100 | 100 | 500 | 100 | 500 | 1000 | >500 | >500 | >500 |
| (A3) | 7.5 | 100 | 100 | 100 | 1000 | 1000 | >500 | >500 | >500 |
| (A4) | 5 | 500 | 500 | 100 | 1000 | 1000 | >500 | >500 | >500 |
| (A5) | 100 | 500 | 500 | 500 | 1000 | 1000 | >500 | >500 | >500 |
| (A6) | 7.5 | 500 | 100 | 100 | 1000 | 1000 | >500 | >500 | >500 |
| Compound | m.p. °C | IR(KBr) (cm−1) δCH=CH | 1H NMR δ (ppm) DMSO–d6 CH2–N |
|---|---|---|---|
| (A1) | 227–230 | 970 | 5.81 s |
| (A2) | 240–243 | 985 | 5.81 s |
| (A3) | 209–212 | 965 | 5.82 s |
| (A4) | 256–259 | 995 | 5.88 s |
| (A5) | 218–221 | 980 | 5.90 s |
| (A6) | 247–249 | 955 | 5.86 s |
| Time | Matrix | Average Value, µg/mL | |||||
|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | A6 | ||
| 1 h | distilled water 3x | 893.8 | 881.9 | 903.1 | 886.4 | 921.9 | 917.4 |
| surface water | 888.1 | 883.4 | 887.9 | 882.7 | 918.0 | 913.6 | |
| wastewater | 871.9 | 876.4 | 879.1 | 878.3 | 913.4 | 909.0 | |
| 7 days | distilled water 3x | 884.5 | 887.1 | 881.7 | 882.6 | 911.9 | 903.5 |
| surface water | 873.1 | 870.2 | 863.8 | 874.5 | 902.5 | 875.1 | |
| wastewater | 860.7 | 864.8 | 861.0 | 860.1 | 864.9 | 860.6 | |
| 28 days | distilled water 3x | 795.2 | 788.1 | 794.1 | 786.9 | 790.0 | 807.6 |
| surface water | 789.1 | 787.5 | 786.5 | 791.0 | 795.8 | 781.5 | |
| wastewater | 781.6 | 780.3 | 780.2 | 783.3 | 777.8 | 763.5 | |
| 1 year | distilled water 3x | 715.3 | 703.7 | 705.0 | 713.4 | 695.1 | 721.2 |
| surface water | 690.1 | 687.8 | 681.1 | 678.9 | 679.3 | 645.5 | |
| wastewater | 686.2 | 679.7 | 696.8 | 686.7 | 701.2 | 680.7 | |
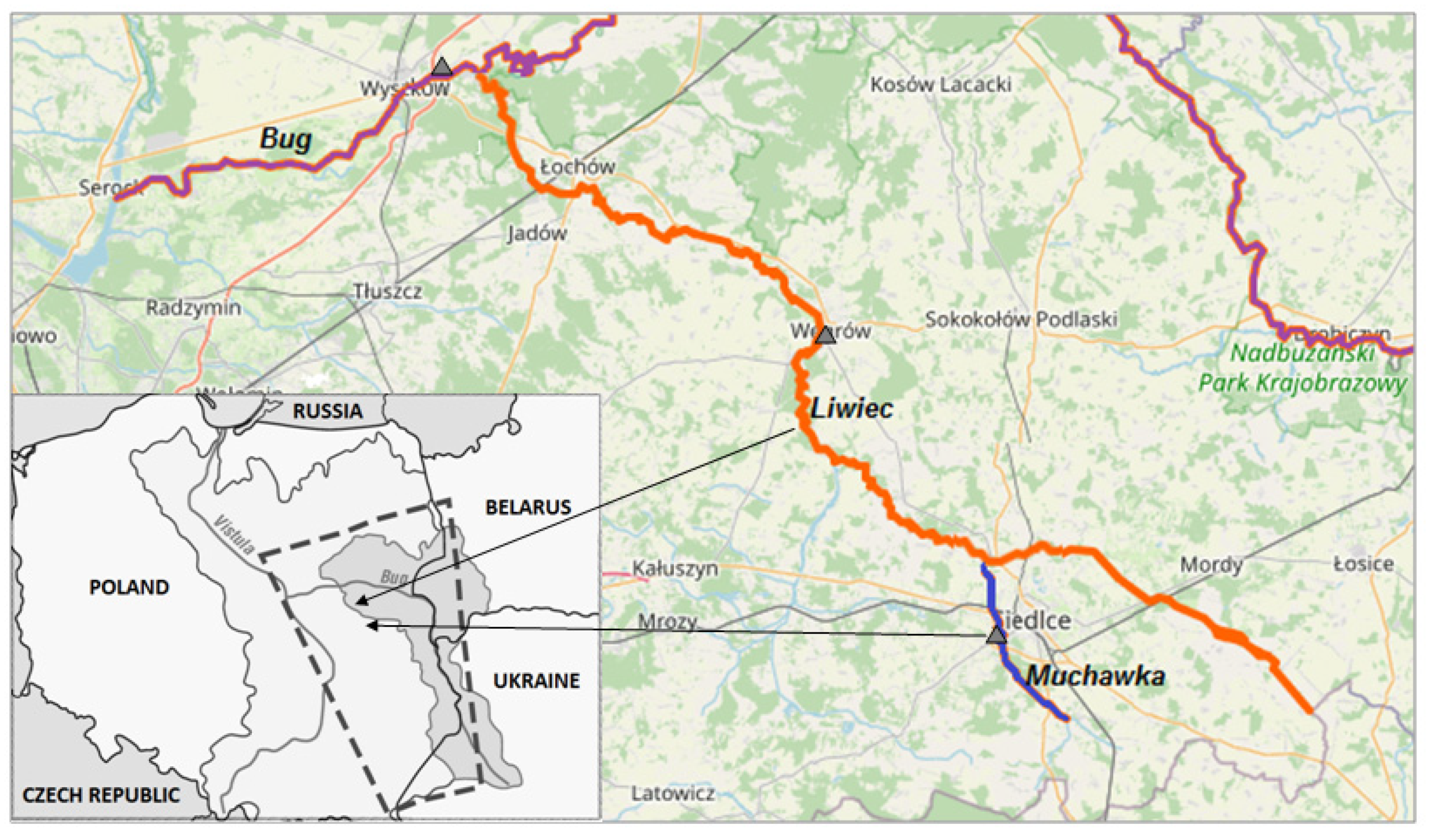
| Times | Water from the River | Average Value (µg/mL), SD (%) | |||||
|---|---|---|---|---|---|---|---|
| (A1) | (A2) | (A3) | (A4) | (A5) | (A6) | ||
| 1 h | Liwiec | 893.1 ± 4.5 | 887.2 ± 3.9 | 829.9 ± 4.0 | 837.4 ± 3.6 | 877.2 ± 4.9 | 849.1 ± 4.6 |
| Muchawka | 867.3 ± 4.1 | 882.7 ± 3.5 | 858.6 ± 4.2 | 823.6 ± 4.6 | 865.7 ± 5.5 | 864.6 ± 5.3 | |
| Bug | 779.7 ± 3.4 | 773.3 ± 3.5 | 863.4 ± 4.1 | 859.1 ± 4.8 | 783.8 ± 3.9 | 854.4 ± 4.6 | |
| 7 days | Liwiec | 783.7 ± 3.2 | 782.3 ± 4.3 | 780.9 ± 3.5 | 768.5 ± 3.6 | 787.3 ± 4.4 | 786.1 ± 3.9 |
| Muchawka | 767.5 ± 4.2 | 764.7 ± 4.1 | 752.1 ± 4.5 | 775.1 ± 4.0 | 774.2 ± 5.2 | 749.1 ± 3.6 | |
| Bug | 681.7 ± 3.6 | 683.4 ± 4.1 | 694.9 ± 3.7 | 700.6 ± 3.9 | 693.6 ± 4.7 | 688.9 ± 4.7 | |
| 28 days | Liwiec | 591.1 ± 4.4 | 596.9 ± 4.1 | 590.3 ± 4.3 | 557.6 ± 4.4 | 587.9 ± 4.3 | 593.3 ± 5.1 |
| Muchawka | 566.5 ± 3.9 | 591.6 ± 3.4 | 595.4 ± 4.5 | 583.5 ± 4.1 | 581.5 ± 4.4 | 590.1 ± 4.0 | |
| Bug | 570.2 ± 4.9 | 587.3 ± 4.2 | 571.8 ± 4.4 | 563.9 ± 4.0 | 567.3 ± 5.5 | 567.8 ± 4.7 | |
| 12 months | Liwiec | 295.2 ± 3.7 | 283.1 ± 3.2 | 295.4 ± 4.4 | 321.2 ± 3.5 | 293.3 ± 4.1 | 301.4 ± 4.8 |
| Muchawka | 281.6 ± 3.8 | 268.9 ± 3.6 | 279.7 ± 3.4 | 345.5 ± 4.7 | 277.9 ± 5.2 | 288.7 ± 3.9 | |
| Bug | 276.8 ± 3.6 | 286.8 ± 4.2 | 301.3 ± 4.6 | 297.7 ± 3.7 | 276.4 ± 3.9 | 299.3 ± 5.1 | |
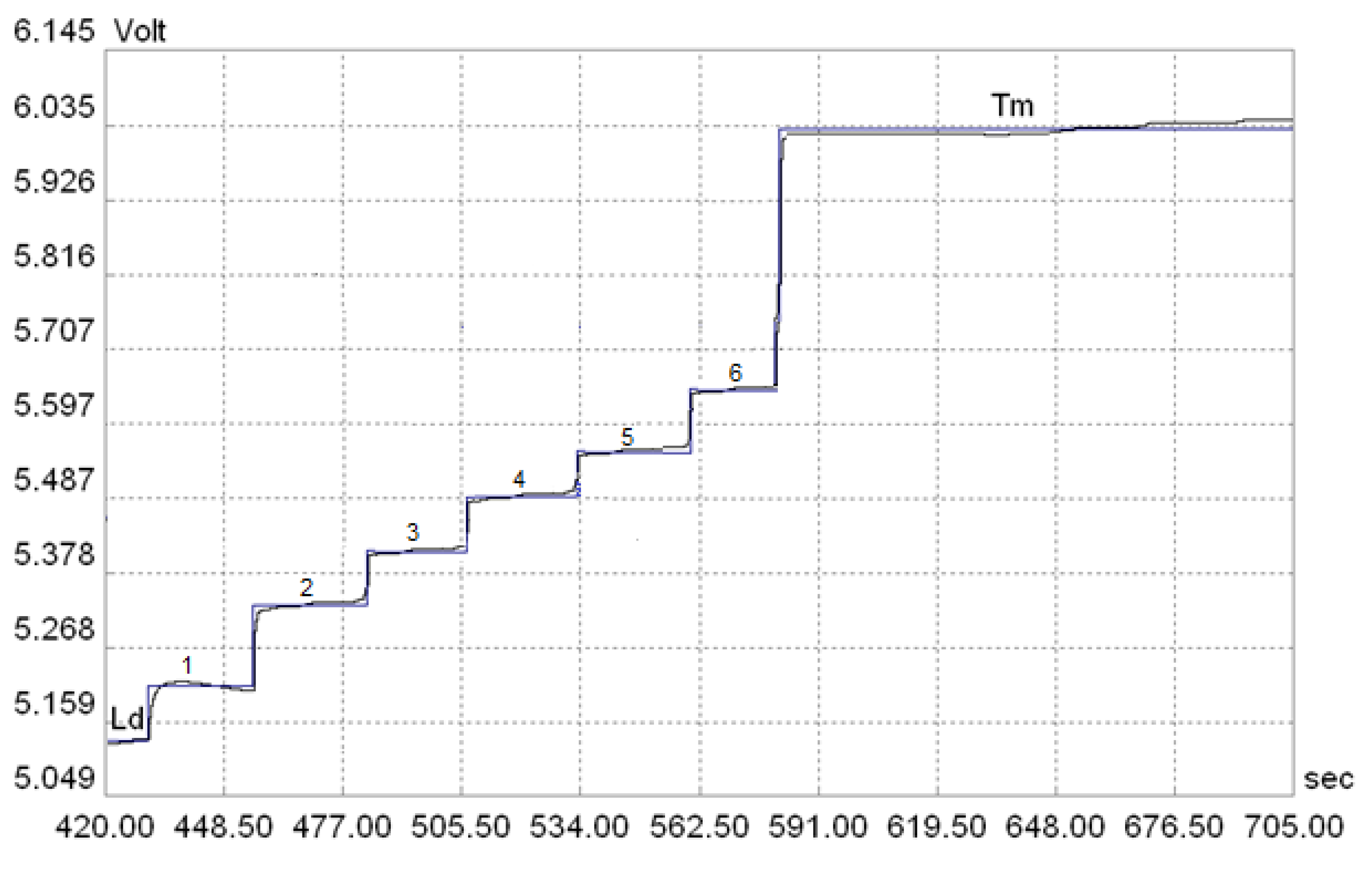
| Considered Parameters | |||||
|---|---|---|---|---|---|
| Stage | Time [s] | Intensity [µA] | Comp [10 mV] | Column | Conductometric Detector |
| 1 | 100 | 100 | 0 | top | |
| 2 | 250 | 250 | 0 | top | X |
| 3 | 65 | 10 | 0 | lower | |
| 4 | 10 | 95 | 0 | lower | |
| 5 | 30 | 75 | 50 | lower | |
| 6 | 250 | 40 | 0 | lower | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kluska, M.; Jabłońska, J.; Prukała, W. Analytics, Properties and Applications of Biologically Active Stilbene Derivatives. Molecules 2023, 28, 4482. https://doi.org/10.3390/molecules28114482
Kluska M, Jabłońska J, Prukała W. Analytics, Properties and Applications of Biologically Active Stilbene Derivatives. Molecules. 2023; 28(11):4482. https://doi.org/10.3390/molecules28114482
Chicago/Turabian StyleKluska, Mariusz, Joanna Jabłońska, and Wiesław Prukała. 2023. "Analytics, Properties and Applications of Biologically Active Stilbene Derivatives" Molecules 28, no. 11: 4482. https://doi.org/10.3390/molecules28114482
APA StyleKluska, M., Jabłońska, J., & Prukała, W. (2023). Analytics, Properties and Applications of Biologically Active Stilbene Derivatives. Molecules, 28(11), 4482. https://doi.org/10.3390/molecules28114482







