Dynamic Stability of Volatile Organic Compounds in Respiratory Air in Schizophrenic Patients and Its Potential Predicting Efficacy of TAAR Agonists
Abstract
1. Introduction
2. Results and Discussion
2.1. Results
2.2. Discussion
3. Materials and Methods
3.1. Participants
3.2. Breath Analysis
3.3. Clinical Investigations
3.4. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef]
- Hafner, H. Is schizophrenia a disease? Epidemiologic data and speculative conclusions. Nervenarzt 1989, 60, 191–199. [Google Scholar]
- McGrath, J.; Saha, S.; Chant, D.; Welham, J. Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 2008, 30, 67–76. [Google Scholar] [CrossRef]
- Girdler, S.J.; Confino, J.E.; Woesner, M.E. Exercise as a Treatment for Schizophrenia: A Review. Psychopharmacol. Bull. 2019, 49, 56–69. [Google Scholar]
- Davison, J.; O’Gorman, A.; Brennan, L.; Cotter, D.R. A systematic review of metabolite biomarkers of schizophrenia. Schizophr. Res. 2018, 195, 32–50. [Google Scholar] [CrossRef]
- Orsolini, L.; Sarchione, F.; Vellante, F.; Fornaro, M.; Matarazzo, I.; Martinotti, G.; Valchera, A.; Di Nicola, M.; Carano, A.; Di Giannantonio, M.; et al. Protein-C Reactive as Biomarker Predictor of Schizophrenia Phases of Illness? A Systematic Review. Curr. Neuropharmacol. 2018, 16, 583–606. [Google Scholar] [CrossRef]
- Kambeitz, J.; Kambeitz-Ilankovic, L.; Leucht, S.; Wood, S.; Davatzikos, C.; Malchow, B.; Falkai, P.; Koutsouleris, N. Detecting neuroimaging biomarkers for schizophrenia: A meta-analysis of multivariate pattern recognition studies. Neuropsychopharmacology 2015, 40, 1742–1751. [Google Scholar] [CrossRef]
- Peng, S.; Li, W.; Lv, L.; Zhang, Z.; Zhan, X. BDNF as a biomarker in diagnosis and evaluation of treatment for schizophrenia and depression. Discov. Med. 2018, 26, 127–136. [Google Scholar]
- Prata, D.; Mechelli, A.; Kapur, S. Clinically meaningful biomarkers for psychosis: A systematic and quantitative review. Neurosci. Biobehav. Rev. 2014, 45, 134–141. [Google Scholar] [CrossRef]
- Bot, M.; Milaneschi, Y.; Al-Shehri, T.; Amin, N.; Garmaeva, S.; Onderwater, G.L.J.; Pool, R.; Thesing, C.S.; Vijfhuizen, L.S.; Vogelzangs, N.; et al. Metabolomics Profile in Depression: A Pooled Analysis of 230 Metabolic Markers in 5283 Cases with Depression and 10,145 Controls. Biol. Psychiatry 2020, 87, 409–418. [Google Scholar] [CrossRef]
- Thorn, R.M.; Reynolds, D.M.; Greenman, J. Multivariate analysis of bacterial volatile compound profiles for discrimination between selected species and strains in vitro. J Microbiol Methods 2011, 84, 258–264. [Google Scholar] [CrossRef]
- Karl, T.; Prazeller, P.; Mayr, D.; Jordan, A.; Rieder, J.; Fall, R.; Lindinger, W. Human breath isoprene and its relation to blood cholesterol levels: New measurements and modeling. J. Appl. Physiol. 2001, 91, 762–770. [Google Scholar] [CrossRef]
- Phillips, M.; Erickson, G.A.; Sabas, M.; Smith, J.P.; Greenberg, J. Volatile organic compounds in the breath of patients with schizophrenia. J. Clin. Pathol. 1995, 48, 466–469. [Google Scholar] [CrossRef]
- Ross, B.M.; Maxwell, R.; Glen, I. Increased breath ethane levels in medicated patients with schizophrenia and bipolar disorder are unrelated to erythrocyte omega-3 fatty acid abundance. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 446–453. [Google Scholar] [CrossRef]
- Popa, C.; Petrus, M.; Bratu, A.M. Ammonia and ethylene biomarkers in the respiration of the people with schizophrenia using photoacoustic spectroscopy. J. Biomed. Opt. 2015, 20, 57006. [Google Scholar] [CrossRef]
- Jiang, C.; Dobrowolny, H.; Gescher, D.M.; Meyer-Lotz, G.; Steiner, J.; Hoeschen, C.; Frodl, T. Volatile organic compounds from exhaled breath in schizophrenia. World J. Biol. Psychiatry 2022, 10, 773–784. [Google Scholar] [CrossRef]
- Hansel, A.; Jordan, A.; Holzinger, R.; Prazeller, P.; Vogel, W.; Lindinger, W. Proton transfer reaction mass spectrometry: On-line trace gas analysis at the ppb level. Int. J. Mass Spectrom. Ion Process. 1995, 149, 609–619. [Google Scholar] [CrossRef]
- Hewitt, C.N.; Hayward, S.; Tani, A. The application of proton transfer reaction-mass spectrometry (PTR-MS) to the monitoring and analysis of volatile organic compounds in the atmosphere. J. Environ. Monit. 2003, 5, 1–7. [Google Scholar] [CrossRef]
- Henderson, B.; Ruszkiewicz, D.M.; Wilkinson, M.; Beauchamp, J.D.; Cristescu, S.M.; Fowler, S.J.; Salman, D.; Francesco, F.D.; Koppen, G.; Langejurgen, J.; et al. A benchmarking protocol for breath analysis: The peppermint experiment. J. Breath Res. 2020, 14, 046008. [Google Scholar] [CrossRef]
- Henning, D.; Lüno, M.; Jiang, C.; Meyer-Lotz, G.; Dobrowolny, H.; Hoeschen, C.; Frodl, T. Gut-brain axis volatile organic compounds derived in breath separate schizophrenia and major depressive disorder. J. Psychiatry Neurosci. 2023, 48, E117–E125. [Google Scholar] [CrossRef]
- Dedic, N.; Dworak, H.; Zeni, C.; Rutigliano, G.; Howes, O.D. Therapeutic Potential of TAAR1 Agonists in Schizophrenia: Evidence from Preclinical Models and Clinical Studies. Int. J. Mol. Sci. 2021, 22, 13185. [Google Scholar] [CrossRef]
- Koblan, K.S.; Kent, J.; Hopkins, S.C.; Krystal, J.H.; Cheng, H.; Goldman, R.; Loebel, A. A Non-D2-Receptor-Binding Drug for the Treatment of Schizophrenia. N. Engl. J. Med. 2020, 382, 1497–1506. [Google Scholar] [CrossRef]
- Castro-Nallar, E.; Bendall, M.L.; Perez-Losada, M.; Sabuncyan, S.; Severance, E.G.; Dickerson, F.B.; Schroeder, J.R.; Yolken, R.H.; Crandall, K.A. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ 2015, 3, e1140. [Google Scholar] [CrossRef]
- Trabue, S.L.; Anhalt, J.C.; Zahn, J.A. Bias of Tedlar bags in the measurement of agricultural odorants. J. Environ. Qual. 2006, 35, 1668–1677. [Google Scholar] [CrossRef]
- Beauchamp, J.; Herbig, J.; Gutmann, R.; Hansel, A. On the use of Tedlar(R) bags for breath-gas sampling and analysis. J. Breath Res. 2008, 2, 046001. [Google Scholar] [CrossRef]
- Ghimenti, S.; Lomonaco, T.; Bellagambi, F.G.; Tabucchi, S.; Onor, M.; Trivella, M.G.; Ceccarini, A.; Fuoco, R.; Di Francesco, F. Comparison of sampling bags for the analysis of volatile organic compounds in breath. J. Breath Res. 2015, 9, 047110. [Google Scholar] [CrossRef]
- Blake, R.S.; Whyte, C.; Hughes, C.O.; Ellis, A.M.; Monks, P.S. Demonstration of proton-transfer reaction time-of-flight mass spectrometry for real-time analysis of trace volatile organic compounds. Anal. Chem. 2004, 76, 3841–3845. [Google Scholar] [CrossRef]
- Sukul, P.; Schubert, J.K.; Zanaty, K.; Trefz, P.; Sinha, A.; Kamysek, S.; Miekisch, W. Exhaled breath compositions under varying respiratory rhythms reflects ventilatory variations: Translating breathomics towards respiratory medicine. Sci. Rep. 2020, 10, 14109. [Google Scholar] [CrossRef]
- Lueno, M.; Dobrowolny, H.; Gescher, D.; Gbaoui, L.; Meyer-Lotz, G.; Hoeschen, C.; Frodl, T. Volatile Organic Compounds from Breath Differ Between Patients with Major Depression and Healthy Controls. Front. Psychiatry 2022, 13, 819607. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Busner, J.; Targum, S.D. The clinical global impressions scale: Applying a research tool in clinical practice. Psychiatry 2007, 4, 28–37. [Google Scholar]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. Manual for the State-Trait Anxiety, Inventory; Consulting Psychologist Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Mundt, J.C.; Marks, I.M.; Shear, M.K.; Greist, J.H. The Work and Social Adjustment Scale: A simple measure of impairment in functioning. Br. J. Psychiatry 2002, 180, 461–464. [Google Scholar] [CrossRef]
- Bernstein, D.P.; Stein, J.A.; Newcomb, M.D.; Walker, E.; Pogge, D.; Ahluvalia, T.; Stokes, J.; Handelsman, L.; Medrano, M.; Desmond, D.; et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abus. Negl. 2003, 27, 169–190. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Sarason, I.G.; Johnson, J.H.; Siegel, J.M. Assessing the impact of life changes: Development of the Life Experiences Survey. J. Consult. Clin. Psychol. 1978, 46, 932–946. [Google Scholar] [CrossRef]
- Norbeck, J.S. Modification of life event questionnaires for use with female respondents. Res. Nurs. Health 1984, 7, 61–71. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Wisniewski, S.R.; Rush, A.J.; Balasubramani, G.K.; Trivedi, M.H.; Nierenberg, A.A.; Investigators, S. Self-rated global measure of the frequency, intensity, and burden of side effects. J. Psychiatr Pract. 2006, 12, 71–79. [Google Scholar] [CrossRef]
- Gardner, D.M.; Murphy, A.L.; O’Donnell, H.; Centorrino, F.; Baldessarini, R.J. International consensus study of antipsychotic dosing. Am. J. Psychiatry 2010, 167, 686–693. [Google Scholar] [CrossRef]
| Baseline SZ | Follow-Up SZ | p (Comparison between Baseline and Follow-Up) | Healthy Subjects | p (Comparison between Patients and Healthy Subjects) | |
|---|---|---|---|---|---|
| PANSS * | 51.20 ± 11.18 | 49.9 ± 10.75 2 missing pieces of data | p = 0.014 | ||
| PANSS positive * | 11.50 ± 3.46 | 10.75 ± 2.92 2 missing pieces of data | p = 0.005 | ||
| PANSS negative * | 13.05 ± 3.49 | 13.05 ± 3.44 2 missing pieces data | p = 0.500 | ||
| BDI-II * | 20.73 ± 13.83 | 16.86 ± 13.02 | p = 0.000384 | 1.91 ± 3.78 | p = 3.1488 × 10−16 |
| Medication group 1/2/3/4 | 7/5/2/7 one missing data | 7/5/2/7 one missing piece of data |
| HC | p | SZ | |||||
|---|---|---|---|---|---|---|---|
| After Awakening | After 30 min | After 60 min | After Awakening | After 30 min | After 60 min | ||
| m/z 19 ↓ | 384,535.21 ± 159,212.53 | 411,666.15 ± 116,909.9 | 434,064.31 ± 114,846.25 | p1 = 0.004 pFDR = 0.011 p2 = 0.093 p3 = 0.896 | 300,524.02 ± 147,925.98 | 346,813.21 ± 134,503.77 | 375,629.56 ± 120,839.35 |
| m/z 32 | 13,733.43 ± 31,038.39 | 4549.32 ± 7067.26 | 3524.65 ± 1945.92 | p1 = 0.974 p2 = 0.032 p3 = 0.839 | 11,342.26 ± 22,900.95 | 6226.55 ± 8595.74 | 3952.63 ± 4174.1 |
| m/z 33 ↓ | 516.51 ± 256.22 | 589.04 ± 252.09 | 615.64 ± 311.97 | p1 = 0.000002 pFDR = 0.000025 p2 = 0.288 p3 = 0.935 | 316.09 ± 256.92 | 349.94 ± 192.1 | 387.64 ± 247.07 |
| m/z 35 | 2.03 ± 2.6 | 7705.57 ± 36,134.6 | 2.59 ± 3.27 | p1 = 0.319 p2 = 0.371 p3 = 0.371 | 1.91 ± 2.99 | 1.09 ± 1.72 | 1.09 ± 1.19 |
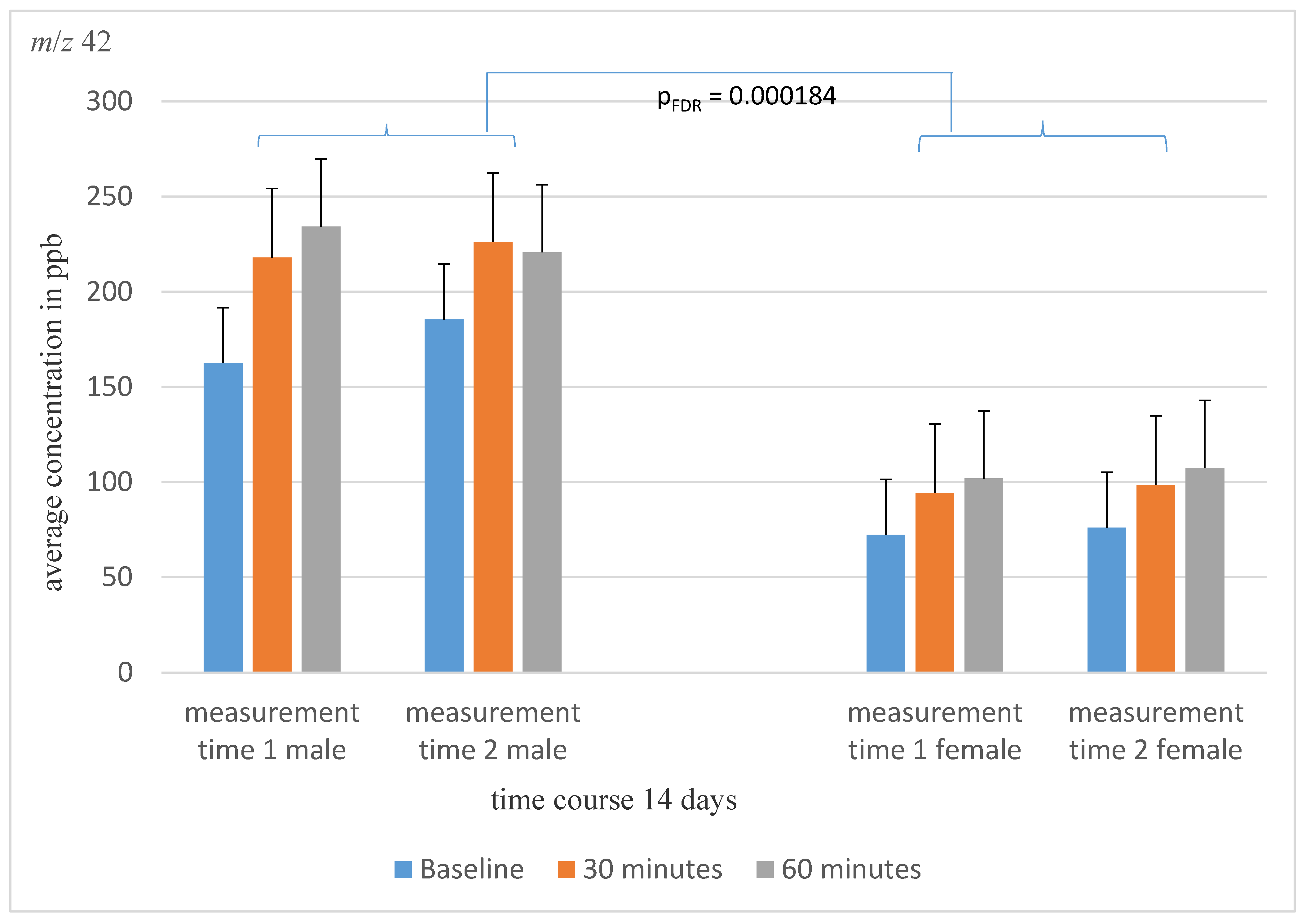
| m/z 42 ↑ | 30.32 ± 39.52 | 25.15 ± 21.87 | 20.64 ± 25.01 | p1 = 0.000000004 pFDR = 0.0000001 p2 = 0.650 p3 = 0.434 | 125.55 ± 109.1 | 167.30 ± 178.09 | 180.00 ± 200.99 |
| m/z 45 | 239.57 ± 375.94 | 131.37 ± 75.08 | 98.70 ± 35.66 | p1 = 0.913 p2 = 0.007 p3 = 0.877 | 212.91 ± 229.99 | 135.30 ± 66.25 | 110.73 ± 58.17 |
| m/z 47 | 132.47 ± 83.38 | 830.64 ± 3397.48 | 322.14 ± 1060.86 | p1 = 0.329 p2 = 0.561 p3 = 0.464 | 240.64 ± 531.82 | 163.32 ± 242.74 | 127.00 ± 105.89 |
| m/z 57 | 31.39 ± 29.78 | 39.31 ± 32.43 | 30.98 ± 49.20 | p1 = 0.138 p2 = 0.642 p3 = 0.678 | 50.64 ± 57.47 | 44.58 ± 41.51 | 38.00 ± 20.56 |
| m/z 59 ↓ | 2265.93 ± 2180.02 | 2173.68 ± 1637.89 | 2344.93 ± 2220.62 | p1 = 0.0009 pFDR = 0.0028 p2 = 0.914 p3 = 0.908 | 1105.18 ± 1233.20 | 1330.55 ± 1336.75 | 1333.00 ± 1274.98 |
| m/z 60 ↓ | 182.49 ± 165.02 | 132.39 ± 63.95 | 130.09 ± 80.03 | p1 = 0.000006 pFDR = 0.00005 p2 = 0.277 p3 = 0.273 | 77.18 ± 50.87 | 77.75 ± 43.07 | 77.00 ± 47.15 |
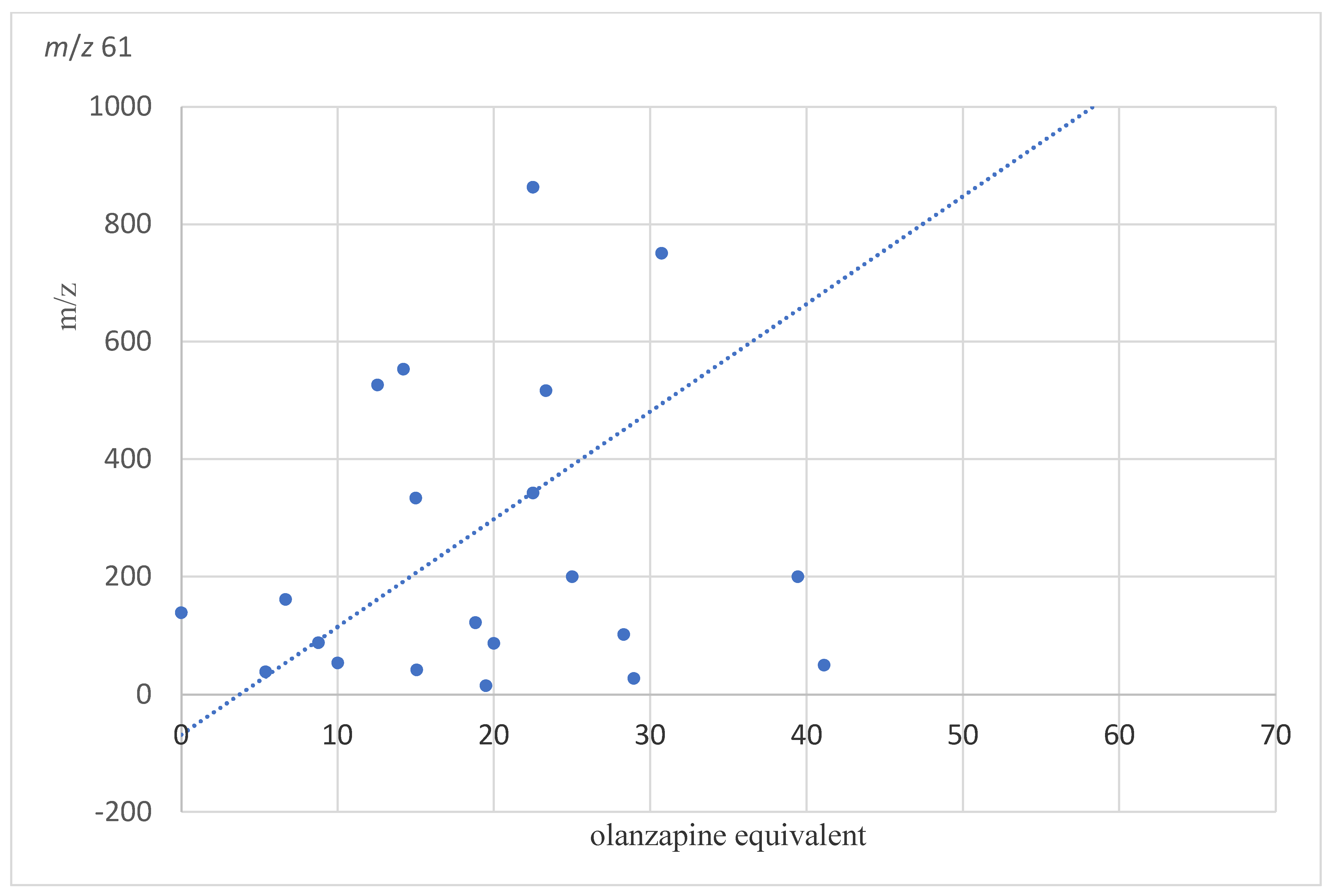
| m/z 61 | 289.09 ± 524.24 | 133.16 ± 50.81 | 171.50 ± 380.04 | p1 = 0.143 p2 = 0.246 p3 = 0.835 | 432.54 ± 800.32 | 309.62 ± 514.23 | 227.09 ± 318.22 |
| m/z 63 | 36.94 ± 16.38 | 36.88 ± 21.44 | 31.77 ± 18.93 | p1 = 0. 0.641 p2 = 0.753 p3 = 0.548 | 29.82 ± 24.54 | 35.77 ± 25.51 | 34.73 ± 21.66 |
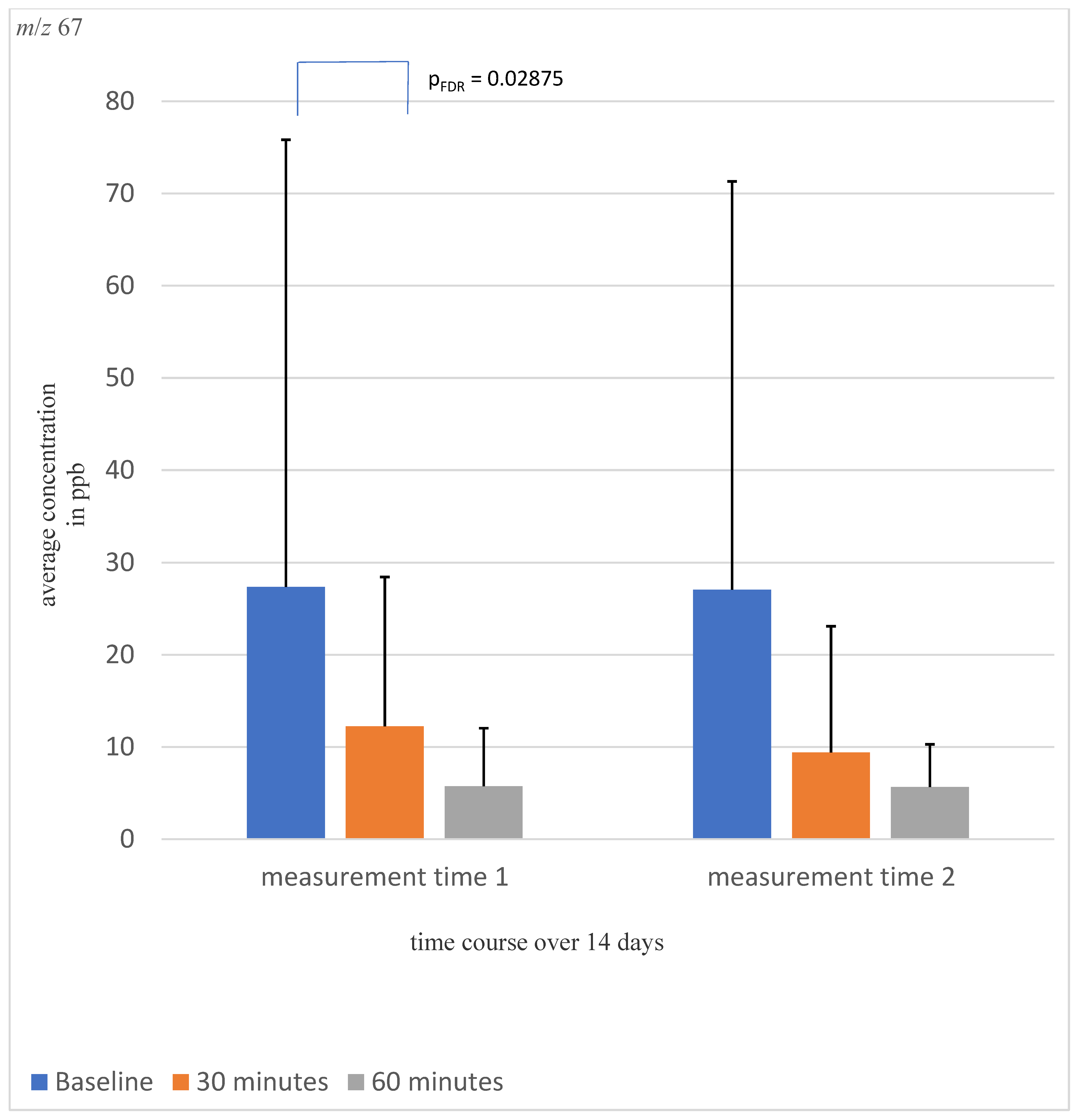
| m/z 67 | 38.22 ± 91.55 | 11.03 ± 17.58 | 7.48 ± 6.49 | p1 = 0.618 p2 = 0.013 p3 = 0.795 | 27.36 ± 48.46 | 12.25 ± 16.19 | 5.73 ± 6.03 |
| m/z 69 ↓ | 370.08 ± 260.74 | 299.69 ± 189.77 | 213.77 ± 127.33 | p1 = 0.00023 pFDR = 0.00096 p2 = 0.022 p3 = 0.264 | 196.00 ± 138.33 | 200.42 ± 139.57 | 155.27 ± 97.86 |
| m/z 71 | 11.47 ± 6.21 | 8.91 ± 4.58 | 8.86 ± 6.85 | p1 = 0.086 p2 = 0.449 p3 = 0.548 | 37.73 ± 101.83 | 31.73 ± 91.44 | 10.73 ± 13.23 |
| m/z 73 | 13.98 ± 16.9 | 10.66 ± 7.42 | 10.57 ± 5.79 | p1 = 0.022 p2 = 0.255 p3 = 0.776 | 27.00 ± 45.23 | 18.62 ± 23.48 | 17.00 ± 10.58 |
| m/z 74 ↑ | 30.87 ± 28.43 | 28.10 ± 17.04 | 29.20 ± 12.20 | p1 = 0.000018 pFDR = 0.00011 p2 = 0.906 p3 = 0.901 | 17.27 ± 8.41 | 17.47 ± 12.12 | 16.27 ± 8.15 |
| m/z 79 | 5.09 ± 7.55 | 2.57 ± 2.45 | 3.14 ± 5.79 | p1 = 0.013 p2 = 0.518 p3 = 0.721 | 10.36 ± 21.07 | 10.00 ± 16.41 | 6.36 ± 8.66 |
| m/z 87 | 126.77 ± 449.60 | 25.11 ± 21.01 | 17.91 ± 11.39 | p1 = 0.483 p2 = 0.265 p3 = 0.392 | 38.91 ± 84.10 | 38.08 ± 80.26 | 22.55 ± 52.17 |
| m/z 89 ↓ | 290.55 ± 293.63 | 312.16 ± 286.39 | 287.77 ± 267.26 | p1 = 0.000033 pFDR = 0.00017 p2 = 0.612 p3 = 0.897 | 98.09 ± 92.99 | 163.11 ± 277.28 | 102.27 ± 62.65 |
| m/z 91 | 5.47 ± 4.32 | 6.13 ± 4.37 | 6.09 ± 5.47 | p1 = 0.029 p2 = 0.405 p3 = 0.873 | 3.36 ± 2.92 | 4.91 ± 3.64 | 4.64 ± 3.67 |
| m/z 93 ↑ | 2.57 ± 2.34 | 0.83 ± 1.60 | 3.05 ± 2.42 | p1 = 0.00081 pFDR = 0.0028 p2 = 0.083 p3 = 0.182 | 6.64 ± 8.20 | 4.09 ± 4.60 | 3.73 ± 4.20 |
| m/z 95 | 708.63 ± 414.87 | 890.45 ± 327.40 | 854.39 ± 348.29 | p1 = 0.197 p2 = 0.068 p3 = 0.376 | 586.64 ± 466.01 | 792.60 ± 467.37 | 896.18 ± 425.54 |
| m/z 101 | 3.60 ± 3.66 | 3.32 ± 4.53 | 2.59 ± 2.39 | p1 = 0.080 p2 = 0.833 p3 = 0.630 | 2.18 ± 3.20 | 2.00 ± 2.05 | 2.36 ± 2.80 |
| Correlation Coefficient in Classes | ||||
|---|---|---|---|---|
| Correlation within the Class | 95%-Confidence Interval | F-Test with True Value 0 | ||
| Lower Limit | Upper Limit | Sig. | ||
| m/z 32 | 0.098 | −1.171 | 0.626 | 0.407 |
| m/z 33 | 0.765 | 0.434 | 0.902 | 0.00081 |
| m/z 35 | 0.708 | 0.298 | 0.879 | 0.00336 |
| m/z 42 | 0.894 | 0.744 | 0.956 | 0.000002 |
| m/z 45 | 0.615 | 0.072 | 0.840 | 0.017 |
| m/z 47 | 0.643 | 0.141 | 0.852 | 0.011 |
| m/z 57 | 0.372 | −0.512 | 0.739 | 0.147 |
| m/z 59 | 0.781 | 0.473 | 0.909 | 0.00049 |
| m/z 60 | 0.801 | 0.534 | 0.920 | 0.00020 |
| m/z 61 | 0.556 | −0.070 | 0.816 | 0.035 |
| m/z 63 | 0.836 | 0.605 | 0.932 | 0.00006 |
| m/z 67 | 0.243 | −0.824 | 0.686 | 0.265 |
| m/z 69 | 0.759 | 0.419 | 0.900 | 0.00097 |
| m/z 71 | 0.553 | −0.077 | 0.814 | 0.036 |
| m/z 73 | 0.475 | −0.264 | 0.782 | 0.074 |
| m/z 74 | 0.346 | −0.576 | 0.728 | 0.169 |
| m/z 79 | 0.701 | 0.280 | 0.876 | 0.003935 |
| m/z 87 | 0.550 | −0.083 | 0.813 | 0.037 |
| m/z 89 | 0.022 | −0.1355 | 0.594 | 0.479 |
| m/z 91 | 0.591 | 0.018 | 0.831 | 0.023 |
| m/z 93 | 0.600 | 0.036 | 0.834 | 0.021 |
| m/z 95 | 0.069 | −1.243 | 0.613 | 0.436 |
| m/z 101 | 0.226 | −0.864 | 0.679 | 0.281 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Held, A.; Henning, D.; Jiang, C.; Hoeschen, C.; Frodl, T. Dynamic Stability of Volatile Organic Compounds in Respiratory Air in Schizophrenic Patients and Its Potential Predicting Efficacy of TAAR Agonists. Molecules 2023, 28, 4385. https://doi.org/10.3390/molecules28114385
Held A, Henning D, Jiang C, Hoeschen C, Frodl T. Dynamic Stability of Volatile Organic Compounds in Respiratory Air in Schizophrenic Patients and Its Potential Predicting Efficacy of TAAR Agonists. Molecules. 2023; 28(11):4385. https://doi.org/10.3390/molecules28114385
Chicago/Turabian StyleHeld, Anna, Dariush Henning, Carina Jiang, Christoph Hoeschen, and Thomas Frodl. 2023. "Dynamic Stability of Volatile Organic Compounds in Respiratory Air in Schizophrenic Patients and Its Potential Predicting Efficacy of TAAR Agonists" Molecules 28, no. 11: 4385. https://doi.org/10.3390/molecules28114385
APA StyleHeld, A., Henning, D., Jiang, C., Hoeschen, C., & Frodl, T. (2023). Dynamic Stability of Volatile Organic Compounds in Respiratory Air in Schizophrenic Patients and Its Potential Predicting Efficacy of TAAR Agonists. Molecules, 28(11), 4385. https://doi.org/10.3390/molecules28114385








