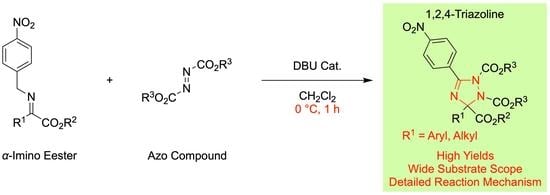
Formal [3 + 2] Cycloaddition of α-Imino Esters with Azo Compounds: Facile Construction of Pentasubstituted 1,2,4-Triazoline Skeletons
Abstract
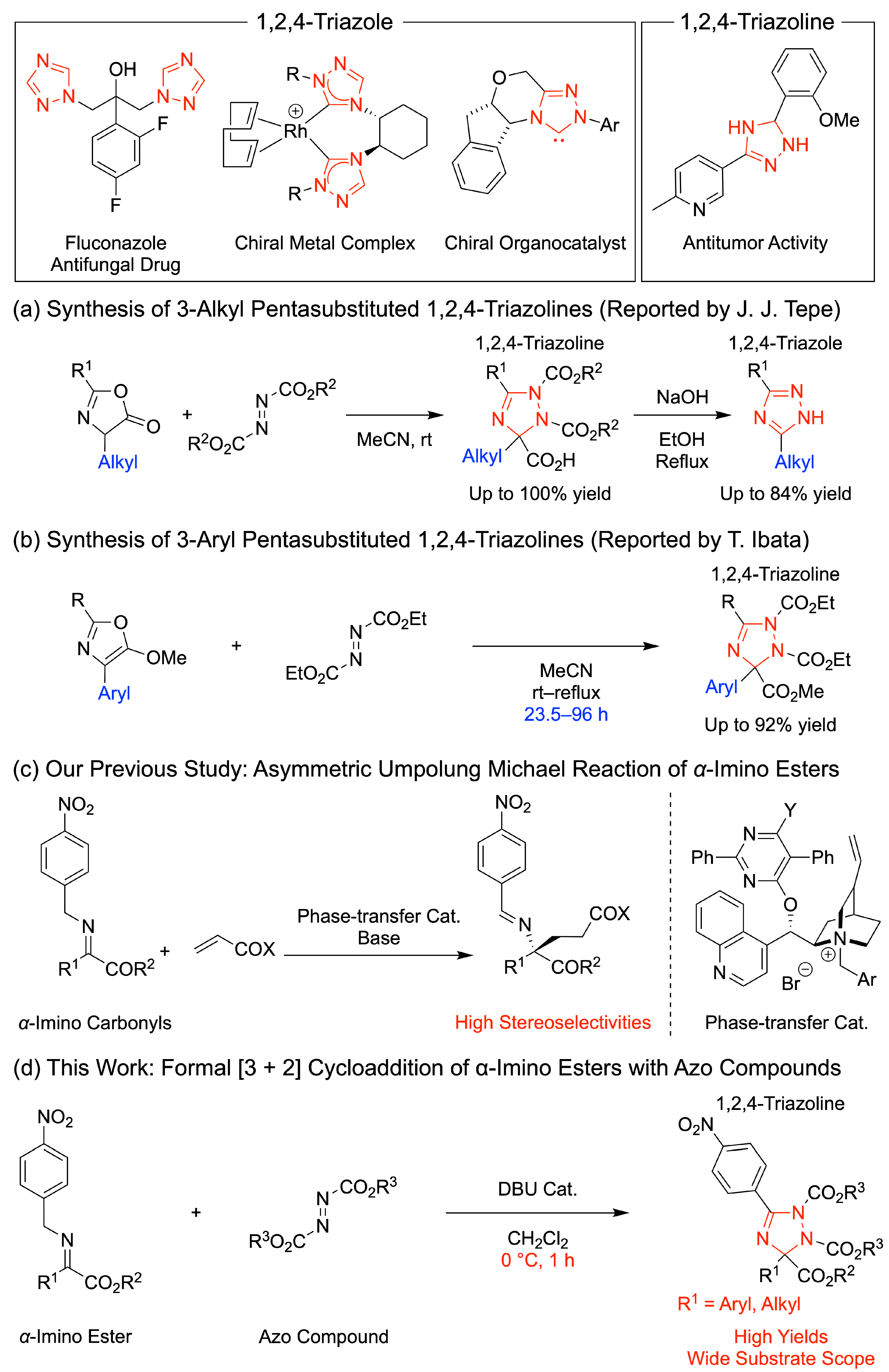
1. Introduction
2. Results and Discussion
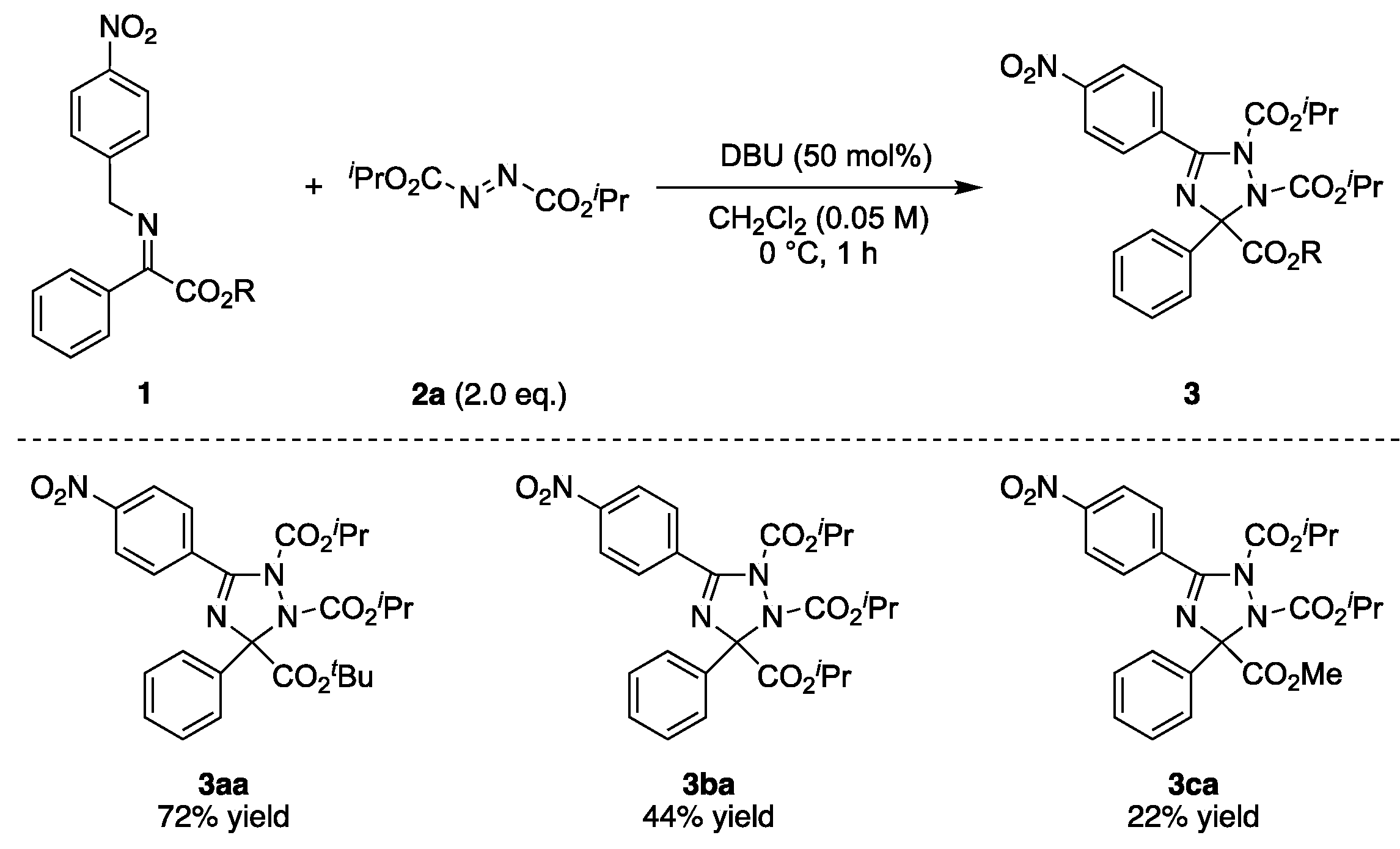
2.1. Reaction Condition Optimization
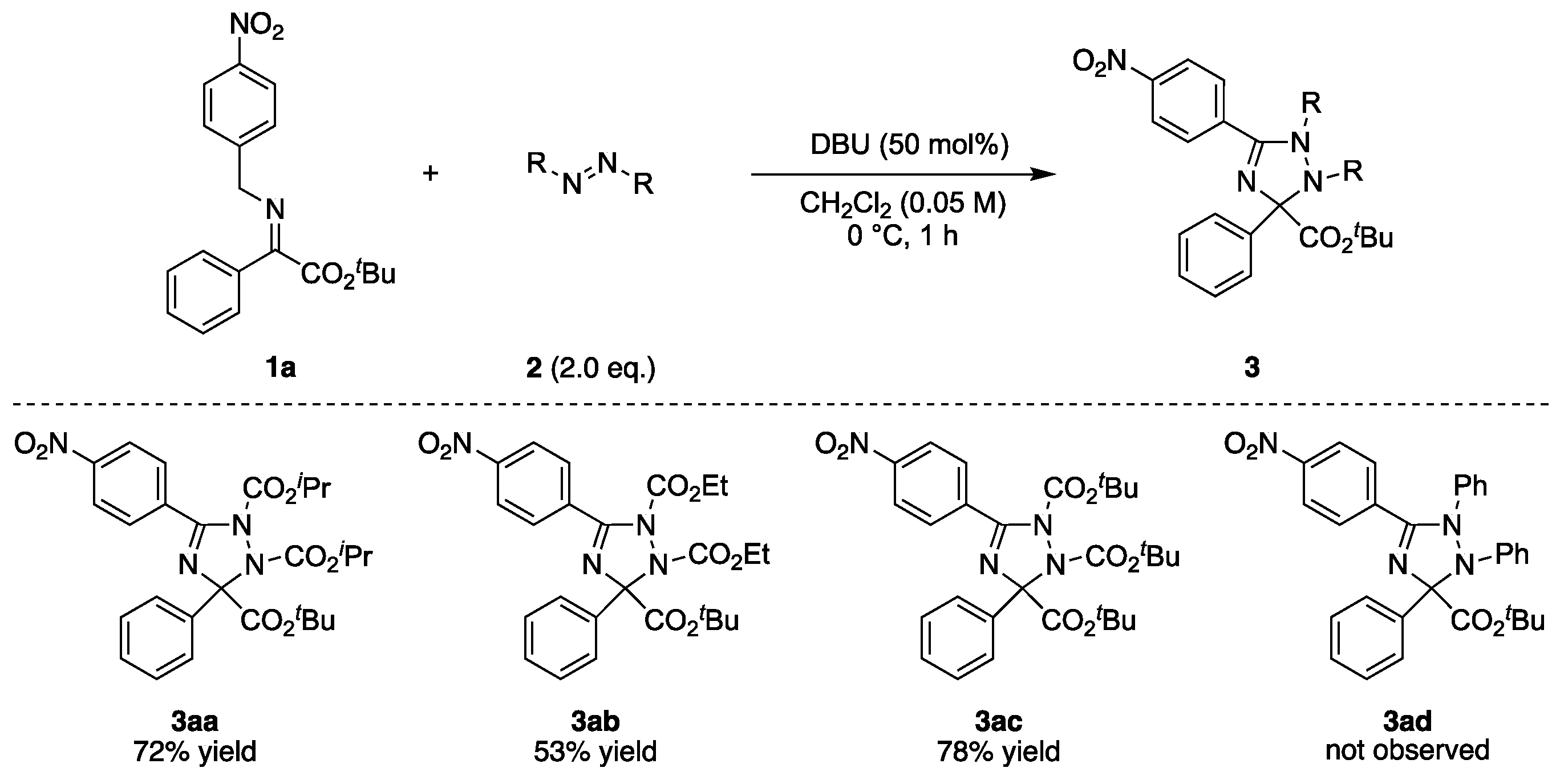
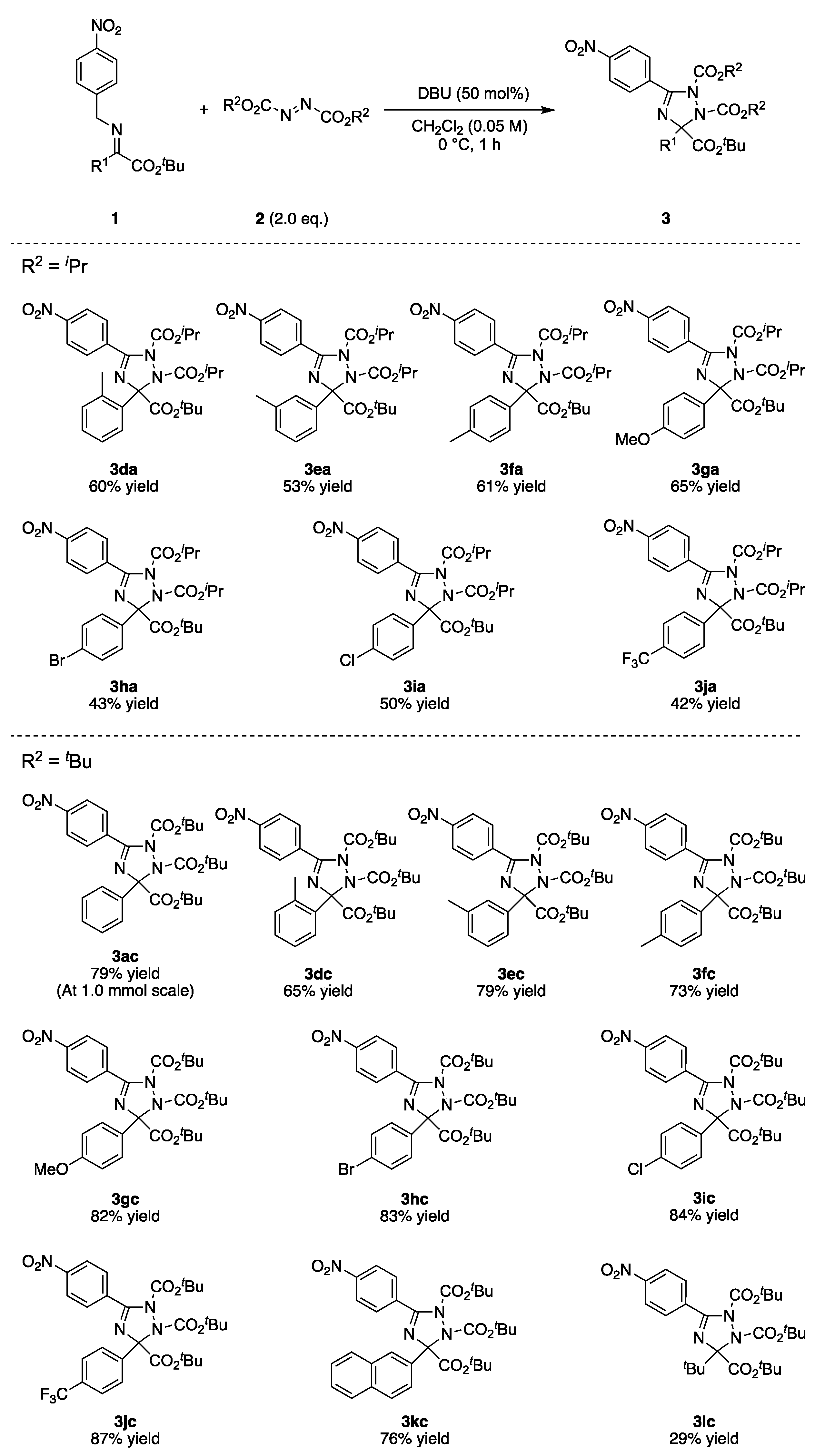
2.2. Substrate Scope
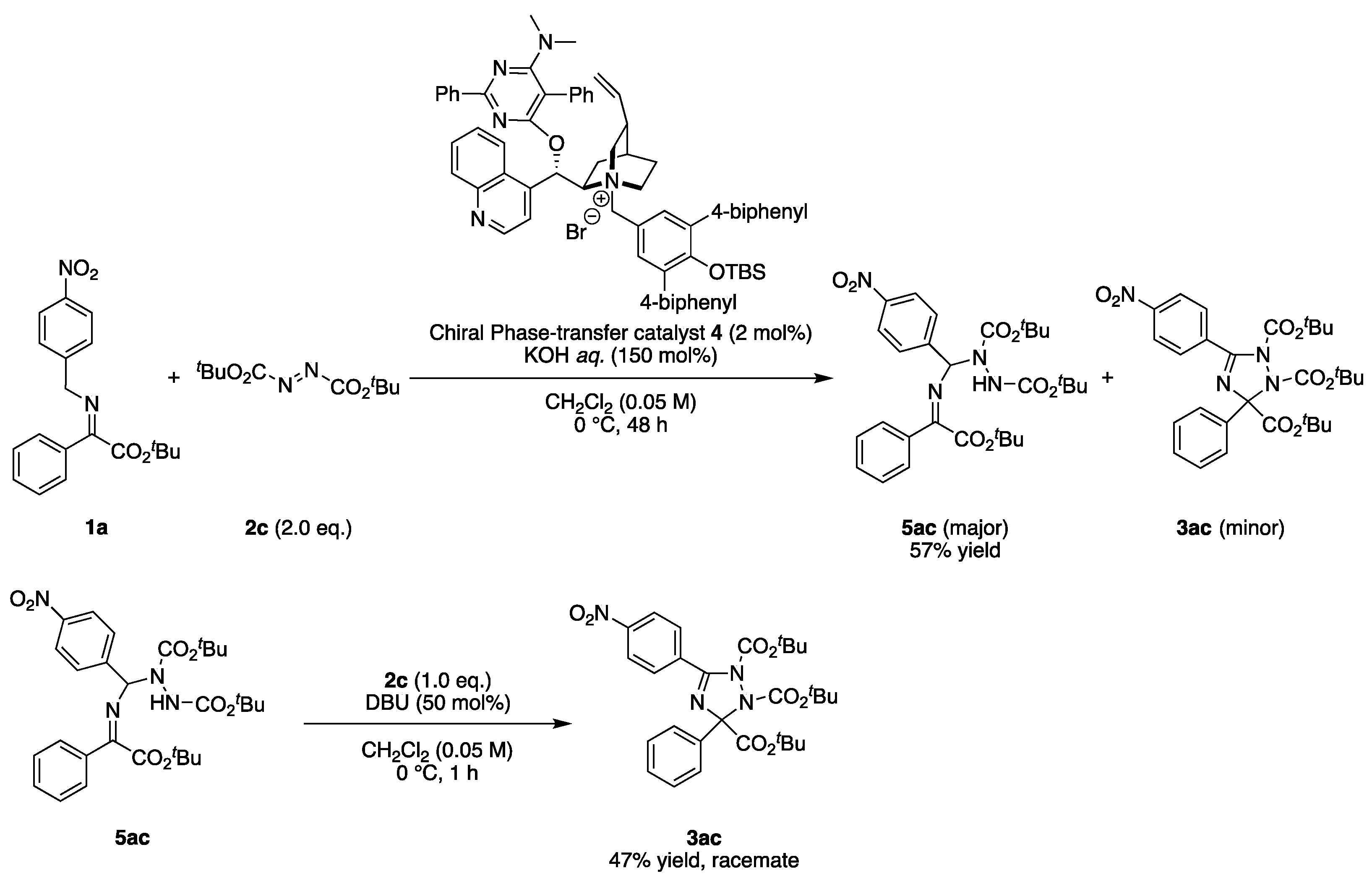
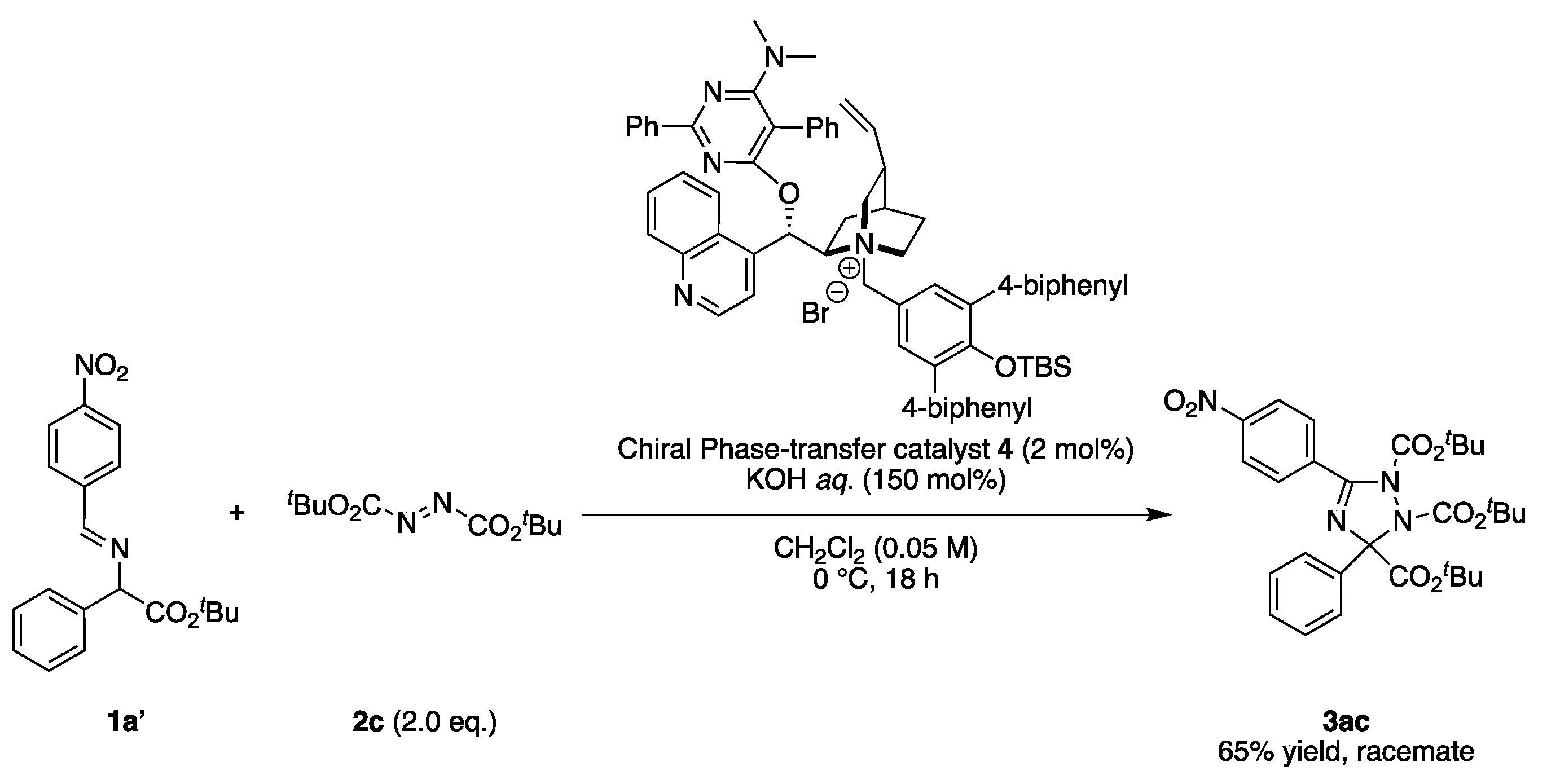
2.3. Asymmetric Synthesis
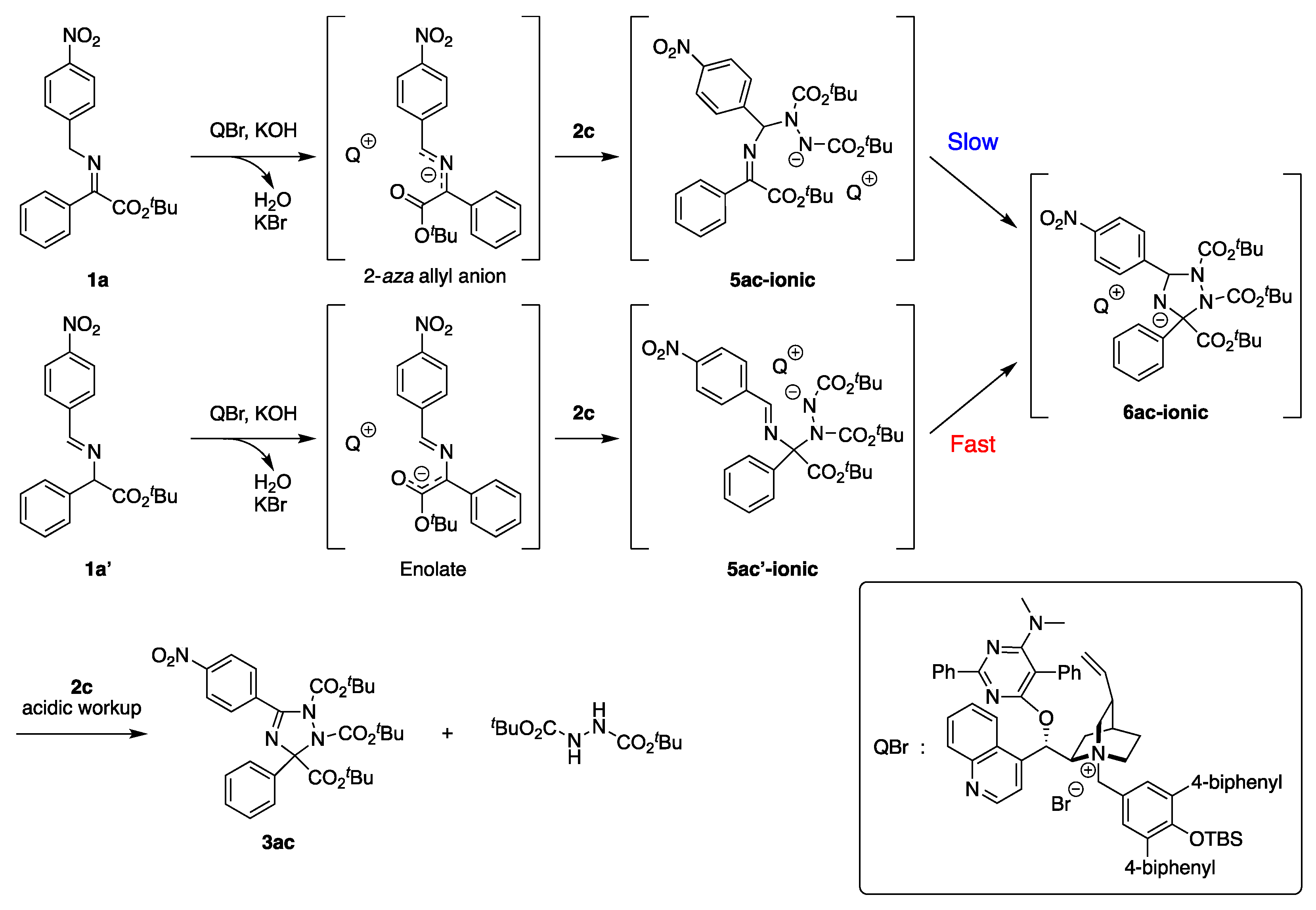
2.4. Reaction Mechanistic Study
3. Materials and Methods
3.1. Synthesis of Substrates and a Catalyst
3.2. Synthesis of 1,2,4-Triazolines
3.2.1. General Procedure for Table 1
3.2.2. General Procedure for Scheme 1, Scheme 2, Scheme 3 and Scheme 4 (Optimized Protocol)
- 3-(tert-butyl) 1,2-diisopropyl 5-(4-nitrophenyl)-3-phenyl-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3aa), White solid, 19.4 mg, 0.036 mmol, 72% yield (0.050 mmol scale reaction). m.p. 68–70 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.26–8.30 (m, 2H), 8.00–8.04 (m, 2H), 7.68–7.72 (m, 2H), 7.34–7.43 (m, 3H), 5.10 (sep, J = 6.2 Hz, 1H), 4.81 (sep, J = 6.2 Hz, 1H), 1.40 (s, 9H), 1.39 (d, J = 6.2 Hz, 3H), 1.36 (d, J = 6.2 Hz, 3H), 1.12 (d, J = 6.2 Hz, 3H), 1.11 (d, J = 6.2 Hz, 3H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 165.5, 156.5, 154.5, 152.2, 149.4, 137.0, 135.0, 130.8, 128.5, 127.8, 127.3, 122.9, 95.3, 83.7, 72.5, 71.4, 27.6, 22.2, 21.8, 21.53, 21.46; HRMS (ESI+ in MeCN) calcd. for C27H33O8N4+ (M + H) 541.2293 found 541.2297; IR (KBr) ν 2982, 1752, 1527, 1349, 1260, 1155, 1102, 849 cm−1.
- tri-isopropyl 5-(4-nitrophenyl)-3-phenyl-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3ba), White solid, 12.8 mg, 0.024 mmol, 44% yield (0.055 mmol scale reaction). m.p. 60–62 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.26–8.30 (m, 2H), 8.02–8.06 (m, 2H), 7.65–7.69 (m, 2H), 7.35–7.44 (m, 3H), 5.08 (sep, J = 6.2 Hz, 1H), 5.03 (sep, J = 6.2 Hz, 1H), 4.84 (sep, J = 6.2 Hz, 1H), 1.37 (d, J = 6.2 Hz, 3H), 1.33 (d, J = 6.2 Hz, 3H), 1.22 (d, J = 6.2 Hz, 3H), 1.18 (d, J = 6.2 Hz, 3H), 1.13 (d, J = 6.2 Hz, 3H), 1.12 (d, J = 6.2 Hz, 3H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 166.4, 156.8, 154.2, 152.1, 149.5, 136.8, 134.7, 130.9, 128.7, 127.9, 127.2, 122.9, 94.6, 72.7, 71.4, 71.1, 22.1, 21.7, 21.52, 21.45; HRMS (ESI+ in MeCN) calcd. for C26H31O8N4+ (M + H) 527.2136 found 527.2241; IR (KBr) ν 2983, 1751, 1527, 1349, 1256, 1183, 1099, 849 cm−1.
- 1,2-diisopropyl 3-methyl 5-(4-nitrophenyl)-3-phenyl-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3ca), White solid, 7.6 mg, 0.016 mmol, 23% yield (0.069 mmol scale reaction). m.p. 60–62 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.26–8.31 (m, 2H), 8.04–8.08 (m, 2H), 7.66–7.70 (m, 2H), 7.37–7.46 (m, 3H), 5.11 (sep, J = 6.4 Hz, 1H), 4.83 (sep, J = 6.4 Hz, 1H), 3.76 (s, 3H), 1.36 (d, J = 6.4 Hz, 3H), 1.32 (d, J = 6.4 Hz, 3H), 1.14 (d, J = 6.4 Hz, 3H), 1.13 (d, J = 6.4 Hz, 3H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 167.5, 157.1, 154.2, 151.9, 149.5, 136.6, 134.4, 131.1, 128.9, 128.1, 127.1, 122.9, 94.3, 72.8, 71.6, 53.6, 22.0, 21.7, 21.54, 21.47; HRMS (ESI+ in MeCN) calcd. for C24H27O8N4+ (M + H) 499.1823 found 499.1828; IR (KBr) ν 2983, 1748, 1526, 1349, 1254, 1184, 1102, 849 cm−1.
- 3-(tert-butyl) 1,2-diisopropyl 5-(4-nitrophenyl)-3-(o-tolyl)-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3da), White solid, 16.6 mg, 0.299 mmol, 60% yield (0.050 mmol scale reaction). m.p. 81–83 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.25–8.29 (m, 2H), 7.96–8.00 (m, 2H), 7.67 (d, J = 7.7 Hz, 1H), 7.25–7.30 (m, 2H), 7.17–7.22 (m, 1H), 5.09 (sep, J = 6.4 Hz, 1H), 4.86 (sep, J = 6.4 Hz, 1H), 2.62 (s, 3H), 1.42 (s, 9H), 1.38 (d, J = 6.4 Hz, 6H), 1.20 (d, J = 6.4 Hz, 3H), 1.19 (d, J = 6.4 Hz, 3H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 165.0, 155.9, 154.5, 152.2, 149.3, 137.5, 135.0, 134.7, 131.6, 130.9, 128.7, 126.7, 125.5, 122.9, 96.9, 83.6, 72.5, 71.4, 27.5, 22.1, 21.99, 21.85, 21.64, 21.58; HRMS (ESI+ in MeCN) calcd. for C28H35O8N4+ (M + H) 555.2449 found 555.2449; IR (KBr) ν 2982, 1744, 1527, 1349, 1257, 1157, 1103, 849 cm−1.
- 3-(tert-butyl) 1,2-diisopropyl 5-(4-nitrophenyl)-3-(m-tolyl)-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3ea), White solid, 14.8 mg, 0.027 mmol, 53% yield (0.050 mmol scale reaction). m.p. 96–98 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.26–8.30 (m, 2H), 8.04–8.00 (m, 2H), 7.50 (s, 1H), 7.48 (d, J = 6.8 Hz, 1H), 7.28–7.32 (m, 1H), 7.18 (d, J = 7.8 Hz, 1H), 5.11 (sep, J = 6.3 Hz, 1H), 4.81 (sep, J = 6.3 Hz, 1H), 2.40 (s, 3H), 1.40 (s, 9H), 1.39 (d, J = 6.3 Hz, 3H), 1.36 (d, J = 6.3 Hz, 3H), 1.14 (d, J = 6.3 Hz, 6H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 165.5, 156.3, 154.5, 152.2, 149.4, 137.4, 136.9, 135.0, 130.8, 129.3, 127.93, 127.81, 124.5, 122.9, 95.4, 83.6, 72.6, 71.3, 27.6, 22.2, 21.8, 21.59, 21.56, 21.48; HRMS (ESI+ in MeCN) calcd. for C28H35O8N4+ (M + H) 555.2449 found 555.2454; IR (KBr) ν 2981, 1747, 1526, 1348, 1253, 1155, 1103, 845 cm−1.
- 3-(tert-butyl) 1,2-diisopropyl 5-(4-nitrophenyl)-3-(p-tolyl)-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3fa), White solid, 16.8 mg, 0.030 mmol, 61% yield (0.050 mmol scale reaction). m.p. 76–78 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.26–8.30 (m, 2H), 7.99–8.03 (m, 2H), 7.55–7.59 (m, 2H), 7.21 (d, J = 8.0 Hz, 2H), 5.09 (sep, J = 6.3 Hz, 1H), 4.81 (sep, J = 6.3 Hz, 1H), 2.37 (s, 3H), 1.40 (s, 9H), 1.39 (d, J = 6.4 Hz, 3H), 1.35 (d, J = 6.4 Hz, 3H), 1.13 (d, J = 6.4 Hz, 3H), 1.20 (d, J = 6.4 Hz, 3H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 165.6, 156.3, 154.5, 152.2, 149.3, 138.4, 135.1, 134.0, 130.7, 128.6, 127.2, 122.9, 95.3, 83.6, 72.6, 71.3, 27.6, 22.2, 21.8, 21.56, 21.45, 21.1; HRMS (ESI+ in MeCN) calcd. for C28H35O8N4+ (M + H) 555.2449 found 555.2455; IR (KBr) ν 2982, 1747, 1526, 1348, 1258, 1155, 1102, 849 cm−1.
- 3-(tert-butyl) 1,2-diisopropyl 3-(4-methoxyphenyl)-5-(4-nitrophenyl)-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3ga), White solid, 18.6 mg, 0.033 mmol, 65% yield (0.050 mmol scale reaction). m.p. 98–100 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.26–8.30 (m, 2H), 8.00–8.04 (m, 2H), 7.59–7.63 (m, 2H), 6.91–6.95 (m, 2H), 5.09 (sep, J = 6.4 Hz, 1H), 4.81 (sep, J = 6.4 Hz, 1H), 3.83 (s, 3H), 1.40 (s, 9H), 1.39 (d, J = 6.4 Hz, 3H), 1.35 (d, J = 6.4 Hz, 3H), 1.13 (d, J = 6.4 Hz, 3H), 1.10 (d, J = 6.4 Hz, 3H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 165.7, 159.7, 156.3, 154.5, 152.2, 149.3, 135.1, 130.7, 129.1, 128.6, 122.9, 113.2, 95.0, 83.6, 72.6, 71.3, 55.2, 27.6, 22.2, 21.8, 21.56, 21.44; HRMS (ESI+ in MeCN) calcd. for C28H35O9N4+ (M + H) 571.2399 found 571.2404; IR (KBr) ν 2980, 1757, 1526, 1348, 1253, 1155, 1102, 849 cm−1.
- 3-(tert-butyl) 1,2-diisopropyl 3-(4-bromophenyl)-5-(4-nitrophenyl)-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3ha), White solid, 13.3 mg, 0.215 mmol, 43% yield (0.050 mmol scale reaction). m.p. 156–158 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.27–8.31 (m, 2H), 7.99–8.03 (m, 2H), 7.51–7.59 (m, 4H), 5.10 (sep, J = 6.3 Hz, 1H), 4.82 (sep, J = 6.3 Hz, 1H), 1.40 (s, 9H), 1.39 (d, J = 6.3 Hz, 3H), 1.36 (d, J = 6.3 Hz, 3H), 1.13 (d, J = 6.3 Hz, 3H), 1.11 (d, J = 6.3 Hz, 3H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 165.0, 156.7, 154.5, 152.0, 149.5, 136.3, 134.8, 131.0, 130.8, 129.1, 122.99, 122.00, 94.8, 84.1, 72.8, 71.6, 27.6, 22.2, 21.8, 21.56, 21.42; HRMS (ESI+ in MeCN) calcd. for C27H32O8N4Br+ (M + H) 619.1398 found 619.1402; IR (KBr) ν 2981, 1751, 1526, 1348, 1257, 1155, 1102, 849 cm−1.
- 3-(tert-butyl) 1,2-diisopropyl 3-(4-chlorophenyl)-5-(4-nitrophenyl)-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3ia), White solid, 14.4 mg, 0.025 mmol, 50% yield (0.050 mmol scale reaction). m.p. 154–156 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.27–8.31 (m, 2H), 7.99–8.03 (m, 2H), 7.61–7.65 (m, 2H), 7.35–7.40 (m, 2H), 5.10 (sep, J = 6.4 Hz, 1H), 4.82 (sep, J = 6.4 Hz, 1H), 1.40 (s, 9H), 1.39 (d, J = 6.4 Hz, 3H), 1.35 (d, J = 6.4 Hz, 3H), 1.13 (d, J = 6.4 Hz, 3H), 1.11 (d, J = 6.4 Hz, 3H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 165.2, 156.7, 154.5, 152.0, 149.4, 135.7, 134.8, 134.5, 130.8, 128.8, 128.0, 123.0, 94.8, 84.1, 72.8, 71.6, 27.6, 22.2, 21.8, 21.54, 21.42; HRMS (ESI+ in MeCN) calcd. for C27H32O8N4Cl+ (M + H) 575.1903 found 575.1910; IR (KBr) ν 2981, 1751, 1527, 1351, 1259, 1155, 1102, 849 cm−1.
- 3-(tert-butyl) 1,2-diisopropyl 5-(4-nitrophenyl)-3-(4-(trifluoromethyl)phenyl)-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3ja), White solid, 12.7 mg, 0.021mmol, 42% yield (0.050 mmol scale reaction). m.p. 77–79 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.27–8.32 (m, 2H), 8.00–8.04 (m, 2H), 7.83 (d, J = 8.0 Hz, 2H), 7.67 (d, J = 8.3 Hz, 2H), 5.12 (sep, J = 6.3 Hz, 1H), 4.82 (sep, J = 6.3 Hz, 1H), 1.40 (s, 9H), 1.39 (d, J = 6.3 Hz, 3H), 1.37 (d, J = 6.3 Hz, 3H), 1.13 (d, J = 6.3 Hz, 3H), 1.10 (d, J = 6.3 Hz, 3H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 165.0, 157.0, 154.5, 151.9, 149.5, 141.1, 134.6, 130.86, 130.71 (q, J = 32.3 Hz), 127.8, 124.8 (q, J = 3.9 Hz), 123.9 (q, J = 272.8 Hz), 123.0, 94.8, 84.3, 72.9, 71.7, 27.6, 22.2, 21.8, 21.54, 21.40; 19F-NMR (376 MHz, CHLOROFORM-D) δ -62.5; HRMS (ESI+ in MeCN) calcd. for C28H32O8N4F3+ (M + H) 609.2167 found 609.2172; IR (KBr) ν 2983, 1752,1528,1326, 1257, 1165, 1102, 850 cm−1.
- 3-(tert-butyl) 1,2-diethyl 5-(4-nitrophenyl)-3-phenyl-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3cb), White solid, 13.6 mg, 0.027 mmol, 53% yield (0.050 mmol scale reaction). m.p. 67–69 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.26–8.30 (m, 2H), 8.01–8.05 (m, 2H), 7.68–7.72 (m, 2H), 7.35–7.45 (m, 3H), 4.38–4.46 (m, 1H), 4.20–4.30 (m, 1H), 4.05–4.18 (m, 2H), 1.40 (s, 9H), 1.36 (t, J = 7.1 Hz, 3H), 1.10 (t, J = 7.1 Hz, 3H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 165.4, 156.2, 154.9, 152.4, 149.4, 136.8, 134.8, 130.8, 128.6, 127.9, 127.3, 123.0, 95.5, 83.9, 64.1, 63.1, 27.6, 14.4, 13.8; HRMS (ESI+ in MeCN) calcd. for C25H29O8N4+ (M + H) 513.1980 found 513.1984; IR (KBr) ν 2980, 1752, 1526, 1351, 1258, 1153, 1022, 845 cm−1.
- tri-tert-butyl 5-(4-nitrophenyl)-3-phenyl-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3ac), White solid, 22.4 mg, 0.039 mmol, 78% yield (0.050 mmol scale reaction). Large-scale synthesis was conducted using 1.0 mmol (340.4 mg) of 1a, and 0.79 mmol (447.5 mg, 79% yield) of 3ac was isolated. m.p. 87–89 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.26–8.30 (m, 2H), 7.99–8.03 (m, 2H), 7.68–7.71 (m, 2H), 7.33–7.44 (m, 3H), 1.58 (s, 9H), 1.41 (s, 9H), 1.29 (s, 9H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 165.9, 156.6, 153.5, 151.0, 149.3, 137.3, 135.4, 130.7, 128.4, 127.8, 127.3, 122.9, 94.8, 84.8, 83.6, 83.0, 28.2, 27.6 (1 peak is overlapped with the other peak); HRMS (ESI+ in MeCN) calcd. for C29H37O8N4+ (M + H) 569.2606 found 569.2615; IR (KBr) ν 2979, 1744, 1527, 1369, 1349, 1253, 1149, 849 cm−1; HPLC (CHIRALPAK AD-H column, hexane/2-propanol = 95/5, flow rate 1.0 mL/min, 25 °C, 254 nm) first peak: tR = 5.8 min and second peak: tR = 6.7 min.
- tri-tert-butyl 5-(4-nitrophenyl)-3-(o-tolyl)-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3dc), White solid, 19.0 mg, 0.033 mmol, 65% yield (0.050 mmol scale reaction). m.p. 99–101 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.25–8.29 (m, 2H), 7.94–7.98 (m, 2H), 7.70 (d, J = 7.5 Hz, 1H), 7.19–7.29 (m, 3H), 2.62 (s, 3H), 1.58 (s, 9H), 1.42 (s, 9H), 1.36 (s, 9H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 165.5, 156.0, 153.7, 151.0, 149.2, 137.5, 135.4, 135.0, 131.6, 130.8, 128.6, 126.6, 125.5, 122.9, 96.4, 84.8, 83.6, 83.0, 28.2, 27.7, 27.5, 22.0; HRMS (ESI+ in MeCN) calcd. for C30H39O8N4+ (M + H) 583.2762 found 583.2767; IR (KBr) ν 2979, 1742, 1527, 1369, 1348, 1254, 1150, 849 cm−1.
- tri-tert-butyl 5-(4-nitrophenyl)-3-(m-tolyl)-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3ec), White solid, 23.0 mg, 0.039 mmol, 79% yield (0.050 mmol scale reaction). m.p. 76–78 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.26–8.30 (m, 2H), 7.99–8.03 (m, 2H), 7.50 (s, 1H), 7.49 (d, J = 7.7 Hz, 1H), 7.28–7.33 (m, 1H), 7.17 (d, J = 7.8 Hz, 1H), 2.41 (s, 3H), 1.58 (s, 9H), 1.42 (s, 9H), 1.31 (s, 9H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 165.9, 156.5, 153.5, 151.0, 149.2, 137.3, 137.1, 135.4, 130.7, 129.2, 127.9, 127.7, 124.5, 122.9, 94.9, 84.7, 83.5, 82.9, 28.2, 27.6, 21.6 (1 peak is overlapped with the other peak); HRMS (ESI+ in MeCN) calcd. for C30H39O8N4+ (M + H) 583.2762 found 583.2770; IR (KBr) ν 2979, 1743, 1526, 1369, 1348, 1257, 1149, 851 cm−1.
- tri-tert-butyl 5-(4-nitrophenyl)-3-(p-tolyl)-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3fc), White solid, 21.4 mg, 0.0367 mmol, 73% yield (0.050 mmol scale reaction). m.p. 96–98 °C;1H-NMR (400 MHz, CHLOROFORM-D) δ 8.25–8.30 (m, 2H), 7.98–8.02 (m, 2H), 7.56–7.59 (m, 2H), 7.22 (d, J = 8.0 Hz, 2H), 2.38 (s, 3H), 1.57 (s, 9H), 1.41 (s, 9H), 1.29 (s, 9H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 166.0, 156.5, 153.4, 151.0, 149.2, 138.2, 135.5, 134.3, 130.7, 128.5, 127.2, 122.9, 94.8, 84.7, 83.5, 82.9, 28.2, 27.6, 21.1 (1 peak is overlapped with the other peak); HRMS (ESI+ in MeCN) calcd. for C30H39O8N4+ (M + H) 583.2762 found 583.2767; IR (KBr) ν 2979, 1744, 1527, 1369, 1348, 1254, 1150, 850 cm−1.
- tri-tert-butyl 3-(4-methoxyphenyl)-5-(4-nitrophenyl)-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3gc), White solid, 24.4 mg, 0.041 mmol, 82% yield (0.050 mmol scale reaction). m.p. 87–89 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.26–8.30 (m, 2H), 7.98–8.02 (m, 2H), 7.59–7.64 (m, 2H), 6.91–6.96 (m, 2H), 3.83 (s, 3H), 1.58 (s, 9H), 1.41 (s, 9H), 1.29 (s, 9H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 166.1, 159.6, 156.5, 153.4, 151.0, 149.2, 135.5, 130.6, 129.4, 128.6, 122.9, 113.2, 94.5, 84.7, 83.5, 82.9, 55.2, 28.2, 27.6 (1 peak is overlapped with the other peak); HRMS (ESI+ in MeCN) calcd. for C30H39O9N4+ (M + H) 599.2712 found 599.2715; IR (KBr) ν 2979, 1744, 1527, 1369, 1348, 1253, 1150, 849 cm−1.
- tri-tert-butyl 3-(4-bromophenyl)-5-(4-nitrophenyl)-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3hc), White solid, 27.0 mg, 0.042 mmol, 83% yield (0.050 mmol scale reaction). m.p. 94–96 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.26–8.30 (m, 2H), 7.97–8.00 (m, 2H), 7.52–7.60 (m, 4H), 1.58 (s, 9H), 1.41 (s, 9H), 1.29 (s, 9H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 165.6, 157.0, 153.4, 150.8, 149.3, 136.6, 135.2, 130.9, 130.7, 129.1, 123.0, 122.7, 94.2, 85.1, 84.2, 83.2, 28.2, 27.6 (1 peak is overlapped with the other peak); HRMS (ESI+ in MeCN) calcd. for C29H36O8N4Br+ (M + H) 647.1711 found 647.1721; IR (KBr) ν 2979, 1751, 1527, 1369, 1348, 1253, 1149, 849 cm−1.
- tri-tert-butyl 3-(4-chlorophenyl)-5-(4-nitrophenyl)-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3ic), White solid, 25.4 mg, 0.042 mmol, 84% yield (0.050 mmol scale reaction). m.p. 86–88 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.26–8.31 (m, 2H), 7.97–8.02 (m, 2H), 7.62–7.66 (m, 2H), 7.36–7.40 (m, 2H), 1.58 (s, 9H), 1.41 (s, 9H), 1.28 (s, 9H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 165.6, 156.9, 153.4, 150.8, 149.3, 136.0, 135.2, 134.4, 130.7, 128.8, 128.0, 123.0, 94.3, 85.0, 84.0, 83.2, 28.2, 27.6 (1 peak is overlapped with the other peak); HRMS (ESI+ in MeCN) calcd. for C29H36O8N4Cl+ (M + H) 603.2216 found 603.2227; IR (KBr) ν 2979, 1752, 1527,1369, 1348, 1255, 1149, 848 cm−1.
- tri-tert-butyl 5-(4-nitrophenyl)-3-(4-(trifluoromethyl)phenyl)-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3jc), White solid, 27.7 mg, 0.044 mmol, 87% yield (0.050 mmol scale reaction). m.p. 106–108 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.27–8.31 (m, 2H), 7.98–8.02 (m, 2H), 7.84 (d, J = 8.2 Hz, 2H), 7.67 (d, J = 8.2 Hz, 2H), 1.59 (s, 9H), 1.41 (s, 9H), 1.28 (s, 9H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 165.5, 157.1, 153.5, 150.7, 149.4, 141.4, 135.1, 130.8, 130.5 (q, J = 33.1 Hz), 127.8, 124.8 (q, J = 3.7 Hz), 124.0 (q, J = 273.1 Hz), 123.0, 94.3, 85.1, 84.2, 83.4, 28.2, 27.6 (1 peak is overlapped with the other peak); 19F-NMR (376 MHz, CHLOROFORM-D) δ -62.5; HRMS (ESI+ in MeCN) calcd. for C30H36O8N4F3+ (M + H) 637.2480 found 637.2484; IR (KBr) ν 2980, 1751, 1528, 1370, 1326, 1253, 1149, 850 cm−1.
- tri-tert-butyl 3-(naphthalen-2-yl)-5-(4-nitrophenyl)-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3kc), White solid, 23.6 mg, 0.038 mmol, 76% yield (0.050 mmol scale reaction). m.p. 108–110 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.26–8.30 (m, 2H), 8.13 (s, 1H), 8.00–8.04 (m, 2H), 7.83–7.91 (m, 4H), 7.46–7.53 (m, 2H), 1.61 (s, 9H), 1.43 (s, 9H), 1.31 (s, 9H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 166.0, 156.8, 153.6, 151.0, 149.3, 135.3, 134.83 133.3, 132.7, 130.8, 128.4, 127.6, 127.3, 126.4, 126.02, 125.96, 125.7, 123.0, 95.0, 84.8, 83.9, 83.1, 28.2, 27.7 (1 peak is overlapped with the other peak); HRMS (ESI+ in MeCN) calcd. for C33H39O8N4+ (M + H) 619.2762 found 619.2767; IR (KBr) ν 2979, 1746, 1526, 1369, 1348, 1252, 1149, 851 cm−1.
- tri-tert-butyl 3-(tert-butyl)-5-(4-nitrophenyl)-1H-1,2,4-triazole-1,2,3(3H)-tricarboxylate (3lc), White solid, 7.92 mg, 0.014 mmol, 29% yield (0.050 mmol scale reaction). m.p. 68–70 °C; 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.27–8.31 (m, 2H), 7.91–7.95 (m, 2H), 1.56 (s, 9H), 1.40 (s, 9H), 1.30 (s, 9H), 1.19 (s, 9H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 164.8, 155.9, 154.6, 150.7, 149.0, 136.4, 130.0, 123.0, 98.7, 84.3, 82.8, 82.6, 39.1, 28.1, 27.76, 27.62, 25.2; HRMS (ESI+ in MeCN) calcd. For C27H41O8N4+ (M + H) 549.2919 found 549.2920; IR (KBr) ν 2979, 1758, 1528, 1370, 1348, 1255, 1149, 850 cm−1.
3.2.3. General Procedure for Scheme 5 (for the Synthesis of 5ac)
- di-tert-butyl (Z)-1-(((2-(tert-butoxy)-2-oxo-1-phenylethylidene)amino)(4-nitrophenyl)methyl)hydrazine-1,2-dicarboxylate (5ac), White solid, 30.3 mg, 0.053 mmol, 53% yield (0.10 mmol scale reaction). m.p. 85–87 °C: 1H-NMR (400 MHz, CHLOROFORM-D) δ 8.15 (d, J = 8.8 Hz, 2H), 7.84–7.86 (m, 2H), 7.64 (d, J = 8.5 Hz, 2H), 7.49–7.53 (m, 1H), 7.42–7.46 (m, 2H), 6.88 (br, 1H), 6.50 (br, 1H), 1.48 (s, 9H), 1.46 (s, 9H), 1.31 (s, 9H); 13C-NMR (101 MHz, CHLOROFORM-D) δ 163.4, 162.2, 154.4, 147.8, 145.4, 133.9, 131.8, 128.9, 128.6, 127.9, 123.0, 84.9, 82.4, 80.9, 28.2, 28.08, 28.01 (2 peaks are overlapped with the other peaks); HRMS (ESI+ in MeCN) calcd. for C29H39O8N4+ (M + H) 571.2762 found 571.2761; IR (KBr) ν 2979, 1727, 1525, 1368, 1346, 1259, 1153, 854 cm−1.
3.2.4. General Procedure for Scheme 5 (for the Synthesis of 3ac)
3.2.5. General Procedure for Scheme 6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Murphy, M.; Bernard, E.M.; Ishimaru, T.; Armstrong, D. Activity of voriconazole (UK-109496) against clinical isolates of Aspergillus species and its effectiveness in an experimental model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 1997, 41, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Moosa, M.-Y.S.; Sobel, J.D.; Elhalis, H.; Du, W.; Akins, R.A. Fungicidal Activity of Fluconazole against Candida Albicans in a Synthetic Vagina-Simulative Medium. Antimicrob. Agents Chemother. 2004, 48, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, A.; Azzouni, S.; Plais, R.; Gaucher, A.; Efrit, M.L.; Prim, D. Recent advances in the chemistry of 1,2,4-triazoles: Synthesis, reactivity and biological activities. Tetrahedron Lett. 2021, 86, 153518. [Google Scholar] [CrossRef]
- Imberg, L.; Platte, S.; Erbacher, C.; Daniliuc, C.G.; Kalinina, S.A.; Dorner, W.; Poso, A.; Karst, U.; Kalinin, D.V. Amide-functionalized 1,2,4-Triazol-5-amines as Covalent Inhibitors of Blood Coagulation Factor XIIa and Thrombin. ACS Pharmacol. Transl. Sci. 2022, 5, 1318–1347. [Google Scholar] [CrossRef] [PubMed]
- Riederer, S.K.U.; Bechlars, B.; Herrmann, W.A.; Kühn, F.E. Chiral N-heterocyclic biscarbenes based on 1,2,4-triazole as ligands for metal-catalyzed asymmetric synthesis. Dalton Trans. 2011, 40, 41–43. [Google Scholar] [CrossRef]
- Yang, X.; Birman, V.B. Acyl transfer catalysis with 1,2,4-triazole anion. Org. Lett. 2009, 11, 1499–1502. [Google Scholar] [CrossRef]
- Kerr, M.S.; Rovis, T. Enantioselective Synthesis of Quaternary Stereocenters via a Catalytic Asymmetric Stetter Reaction. J. Am. Chem. Soc. 2004, 126, 8876–8877. [Google Scholar] [CrossRef]
- Huang, H.; Guo, W.; Wu, W.; Li, C.-J.; Jiang, H. Copper-Catalyzed Oxidative C(sp3)-H Functionalization for Facile Synthesis of 1,2,4-Triazoles and 1,3,5-Triazines from Amidines. Org. Lett. 2015, 17, 2894–2897. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Zheng, L.; Liu, Z.-Q. Recent Advances in the Synthesis of Nitrogen Heterocycles Using Arenediazonium Salts as Nitrogen Sources. Adv. Synth. Catal. 2020, 362, 4876–4895. [Google Scholar] [CrossRef]
- Liu, J.Q.; Shen, X.Y.; Wang, Y.H.; Wang, X.S.; Bi, X.H. [3 + 2] Cycloaddition of Isocyanides with Aryl Diazonium Salts: Catalyst-Dependent Regioselective Synthesis of 1,3- and 1,5-Disubstituted 1,2,4-Triazoles. Org. Lett. 2018, 20, 6930–6933. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Z.; Chen, N.; Chen, Z.; Zhang, F. Intramolecular electrochemical dehydrogenative N–N bond formation for the synthesis of 1,2,4-triazolo [1,5-a]pyridines. Green Chem. 2019, 21, 4035–4039. [Google Scholar] [CrossRef]
- Ueda, S.; Nagasawa, H. Facile Synthesis of 1,2,4-Triazoles via a Copper-Catalyzed Tandem Addition–Oxidative Cyclization. J. Am. Chem. Soc. 2009, 131, 15080–15081. [Google Scholar] [CrossRef]
- Shang, E.; Zhang, J.; Bai, J.; Wang, Z.; Li, X.; Zhu, B.; Lei, X. Syntheses of [1,2,4]Triazolo [1,5-a]benzazoles Enabled by the Transition-Metal-Free Oxidative N–N Bond Formation. Chem. Commun. 2016, 52, 7028–7031. [Google Scholar] [CrossRef]
- Eliseeva, A.I.; Nesterenko, O.O.; Slepukhin, P.A.; Benassi, E.; Belskaya, N.P. Synthesis and Fluorescent Behaviour of 2-Aryl-4,5-dihydro-1H-1,2,4-triazoles. J. Org. Chem. 2017, 82, 86–100. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, F.-G.; Ma, J.-A. Cu-Catalysed three-component reaction of aryldiazonium salts with fluorinated diazo reagents and nitriles: Access to difluoro- and trifluoromethylated N1-aryl-1,2,4-triazoles. Adv. Synth. Catal. 2020, 362, 4432–4437. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Takeda, N.; Yasui, M.; Okazaki, M.; Suzuki, S.; Ueda, M. Synthesis of multi-substituted 1,2,4-triazoles utilising the ambiphilic reactivity of hydrazones. Chem. Commun. 2021, 57, 12187–12190. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Jiang, W.; Lu, H.; Liu, J.; Lin, Y.; Cao, H. Cu(II)-catalyzed C–H amidation/cyclization of azomethine imines with dioxazolones via acyl nitrenes: A direct access to diverse 1,2,4-triazole derivatives. Org. Lett. 2022, 24, 613–618. [Google Scholar] [CrossRef]
- Hirayama, T.; Watanabe, M.; Akazawa, C.; Ishigami, M.; Fujikawa, F.; Kasahara, T.; Otsuka, M.; Nishida, N.; Mizuno, D. Anti-tumor Activities of Some Heterocyclic and Nitrogen-containing Compounds. Yakugaku Zazzhi 1980, 100, 1225–1234. [Google Scholar] [CrossRef]
- Saleem, R.S.Z.; Tepe, J.J. Synthesis of 1,2,4-Triazolines and Triazoles Utilizing Oxazolones. J. Org. Chem. 2010, 75, 4330–4332. [Google Scholar] [CrossRef]
- Ibata, T.; Suga, H.; Isogami, Y.; Tamura, H.; Shi, X. Abnormal Diels–Alder Reaction of Oxazoles with 4-Phenyl-3H-1,2,4-triazole-3,5(4H)-dione and Diethyl Azodicarboxylate, and X-Ray Crystal Structure of an Adduct. Bull. Chem. Soc. Jpn. 1992, 65, 2998–3007. [Google Scholar] [CrossRef]
- Monge, D.; Jensen, K.L.; Marín, I.; Jørgensen, K.A. Synthesis of 1,2,4-Triazolines: Base-Catalyzed Hydrazination/Cyclization Cascade of α-Isocyano Esters and Amides. Org. Lett. 2011, 13, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, X.-H.; He, P.; Lin, L.-L.; Feng, X.-M. Enantioselective synthesis of 1,2,4-triazolines by chiral iron(ii)-complex catalyzed cyclization of α-isocyano esters and azodicarboxylates. Chem. Commun. 2013, 49, 2572–2574. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-X.; Bi, H.-L.; Zhou, H.; Yang, H.; Shi, M. Cinchona Alkaloid Squaramide Catalyzed Enantioselective Hydrazination/Cyclization Cascade Reaction of α-Isocyanoacetates and Azodicarboxylates. J. Org. Chem. 2013, 78, 9377–9382. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Chen, J.; Tu, M.; Piotrowski, D.W.; Huang, Y. Enantioselective Synthesis of 1,2,4-Triazolines Catalyzed by a Cinchona Alkaloid-derived Organocatalyst. Chem. Commun. 2013, 49, 1098–11100. [Google Scholar] [CrossRef]
- Ma, B.-W.; Luo, W.-W.; Lin, L.-L.; Liu, X.-H.; Feng, X.-M. A chiral cobalt(ii) complex catalyzed asymmetric formal [3 + 2] cycloaddition for the synthesis of 1,2,4-triazoline. Chem. Commun. 2017, 53, 4077–4079. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Y.; Wang, K.; Man, Y.; Xiang, Y.; Li, N.; Tang, B. Visible light-induced cyclization reactions for the synthesis of 1,2,4-triazolines and 1,2,4-triazoles. Chem. Commun. 2017, 53, 9644–9647. [Google Scholar] [CrossRef]
- Hashimoto, T.; Maruoka, K. Recent Development and Application of Chiral Phase-Transfer Catalysts. Chem. Rev. 2007, 107, 5656–5682. [Google Scholar] [CrossRef]
- Lasch, R.; Heinrich, M.R. Cycloaddition reactions of glycine imine anions to phenylazocarboxylic esters—A new access to 1,3,5-trisubstituted 1,2,4-triazoles. Tetrahedron 2015, 71, 4282–4295. [Google Scholar] [CrossRef]
- Eftekhari-Sis, B.; Zirak, M. α-Imino Esters in Organic Synthesis: Recent Advances. Chem. Rev. 2017, 117, 8326–8419. [Google Scholar] [CrossRef]
- Mazuela, J.; Antonsson, T.; Johansson, M.J.; Knerr, L.; Marsden, S.P. Direct Synthesis of N-Alkyl Arylglycines by Organocatalytic Asymmetric Transfer Hydrogenation of N-Alkyl Aryl Imino Esters. Org. Lett. 2017, 19, 5541–5544. [Google Scholar] [CrossRef]
- Chen, J.; Li, F.; Wang, F.; Hu, Y.; Zhang, Z.; Zhao, M.; Zhang, W. Pd(OAc)2-Catalyzed Asymmetric Hydrogenation of α-Iminoesters. Org. Lett. 2019, 21, 9060–9065. [Google Scholar] [CrossRef]
- Hu, X.H.; Hu, X.P. Ir-Catalyzed Asymmetric Hydrogenation of α-Imino Esters with Chiral Ferrocenylphosphine-Phosphoramidite Ligands. Adv. Synth. Catal. 2019, 361, 5063–5068. [Google Scholar] [CrossRef]
- Kim, J.; Shin, M.; Cho, S.H. Copper-catalyzed diastereoselective and enantioselective addition of 1, 1-diborylalkanes to cyclic ketimines and α-imino esters. ACS Catal. 2019, 9, 8503–8508. [Google Scholar] [CrossRef]
- Liu, D.; Li, B.; Chen, J.; Gridnev, I.D.; Yan, D.; Zhang, W. Ni-catalyzed asymmetric hydrogenation of N-aryl imino esters for the efficient synthesis of chiral α-aryl glycines. Nat. Commun. 2020, 11, 5935. [Google Scholar] [CrossRef]
- Cabré, A.; Verdaguer, X.; Riera, A. Recent Advances in the Enantioselective Synthesis of Chiral Amines via Transition Metal-Catalyzed Asymmetric Hydrogenation. Chem. Rev. 2022, 122, 269–339. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, L.; Li, Z.; Deng, L. Catalytic asymmetric umpolung reactions of imines. Nature 2015, 523, 445–450. [Google Scholar] [CrossRef]
- Mizota, I.; Shimizu, M. Umpolung Reactions of α-Imino Esters: Useful Methods for the Preparation of α-Amino Acid Frameworks. Chem. Rec. 2016, 16, 688–702. [Google Scholar] [CrossRef]
- Chen, P.; Yue, Z.; Zhang, J.; Lv, X.; Wang, L.; Zhang, J. Phosphine-Catalyzed Asymmetric Umpolung Addition of Trifluoromethyl Ketimines to Morita–Baylis–Hillman Carbonates. Angew. Chem. Int. Ed. 2016, 55, 13316–13320. [Google Scholar] [CrossRef]
- Su, Y.-L.; Li, Y.-H.; Chen, Y.-G.; Han, Z.-Y. Ir/PTC Cooperatively Catalyzed Asymmetric Umpolung Allylation of α-Imino Ester Enabled Synthesis of α-Quaternary Amino Acid Derivatives Bearing Two Vicinal Stereocenters. Chem. Commun. 2017, 53, 1985–1988. [Google Scholar] [CrossRef]
- Feng, B.; Lu, L.-Q.; Chen, J.-R.; Feng, G.; He, B.-Q.; Lu, B.; Xiao, W.-J. Umpolung of Imines Enables Catalytic Asymmetric Regio-reversed [3 + 2] Cycloadditions of Iminoesters with Nitroolefins. Angew. Chem. Int. Ed. 2018, 57, 5888–5892. [Google Scholar] [CrossRef]
- Yoshida, Y.; Mino, T.; Sakamoto, M. Organocatalytic Highly Regio- and Enantioselective Umpolung Michael Addition Reaction of α-Imino Esters. Chem. Eur. J. 2017, 23, 12749–12753. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Moriya, Y.; Mino, T.; Sakamoto, M. Regio- and Enantioselective Synthesis of α-Amino-δ-Ketoesters Through Catalytic Umpolung Reaction of α-Iminoesters with Enones. Adv. Synth. Catal. 2018, 360, 4142–4146. [Google Scholar] [CrossRef]
- Yoshida, Y.; Hiroshige, T.; Omori, K.; Mino, T.; Sakamoto, M. Chemo- and Regioselective Asymmetric Synthesis of Cyclic Enamides through the Catalytic Umpolung Organocascade Reaction of α-Imino Amides. J. Org. Chem. 2019, 84, 7362–7371. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Omori, K.; Hiroshige, T.; Mino, T.; Sakamoto, M. Chemoselective Catalytic Asymmetric Synthesis of Functionalized Aminals Through the Umpolung Organocascade Reaction of α-Imino Amides. Chem.-Asian J. 2019, 14, 2737–2743. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Kukita, M.; Omori, K.; Mino, T.; Sakamoto, M. Iminophosphorane-mediated regioselective umpolung alkylation reaction of α-iminoesters, Org. Biomol. Chem. 2021, 19, 4551–4564. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, Y.; Deng, L. Cinchonium Betaines as Efficient Catalysts for Asymmetric Proton Transfer Catalysis: The Development of a Practical Enantioselective Isomerization of Trifluoromethyl Imines. J. Am. Chem. Soc. 2016, 138, 12297–12302. [Google Scholar] [CrossRef]
- Wang, G.; Piva de Silva, G.; Wiebe, N.E.; Fehr, G.M.; Davis, R.L. Development of a Metal–Free Amine Oxidation Method Utilizing DEAD Chemistry. RSC Adv. 2017, 7, 48848–48852. [Google Scholar] [CrossRef]

 | |||||
|---|---|---|---|---|---|
| Entry | Solvent | X | Temp. (°C) | Time (h) | Yield (%) b |
| 1 | toluene | 50 | −40 | 18 | 9 |
| 2 | Et2O | 50 | −40 | 18 | 4 |
| 3 | CH2Cl2 | 50 | −40 | 18 | 43 |
| 4 | tetrahydrofuran | 50 | −40 | 18 | 28 |
| 5 | MeOH | 50 | −40 | 18 | 0 |
| 6 | CH2Cl2 | 100 | −40 | 18 | 43 |
| 7 | CH2Cl2 | 150 | −40 | 18 | 42 |
| 8 | CH2Cl2 | 50 | −20 | 18 | 53 |
| 9 | CH2Cl2 | 50 | 0 | 18 | 61 |
| 10 | CH2Cl2 | 50 | r.t | 18 | 51 |
| 11 | CH2Cl2 | 50 | 0 | 1 | 64 |
| 12 | CH2Cl2 | 50 | 0 | 53 | 64 |
| 13 c | CH2Cl2 | 50 | 0 | 1 | 88 (72 d) |
| 14 c,e | CH2Cl2 | 50 | 0 | 1 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, Y.; Ida, H.; Mino, T.; Sakamoto, M. Formal [3 + 2] Cycloaddition of α-Imino Esters with Azo Compounds: Facile Construction of Pentasubstituted 1,2,4-Triazoline Skeletons. Molecules 2023, 28, 4339. https://doi.org/10.3390/molecules28114339
Yoshida Y, Ida H, Mino T, Sakamoto M. Formal [3 + 2] Cycloaddition of α-Imino Esters with Azo Compounds: Facile Construction of Pentasubstituted 1,2,4-Triazoline Skeletons. Molecules. 2023; 28(11):4339. https://doi.org/10.3390/molecules28114339
Chicago/Turabian StyleYoshida, Yasushi, Hidetoshi Ida, Takashi Mino, and Masami Sakamoto. 2023. "Formal [3 + 2] Cycloaddition of α-Imino Esters with Azo Compounds: Facile Construction of Pentasubstituted 1,2,4-Triazoline Skeletons" Molecules 28, no. 11: 4339. https://doi.org/10.3390/molecules28114339
APA StyleYoshida, Y., Ida, H., Mino, T., & Sakamoto, M. (2023). Formal [3 + 2] Cycloaddition of α-Imino Esters with Azo Compounds: Facile Construction of Pentasubstituted 1,2,4-Triazoline Skeletons. Molecules, 28(11), 4339. https://doi.org/10.3390/molecules28114339