Chemical Variability and Chemotype Concept of Essential Oils from Algerian Wild Plants
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of Essential Oils
2.2. Influence of the Vegetative Cycle on the Yield of Essential Oil
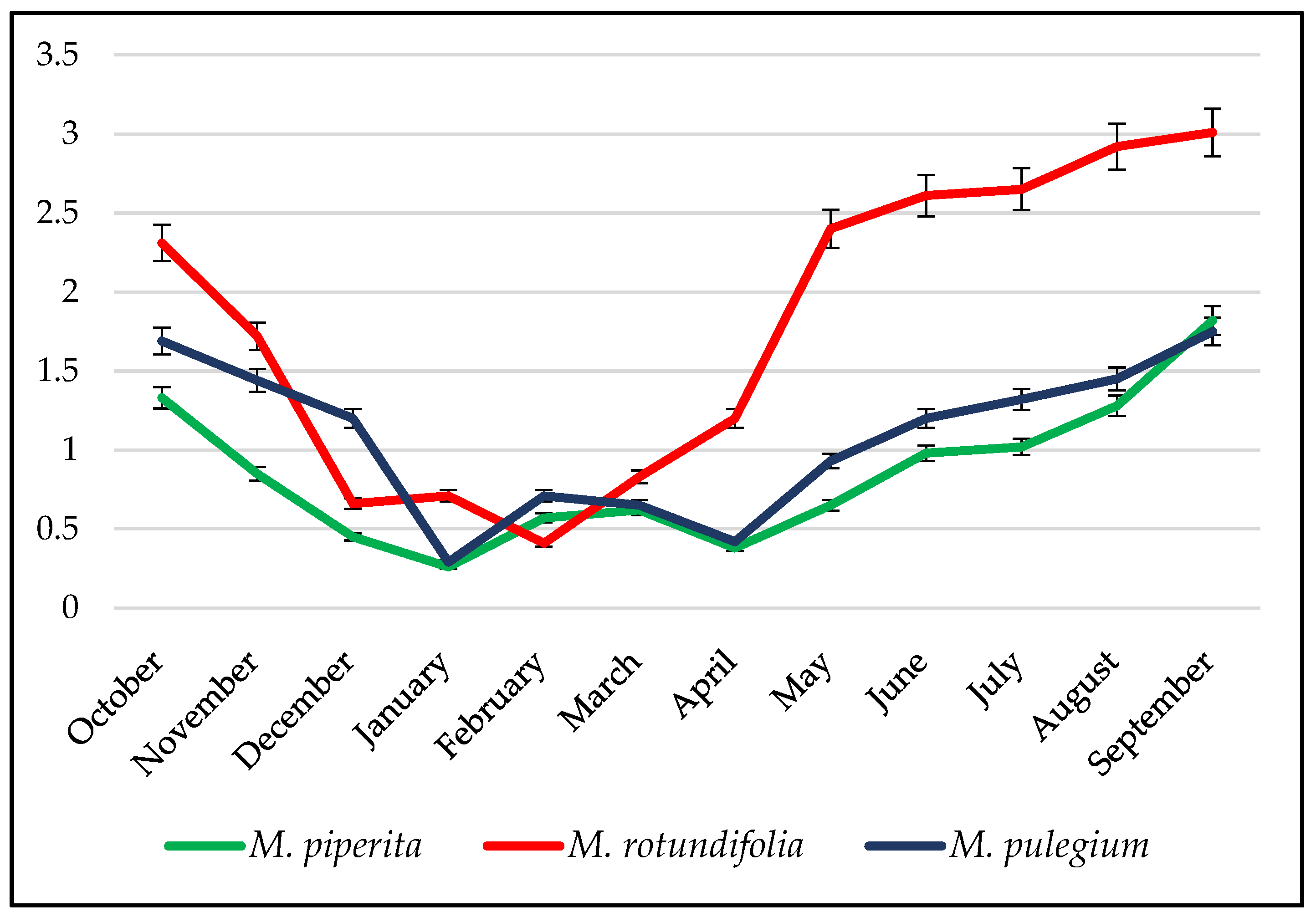
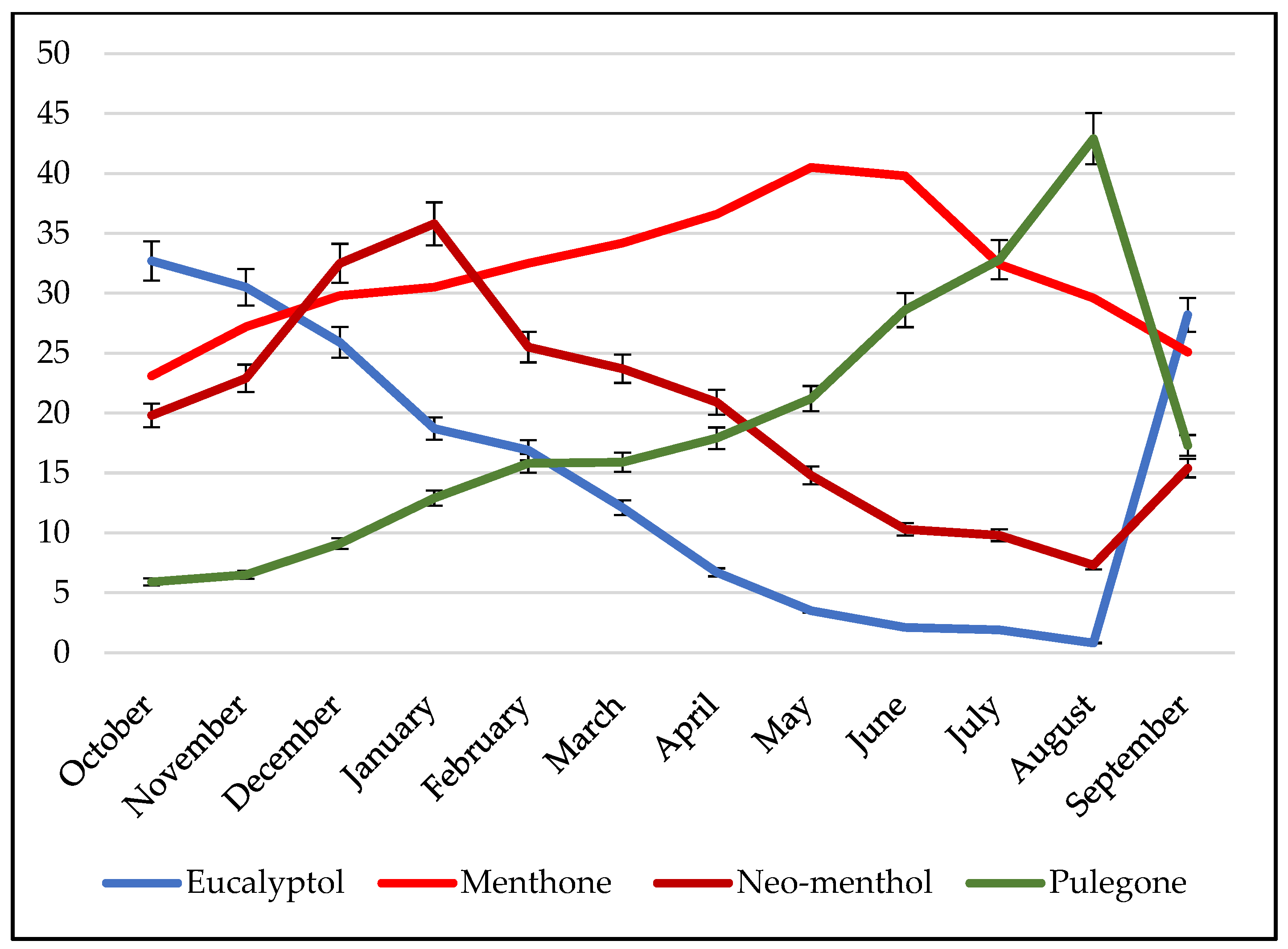
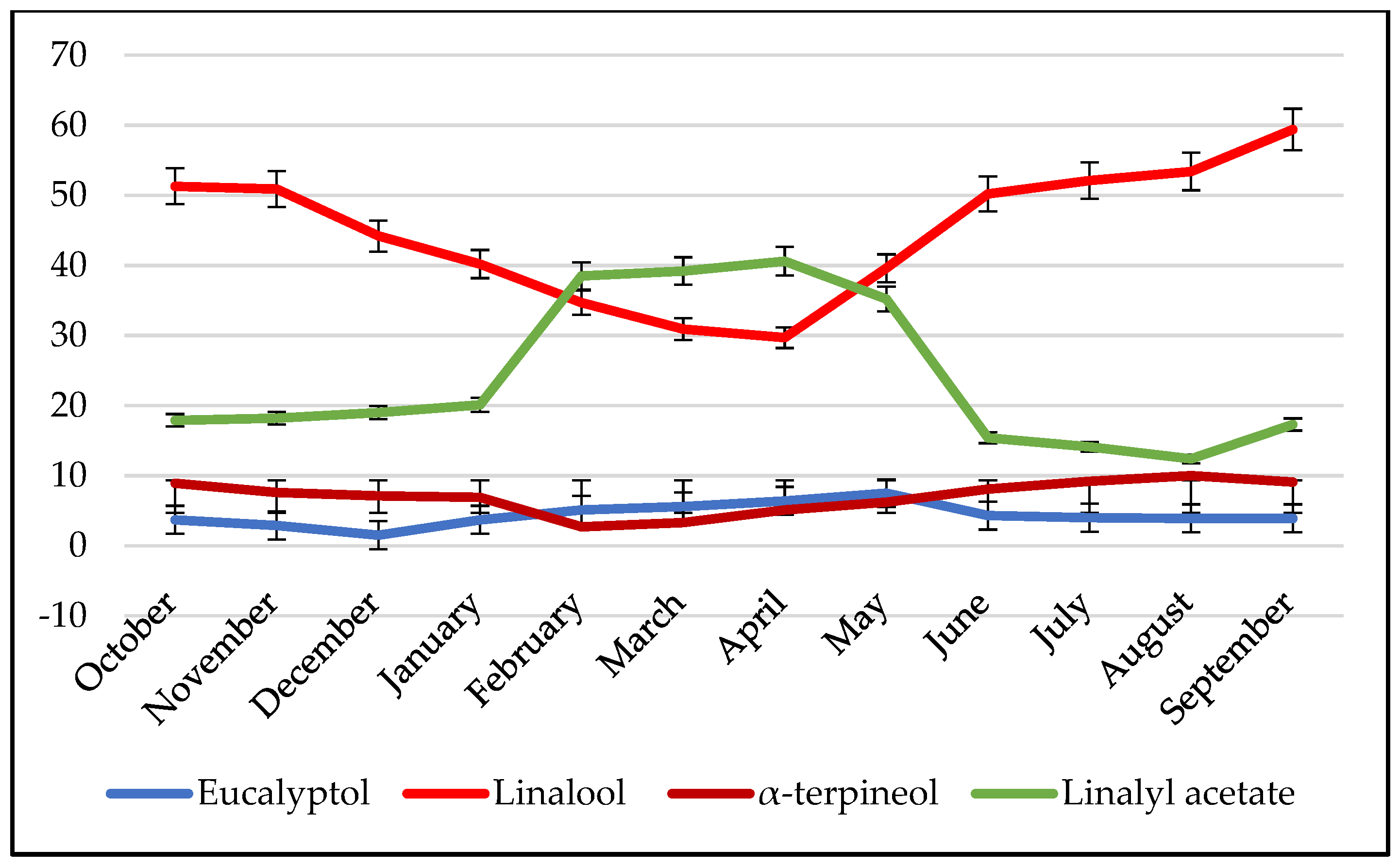
2.3. Variability of Chemical Composition as a Function of Time
2.4. Variability of Chemical Composition within a Species
2.5. Polymorphism and Chemical Composition
2.6. Variability between the Species of a Genus
3. Discussion
3.1. Essential Oils International Standards
3.2. Impact of Chemical Variability on Therapeutic Application
3.3. Parameters Influencing the Chemical Variability of Essential Oils
3.3.1. Mints
3.3.2. Rosemary
3.3.3. Eucalyptus
3.3.4. Thymus
4. Materials and Methods
4.1. Plants and Extraction of Essential Oils
4.2. Identification of Compounds
4.3. Quantification of Compounds
4.4. GC Analysis
4.5. GC-MS Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Achiri, R.; Mesli, F.; Benomari, F.Z.; Djabou, N.; Tabti, B.; Muselli, A.; Dib, M.A. Chemical composition/pharmacophore modelling-based, virtual screening, molecular docking and dynamic simulation studies for the discovery of novel superoxide dismutase (SODs) of bioactive molecules from aerial parts of Inula Montana as antioxydant’s agents. J. Biomol. Struct. Dyn. 2021, 2, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Willem, J.P. Huiles Essentielles Antivirales, la Solution Naturelle Pour Lutter Contre les Infections, 1st ed.; Trédaniel La Maisnie: Paris, France, 2015. [Google Scholar]
- Fernandez, X.; Chemat, F. La Chimie des Huiles Essentielles: Tradition et Innovation, 1st ed.; De Boeck Sup: Paris, France, 2012. [Google Scholar]
- Deschepper, R. Variabilité de la Composition des Huiles Essentielles et Intérêt de la Notion de Chémotype en Aromathérapie. Ph.D. Thesis, University of Aix-Marseille, Aix-Marseille, France, 2017. [Google Scholar]
- Fernandez, X.; Chemat, F. Les Huiles Essentielles: Vertus et Applications, 1st ed.; Vuibert: Paris, France, 2012. [Google Scholar]
- Tabti, L.; Dib, M.A.; Djabou, N.; Guaouar, N.; Paolini, J.; Costa, J.; Muselli, A. Control of fungal pathogens of Citrus sinensis L. by essential oil and hydrosol of Thymus capitatus. J. Appl. Bot. Food Qual. 2014, 87, 279–285. [Google Scholar]
- Djabou, N.; Andreani, S.; Varesi, L.; Tomi, F.; Costa, J.; Muselli, A. Analysis of the volatile fraction of Teucrium marum L. Flavour Fragr. J. 2013, 28, 14–24. [Google Scholar] [CrossRef]
- Djabou, N.; Dib, M.A.; Tabti, L.; Costa, J.; Muselli, A. Chemical composition and antioxidant activity of hydrosol extracts obtained by liquid-liquid extraction (LLE) of Daucus muricatus L. J. Essent. Oil Res. 2014, 26, 393–399. [Google Scholar] [CrossRef]
- Fekih, N.; Allali, H.; Merghache, S.; Chaïb, F.; Merghache, D.; Dib, M.A.; Djabou, N.; Muselli, A.; Tabti, B.; Costa, J. Chemical composition and antibacterial activity of Pinus halepensis Miller growing in West Northern of Algeria. Asian Pac. J. Trop. Dis. 2014, 4, 97–103. [Google Scholar] [CrossRef]
- Tabti, L.; Dib, M.A.; Benyelles, N.G.; Djabou, N.; Alam, S.B.; Paolini, J.; Costa, J.; Muselli, A. Fatty-acid composition and antifungal activity of extracts of Thymus capitatus. J. Herbs Spices Med. Plants 2015, 21, 203–210. [Google Scholar] [CrossRef]
- Bouayad-Alam, S.; Dib, M.A.; Djabou, N.; Tabti, B.; Benyelles, N.G.; Costa, J.; Muselli, A. Essential oils as biocides for the control of fungal infections and devastating pest (Tutaabsoluta) of Tomato (Lycopersicon esculentum. Mill.). Chem. Biodivers. 2017, 14, 1700065. [Google Scholar] [CrossRef]
- Tabet Zatla, A.; Dib, M.A.; Djabou, N.; Tabti, B.; Meliani, N.; Costa, J.; Muselli, A. Chemical variability of essential oil of Daucus carota subsp. sativus from Algeria. J. Herbs Spices Med. Plants 2017, 23, 3. [Google Scholar] [CrossRef]
- Medbouhi, A.; Benbelaïd, F.; Djabou, N.; Beaufay, C.; Bendahou, M.; Quetin-Leclercq, J.; Tintaru, A.; Costa, J.; Muselli, A. Essential oil of Algerian Eryngium campestre: Chemical variability and evaluation of biological activities. Molecules 2019, 24, 2575. [Google Scholar] [CrossRef]
- Mejdoub, K.; Benomari, F.Z.; Djabou, N.; Dib, M.A.; Benyelles, N.G.; Costa, J.; Muselli, A. Antifungal and insecticidal activities of essential oils of four Mentha species. Jundishapur J. Nat. Pharm. Prod. 2019, 14, 1. [Google Scholar]
- De Carvalho Augusto, R.; Merad, N.; Rognon, A.; Gourbal, B.; Bertrand, C.; Djabou, N.; Duval, D.R. Molluscicidal and parasiticidal activities of Eryngium triquetrum essential oil on Schistosoma mansoni and its intermediate snail host Biomphalaria glabrata, a double impact. Parasit Vectors 2020, 13, 486. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Shukla, R.; Singh, P.; Prasad, C.S.; Dubey, N.K. Assessment of Thymus vulgaris L. essential oil as a safe botanical preservative against post-harvest fungal infestation of food commodities. Innov. Food Sci. Emerg. Technol. 2008, 9, 575–580. [Google Scholar] [CrossRef]
- Benomari, F.Z.; Andreu, V.; Kotarba, J.; Dib, M.A.; Bertrand, C.; Muselli, A.; Costa, J.; Djabou, N. Essential oils from Algerian species of Mentha as new bio-control agents against phytopathogen strains. Environ. Sci. Pollut. Res. 2018, 25, 29889–29900. [Google Scholar] [CrossRef] [PubMed]
- Kerdchoechuen, O.; Laohakunjit, N.; Singkornard, S.; Matta, F.B. Essential oils from six herbal plants for biocontrol of the maize weevil. Hort. Sci. 2010, 45, 592–598. [Google Scholar] [CrossRef]
- Benomari, F.Z.; Djabou, N.; Moumani, M.; Hassani, F.; Muselli, A.; Costa, J. Chemical variability of essential oils of three subspecies of Thymus munbyanus Boiss. & Reut. from Western Algeria. J. Essent. Oil Res. 2020, 32, 474–484. [Google Scholar]
- Morel, J.M.; Bruel, P.; Labouyrie, V.; Priolet, P.L.; Liégard, L. Intérêt des huiles essentielles GAE dans la prise en charge des affections virales des voies respiratoires en officine. Phytothérapie 2010, 8, 21–25. [Google Scholar] [CrossRef]
- Bouayad, S.; Benyelles, N.G.; Dib, M.A.; Djabou, N.; Tabti, L.; Paolini, J.; Muselli, A.; Costa, J. Antifungal activity of essential oils of tree aromatic plants from western Algeria against five fungal pathogens of tomato (Lycopersicon esculentum. Mill). J. Appl. Bot. Food Qual. 2014, 87, 56–61. [Google Scholar]
- Franchomme, P.; Jollois, R.; Pénoël, D. L’aromatherapie Exactement-Encyclopédie de L’utilisation Thérapeutique des Extraits Aromatiques, 1st ed.; Roger Jollois: Paris, France, 2001. [Google Scholar]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Djabou, N.; Battesti, M.J.; Allali, H.; Desjobert, J.M.; Varesi, L.; Costa, J.; Muselli, A. Chemical and genetic differentiation of Corsican subspecies of Teucrium flavum L. Phytochemistry 2011, 72, 1390–1399. [Google Scholar] [CrossRef]
- Djabou, N.; Muselli, A.; Allali, H.; Dib, M.A.; Tabti, B.; Varesi, L.; Costa, J. Chemical and genetic diversity of two Mediterranean subspecies of Teurciumpolium L. Phytochemistry 2012, 83, 51–62. [Google Scholar] [CrossRef]
- Djabou, N.; Allali, H.; Battesti, M.J.; Tabti, B.; Costa, J.; Muselli, A.; Varesi, L. Chemical and genetic differentiation of two Mediterranean subspecies of Teucrium scorodonia L. Phytochemistry 2012, 74, 123–132. [Google Scholar] [CrossRef]
- Dib, M.A.; Djabou, N.; Desjobert, J.M.; Allali, H.; Tabti, B.; Muselli, A.; Costa, J. Characterization of volatile compounds of Daucus crinitus Desf. Headspace solid phase micro extraction as alternative technique to hydrodistillation. Chem. Cent. J. 2010, 4, 16. [Google Scholar] [CrossRef]
- Younes, K.; Merghache, S.; Djabou, N.; Merghache, D.; Muselli, A.; Tabti, B.; Costa, J. Chemical composition, antibacterial and antioxidant activities of a new essential oil chemotype of Algerian Artemisia arborescens L. Afr. J. Pharm. Pharmacol. 2012, 6, 2912–2921. [Google Scholar] [CrossRef]
- Bendiabdellah, A.; Dib, M.A.; Meliani, N.; Muselli, A.; Djabou, N.; Tabti, B.; Costa, J. Antibacterial activity of Daucus crinitus essential oils along the vegetable life of the plant. J. Chem. 2013, 2013, 149502. [Google Scholar] [CrossRef]
- Meliani, N.; Dib, M.A.; Djabou, N.; Costa, J.; Allali, H.; Tabti, B.; Muselli, A. Chemical composition and antimicrobial activity of Daucus aureus essential oils from Algeria. Nat. Prod. Commun. 2013, 8, 835–840. [Google Scholar]
- Selles, C.; Dib, M.A.; Djabou, N.; Beddou, F.; Muselli, A.; Tabti, B.; Costa, J.; Hammouti, B. Antimicrobial activity and evolution of the composition of essential oil from Algerian Anacyclus pyrethrum L. through the vegetable cycle. Nat. Prod. Res. 2013, 27, 2231–2234. [Google Scholar] [CrossRef] [PubMed]
- Bendiabdellah, A.; Dib, M.A.; Djabou, N.; Hassani, F.; Paolini, J.; Tabti, B.; Costa, J.; Muselli, A. Daucus carota ssp. HispanicusGouan essential oils: Chemical variability and fungitoxic activity. J. Essent. Oil Res. 2014, 26, 427–440. [Google Scholar] [CrossRef]
- Benyelles, B.; Allali, H.; Dib, M.A.; Djabou, N.; Costa, J. Essential oil from Rhaponticumacaule L. roots: Comparative study using HS-SPME/GC/GC–MS and hydrodistillation techniques. J. Saudi Chem. Soc. 2014, 18, 972–976. [Google Scholar] [CrossRef]
- Chikhi, I.; Allali, H.; Bechlaghem, K.; Fekih, N.; Muselli, A.; Djabou, N.; Dib, M.A.; Tabti, B.; Halla, N.; Costa, J. Assessment of in vitro antimicrobial potency and free radical scavenging capacity of the essential oil and ethanol extract of Calycotomevillosa subsp. intermedia growing in Algeria. Asian Pac. J. Trop. Dis. 2014, 4, 356–362. [Google Scholar] [CrossRef]
- Benyelles, B.; Allali, H.; Fekih, N.; Touaibia, M.; Muselli, A.; Djabou, N.; Dib, M.A.; Tabti, B.; Costa, J. Chemical composition of the volatile components of Tropaeolum majus L. (Garden Nasturtium) from North Western Algeria. Phytochem. Bioact. Subst. J. 2015, 9, 92–97. [Google Scholar]
- Dib, M.A.; Djabou, N.; Allali, H.; Paolini, J.; Tabti, B.; Costa, J.; Muselli, A. Chemical composition of essential oils and hydrosol extracts of Daucus muricatus and assessment of its antioxidant activity. J. Herbs Spices Med. Plants 2015, 21, 23–37. [Google Scholar]
- Younes, K.; Merghache, S.; Djabou, N.; Selles, C.; Muselli, A.; Tabti, B.; Costa, J. Chemical composition and free radical scavenging activity of essential oils and extracts of Algerian Cardariadraba (L.) Desv. J. Essent. Oil-Bear. Plants 2015, 18, 1448–1458. [Google Scholar] [CrossRef]
- Benomari, F.Z.; Djabou, N.; Medbouhi, A.; Khadir, A.; Bendahou, M.; Selles, C.; Desjobert, J.M.; Costa, J.; Muselli, A. Chemical variability andbiologicalactivities of essential oils of Micromeriainodora (Desf.) Benth. from Algeria. Chem. Biodivers. 2016, 13, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Belabbes, R.; Dib, M.A.; Djabou, N.; Ilias, F.; Tabti, B.; Costa, J.; Muselli, A. Chemical variability, antioxidant and antifungal activities of essential oils and hydrosol extract of Calendula arvensis L. from western Algeria. Chem. Biodivers. 2017, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Tabet Zatla, A.; Dib, M.A.; Djabou, N.; Ilias, F.; Costa, J.; Muselli, A. Antifungal activities of essential oils and hydrosol extracts of Daucus carota subsp. sativus for the control of fungal pathogens, in particular gray rot of strawberry during storage. J. Essent. Oil Res. 2017, 29, 391–399. [Google Scholar] [CrossRef]
- Medbouhi, A.; Merad, N.; Khadir, A.; Bendahou, M.; Djabou, N.; Costa, J.; Muselli, A. Chemical composition and biological investigations of Eryngium triquetrum essential oil from Algeria. Chem. Biodivers. 2018, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Medbouhi, A.; Tintaru, A.; Beaufay, C.; Naubron, J.V.; Djabou, N.; Costa, J.; Quetin-Leclercq, J.; Muselli, A. Structure elucidation and cytotoxicity of a new 17-membered ring lactone from Algerian Eryngium campestre. Molecules 2018, 23, 3250. [Google Scholar] [CrossRef] [PubMed]
- Benomari, F.Z.; Dib, M.A.; Muselli, A.; Costa, J.; Djabou, N. Comparative study of chemical composition of essential oils for two species of Asteriscus genus from Western Algeria. J. Essent. Oil Res. 2019, 31, 414–424. [Google Scholar] [CrossRef]
- Mejdoub, K.; Mami, I.R.; Belabbes, R.; Dib, M.A.; Djabou, N.; Tabti, B.; Benyelles, N.G.; Costa, J.; Muselli, A. Chemical variability of Atractylisgummifera essential oils at three developmental stages and investigation of their antioxidant, antifungal and insecticidal activities. Curr. Bioact. Compd. 2020, 16, 489–497. [Google Scholar] [CrossRef]
- Merad, N.; Andreu, V.; Chaib, S.; Augusto, R.C.; Duval, D. Essential oils from two Apiaceae species as potential agents in organic crops protection. Antibiotics 2021, 10, 636. [Google Scholar] [CrossRef]
- ISO/TC 54; Normalisation des Méthodes D’Analyse et des Spécifications des Huiles Essentielles. ISO: Geneva, Switzerland. Available online: https://www.iso.org/fr/committee/48956 (accessed on 28 March 2023).
- Eaissi, A.; Medini, H.; Simmonds, M.; Lynen, F.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F.; Khouja, M.L. Variation in volatile leaf oils of seven Eucalyptus species harvested from Zerniza Arboreta (Tunisia). Chem. Biodivers. 2011, 8, 362–372. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Biserta, S.; Bolelho, D.; Bruze, M. RIFM fragrance ingredient safety assessment, pulegone, CAS Registry Number 89-82-7. Food Chem. Toxicol. 2019, 127, S200–S207. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, B.; Madyastha, P.; Madyastha, K.M. Hepatotoxicity of pulegone in rats: Its effects on microsomal enzymes, in vivo. Toxicology 1989, 55, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Amrouni, S.; Touati, M.; Hadef, Y.; Djahoudi, A. Effet de l’huile essentielle d’Origanumvulgare et de Thymus ciliates sur Pseudomonas aeruginosa VIM-2 carbapénèmase. Phytothérapie 2014, 12, 309–313. [Google Scholar] [CrossRef]
- Bremness, L. Plantes Aromatiques et Médicinales, 1st ed.; Larousse: Paris, France, 2012. [Google Scholar]
- De la Charie, T. Se Soigner par les Huiles Essentielles: Pourquoi et Comment ça Marche? 1st ed.; Édition du Rocher: Paris, France, 2019. [Google Scholar]
- Council of Europe. European Pharmacopoeia; Council of Europe: Strasbourg, France, 1997. [Google Scholar]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography, 1st ed.; Academic Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Konig, W.A.; Hochmuth, D.H.; Joulain, D. Terpenoids and Related Constituents of Essential Oils: Library of Mass Finder; Institute of Organic Chemistry: Hamburg, Germany, 2001. [Google Scholar]
- McLafferty, F.W.; Stauffer, D.B. Wiley/NBS Registry of Mass Spectral Data; Wiley–Blackwell: New York, NY, USA, 1989. [Google Scholar]
- Costa, R.; Zellner, B.A.; Crupi, M.L.; De Fina, M.R.; Valentino, M.R.D.; Mondello, L. GC-MS, GC-O and enantio-GC investigation oil of Trachonanthus camphorates L. Flavour Fragr. J. 2008, 3, 40–48. [Google Scholar] [CrossRef]
| Compound Name and Class | Thymus | Thymus Munbyanus subsp. | Mentha | Rosmarinus | Lavandula | Eucalyptus | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Capitatus | Abylaeus | Ciliatus | Coloratus | Pulegium | Piperita | Rotundifulia | Officinalis | Stoechas | Aspic | Radiata | Globulus | Polybractea | ||
| Total Identification % | 99.5 | 99.3 | 99.7 | 98.2 | 98.5 | 98.8 | 98.9 | 93.6 | 95.3 | 95.1 | 95.2 | 95.7 | 94.5 | 95.2 |
| Hydrocarbon compounds | 24.7 | 17 | 31.3 | 55.1 | 2.7 | 4.8 | 6.5 | 21.2 | 60.9 | 7.6 | 17.5 | 13.7 | 11.7 | 41.6 |
| Monoterpene hydrocarbons | 23 | 14.2 | 28.9 | 37.9 | 2 | 2.8 | 4.9 | 20.4 | 58 | 6.8 | 16.4 | 13.7 | 11.4 | 41.6 |
| Sesquiterpene hydrocarbons | 1.7 | 2.8 | 2.1 | 17.2 | 0.7 | 2 | 1.6 | 0.8 | 2.9 | 0.8 | 1.1 | trace | 0.3 | trace |
| Diterpene hydrocarbons | - | 0.3 | - | |||||||||||
| Oxygenated compounds | 74.8 | 82.3 | 68.4 | 43.1 | 95.8 | 94 | 92.4 | 72.4 | 34.4 | 87.5 | 77.7 | 82 | 82.8 | 53.6 |
| Oxygenated monoterpenes | 74.2 | 81 | 68.4 | 40.3 | 94.2 | 92.5 | 91.3 | 72 | 34.4 | 84.9 | 77.1 | 81.8 | 82.8 | 51.3 |
| Oxygenated sesquiterpenes | 0.1 | 0.5 | - | 2 | 0 | 0.8 | 0 | 0.2 | 0 | 2.6 | 0.6 | 0.2 | trace | 2.3 |
| Non-terpenic oxygenated compounds | 0.5 | 0.8 | - | 0.8 | 1.6 | 0.7 | 1.1 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benomari, F.Z.; Sarazin, M.; Chaib, D.; Pichette, A.; Boumghar, H.; Boumghar, Y.; Djabou, N. Chemical Variability and Chemotype Concept of Essential Oils from Algerian Wild Plants. Molecules 2023, 28, 4439. https://doi.org/10.3390/molecules28114439
Benomari FZ, Sarazin M, Chaib D, Pichette A, Boumghar H, Boumghar Y, Djabou N. Chemical Variability and Chemotype Concept of Essential Oils from Algerian Wild Plants. Molecules. 2023; 28(11):4439. https://doi.org/10.3390/molecules28114439
Chicago/Turabian StyleBenomari, Fatima Zahra, Mathieu Sarazin, Djamel Chaib, André Pichette, Hinane Boumghar, Yacine Boumghar, and Nassim Djabou. 2023. "Chemical Variability and Chemotype Concept of Essential Oils from Algerian Wild Plants" Molecules 28, no. 11: 4439. https://doi.org/10.3390/molecules28114439
APA StyleBenomari, F. Z., Sarazin, M., Chaib, D., Pichette, A., Boumghar, H., Boumghar, Y., & Djabou, N. (2023). Chemical Variability and Chemotype Concept of Essential Oils from Algerian Wild Plants. Molecules, 28(11), 4439. https://doi.org/10.3390/molecules28114439





