Achieving Boron–Carbon–Nitrogen Heterostructures by Collision Fusion of Carbon Nanotubes and Boron Nitride Nanotubes
Abstract
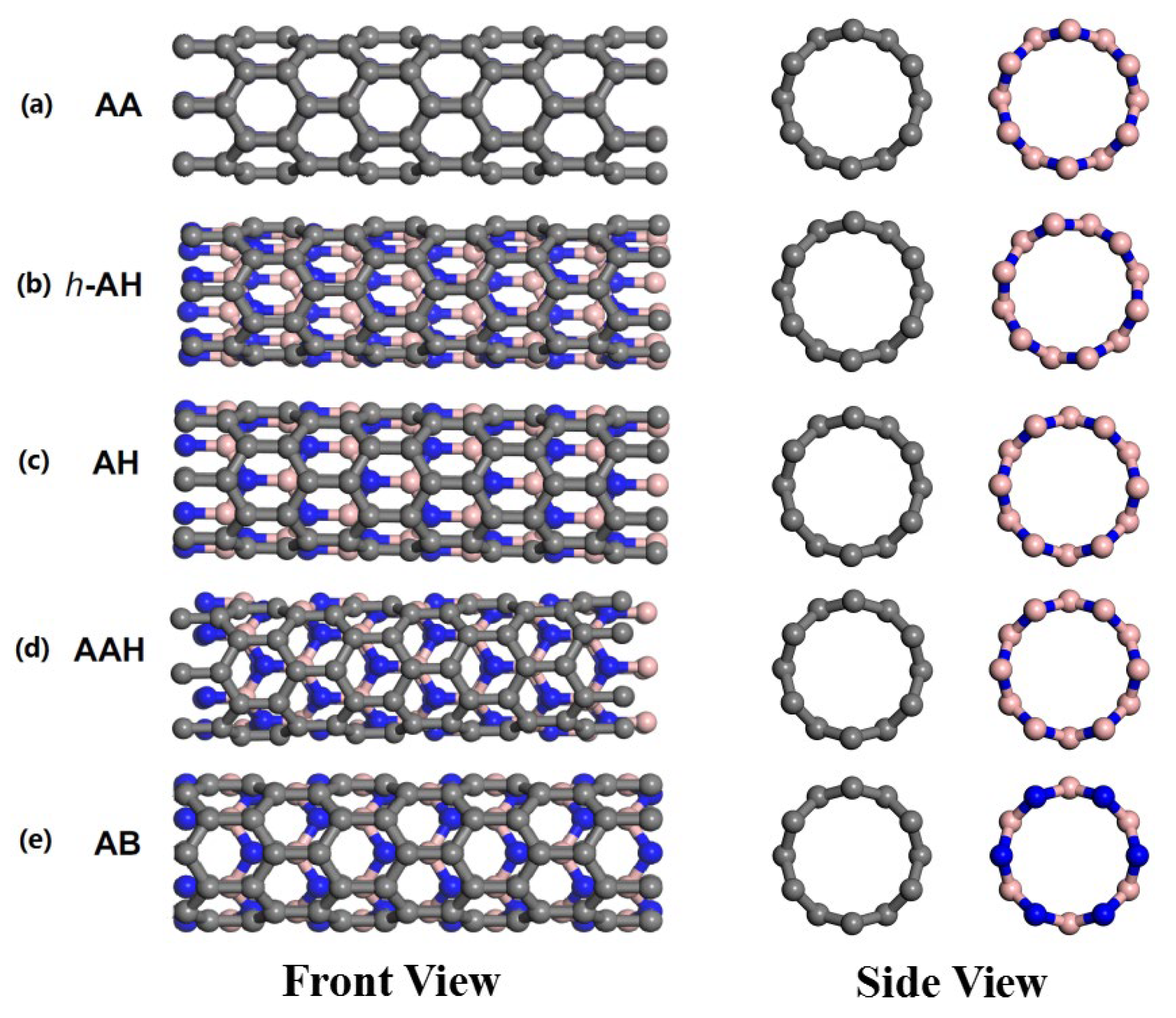
1. Introduction
2. Collision Results
3. Discussion
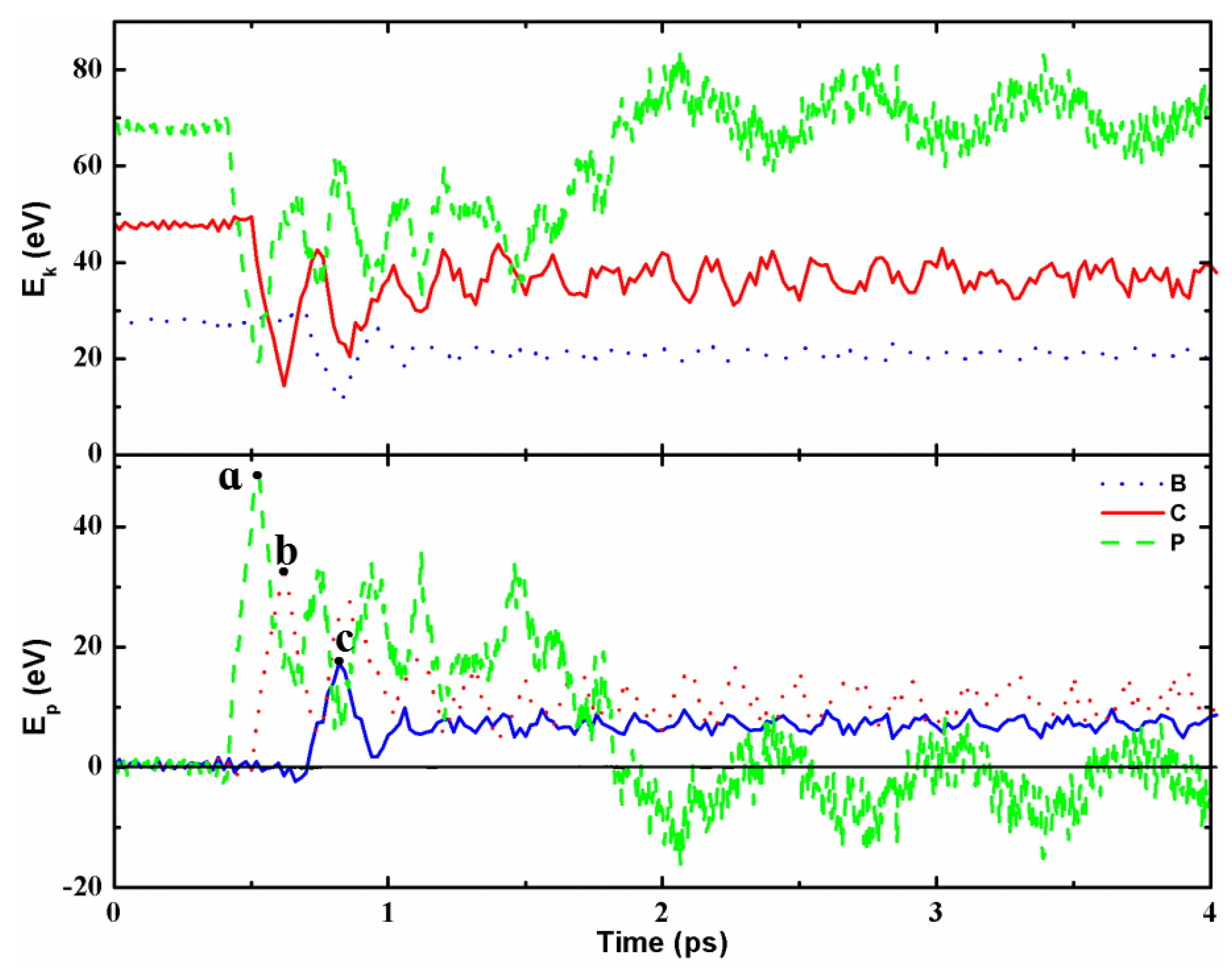
3.1. Collision Energetics
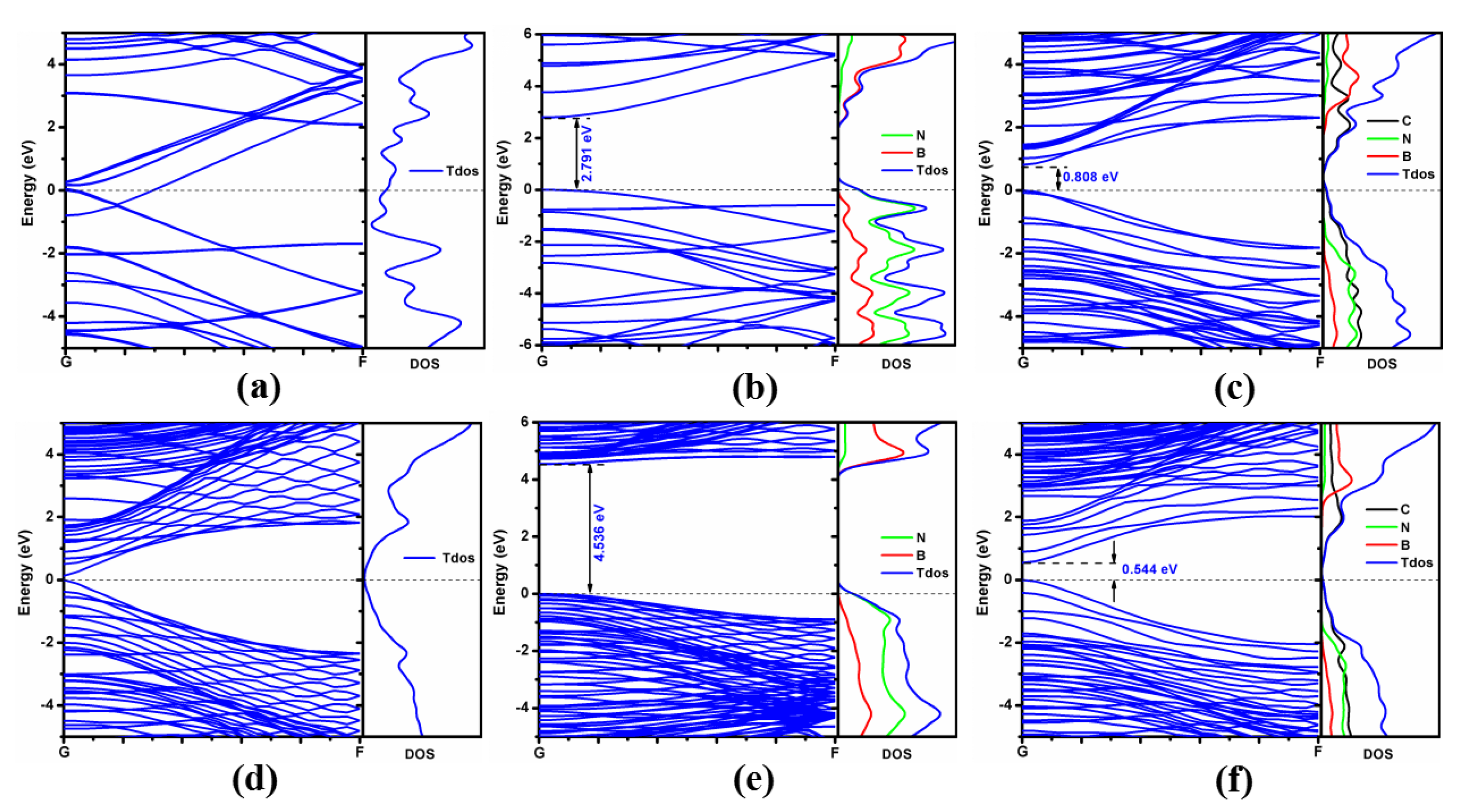
3.2. Electronic Structures
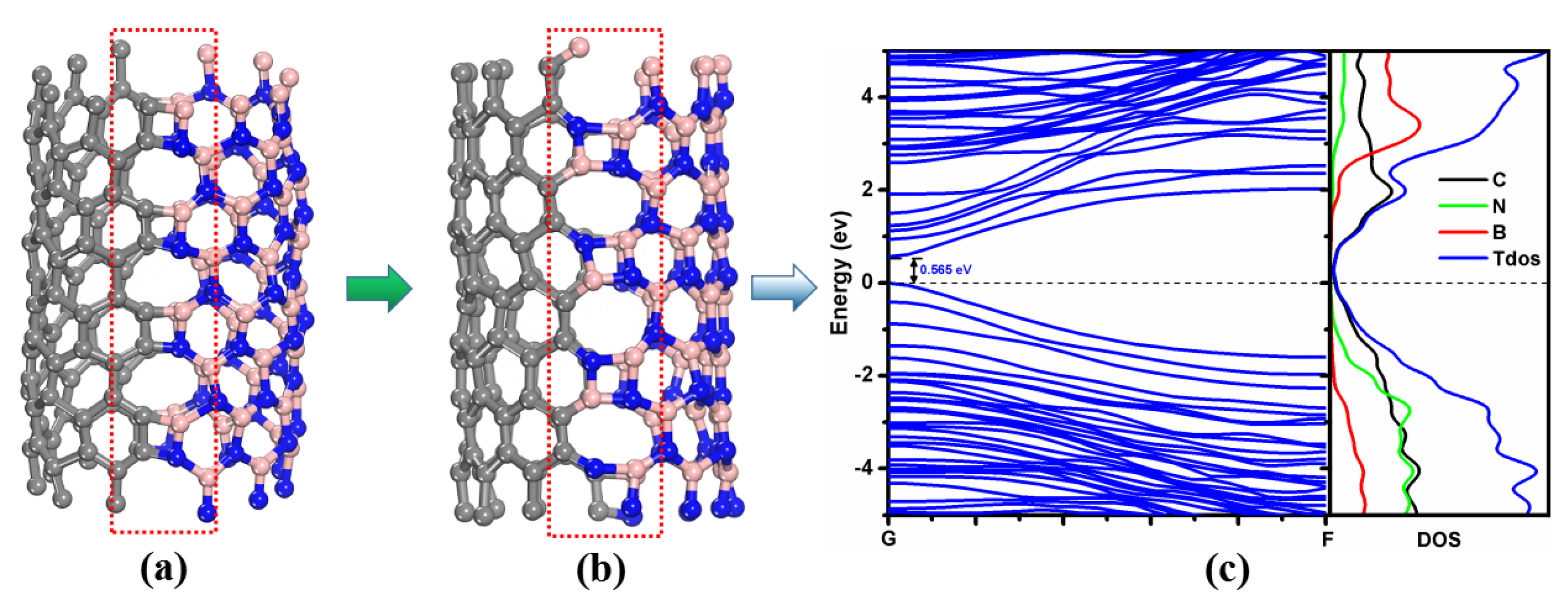
3.3. Defects
4. Computational Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.; Wang, Y.J.; Xu, Y.F.; Sivakumar, P.K.; Pasco, K.; Filippozzi, U.; Parkin, S.S.P.; Zeng, Y.J.; McQueen, T.; Ali, M.N. The field-free Josephson diode in a van der Waals heterostructure. Nature 2022, 604, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.J.; Ouyang, Y.L.; Jiang, P.F.; Yu, C.Q.; He, J.; Chen, J. The Impact of Interlayer Rotation on Thermal Transport Across Graphene/Hexagonal Boron Nitride van der Waals Heterostructure. Nano Lett. 2021, 21, 2634–2641. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.W.; Chen, Q.G.; Xia, X.H.; Chen, M.H. Emerging of Heterostructure Materials in Energy Storage: A Review. Adv. Mater. 2021, 33, 2100855. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Z.; Wang, Z.H.; Lim, Y.V.; Wang, Y.; Li, Y.; Zhang, D.H.; Yang, H.Y. Recent Advances in Heterostructure Engineering for Lithium–Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2003689. [Google Scholar] [CrossRef]
- Tang, F.H.; He, D.X.; Jiang, H.; Wang, R.S.; Li, Z.L.; Xue, W.D.; Zhao, R. The coplanar graphene oxide/graphite heterostructure-based electrodes for electrochemical supercapacitors. Carbon 2022, 197, 163–170. [Google Scholar] [CrossRef]
- Yuan, K.; Hao, P.J.; Zhou, Y.; Hu, X.C.; Zhang, J.B.; Zhong, S.W. A two-dimensional MXene/BN van der Waals heterostructure as an anode material for lithium-ion batteries. Phys. Chem. Chem. Phys. 2022, 24, 13713–13719. [Google Scholar] [CrossRef]
- Liu, C.; Lu, Y.H.; Yu, X.T.; Shen, R.J.; Wu, Z.M.; Yang, Z.S.; Yan, Y.F.; Feng, L.X.; Lin, S.S. Hot carriers assisted mixed-dimensional graphene/MoS2/p-GaN light emitting diode. Carbon 2022, 197, 192–199. [Google Scholar] [CrossRef]
- Wang, B.A.; Yuan, H.K.; Yang, T.; Wang, P.; Xu, X.H.; Chang, J.L.; Kuang, M.Q.; Chen, H. A two-dimensional PtS2/BN heterostructure as an S-scheme photocatalyst with enhanced activity for overall water splitting. Phys. Chem. Chem. Phys. 2022, 24, 26908–26914. [Google Scholar] [CrossRef]
- Song, J.; Jiang, M.J.; Wan, C.; Li, H.J.; Zhang, Q.; Chen, Y.H.; Wu, X.H.; Yin, X.M.; Liu, J.F. Defective graphene/SiGe heterostructures as anodes of Li-ion batteries: A first-principles calculation study. Phys. Chem. Chem. Phys. 2022, 25, 617–624. [Google Scholar] [CrossRef]
- Sun, X.X.; Zhu, C.G.; Yi, J.L.; Xiang, L.; Ma, C.; Liu, H.W.; Zheng, B.Y.; Liu, Y.; You, W.X.; Zhang, W.J.; et al. Reconfigurable logic-in-memory architectures based on a two-dimensional van der Waals heterostructure device. Nat. Electron. 2022, 5, 752–760. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhuang, Y.C.; Sun, Q.F.; He, L. Coexistence of electron whispering-gallery modes and atomic collapse states in graphene/WSe2 heterostructure quantum dots. Nat. Commun. 2022, 13, 1597. [Google Scholar] [CrossRef] [PubMed]
- Chepkasov, I.V.; Smet, J.H.; Krasheninnikov, A.V. Single- and Multilayers of Alkali Metal Atoms inside Graphene/MoS2 Heterostructures: A Systematic First-Principles Study. J. Phys. Chem. C 2022, 126, 15558–15564. [Google Scholar] [CrossRef]
- Yang, Z.H.; Wu, M.S.; Luo, W.W.; Liu, G.; Xu, B. Structural, Electronic, and Transport Properties of Phosphorene–Graphene Lateral Heterostructure Anodes: Insights from First-Principles Calculations. J. Phys. Chem. C 2022, 126, 8928–8937. [Google Scholar] [CrossRef]
- Kuang, H.F.; Zhang, H.Q.; Liu, X.H.; Chen, Y.D.; Zhang, W.G.; Chen, H.; Ling, Q.D. Microwave-assisted synthesis of NiCo-LDH/graphene nanoscrolls composite for supercapacitor. Carbon 2022, 190, 57–67. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.M.; Lin, Y.P.; Wang, X.H.; Li, J.M.; Li, Y.; Gao, L.J.; Zhang, L.B.; Chao, D.L.; Xiao, X.; et al. Electronic Modulation of Non-van der Waals 2D Electrocatalysts for Efficient Energy Conversion. Adv. Mater. 2021, 33, 2008422. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Gong, J.; Jiang, X.W.; Yang, S.Y. Influence of the interface structure and strain on the rectification performance of lateral MoS2/graphene heterostructure devices. Phys. Chem. Chem. Phys. 2022, 24, 2265–2274. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Han, L.Y.; Xiao, C.X.; Song, Q.; Yin, X.M.; Li, W.; Li, K.Z.; Li, Y.Y. Nano-interface effect of graphene on carbon nanotube reinforced carbon/carbon composites. Carbon 2022, 190, 422–429. [Google Scholar] [CrossRef]
- Zhang, S.; Pang, J.B.; Li, Y.F.; Yang, F.; Gemming, T.; Wang, K.; Wang, X.; Peng, S.G.; Liu, X.Y.; Chang, B.; et al. Emerging Internet of Things driven carbon nanotubes-based devices. Nano Res. 2022, 15, 4613–4637. [Google Scholar] [CrossRef]
- Wang, R.R.; Wu, R.B.; Yan, X.X.; Liu, D.; Guo, P.F.; Li, W.; Pan, H.G. Implanting Single Zn Atoms Coupled with Metallic Co Nanoparticles into Porous Carbon Nanosheets Grafted with Carbon Nanotubes for High-Performance Lithium-Sulfur Batteries. Adv. Funct. Mater. 2022, 32, 2200424. [Google Scholar] [CrossRef]
- Zhu, R.F.; Wang, D.; Liu, Y.M.; Liu, M.M.; Fu, S.H. Bifunctional superwetting carbon nanotubes/cellulose composite membrane for solar desalination and oily seawater purification. Chem. Eng. J. 2022, 433, 133510. [Google Scholar] [CrossRef]
- Poggioli, A.R.; Limmer, D.T. Distinct Chemistries Explain Decoupling of Slip and Wettability in Atomically Smooth Aqueous Interfaces. J. Phys. Chem. Lett. 2021, 12, 9060–9067. [Google Scholar] [CrossRef] [PubMed]
- Stern, H.L.; Gu, Q.S.; Jarman, J.; Barker, S.E.; Mendelson, N.; Chugh, D.; Schott, S.; Tan, H.H.; Sirringhaus, H.; Aharonovich, I.; et al. Room-temperature optically detected magnetic resonance of single defects in hexagonal boron nitride. Nat. Commun. 2022, 13, 618. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.V.; Bodepudi, S.C.; Shehzad, K.; Liu, Y.; Xu, Y.; Yu, B.; Duan, X.F. 2D Heterostructures for Ubiquitous Electronics and Optoelectronics: Principles, Opportunities, and Challenges. Chem. Rev. 2022, 122, 6514–6613. [Google Scholar] [CrossRef]
- Turiansky, M.E.; Alkauskas, A.; van de Walle, C.G. Spinning up quantum defects in 2D materials. Nat. Mater. 2020, 19, 487–489. [Google Scholar] [CrossRef]
- Mirzayev, M.N. Heat transfer of hexagonal boron nitride (h-BN) compound up to 1 MeV neutron energy: Kinetics of the release of wigner energy. Radiat. Phys. Chem. 2021, 180, 109244. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, K.; Cai, Q.R.; Wang, N.Y.; Wu, L.Y.; He, Q.; Wang, H.; Zhang, Y.; Xie, Y.F.; Yao, Y.G.; et al. Advances in synthesis and applications of boron nitride nanotubes: A review. Chem. Eng. J. 2022, 431, 134118. [Google Scholar] [CrossRef]
- Qi, R.S.; Li, N.; Du, J.L.; Shi, R.C.; Huang, Y.; Yang, X.X.; Liu, L.; Xu, Z.; Dai, Q.; Yu, D.P.; et al. Four-dimensional vibrational spectroscopy for nanoscale mapping of phonon dispersion in BN nanotubes. Nat. Commun. 2021, 12, 1179. [Google Scholar] [CrossRef]
- Konabe, S. Exciton effect on shift current in single-walled boron-nitride nanotubes. Phys. Rev. B 2021, 103, 075402. [Google Scholar] [CrossRef]
- Feng, Y.; Li, H.N.; Hou, B.; Kataura, H.; Inoue, T.; Chiashi, S.; Xiang, R.; Maruyama, S. Zeolite-supported synthesis, solution dispersion, and optical characterizations of single-walled carbon nanotubes wrapped by boron nitride nanotubes. J. Appl. Phys. 2021, 129, 015101. [Google Scholar] [CrossRef]
- Jones, R.S.; Maciejewska, B.; Grobert, N. Synthesis, characterisation and applications of core–shell carbon–hexagonal boron nitride nanotubes. Nanoscale Adv. 2020, 2, 4996–5014. [Google Scholar] [CrossRef]
- Stephan, O.; Ajayan, P.M.; Colliex, C.; Redlich, P.; Lambert, J.M.; Bernier, P.; Lefin, P. Doping Graphitic and Carbon Nanotube Structures with Boron and Nitrogen. Science 1994, 266, 1683–1685. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, G.; Zhang, J.; Shao, Q.Y. Tunable electronic properties of ultra-thin boron-carbon-nitrogen heteronanotubes for various compositions. J. Mol. Model. 2014, 20, 2371–2378. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, P.; Lima, C.N.; Frota, H.O.; Ghosh, A. First-principles study of nanotubes of carbon, boron and nitrogen. Appl. Surf. Sci. 2019, 490, 242–250. [Google Scholar] [CrossRef]
- Belgacem, A.B.; Hinkov, I.; Yahia, S.B.; Brinza, O.; Farhat, S. Arc discharge boron nitrogen doping of carbon nanotubes. Mater. Today Commun. 2016, 8, 183–195. [Google Scholar] [CrossRef]
- Campbell, E.E.B.; Schyja, V.; Ehlich, R.; Hertel, I.V. Observation of molecular fusion and deep inelastic scattering in C602+ + C60 collisions. Phys. Rev. Lett. 1993, 70, 263. [Google Scholar] [CrossRef] [PubMed]
- Rohmund, F.; Campbell, E.E.B. Charge transfer collisions between C602+ and C60. Chem. Phys. Lett. 1995, 245, 237–243. [Google Scholar] [CrossRef]
- Rohmund, F.; Glotov, A.; Hansen, K.; Campbell, E.E.B. Experimental studies of fusion and fragmentation of fullerenes. J. Phys. B 1996, 29, 5143–5161. [Google Scholar] [CrossRef]
- Lee, G.D.; Wang, C.Z.; Yoon, E.; Hwang, N.M.; Ho, K.M. The role of pentagon–heptagon pair defect in carbon nanotube: The center of vacancy reconstruction. Appl. Phys. Lett. 2010, 97, 093106. [Google Scholar] [CrossRef]
- Stone, A.J.; Wales, D.J. Theoretical studies of icosahedral C60 and some related species. Chem. Phys. Lett. 1986, 128, 501–503. [Google Scholar] [CrossRef]
- Lusk, M.T.; Wu, D.T.; Carr, L.D. Graphene nanoengineering and the inverse Stone-Thrower-Wales defect. Phys. Rev. B 2010, 81, 155444. [Google Scholar] [CrossRef]
- Odom, T.W.; Huang, J.L.; Kim, P.; Lieb, C.M. Atomic structure and electronic properties of single-walled carbon nanotubes. Nature 1998, 391, 62–64. [Google Scholar] [CrossRef]
- Rubio, A.; Corkill, J.L.; Cohen, M.L. Theory of graphitic boron nitride nanotubes. Phys. Rev. B 1994, 49, 5081–5084. [Google Scholar] [CrossRef] [PubMed]
- Blasé, X.; Rubio, A.; Louie, S.G.; Cohen, M.L. Stability and Band Gap Constancy of Boron Nitride Nanotubes. Europhys. Lett. 1994, 28, 335–340. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Gweon, G.H.; Fedorov, A.V.; First, P.N.; De Heer, W.A.; Lee, D.H.; Guinea, F.; Castro Neto, A.H.; Lanzara, A. Substrate-induced bandgap opening in epitaxial graphene. Nat. Mater. 2007, 6, 770–775. [Google Scholar] [CrossRef]
- Son, Y.W.; Cohen, M.L.; Louie, S.G. Energy Gaps in Graphene Nanoribbons. Phys. Rev. Lett. 2006, 97, 216803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Guo, W.L. Energy-gap modulation of BN ribbons by transverse electric fields: First-principles calculations. Phys. Rev. B 2008, 77, 075403. [Google Scholar] [CrossRef]
- Lambin, P.; Vigneron, J.P.; Fonseca, A.; Nagy, J.B.; Lucas, A.A. Atomic structure and electronic properties of a bent carbon nanotube. Synth. Met. 1996, 77, 249–252. [Google Scholar] [CrossRef]
- Terrones, M.; Terrones, H.; Banhart, F.; Charlier, J.-C.; Ajayan, P.M. Coalescence of Single-Walled Carbon Nanotubes. Science 2000, 288, 1226–1229. [Google Scholar] [CrossRef]
- Zhang, C.; Mao, F.; Meng, X.R.; Wang, D.Q.; Zhang, F.S. Collision-induced fusion of two single-walled carbon nanotubes: A quantitative study. Chem. Phys. Lett. 2016, 657, 184–189. [Google Scholar] [CrossRef]
- Aradi, B.; Hourahine, B.; Frauenheim, T. DFTB+, a Sparse Matrix-Based Implementation of the DFTB Method. J. Phys. Chem. A 2007, 111, 5678–5684. [Google Scholar] [CrossRef] [PubMed]
- Enyashin, A.N.; Ivanovskii, A.L. Mechanical and electronic properties of a C/BN nanocable under tensile deformation. Nanotechnology 2005, 16, 1304–1310. [Google Scholar] [CrossRef]
- Enyashin, A.N.; Seifert, G.; Ivanovskii, A.L. Calculation of the Electronic and Thermal Properties of C/BN Nanotubular Heterostructures. Inorg. Mater. 2005, 41, 595–603. [Google Scholar] [CrossRef]
- Swope, W.C.; Andersen, H.C.; Berens, P.H.; Wilson, K.R. A computer simulation method for the calculation of equilibrium constants for the formation of physical clusters of molecules: Application to small water clusters. J. Chem. Phys. 1982, 76, 637–649. [Google Scholar] [CrossRef]
- Kraska, T. Molecular-dynamics simulation of argon nucleation from supersaturated vapor in the NVE ensemble. J. Chem. Phys. 2006, 124, 054507. [Google Scholar] [CrossRef] [PubMed]
- Jakowski, J.; Irle, S.; Morokuma, K. Collision-induced fusion of two C60 fullerenes: Quantum chemical molecular dynamics simulations. Phys. Rev. B 2010, 82, 125443. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter. 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]

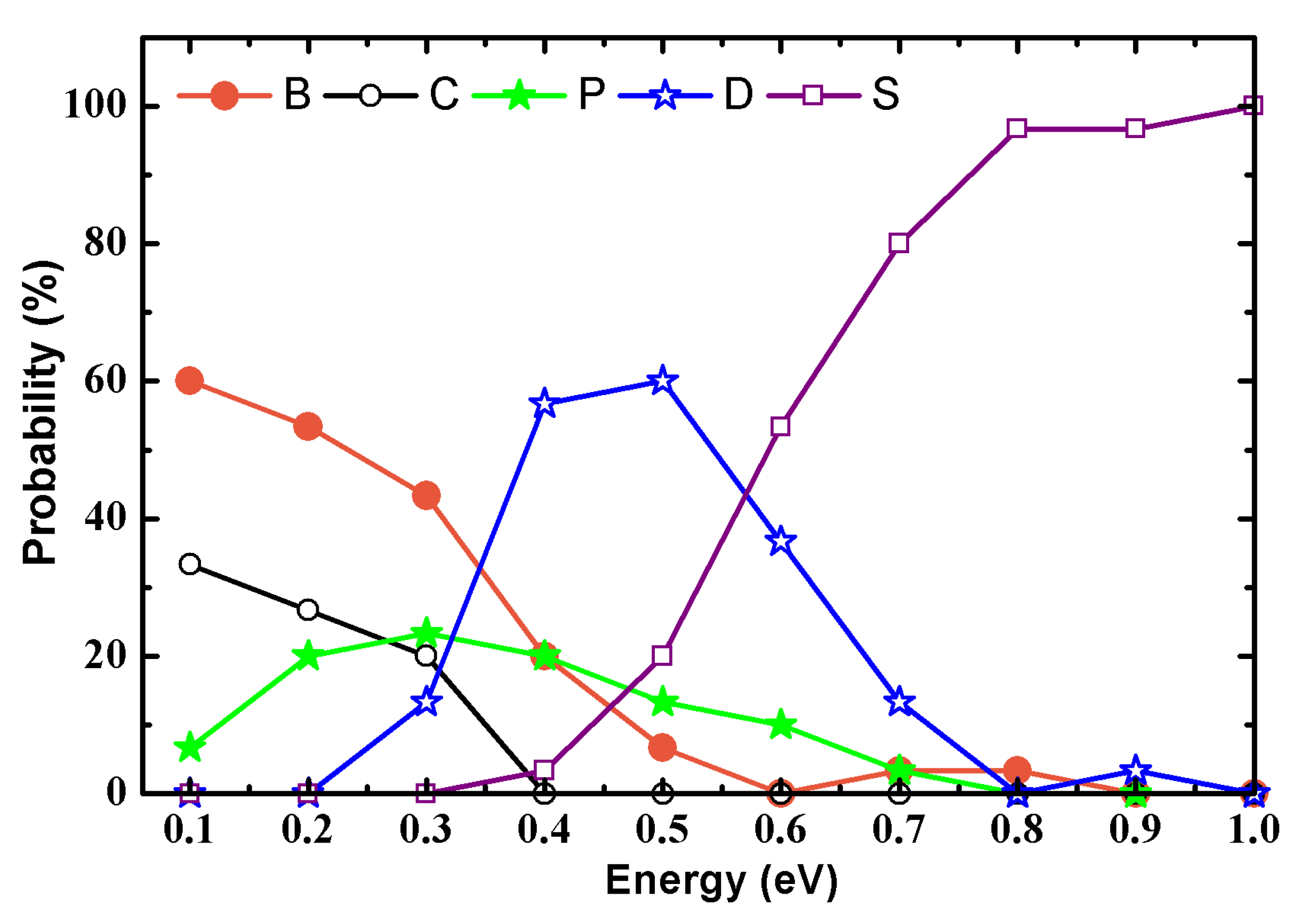

| Model | AA | h-AH | AH | AAH | AB | ||
|---|---|---|---|---|---|---|---|
| Result | |||||||
| En (eV) | |||||||
| 0.1 | B, C, P | B, C, P | B, C | B | B, C | ||
| 0.2 | B, C | B, C, P | B, P | B | B, C, P | ||
| 0.3 | B, C | B, P, D | B, P, D | B, C, P, D | B, C, P | ||
| 0.4 | B, P, D | P, D | B, D | D | B, P, D | ||
| 0.5 | D | D, S | B, P, D | B, D, S | P, D, S | ||
| 0.6 | D, S | D, S | P, D, S | D, S | P, D, S | ||
| 0.7 | S | S | B, D, S | S | P, D, S | ||
| 0.8 | S | S | S | D, S | S | ||
| 0.9 | S | S | D, S | D, S | S | ||
| 1.0 | S | S | S | S | S | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Xu, J.; Song, H.; Ren, K.; Yu, Z.G.; Zhang, Y.-W. Achieving Boron–Carbon–Nitrogen Heterostructures by Collision Fusion of Carbon Nanotubes and Boron Nitride Nanotubes. Molecules 2023, 28, 4334. https://doi.org/10.3390/molecules28114334
Zhang C, Xu J, Song H, Ren K, Yu ZG, Zhang Y-W. Achieving Boron–Carbon–Nitrogen Heterostructures by Collision Fusion of Carbon Nanotubes and Boron Nitride Nanotubes. Molecules. 2023; 28(11):4334. https://doi.org/10.3390/molecules28114334
Chicago/Turabian StyleZhang, Chao, Jiangwei Xu, Huaizhi Song, Kai Ren, Zhi Gen Yu, and Yong-Wei Zhang. 2023. "Achieving Boron–Carbon–Nitrogen Heterostructures by Collision Fusion of Carbon Nanotubes and Boron Nitride Nanotubes" Molecules 28, no. 11: 4334. https://doi.org/10.3390/molecules28114334
APA StyleZhang, C., Xu, J., Song, H., Ren, K., Yu, Z. G., & Zhang, Y.-W. (2023). Achieving Boron–Carbon–Nitrogen Heterostructures by Collision Fusion of Carbon Nanotubes and Boron Nitride Nanotubes. Molecules, 28(11), 4334. https://doi.org/10.3390/molecules28114334








