Facile Self-Assembly of Exfoliated Graphene/PANI Film for High-Energy Zn-Ion Micro-Supercapacitors
Abstract
1. Introduction
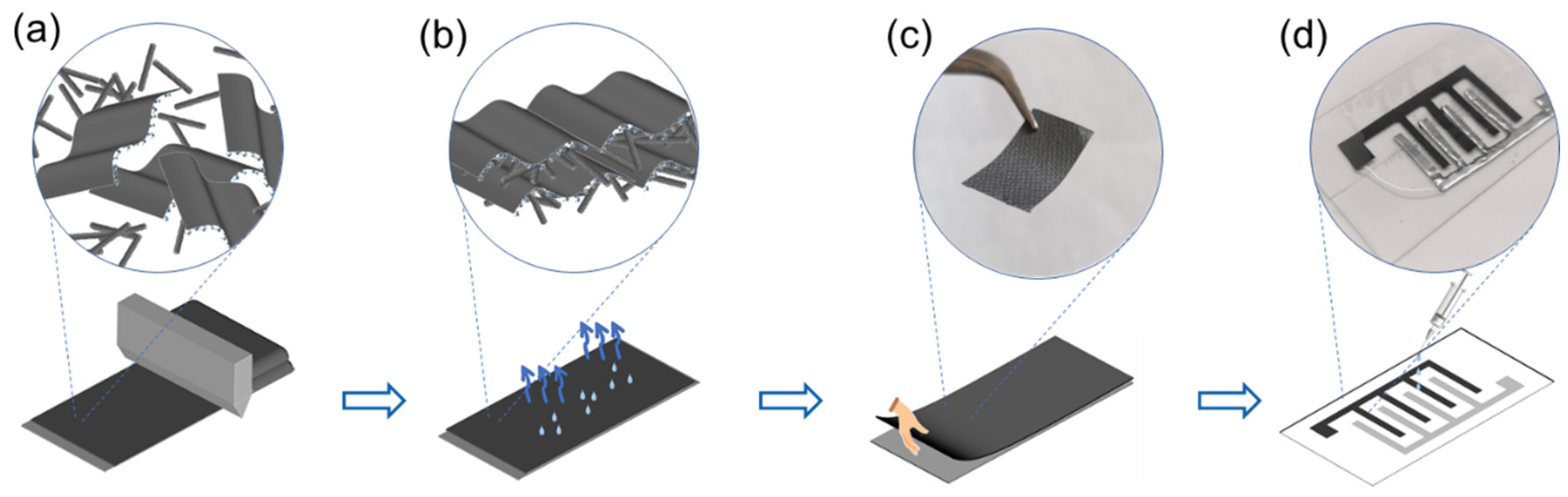
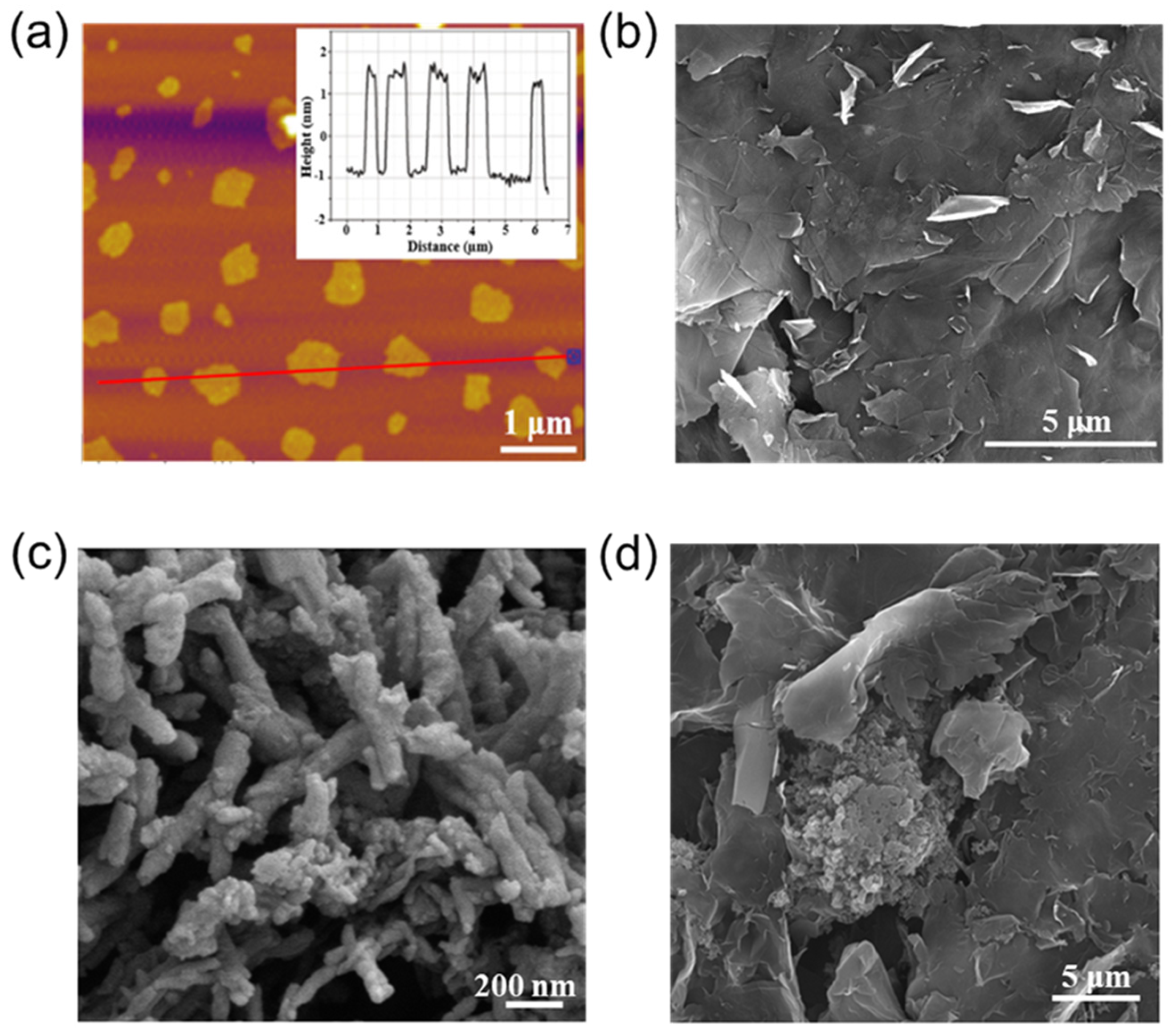
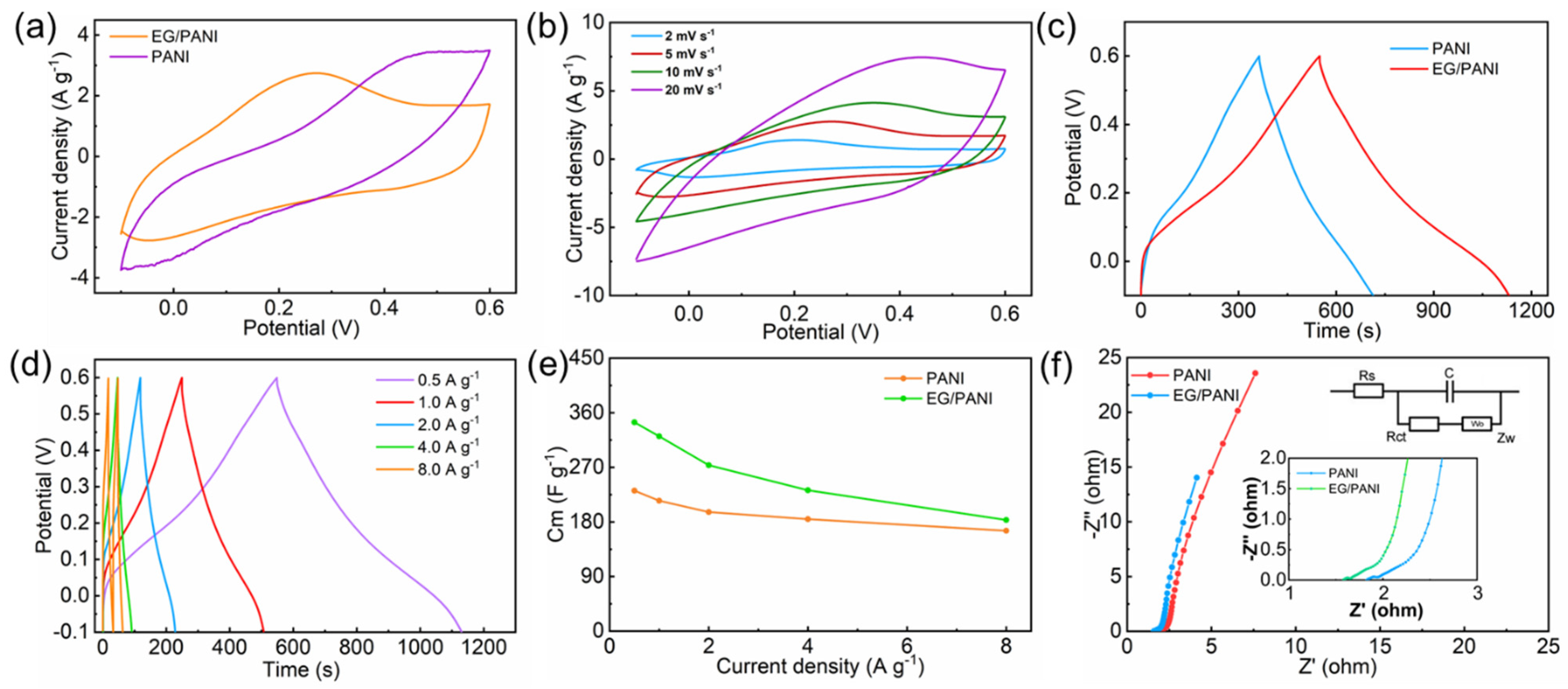
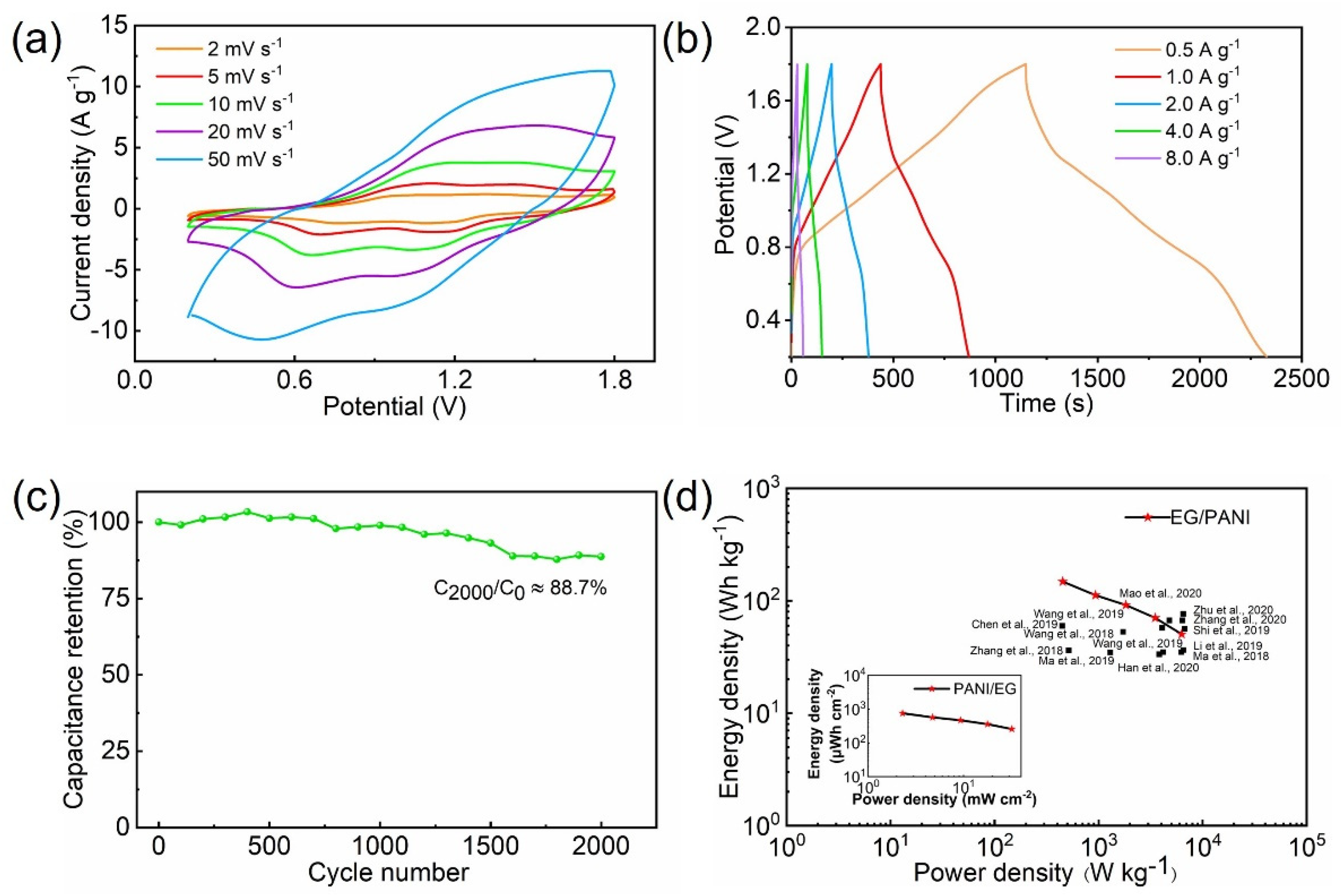
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liang, Z.W.; Chen, J.; Tian, W.S.; Liu, Y.; Chen, M.M.; Cao, D.W. Preparation of multi-function graphene materials through electrode-distance controlled electrochemical exfoliation. Nanotechnology 2022, 33, 375601. [Google Scholar] [CrossRef]
- Hu, X.; Zhong, G.; Li, J.; Liu, Y.; Yuan, J.; Chen, J.; Zhan, H.; Wen, Z. Hierarchical porous carbon nanofibers for compatible anode and cathode of potassium-ion hybrid capacitor. Energy Environ. Sci. 2020, 13, 2431–2440. [Google Scholar] [CrossRef]
- Zhang, W.; Li, W.; Li, S. Self-template activated carbons for aqueous supercapacitors. Sustain. Mater. Technol. 2023, 36, e00582. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Y.; Huang, J.; Xu, H. Fabricating dual redox electrolyte to achieve ultrahigh specific capacitance and reasonable Coulombic efficiency for biomass activated carbon. Electrochim. Acta 2022, 414, 140215. [Google Scholar] [CrossRef]
- Mao, C.; Chang, Y.; Zhao, X.; Dong, X.; Geng, Y.; Zhang, N.; Dai, L.; Wu, X.; Wang, L.; He, Z. Functional carbon materials for high-performance Zn metal anodes. J. Energy Chem. 2022, 75, 135–153. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Tang, Y. A novel zinc-ion hybrid supercapacitor for long-life and low-cost energy storage applications. Energy Storage Mater. 2018, 13, 1–7. [Google Scholar]
- Li, R.; Shen, X.; Ji, Z.; Xue, Y.; Song, P.; Zhou, H.; Kong, L.; Zeng, S.; Chen, C. Ultralight coaxial fiber-shaped zinc-ion hybrid supercapacitor with high specific capacitance and energy density for wearable electronics. Chem. Eng. J. 2023, 457, 141266. [Google Scholar] [CrossRef]
- Dong, L.; Ma, X.; Li, Y.; Zhao, L.; Liu, W.; Cheng, J.; Xu, C.; Li, B.; Yang, Q.-H.; Kang, F. Extremely safe, high-rate and ultralong-life zinc-ion hybrid supercapacitors. Energy Storage Mater. 2018, 13, 96–102. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, L. Recent advances of cathode materials for zinc-ion hybrid capacitors. Nano Energy 2023, 109, 108290. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Zhang, Q.; Cheng, Y.; Zhao, B.; Dai, S.; Wu, H.-H.; Zhang, K.; Ding, D.; Wu, Y.; et al. Anion and cation substitution in transition-metal oxides nanosheets for high-performance hybrid supercapacitors. Nano Energy 2018, 57, 22–33. [Google Scholar] [CrossRef]
- Wang, H.R.; Xue, Y.Q.; Song, X.; Lei, S.L.; Yu, H.; Du, C.F.; Ren, Z.W.; Guo, R.S.; Zhou, F. Solid solution reinforced V3CrC3Tx MXene cathodes for Zn-ion micro-supercapacitors with high areal energy density and superior flexibility. J. Mater. Chem. A 2022, 10, 20953–20963. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Yang, T.; Zhang, P.; Wei, X.; Zhang, L.; Li, H. Polyaniline/graphene hybrid fibers as electrodes for flexible supercapacitors. Synth. Met. 2020, 268, 116484. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Guo, X.; Ruan, L.; Wei, N.; Ma, Y.; Li, J.; Wang, M.; Li, W.; Zeng, W. MXene-Reduced Graphene Oxide Aerogel for Aqueous Zinc-Ion Hybrid Supercapacitor with Ultralong Cycle Life. Adv. Electron. Mater. 2019, 5, 1900537. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, M.; Ding, X.; Liu, Z.; Tian, H.; Shen, H.; Zu, X.; Li, S.; Qiao, L. One-step colloid fabrication of nickel phosphides nanoplate/nickel foam hybrid electrode for high-performance asymmetric supercapacitors. Chem. Eng. J. 2019, 373, 1132–1143. [Google Scholar] [CrossRef]
- Tang, H.; Yao, J.; Zhu, Y. Recent Developments and Future Prospects for Zinc-Ion Hybrid Capacitors: A Review. Adv. Energy Mater. 2021, 11, 2003994. [Google Scholar] [CrossRef]
- Li, Z.; An, Y.; Dong, S.; Chen, C.; Wu, L.; Sun, Y.; Zhang, X. Progress on zinc ion hybrid supercapacitors: Insights and challenges. Energy Storage Mater. 2020, 31, 252–266. [Google Scholar] [CrossRef]
- Li, Z.N.; Gadipelli, S.; Li, H.C.; Howard, C.A.; Brett, D.J.L.; Shearing, P.R.; Guo, Z.X.; Parkin, I.P.; Li, F. Tuning the interlayer spacing of graphene laminate films for efficient pore utilization towards compact capacitive energy storage. Nat. Energy 2020, 5, 160–168. [Google Scholar] [CrossRef]
- Li, N.; Huang, X.K.; Zhang, H.Y.; Li, Y.Y.; Wang, C.X. Transparent and Self-Supporting Graphene Films with Wrinkled- Graphene-Wall-Assembled Opening Polyhedron Building Blocks for High Performance Flexible/Transparent Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 9763–9771. [Google Scholar] [CrossRef]
- Meng, Q.; Du, C.; Xu, Z.; Nie, J.; Hong, M.; Zhang, X.; Chen, J. Siloxene-reduced graphene oxide composite hydrogel for supercapacitors. Chem. Eng. J. 2020, 393, 124684. [Google Scholar] [CrossRef]
- Chen, D.; Wang, F.; Li, Y.; Wang, W.-W.; Huang, T.-X.; Li, J.-F.; Novoselov, K.S.; Tian, Z.-Q.; Zhan, D. Programmed electrochemical exfoliation of graphite to high quality graphene. Chem. Commun. 2019, 55, 3379–3382. [Google Scholar] [CrossRef]
- Wu, J.F.; Zhang, Q.; Wang, J.J.; Huang, X.P.; Bai, H. A self-assembly route to porous polyaniline/reduced graphene oxide com-posite materials with molecular-level uniformity for high-performance supercapacitors. Energy Environ. Sci. 2018, 11, 1280–1286. [Google Scholar] [CrossRef]
- Al-Gaashania, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Yang, J.; Cao, J.; Peng, Y.; Bissett, M.; Kinloch, I.A.; Dryfe, R.A. Unlocking the energy storage potential of polypyrrole via electrochemical graphene oxide for high performance zinc-ion hybrid supercapacitors. J. Power Sources 2021, 516, 230663. [Google Scholar] [CrossRef]
- Salverda, M.; Thiruppathi, A.R.; Pakravan, F.; Wood, P.C.; Chen, A. Electrochemical Exfoliation of Graphite to Graphene-Based Nanomaterials. Molecules 2022, 27, 8643. [Google Scholar] [CrossRef]
- Sadak, O.; Prathap, M.A.; Gunasekaran, S. Facile fabrication of highly ordered polyaniline–exfoliated graphite composite for enhanced charge storage. Carbon 2018, 144, 756–763. [Google Scholar] [CrossRef]
- Sun, Y.; Jiao, L.; Han, D.; Wang, F.; Zhang, P.; Li, H.; Niu, L. Hierarchical architecture of polyaniline nanoneedle arrays on electrochemically exfoliated graphene for supercapacitors and sodium batteries cathode. Mater. Des. 2019, 188, 108440. [Google Scholar] [CrossRef]
- Liu, W.-J.; Yuan, M.; Lian, J.-B.; Li, G.-C.; Li, Q.-P.; Qiao, F.; Zhao, Y. Embedding partial sulfurization of iron–cobalt oxide nanoparticles into carbon nanofibers as an efficient electrode for the advanced asymmetric supercapacitor. Tungsten 2022, 5, 118–129. [Google Scholar] [CrossRef]
- Fan, T.; Tong, S.; Zeng, W.; Niu, Q.; Liu, Y.; Kao, C.-Y.; Liu, J.; Huang, W.; Min, Y.; Epstein, A.J. Self-assembling sulfonated graphene/polyaniline nanocomposite paper for high performance supercapacitor. Synth. Met. 2015, 199, 79–86. [Google Scholar] [CrossRef]
- Nie, Z.G.; Wang, Y.; Wang, H. Research progress on critical materials for supercapacitors. Modern Chem. Ind. 2021, 41, 33–36+41. [Google Scholar]
- Song, T.L.; Hao, H.L.; Zhao, Y.; Wang, X.; Li, C.W.; Li, W.Y. High-performance Zn-ion hybrid supercapacitor enabled by the hier-archical N/S co-doped graphene/polyaniline cathode. J. Alloys Compd. 2022, 924, 166493. [Google Scholar]
- Zhang, W.W.; Jiang, H.L.; Li, Y.H.; Ma, W.J.; Yang, X.Y.; Zhang, J.F. Pizza-like heterostructured Ti3C2Tx/Bi2S3@N-C with ultra-high specific capacitance as a potential electrode material for aqueous zinc-ion hybrid supercapacitors. J. Alloys Compd. 2021, 883, 160881. [Google Scholar] [CrossRef]
- Long, Y.; Huang, X.; Li, Y.; Yi, M.; Hou, J.; Zhou, X.; Hu, Q.; Zheng, Q.; Lin, D. In-situ regulation of zinc metal surface for Dendrite-Free Zinc-ion hybrid supercapacitors. J. Colloid Interface Sci. 2022, 614, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, D.D.; Wang, G.W.; Xu, Y.T.; Li, H.X.; Yan, X.B. An aqueous zinc-ion hybrid super-capacitor for achieving ultra-high-volumetric energy density. Chin. Chem. Lett. 2020, 32, 926–931. [Google Scholar] [CrossRef]
- Zhu, Y.; Ye, X.; Jiang, H.; Xia, J.; Yue, Z.; Wang, L.; Wan, Z.; Jia, C.; Yao, X. Controlled swelling of graphene films towards hierarchical structures for supercapacitor electrodes. J. Power Sources 2020, 453, 227851. [Google Scholar] [CrossRef]
- Chen, S.; Ma, L.; Zhang, K.; Kamruzzaman, M.; Zhi, C.; Zapien, J.A. A flexible solid-state zinc ion hybrid supercapacitor based on co-polymer derived hollow carbon spheres. J. Mater. Chem. A 2019, 7, 7784–7790. [Google Scholar] [CrossRef]
- Li, G.; Jiang, X.; Liu, C.; Song, M.; Yang, S.; Lian, J.; Lee, J.Y. A microporous carbon derived from metal-organic frameworks for long-life lithium sulfur batteries. Int. J. Energy Res. 2019, 44, 2126–2136. [Google Scholar] [CrossRef]
- Zhang, P.P.; Li, Y.; Wang, G.; Wang, F.X.; Yang, S.; Zhu, F.; Zhuang, X.D.; Schmidt, O.G.; Feng, X.L. Zn-Ion Hybrid Micro-Supercapacitors with Ultrahigh Areal Energy Density and Long-Term Durability. Adv. Mater. 2018, 31, 1806005. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, J.; Dong, L.; Liu, W.; Mou, J.; Zhao, L.; Wang, J.; Ren, D.; Wu, J.; Xu, C.; et al. Multivalent ion storage towards high-performance aqueous zinc-ion hybrid supercapacitors. Energy Storage Mater. 2018, 20, 335–342. [Google Scholar] [CrossRef]
- Ma, X.P.; Wang, J.J.; Wang, X.L.; Zhao, L.; Xu, C.J. Aqueous V2O5/activated carbon zinc-ion hybrid capacitors with high energy density and excellent cycling stability. J. Mater. Sci. Mater. Electron. 2019, 30, 5478–5486. [Google Scholar] [CrossRef]
- Mao, K.; Shi, J.; Zhang, Q.; Hou, Y.; Wen, L.; Liu, Z.; Long, F.; Niu, K.; Liu, N.; Gao, Y. High-capacitance MXene anode based on Zn-ion pre-intercalation strategy for degradable micro Zn-ion hybrid supercapacitors. Nano Energy 2022, 103, 107791. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Zeng, W.; Wang, M.; Ruan, L.; Ma, Y. A New Free-Standing Aqueous Zinc-Ion Capacitor Based on MnO2–CNTs Cathode and MXene Anode. Nano-Micro Lett. 2019, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wu, S.; Zhang, S.; Deng, Y.; Cui, C.; Zhang, L.; Long, Y.; Li, H.; Tao, Y.; Weng, Z.; et al. A Corrosion-Resistant and Dendrite-Free Zinc Metal Anode in Aqueous Systems. Small 2020, 16, e2001736. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.J.; Wang, S.L.; Wang, Q.; Chen, X.; Du, X.Y.; Wang, M.; Zhao, Y.J.; Dong, C.; Ruan, L.M.; Zeng, W. A new flexible zinc-ion capacitor based on δ-MnO2@Carbon cloth battery-type cathode and MXene@Cotton cloth capacitor-type anode. J. Power Sources 2019, 446, 227345. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Moore, J.L.; Nagaiah, N.; Zhai, L.; Jung, Y.; Thomas, J.Y. Asymmetric supercapacitor electrodes and devices. Adv. Mater. 2017, 29, 1605336. [Google Scholar]
- Pour, B.G.; Aval, F.L.; Mirzaee, M. CNTs Supercapacitor Based on the PVDF/PVA Gel Electrolytes. Recent Pat. Nanotechnol. 2020, 14, 163–170. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Niu, J. Facile Self-Assembly of Exfoliated Graphene/PANI Film for High-Energy Zn-Ion Micro-Supercapacitors. Molecules 2023, 28, 4470. https://doi.org/10.3390/molecules28114470
Wang Y, Niu J. Facile Self-Assembly of Exfoliated Graphene/PANI Film for High-Energy Zn-Ion Micro-Supercapacitors. Molecules. 2023; 28(11):4470. https://doi.org/10.3390/molecules28114470
Chicago/Turabian StyleWang, Yili, and Jin Niu. 2023. "Facile Self-Assembly of Exfoliated Graphene/PANI Film for High-Energy Zn-Ion Micro-Supercapacitors" Molecules 28, no. 11: 4470. https://doi.org/10.3390/molecules28114470
APA StyleWang, Y., & Niu, J. (2023). Facile Self-Assembly of Exfoliated Graphene/PANI Film for High-Energy Zn-Ion Micro-Supercapacitors. Molecules, 28(11), 4470. https://doi.org/10.3390/molecules28114470






