Review of Recent Developments in the Fabrication of ZnO/CdS Heterostructure Photocatalysts for Degradation of Organic Pollutants and Hydrogen Production
Abstract
1. Introduction
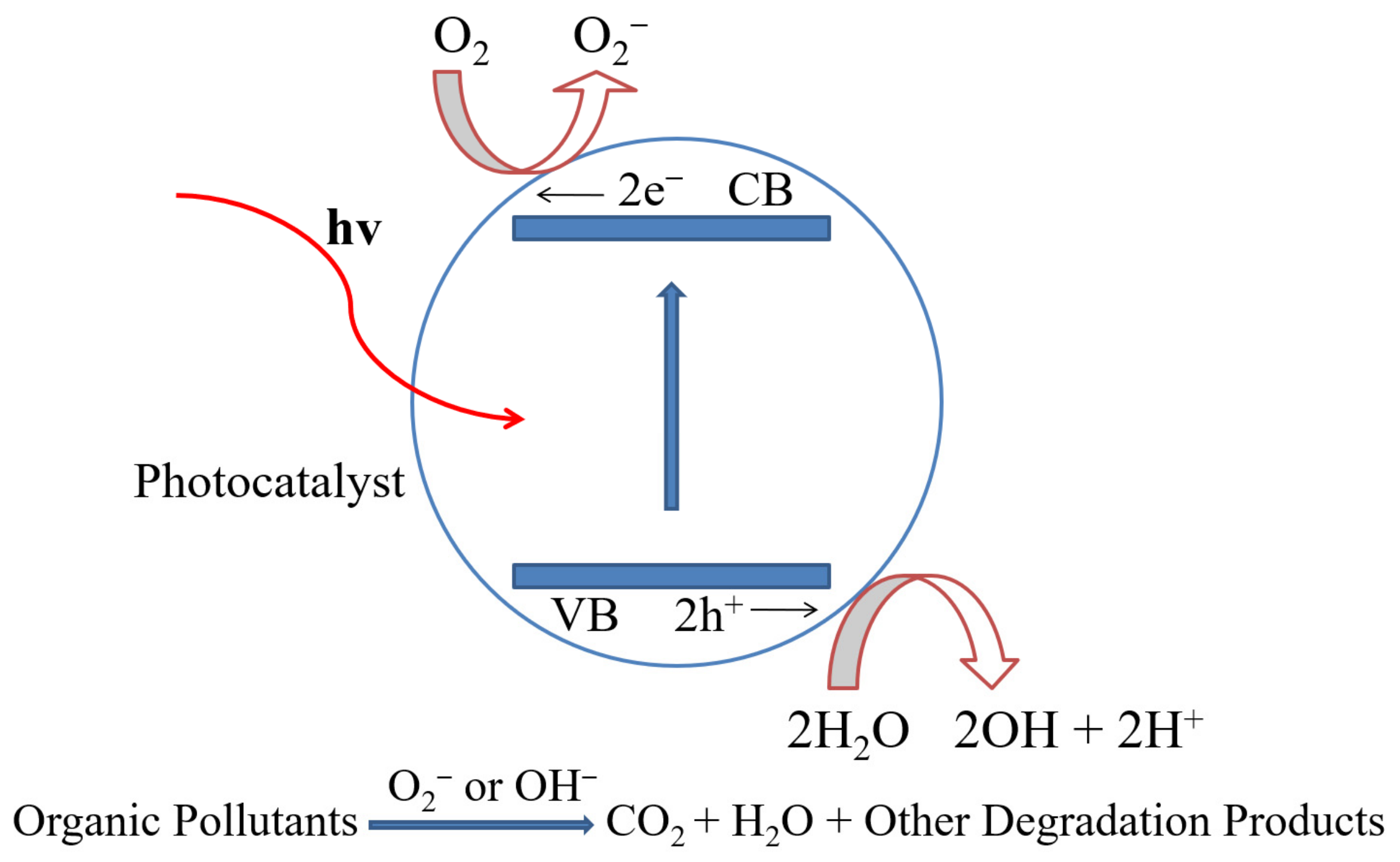
2. Development of ZnO/CdS Heterostructure Photocatalysts
2.1. Chemical CoPrecipitation/Microwave Assisted CoPrecipitation Method
2.2. Microwave Method
2.3. Hydrothermal Method
2.4. Solvothermal Method
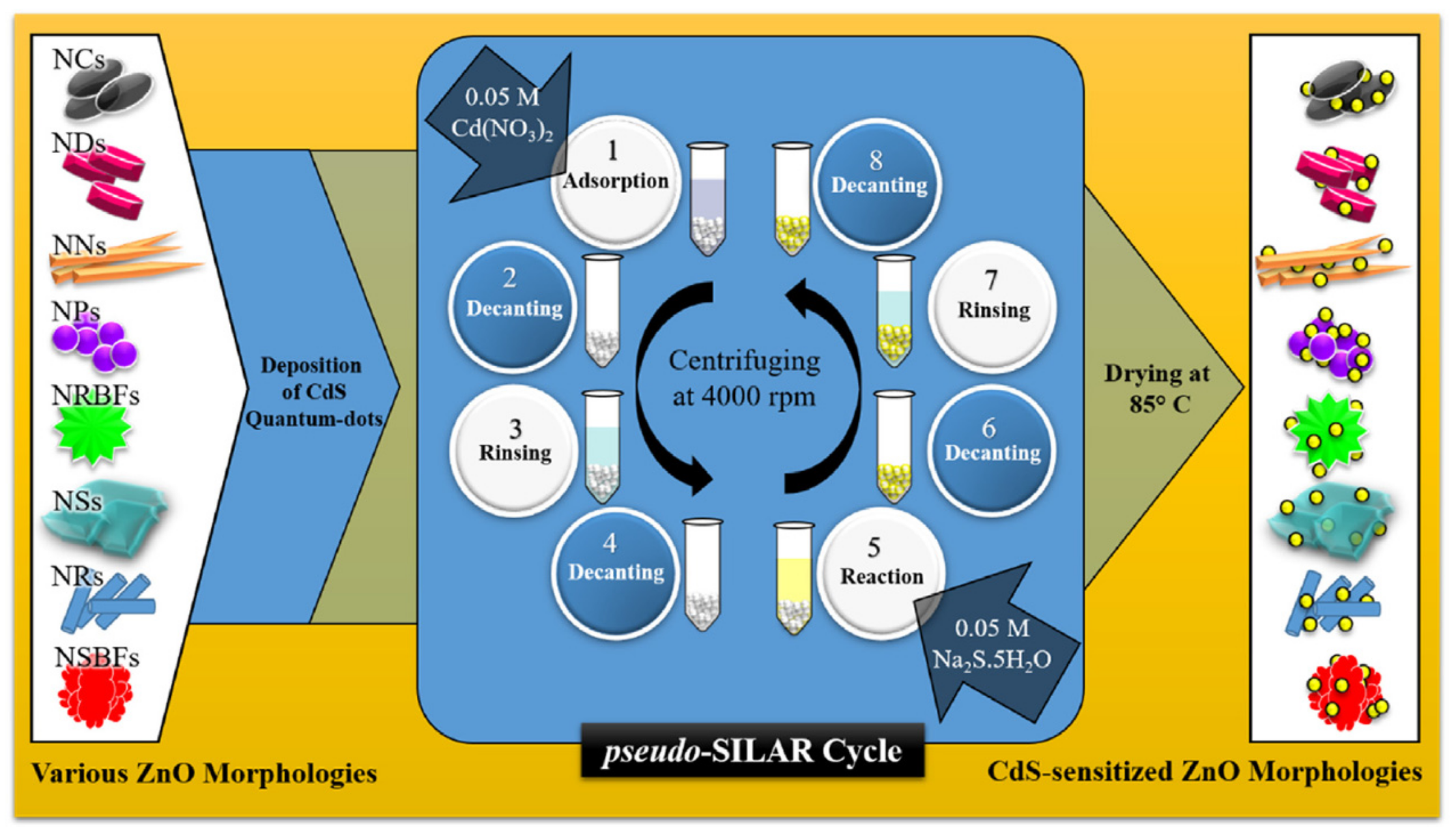
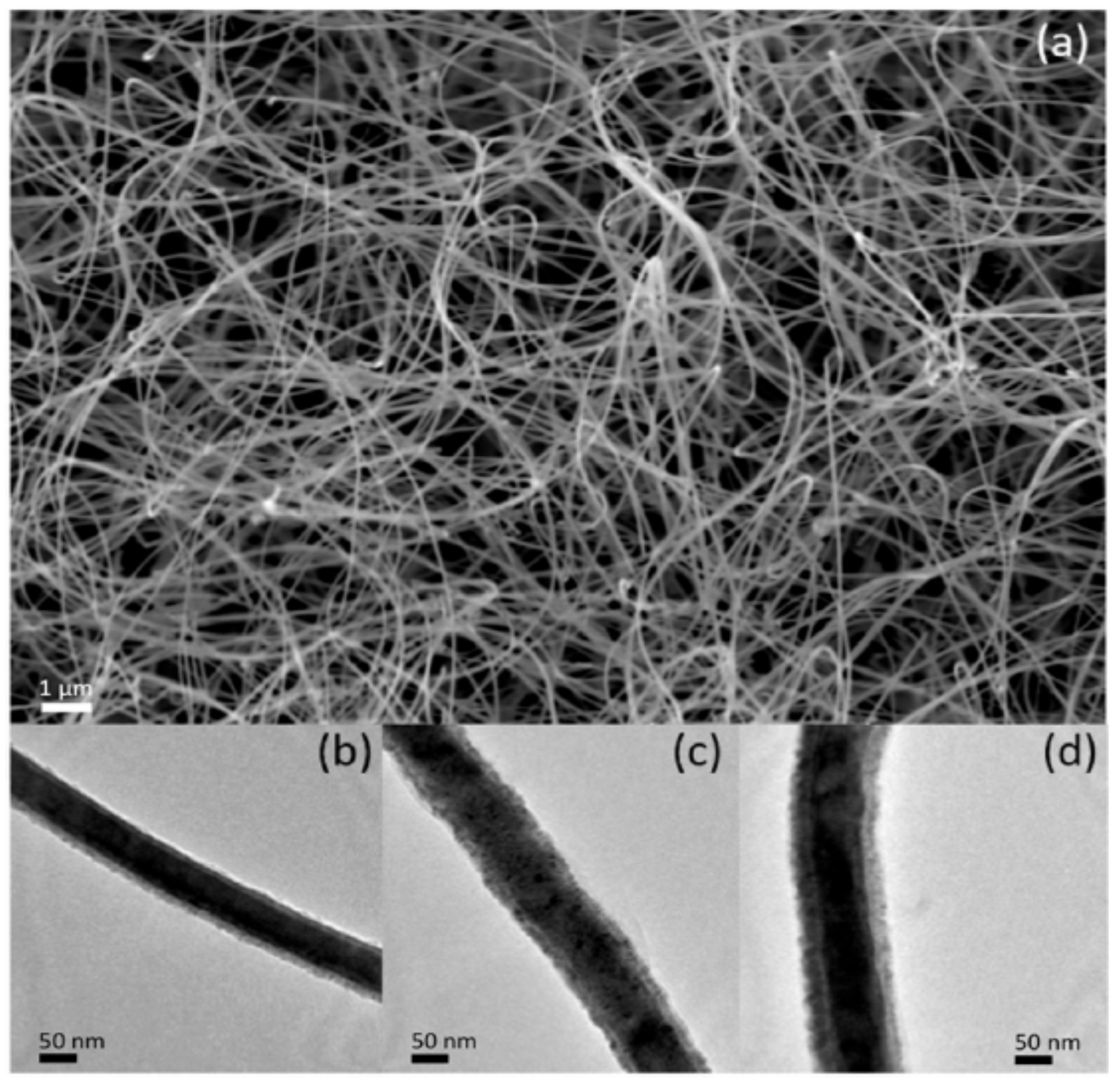
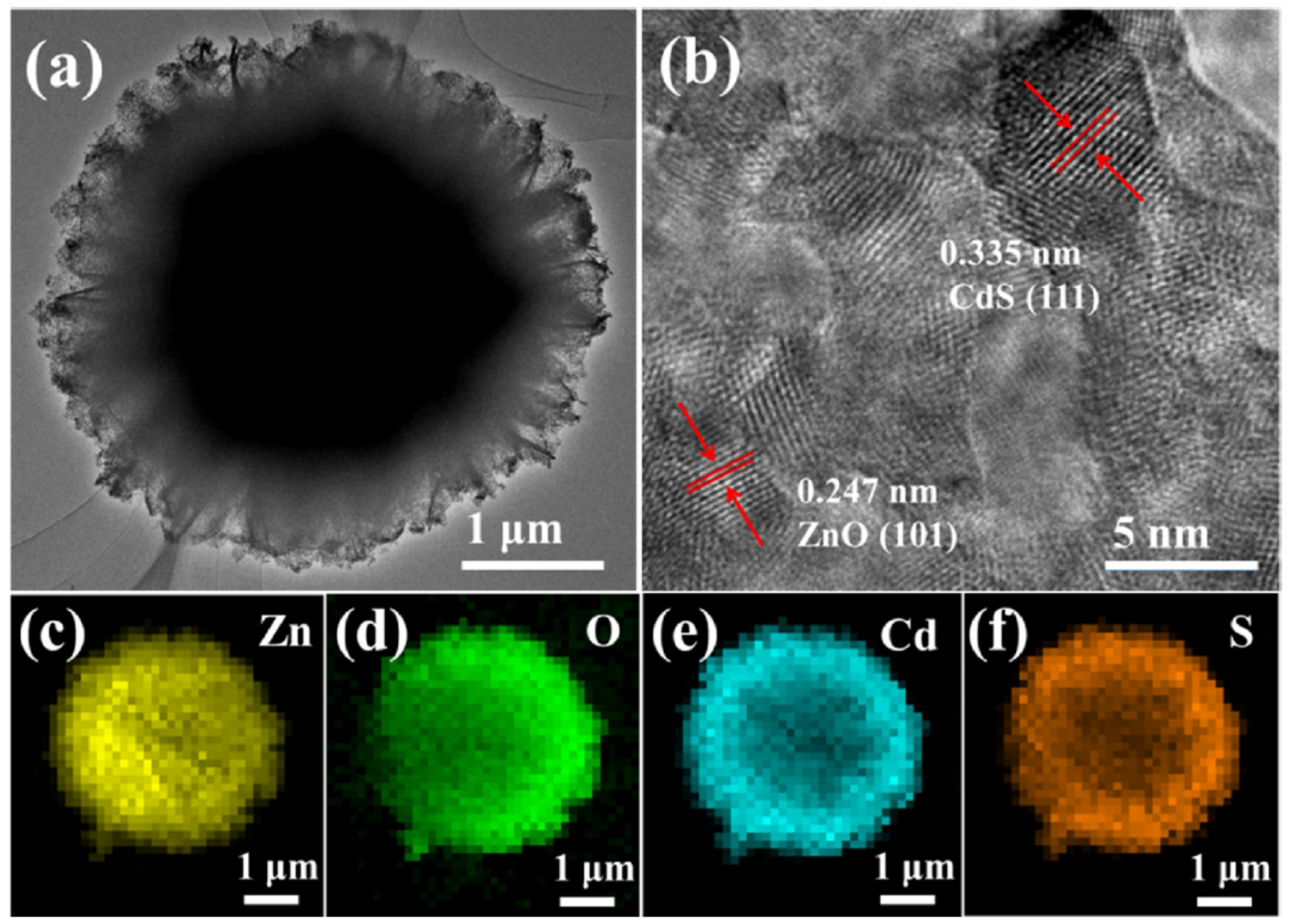
2.5. Successive Ionic Layer Adsorption-Reaction (SILAR) Method
| Catalyst | Main Precursors | Method | Temperature | Reaction Time | Morphology | Ref | |
|---|---|---|---|---|---|---|---|
| Zinc | Cadmium | ||||||
| CdS@ZnO | Zn(NO3)2 6H2O | Cd(CH3COO)2 2H2O | Atomic layer | 250 °C | - | Nanospheres | [65] |
| ZnO/CdS | Zn(CH3COO)2·2H2O | Cd(NO3)2 4H2O | Chemical bath deposition | 60 °C | 12 h | Nanofibers | [64] |
| ZnO/CdS | Zn(NO3)2 6H2O | CdSO4 | Chemical bath deposition | 70 °C | - | Nanorods | [80] |
| ZnO/CdS/CuS | Zn(CH3COO)2·2H2O | CdCl2 2H2O | Chemical solution deposition | 120 °C | 4 h | - | [81] |
| CdS/ZnO | Zn(CH3COO)2·2H2O | Cd(NO3)2 4H2O | Hydrothermal | 300 °C | 3 h | Nanosheet | [82] |
| ZnO/CdS | ZnCl2 | Cd(NO3)2 4H2O | Hydrothermal | 200 °C | 24 h | Nanoparticles | [38] |
| ZnO/CdS | Zn(NO3)2 6H2O | Cd(NO3)2 4H2O | Hydrothermal | 120 °C | 12 h | - | [72] |
| ZnO/CdS | Zn(CH3COO)2·2H2O | CdCl2 | Hydrothermal | 40 °C | 20 min | Nanosheets | [83] |
| ZnO/CdS | Zn(NO3)2 6H2O | Cd(CH3COO)2 2H2O | Hydrothermal | 120 °C | 24 h | - | [60] |
| ZnO/CdS | Zn(NO3)2 6H2O | CdCl2 5H2O | In situ | 90 °C | 1.5 h | Nanorods | [84] |
| CdS/ZnO | Zn(NO3)2 | Cd(CH3COO)2 2H2O | Microwave | - | 20 min | Nanorods | [69] |
| ZnO/CdS | Zn(CH3COO)2·2H2O | Cd(CH3COO)2 2H2O | Microwave | 100 °C | 2 h | Nanosheets | [59] |
| GO/CdS/ZnO | Zn(NO3)2 6H2O | Cd(CH3COO)2 2H2O | Microwave-assisted co-precipitation | 240 W | 30 min | Nanorods | [58] |
| ZnO/CdS | Zn(NO3)2 6H2O | Cd(CH3COO)2 2H2O | One-pot | RT | - | Nanostructures | [85] |
| CdS/ZnO | Zn(CH3COO)2·2H2O | Cd(NO3)2 4H2O | Photodeposition technique | RT | 30 min | Nanorods | [73] |
| CdS/ZnO | Zn(CH3COO)2·2H2O | Cd(CH3COO)2 2H2O | Precipitation | 90 °C | 1 h | Nanoflowers | [37] |
| ZnO/CdS | Zn(NO3)2 | Cd(NO3)2 | Silar | 250 °C | 10 min | Nanorods | [76] |
| PbS/CdS/ZnO | Zn(CH3COO)2·2H2O | CdCl2 | Silar | 110 °C | 4 h | Nanowire | [77] |
| ZnO/CdS/CdSe | Zn(NO3)2 6H2O | Cd(NO3)2 4H2O | Silar | RT | - | Nanorods | [86] |
| ZnO/CdS | Zn(CH3COO)2·2H2O | CdCl2 5H2O | Silar | 500 °C | 2 h | Nanofilm | [78] |
| ZnO-CdS | Zn(CH3COO)2 | Cd(NO3)2 | Silar | 300 °C | 2 h | Nanorods | [63] |
| CdS@ZnO | Zn(CH3COO)2·2H2O | Cd(CH3COO)2 2H2O | Solvothermal | 80 °C | 48 h | - | [61] |
| ZnO/CdS | Zn(CH3COO)2·2H2O | Cd(CH3COO)2 2H2O | Ultra-sonication | 80 °C | 2 h | Nanostructures | [62] |
| ZnO-CdS | Zn(NO3)2 6H2O | Cd(NO3)2 4H2O | Wet chemical | 100 °C | 2 h | Nanoflower | [50] |
| ZnO-CdS | Zn(CH3COO)2·2H2O | Cd(CH3COO)2 2H2O | Wet chemical coprecipitation | RT | 2 h | Polycrystalline | [66] |
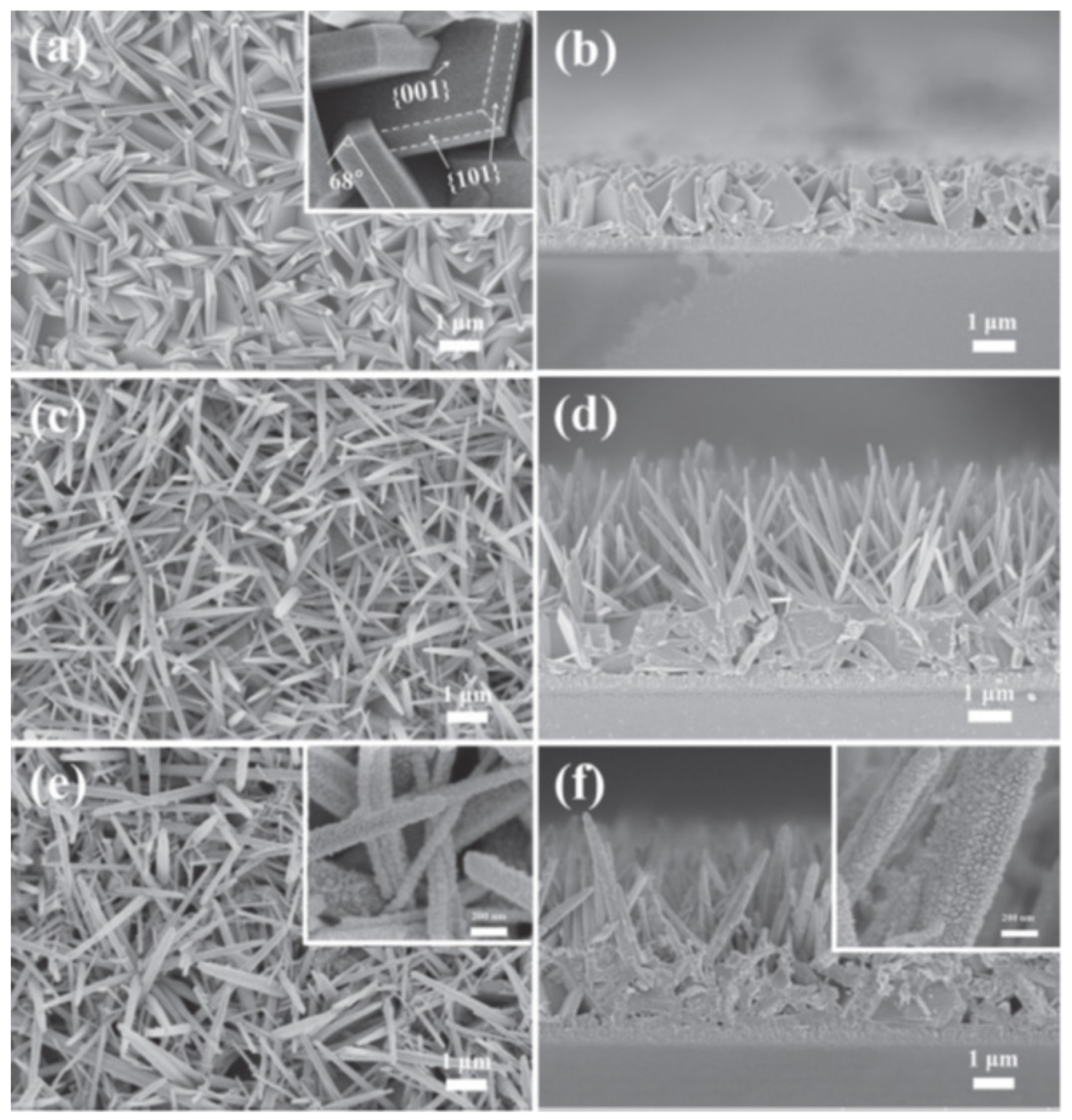
3. Morphology Control of ZnO/CdS Heterostructures
3.1. Binary Heterostructured Photocatalysts
| Photocatalyst | Type of Heterostructure | Bandgap | Pollutant | Dosage | Light Source | Efficiency | Ref |
|---|---|---|---|---|---|---|---|
| ZnO/CdS | -- | 2.4 eV | MB | -- | UV–visible | 99% in 110 min | [66] |
| ZnO/CdS | Type-II | 3.05 eV | RhB | 5 mg/60 mL | Visible | 72.4% in 120 min | [81] |
| MO | 88.5% in 120 min | ||||||
| ZnO/CdS | -- | 3.37 eV | RhB | 5 mg/20 mL | UV–visible | 90% in 80 min | [50] |
| CdS/ZnO | -- | 1.78 eV | RhB | 15 mg/L | UV–visible | 91.5% in 150 min | [90] |
| CdS/ZnO | Type-II | 3.04 eV | RhB | - | Visible | 85% in 30 min | [73] |
| RGO/ZnO/CdS | -- | -- | Aqueous chromium | 20 mg/L | Visible | 93.2% in 30 min | [72] |
| ZnO/CdS | Hierarchical | - | Bisphenol—A | 25 mg/50 mL | Visible | 55% 30 min | [64] |
| Visible | 100% 30 min | ||||||
| ZnO/CdS | Core–shell | - | H2 production | 100 mg/200 mL | Visible | 6.696 mmol/g/h | [38] |
| CdS/ZnO | Core–shell | 2.68 eV | H2 production | 20 mg/80 mL | Visible | 7.94 mmol/g/h | [61] |
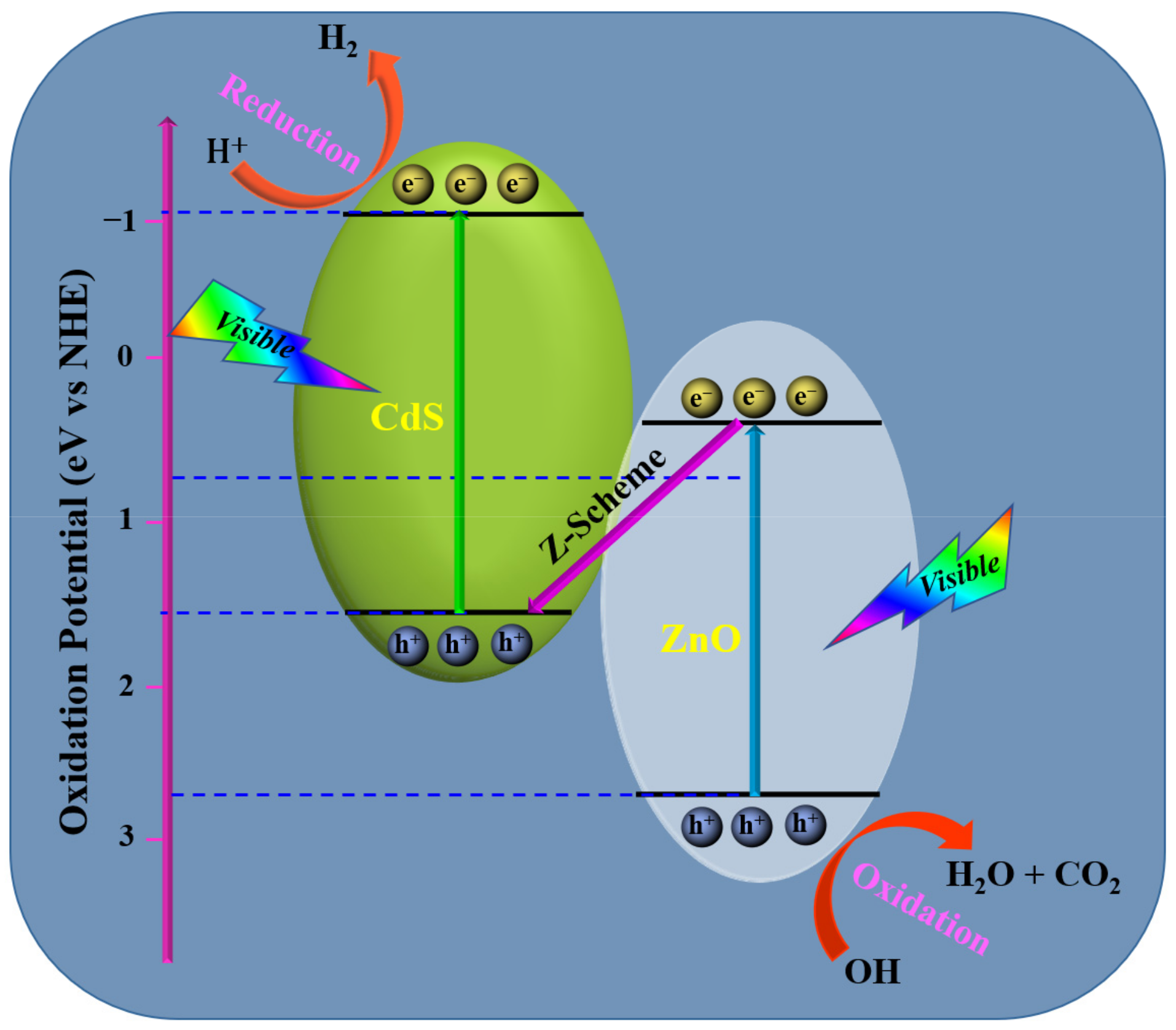
| ZnO/CdS | Z-scheme | - | H2 production | 50 mg/80 mL | Visible | 4134 μmol/g/h | [83] |
| CdS/ZnO | -- | 2.62 eV | H2 production | 50 mg/100 mL | Visible | 4076 μmol/g/h | [69] |
| ZnO/CdS | Z-scheme | 2.81 eV | H2 production | 20 mg | Solar | 1545 ± 0.3 μmol/g/h | [59] |
| CdS/ZnO | Core–shell | 2.4 eV | H2 production | 10 mg/100 mL | Visible | 11.13 mmol/g/h | [65] |
3.2. Ternary Heterostructured Photocatalysts
| Photocatalyst | Type of Heterostructure | Bandgap | Pollutant | Dosage | Light Source | Efficiency | Ref |
|---|---|---|---|---|---|---|---|
| GO/ZnO/CdS | -- | 2.34 eV | H2 production | 0.40 g/L | Visible | 6511 μmol/g/h | [58] |
| CdS/ZnO/TiO2 | Hierarchical | 2.34 eV | Nitrite in water | 3% | Visible | 92.58% | [93] |
| ZnO/CdS/CdSe | -- | 1.754 | -- | -- | Visible | 6.244 mA/cm2 | [86] |
| ZnO/CdS/CuS | -- | 2.97 | RhB | 5 mg/60 mL | Visible | 82% | [81] |
| CuSeZnO/rGO/CdS | -- | 2.2 eV | Hydrogen evolution | 1 g | Visible | 1073 mmol/g/h | [108] |
| MoSe2-CdS-ZnO | Z-scheme | -- | Hydrogen evolution | -- | Visible | 116.4 μmol/cm2 | [97] |
| ZnO@ZnFe2O4/CdS | Hierarchical | -- | CO2 reduction | 5 mg | Visible | 95.84 μmol/g/h | [101] |
| ZnO/CdS/MoS2 | -- | 2.24 eV | Amoxicillin | -- | Visible | 94% in 60 min | [100] |
| ZnO/CdS/MoS2 | S-scheme | -- | Hydrogen evolution | 15 mg | Visible | 10,247.4 μmol/g/h | [99] |
| CdS/ZnS/ZnO | -- | 2.4 eV | Hydrogen evolution | 10 mg | Visible | 51.45 mmol//g/h | [102] |
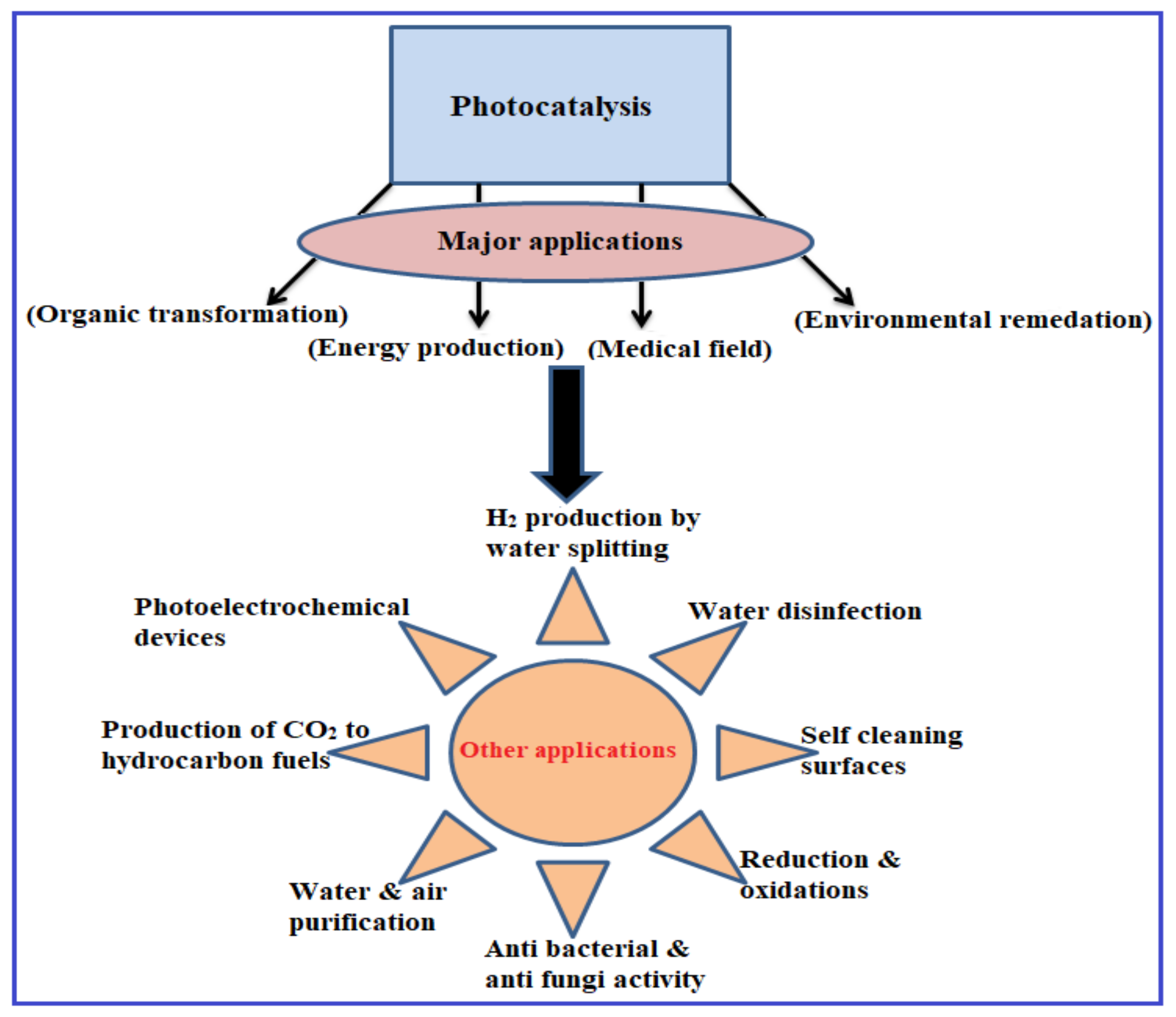
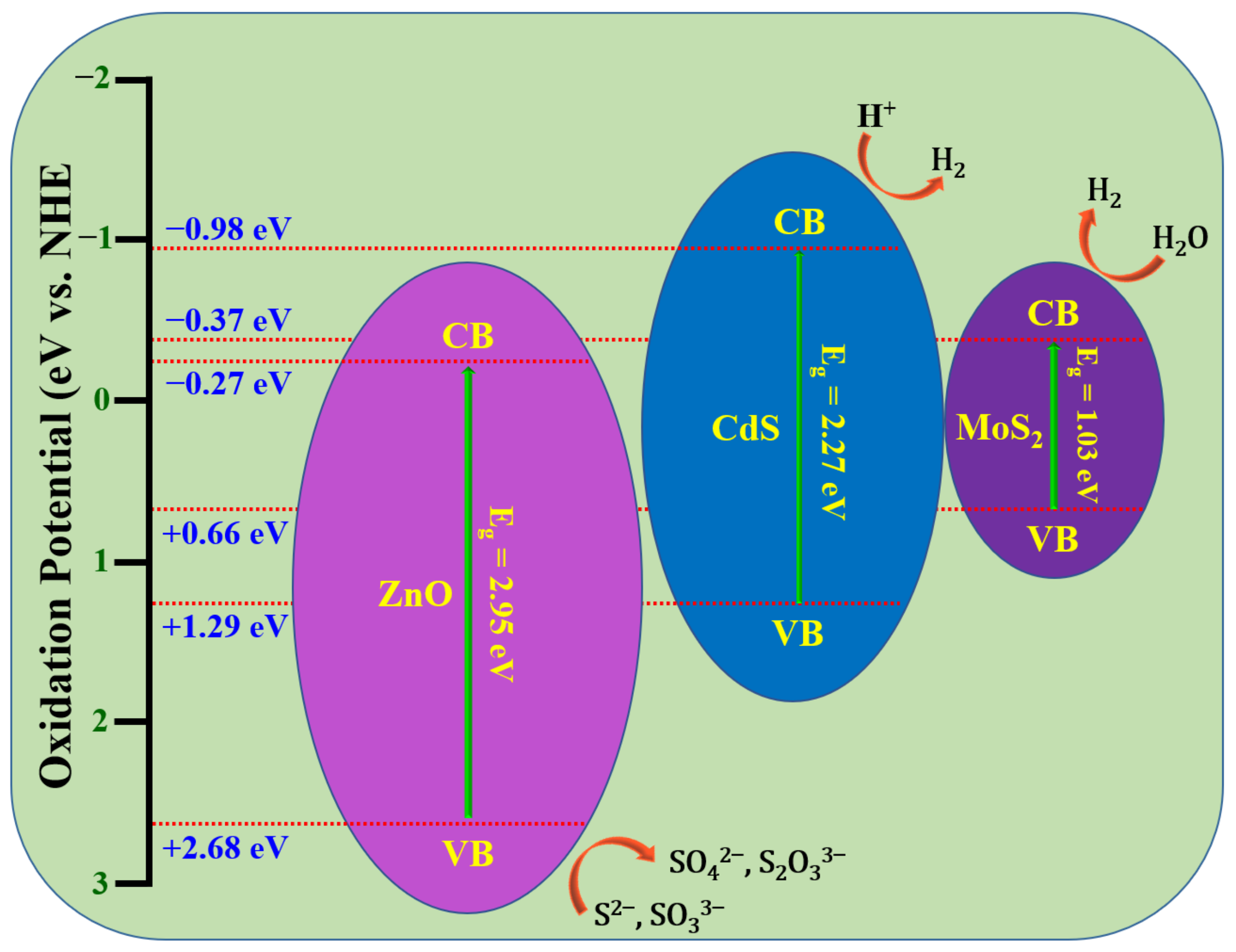
4. Photocatalytic Mechanism and Applications
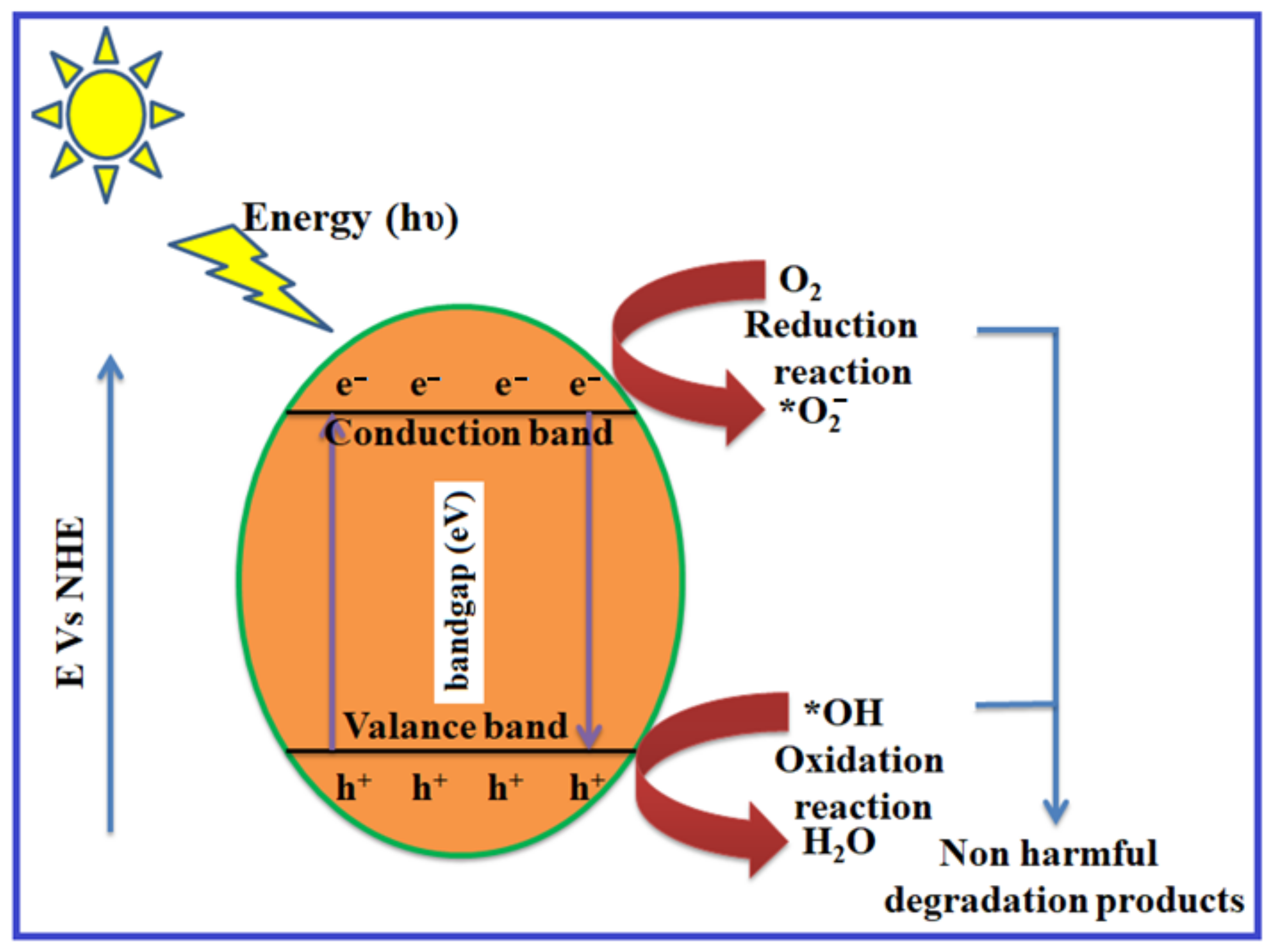
4.1. Photodegradation Mechanism in Binary Heterostructures
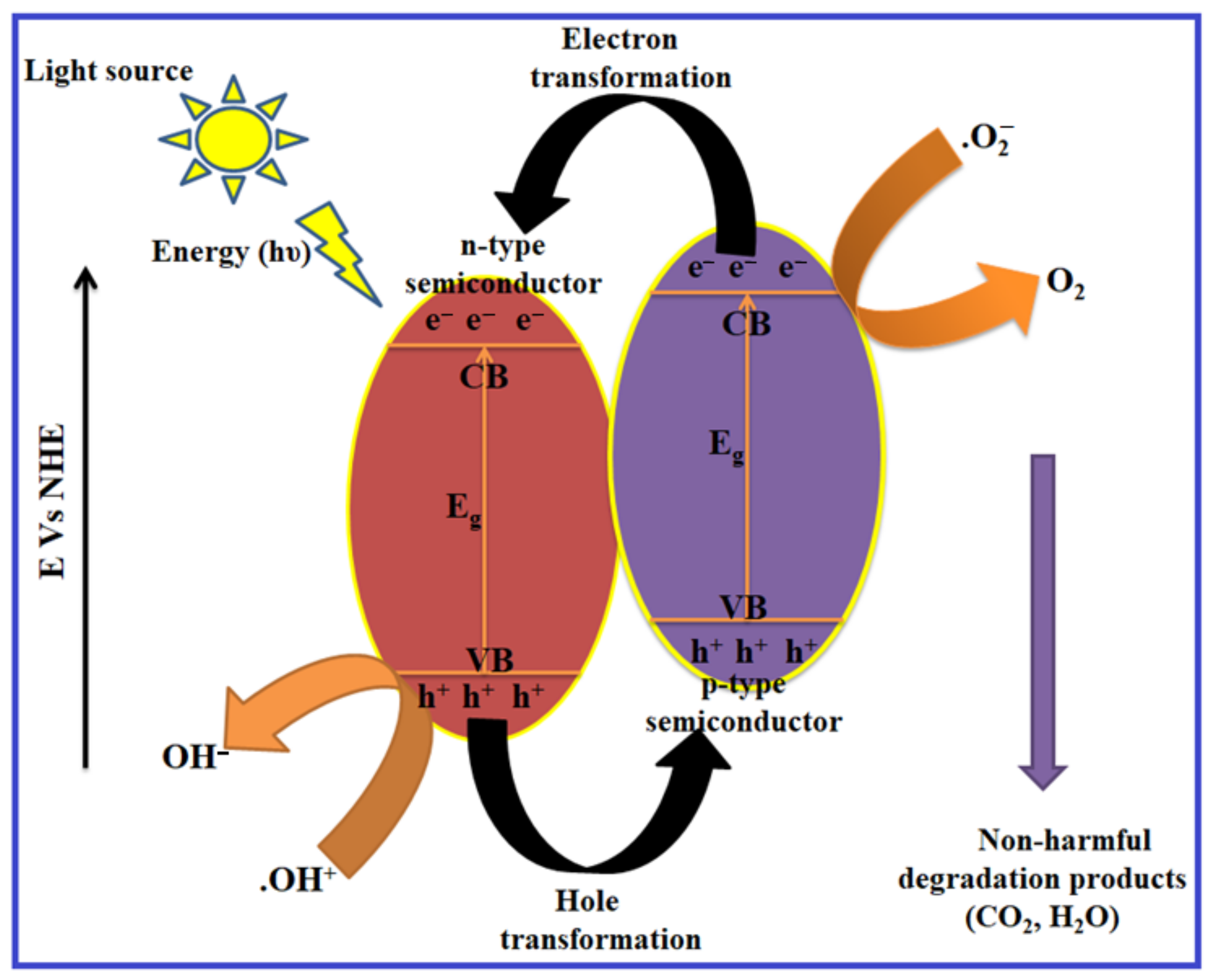
4.2. Photodegradation Mechanism in Ternary Heterostructures
5. Current State of Research on ZnO/CdS Heterostructures
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zarrin, S.; Heshmatpour, F. Facile preparation of new nanohybrids for enhancing photocatalytic activity toward removal of organic dyes under visible light irradiation. J. Phys. Chem. Solids 2020, 140, 109271. [Google Scholar] [CrossRef]
- Sun, C.; Yang, J.; Xu, M.; Cui, Y.; Ren, W.; Zhang, J.; Zhao, H.; Liang, B. Recent intensification strategies of SnO2-based photocatalysts: A review. Chem. Eng. J. 2022, 427, 131564. [Google Scholar] [CrossRef]
- Najafidoust, A.; Abbasi Asl, E.; Kazemi Hakki, H.; Sarani, M.; Bananifard, H.; Sillanpaa, M.; Etemadi, M. Sequential impregnation and sol-gel synthesis of Fe-ZnO over hydrophobic silica aerogel as a floating photocatalyst with highly enhanced photodecomposition of BTX compounds from water. Sol. Energy 2021, 225, 344–356. [Google Scholar] [CrossRef]
- Sreeram, N.; Aruna, V.; Koutavarapu, R.; Lee, D.-Y.; Shim, J. Novel Indium Vanadium Oxide Nanosheet-Supported Nickel Iron Oxide Nanoplate Heterostructure for Synergistically Enhanced Photocatalytic Degradation of Tetracycline. Catalysts 2022, 12, 1471. [Google Scholar] [CrossRef]
- Gong, W.; Wei, X.; Han, Y.; Subhan, S.; Yu, X.; Ji, T.; Sun, W.; Zhang, Y.; Shi, Z.; Zhao, Z.; et al. Photon localization-assisted visible light photocatalysis of photonic crystal CdS-N/ZnO heterojunction for efficient photodegradation tetracycline hydrochloride. Sep. Purif. Technol. 2023, 316, 123795. [Google Scholar] [CrossRef]
- Rajput, R.B.; Shaikh, R.; Sawant, J.; Kale, R.B. Recent developments in ZnO-based heterostructures as photoelectrocatalysts for wastewater treatment: A review. Environ. Adv. 2022, 9, 100264. [Google Scholar] [CrossRef]
- Tamtam, M.R.; Koutavarapu, R.; Shim, J. InVO4 nanosheets decorated with ZnWO4 nanorods: A novel composite and its enhanced photocatalytic performance under solar light. Environ. Res. 2023, 227, 115735. [Google Scholar] [CrossRef]
- Han, W.; Xiang, W.; Meng, Z.; Dong, S.; Lv, Y. A novel Cr-doped CdS/ZnO nanocomposite for efficient photocatalytic hydroxylation of benzene to phenol. Colloids Surf. A Physicochem. Eng. Asp. 2023, 670, 131529. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T.; Nadhari, W.N.A.W.; Ahmad, M.; Khanday, W.A.; Ziyang, L.; Pin, Z. Optimization of banana trunk-activated carbon production for methylene blue-contaminated water treatment. Appl. Water Sci. 2018, 8, 9. [Google Scholar] [CrossRef]
- Ma, D.; Shi, J.-W.; Zou, Y.; Fan, Z.; Ji, X.; Niu, C. Highly Efficient Photocatalyst Based on a CdS Quantum Dots/ZnO Nanosheets 0D/2D Heterojunction for Hydrogen Evolution from Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 25377–25386. [Google Scholar] [CrossRef]
- Koutavarapu, R.; Jang, W.Y.; Rao, M.C.; Arumugam, M.; Shim, J. Novel BiVO4-nanosheet-supported MoS2-nanoflake-heterostructure with synergistic enhanced photocatalytic removal of tetracycline under visible light irradiation. Chemosphere 2022, 305, 135465. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, J. A synergistic effect between ZnO/CdS S-scheme heterojunction and GO cocatalyst for boosting photocatalytic performance. Opt. Mater. 2023, 139, 113726. [Google Scholar] [CrossRef]
- Kalaiarasan, S.; Uthirakumar, P.; Shin, D.-Y.; Lee, I.-H. The degradation profile of high molecular weight textile reactive dyes: A daylight induced photocatalytic activity of ZnO/carbon quantum dot photocatalyst. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100423. [Google Scholar] [CrossRef]
- Goktas, S.; Goktas, A. A comparative study on recent progress in efficient ZnO based nanocomposite and heterojunction photocatalysts: A review. J. Alloys Compd. 2021, 863, 158734. [Google Scholar] [CrossRef]
- Ramanathan, S.; Radhika, N.; Padmanabhan, D.; Durairaj, A.; Paul Selvin, S.; Lydia, S.; Kavitha, S.; Vasanthkumar, S. Eco-friendly Synthesis of CRGO and CRGO/SnO2 Nanocomposite for Photocatalytic Degradation of Methylene Green Dye. ACS Omega 2020, 5, 158–169. [Google Scholar] [CrossRef]
- Adeel, M.; Saeed, M.; Khan, I.; Muneer, M.; Akram, N. Synthesis and Characterization of Co–ZnO and Evaluation of Its Photocatalytic Activity for Photodegradation of Methyl Orange. ACS Omega 2021, 6, 1426–1435. [Google Scholar] [CrossRef]
- Silva, E.; Alvarado-Beltrán, C.G.; Gaxiola, A.; Orozco-Carmona, V.M.; Luque, P.A.; Castro-Beltrán, A. A new green procedure to obtain and photosensitize SnO2, in one step, for solar photocatalysis using natural dyes. Ceram. Int. 2023, 49, 16732–16739. [Google Scholar] [CrossRef]
- Kannan, K.; Radhika, D.; Kasai, R.D.; Gnanasangeetha, D.; Palani, G.; Gurushankar, K.; Koutavarapu, R.; Lee, D.-Y.; Shim, J. Facile fabrication of novel ceria-based nanocomposite (CYO-CSO) via co-precipitation: Electrochemical, photocatalytic and antibacterial performances. J. Mol. Struct. 2022, 1256, 132519. [Google Scholar] [CrossRef]
- Koutavarapu, R.; Peera, S.G.; Lee, T.G.; Myla, C.R.; Lee, D.-Y.; Shim, J.; Balasingam, S.K. Recent Trends in Graphitic Carbon Nitride-Based Binary and Ternary Heterostructured Electrodes for Photoelectrochemical Water Splitting. Processes 2021, 9, 1959. [Google Scholar] [CrossRef]
- Wang, R.; Hao, Q.; Feng, J.; Wang, G.-C.; Ding, H.; Chen, D.; Ni, B. Enhanced separation of photogenerated charge carriers and catalytic properties of ZnO-MnO2 composites by microwave and photothermal effect. J. Alloys Compd. 2019, 786, 418–427. [Google Scholar] [CrossRef]
- Seo, Y.S.; Oh, S.-G. Controlling the recombination of electron-hole pairs by changing the shape of ZnO nanorods via sol-gel method using water and their enhanced photocatalytic properties. Korean J. Chem. Eng. 2019, 36, 2118–2124. [Google Scholar] [CrossRef]
- Arumugam, M.; Koutavarapu, R.; Seralathan, K.-K.; Praserthdam, S.; Praserthdam, P. Noble metals (Pd, Ag, Pt, and Au) doped bismuth oxybromide photocatalysts for improved visible light-driven catalytic activity for the degradation of phenol. Chemosphere 2023, 324, 138368. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, C.; Xun, Q.-N.; Liu, X.; Xing, W.-F.; Pu, C.; Yao, Y.; Chen, M.-J.; Chang, G.-G. Metal-organic framework derived hierarchical ZnO nanosheets/CdS composites for high photocatalytic activity under solar radiation. Chem. Phys. Lett. 2023, 821, 140464. [Google Scholar] [CrossRef]
- Lavudya, P.; Pant, H.; Srikanth, V.V.S.S.; Ammanabrolu, R. Mesoporous and phase pure anatase TiO2 nanospheres for enhanced photocatalysis. Inorg. Chem. Commun. 2023, 152, 110699. [Google Scholar] [CrossRef]
- Singh, K.; Kaur, H.; Sharma, P.K.; Singh, G.; Singh, J. ZnO and cobalt decorated ZnO NPs: Synthesis, photocatalysis and antimicrobial applications. Chemosphere 2023, 313, 137322. [Google Scholar] [CrossRef]
- Babu, B.; Talluri, B.; Gurugubelli, T.R.; Kim, J.; Yoo, K. Effect of annealing environment on the photoelectrochemical water oxidation and electrochemical supercapacitor performance of SnO2 quantum dots. Chemosphere 2022, 286, 131577. [Google Scholar] [CrossRef]
- Rodrigues, M.H.d.M.; Borges, K.C.M.; Tello, A.C.M.; Roca, R.A.; Gonçalves, R.d.F.; da Silva, A.B.F.; Longo, E.; Godinho, M.J. Effect of pH on the synthesis of BiVO4 to improve photocatalysis and antimicrobial properties. Mater. Chem. Phys. 2023, 296, 127198. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, Y.; Fan, Y.; Wang, R.; Zhu, R.; Tang, Y.; Yin, Z.; Zeng, Z. InVO4-based photocatalysts for energy and environmental applications. Chem. Eng. J. 2022, 428, 131145. [Google Scholar] [CrossRef]
- Zhao, L.; Xi, X.; Liu, Y.; Ma, L.; Nie, Z. Facile synthesis of WO3 micro/nanostructures by paper-assisted calcination for visible-light-driven photocatalysis. Chem. Phys. 2020, 528, 110515. [Google Scholar] [CrossRef]
- Bosso, P.; Del Sole, R.; Milella, A.; Mengucci, P.; Barucca, G.; Armenise, V.; Bianco, G.V.; Fracassi, F.; Palumbo, F. Nanostructured iron oxide thin films deposited by RF sputtering as catalysts for the heterogeneous solar photo-Fenton reaction. Vacuum 2023, 207, 111646. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Xia, Y.; Dong, M.; Zhou, Z.; Zhang, G.; Li, L.; Hu, Q.; Zhu, X.; Yi, J. The role of facet engineered surface and interface in CdS nanostructures toward solar driven hydrogen evolution. Appl. Surf. Sci. 2023, 615, 156402. [Google Scholar] [CrossRef]
- Alwany, A.B.; Alnakhlani, A.; Youssef, G.M.; Algradee, M.A.; Hassan, B. Effect of Li+ concentration on the structural and optical properties of chemically synthesized ZnS nanoparticles. Results Opt. 2023, 12, 100424. [Google Scholar] [CrossRef]
- Katoch, V.; Singh, M.; Katoch, A.; Prakash, B. Cost-effective microreactors for the synthesis of SnS nanoparticles and inline photocatalytic degradation of azo dyes. Mater. Lett. 2023, 333, 133677. [Google Scholar] [CrossRef]
- Wang, D.; Wen, W.; Li, W.; He, G.; Zhang, C. The doping of B in ZnO/CdS for enhanced visible-light H2 production. New J. Chem. 2022, 46, 14840–14848. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, L.; Li, P.; Zhang, L.; Chen, X.; Chu, S.; Gao, Y.; Xie, S.; Jiang, J.; Wang, H. In situ creation of ZnO@CdS nanoflowers on ITO electrodes for sensitive photoelectrochemical detection of copper ions in blood. J. Mater. Chem. B 2021, 9, 5869–5876. [Google Scholar] [CrossRef]
- Sreeram, N.; Aruna, V.; Koutavarapu, R.; Lee, D.-Y.; Rao, M.C.; Shim, J. Fabrication of InVO4/SnWO4 heterostructured photocatalyst for efficient photocatalytic degradation of tetracycline under visible light. Environ. Res. 2023, 220, 115191. [Google Scholar] [CrossRef]
- He, Y.; Hu, H.; Wang, J.; Wang, X.; Sun, M.; Tian, C.; Deng, C. Fabrication of multi-scale CdS/ZnO heteroarchitectures with boosted dual photocatalytic activities for hydrogen generation and organic dye degradation under solar light. Mater. Res. Bull. 2023, 162, 112180. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Yan, J.; Liu, S. Heteroepitaxial growth of core-shell ZnO/CdS heterostructure for efficient and stable photocatalytic hydrogen generation. Int. J. Hydrogen Energy 2022, 47, 34410–34420. [Google Scholar] [CrossRef]
- Arun, V.; Manikandan, V.; AlSalhi, M.S.; Devanesan, S.; Priyadharsan, A.; Ka, R.K.; Maadeswaran, P. An efficient optical properties of Sn doped ZnO/CdS based solar light driven nanocomposites for enhanced photocatalytic degradation applications. Chemosphere 2022, 300, 134460. [Google Scholar] [CrossRef]
- Gurugubelli, T.R.; Ravikumar, R.V.S.S.N.; Koutavarapu, R. Enhanced Photocatalytic Activity of ZnO–CdS Composite Nanostructures towards the Degradation of Rhodamine B under Solar Light. Catalysts 2022, 12, 84. [Google Scholar] [CrossRef]
- Zou, M.; Tan, C.; Zhang, Y.; Hu, J.; Ma, Z.; Yuan, Z.; Zhang, L.; Wu, M.; Zhou, H. Exploring the potential of flexible CdS/ZnO/Polyurethane nanocomposite membrane for wastewater remediation. J. Environ. Chem. Eng. 2023, 110135, In press. [Google Scholar] [CrossRef]
- Maddi, L.; Vinukonda, K.; Gurugubelli, T.R.; Koutavarapu, R. One-Step, In Situ Hydrothermal Fabrication of Cobalt-Doped ZnO/CdS Nanosheets for Optoelectronic Applications. Electronics 2023, 12, 1245. [Google Scholar] [CrossRef]
- Sun, G.; Xiao, B.; Zheng, H.; Shi, J.-W.; Mao, S.; He, C.; Li, Z.; Cheng, Y. Ascorbic acid functionalized CdS–ZnO core–shell nanorods with hydrogen spillover for greatly enhanced photocatalytic H2 evolution and outstanding photostability. J. Mater. Chem. A 2021, 9, 9735–9744. [Google Scholar] [CrossRef]
- You, D.; Xu, C.; Wang, X.; Wang, J.; Su, W.; Wang, R.; Chen, T.; Wang, R.; Shi, Z. A core@dual-shell nanorod array with a cascading band configuration for enhanced photocatalytic properties and anti-photocorrosion. J. Mater. Chem. A 2020, 8, 3726–3734. [Google Scholar] [CrossRef]
- Hao, D.; Liu, Y.; Gao, S.; Arandiyan, H.; Bai, X.; Kong, Q.; Wei, W.; Shen, P.K.; Ni, B.-J. Emerging artificial nitrogen cycle processes through novel electrochemical and photochemical synthesis. Mater. Today 2021, 46, 212–233. [Google Scholar] [CrossRef]
- Yu, Z.J.; Kumar, M.R.; Chu, Y.; Hao, H.X.; Wu, Q.Y.; Xie, H.D. Photocatalytic Decomposition of RhB by Newly Designed and Highly Effective CF@ZnO/CdS Hierarchical Heterostructures. ACS Sustain. Chem. Eng. 2018, 6, 155–164. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.; Zhang, S.; Wan, J.; Wang, X.; Deng, L.; Kan, J.; Wu, G. Clustered tubular S-scheme ZnO/CdS heterojunctions for enhanced photocatalytic hydrogen production. Mater. Sci. Eng. B 2023, 289, 116282. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, F.; Qiang, Q.; Liu, T.; Wang, Y. Fabrication of selective interface of ZnO/CdS heterostructures for more efficient photocatalytic hydrogen evolution. Dalton Trans. 2018, 47, 12162–12171. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Sadhasivam, T.; Oh, T.H. Electrochemically synthesized Cd doped ZnO nanorods and integrated atomic Cd-S bridged Cd:ZnO/CdS heterostructure photoanode for enhanced visible light responsive water oxidation applications. J. Electroanal. Chem. 2023, 934, 117289. [Google Scholar] [CrossRef]
- Zgura, I.; Preda, N.; Socol, G.; Ghica, C.; Ghica, D.; Enculescu, M.; Negut, I.; Nedelcu, L.; Frunza, L.; Ganea, C.P.; et al. Wet chemical synthesis of ZnO-CdS composites and their photocatalytic activity. Mater. Res. Bull. 2018, 99, 174–181. [Google Scholar] [CrossRef]
- Wang, C.; Dai, J.; Guo, S.; Sun, R.; Zhang, C.; Zhao, X.; Zhou, L.; Zhang, F.; Li, N.; Wang, M.; et al. Efficient photoelectrochemical sensor of Cu2+ based on ZnO-graphene nanocomposite sensitized with hexagonal CdS by calcination method. J. Electroanal. Chem. 2021, 893, 115330. [Google Scholar] [CrossRef]
- Kim, W.; Monllor-Satoca, D.; Chae, W.-S.; Mahadik, M.A.; Jang, J.S. Enhanced photoelectrochemical and hydrogen production activity of aligned CdS nanowire with anisotropic transport properties. Appl. Surf. Sci. 2019, 463, 339–347. [Google Scholar] [CrossRef]
- Mahadik, M.A.; Chung, H.-S.; Ryu, H.I.; Chae, W.-S.; Cho, M.; Jang, J.S. Efficient Way To Assemble CdS Nanorose-Decorated CdSe-Tetrakaidecahedron Heterojunction Photoanodes for High-Photoelectrochemical Performance. ACS Sustain. Chem. Eng. 2019, 7, 19708–19719. [Google Scholar] [CrossRef]
- Xie, X.; Wang, R.; Ma, Y.; Chen, J.; Shi, Z.; Cui, Q.; Li, Z.; Xu, C. Sulfate-Functionalized Core–Shell ZnO/CdS/Ag2S Nanorod Arrays with Dual-Charge-Transfer Channels for Enhanced Photoelectrochemical Performance. ACS Appl. Energy Mater. 2022, 5, 6228–6237. [Google Scholar] [CrossRef]
- Zhu, L.; Yin, Z.; Lv, Z.; Li, M.; Tang, D. Ultrasensitive photoelectrochemical immunoassay for prostate-specific antigen based on silver nanoparticle-triggered ion-exchange reaction with ZnO/CdS nanorods. Analyst 2021, 146, 4487–4494. [Google Scholar] [CrossRef]
- Cao, J.-T.; Wang, B.; Dong, Y.-X.; Wang, Q.; Ren, S.-W.; Liu, Y.-M.; Zhao, W.-W. Photogenerated Hole-Induced Chemical Redox Cycling on Bi2S3/Bi2Sn2O7 Heterojunction: Toward General Amplified Split-Type Photoelectrochemical Immunoassay. ACS Sens. 2018, 3, 1087–1092. [Google Scholar] [CrossRef]
- Tak, Y.; Hong, S.J.; Lee, J.S.; Yong, K. Fabrication of ZnO/CdS core/shell nanowire arrays for efficient solar energy conversion. J. Mater. Chem. 2009, 19, 5945–5951. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Y.; Huo, S. Preparation and photocatalytic H2 production property of graphene oxide/CdS/single crystal ZnO nanorod ternary hybrids. Vacuum 2022, 205, 111467. [Google Scholar] [CrossRef]
- Revathi, M.; Pricilla, A.; Saravanan, S.P. Design and Fabrication of ZnO/CdS heterostructured nanocomposites for enhanced hydrogen evolution from solar water splitting. Inorg. Chem. Commun. 2021, 134, 109056. [Google Scholar] [CrossRef]
- Senasu, T.; Chankhanittha, T.; Hemavibool, K.; Nanan, S. Visible-light-responsive photocatalyst based on ZnO/CdS nanocomposite for photodegradation of reactive red azo dye and ofloxacin antibiotic. Mater. Sci. Semicond. Process. 2021, 123, 105558. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, R.; Ma, Y.; Liu, W.; Zhu, A.; Tan, P.; Bian, Y.; Xiong, X.; Li, E.; Pan, J. Improved photocatalytic hydrogen evolution by facet engineering of core-shell structural CdS@ZnO. Int. J. Hydrogen Energy 2019, 44, 25599–25606. [Google Scholar] [CrossRef]
- Ansir, R.; Shah, S.M.; Ullah, N.; Hussain, M.N. Performance of pyrocatechol violet and carminic acid sensitized ZnO/CdS nanostructured photoactive materials for dye sensitized solar cell. Solid-State Electron. 2020, 172, 107886. [Google Scholar] [CrossRef]
- Kolaei, M.; Tayebi, M.; Masoumi, Z.; Lee, B.-K. A novel approach for improving photoelectrochemical water splitting performance of ZnO-CdS photoanodes: Unveiling the effect of surface roughness of ZnO nanorods on distribution of CdS nanoparticles. J. Alloys Compd. 2022, 906, 164314. [Google Scholar] [CrossRef]
- Zhang, C.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. The ultrasonic-induced-piezoelectric enhanced photocatalytic performance of ZnO/CdS nanofibers for degradation of bisphenol A. J. Alloys Compd. 2021, 885, 160987. [Google Scholar] [CrossRef]
- Ma, D.; Shi, J.-W.; Zou, Y.; Fan, Z.; Ji, X.; Niu, C.; Wang, L. Rational design of CdS@ZnO core-shell structure via atomic layer deposition for drastically enhanced photocatalytic H2 evolution with excellent photostability. Nano Energy 2017, 39, 183–191. [Google Scholar] [CrossRef]
- Saxena, N.; Sondhi, H.; Sharma, R.; Joshi, M.; Amirthapandian, S.; Rajput, P.; Sinha, O.P.; Krishna, R. Equimolar ZnO-CdS nanocomposite for enhanced photocatalytic performance. Chem. Phys. Impact 2022, 5, 100119. [Google Scholar] [CrossRef]
- Rao, G.T.; Ravikumar, R.V.S.S.N. Novel Fe-doped ZnO-CdS nanocomposite with enhanced visible light-driven photocatalytic performance. Mater. Res. Innov. 2021, 25, 215–220. [Google Scholar] [CrossRef]
- Sanchez, H.E.; Esparza, D.; Lopez-Luke, T.; Castañeda-Contreras, J.; Marañon-Ruiz, V.F.; Zarazúa, I.; Rodriguez, R.A. Effect of Al3+ doping concentration and film thickness of ZnO nanoparticles over the TiO2 photoelectrode in CdS quantum dots sensitized solar cells. Sol. Energy 2020, 197, 154–162. [Google Scholar] [CrossRef]
- Huo, S.; Chen, C. One-step synthesis CdS/single crystal ZnO nanorod heterostructures with high photocatalytic H2 production ability. Inorg. Chem. Commun. 2021, 132, 108841. [Google Scholar] [CrossRef]
- Bathula, B.; Gurugubelli, T.R.; Yoo, J.; Yoo, K. Recent Progress in the Use of SnO2 Quantum Dots: From Synthesis to Photocatalytic Applications. Catalysts 2023, 13, 765. [Google Scholar] [CrossRef]
- Ren, W.; Yang, J.; Zhang, J.; Li, W.; Sun, C.; Zhao, H.; Wen, Y.; Sha, O.; Liang, B. Recent progress in SnO2/g-C3N4 heterojunction photocatalysts: Synthesis, modification, and application. J. Alloys Compd. 2022, 906, 164372. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, L.; Zuo, Y.; He, G.; Chen, Q.; Meng, Q.; Chen, H. Reduced graphene oxide supported ZnO/CdS heterojunction enhances photocatalytic removal efficiency of hexavalent chromium from aqueous solution. Chemosphere 2022, 286, 131738. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, K.A.; Iqbal, M.; Louis, H.; Bello, O.S. Synthesis, characterization and application of CdS/ZnO nanorod heterostructure for the photodegradation of Rhodamine B dye. Mater. Sci. Energy Technol. 2019, 2, 329–336. [Google Scholar] [CrossRef]
- Zhai, C.; Sun, M.; Zhu, M.; Zhang, K.; Du, Y. Insights into photo-activated electrode for boosting electrocatalytic methanol oxidation based on ultrathin MoS2 nanosheets enwrapped CdS nanowires. Int. J. Hydrogen Energy 2017, 42, 5006–5015. [Google Scholar] [CrossRef]
- Liang, S.; Sui, G.; Li, J.; Guo, D.; Luo, Z.; Xu, R.; Yao, H.; Wang, C.; Chen, S. ZIF-L-derived porous C-doped ZnO/CdS graded nanorods with Z-scheme heterojunctions for enhanced photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 11190–11202. [Google Scholar] [CrossRef]
- Wang, S.; Liu, P.; Meng, C.; Wang, Y.; Zhang, L.; Pan, L.; Yin, Z.; Tang, N.; Zou, J.-J. Boosting photoelectrochemical water splitting by Au@Pt modified ZnO/CdS with synergy of Au-S bonds and surface plasmon resonance. J. Catal. 2022, 408, 196–205. [Google Scholar] [CrossRef]
- Holi, A.M.; Al-Zahrani, A.A.; Najm, A.S.; Chelvanathan, P.; Amin, N. PbS/CdS/ZnO nanowire arrays: Synthesis, structural, optical, electrical, and photoelectrochemical properties. Chem. Phys. Lett. 2020, 750, 137486. [Google Scholar] [CrossRef]
- Lai, Y.; Chi, L.; Liu, B.; Li, Z. Facile fabrication and optimization of bowl-like ZnO/CdS nano-composite thin films with hierarchical nanopores and nano-cracks for high-performance photoelectrochemistry. Int. J. Hydrogen Energy 2018, 43, 22046–22054. [Google Scholar] [CrossRef]
- Khan, M.; Irfan, M.H.; Israr, M.; Rehman, N.; Park, T.J.; Basit, M.A. Comparative investigation of ZnO morphologies for optimal CdS quantum-dot deposition via pseudo-SILAR method. Chem. Phys. Lett. 2020, 744, 137223. [Google Scholar] [CrossRef]
- Rokade, A.; Rondiya, S.; Date, A.; Sharma, V.; Prasad, M.; Pathan, H.; Jadkar, S. Electrochemical Synthesis of Core-shell ZnO/CdS Nanostructure for Photocatalytic Water Splitting Application. Energy Procedia 2017, 110, 121–127. [Google Scholar] [CrossRef]
- Nandi, P.; Das, D. ZnO/CdS/CuS heterostructure: A suitable candidate for applications in visible-light photocatalysis. J. Phys. Chem. Solids 2022, 160, 110344. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, B.; Zhang, L.; Fan, J.; Yu, J. 0D/2D CdS/ZnO composite with n-n heterojunction for efficient detection of triethylamine. J. Colloid Interface Sci. 2021, 600, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, B.; Liu, M.; Zhang, L.; Yu, J.; Zhou, M. Direct Z-scheme ZnO/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Catal. B Environ. 2019, 243, 19–26. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, Y.; Alba-Cabañas, J.; Cruzata, O.; Bianco, S.; Tresso, E.; Rossi, F.; Vaillant-Roca, L. In-situ pulsed laser induced growth of CdS nanoparticles on ZnO nanorods surfaces. Mater. Res. Bull. 2020, 125, 110790. [Google Scholar] [CrossRef]
- Radhika, P.; Achary, K.M.R.; Sreekanth, M. A study of tunable optical properties of ZnO/CdS heterostructures with varying shell thickness structures. Mater. Today Proc. 2021, 46, 903–907. [Google Scholar] [CrossRef]
- Li, C.; Chen, S.; Gao, X.; Zhang, W.; Wang, Y. Fabrication, characterization and photoelectrochemical properties of CdS/CdSe nanofilm co-sensitized ZnO nanorod arrays on Zn foil substrate. J. Colloid Interface Sci. 2021, 588, 269–282. [Google Scholar] [CrossRef]
- Tso, S.; Li, W.-S.; Wu, B.-H.; Chen, L.-J. Enhanced H2 production in water splitting with CdS-ZnO core-shell nanowires. Nano Energy 2018, 43, 270–277. [Google Scholar] [CrossRef]
- Bai, L.; Li, S.; Ding, Z.; Wang, X. Wet chemical synthesis of CdS/ZnO nanoparticle/nanorod hetero-structure for enhanced visible light disposal of Cr(VI) and methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125489. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, J.; Ren, W.; Wang, H.; Zhang, R.; Xie, Y.; Chen, Y. Construction of ZnO/CdS three-dimensional hierarchical photoelectrode for improved photoelectrochemical performance. Renew. Energy 2020, 153, 241–248. [Google Scholar] [CrossRef]
- Venkatareddy, C.; Bandaru, N.; Neelakanta Reddy, I.; Shim, J.; Yoo, K. UV–Visible light driven photocatalytic activities of CdS nanoparticles supported ZnO layers. Mater. Sci. Eng. B 2018, 232, 68–75. [Google Scholar] [CrossRef]
- Zhao, R.; Zhong, J.; Ji, C.; Zhao, J.; Lu, H. Three-dimensional ZnO/ZnxCd1−xS/CdS nanostructures modified by microwave hydrothermal reaction-deposited CdSe quantum dots for chemical solar cells. Sol. Energy 2019, 191, 78–83. [Google Scholar] [CrossRef]
- BiBi, S.; Shah, M.Z.U.; Sajjad, M.; Shafi, H.Z.; Amin, B.; Bajaber, M.A.; Shah, A. A new ZnO-ZnS-CdS heterostructure on Ni substrate: A binder-free electrode for advanced asymmetric supercapacitors with improved performance. Electrochim. Acta 2022, 430, 141031. [Google Scholar] [CrossRef]
- Zhao, G.; Sun, M.; Liu, X.; Xuan, J.; Kong, W.; Zhang, R.; Sun, Y.; Jia, F.; Yin, G.; Liu, B. Fabrication of CdS quantum dots sensitized ZnO nanorods/TiO2 nanosheets hierarchical heterostructure films for enhanced photoelectrochemical performance. Electrochim. Acta 2019, 304, 334–341. [Google Scholar] [CrossRef]
- Hashem, E.M.; Hamza, M.A.; El-Shazly, A.N.; Abd El-Rahman, S.A.; El-Tanany, E.M.; Mohamed, R.T.; Allam, N.K. Novel Z-Scheme/Type-II CdS@ZnO/g-C3N4 ternary nanocomposites for the durable photodegradation of organics: Kinetic and mechanistic insights. Chemosphere 2021, 277, 128730. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Qian, L.; Zheng, G. Photoelectrochemical CO2 reduction to syngas by a ZnO–CdS–Cu nanocomposite. Mol. Catal. 2020, 492, 110953. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Zhou, Y.; Zou, Z. Al-ZnO/CdS Photoanode Modified with a Triple Functions Conformal TiO2 Film for Enhanced Photoelectrochemical Efficiency and Stability. Appl. Catal. B Environ. 2019, 255, 117738. [Google Scholar] [CrossRef]
- Li, L.; Shi, H.; Yu, H.; Tan, X.; Wang, Y.; Ge, S.; Wang, A.; Cui, K.; Zhang, L.; Yu, J. Ultrathin MoSe2 nanosheet anchored CdS-ZnO functional paper chip as a highly efficient tandem Z-scheme heterojunction photoanode for scalable photoelectrochemical water splitting. Appl. Catal. B Environ. 2021, 292, 120184. [Google Scholar] [CrossRef]
- Cao, J.-T.; Liao, X.-J.; Wang, Y.-L.; Liu, Y.-M. A novel photoelectrochemical strategy for lead ion detection based on CdSe quantum dots co-sensitized ZnO-CdS nanostructure. J. Electroanal. Chem. 2021, 880, 114828. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Z.; Qiao, X.-Q.; Huang, L.; Gan, S.; Hou, D.; Zhao, J.; Sun, C.; Li, D.-S. A synergistic effect between S-scheme heterojunction and Noble-metal free cocatalyst to promote the hydrogen evolution of ZnO/CdS/MoS2 photocatalyst. Chem. Eng. J. 2021, 424, 130368. [Google Scholar] [CrossRef]
- Tang, Y.; Zheng, Z.; Sun, X.; Li, X.; Li, L. Ternary CdS-MoS2 coated ZnO nanobrush photoelectrode for one-dimensional acceleration of charge separation upon visible light illumination. Chem. Eng. J. 2019, 368, 448–458. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Han, W.; Liang, S.; Jiao, Y.; Tian, G. ZIF-8 derived hierarchical ZnO@ZnFe2O4 hollow polyhedrons anchored with CdS for efficient photocatalytic CO2 reduction. Sep. Purif. Technol. 2023, 309, 122970. [Google Scholar] [CrossRef]
- Sun, D.; Shi, J.-W.; Ma, D.; Zou, Y.; Sun, G.; Mao, S.; Sun, L.; Cheng, Y. CdS/ZnS/ZnO ternary heterostructure nanofibers fabricated by electrospinning for excellent photocatalytic hydrogen evolution without co-catalyst. Chin. J. Catal. 2020, 41, 1421–1429. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, H.; Tang, Y.; Xiang, W.; Wang, C.; Xu, T.; Wang, X.; Xiao, M.; Zhang, J. High-performance CdSe/CdS@ZnO quantum dots enabled by ZnO sol as surface ligands: A novel strategy for improved optical properties and stability. Chem. Eng. J. 2022, 428, 131159. [Google Scholar] [CrossRef]
- Das, D.; Nandi, P. Synthesis of CdS/GO modified ZnO heterostructure for visible light dye degradation applications. Appl. Surf. Sci. 2021, 570, 151260. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, L.; Xin, Z.; Yu, Y.; Wang, L.; Zhang, W. Visible light response and heterostructure of composite CdS@ZnS–ZnO to enhance its photocatalytic activity. J. Alloys Compd. 2020, 813, 152190. [Google Scholar] [CrossRef]
- Liu, C.; Qiu, Y.; Zhang, J.; Liang, Q.; Mitsuzaki, N.; Chen, Z. Construction of CdS quantum dots modified g-C3N4/ZnO heterostructured photoanode for efficient photoelectrochemical water splitting. J. Photochem. Photobiol. A Chem. 2019, 371, 109–117. [Google Scholar] [CrossRef]
- Kong, W.; Zhao, Y.; Xuan, J.; Gao, Z.; Wang, J.; Tan, S.; Jia, F.; Teng, Z.; Sun, M.; Yin, G. Synchronous etching and W-doping for 3D CdS/ZnO/TiO2 hierarchical heterostructure photoelectrodes to significantly enhance the photoelectrochemical performance. Appl. Surf. Sci. 2021, 537, 147998. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Xu, H.; Gan, L.; Ji, X.; Liu, H.; Zhang, R. CuS-modified ZnO rod/reduced graphene oxide/CdS heterostructure for efficient visible-light photocatalytic hydrogen generation. Int. J. Hydrogen Energy 2020, 45, 28394–28403. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Rahim Pouran, S. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Masar, M.; Ali, H.; Guler, A.C.; Urbanek, M.; Urbanek, P.; Hanulikova, B.; Pistekova, H.; Annusova, A.; Machovsky, M.; Kuritka, I. Multifunctional bandgap-reduced ZnO nanocrystals for photocatalysis, self-cleaning, and antibacterial glass surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130447. [Google Scholar] [CrossRef]
- Bai, X.; Sun, B.; Wang, X.; Zhang, T.; Hao, Q.; Ni, B.-J.; Zong, R.; Zhang, Z.; Zhang, X.; Li, H. Defective crystal plane-oriented induced lattice polarization for the photocatalytic enhancement of ZnO. CrystEngComm 2020, 22, 2709–2717. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
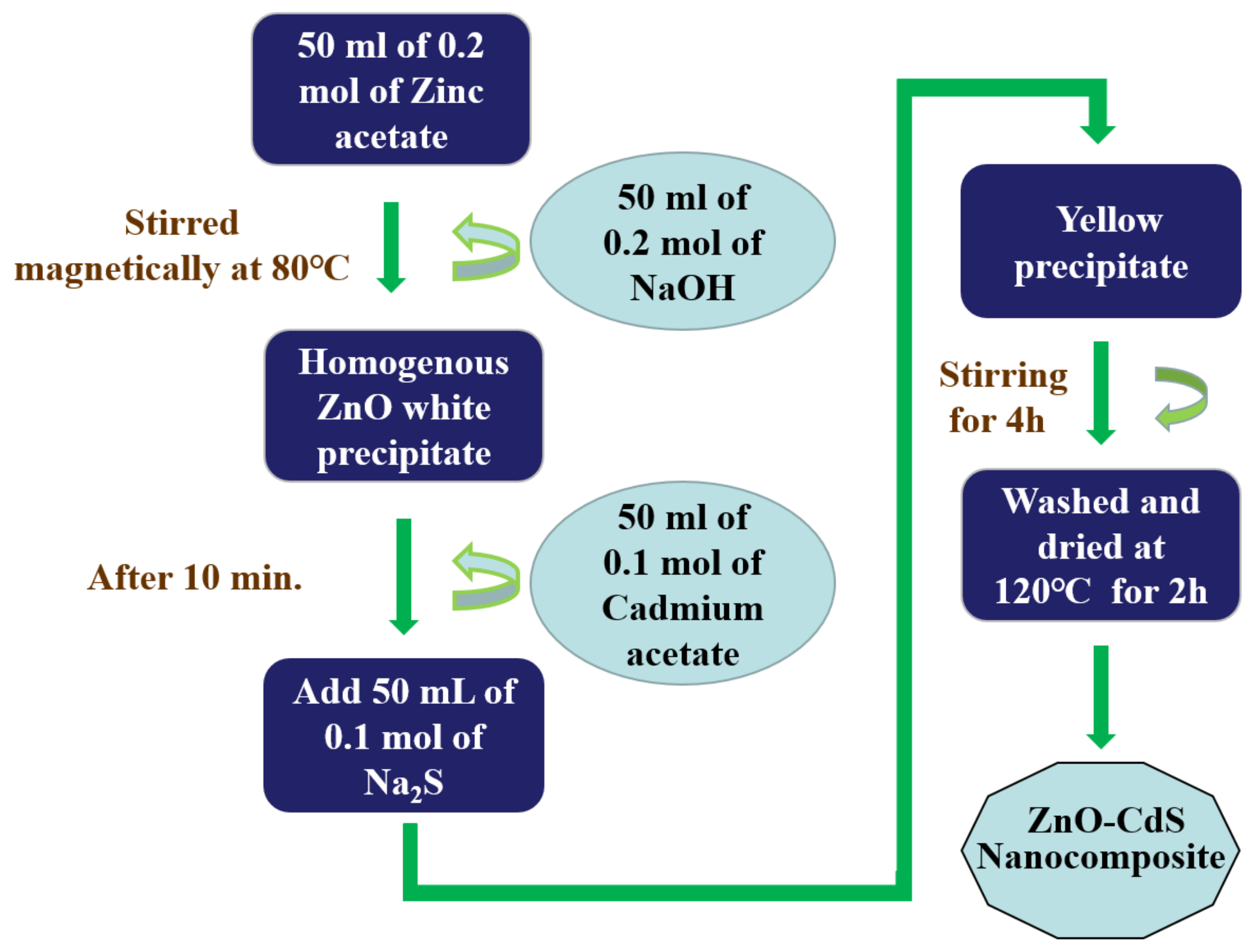

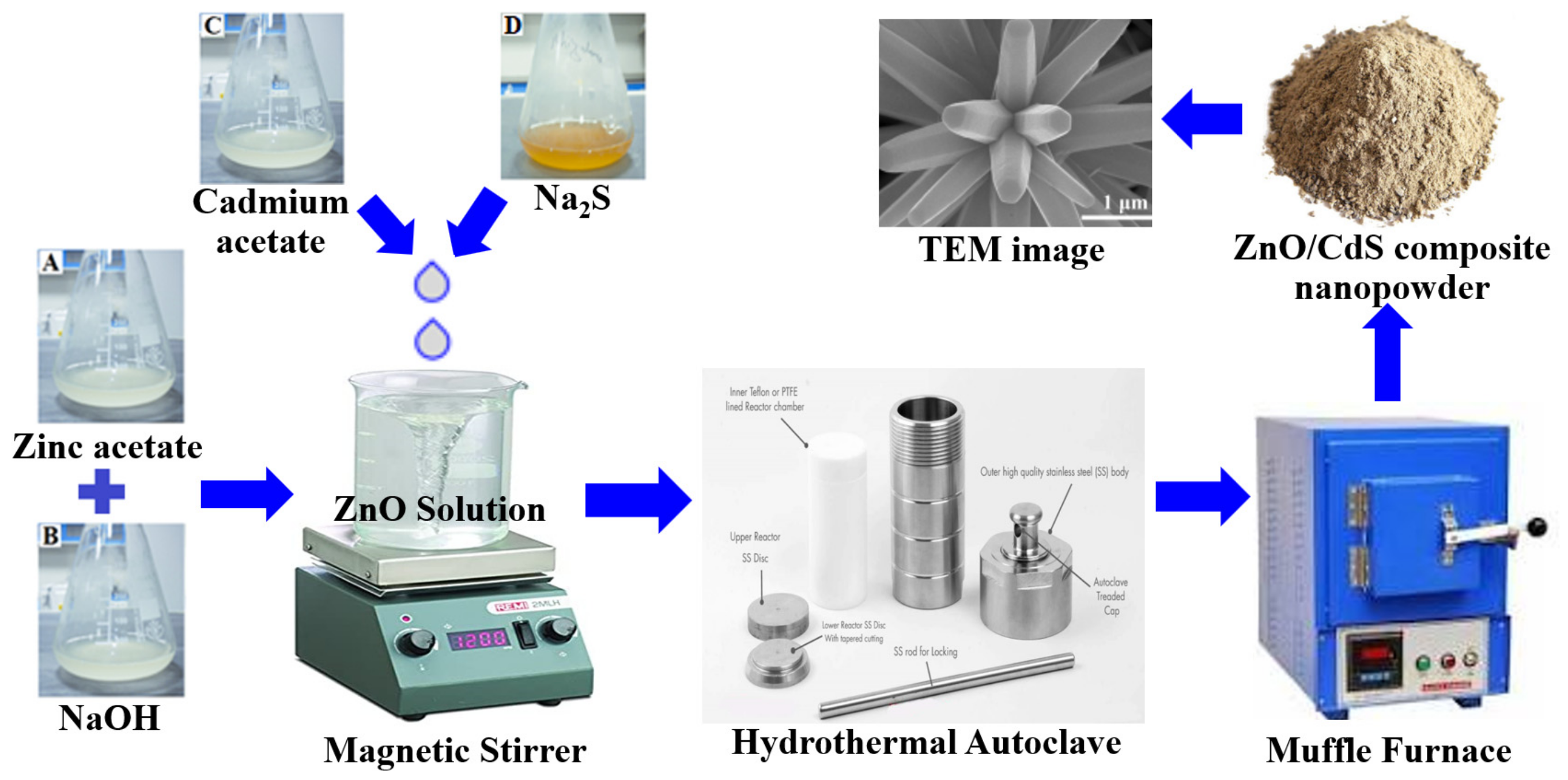
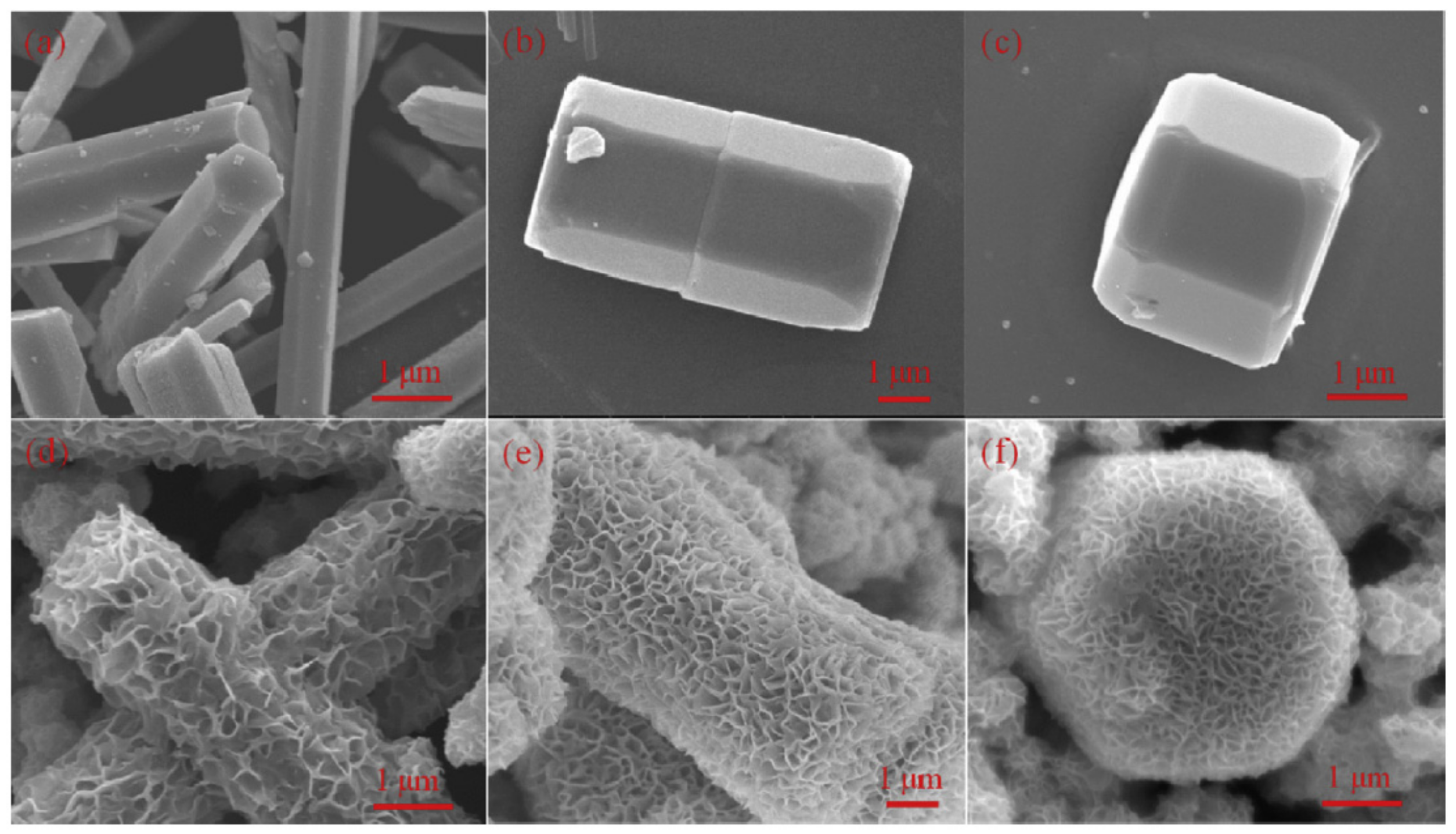

| Synthesis Method | Advantages | Disadvantages |
|---|---|---|
| Coprecipitation | High yield, low cost, good control over composition, uniform particle size distribution, enhanced reactivity, easy scalability, and versatility. | Difficulty in controlling the particle size, sensitivity to reaction conditions, formation of secondary phase, and particles agglomeration. |
| Microwave | Fast reaction rate, high energy efficiency, scalability, high purity, and homogeneity. | Uneven heating, limited control over reaction conditions, defect formation, and risk of operation. |
| Hydrothermal | Controlled size and morphology, high crystallinity, low temperature synthesis, enhanced photocatalytic activity, and easy scalability. | Limited material options, a complex experimental setup, slow reaction rates, difficulty in controlling stoichiometry, and limited control over crystal orientation. |
| Solvothermal | Controlled size and morphology, high purity, enhanced photocatalytic activity, versatility, and easy scalability. | High temperature and pressure requirements, use of hazardous solvents, long synthesis time, difficulty in controlling stoichiometry, and high energy consumption. |
| SILAR | Low cost, good control over film thickness, large surface area, high purity, and room temperature synthesis. | Slow deposition rate, limited film thickness, poor control over composition, difficulty in achieving uniformity, and limited applicability to more complex nanocomposites. |
| Type of Heterostructure | Advantages | Disadvantages |
|---|---|---|
| p-n | Efficient charge separation, simple fabrication, and enhanced optoelectronic device performance. | Limited light absorption and band alignment challenges. |
| Type-I | Efficient charge separation, simple fabrication, and potential applications in the fields of photocatalysis, solar cells, and optoelectronic devices. | Limited light absorption and possibility of recombination of charge carriers. |
| Type-II | Broad spectrum light absorption, efficient charge separation, improved photocatalytic efficiency, optoelectronic performance, and solar cell efficiency. | Reduced carrier mobility, requiring precise control over band alignment and interface structures. |
| Type-III | Unique band alignment, enhanced charge separation, and potential for high-performance devices. | Limited light absorption, complex design, and challenging fabrication. |
| Z-Scheme | Efficient charge transfer, expanded light absorption, and versatile applications in photocatalysis, solar cells, and other energy conversion devices due to their efficient charge transfer and enhanced performance. | Complex design and fabrication, performance-dependence on mediator materials. |
| S-Scheme | Simple design compared to the Z-scheme, direct hole transfer, enhanced photocatalytic and photovoltaic characteristics | Limited charge transport and potential band alignment challenges. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadikatla, S.K.; Chintada, V.B.; Gurugubelli, T.R.; Koutavarapu, R. Review of Recent Developments in the Fabrication of ZnO/CdS Heterostructure Photocatalysts for Degradation of Organic Pollutants and Hydrogen Production. Molecules 2023, 28, 4277. https://doi.org/10.3390/molecules28114277
Nadikatla SK, Chintada VB, Gurugubelli TR, Koutavarapu R. Review of Recent Developments in the Fabrication of ZnO/CdS Heterostructure Photocatalysts for Degradation of Organic Pollutants and Hydrogen Production. Molecules. 2023; 28(11):4277. https://doi.org/10.3390/molecules28114277
Chicago/Turabian StyleNadikatla, Santhosh Kumar, Vinod Babu Chintada, Thirumala Rao Gurugubelli, and Ravindranadh Koutavarapu. 2023. "Review of Recent Developments in the Fabrication of ZnO/CdS Heterostructure Photocatalysts for Degradation of Organic Pollutants and Hydrogen Production" Molecules 28, no. 11: 4277. https://doi.org/10.3390/molecules28114277
APA StyleNadikatla, S. K., Chintada, V. B., Gurugubelli, T. R., & Koutavarapu, R. (2023). Review of Recent Developments in the Fabrication of ZnO/CdS Heterostructure Photocatalysts for Degradation of Organic Pollutants and Hydrogen Production. Molecules, 28(11), 4277. https://doi.org/10.3390/molecules28114277






