The Transport of Charged Molecules across Three Lipid Membranes Investigated with Second Harmonic Generation
Abstract
1. Introduction
2. Results and Discussion
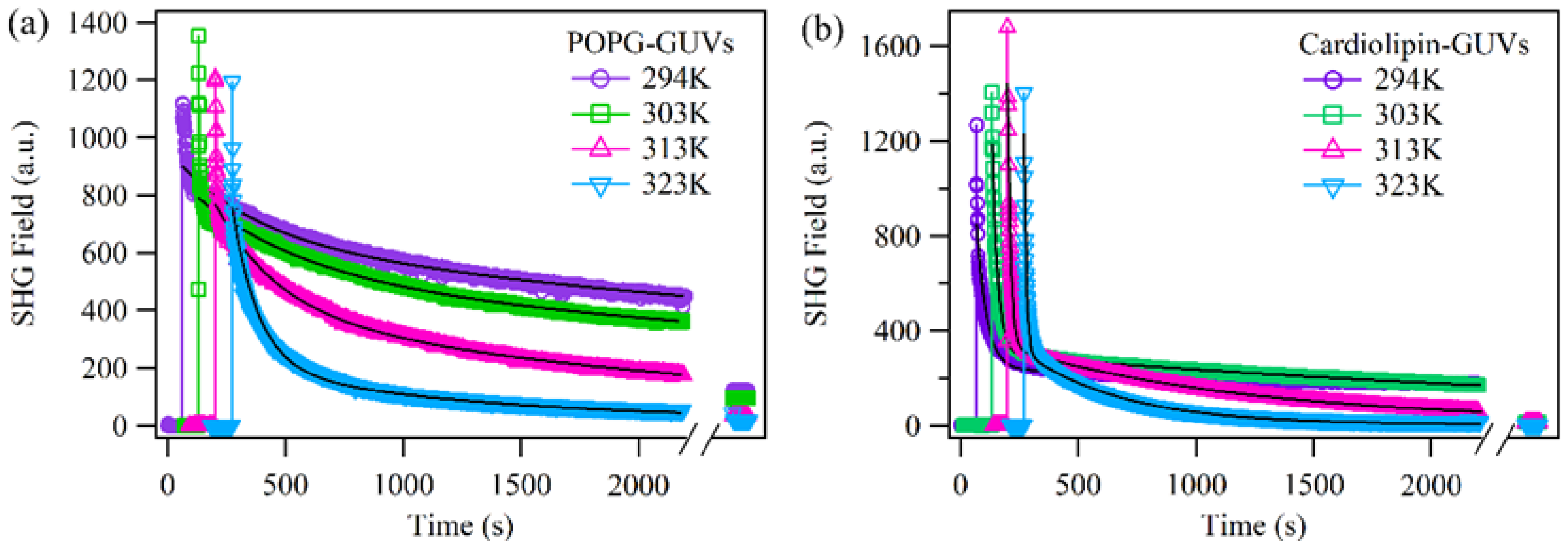
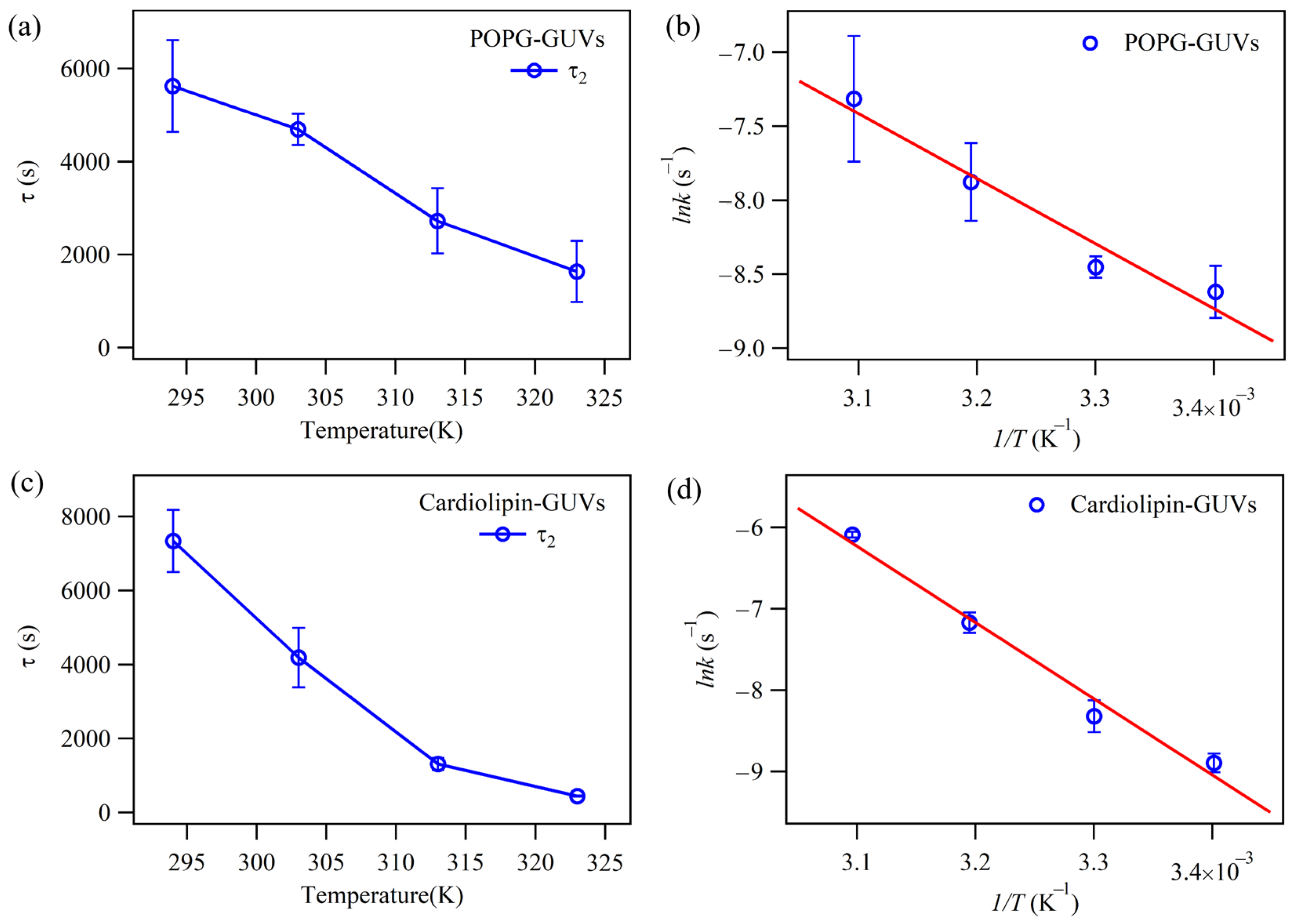
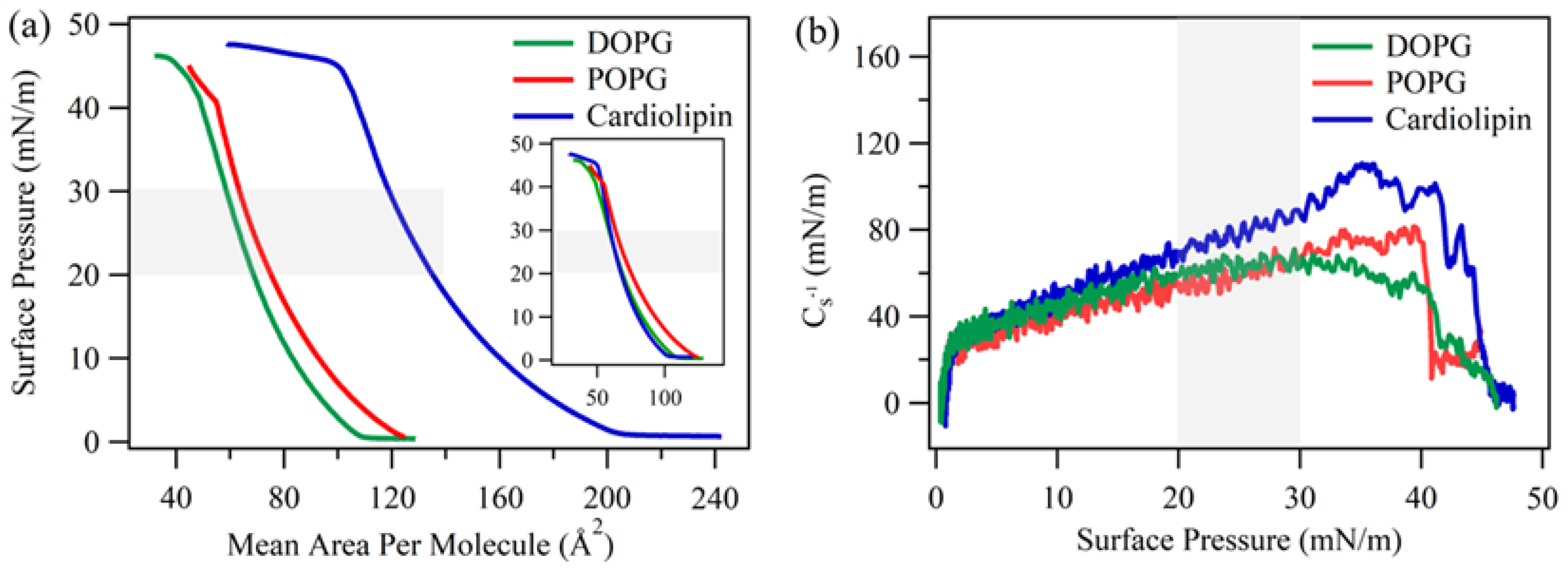
2.1. The Permeability of D289 across Membranes of Different Lipids with GUVs (Giant Unilamellar Vesicles) as a Model
2.2. The Effect of Cholesterol on the Permeability of Lipid Bilayers
2.3. The Effect of Surface Curvature on the Permeabilities of Different Lipids with SUVs as a Model
3. Experimental
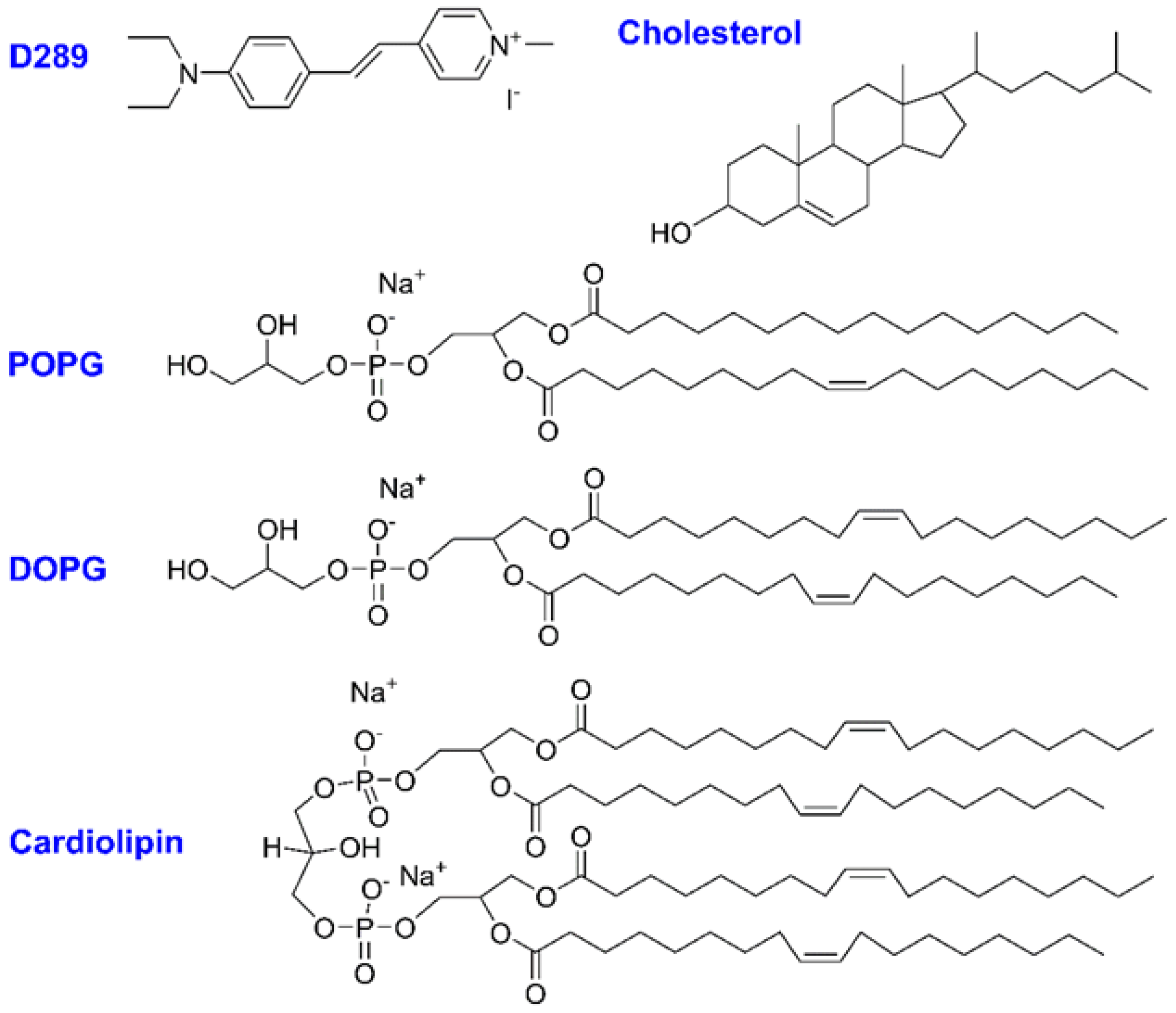
3.1. Materials
3.2. Vesicles Preparation and Characterization
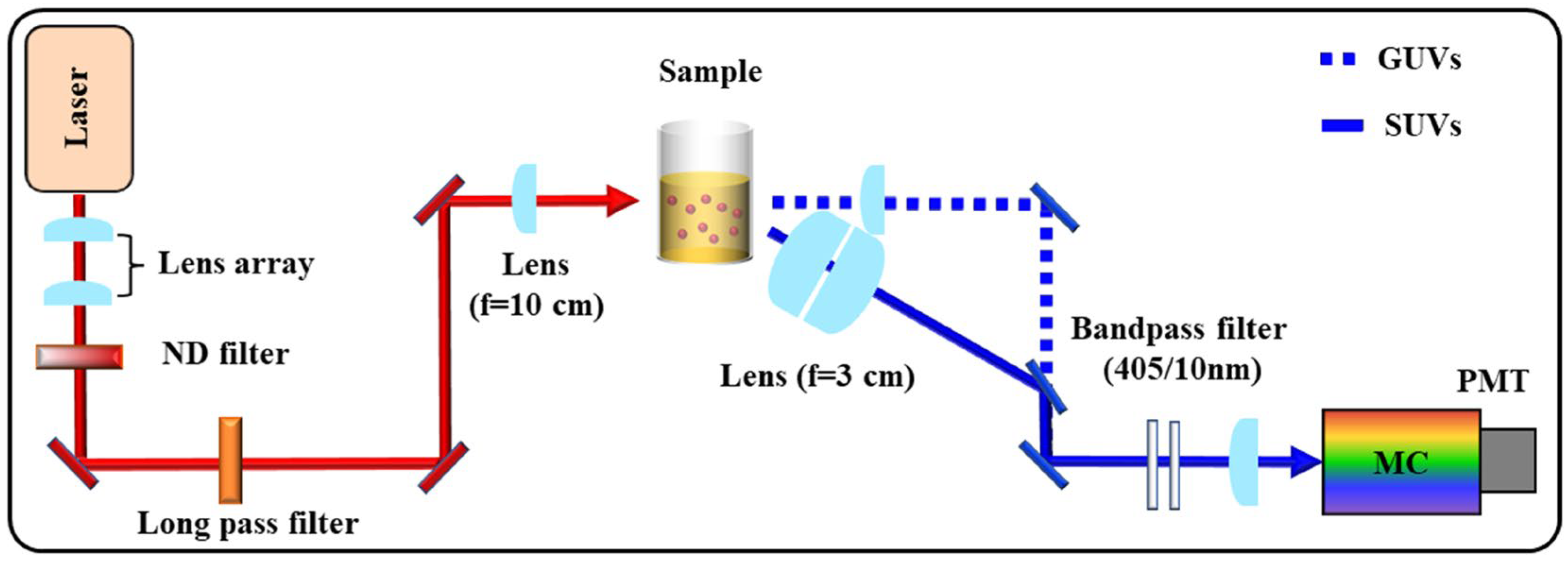
3.3. Second Harmonic Generation (SHG) Measurements
3.4. Surface Pressure-Area Isotherm of Lipid Monolayers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Burns, J.R.; Seifert, A.; Fertig, N.; Howorka, S. A biomimetic DNA-based channel for the ligand-controlled transport of charged molecular cargo across a biological membrane. Nat. Nanotechnol. 2016, 11, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Okur, H.I.; Tarun, O.B.; Roke, S. Chemistry of Lipid Membranes from Models to Living Systems: A Perspective of Hydration, Surface Potential, Curvature, Confinement and Heterogeneity. J. Am. Chem. Soc. 2019, 141, 12168–12181. [Google Scholar] [CrossRef]
- Si, W.; Li, Z.-T.; Hou, J.-L. Voltage-Driven Reversible Insertion into and Leaving from a Lipid Bilayer: Tuning Transmembrane Transport of Artificial Channels. Angew. Chem. Int. Ed. 2014, 53, 4578–4581. [Google Scholar] [CrossRef]
- Wu, T.; Wilhelm, M.J.; Ma, J.; Li, Y.; Wu, Y.; Dai, H.-L. Influence of Phase Transitions on Diffusive Molecular Transport Across Biological Membranes. Angew. Chem. Int. Ed. 2022, 61, e202205608. [Google Scholar] [CrossRef]
- Hannesschlaeger, C.; Horner, A.; Pohl, P. Intrinsic Membrane Permeability to Small Molecules. Chem. Rev. 2019, 119, 5922–5953. [Google Scholar] [CrossRef]
- Sharifian, G.M. Recent Experimental Developments in Studying Passive Membrane Transport of Drug Molecules. Mol. Pharm. 2021, 18, 2122–2141. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Hu, J.; Cochrane, W.G.; Jones, A.X.; Blackmond, D.G.; Paegel, B.M. Chiral lipid bilayers are enantioselectively permeable. Nat. Chem. 2021, 13, 786–791. [Google Scholar] [CrossRef]
- Seo, P.R.; Teksin, Z.S.; Kao, J.P.Y.; Polli, J.E. Lipid composition effect on permeability across PAMPA. Eur. J. Pharm. Sci. 2006, 29, 259–268. [Google Scholar] [CrossRef]
- Tse, C.H.; Comer, J.; Wang, Y.; Chipot, C. Link between Membrane Composition and Permeability to Drugs. J. Chem. Theory Comput. 2018, 14, 2895–2909. [Google Scholar] [CrossRef]
- Blok, M.C.; Van Der Neut-Kok, E.C.M.; Van Deenen, L.L.M.; De Gier, J. The effect of chain length and lipid phase transitions on the selective permeability properties of liposomes. Biochim. Biophys. Acta Biomembr. 1975, 406, 187–196. [Google Scholar] [CrossRef]
- Howe, A.; Sofou, S. Daptomycin-Induced Lipid Phases on Model Lipid Bilayers: Effect of Lipid Type and of Lipid Leaflet Order on Membrane Permeability. J. Phys. Chem. B 2021, 125, 5775–5785. [Google Scholar] [CrossRef]
- In’t Veld, G.; Driessen, A.J.M.; Konings, W.N. Effect of the unsaturation of phospholipid acyl chains on leucine transport of Lactococcus lactis and membrane permeability. Biochim. Biophys. Acta Biomembr. 1992, 1108, 31–39. [Google Scholar] [CrossRef]
- Baccouch, R.; Shi, Y.; Vernay, E.; Mathelié-Guinlet, M.; Taib-Maamar, N.; Villette, S.; Feuillie, C.; Rascol, E.; Nuss, P.; Lecomte, S.; et al. The impact of lipid polyunsaturation on the physical and mechanical properties of lipid membranes. Biochim. Biophys. Acta Biomembr. 2023, 1865, 184084. [Google Scholar] [CrossRef]
- Martins, P.T.; Velazquez-Campoy, A.; Vaz, W.L.C.; Cardoso, R.M.S.; Valério, J.; Moreno, M.J. Kinetics and Thermodynamics of Chlorpromazine Interaction with Lipid Bilayers: Effect of Charge and Cholesterol. J. Am. Chem. Soc. 2012, 134, 4184–4195. [Google Scholar] [CrossRef]
- Dahley, C.; Garessus, E.D.G.; Ebert, A.; Goss, K.-U. Impact of cholesterol and sphingomyelin on intrinsic membrane permeability. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183953. [Google Scholar] [CrossRef]
- Sugano, K.; Kansy, M.; Artursson, P.; Avdeef, A.; Bendels, S.; Di, L.; Ecker, G.F.; Faller, B.; Fischer, H.; Gerebtzoff, G.; et al. Coexistence of passive and carrier-mediated processes in drug transport. Nat. Rev. Drug Discov. 2010, 9, 597–614. [Google Scholar] [CrossRef]
- Liu, J.; Subir, M.; Nguyen, K.; Eisenthal, K.B. Second Harmonic Studies of Ions Crossing Liposome Membranes in Real Time. J. Phys. Chem. B 2008, 112, 15263–15266. [Google Scholar] [CrossRef]
- Xu, B.; Chen, S.-L.; Zhang, Y.; Li, B.; Yuan, Q.; Gan, W. Evaluating the cross-membrane dynamics of a charged molecule on lipid films with different surface curvature. J. Colloid Interface Sci. 2022, 610, 376–384. [Google Scholar] [CrossRef]
- Karve, S.; Bajagur Kempegowda, G.; Sofou, S. Heterogeneous Domains and Membrane Permeability in Phosphatidylcholine−Phosphatidic Acid Rigid Vesicles As a Function of pH and Lipid Chain Mismatch. Langmuir 2008, 24, 5679–5688. [Google Scholar] [CrossRef]
- Shang, X.; Liu, Y.; Yan, E.; Eisenthal, K.B. Effects of Counterions on Molecular Transport Across Liposome Bilayer: Probed by Second Harmonic Generation. J. Phys. Chem. B 2001, 105, 12816–12822. [Google Scholar] [CrossRef]
- Kumal, R.R.; Nguyenhuu, H.; Winter, J.E.; McCarley, R.L.; Haber, L.H. Impacts of Salt, Buffer, and Lipid Nature on Molecular Adsorption and Transport in Liposomes As Observed by Second Harmonic Generation. J. Phys. Chem. C 2017, 121, 15851–15860. [Google Scholar] [CrossRef]
- Srivastava, A.; Eisenthal, K.B. Kinetics of molecular transport across a liposome bilayer. Chem. Phys. Lett. 1998, 292, 345–351. [Google Scholar] [CrossRef]
- Frallicciardi, J.; Melcr, J.; Siginou, P.; Marrink, S.J.; Poolman, B. Membrane thickness, lipid phase and sterol type are determining factors in the permeability of membranes to small solutes. Nat. Commun. 2022, 13, 1605. [Google Scholar] [CrossRef] [PubMed]
- Fettiplace, R. The influence of the lipid on the water permeability of artificial membranes. Biochim. Biophys. Acta Biomembr. 1978, 513, 1–10. [Google Scholar] [CrossRef]
- Olbrich, K.; Rawicz, W.; Needham, D.; Evans, E. Water Permeability and Mechanical Strength of Polyunsaturated Lipid Bilayers. Biophys. J. 2000, 79, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Ytzhak, S.; Weitman, H.; Ehrenberg, B. The Effect of Lipid Composition on the Permeability of Fluorescent Markers from Photosensitized Membranes. Photochem. Photobiol. 2013, 89, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, M.W. Temperature effect on the transport dynamics of a small molecule through a liposome bilayer. Eur. Phys. J. E 2007, 23, 313–317. [Google Scholar] [CrossRef]
- Chen, S.L.; Liang, Y.Z.; Hou, Y.; Wang, H.; Wu, X.; Gan, W.; Yuan, Q. Simple physics in and easy manipulating of the interfacial behavior of charged molecules on drug delivery vesicles. Mater. Today Phys. 2019, 9, 100092. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, S.-L.; Gan, W.; Ma, X.; Yuan, Q. Understanding the Dynamic Behavior of an Anticancer Drug, Doxorubicin, on a Lipid Membrane Using Multiple Spectroscopic Techniques. J. Phys. Chem. B 2019, 123, 3756–3762. [Google Scholar] [CrossRef]
- Hou, Y.; Xu, B.; Chen, S.-l.; Gan, W.; Yuan, Q.; Lin, X. Understanding the different cross-membrane transport kinetics of two charged molecules on the DOPG lipid surface with second harmonic generation and MD simulation. Soft Matter 2022, 18, 4305–4314. [Google Scholar] [CrossRef]
- Xiang, T.X.; Anderson, B.D. Permeability of acetic acid across gel and liquid-crystalline lipid bilayers conforms to free-surface-area theory. Biophys. J. 1997, 72, 223–237. [Google Scholar] [CrossRef]
- Nilam, M.; Collin, S.; Karmacharya, S.; Hennig, A.; Nau, W.M. Membrane Permeability and Its Activation Energies in Dependence on Analyte, Lipid, and Phase Type Obtained by the Fluorescent Artificial Receptor Membrane Assay. ACS Sens. 2021, 6, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Scheidt, H.A.; Kaur, N.; Kang, T.S.; Gahlay, G.K.; Huster, D.; Mithu, V.S. Effect of the Alkyl Chain Length of Amphiphilic Ionic Liquids on the Structure and Dynamics of Model Lipid Membranes. Langmuir 2019, 35, 12215–12223. [Google Scholar] [CrossRef] [PubMed]
- Prislan, I.; Lokar, M.; Zirdum, M.; Valant, J.; Poklar Ulrih, N. Contribution of headgroup and chain length of glycerophospholipids to thermal stability and permeability of liposomes loaded with calcein. Chem. Phys. Lipids 2019, 225, 104807. [Google Scholar] [CrossRef] [PubMed]
- Guglielmelli, A.; Bartucci, R.; Rizzuti, B.; Palermo, G.; Guzzi, R.; Strangi, G. The interaction of tryptophan enantiomers with model membranes is modulated by polar head type and physical state of phospholipids. Colloids Surf. B Biointerfaces 2023, 224, 113216. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Nakano, M.; Nochi, K.; Yamashita, T.; Morita, K.; Teramae, N. Longitudinal diffusion behavior of hemicyanine dyes across phospholipid vesicle membranes as studied by second-harmonic generation and fluorescence spectroscopies. Anal. Bioanal. Chem. 2006, 386, 627–632. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, E.C.Y.; Eisenthal, K.B. Effects of Bilayer Surface Charge Density on Molecular Adsorption and Transport across Liposome Bilayers. Biophys. J. 2001, 80, 1004–1012. [Google Scholar] [CrossRef]
- Troiano, J.M.; McGeachy, A.C.; Olenick, L.L.; Fang, D.; Liang, D.; Hong, J.; Kuech, T.R.; Caudill, E.R.; Pedersen, J.A.; Cui, Q.; et al. Quantifying the Electrostatics of Polycation–Lipid Bilayer Interactions. J. Am. Chem. Soc. 2017, 139, 5808–5816. [Google Scholar] [CrossRef]
- Li, B.; Li, J.; Gan, W.; Tan, Y.; Yuan, Q. Unveiling the Molecular Dynamics in a Living Cell to the Subcellular Organelle Level Using Second-Harmonic Generation Spectroscopy and Microscopy. Anal. Chem. 2021, 93, 14146–14152. [Google Scholar] [CrossRef] [PubMed]
- Yan, E.C.Y.; Eisenthal, K.B. Effect of Cholesterol on Molecular Transport of Organic Cations across Liposome Bilayers Probed by Second Harmonic Generation. Biophys. J. 2000, 79, 898–903. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Goor, O.J.G.M.; Han, S. Quantitative Analysis of Molecular Transport across Liposomal Bilayer by J-Mediated 13C Overhauser Dynamic Nuclear Polarization. Anal. Chem. 2012, 84, 8936–8940. [Google Scholar] [CrossRef] [PubMed]
- Varshney, G.K.; Saini, R.K.; Gupta, P.K.; Das, K. Effect of Curcumin on the Diffusion Kinetics of a Hemicyanine Dye, LDS-698, across a Lipid Bilayer Probed by Second Harmonic Spectroscopy. Langmuir 2013, 29, 2912–2918. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shang, X.; Pompano, R.; Eisenthal, K.B. Antibiotic assisted molecular ion transport across a membrane in real time. Faraday Discuss. 2005, 129, 291–299. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Liu, X.; Ruan, Y.; Chen, S.-L.; Yuan, Q.; Gan, W. The effect of side group on the dynamic behavior of anthracyclines on DOPG lipid membranes revealed by second harmonic generation and fluorescence. Chem. Phys. 2021, 541, 111036. [Google Scholar] [CrossRef]
- Boyd, M.A.; Thavarajah, W.; Lucks, J.B.; Kamat, N.P. Robust and tunable performance of a cell-free biosensor encapsulated in lipid vesicles. Sci. Adv. 2023, 9, eadd6605. [Google Scholar] [CrossRef]
- Hamal, P.; Nguyenhuu, H.; Subasinghege Don, V.; Kumal, R.R.; Kumar, R.; McCarley, R.L.; Haber, L.H. Molecular Adsorption and Transport at Liposome Surfaces Studied by Molecular Dynamics Simulations and Second Harmonic Generation Spectroscopy. J. Phys. Chem. B 2019, 123, 7722–7730. [Google Scholar] [CrossRef]
- Boyd, R.W. Nonlinear Optics; Academic Press: San Diego, CA, USA, 1992; p. 155. [Google Scholar]
- Eisenthal, K.B. Liquid Interfaces Probed by Second-Harmonic and Sum-Frequency Spectroscopy. Chem. Rev. 1996, 96, 1343–1360. [Google Scholar] [CrossRef] [PubMed]
- Elmer-Dixon, M.M.; Xie, Z.; Alverson, J.B.; Priestley, N.D.; Bowler, B.E. Curvature-Dependent Binding of Cytochrome c to Cardiolipin. J. Am. Chem. Soc. 2020, 142, 19532–19539. [Google Scholar] [CrossRef]
- Elmer-Dixon, M.M.; Hoody, J.; Steele, H.B.B.; Becht, D.C.; Bowler, B.E. Cardiolipin Preferentially Partitions to the Inner Leaflet of Mixed Lipid Large Unilamellar Vesicles. J. Phys. Chem. B 2019, 123, 9111–9122. [Google Scholar] [CrossRef]
- Beltrán-Heredia, E.; Tsai, F.-C.; Salinas-Almaguer, S.; Cao, F.J.; Bassereau, P.; Monroy, F. Membrane curvature induces cardiolipin sorting. Commun. Biol. 2019, 2, 225. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.; Xu, B.; Dai, H.-L. Activation of Thiols at a Silver Nanoparticle Surface. Angew. Chem. Int. Ed. 2011, 50, 6622–6625. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, B.; Hou, Y.; Zeb, J.; Yuan, Q.; Gan, W. Measuring the activation energy of the structural evolution in vesicle formation with combined spectroscopic methods and revealing the different ionic effects from Na+ and Ca2+. Colloids Surf. Physicochem. Eng. Asp. 2023, 662, 130940. [Google Scholar] [CrossRef]
- Varshney, G.K.; Kintali, S.R.; Das, K. Effect of Curcumin Addition on the Adsorption and Transport of a Cationic Dye across DPPG-POPG Liposomes Probed by Second Harmonic Spectroscopy. Langmuir 2017, 33, 8302–8310. [Google Scholar] [CrossRef] [PubMed]
- Eremchev, M.; Roesel, D.; Poojari, C.S.; Roux, A.; Hub, J.S.; Roke, S. Passive transport of Ca2+ ions through lipid bilayers imaged by widefield second harmonic microscopy. Biophys. J. 2023, 122, 624–631. [Google Scholar] [CrossRef]
- Cebecauer, M.; Amaro, M.; Jurkiewicz, P.; Sarmento, M.J.; Šachl, R.; Cwiklik, L.; Hof, M. Membrane Lipid Nanodomains. Chem. Rev. 2018, 118, 11259–11297. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Guha, P.; Chen, S.-L.; Yuan, Q.; Gan, W. Observing the structural variations on binary complex vesicle surfaces and the influence on molecular transportation. Chem. Phys. 2021, 548, 111250. [Google Scholar] [CrossRef]
- Castro, A.; Bhattacharyya, K.; Eisenthal, K.B. Energetics of adsorption of neutral and charged molecules at the air/water interface by second harmonic generation: Hydrophobic and solvation effects. J. Chem. Phys. 1991, 95, 1310–1315. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, S.; Wu, W.; Yang, F.; Gan, W.; Jia, H.; Chen, S.; Zhu, X.; Yuan, Q. Lyophobicity may not be the main driving force for long chain surfactants from the bulk phase to the interface. Phys. Chem. Chem. Phys. 2018, 20, 10165–10172. [Google Scholar] [CrossRef]
- Ward, A.F.H.; Tordai, L. Standard Entropy of Adsorption. Nature 1946, 158, 416. [Google Scholar] [CrossRef]
- Varga, I.; Mészáros, R.; Gilányi, T. Adsorption of Sodium Alkyl Sulfate Homologues at the Air/Solution Interface. J. Phys. Chem. B 2007, 111, 7160–7168. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.M.; Mills, D.; Grzybek, M.; Howard, J. Measurement of the membrane curvature preference of phospholipids reveals only weak coupling between lipid shape and leaflet curvature. Proc. Natl. Acad. Sci. USA 2009, 106, 22245–22250. [Google Scholar] [CrossRef] [PubMed]
- Virden, J.W.; Berg, J.C. Sodium chloride-induced aggregation of dipalmitoylphoshpatidylglycerol small unilamellar vesicles with varying amounts of incorporated cholesterol. Langmuir 1992, 8, 1532–1537. [Google Scholar] [CrossRef]
- McIntosh, T.J.; Simon, S.A. Area per molecule and distribution of water in fully hydrated dilauroylphosphatidylethanolamine bilayers. Biochemistry 1986, 25, 4948–4952. [Google Scholar] [CrossRef]
- Broemstrup, T.; Reuter, N. Molecular Dynamics Simulations of Mixed Acidic/Zwitterionic Phospholipid Bilayers. Biophys. J. 2010, 99, 825–833. [Google Scholar] [CrossRef]
- Pan, J.; Cheng, X.; Sharp, M.; Ho, C.-S.; Khadka, N.; Katsaras, J. Structural and mechanical properties of cardiolipin lipid bilayers determined using neutron spin echo, small angle neutron and X-ray scattering, and molecular dynamics simulations. Soft Matter 2015, 11, 130–138. [Google Scholar] [CrossRef]
- Kučerka, N.; Holland, B.W.; Gray, C.G.; Tomberli, B.; Katsaras, J. Scattering Density Profile Model of POPG Bilayers As Determined by Molecular Dynamics Simulations and Small-Angle Neutron and X-ray Scattering Experiments. J. Phys. Chem. B 2012, 116, 232–239. [Google Scholar] [CrossRef]
- Pan, J.; Heberle, F.A.; Tristram-Nagle, S.; Szymanski, M.; Koepfinger, M.; Katsaras, J.; Kučerka, N. Molecular structures of fluid phase phosphatidylglycerol bilayers as determined by small angle neutron and X-ray scattering. Biochim. Biophys. Acta Biomembr. 2012, 1818, 2135–2148. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Fu, L.; Leblanc, R.M.; Yan, E.C.Y. Lipid Compositions Modulate Fluidity and Stability of Bilayers: Characterization by Surface Pressure and Sum Frequency Generation Spectroscopy. Langmuir 2013, 29, 15022–15031. [Google Scholar] [CrossRef]
- Maltseva, D.; Gonella, G.; Ruysschaert, J.-M.; Bonn, M. Phospholipid acyl tail affects lipid headgroup orientation and membrane hydration. J. Chem. Phys. 2022, 156, 234706. [Google Scholar] [CrossRef]
- McMahon, H.T.; Gallop, J.L. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 2005, 438, 590–596. [Google Scholar] [CrossRef]
- Sharifian, G.M.; Wilhelm, M.J.; Moore, M.; Dai, H.-L. Spatially Resolved Membrane Transport in a Single Cell Imaged by Second Harmonic Light Scattering. Biochemistry 2019, 58, 1841–1844. [Google Scholar] [CrossRef] [PubMed]
- Bresseleers, G.J.M.; Goderis, H.L.; Tobback, P.P. Measurement of the glucose permeation rate across phospholipid bilayers using small unilamellar vesicles Effect of membrane composition and temperature. Biochim. Biophys. Acta Biomembr. 1984, 772, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Reeves, J.P.; Dowben, R.M. Formation and properties of thin-walled phospholipid vesicles. J. Cell Physiol. 1969, 73, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Hope, M.J.; Bally, M.B.; Webb, G.; Cullis, P.R. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta Biomembr. 1985, 812, 55–65. [Google Scholar] [CrossRef]


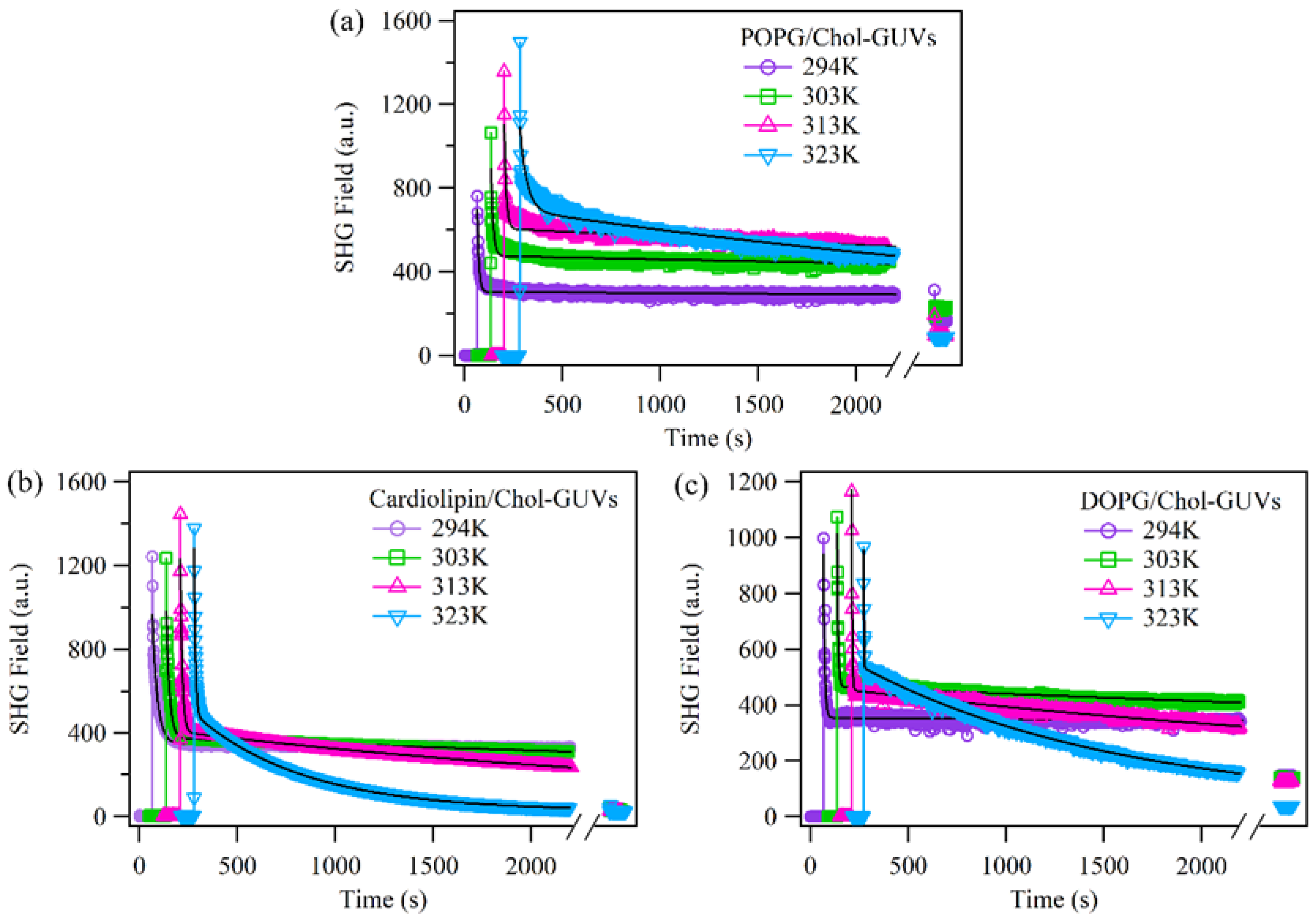
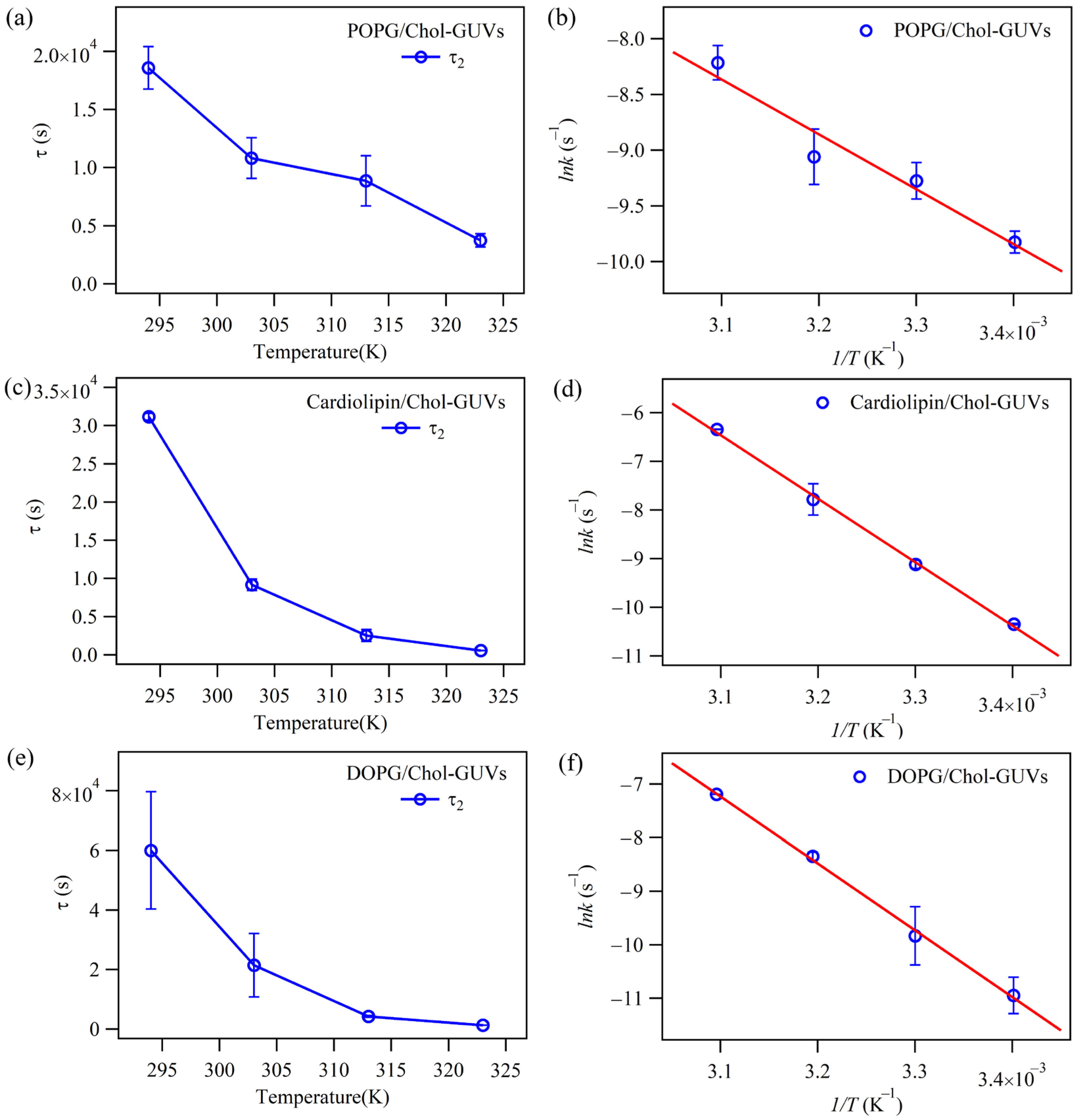
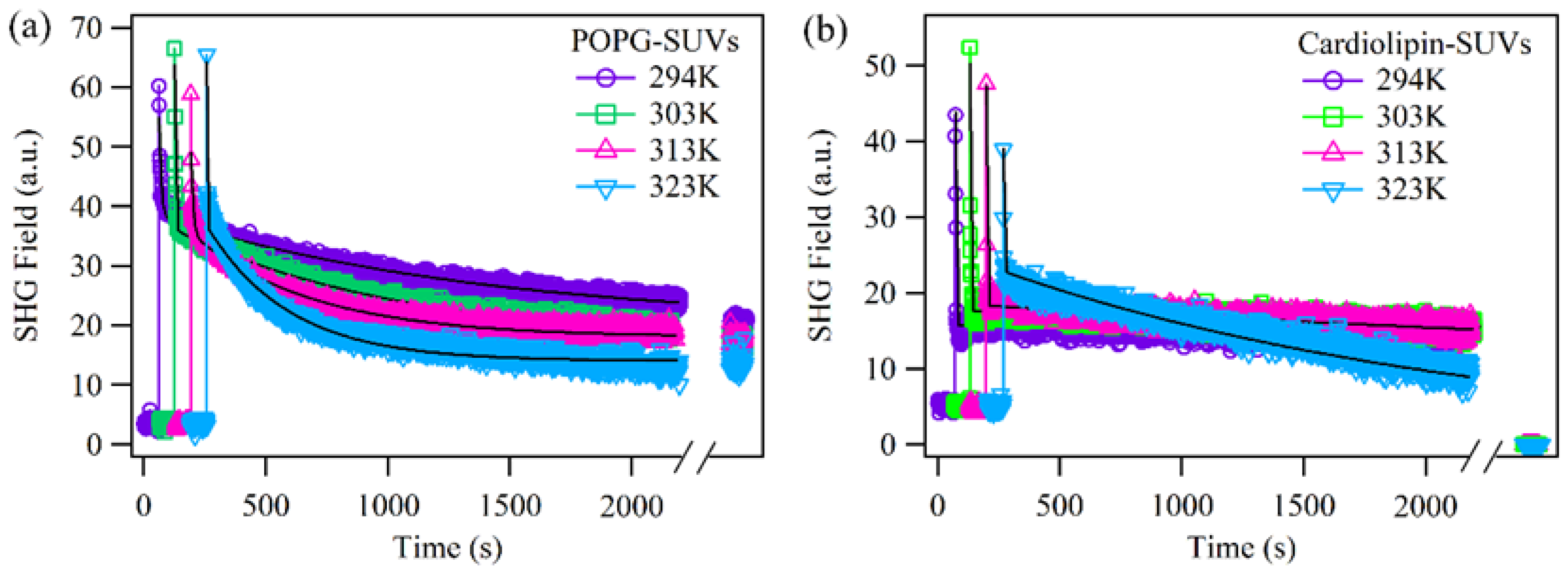


| Lipids | (kJ/mol) GUVs—Non Chol | (kJ/mol) GUVs—Chol | (kJ/mol) SUVs—Non Chol |
|---|---|---|---|
| POPG | 36.5 ± 7 | 40.9 ± 2 | 45.6 ± 7 |
| cardiolipin | 77.9 ± 3 | 108.6 ± 2 | 60.9 ± 6 |
| DOPG | 68 a | 103.8 ± 12 | 116 a |
| Lipids | SUVs | GUVs | ||
|---|---|---|---|---|
| Non-Chol | With-Chol | Non-Chol | With-Chol | |
| POPG | 94 ± 3 | 99 ± 3 | 1320 ± 150 | 1380 ± 280 |
| cardiolipin | 89 ± 2 | 97 ± 2 | 1450 ± 180 | 1450 ± 170 |
| DOPG | 97 ± 3 | 100 ± 1 | 1230 ± 170 | 1290 ± 190 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Li, J.; Zhang, S.; Zeb, J.; Chen, S.; Yuan, Q.; Gan, W. The Transport of Charged Molecules across Three Lipid Membranes Investigated with Second Harmonic Generation. Molecules 2023, 28, 4330. https://doi.org/10.3390/molecules28114330
Xu B, Li J, Zhang S, Zeb J, Chen S, Yuan Q, Gan W. The Transport of Charged Molecules across Three Lipid Membranes Investigated with Second Harmonic Generation. Molecules. 2023; 28(11):4330. https://doi.org/10.3390/molecules28114330
Chicago/Turabian StyleXu, Baomei, Jianhui Li, Shuai Zhang, Johar Zeb, Shunli Chen, Qunhui Yuan, and Wei Gan. 2023. "The Transport of Charged Molecules across Three Lipid Membranes Investigated with Second Harmonic Generation" Molecules 28, no. 11: 4330. https://doi.org/10.3390/molecules28114330
APA StyleXu, B., Li, J., Zhang, S., Zeb, J., Chen, S., Yuan, Q., & Gan, W. (2023). The Transport of Charged Molecules across Three Lipid Membranes Investigated with Second Harmonic Generation. Molecules, 28(11), 4330. https://doi.org/10.3390/molecules28114330







