Isotope Fingerprinting as a Backup for Modern Safety and Traceability Systems in the Animal-Derived Food Chain
Abstract
1. Introduction
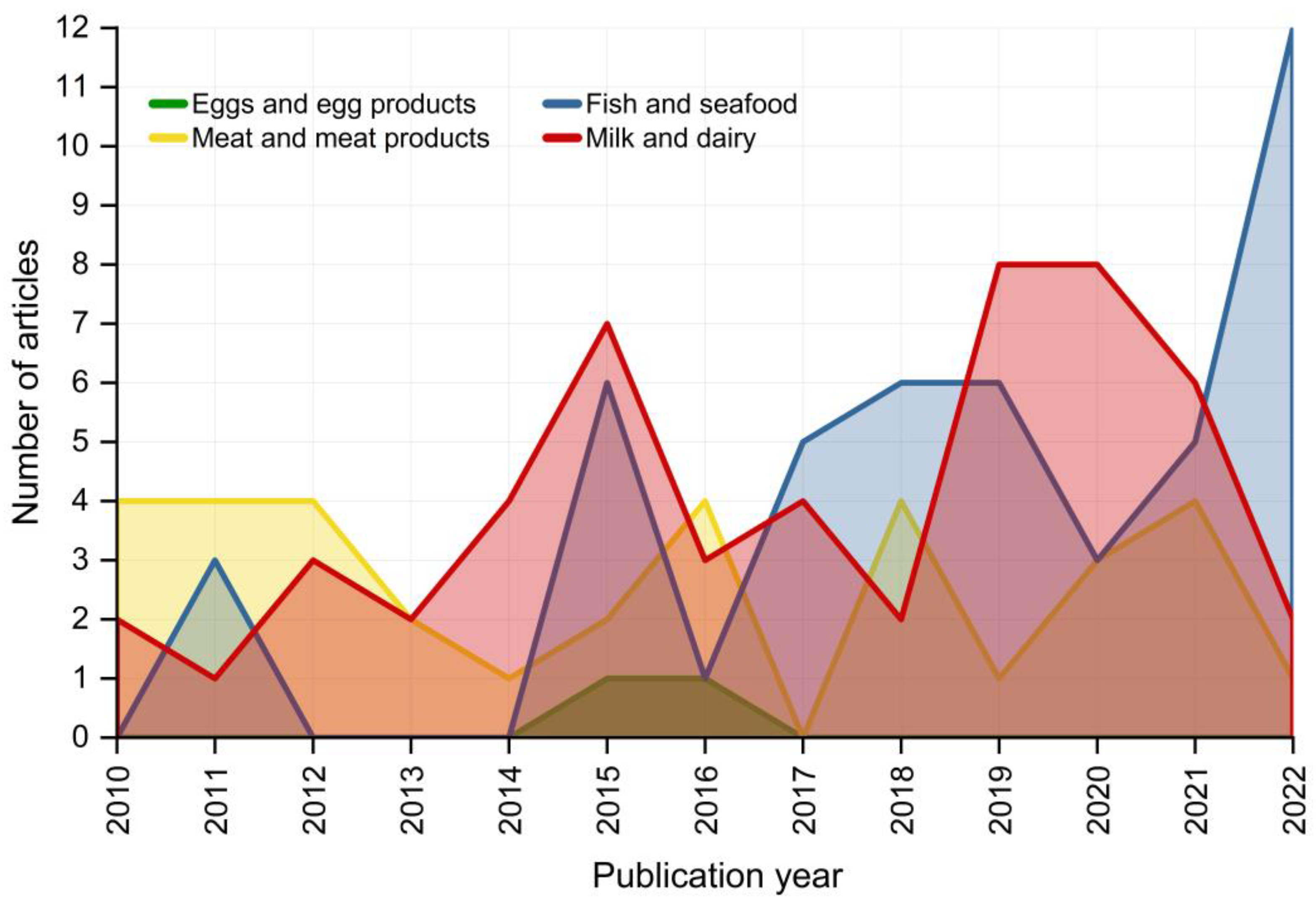
2. A General Picture of Trends and Tendencies in Using Isotopic Ratios to Trace Foods of Animal Origin
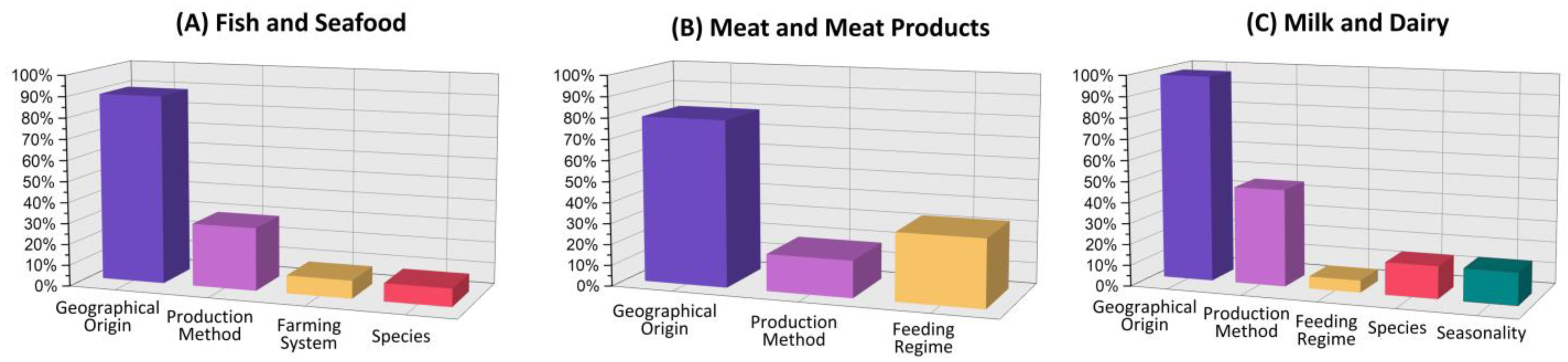
3. Applications and Motivations of Stable Isotope Fingerprinting to Animal-Derived Foods
3.1. Fish and Seafood
3.2. Meat and Meat Products
3.3. Milk and Dairy Products
3.4. Eggs and Egg Products
4. Charting Future Research Directions
5. Materials and Methods
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A
| Product | Tissue | Purpose * | Isotopes | Other Markers | Ref. |
|---|---|---|---|---|---|
| Cnidarian | |||||
| Flame jellyfish (Rhopilema esculentum), Nomura’s jellyfish (Nemopilema nomurai) | Bell tissues | GO, PM | δ13C, δ15N | Fatty acids | [27] |
| Crustaceans | |||||
| Swimming crab (Portunus trituberculatus) | Gills, claw, hepatopancreas, abdominal muscle | GO | δ13C, δ15N | Na, Mg, Al, K, Mn, Fe, Co, Cu, Zn, As, Se, Rb, Ag, Ba | [31] |
| Louisiana crawfish (Procambarus clarkii) | Abdominal muscle | GO | δ13C, δ15N | Na, Mg, Al, K, Ca, Mn, Zn, Cu, Fe, Sr, Ba, As, Se, Cd | [106] |
| Pacific white shrimp (Litopenaeus vannamei) | Muscle | PM, FR | δ13C, δ15N | - | [107] |
| Black tiger prawn (Penaeus monodon) | Abdominal muscle | GO, PM | δ13C, δ15N | Mg, Al, Si, P, S, Cl, K, Ca, Ti, Cr, Mn, Fe, Ni, Cu, Zn, As, Se, Br, Rb, Sr, Y, Zr, Cd, Sn, Sb, Nd, Hf, Pb, Bi, At, U | [108] |
| Chinese mitten crab (Eriocheir sinensis) | Muscle | GO | δ13C, δ15N | Na, Mg, Al, K, Ca, Mn, Cu, Zn, Sr, Ba | [109] |
| Pacific white shrimp (Litopenaeus vannamei) | Muscle | GO | δ13C, δ15N | - | [110] |
| Shrimp (Penaeus monodon, Litopenaeus vannamei, Fenneropenaeus indicus, Fenneropenaeus merguiensis, Farfantepenaeus notialis, Pleoticus muelleri, Pandalus borealis) | Muscle | GO, PM, SP | δ13C, δ15N | Pb, Cd, As, P, S | [34] |
| Shrimp (Pandalus borealis, Marsupenaeus japonicus, Fenneropenaeus chinensis, Litopenaeus vannamei, Penaeus monodon, Solenocera crassicornis) | Muscle | GO | δ13C, δ15N | Cytochrome oxidase I (COI) | [111] |
| Tiger prawn (Penaeidae family) | Muscle, chitin, recovered water | GO | δ2H, δ13C δ15N, δ18O | Li, B, Al, Ti, V, Mo, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Sr, Cd, Hg, K | [112] |
| Echinoderms | |||||
| Sea urchin (Mesocentrotus nudus) | Gonads, spines | GO | δ13C δ15N, δ18O | [113] | |
| Sea cucumber (Apostichopus japonicus) | Body wall | GO | δ13C, δ15N | Ba, Li, Ca, K, Na, Mg, Al, Fe, Mn, Cu, Zn, Se, Cr, Co, Ni, As, Sr, Cd, Pb, Sn, V, Ag | [114] |
| Sea cucumber (Apostichopus japonicus) | Body wall | GO, PM | δ13C | Amino acids | [115] |
| Sea cucumber (Apostichopus japonicus) | Body wall | GO | δ13C, δ15N | Fatty acids | [116] |
| Fish | |||||
| Flathead grey mullet (Mugil cephalus)—salted/dried | Roes (ovaries) | GO | δ13C, δ15N δ34S | - | [46] |
| Silverfish (Trachinotus ovatus) and silver pomfret (Pampus argenteus) | Muscle | GO | δ13C, δ15N | K, Ca, Na, Al, Ti, Mn, Fe, Cu, Zn, Se, Rb, Sr, and Sn | [117] |
| Flathead grey mullet (Mugil cephalus)—raw, salted/dried | Roes (ovaries) | GO, PM, SP | δ13C, δ15N | DNA/RNA | [45] |
| Atlantic salmon (Salmo salar), rainbow trout (Oncorhynchus mykiss) | Muscle | GO, PM | δ2H, δ18O | - | [41] |
| Atlantic bluefin tuna (Thunnus thynnus), albacore (Thunnus alalunga), bullet tuna (Auxis rochei), Atlantic mackerel (Scomber scombrus) | Muscle | GO, SP | δ13C, δ15N | Mitochondrial DNA, fatty acids | [32] |
| European sea bass (Dicentrarchus labrax) | Muscle | GO, FS | δ2H, δ13C, δ15N, δ18O | - | [30] |
| Black carp (Mylopharyngodon piceus), silver carp (Hypophthalmichthys molitrix), grass carp (Ctenopharyngodon idella), common carp (Cyprinus carpio) | Muscle | PM, FS | δ13C, δ15N | Li, Be, Na, Mg, Al, K, Ca, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Rb, Sr, Mo, Ag, Cd, In, Cs, Ba, Tl, Pb, Bi | [118] |
| European sea bass (Dicentrarchus labrax) | Muscle | GO, PM | δ13C, δ15N | La, Eu, Ho, Er, Lu, Tb | [28] |
| European eel (Anguilla anguilla) | Muscle | GO, FS, FR | δ13C, δ15N | Fatty acids | [119] |
| Crucian carp (Carassius carassius), snakehead (Channa argus), carp (Cyprinus carpio), silver carp (Hypophthalmichthys molitrix), weever (Lateolabrax japonicus), redeye mullet (Liza haematocheila), wuchang (Megalobrama amblycephala), flathead grey mullet (Mugil cephalus), yellow catfish (Pelteobagrus fulvidraco), catfish (Silurus asotus), javelin gobi (Synechogobius hasta) | Muscle | SP | δ13C, δ15N | As, Hg, V | [33] |
| Atlantic salmon (Salmo salar) Pacific salmon (Oncorhynchus gorbuscha, Oncorhynchus nerka) | Muscle | GO, PM, FR | δ13C, δ15N | Phe, Lys, Leu, His, Gly, Asx, Ser | [120] |
| Rainbow trout (Oncorhynchus mykiss) | Muscle | GO, FR | δ2H, δ13C, δ15N, δ18O, δ34S | - | [40] |
| European sea bass (Dicentrarchus labrax) | Muscle | GO, PM, FS | δ13C, δ15N | Biometric traits, fatty acids, elements | [121] |
| Hake (Merlucciidae family) | Muscle | GO | δ13C, δ15N | - | [122] |
| Largemouth bass (Micropterus salmoides) | Muscle, liver | FR | δ2H, δ18O | - | [123] |
| Meagre (Argyrosomus regius) | Muscle | PM | δ13C, δ15N | Moisture, fatty acids, S, Cl, K, Ca, Fe, Zn, As, Se, Br, Sr | [124] |
| European sea bass (Dicentrarchus labrax) | Collagen (scales) | GO | δ13C, δ15N | - | [125] |
| Atlantic salmon (Salmo salar), Pacific salmon (Oncorhynchus nerka), Brown trout (Salmo trutta)*—* Raw, smoked, graved | Muscle | PM | δ13C, δ15N | Lipid content, carotenoids | [126] |
| Croaker (Micropogonias furnieri) | Muscle | GO, SEAS | δ13C, δ15N | Moisture, protein content, lipid content, S, Cl, K, Ca, Fe, Zn, As, Se, Br, Sr, Hg | [127] |
| Mackerel (Scomber japonicas), Yellow Croaker (Larimichthys polyactis, Larimichthys crocea), pollock (Theragra chalcogramma) | Muscle | GO | δ13C, δ15N | - | [128] |
| Hairtail (Trichiurus japonicus, Trichiurus lepturus) | Muscle | GO | δ13C, δ15N | Cytochrome oxidase I (COI) | [111] |
| Atlantic cod (Gadus morhua) *, saithe (Pollachius virens)*—*salted | Muscle, bone, skin | SP | δ13C, δ15N | - | [129] |
| Sea bream (Sparus aurata) | Muscle | GO | δ13C, δ15N | RNA, DNA, cytochrome-c-oxidase, citrate synthase, | [130] |
| Cachara (Pseudoplatystoma fasciatum) | Muscle | PM, SEAS | δ13C, δ15N | Protein content, lipid content, ash, moisture | [131] |
| Mollusks | |||||
| Manila clams (Ruditapes philippinarum) | Soft tissue, shell | GO | δ13C, δ15N, δ18O δ34S, 87Sr/86Sr, | - | [39] |
| Manila clams (Ruditapes philippinarum) | Adductor muscle | GO | δ13C, δ15N | Fatty acids | [51] |
| Manila clams (Ruditapes philippinarum) | Soft tissue | GO | 143Nd/144Nd | - | [48] |
| Mediterranean mussel (Mytilus galloprovincialis) | Soft tissue, shell | GO | δ13C, δ15N | B, Al, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Cd, Ba, Pb | [29] |
| Mussels (Mytilus edulis) | Soft tissue | GO | δ2H, δ13C, δ15N, δ18O | - | [38] |
| Manila clam (Ruditapes philippinarum) | Soft tissue, shell | GO | δ13C, δ15N | - | [132] |
| Manila Clams (Ruditapes philippinarum) | Adductor muscle | GO | δ2H, δ13C, δ15N, δ18O δ34S, 87Sr/86Sr, 143Nd/144Nd | - | [36] |
| Mussels (Mytilus spp.) | Shell | GO | 143Nd/144Nd | - | [47] |
| Yesso scallop (Patinopecten yessoensis) | Adductor muscle | GO | δ13C, δ15N | Ala, Gly, Val, Leu, Ser, Phe, Fuc, Rha, Glc, Man | [50] |
| Scallops (Patinopecten yessoensis, Chlamys farreri, Argopecten irradians) | Adductor muscle | GO | δ13C | Fatty acids | [133] |
| Octopus (Octopus berrima, Octopus pallidus, Octopus pallidus, Amphioctopus aegina) | Soft tissue | GO | δ13C, δ18O | Al, Si, P, S, Cl, K, Ca, Ti, V, Cr, Mn, Fe, Ni, Cu, Zn, As, Br, Rb, Sr, Y, Zr, Sb, Ba, Ce, Sm, Pb | [134] |
| Jumbo squid (Dosidicus gigas) | Soft tissue, cartilage | GO | δ13C, δ15N | Fatty acids | [135] |
| Product | Tissue | Purpose * | Isotopes | Other Markers | Ref. |
|---|---|---|---|---|---|
| Bovine | |||||
| Zebu | Muscle (Longissimus thoracis) | GO, PM | δ2H, δ13C, δ15N, δ18O, δ34S | Fatty acids | [136] |
| Yak | Muscle (back, hip leg) | GO | δ13C, δ15N, δ34S | - | [137] |
| Beef | Bone | GO | δ13C, δ15N, δ18O | Na, Mg, K, V, Cr, Mn, Fe, Co, Ni, Zn, Se, Sr, Mo, Sn, Ba, Pb, Th, U | [138] |
| Beef | Kidney, liver | GO | 206Pb/204Pb, 207Pb/204Pb, 08Pb/204Pb, 207Pb/206Pb, 208Pb/206Pb | - | |
| Beef | Muscle | GO | δ13C, δ15N | Be, Na, Mg, K, Ca, Ti, V, Mn, Fe, Co, Ni, Cu, Zn, Ga, Se, Rb, Sr, Zr, Mo, Sn, Sb, Ba, Bi | [139] |
| Beef—processed | Muscle | FR | δ13C, δ15N | - | [140] |
| Beef | Muscle (rib) | FR | δ13C, 14C | - | [141] |
| Beef | Muscle (patties) | GO | δ13C, δ15N | - | [56] |
| Beef | Muscle | GO, FR | δ2H, δ13C, δ15N, δ18O, δ34S | - | [61] |
| Beef | Muscle | FR | δ2H, δ13C, δ15N, δ18O, δ34S | [66] | |
| Beef | Muscle (neck) | GO | δ13C, δ15N, δ18O, δ34S, 87Sr/86Sr | Li, B, Na, Mg, Al, K, As, Fe, Ca, V, Mn, Co, Ni, Cu, Zn, Ga, Se, Rb, Sr, Mo, Cd, Cs, Ba, La, Ce, Nd, Sm, Eu, Yb, Lu, Tl, Pb, U | [142] |
| Beef | Muscle (rib, sirloin) | GO | δ13C, δ15N, δ18O | [143] | |
| Beef | Muscle | GO | δ2H, δ13C, δ15N | - | [144] |
| Beef—processed (roasted) | Muscle water, minced muscle water | GO | δ2H, δ18O | - | [145] |
| Ovine and caprine | |||||
| Lamb | Muscle | GO | δ2H, δ13C, δ15N, δ18O, | Sr, Y, Mo, La, Ce, Nd, Pb, Na, Mg, Al, K, Zn, Rb, fatty acids | [146] |
| Lamb | Muscle | GO | δ13C, δ15N | Mn, Fe, Cu, Zn, As, Rb, Sr, Mo, Cs | [147] |
| Lamb | Muscle (longissimus lumborum) | GO | δ13C, δ15N | - | [148] |
| Lamb | Muscle (longissimus lumborum) | GO, FR | δ13C, δ15N | - | [149] |
| Lamb | Muscle | GO, FR | δ2H, δ13C, δ15N | - | [58] |
| Lamb | Muscle (longissimus dorsi) | GO, PM | δ2H, δ13C, δ15N, δ18O, δ34S | Fatty acids | [65] |
| Mutton | Kidney, liver | GO | 206Pb/204Pb, 207Pb/204Pb, 208Pb/204Pb, 207Pb/206Pb, 208Pb/206Pb | - | [70] |
| Lamb | Muscle (longissimus thoracis) | FR | δ15N | - | [55] |
| Poultry | |||||
| Chicken | Muscle (breast) | GO | δ2H, δ13C, δ15N, δ18O | - | [150] |
| Chicken, turkey | Muscle | GO | δ2H, δ13C, δ15N, δ18O, δ34S | Li, Be, B, Na, Mg, Al, Ca, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, As, Se, Rb, Sr, Mo, Ag, Cd, Te, Ba, Tl, Pb | [57] |
| Chicken | Kidney, liver | GO | 206Pb/204Pb, 207Pb/204Pb, 208Pb/204Pb, 207Pb/206Pb, 208Pb/206Pb | - | [70] |
| Chicken | Muscle (breast) | PM, FR | δ13C, δ15N | - | [151] |
| Chicken | Muscle (breast, thigh, drumstick, wing) | FR | δ13C, δ15N | - | [152] |
| Chicken | Muscle (breast) | FR | δ13C | - | [54] |
| Swine | |||||
| Pig | Muscle water (tenderloin) | GO, FR | δ2H, δ13C, δ18O | Na, Mg, K, P, Zn, Fe, Cu, Mn, Cr, Pb, Cd | [153] |
| Pig | Muscle | GO, PM | δ2H, δ13C, δ15N, δ18O | K, Na, Mg, Ca, Fe, Cu, Se | [154] |
| Pig | Muscle | GO | 7Li/6Li, 11B/10B, 53Cr/52Cr, 52Cr/50Cr, 60Ni/58Ni, 65Cu/64Cu, 71Ga/69Ga, 88Sr/86Sr, 87Sr/84Sr, 88Sr/84Sr, 87Sr/86Sr, 86Sr/84Sr, 97Mo/95Mo, 97Mo/94Mo, 95Mo/94Mo, 109Ag/107Ag, 114Cd/113Cd, 114Cd/112Cd, 114Cd/111Cd, 114Cd/110Cd, 113Cd/112Cd, 13Cd/111Cd, 113Cd/110Cd, 112Cd/111Cd, 112Cd/110Cd, 111Cd/110Cd | - | [72] |
| Pig—processed (dry-cured hams) | Muscle (hind) | GO, PM | 87Sr/86Sr | Trace and ultra-trace elements; Rb/Sr | [68] |
| Pig | Muscle (sirloin) | GO | δ13C, δ15N, 11B/10B, 53Cr/50Cr, 52Cr/50Cr, 60Ni/58Ni, 65Cu/63Cu, 66Zn/64Zn, 71Ga/69Ga, 88Sr/86Sr, 88Sr/84Sr, 87Sr/86Sr, 87Sr/84Sr, 86Sr/84Sr, 97Mo/95Mo, 97Mo/94Mo, 95Mo/94Mo, 109Ag/107Ag, 208Pb/207Pb, 208Pb/206Pb, 114Cd/111Cd, 114Cd/110Cd, 113Cd/112Cd, 113Cd/111Cd, 113Cd/110Cd, 112Cd/111Cd, 112Cd/110Cd, 111Cd/110Cd | V, Mn, Co, As, Se, Rb, Cs | [71] |
| Pig | Muscle | GO | δ2H, δ13C, δ15N, δ18O, 87Sr/86Sr | [155] | |
| Pig | Muscle (longissimus dorsi) | PM | δ13C, δ15N | - | [156] |
| Pig—processed (dry-cured hams) | Muscle (biceps femoris), subcutaneous fat | GO | δ2H, δ13C, δ15N, δ18O, δ34S | - | [67] |
| Species | Product | Fraction | Purpose * | Isotopes | Other Markers | Ref. |
|---|---|---|---|---|---|---|
| Bovine | ||||||
| Cow | Milk, cheese | Fatty acids | PM | δ13C | - | [76] |
| Cow | Milk | Lactose, milk water | GO | δ2H, δ13C, δ18O | - | [157] |
| Cow | Milk | Whole, fat, casein, whey | GO | δ2H, δ13C, δ15N, δ18O | Li, Al, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Sr, Cd, Ba, Pb, Bi | [158] |
| Cow | Milk | Whole | GO | δ13C, δ15N, δ18O | 51 elements, 35 fatty acids | [100] |
| Cow | Milk, infant formulae | Whole | GO | δ2H, δ13C, δ15N, δ18O, δ34S, 87Sr/86Sr | N, C, S | [93] |
| Cow | Milk | Whole, casein | GO | δ2H, δ13C, δ15N, δ18O, δ34S, 87Sr/86Sr | Ca, Cl, K, P, S, Br, Rb, Sr | [90] |
| Cow | Milk | Milk water | GO | δ2H, δ18O | - | [60] |
| Cow | Milk | Whole, fat, casein | PM | δ13C, δ15N, δ18O, δ34S | - | [95] |
| Cow | Milk | Whole, casein | GO | δ13C, δ15N | Si, Se, Li, B, Rb, Ba, P, Mn, Mo, Pb | [159] |
| Cow | Milk | Milk water, casein, lactose | GO, SP, SEAS | δ2H, δ18O | - | [59] |
| Cow | Milk | Casein | GO, SEAS | δ2H, δ13C, δ15N, δ18O | - | [160] |
| Cow | Milk | Fat | GO, SEAS | δ13C | Fatty acids | [98] |
| Cow | Milk | Whole | GO | δ2H, δ13C, δ15N, δ18O | Asp, Glu, His, Ser, Arg, Gly, Thr, Pro, Ala, Val, Met, Cys, Ile, Leu, Phe, Lys, Tyr, Na, Mg, Al, K, Ca, Sc, Ti, Mn, Fe, Zn, Se, Rb, Sr, Mo | [101] |
| Cow | Milk | Whole | GO | δ2H, δ13C, δ15N, δ18O | - | [86] |
| Cow | Milk | Casein | GO, SEAS | δ2H, δ13C, δ15N, δ18O, δ34S | P, S, Cl, K, Ca, Zn, Br, Rb, Sr | [161] |
| Cow | Milk | Whole | GO, PM | δ13C, δ15N, δ18O, δ34S | - | [99] |
| Cow | Milk | Whole | GO | δ2H, δ13C, δ15N, δ18O | - | [162] |
| Cow | Cheese | Casein | GO, PM | δ34S | - | [63] |
| Cow | Cheese | Casein | GO, SP, PM | δ13C | Co, P, As, Mn, K, Li, Mg, Ga, Ca, Na, Zn, Rb | [163] |
| Cow | Milk | Whole, fatty acids, amino acids | PM | δ13C, δ15N | - | [164] |
| Cow | Milk | Whole | GO | δ2H, δ13C, δ15N, δ18O | Ag, Al, As, B, Ba, Be, Ca, Cd, Co, Cr, Cu, Fe, Hg, K, Li, Mg, Mn, Mo, Na, Ni, Pb, Rb, Se, Sr, V, Zn | [165] |
| Cow | Milk | Whole | SEAS | δ13C, δ15N, δ18O | - | [166] |
| Cow | Milk | Whole | PM | δ13C, δ15N | Fatty acids, vitamin E | [79] |
| Cow | Milk, skimmed milk, chocolate milk | Whole, fat | GO | δ13C, δ15N | - | [167] |
| Cow | Cheese | Whole, casein, fat | GO, PM | δ13C, δ15N | - | [82] |
| Cow | Milk | Whole | GO | δ13C, δ15N | - | [168] |
| Cow | UHT milk | Casein | GO | δ13C, δ15N | - | [169] |
| Cow | Milk, cheese | Casein | GO | δ13C, δ15N, δ18O, δ34S | P, S, Cl, K, Ca, Zn, Br, Rb, Sr | [96] |
| Cow | Milk | Whole | GO, SP | δ2H, δ13C, δ18O | Al, Ag, As, Ba, Be, Bi, Ca, Cd, Co, Cr, Cs, Cu, Fe, Ga, In, K, Li, Mg, Mn, Na, Ni, Pb, Rb, Se, Sr, Tl, V, U, Zn | [170] |
| Cow | Milk, UHT milk | Whole | GO, PM, SEAS | δ13C, δ15N | - | [171] |
| Cow | Milk | Proteins, milk water | GO | δ2H, δ13C, δ15N, δ18O | - | [62] |
| Cow | Milk, pasteurized milk, cheese | Dry matter, fat | PM | δ13C, δ15N | - | [75] |
| Cow | Milk, pasteurized milk | Casein, fat | PM | δ13C, δ15N | α-linolenic acid | [102] |
| Cow | Skimmed milk, milk powder | Fatty acids | GO | δ2H, δ13C, δ15N | Fatty acids | [172] |
| Cow | Milk | Fat | GO, FR | δ2H, δ13C, δ15N, δ18O | - | [172] |
| Cow | Milk, cheese | Whole | GO | δ2H, δ13C, δ15N, δ18O 87Sr/86Sr | - | [77] |
| Cow | Cheese | Whole | GO | δ2H, δ13C, δ15N, δ34S | Li, Na, Mn, Fe, Cu, Se, Rb, Sr, Mo, Ba, Re, Bi, U | [173] |
| Cow | Milk | Fat | FR | δ13C | Phytanic acid | [74] |
| Cow | Milk | Whole | PM | δ13C, δ15N | - | [78] |
| Cow | Milk | Fat | PM | δ13C | Fatty acids | [81] |
| Cow | Milk powder | Fatty acids | GO | δ2H, δ13C | - | [174] |
| Cow | Milk, cheese | Casein | GO, PM | δ2H, δ13C, δ15N, δ18O | - | [87] |
| Cow | Pasteurized milk | Protein, fat | PM | δ13C, δ15N, δ34S | - | [80] |
| Cow | Cheese | Casein | GO, PM | δ2H, δ13C, δ15N, δ34S | Li, Be, B, Na, Mg, P, K, Ca, V, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Rb, Sr, Y, Mo, Pd, Ag, Cd, Sn, Sb, Te, Cs, Ba, La, Ce, Pr, Nd, Sm, Eu, Gd, Dy, Ho, Er, Tm, Yb, Re, Ir, Au, Hg, Pb, Bi, U | [84] |
| Cow | Raw milk, pasteurized milk | Casein, fat | GO, PM | δ13C, δ15N | - | [85] |
| Cow | Cheese | Casein, glycerol | GO | δ13C, δ15N, δ18O, δ34S | Al, B, Ba, Ca, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, Sr, Zn, Ag, Be, Cd, Ce, Co, Cs, Dy, Er, Eu, Ga, Gd, Ge, Ho, Ir, La, Li, Lu, Nb, Nd, Pb, Pr, Pt, Rb, Re, Ru, Sb, Sm, Ta, Te, Tl, Tm, U, V, Yb | [94] |
| Cow | Milk | Whole | GO | δ2H, δ18O | - | [175] |
| Buffalo | Milk, cheese | Milk, casein (cheese) | GO, PM | δ13C, δ15N, δ18O, | - | [176] |
| Buffalo | Milk, cheese | Whole | GO, PM | δ2H, δ13C, δ15N, δ18O, δ34S | Li, Be, B, Na, Mg, Al, P, K, Ca, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Rb, Sr, Y, Mo, Pd, Ag, Cd, In, Sn, Sb, Te, Cs, Ba, La, Ce, Pr, Nd, Sm, Eu, Gd, Dy, Ho, Er, Tm, Yb, Re, Ir, Pt, Au, Hg, Tl, Pb, Bi, Th, U | [97] |
| Buffalo | Milk, cheese, cream, butter | Whole | GO | δ2H, δ13C, δ15N, δ18O | - | [177] |
| Ovine and caprine | ||||||
| Sheep | Milk, cheese | Whole | GO | δ13C | - | [76] |
| Sheep | Milk | Milk water, casein, lactose | GO, SP, SEAS | δ2H, δ18O | - | [59] |
| Sheep | Milk | Casein, fat | GO | δ2H, δ13C, δ15N, δ18O | Cr, Mn, Fe, Ni, Cu, Zn, Se, Rb, Sr, Cd, Ba | [178] |
| Sheep | Cheese | Whole, casein, fat | GO, PM | δ13C, δ15N | - | [82] |
| Sheep | Cheese | Whole | GO, FR | δ2H, δ13C, δ15N, δ18O, δ34S | - | [83] |
| Sheep | Milk, cheese | Casein | GO, SP | δ13C, δ15N, δ18O, δ34S | P, S, Cl, K, Ca, Zn, Br, Rb, Sr | [96] |
| Sheep | Raw milk | Casein, fat | PM | δ13C, δ15N | α-linolenic acid | [102] |
| Sheep | Milk | Whole | GO, SP | δ2H, δ13C, δ18O | Al, As, Ba, Be, Bi, Ca, Cd, Co, CR, Cs, Cu, Fe, Ga, In, K, Li, Mg, Mn, Ni, Pb, Rb, Se, Na, Ag, Sr, Tl, V, U, Zn | [170] |
| Goat | Milk | Milk water, casein, lactose | GO, SP, SEAS | δ2H, δ18O | - | [59] |
| Goat | Cheese | Casein | GO, SP, PM | δ13C | Co, P, As, Mn, K, Li, Mg, Ga, Ca, Na, Zn, Rb | [163] |
| Goat | Milk, cheese | Casein | GO | δ13C, δ15N, δ18O, δ34S | P, S, Cl, K, Ca, Zn, Br, Rb, Sr | [96] |
| Goat | Raw milk | Casein, fat | PM | δ13C, δ15N | α-linolenic acid | [102] |
| Goat | Milk, cheese | Whole | GO | δ2H, δ13C, δ15N, δ18O, 87Sr/86Sr | - | [77] |
| Goat | Milk powder | - | GO, PM | δ13C, δ15N | Li, Na, Mg, K, Ca, Mn, Cu, Zn, Rb, Sr, Mo, Cs, Ba | [179] |
| Product | Fraction | Purpose * | Isotopes | Other Markers | Ref. |
|---|---|---|---|---|---|
| Chicken egg | Albumen | GO, PM | δ13C, δ15N | - | [103] |
| Chicken egg | Albumen, yolk | FR | δ13C, δ15N | - | [104] |
References
- Zhao, Y.; Zhang, B.; Chen, G.; Chen, A.; Yang, S.; Ye, Z. Recent Developments in Application of Stable Isotope Analysis on Agro-Product Authenticity and Traceability. Food Chem. 2014, 145, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Yang, J.; Xiao, G.; Zhao, Y.; Li, Z.; Bai, W.; Zeng, X.; Dong, H. A Comprehensive Overview of Emerging Techniques and Chemometrics for Authenticity and Traceability of Animal-Derived Food. Food Chem. 2023, 402, 134216. [Google Scholar] [CrossRef] [PubMed]
- Pelegrino, B.O.; Silva, R.; Guimarães, J.T.; Coutinho, N.F.; Pimentel, T.C.; Castro, B.G.; Freitas, M.Q.; Esmerino, E.A.; Sant’Ana, A.S.; Silva, M.C.; et al. Traceability: Perception and Attitudes of Artisanal Cheese Producers in Brazil. J. Dairy Sci. 2020, 103, 4874–4879. [Google Scholar] [CrossRef] [PubMed]
- Drivelos, S.A.; Georgiou, C.A. Multi-Element and Multi-Isotope-Ratio Analysis to Determine the Geographical Origin of Foods in the European Union. TrAC Trends Anal. Chem. 2012, 40, 38–51. [Google Scholar] [CrossRef]
- Fanelli, V.; Mascio, I.; Miazzi, M.M.; Savoia, M.A.; De Giovanni, C.; Montemurro, C. Molecular Approaches to Agri-Food Traceability and Authentication: An Updated Review. Foods 2021, 10, 1644. [Google Scholar] [CrossRef]
- Vinci, G.; Preti, R.; Tieri, A.; Vieri, S. Authenticity and Quality of Animal Origin Food Investigated by Stable-Isotope Ratio Analysis. J. Sci. Food Agric. 2013, 93, 439–448. [Google Scholar] [CrossRef]
- Camin, F.; Bontempo, L.; Perini, M.; Piasentier, E. Stable Isotope Ratio Analysis for Assessing the Authenticity of Food of Animal Origin. Compr. Rev. Food Sci. Food Saf. 2016, 15, 868–877. [Google Scholar] [CrossRef]
- Prache, S.; Martin, B.; Coppa, M. Authentication of Grass-Fed Meat and Dairy Products from Cattle and Sheep. Animal 2020, 14, 854–863. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M. Food Frauds: Global Incidents and Misleading Situations. Trends Food. Sci. Technol. 2021, 114, 424–442. [Google Scholar] [CrossRef]
- European Commission Knowledge Centre for Food Fraud and Quality Monthly Food Fraud Summary Reports (January 2023, February 2023, March 2023). Available online: https://knowledge4policy.ec.europa.eu/food-fraud-quality/monthly-food-fraud-summary-reports_en#year2023 (accessed on 15 April 2023).
- European Parliament; Council of the European Union. Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying down Procedures in Matters of Food Safety. Off. J. Eur. Union 2002, L31/1. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32002R0178 (accessed on 28 February 2023).
- European Parliament; Council of the European Union. Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on Official Controls and Other Official Activities Performed to Ensure the Application of Food and Feed Law, Rules on Animal Health and Welfare, Plant Health and Plant Protection Products. Off. J. Eur. Union. 2017, L 95/1. Available online: https://eur-lex.europa.eu/eli/reg/2017/625/oj (accessed on 28 February 2023).
- Camin, F.; Boner, M.; Bontempo, L.; Fauhl-Hassek, C.; Kelly, S.D.; Riedl, J.; Rossmann, A. Stable Isotope Techniques for Verifying the Declared Geographical Origin of Food in Legal Cases. Trends Food Sci. Technol. 2017, 61, 176–187. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food Authentication: Techniques, Trends & Emerging Approaches. TrAC-Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar] [CrossRef]
- Katerinopoulou, K.; Kontogeorgos, A.; Salmas, C.E.; Patakas, A.; Ladavos, A. Geographical Origin Authentication of Agri-Food Products: A Review. Foods 2020, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Danezis, G.P.; Tsagkaris, A.S.; Brusic, V.; Georgiou, C.A. Food Authentication: State of the Art and Prospects. Curr. Opin. Food Sci. 2016, 10, 22–31. [Google Scholar] [CrossRef]
- Ghidini, S.; Ianieri, A.; Zanardi, E.; Conter, M.; Boschetti, T.; Iacumin, P.; Bracchi, P.G. Stable Isotopes Determination in Food Authentication: A Review. Ann. Fac. Medic. Vet. Parma 2006, XXVI, 193–204. [Google Scholar]
- Varrà, M.O.; Ghidini, S.; Husáková, L.; Ianieri, A.; Zanardi, E. Advances in Troubleshooting Fish and Seafood Authentication by Inorganic Elemental Composition. Foods 2021, 10, 270. [Google Scholar] [CrossRef]
- Borràs, E.; Ferré, J.; Boqué, R.; Mestres, M.; Aceña, L.; Busto, O. Data Fusion Methodologies for Food and Beverage Authentication and Quality Assessment—A Review. Anal. Chim. Acta 2015, 891, 1–14. [Google Scholar] [CrossRef]
- van Leeuwen, K.A.; Prenzler, P.D.; Ryan, D.; Camin, F. Gas Chromatography-Combustion-Isotope Ratio Mass Spectrometry for Traceability and Authenticity in Foods and Beverages. Compr. Rev. Food Sci. Food Saf. 2014, 13, 814–837. [Google Scholar] [CrossRef]
- Meier-Augenstein, W. Applied Gas Chromatography Coupled to Isotope Ratio Mass Spectrometry. J. Chromatogr. A 1999, 842, 351–357. [Google Scholar] [CrossRef]
- Perini, M.; Bontempo, L. Liquid Chromatography Coupled to Isotope Ratio Mass Spectrometry (LC-IRMS): A Review. TrAC Trends Anal. Chem. 2022, 147, 116515. [Google Scholar] [CrossRef]
- Yang, L. Accurate and Precise Determination of Isotopic Ratios by Mc-Icp-Ms: A Review. Mass Spectrom. Rev. 2009, 28, 990–1011. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tong, S.; Zhou, L.; Hu, Z.; Mester, Z.; Meija, J. A Critical Review on Isotopic Fractionation Correction Methods for Accurate Isotope Amount Ratio Measurements by MC-ICP-MS. J. Anal. At. Spectrom. 2018, 33, 1849–1861. [Google Scholar] [CrossRef]
- Becker, J.S. State-of-the-Art and Progress in Precise and Accurate Isotope Ratio Measurements by ICP-MS and LA-ICP-MS. J. Anal. At. Spectrom. 2002, 17, 1172–1185. [Google Scholar] [CrossRef]
- Jézéquel, T.; Joubert, V.; Giraudeau, P.; Remaud, G.S.; Akoka, S. The New Face of Isotopic NMR at Natural Abundance. Magn. Reson. Chem. 2017, 55, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gong, Y.; Zhang, J.; Tang, Y.; Shi, X.; Shi, J. Intra- and Inter-Specific Variation in Edible Jellyfish Biomarkers and Implications for Origin Traceability and Authentication. Front. Mar. Sci. 2021, 8, 755048. [Google Scholar] [CrossRef]
- Varrà, M.O.; Ghidini, S.; Zanardi, E.; Badiani, A.; Ianieri, A. Authentication of European Sea Bass According to Production Method and Geographical Origin by Light Stable Isotope Ratio and Rare Earth Elements Analyses Combined with Chemometrics. Ital. J. Food Saf. 2019, 8, 21–25. [Google Scholar] [CrossRef] [PubMed]
- del Rio-Lavín, A.; Weber, J.; Molkentin, J.; Jiménez, E.; Artetxe-Arrate, I.; Pardo, M.Á. Stable Isotope and Trace Element Analysis for Tracing the Geographical Origin of the Mediterranean Mussel (Mytilus Galloprovincialis) in Food Authentication. Food Control 2022, 139, 109069. [Google Scholar] [CrossRef]
- Tulli, F.; Moreno-Rojas, J.M.; Messina, C.M.; Trocino, A.; Xiccato, G.; Muñoz-Redondo, J.M.; Santulli, A.; Tibaldi, E. The Use of Stable Isotope Ratio Analysis to Trace European Sea Bass (D. labrax) Originating from Different Farming Systems. Animals 2020, 10, 2042. [Google Scholar] [CrossRef]
- Xu, Y.; Peng, K.; Jiang, F.; Cui, Y.M.; Han, D.; Liu, H.; Hong, H.; Tian, X. Geographical Discrimination of Swimming Crabs (Portunus Trituberculatus) Using Stable Isotope and Multi-Element Analyses. J. Food Compos. Anal. 2022, 11, 3060. [Google Scholar] [CrossRef]
- Rampazzo, F.; Tosi, F.; Tedeschi, P.; Gion, C.; Arcangeli, G.; Brandolini, V.; Giovanardi, O.; Maietti, A.; Berto, D. Preliminary Multi Analytical Approach to Address Geographic Traceability at the Intraspecific Level in Scombridae Family. Isotopes Environ. Health Stud. 2020, 56, 260–279. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Yuan, Z.; Liu, H.; Lam, P.K.S. Heavy Metals (As, Hg and V) and Stable Isotope Ratios (Δ13C and Δ15N) in Fish from Yellow River Estuary, China. Sci. Total Environ. 2018, 613–614, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Ortea, I.; Gallardo, J.M. Investigation of Production Method, Geographical Origin and Species Authentication in Commercially Relevant Shrimps Using Stable Isotope Ratio and/or Multi-Element Analyses Combined with Chemometrics: An Exploratory Analysis. Food Chem. 2015, 170, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.X.; Hobson, K.A.; Wassenaar, L.I. Using Hydrogen Isotopes of Freshwater Fish Tissue as a Tracer of Provenance. Ecol. Evol. 2016, 6, 7776–7782. [Google Scholar] [CrossRef]
- Won, E.J.; Kim, S.H.; Go, Y.S.; Kumar, K.S.; Kim, M.S.; Yoon, S.H.; Bayon, G.; Kim, J.H.; Shin, K.H. A Multi-Elements Isotope Approach to Assess the Geographic Provenance of Manila Clams (Ruditapes philippinarum) via Recombining Appropriate Elements. Foods 2021, 10, 646. [Google Scholar] [CrossRef]
- Martino, J.C.; Trueman, C.N.; Mazumder, D.; Crawford, J.; Doubleday, Z.A. The Universal Imprint of Oxygen Isotopes Can Track the Origins of Seafood. Fish Fish. 2022, 23, 1455–1468. [Google Scholar] [CrossRef]
- Kang, X.; Zhao, Y.; Tan, Z.; Ning, J.; Zhai, Y.; Zheng, G. Evaluation of Multivariate Data Analysis for Marine Mussels Mytilus Edulis Authentication in China: Based on Stable Isotope Ratio and Compositions of C, N, O and H. J. Food Compos. Anal. 2022, 111, 104627. [Google Scholar] [CrossRef]
- Brombin, V.; Natali, C.; Frijia, G.; Schmitt, K.; Casalini, M.; Bianchini, G. Isotope Geochemistry for Seafood Traceability and Authentication: The Northern Adriatic Manila Clams Case Study. Foods 2022, 11, 3054. [Google Scholar] [CrossRef]
- Camin, F.; Perini, M.; Bontempo, L.; Galeotti, M.; Tibaldi, E.; Piasentier, E. Stable Isotope Ratios of H, C, O, N and S for the Geographical Traceability of Italian Rainbow Trout (Oncorhynchus Mykiss). Food Chem. 2018, 267, 288–295. [Google Scholar] [CrossRef]
- Han, C.; Dong, S.; Li, L.; Gao, Q. Efficacy of Using Stable Isotopes Coupled with Chemometrics to Differentiate the Production Method and Geographical Origin of Farmed Salmonids. Food Chem. 2021, 364, 130364. [Google Scholar] [CrossRef]
- Shipley, O.N.; Newton, A.L.; Frisk, M.G.; Henkes, G.A.; LaBelle, J.S.; Camhi, M.D.; Hyatt, M.W.; Walters, H.; Olin, J.A. Telemetry-Validated Nitrogen Stable Isotope Clocks Identify Ocean-to-Estuarine Habitat Shifts in Mobile Organisms. Methods Ecol. Evol. 2021, 12, 897–908. [Google Scholar] [CrossRef]
- Phillips, D.L.; Eldridge, P.M. Estimating the Timing of Diet Shifts Using Stable Isotopes. Oecologia 2006, 147, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Busst, G.M.A.; Britton, J.R. Tissue-Specific Turnover Rates of the Nitrogen Stable Isotope as Functions of Time and Growth in a Cyprinid Fish. Hydrobiologia 2018, 805, 49–60. [Google Scholar] [CrossRef]
- Chen, H.L.; Chang, N.N.; Hsiao, W.V.; Chen, W.J.; Wang, C.H.; Shiao, J.C. Using Molecular Phylogenetic and Stable Isotopic Analysis to Identify Species, Geographical Origin and Production Method of Mullet Roes. Food Control 2022, 141, 109206. [Google Scholar] [CrossRef]
- Thomatou, A.A.; Psarra, E.; Mazarakioti, E.C.; Katerinopoulou, K.; Tsirogiannis, G.; Zotos, A.; Kontogeorgos, A.; Patakas, A.; Ladavos, A. Stable Isotope Analysis for the Discrimination of the Geographical Origin of Greek Bottarga ‘Avgotaracho Messolongiou’: A Preliminary Research. Foods 2022, 11, 2960. [Google Scholar] [CrossRef]
- Zhao, L.; Tanaka, K.; Tazoe, H.; Iizuka, T.; Kubota, K.; Murakami-Sugihara, N.; Shirai, K. Determination of the Geographical Origin of Marine Mussels (Mytilus Spp.) Using 143Nd/144Nd Ratios. Mar. Environ. Res. 2019, 148, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Zhao, L.; Tazoe, H.; Iizuka, T.; Murakami-Sugihara, N.; Toyama, K.; Yamamoto, T.; Yorisue, T.; Shirai, K. Using Neodymium Isotope Ratio in Ruditapes philippinarum Shells for Tracking the Geographical Origin. Food Chem. 2022, 382, 13914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, J.; Han, D.; Zhao, X.; Chen, X.; Liu, Y. Geographical Origin Traceability and Species Identification of Three Scallops (Patinopecten Yessoensis, Chlamys Farreri, and Argopecten Irradians) Using Stable Isotope Analysis. Food Chem. 2019, 299, 125107. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Wang, G.; Tao, W.; Lou, Y.; Li, N.; Liu, Y. Tracing the Geographical Origins of Yesso Scallop (Patinopecten Yessoensis) by Using Compound-Specific Isotope Analysis: An Approach for Overcoming the Seasonal Effect. Food Control 2019, 102, 38–45. [Google Scholar] [CrossRef]
- Go, Y.S.; Won, E.J.; Kim, S.H.; Lee, D.H.; Kang, J.H.; Shin, K.H. Stepwise Approach for Tracing the Geographical Origins of the Manila Clam Ruditapes philippinarum Using Dual-Element Isotopes and Carbon Isotopes of Fatty Acids. Foods 2022, 11, 1965. [Google Scholar] [CrossRef]
- Dang, C.; De Montaudouin, X.; Savoye, N.; Caill-Milly, N.; Martinez, P.; Sauriau, P.G. Stable Isotopes Changes in the Adductor Muscle of Diseased Bivalve Ruditapes philippinarum. Mar. Biol. 2009, 156, 611–618. [Google Scholar] [CrossRef]
- Pianezze, S.; Camin, F.; Perini, M.; Corazzin, M.; Piasentier, E. Tracing Lamb Meat with Stable Isotope Ratio Analysis: A Review. Small Rumin. Res. 2021, 203, 106482. [Google Scholar] [CrossRef]
- Rhodes, C.N.; Lofthouse, J.H.; Hird, S.; Rose, P.; Reece, P.; Christy, J.; Macarthur, R.; Brereton, P.A. The Use of Stable Carbon Isotopes to Authenticate Claims That Poultry Have Been Corn-Fed. Food Chem. 2010, 118, 927–932. [Google Scholar] [CrossRef]
- Devincenzi, T.; Delfosse, O.; Andueza, D.; Nabinger, C.; Prache, S. Dose-Dependent Response of Nitrogen Stable Isotope Ratio to Proportion of Legumes in Diet to Authenticate Lamb Meat Produced from Legume-Rich Diets. Food Chem. 2014, 152, 456–461. [Google Scholar] [CrossRef]
- Martinelli, L.A.; Nardoto, G.B.; Chesson, L.A.; Rinaldi, F.D.; Ometto, J.P.H.B.; Cerling, T.E.; Ehleringer, J.R. Worldwide Stable Carbon and Nitrogen Isotopes of Big Mac® Patties: An Example of a Truly “Glocal” Food. Food Chem. 2011, 127, 1712–1718. [Google Scholar] [CrossRef]
- Rees, G.; Kelly, S.D.; Cairns, P.; Ueckermann, H.; Hoelzl, S.; Rossmann, A.; Scotter, M.J. Verifying the Geographical Origin of Poultry: The Application of Stable Isotope and Trace Element (SITE) Analysis. Food Control 2016, 67, 144–154. [Google Scholar] [CrossRef]
- Sun, S.; Guo, B.; Wei, Y. Origin Assignment by Multi-Element Stable Isotopes of Lamb Tissues. Food Chem. 2016, 213, 675–681. [Google Scholar] [CrossRef]
- Gregorčič, S.H.; Potočnik, D.; Camin, F.; Ogrinc, N. Milk Authentication: Stable Isotope Composition of Hydrogen and Oxygen in Milks and Their Constituents. Molecules 2020, 25, 4000. [Google Scholar] [CrossRef] [PubMed]
- Boito, M.; Iacumin, P.; Rossi, M.; Ogrinc, N.; Venturelli, G. Isotope Partitioning between Cow Milk and Farm Water: A Tool for Verification of Milk Provenance. Rapid Commun. Mass Spectrom. 2021, 35, e9160. [Google Scholar] [CrossRef]
- Osorio, M.T.; Moloney, A.P.; Schmidt, O.; Monahan, F.J. Beef Authentication and Retrospective Dietary Verification Using Stable Isotope Ratio Analysis of Bovine Muscle and Tail Hair. J. Agric. Food Chem. 2011, 59, 3295–3305. [Google Scholar] [CrossRef]
- Luo, D.; Dong, H.; Luo, H.; Xian, Y.; Guo, X.; Wu, Y. Multi-Element (C, N, H, O) Stable Isotope Ratio Analysis for Determining the Geographical Origin of Pure Milk from Different Regions. Food Anal. Methods 2016, 9, 437–442. [Google Scholar] [CrossRef]
- Pianezze, S.; Bontempo, L.; Perini, M.; Tonon, A.; Ziller, L.; Franceschi, P.; Camin, F. δ34S for Tracing the Origin of Cheese and Detecting Its Authenticity. J. Mass Spectrom. 2020, 55, e4451. [Google Scholar] [CrossRef] [PubMed]
- Zazzo, A.; Monahan, F.J.; Moloney, A.P.; Green, S.; Schmidt, O. Sulphur Isotopes in Animal Hair Track Distance to Sea. Rapid Commun. Mass Spectrom. 2011, 25, 2371–2378. [Google Scholar] [CrossRef] [PubMed]
- Mekki, I.; Camin, F.; Perini, M.; Smeti, S.; Hajji, H.; Mahouachi, M.; Piasentier, E.; Atti, N. Differentiating the Geographical Origin of Tunisian Indigenous Lamb Using Stable Isotope Ratio and Fatty Acid Content. J. Food Compos. Anal. 2016, 53, 40–48. [Google Scholar] [CrossRef]
- Osorio, M.T.; Moloney, A.P.; Schmidt, O.; Monahan, F.J. Multielement Isotope Analysis of Bovine Muscle for Determination of International Geographical Origin of Meat. J. Agric. Food Chem. 2011, 59, 3285–3294. [Google Scholar] [CrossRef]
- Perini, M.; Camin, F.; Sánchez Del Pulgar, J.; Piasentier, E. Effect of Origin, Breeding and Processing Conditions on the Isotope Ratios of Bioelements in Dry-Cured Ham. Food Chem. 2013, 136, 1543–1550. [Google Scholar] [CrossRef]
- Epova, E.N.; Bérail, S.; Zuliani, T.; Malherbe, J.; Sarthou, L.; Valiente, M.; Donard, O.F.X. 87Sr/86Sr Isotope Ratio and Multielemental Signatures as Indicators of Origin of European Cured Hams: The Role of Salt. Food Chem. 2018, 246, 313–322. [Google Scholar] [CrossRef]
- Coelho, I.; Castanheira, I.; Bordado, J.M.; Donard, O.; Silva, J.A.L. Recent Developments and Trends in the Application of Strontium and Its Isotopes in Biological Related Fields. TrAC Trends Anal. Chem. 2017, 90, 45–61. [Google Scholar] [CrossRef]
- Evans, J.A.; Pashley, V.; Richards, G.J.; Brereton, N.; Knowles, T.G. Geogenic Lead Isotope Signatures from Meat Products in Great Britain: Potential for Use in Food Authentication and Supply Chain Traceability. Sci. Total Environ. 2015, 537, 447–452. [Google Scholar] [CrossRef]
- Park, Y.M.; Lee, C.M.; Hong, J.H.; Jamila, N.; Khan, N.; Jung, J.H.; Jung, Y.C.; Kim, K.S. Origin Discrimination of Defatted Pork via Trace Elements Profiling, Stable Isotope Ratios Analysis, and Multivariate Statistical Techniques. Meat Sci. 2018, 143, 93–103. [Google Scholar] [CrossRef]
- Nho, E.Y.; Choi, J.Y.; Lee, C.M.; Dang, Y.M.; Khan, N.; Jamila, N.; Kim, K.S. Origin Authentication of Pork Fat via Elemental Composition, Isotope Ratios, and Multivariate Chemometric Analyses. Anal. Lett. 2019, 52, 1445–1461. [Google Scholar] [CrossRef]
- Besser, A.C.; Elliott Smith, E.A.; Newsome, S.D. Assessing the Potential of Amino Acid δ13C and δ15N Analysis in Terrestrial and Freshwater Ecosystems. J. Ecol. 2022, 110, 935–950. [Google Scholar] [CrossRef]
- Kaffarnik, S.; Schröder, M.; Lehnert, K.; Baars, T.; Vetter, W. Δ13C Values and Phytanic Acid Diastereomer Ratios: Combined Evaluation of Two Markers Suggested for Authentication of Organic Milk and Dairy Products. Eur. Food Res. Technol. 2014, 238, 819–827. [Google Scholar] [CrossRef]
- Capici, C.; Mimmo, T.; Kerschbaumer, L.; Cesco, S.; Scampicchio, M. Determination of Cheese Authenticity by Carbon and Nitrogen Isotope Analysis: Stelvio Cheese as a Case Study. Food Anal. Methods 2015, 8, 2157–2162. [Google Scholar] [CrossRef]
- Schipilliti, L.; Bonaccorsi, I.; Consolo, G.; Mondello, L. Isotopic and Statistical Methods for the Traceability of Milk and Dairy Products. Food Anal. Methods 2022, 15, 1936–1944. [Google Scholar] [CrossRef]
- Stevenson, R.; Desrochers, S.; Hélie, J.F. Stable and Radiogenic Isotopes as Indicators of Agri-Food Provenance: Insights from Artisanal Cheeses from Quebec, Canada. Int. Dairy J. 2015, 49, 37–45. [Google Scholar] [CrossRef]
- Chung, I.M.; Park, I.; Yoon, J.Y.; Yang, Y.S.; Kim, S.H. Determination of Organic Milk Authenticity Using Carbon and Nitrogen Natural Isotopes. Food Chem. 2014, 160, 214–218. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, J.K.; Lee, K.J.; Son, N.Y.; An, M.J.; Lee, J.H.; An, Y.J.; Kim, S.H. Discrimination of Organic Milk by Stable Isotope Ratio, Vitamin E, and Fatty Acid Profiling Combined with Multivariate Analysis: A Case Study of Monthly and Seasonal Variation in Korea for 2016–2017. Food Chem. 2018, 261, 112–123. [Google Scholar] [CrossRef]
- Molkentin, J.; Giesemann, A. Follow-up of Stable Isotope Analysis of Organic versus Conventional Milk. Anal. Bionanal. Chem. 2010, 398, 1493–1500. [Google Scholar] [CrossRef]
- Molkentin, J. Applicability of Organic Milk Indicators to the Authentication of Processed Products. Food Chem. 2013, 137, 25–30. [Google Scholar] [CrossRef]
- Faberi, A.; Compagnone, D.; Fuselli, F.; La Mantia, A.; Mascini, M.; Montesano, C.; Rocchi, R.; Sergi, M. Italian Cheeses Discrimination by Means of δ13C and δ15N Isotopic Ratio Mass Spectrometry. Food Anal. Methods 2018, 11, 1467–1475. [Google Scholar] [CrossRef]
- Valenti, B.; Biondi, L.; Campidonico, L.; Bontempo, L.; Luciano, G.; Di Paola, F.; Copani, V.; Ziller, L.; Camin, F. Changes in Stable Isotope Ratios in PDO Cheese Related to the Area of Production and Green Forage Availability. The Case Study of Pecorino Siciliano. Rapid Commun. Mass Spectrom. 2017, 31, 737–744. [Google Scholar] [CrossRef]
- Camin, F.; Wehrens, R.; Bertoldi, D.; Bontempo, L.; Ziller, L.; Perini, M.; Nicolini, G.; Nocetti, M.; Larcher, R. H, C, N and S Stable Isotopes and Mineral Profiles to Objectively Guarantee the Authenticity of Grated Hard Cheeses. Anal. Chim. Acta 2012, 711, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Scampicchio, M.; Mimmo, T.; Capici, C.; Huck, C.; Innocente, N.; Drusch, S.; Cesco, S. Identification of Milk Origin and Process-Induced Changes in Milk by Stable Isotope Ratio Mass Spectrometry. J. Agric. Food Chem. 2012, 60, 11268–11273. [Google Scholar] [CrossRef] [PubMed]
- Behkami, S.; Gholami, R.; Gholami, M.; Roohparvar, R. Precipitation Isotopic Information: A Tool for Building the Data Base to Verify Milk Geographical Origin Traceability. Food Control 2020, 107, 106780. [Google Scholar] [CrossRef]
- Bontempo, L.; Lombardi, G.; Paoletti, R.; Ziller, L.; Camin, F. H, C, N and O Stable Isotope Characteristics of Alpine Forage, Milk and Cheese. Int. Dairy J. 2012, 23, 99–104. [Google Scholar] [CrossRef]
- Vander Zanden, H.B.; Soto, D.X.; Bowen, G.J.; Hobson, K.A. Expanding the Isotopic Toolbox: Applications of Hydrogen and Oxygen Stable Isotope Ratios to Food Web Studies. Front. Ecol. Evol. 2016, 4, 20. [Google Scholar] [CrossRef]
- Krivachy Tanz, N.; Rossmann, A.; Schmidt, H.L. Potentials and Caveats with Oxygen and Sulfur Stable Isotope Analyses in Authenticity and Origin Checks of Food and Food Commodities. Food Control 2015, 48, 143–150. [Google Scholar] [CrossRef]
- Gregorčič, S.H.; Ogrinc, N.; Frew, R.; Nečemer, M.; Strojnik, L.; Zuliani, T. The Provenance of Slovenian Milk Using 87sr/86sr Isotope Ratios. Foods 2021, 10, 1729. [Google Scholar] [CrossRef]
- Baffi, C.; Trincherini, P.R. Food Traceability Using the 87Sr/86Sr Isotopic Ratio Mass Spectrometry. Eur. Food Res. Technol. 2016, 242, 1411–1439. [Google Scholar] [CrossRef]
- Flockhart, D.T.T.; Kyser, T.K.; Chipley, D.; Miller, N.G.; Norris, D.R. Experimental Evidence Shows No Fractionation of Strontium Isotopes (87Sr/86Sr) among Soil, Plants, and Herbivores: Implications for Tracking Wildlife and Forensic Science. Isotopes Environ. Health Stud. 2015, 51, 372–381. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, Z.; Jin, B.; Wu, Y.; Xie, L.; Chen, H.; Lin, G.; Zhao, Y.; Rogers, K.M.; Wu, H. Origin Verification of Imported Infant Formula and Fresh Milk into China Using Stable Isotope and Elemental Chemometrics. Food Control 2021, 128, 108165. [Google Scholar] [CrossRef]
- Bontempo, L.; Larcher, R.; Camin, F.; Hölzl, S.; Rossmann, A.; Horn, P.; Nicolini, G. Elemental and Isotopic Characterisation of Typical Italian Alpine Cheeses. Int. Dairy J. 2011, 21, 441–446. [Google Scholar] [CrossRef]
- O’Sullivan, R.; Monahan, F.J.; Bahar, B.; Kirwan, L.; Pierce, K.; O’Shea, A.; Mc Elroy, S.; Malone, F.; Hanafin, B.; Molloy, S.; et al. Stable Isotope Profile (C, N, O, S) of Irish Raw Milk: Baseline Data for Authentication. Food Control 2021, 121, 107643. [Google Scholar] [CrossRef]
- Nečemer, M.; Potočnik, D.; Ogrinc, N. Discrimination between Slovenian Cow, Goat and Sheep Milk and Cheese According to Geographical Origin Using a Combination of Elemental Content and Stable Isotope Data. J. Food Compos. Anal. 2016, 52, 16–23. [Google Scholar] [CrossRef]
- Bontempo, L.; Barbero, A.; Bertoldi, D.; Camin, F.; Larcher, R.; Perini, M.; Sepulcri, A.; Zicarelli, L.; Piasentier, E. Isotopic and Elemental Profiles of Mediterranean Buffalo Milk and Cheese and Authentication of Mozzarella Di Bufala Campana PDO: An Initial Exploratory Study. Food Chem. 2019, 285, 316–323. [Google Scholar] [CrossRef]
- Potočnik, D.; Strojnik, L.; Eftimov, T.; Levart, A.; Ogrinc, N. Fatty Acid and Stable Carbon Isotope Composition of Slovenian Milk: Year, Season, and Regional Variability. Molecules 2020, 25, 2892. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, J.K.; Yang, Y.J.; An, Y.J.; Kim, S.Y.; Kwon, C.; Kim, S.H. A Case Study for Geographical Indication of Organic Milk in Korea Using Stable Isotope Ratios-Based Chemometric Analysis. Food Control 2020, 107, 106755. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, C.; Deng, X.; Zhang, R.; Qu, L.; Wang, M.; Ren, S.; Wu, H.; Yue, Z.; Niu, B. Determining the Geographical Origin of Milk by Multivariate Analysis Based on Stable Isotope Ratios, Elements and Fatty Acids. Anal. Methods 2021, 13, 2537–2548. [Google Scholar] [CrossRef]
- Xie, L.; Zhao, S.; Rogers, K.M.; Xia, Y.; Zhang, B.; Suo, R.; Zhao, Y. A Case of Milk Traceability in Small-Scale Districts-Inner Mongolia of China by Nutritional and Geographical Parameters. Food Chem. 2020, 316, 126332. [Google Scholar] [CrossRef]
- Erich, S.; Schill, S.; Annweiler, E.; Waiblinger, H.U.; Kuballa, T.; Lachenmeier, D.W.; Monakhova, Y.B. Combined Chemometric Analysis of 1H NMR, 13C NMR and Stable Isotope Data to Differentiate Organic and Conventional Milk. Food Chem. 2015, 188, 1–7. [Google Scholar] [CrossRef]
- Rogers, K.M.; Van Ruth, S.; Alewijn, M.; Philips, A.; Rogers, P. Verification of Egg Farming Systems from the Netherlands and New Zealand Using Stable Isotopes. J. Agric. Food Chem. 2015, 63, 8372–8380. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.M.; Shi, G.Y.; Wang, H.W. Differentiation of Pigment in Eggs Using Carbon (13C/12C) and Nitrogen (15N/14N) Stable Isotopes. J. AOAC Int. 2016, 99, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Donarski, J.; Camin, F.; Fauhl-Hassek, C.; Posey, R.; Sudnik, M. Sampling Guidelines for Building and Curating Food Authenticity Databases. Trends Food Sci. Technol. 2019, 90, 187–193. [Google Scholar] [CrossRef]
- Xia, Y.; Jia, L.; Zhang, K.; Xie, J.; Yu, E.; Tian, J.; Gong, W.; Li, Z.; Li, H.; Wang, G.; et al. Geographical Origin Traceability of Procambarus Clarkii Based on Mineral Elements and Stable Isotopes. Foods 2022, 11, 3060. [Google Scholar] [CrossRef]
- Li, L.; Kokkuar, N.; Han, C.; Ren, W.; Dong, S. Effects of Dietary Shifts on the Stable Isotope Signature of Pacific White Shrimp Litopenaeus Vannamei and Implications for Traceability. Mar. Freshw. Res. 2020, 71, 1294–1300. [Google Scholar] [CrossRef]
- Gopi, K.; Mazumder, D.; Sammut, J.; Saintilan, N.; Crawford, J.; Gadd, P. Combined Use of Stable Isotope Analysis and Elemental Profiling to Determine Provenance of Black Tiger Prawns (Penaeus Monodon). Food Control 2019, 95, 242–248. [Google Scholar] [CrossRef]
- Luo, R.; Jiang, T.; Chen, X.; Zheng, C.; Liu, H.; Yang, J. Determination of Geographic Origin of Chinese Mitten Crab (Eriocheir Sinensis) Using Integrated Stable Isotope and Multi-Element Analyses. Food Chem. 2019, 274, 1–7. [Google Scholar] [CrossRef]
- Li, L.; Ren, W.; Dong, S.; Feng, J. Investigation of Geographic Origin, Salinity and Feed on Stable Isotope Profile of Pacific White Shrimp (Litopenaeus Vannamei). Aquac. Res. 2018, 49, 1029–1036. [Google Scholar] [CrossRef]
- Kim, H.; Kumar, K.S.; Hwang, S.Y.; Kang, B.C.; Moon, H.B.; Shin, K.H. Utility of Stable Isotope and Cytochrome Oxidase I Gene Sequencing Analyses in Inferring Origin and Authentication of Hairtail Fish and Shrimp. J. Agric. Food Chem. 2015, 63, 5548–5556. [Google Scholar] [CrossRef]
- Carter, J.F.; Tinggi, U.; Yang, X.; Fry, B. Stable Isotope and Trace Metal Compositions of Australian Prawns as a Guide to Authenticity and Wholesomeness. Food Chem. 2015, 170, 241–248. [Google Scholar] [CrossRef]
- Dong, X.; Han, C.; Li, L. Stable Isotope Ratio Analysis for the Authentication of Sea Urchin (Mesocentrotus Nudus) from Different Culture Areas in the North Yellow Sea, China. Aquaculture 2022, 561, 738637. [Google Scholar] [CrossRef]
- Kang, X.; Zhao, Y.; Liu, W.; Ding, H.; Zhai, Y.; Ning, J.; Sheng, X. Geographical Traceability of Sea Cucumbers in China via Chemometric Analysis of Stable Isotopes and Multi-Elements. J. Food Compos. Anal. 2021, 99, 103852. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Li, Y.; Zhang, X.; Qi, H. Authentication of the Sea Cucumber (Apostichopus Japonicus) Using Amino Acids Carbon Stable Isotope Fingerprinting. Food Control 2018, 91, 128–137. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Li, Y.; Zhao, X. Identification of the Geographical Origins of Sea Cucumber (Apostichopus Japonicus) in Northern China by Using Stable Isotope Ratios and Fatty Acid Profiles. Food Chem. 2017, 218, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Li, X.; Ran, G.; Chen, J.; Jiang, X.; Sun, J.; Bai, W. Determination of the Geographical Origin of Trachinotus Ovatus and Pampus Argenteus in China by Multi-Element and Stable Isotope Analysis. Food Chem. 2022, 394, 133457. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yuan, Y.; Zhao, Y.; Zhang, Y.; Nie, J.; Shao, S.; Rogers, K.M. Differentiating Wild, Lake-Farmed and Pond-Farmed Carp Using Stable Isotope and Multi-Element Analysis of Fish Scales with Chemometrics. Food Chem. 2020, 328, 127115. [Google Scholar] [CrossRef]
- Vasconi, M.; Lopez, A.; Galimberti, C.; Moreno Rojas, J.M.; Muñoz Redondo, J.M.; Bellagamba, F.; Moretti, V.M. Authentication of Farmed and Wild European Eel (Anguilla Anguilla) by Fatty Acid Profile and Carbon and Nitrogen Isotopic Analyses. Food Control 2019, 102, 112–121. [Google Scholar] [CrossRef]
- Wang, Y.V.; Wan, A.H.L.; Lock, E.J.; Andersen, N.; Winter-Schuh, C.; Larsen, T. Know Your Fish: A Novel Compound-Specific Isotope Approach for Tracing Wild and Farmed Salmon. Food Chem. 2018, 256, 380–389. [Google Scholar] [CrossRef]
- Farabegoli, F.; Pirini, M.; Rotolo, M.; Silvi, M.; Testi, S.; Ghidini, S.; Zanardi, E.; Remondini, D.; Bonaldo, A.; Parma, L.; et al. Toward the Authentication of European Sea Bass Origin through a Combination of Biometric Measurements and Multiple Analytical Techniques. J. Agric. Food Chem. 2018, 66, 6822–6831. [Google Scholar] [CrossRef]
- Carrera, M.; Gallardo, J.M. Determination of the Geographical Origin of All Commercial Hake Species by Stable Isotope Ratio (SIR) Analysis. J. Agric. Food Chem. 2017, 65, 1070–1077. [Google Scholar] [CrossRef]
- Coulter, D.P.; Bowen, G.J.; Höök, T.O. Influence of Diet and Ambient Water on Hydrogen and Oxygen Stable Isotope Ratios in Fish Tissue: Patterns within and among Tissues and Relationships with Growth Rates. Hydrobiologia 2017, 799, 111–121. [Google Scholar] [CrossRef]
- Chaguri, M.P.; Maulvault, A.L.; Costa, S.; Gonçalves, A.; Nunes, M.L.; Carvalho, M.L.; Sant’Ana, L.S.; Bandarra, N.; Marques, A. Chemometrics Tools to Distinguish Wild and Farmed Meagre (Argyrosomus Regius). J. Food Process. Preserv. 2017, 41, e13312. [Google Scholar] [CrossRef]
- Cambiè, G.; Kaiser, M.J.; Marriott, A.L.; Fox, J.; Lambert, G.; Hiddink, J.G.; Overy, T.; Bennet, S.A.; Leng, M.J.; Mc Carthy, I.D. Stable Isotope Signatures Reveal Small-Scale Spatial Separation in Populations of European Sea Bass. Mar. Ecol. Prog. Ser. 2016, 546, 213–223. [Google Scholar] [CrossRef]
- Molkentin, J.; Lehmann, I.; Ostermeyer, U.; Rehbein, H. Traceability of Organic Fish—Authenticating the Production Origin of Salmonids by Chemical and Isotopic Analyses. Food Control 2015, 53, 55–66. [Google Scholar] [CrossRef]
- Chaguri, M.P.; Maulvault, A.L.; Nunes, M.L.; Santiago, D.A.; Denadai, J.C.; Fogaça, F.H.; Sant’Ana, L.S.; Ducatti, C.; Bandarra, N.; Carvalho, M.L.; et al. Different Tools to Trace Geographic Origin and Seasonality of Croaker (Micropogonias Furnieri). LWT-Food Sci. Technol. 2015, 61, 194–200. [Google Scholar] [CrossRef]
- Kim, H.; Suresh Kumar, K.; Shin, K.H. Applicability of Stable C and N Isotope Analysis in Inferring the Geographical Origin and Authentication of Commercial Fish (Mackerel, Yellow Croaker and Pollock). Food Chem. 2015, 172, 523–527. [Google Scholar] [CrossRef]
- Monteiro Oliveira, E.J.V.; Sant’Ana, L.S.; Ducatti, C.; Denadai, J.C.; de Souza Kruliski, C.R. The Use of Stable Isotopes for Authentication of Gadoid Fish Species. Europ.Food Res. Technol. 2011, 232, 97–101. [Google Scholar] [CrossRef]
- Martín-Pérez, M.; Fernández-Borràs, J.; Ibarz, A.; Felip, O.; Gutiérrez, J.; Blasco, J. Stable Isotope Analysis Combined with Metabolic Indices Discriminates between Gilthead Sea Bream (Sparus Aurata) Fingerlings Produced in Various Hatcheries. J. Agric. Food Chem. 2011, 59, 10261–10270. [Google Scholar] [CrossRef]
- Sant’Ana, L.S.; Ducatti, C.; Ramires, D.G. Seasonal Variations in Chemical Composition and Stable Isotopes of Farmed and Wild Brazilian Freshwater Fish. Food Chem. 2010, 122, 74–77. [Google Scholar] [CrossRef]
- Bianchini, G.; Brombin, V.; Carlino, P.; Mistri, E.; Natali, C.; Salani, G.M. Traceability and Authentication of Manila Clams from North-Western Adriatic Lagoons Using C and N Stable Isotope Analysis. Molecules 2021, 26, 1859. [Google Scholar] [CrossRef]
- Zhang, X.; Han, D.; Chen, X.; Zhao, X.; Cheng, J.; Liu, Y. Combined Use of Fatty Acid Profile and Fatty Acid δ13C Fingerprinting for Origin Traceability of Scallops (Patinopecten Yessoensis, Chlamys Farreri, and Argopecten Irradians). Food Chem. 2019, 298, 124966. [Google Scholar] [CrossRef] [PubMed]
- Martino, J.C.; Mazumder, D.; Gadd, P.; Doubleday, Z.A. Tracking the Provenance of Octopus Using Isotopic and Multi-Elemental Analysis. Food Chem. 2022, 371, 131133. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, Y.; Chen, X.; Chen, L. Potential Use of Stable Isotope and Fatty Acid Analyses for Traceability of Geographic Origins of Jumbo Squid (Dosidicus Gigas). Rapid Commun. Mass Spectrom. 2018, 32, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Perini, M.; Nfor, M.B.; Camin, F.; Pianezze, S.; Piasentier, E. Using Bioelements Isotope Ratios and Fatty Acid Composition to Deduce Beef Origin and Zebu Feeding Regime in Cameroon. Molecules 2021, 26, 2155. [Google Scholar] [CrossRef]
- Nie, J.; Shao, S.; Xia, W.; Liu, Z.; Yu, C.; Li, R.; Wang, W.; Li, J.; Yuan, Y.; Rogers, K.M. Stable Isotopes Verify Geographical Origin of Yak Meat from Qinghai-Tibet Plateau. Meat Sci. 2020, 165, 108113. [Google Scholar] [CrossRef]
- Jiang, D.; Du, L.; Guo, Y.; Ma, J.; Li, X.; Han, L.; Xu, Y.; Qian, Y. Potential Use of Stable Isotope and Multi-Element Analyses for Regional Geographical Traceability of Bone Raw Materials for Gelatin Production. Food Anal. Methods 2020, 13, 762–769. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, B.; Chen, G.; Chen, A.; Yang, S.; Ye, Z. Tracing the Geographic Origin of Beef in China on the Basis of the Combination of Stable Isotopes and Multielement Analysis. J. Agric. Food Chem. 2013, 61, 7055–7060. [Google Scholar] [CrossRef]
- Yanagi, Y.; Hirooka, H.; Oishi, K.; Choumei, Y.; Hata, H.; Arai, M.; Kitagawa, M.; Gotoh, T.; Inada, S.; Kumagai, H. Stable Carbon and Nitrogen Isotope Analysis as a Tool for Inferring Beef Cattle Feeding Systems in Japan. Food Chem. 2012, 134, 502–506. [Google Scholar] [CrossRef]
- Kim, S.H.; Cruz, G.D.; Fadel, J.G.; Clifford, A.J. Food Authenticity Using Natural Carbon Isotopes (12C, 13C, 14C) in Grass-Fed and Grain-Fed Beef. Food Sci. Biotechnol. 2012, 21, 295–298. [Google Scholar] [CrossRef]
- Baroni, M.V.; Podio, N.S.; Badini, R.G.; Inga, M.; Ostera, H.A.; Cagnoni, M.; Gallegos, E.; Gautier, E.; Peral-García, P.; Hoogewerff, J.; et al. How Much Do Soil and Water Contribute to the Composition of Meat? A Case Study: Meat from Three Areas of Argentina. J. Agric. Food Chem. 2011, 59, 11117–11128. [Google Scholar] [CrossRef]
- Bong, Y.S.; Shin, W.J.; Lee, A.R.; Kim, Y.S.; Kim, K.; Lee, K.S. Tracing the Geographical Origin of Beefs Being Circulated in Korean Markets Based on Stable Isotopes. Rapid Commun. Mass Spectrom. 2010, 24, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Horacek, M.; Min, J.S. Discrimination of Korean Beef from Beef of Other Origin by Stable Isotope Measurements. Food Chem. 2010, 121, 517–520. [Google Scholar] [CrossRef]
- Horacek, M.; Eisinger, E.; Papesch, W. Reliability of Stable Isotope Values from Meat Juice for the Determination of the Meat Origin. Food Chem. 2010, 118, 910–914. [Google Scholar] [CrossRef]
- Qie, M.; Zhang, B.; Li, Z.; Zhao, S.; Zhao, Y. Data Fusion by Ratio Modulation of Stable Isotope, Multi-Element, and Fatty Acids to Improve Geographical Traceability of Lamb. Food Control 2021, 120, 107549. [Google Scholar] [CrossRef]
- Liu, H.; Qin, Y.; Ma, Q.; Zhao, Q.; Guo, X.; Ma, L.; Gou, C.; Xia, Y.; Gan, R.Y.; Zhang, J. Discrimination the Geographical Origin of Yanchi Tan Lamb with Different Muscle Sections by Stable Isotopic Ratios and Elemental Profiles. Int. J. Food Sci. Technol. 2021, 56, 2604–2611. [Google Scholar] [CrossRef]
- Erasmus, S.W.; Muller, M.; Butler, M.; Hoffman, L.C. The Truth Is in the Isotopes: Authenticating Regionally Unique South African Lamb. Food Chem. 2018, 239, 926–934. [Google Scholar] [CrossRef]
- Erasmus, S.W.; Muller, M.; Van Der Rijst, M.; Hoffman, L.C. Stable Isotope Ratio Analysis: A Potential Analytical Tool for the Authentication of South African Lamb Meat. Food Chem. 2016, 192, 997–1005. [Google Scholar] [CrossRef]
- Zhaxi, C.; Zhao, S.; Zhang, T.; Dong, H.; Liu, H.; Zhao, Y. Stable Isotopes Verify Geographical Origin of Tibetan Chicken. Food Chem. 2021, 358, 129893. [Google Scholar] [CrossRef]
- Coletta, L.D.; Pereira, A.L.; Coelho, A.A.D.; Savino, V.J.M.; Menten, J.F.M.; Correr, E.; Frana, L.C.; Martinelli, L.A. Barn vs. Free-Range Chickens: Differences in Their Diets Determined by Stable Isotopes. Food Chem. 2012, 131, 155–160. [Google Scholar] [CrossRef]
- Cruz, V.C.; Araújo, P.C.; Sartori, J.R.; Pezzato, A.C.; Denadai, J.C.; Polycarpo, G.V.; Zanetti, L.H.; Ducatti, C. Poultry Offal Meal in Chicken: Traceability Using the Technique of Carbon (13C12C)- and Nitrogen (15N/14N)-Stable Isotopes. Poult. Sci. 2012, 91, 478–486. [Google Scholar] [CrossRef]
- Cristea, G.; Voica, C.; Feher, I.; Puscas, R.; Magdas, D.A. Isotopic and Elemental Characterization of Romanian Pork Meat in Corroboration with Advanced Chemometric Methods: A First Exploratory Study. Meat Sci. 2022, 189, 108825. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tu, T.; Tang, X.; Zhao, S.; Qie, M.; Chen, A.; Yang, S. Authentication of Organic Pork and Identification of Geographical Origins of Pork in Four Regions of China by Combined Analysis of Stable Isotopes and Multi-Elements. Meat Sci. 2020, 165, 108129. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.J.; Choi, S.H.; Ryu, J.S.; Song, B.Y.; Song, J.H.; Park, S.; Min, J.S. Discrimination of the Geographic Origin of Pork Using Multi-Isotopes and Statistical Analysis. Rapid Commun. Mass Spectrom. 2018, 32, 1843–1850. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, S.; Wang, D. Stable Carbon and Nitrogen Isotopes as a Potential Tool to Differentiate Pork from Organic and Conventional Systems. J. Sci. Food Agric. 2016, 96, 3950–3955. [Google Scholar] [CrossRef]
- Perini, M.; Thomas, F.; Cabañero Ortiz, A.I.; Simoni, M.; Camin, F. Stable Isotope Ratio Analysis of Lactose as a Possible Potential Geographical Tracer of Milk. Food Control 2022, 139, 109051. [Google Scholar] [CrossRef]
- Kalpage, M.; Dissanayake, C.; Diyabalanage, S.; Chandrajith, R.; Frew, R.; Fernando, R. Stable Isotope and Element Profiling for Determining the Agroclimatic Origin of Cow Milk within a Tropical Country. Foods 2022, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Bay, L.J.; Goh, G.; Ang, T.H.; Kong, K.; Chew, P.; Koh, S.P.; Ch’ng, A.L.; Phang, H.; Chiew, P. Multivariate Statistical Analysis of Stable Isotope Signatures and Element Concentrations to Differentiate the Geographical Origin of Retail Milk Sold in Singapore. Food Control 2021, 123, 107736. [Google Scholar] [CrossRef]
- Wijenayake, K.; Frew, R.; Mc Comb, K.; Van Hale, R.; Clarke, D. Feasibility of Casein to Record Stable Isotopic Variation of Cow Milk in New Zealand. Molecules 2020, 25, 3658. [Google Scholar] [CrossRef]
- Potočnik, D.; Nečemer, M.; Perišić, I.; Jagodic, M.; Mazej, D. Geographical Verification of Slovenian Milk Using Stable Isotope Ratio, Multi- Element and Multivariate Modelling Approaches. Food Chem. 2020, 326, 126958. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, Y.; Rogers, K.M.; Chen, G.; Chen, A.; Yang, S. Application of Multi-Element (C, N, H, O) Stable Isotope Ratio Analysis for the Traceability of Milk Samples from China. Food Chem. 2020, 310, 125826. [Google Scholar] [CrossRef]
- Magdas, D.A.; Feher, I.; Cristea, G.; Voica, C.; Tabaran, A.; Mihaiu, M.; Cordea, D.V.; Bâlteanu, V.A.; Dan, S.D. Geographical Origin and Species Differentiation of Transylvanian Cheese. Comparative Study of Isotopic and Elemental Profiling vs. DNA Results Food Chem. 2019, 277, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Kim, J.K.; Yarnes, C.T.; An, Y.J.; Kwon, C.; Kim, S.Y.; Yang, Y.J.; Chi, H.Y.; Kim, S.H. Fatty Acid- and Amino Acid-Specific Isotope Analysis for Accurate Authentication and Traceability in Organic Milk. J. Agric. Food Chem. 2019, 67, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Griboff, J.; Baroni, M.V.; Horacek, M.; Wunderlin, D.A.; Monferran, M.V. Multielemental + isotopic Fingerprint Enables Linking Soil, Water, Forage and Milk Composition, Assessing the Geographical Origin of Argentinean Milk. Food Chem. 2019, 283, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Garbaras, A.; Skipitytė, R.; Šapolaitė, J.; Ežerinskis, Ž.; Remeikis, V. Seasonal Variation in Stable Isotope Ratios of Cow Milk in Vilnius Region, Lithuania. Animals 2019, 9, 69. [Google Scholar] [CrossRef]
- Bostic, J.N.; Hagopian, W.M.; Jahren, A.H. Carbon and Nitrogen Stable Isotopes in U.S. Milk: Insight into Production Process. Rapid Commun. Mass Spectrom. 2018, 32, 561–566. [Google Scholar] [CrossRef]
- Behkami, S.; Zain, S.M.; Gholami, M.; Bakirdere, S. Isotopic Ratio Analysis of Cattle Tail Hair: A Potential Tool in Building the Database for Cow Milk Geographical Traceability. Food Chem. 2017, 217, 438–444. [Google Scholar] [CrossRef]
- Dong, H.; Xiao, K.; Luo, D. Stability of Carbon and Nitrogen Isotopic Compositions of the Protein Extracted from Milk and Their Potential as “Fingerprints” of Geographical Origin. RSC Adv. 2017, 7, 18946–18952. [Google Scholar] [CrossRef]
- Magdas, D.A.; Dehelean, A.; Feher, I.; Cristea, G.; Puscas, R.; Dan, S.D.; Cordea, D.V. Discrimination Markers for the Geographical and Species Origin of Raw Milk within Romania. Int. Dairy J. 2016, 61, 135–141. [Google Scholar] [CrossRef]
- Scampicchio, M.; Eisenstecken, D.; De Benedictis, L.; Capici, C.; Ballabio, D.; Mimmo, T.; Robatscher, P.; Kerschbaumer, L.; Oberhuber, M.; Kaser, A.; et al. Multi-Method Approach to Trace the Geographical Origin of Alpine Milk: A Case Study of Tyrol Region. Food Anal. Methods 2016, 9, 1262–1273. [Google Scholar] [CrossRef]
- Ehtesham, E.; Hayman, A.; Van Hale, R.; Frew, R. Influence of Feed and Water on the Stable Isotopic Composition of Dairy Milk. Int. Dairy J. 2015, 47, 37–45. [Google Scholar] [CrossRef]
- Camin, F.; Bertoldi, D.; Santato, A.; Bontempo, L.; Perini, M.; Ziller, L.; Stroppa, A.; Larcher, R. Validation of Methods for H, C, N and S Stable Isotopes and Elemental Analysis of Cheese: Results of an International Collaborative Study. Rapid Commun. Mass Spectrom. 2015, 29, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Ehtesham, E.; Hayman, A.R.; Mc Comb, K.A.; Van Hale, R.; Frew, R.D. Correlation of Geographical Location with Stable Isotope Values of Hydrogen and Carbon of Fatty Acids from New Zealand Milk and Bulk Milk Powder. J. Agric. Food Chem. 2013, 61, 8914–8923. [Google Scholar] [CrossRef] [PubMed]
- Chesson, L.A.; Valenzuela, L.O.; O’Grady, S.P.; Cerling, T.E.; Ehleringer, J.R. Hydrogen and Oxygen Stable Isotope Ratios of Milk in the United States. J. Agric. Food Chem. 2010, 58, 2358–2363. [Google Scholar] [CrossRef] [PubMed]
- Altieri, S.; Saiano, K.; Biondi, M.; Ricci, P.; Lubritto, C. Traceability of ‘Mozzarella Di Bufala Campana’ Production Chain by Means of Carbon, Nitrogen and Oxygen Stable Isotope Ratios. J. Sci. Food Agric. 2020, 100, 995–1003. [Google Scholar] [CrossRef]
- Silva, A.V.; Hélie, J.F.; de Andrade Caxito, F.; Monardes, H.; Mustafa, A.F.; Stevenson, R. Multi-Stable Isotope Analysis as a Tool for Assessing the Geographic Provenance of Dairy Products: A Case Study Using Buffalo’s Milk and Cheese Samples from the Amazon Basin, Brazil. Int. Dairy J. 2014, 35, 107–110. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Q.; Guo, X.; Tang, C.; Yu, X. Application of Isotopic and Elemental Fingerprints in Identifying the Geographical Origin of Goat Milk in China. Food Chem. 2019, 277, 448–454. [Google Scholar] [CrossRef]
- Mc Leod, R.J.; Prosser, C.G.; Wakefield, J.W. Identification of Goat Milk Powder by Manufacturer Using Multiple Chemical Parameters. J. Dairy Sci. 2016, 99, 982–993. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varrà, M.O.; Zanardi, E.; Serra, M.; Conter, M.; Ianieri, A.; Ghidini, S. Isotope Fingerprinting as a Backup for Modern Safety and Traceability Systems in the Animal-Derived Food Chain. Molecules 2023, 28, 4300. https://doi.org/10.3390/molecules28114300
Varrà MO, Zanardi E, Serra M, Conter M, Ianieri A, Ghidini S. Isotope Fingerprinting as a Backup for Modern Safety and Traceability Systems in the Animal-Derived Food Chain. Molecules. 2023; 28(11):4300. https://doi.org/10.3390/molecules28114300
Chicago/Turabian StyleVarrà, Maria Olga, Emanuela Zanardi, Matteo Serra, Mauro Conter, Adriana Ianieri, and Sergio Ghidini. 2023. "Isotope Fingerprinting as a Backup for Modern Safety and Traceability Systems in the Animal-Derived Food Chain" Molecules 28, no. 11: 4300. https://doi.org/10.3390/molecules28114300
APA StyleVarrà, M. O., Zanardi, E., Serra, M., Conter, M., Ianieri, A., & Ghidini, S. (2023). Isotope Fingerprinting as a Backup for Modern Safety and Traceability Systems in the Animal-Derived Food Chain. Molecules, 28(11), 4300. https://doi.org/10.3390/molecules28114300








