Bis(2,6-pyrazolyl)pyridines as a New Scaffold for Coordination Polymers
Abstract
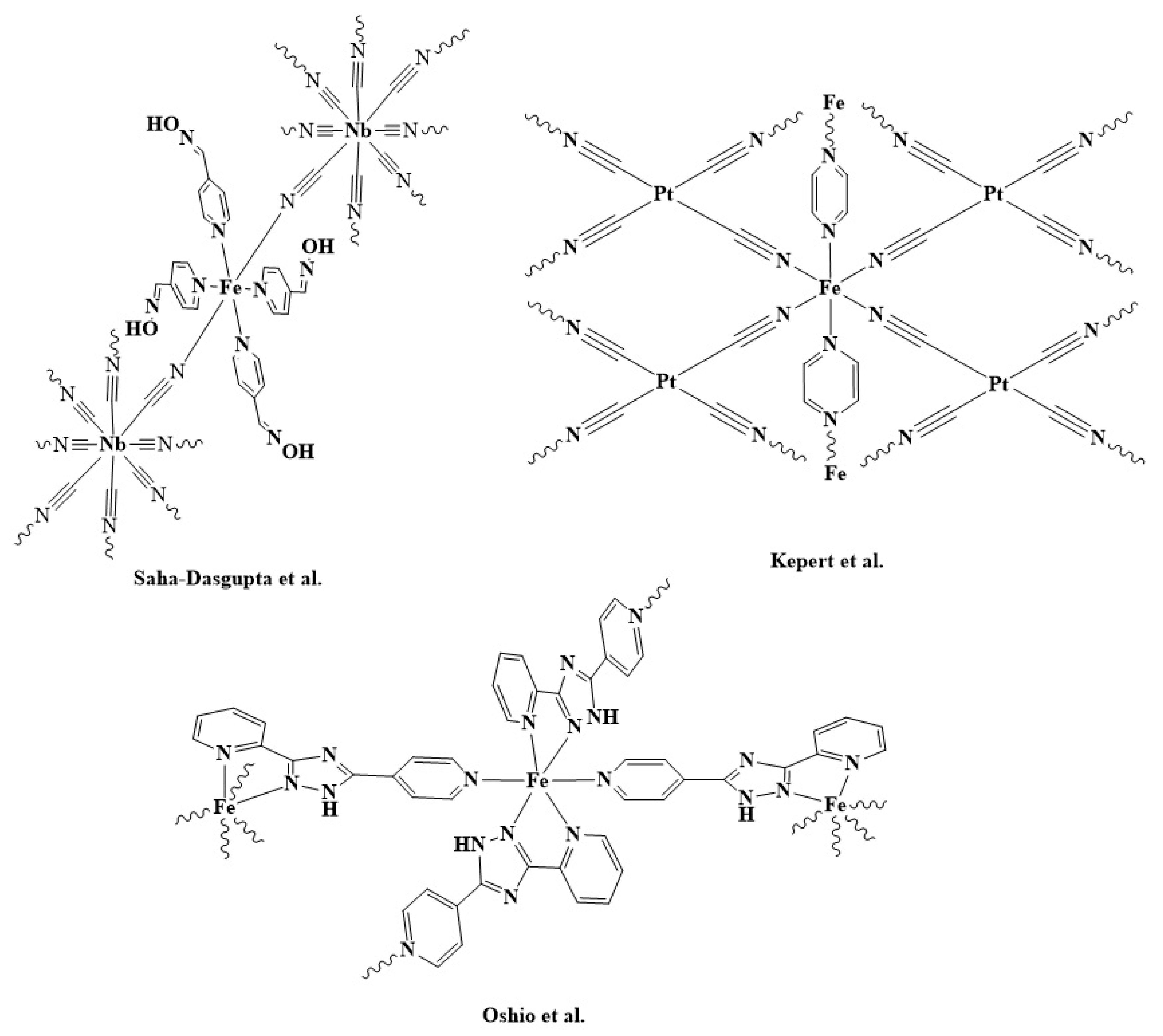
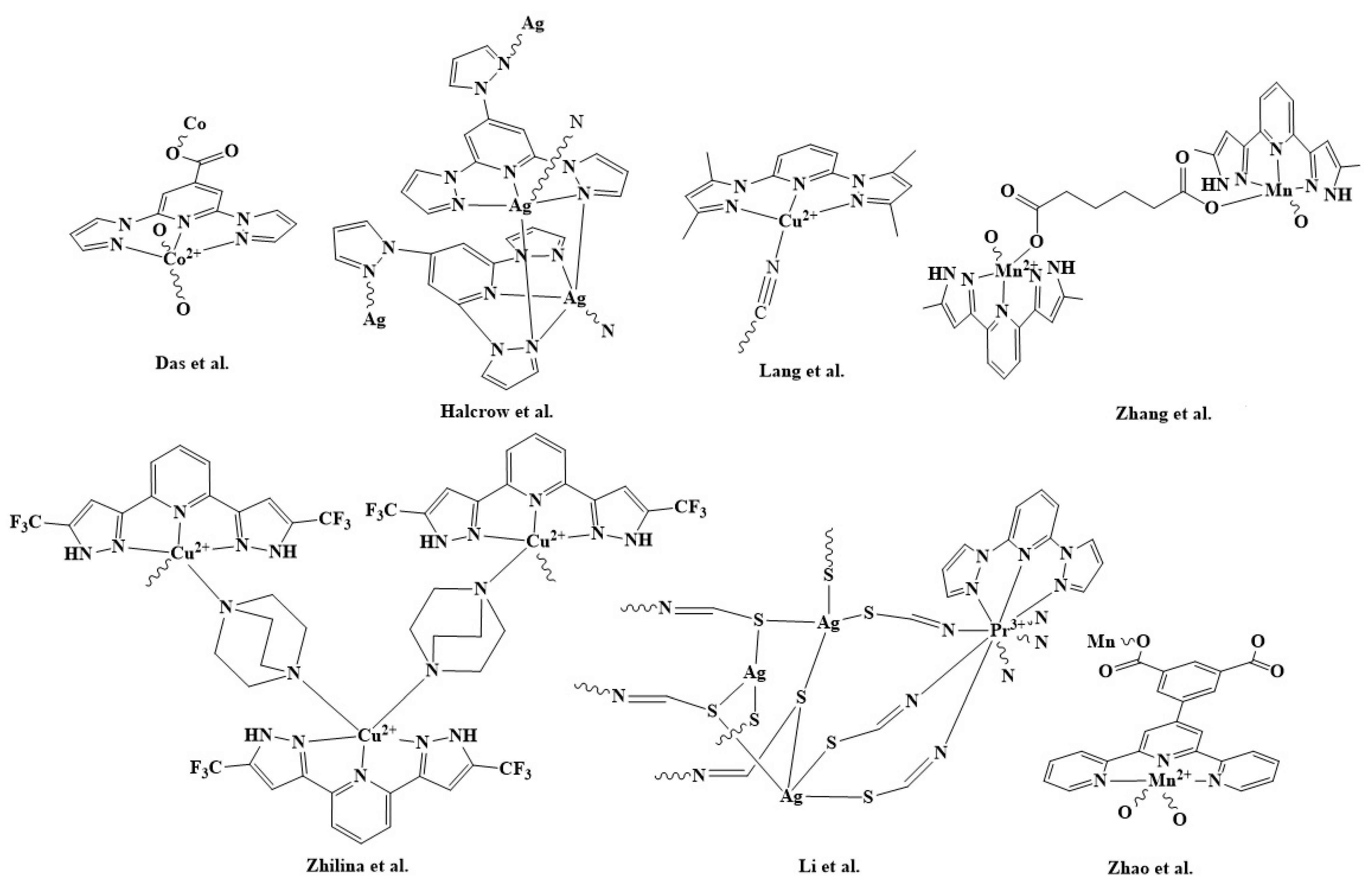
1. Introduction
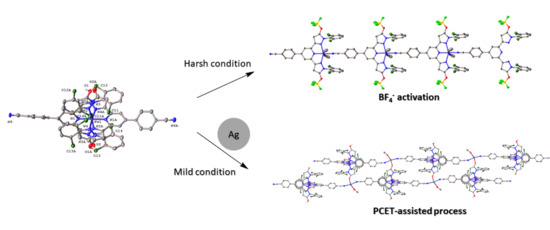
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Freund, R.; Zaremba, O.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Dincă, M.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; et al. The Current Status of MOF and COF Applications. Angew. Chem. Int. Ed. 2021, 60, 23975–24001. [Google Scholar] [CrossRef]
- Cheng, W.; Tang, X.; Zhang, Y.; Wu, D.; Yang, W. Applications of Metal-Organic Framework (MOF)-Based Sensors for Food Safety: Enhancing Mechanisms and Recent Advances. Trends Food Sci. Technol. 2021, 112, 268–282. [Google Scholar] [CrossRef]
- Ali, M.; Pervaiz, E.; Noor, T.; Rabi, O.; Zahra, R.; Yang, M. Recent advancements in MOF-based catalysts for applications in electrochemical and photoelectrochemical water splitting: A review-Ali-2021. Int. J. Energy Res.-Wiley Online Libr. 2021, 45, 1190–1226. Available online: https://onlinelibrary.wiley.com/doi/10.1002/er.5807 (accessed on 3 April 2023). [CrossRef]
- Qian, H.Y. Synthesis Characterization, Crystal Structures, and Antibacterial Activity of 8-Hydroxyquinoline-Coordinated Oxidovanadium(V) Complexes with Tridentate Hydrazone Ligands. Russ. J. Coord. Chem. 2017, 43, 780–786. [Google Scholar] [CrossRef]
- Castellanos, S.; Kapteijn, F.; Gascon, J. Photoswitchable Metal Organic Frameworks: Turn on the Lights and Close the Windows. CrystEngComm 2016, 18, 4006–4012. [Google Scholar] [CrossRef]
- Manrique-Juárez, M.D.; Rat, S.; Salmon, L.; Molnár, G.; Quintero, C.M.; Nicu, L.; Shepherd, H.J.; Bousseksou, A. Switchable Molecule-Based Materials for Micro- and Nanoscale Actuating Applications: Achievements and Prospects. Coord. Chem. Rev. 2016, 308, 395–408. [Google Scholar] [CrossRef]
- Bigdeli, F.; Lollar, C.T.; Morsali, A.; Zhou, H.-C. Switching in Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2020, 59, 4652–4669. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.W.; Henderson, B.L.; Kiesz, M.D.; Whalley, A.C.; Morris, W.; Grunder, S.; Deng, H.; Furukawa, H.; Zink, J.I.; Stoddart, J.F.; et al. Photophysical Pore Control in an Azobenzene-Containing Metal–Organic Framework. Chem. Sci. 2013, 4, 2858–2864. [Google Scholar] [CrossRef]
- Müller, K.; Knebel, A.; Zhao, F.; Bléger, D.; Caro, J.; Heinke, L. Switching Thin Films of Azobenzene-Containing Metal–Organic Frameworks with Visible Light. Chem.– Eur. J. 2017, 23, 5434–5438. [Google Scholar] [CrossRef]
- Park, J.; Feng, D.; Yuan, S.; Zhou, H.-C. Photochromic Metal–Organic Frameworks: Reversible Control of Singlet Oxygen Generation. Angew. Chem. 2015, 127, 440–445. [Google Scholar] [CrossRef]
- Luo, F.; Fan, C.B.; Luo, M.B.; Wu, X.L.; Zhu, Y.; Pu, S.Z.; Xu, W.-Y.; Guo, G.-C. Photoswitching CO2 Capture and Release in a Photochromic Diarylethene Metal–Organic Framework. Angew. Chem. 2014, 126, 9452–9455. [Google Scholar] [CrossRef]
- Nagata, S.; Kokado, K.; Sada, K. Metal–Organic Framework Tethering PNIPAM for ON–OFF Controlled Release in Solution. Chem. Commun. 2015, 51, 8614–8617. [Google Scholar] [CrossRef]
- Sun, J.-K.; Cai, L.-X.; Chen, Y.-J.; Li, Z.-H.; Zhang, J. Reversible Luminescence Switch in a Photochromic Metal–Organic Framework. Chem. Commun. 2011, 47, 6870–6872. [Google Scholar] [CrossRef]
- Ogihara, N.; Ohba, N.; Kishida, Y. On/off switchable electronic conduction in intercalated metal-organic frameworks. Sci. Adv. 2017, 3, e1603103. [Google Scholar] [CrossRef]
- Niel, V.; Thompson, A.L.; Muñoz, M.C.; Galet, A.; Goeta, A.E.; Real, J.A. Crystalline-State Reaction with Allosteric Effect in Spin-Crossover, Interpenetrated Networks with Magnetic and Optical Bistability. Angew. Chem. Int. Ed. 2003, 42, 3760–3763. [Google Scholar] [CrossRef]
- Liu, F.-L.; Li, D.; Su, L.-J.; Tao, J. Reversible Three Equal-Step Spin Crossover in an Iron(II) Hofmann-Type Metal–Organic Framework. Dalton Trans. 2018, 47, 1407–1411. [Google Scholar] [CrossRef]
- Halcrow, M.A. The Effect of Ligand Design on Metal Ion Spin State—Lessons from Spin Crossover Complexes. Crystals 2016, 6, 58. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Imoto, K.; Tsunobuchi, Y.; Takano, S.; Tokoro, H. Light-induced spin-crossover magnet|Nature Chemistry. Nat. Chem. 2011, 3, 564–569. Available online: https://www.nature.com/articles/nchem.1067 (accessed on 3 April 2023). [CrossRef]
- Tarafder, K.; Kanungo, S.; Oppeneer, P.M.; Saha-Dasgupta, T. Pressure and Temperature Control of Spin-Switchable Metal-Organic Coordination Polymers from Ab Initio Calculations. Phys. Rev. Lett. 2012, 109, 077203. [Google Scholar] [CrossRef]
- Gong, L.L.; Feng, X.F.; Luo, F.; Yi, X.F.; Zheng, A.M. Removal and Safe Reuse of Highly Toxic Allyl Alcohol Using a Highly Selective Photo-Sensitive Metal–Organic Framework. Green Chem. 2016, 18, 2047–2055. [Google Scholar] [CrossRef]
- Halcrow, M.A. Spin-Crossover Materials: Properties and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2013. [Google Scholar]
- Southon, P.D.; Liu, L.; Fellows, E.A.; Price, D.J.; Halder, G.J.; Chapman, K.W.; Moubaraki, B.; Murray, K.S.; Létard, J.-F.; Kepert, C.J. Dynamic Interplay between Spin-Crossover and Host−Guest Function in a Nanoporous Metal−Organic Framework Material. J. Am. Chem. Soc. 2009, 131, 10998–11009. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Liu, J.-L.; Leng, J.-D.; Lin, Z.; Tong, M.-L.; Nihei, M.; Oshio, H. Spin Crossover versus Low-Spin Behaviour Exhibited in 2D and 3D Supramolecular Isomers of [FeII(2,4-Bpt)2]⋅Guest. Chem.– Eur. J. 2010, 16, 7973–7978. [Google Scholar] [CrossRef] [PubMed]
- Halcrow, M.A. Structure:Function Relationships in Molecular Spin-Crossover Complexes. Chem. Soc. Rev. 2011, 40, 4119–4142. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Nikovskiy, I.; Aleshin, D.Y.; Novikov, V.V.; Polezhaev, A.V.; Khakina, E.A.; Melnikova, E.K.; Nelyubina, Y.V. Selective Pathway toward Heteroleptic Spin-Crossover Iron(II) Complexes with Pyridine-Based N -Donor Ligands. Inorg. Chem. 2022, 61, 20866–20877. [Google Scholar] [CrossRef]
- Bommakanti, S.; Venkataramudu, U.; Das, S.K. Functional Coordination Polymers from a Bifunctional Ligand: A Quantitative Transmetalation via Single Crystal to Single Crystal Transformation. Cryst. Growth Des. 2019, 19, 1155–1166. [Google Scholar] [CrossRef]
- Berdiell, I.C.; Warriner, S.L.; Halcrow, M.A. Silver (I) complexes of bis-and tris-(pyrazolyl) azine derivatives–dimers, coordination polymers and a pentametallic assembly. Dalton Trans. 2018, 47, 5269–5278. [Google Scholar] [CrossRef]
- Li, L.-L.; Liu, L.-L.; Ren, Z.-G.; Li, H.-X.; Zhang, Y.; Lang, J.-P. Solvothermal Assembly of a Mixed-Valence Cu(I,II) Cyanide Coordination Polymer [Cu(II)Cu(I)2(µ-Br)2(µ-CN)2(Bdmpp)]n by C–C Bond Cleavage of Acetonitrile. CrystEngComm 2009, 11, 2751–2756. [Google Scholar] [CrossRef]
- Liu, G.-F.; Ren, Z.-G.; Li, H.-X.; Chen, Y.; Li, Q.-H.; Zhang, Y.; Lang, J.-P. Homo- and Heterometallic Coordination Oligomers and Polymers Derived from the Preformed Complexes [Cu(Bdmpp)(MeCN)2](ClO4)2, [Cu(Bdmpp)(N3)2], and [Cu(Bdmpp)(N3)(μ-N3)]2 [Bdmpp = 2,6-Bis(3,5-Dimethyl-1H-Pyrazol-1-Yl)Pyridine]: Syntheses, Structures, and Redox Properties. Eur. J. Inorg. Chem. 2007, 2007, 5511–5522. [Google Scholar] [CrossRef]
- Lazarou, K.N.; Chadjistamatis, I.; Terzis, A.; Perlepes, S.P.; Raptopoulou, C.P. Complexes Derived from the Copper(II)/Succinamic Acid/N,N′,N″-Chelate Tertiary Reaction Systems: Synthesis, Structural and Spectroscopic Studies. Polyhedron 2010, 29, 1870–1879. [Google Scholar] [CrossRef]
- Wang, X.; Bai, F.Y.; Xing, Y.H.; Wan, L.J.; Guan, Q.L.; Hou, Y.N.; Zhang, R. Experimental, Characteristic Evidence and Surface Photovoltage Properties of a Series of Cu/Mn Complexes with Bipyrazolyl-Pyridine and Different Spanning Dicarboxylate. Inorg. Chim. Acta 2014, 416, 171–179. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, N.; Bai, F.; Wan, L.; Shan, H.; Hou, Y.; Xing, Y.; Shi, Z. Multi-Functional D10 Metal–Organic Materials Based on Bis-Pyrazole/Pyridine Ligands Supported by a 2,6-Di(3-Pyrazolyl)Pyridine with Different Spanning Flexible Dicarboxylate Ligands: Synthesis, Structure, Photoluminescent and Catalytic Properties. CrystEngComm 2013, 15, 9135–9147. [Google Scholar] [CrossRef]
- Elahi, S.M.; Raizada, M.; Sahu, P.K.; Konar, S. Terpyridine-Based 3D Metal–Organic-Frameworks: A Structure–Property Correlation. Chem.– Eur. J. 2021, 27, 5858–5870. [Google Scholar] [CrossRef]
- Zhilina, E.F.; Chizhov, D.L.; Sidorov, A.A.; Aleksandrov, G.G.; Kiskin, M.; Slepukhin, P.A.; Fedin, M.; Starichenko, D.V.; Korolev, A.V.; Shvachko, Y.N.; et al. Neutral Tetranuclear Cu(II) Complex of 2,6-Di(5-Trifluoromethylpyrazol-3-Yl)Pyridine: Synthesis, Characterization and Its Transformation with Selected Aza-Ligands. Polyhedron 2013, 53, 122–131. [Google Scholar] [CrossRef]
- Yang, Z.N.; Sun, T.T. Catena-Poly[[[2,6-Bis-(Pyrazol-1-Yl-ΚN)Pyridine-ΚN](Nitrato-ΚO,O’)Cadmium(II)]-μ-Thio-Cyanato-ΚN:S]. Acta Cryst. Sect E Struct Rep. Online 2008, 64 Pt 11, m1386. [Google Scholar] [CrossRef]
- Wu, Q.; Xing, N.; Liu, X.; Xu, L.; Ma, X.; Yan, Z.; Xing, Y. Two Novel Cu(II) Complexes: Synthesis, Structure and Application in C–H Bond Activation. Polyhedron 2015, 87, 390–397. [Google Scholar] [CrossRef]
- Cui, H.-L.; Zhan, S.-Z.; Li, M.; Ng, S.W.; Li, D. Luminescent Isomeric Pr–Ag Coordination Polymers Immobilized with Organic Sensitizer and Ag–S Clusters. Dalton Trans. 2011, 40, 6490–6493. [Google Scholar] [CrossRef]
- Kang, X.M.; Wang, W.M.; Yao, L.H.; Ren, H.X.; Zhao, B. Solvent-dependent variations of both structure and catalytic performance in three manganese coordination polymers. Dalton Trans. 2018, 47, 6986–6994. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, W.; Wang, D. Mononuclear, Dinuclear, Hexanuclear, and One-Dimensional Polymeric Silver Complexes Having Ligand-Supported and Unsupported Argentophilic Interactions Stabilized by Pincer-like 2,6-Bis(5-Pyrazolyl)Pyridine Ligands. Dalton Trans. 2008, 1444–1453. [Google Scholar] [CrossRef]
- Cook, B.J.; Pink, M.; Chen, C.-H.; Caulton, K.G. Electrophile Recruitment as a Structural Element in Bis-Pyrazolate Pyridine Complex Aggregation. Eur. J. Inorg. Chem. 2018, 2018, 5160–5166. [Google Scholar] [CrossRef]
- Cook, B.J.; Polezhaev, A.V.; Chen, C.-H.; Pink, M.; Caulton, K.G. Deprotonation, Chloride Abstraction, and Dehydrohalogenation as Synthetic Routes to Bis-Pyrazolate Pyridyl Iron (II) Complexes. Eur. J. Inorg. Chem. 2017, 2017, 3999–4012. [Google Scholar] [CrossRef]
- Nikovskiy, I.; Polezhaev, A.V.; Novikov, V.V.; Aleshin, D.; Pavlov, A.A.; Saffiulina, E.; Aysin, R.R.; Dorovatovskii, P.; Nodaraki, L.; Tuna, F.; et al. Towards Molecular Design of Spin-Crossover Complexes of 2,6-Bis(Pyrazol-3-Yl)Pyridines. Chem. Eur. J. 2020, 26, 5629–5638. [Google Scholar] [CrossRef]
- Aleshin, D.Y.; Nikovskiy, I.; Novikov, V.V.; Polezhaev, A.V.; Melnikova, E.K.; Nelyubina, Y.V. Room-Temperature Spin Crossover in a Solution of Iron(II) Complexes with N,N′-Disubstituted Bis(Pyrazol-3-Yl)Pyridines. ACS Omega 2021, 6, 33111–33121. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, D.R.; Gagliardi, C.J.; Hull, J.F.; Murphy, C.F.; Kent, C.A.; Westlake, B.C.; Paul, A.; Ess, D.H.; McCafferty, D.G.; Meyer, T.J. Proton-Coupled Electron Transfer. Chem. Rev. 2012, 112, 4016–4093. [Google Scholar] [CrossRef]
- Tyburski, R.; Liu, T.; Glover, S.D.; Hammarström, L. Proton-Coupled Electron Transfer Guidelines, Fair and Square. J. Am. Chem. Soc. 2021, 143, 560–576. [Google Scholar] [CrossRef]
- Gentry, E.C.; Knowles, R.R. Synthetic Applications of Proton-Coupled Electron Transfer. Acc. Chem. Res. 2016, 49, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.C.; Tarantino, K.T.; Knowles, R.R. Proton-Coupled Electron Transfer in Organic Synthesis: Fundamentals, Applications, and Opportunities. Top. Curr. Chem. (Z) 2016, 374, 30. [Google Scholar] [CrossRef]
- Yang, J.-D.; Ji, P.; Xue, X.-S.; Cheng, J.-P. Recent Advances and Advisable Applications of Bond Energetics in Organic Chemistry. J. Am. Chem. Soc. 2018, 140, 8611–8623. [Google Scholar] [CrossRef]
- Rono, L.J.; Yayla, H.G.; Wang, D.Y.; Armstrong, M.F.; Knowles, R.R. Enantioselective Photoredox Catalysis Enabled by Proton-Coupled Electron Transfer: Development of an Asymmetric Aza-Pinacol Cyclization. J. Am. Chem. Soc. 2013, 135, 17735–17738. [Google Scholar] [CrossRef]
- Brewer, C.; Brewer, G.; Luckett, C.; Marbury, G.S.; Viragh, C.; Beatty, A.M.; Scheidt, W.R. Proton Control of Oxidation and Spin State in a Series of Iron Tripodal Imidazole Complexes. Inorg. Chem. 2004, 43, 2402–2415. [Google Scholar] [CrossRef]
- Kirov, G.K. Theory of Diffusion Methods of Growing Crystals. In Рoст Кристаллoь/Rost Kristallov/Growth of Crystals; Chernov, A.A., Ed.; Springer: Boston, MA, USA, 1984. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, K.; Li, J. Solvothermal Synthesis of Multifunctional Coordination Polymers. Z. Für Nat. B 2010, 65, 976–998. Available online: https://www.degruyter.com/document/doi/10.1515/znb-2010-0804/html (accessed on 3 April 2023). [CrossRef]
- Luz, I.; Toy, L.; Rabie, F.; Lail, M.; Soukri, M. Synthesis of Soluble Metal Organic Framework Composites for Mixed Matrix Membranes. ACS Appl. Mater. Interfaces 2019, 11, 15638–15645. [Google Scholar] [CrossRef]
- Dahl, E.W.; Szymczak, N.K. Hydrogen Bonds Dictate the Coordination Geometry of Copper: Characterization of a Square-Planar Copper(I) Complex. Angew. Chem. Int. Ed. 2016, 55, 3101–3105. [Google Scholar] [CrossRef]
- Alvarez, S. Distortion Pathways of Transition Metal Coordination Polyhedra Induced by Chelating Topology. Chem. Rev. 2015, 115, 13447–13483. [Google Scholar] [CrossRef]
- Gravogl, L.; Heinemann, F.W.; Munz, D.; Meyer, K. An Iron Pincer Complex in Four Oxidation States. Inorg. Chem. 2020, 59, 5632–5645. [Google Scholar] [CrossRef]
- Muthaiah, S.; Bhatia, A.; Kannan, M.; Muthaiah, S.; Bhatia, A.; Kannan, M. Stability of Metal Complexes; IntechOpen Limited: London, UK, 2020. [Google Scholar] [CrossRef]
- Geng, C.; Li, J.; Weiske, T.; Schwarz, H. Thermal O–H Bond Activation of Water As Mediated by Heteronuclear [Al2Mg2O5]•+: Evidence for Oxygen-Atom Scrambling. J. Am. Chem. Soc. 2018, 140, 9275–9281. [Google Scholar] [CrossRef]
- Beck, M.T. Critical Survey of Stability Constants of Cyano Complexes. Pure Appl. Chem. 1987, 59, 1703–1720. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta Cryst. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.; Aromí, G.; Teat, S.J.; Wernsdorfer, W.; Winpenny, R.E.P. Synthesis and Characterisation of a {Ni8} Single Molecule Magnet and Another Octanuclear Nickel Cage. Chem. Commun. 2005, 2808–2810. [Google Scholar] [CrossRef] [PubMed]
- Aromí, G.; Bell, A.; Teat, S.J.; Winpenny, R.E.P. Synthesis and Characterisation of a {Ni21Ag} Cage. Chem. Commun. 2005, 2927–2929. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.J.; Trzop, E.; Müller-Bunz, H.; Dîrtu, M.M.; Garcia, Y.; Collet, E.; Morgan, G.G. Electronic vs. Structural Ordering in a Manganese (iii) Spin Crossover Complex. Chem. Commun. 2015, 51, 17540–17543. [Google Scholar] [CrossRef]
- Boduszek, B.; Shine, H.J. Preparation of Solid Thianthrene Cation Radical Tetrafluoroborate. J. Org. Chem. 1988, 53, 5142–5143. [Google Scholar] [CrossRef]
- Chalkley, M.J.; Garrido-Barros, P.; Peters, J.C. A Molecular Mediator for Reductive Concerted Proton-Electron Transfers via Electrocatalysis. Science 2020, 369, 850–854. [Google Scholar] [CrossRef] [PubMed]
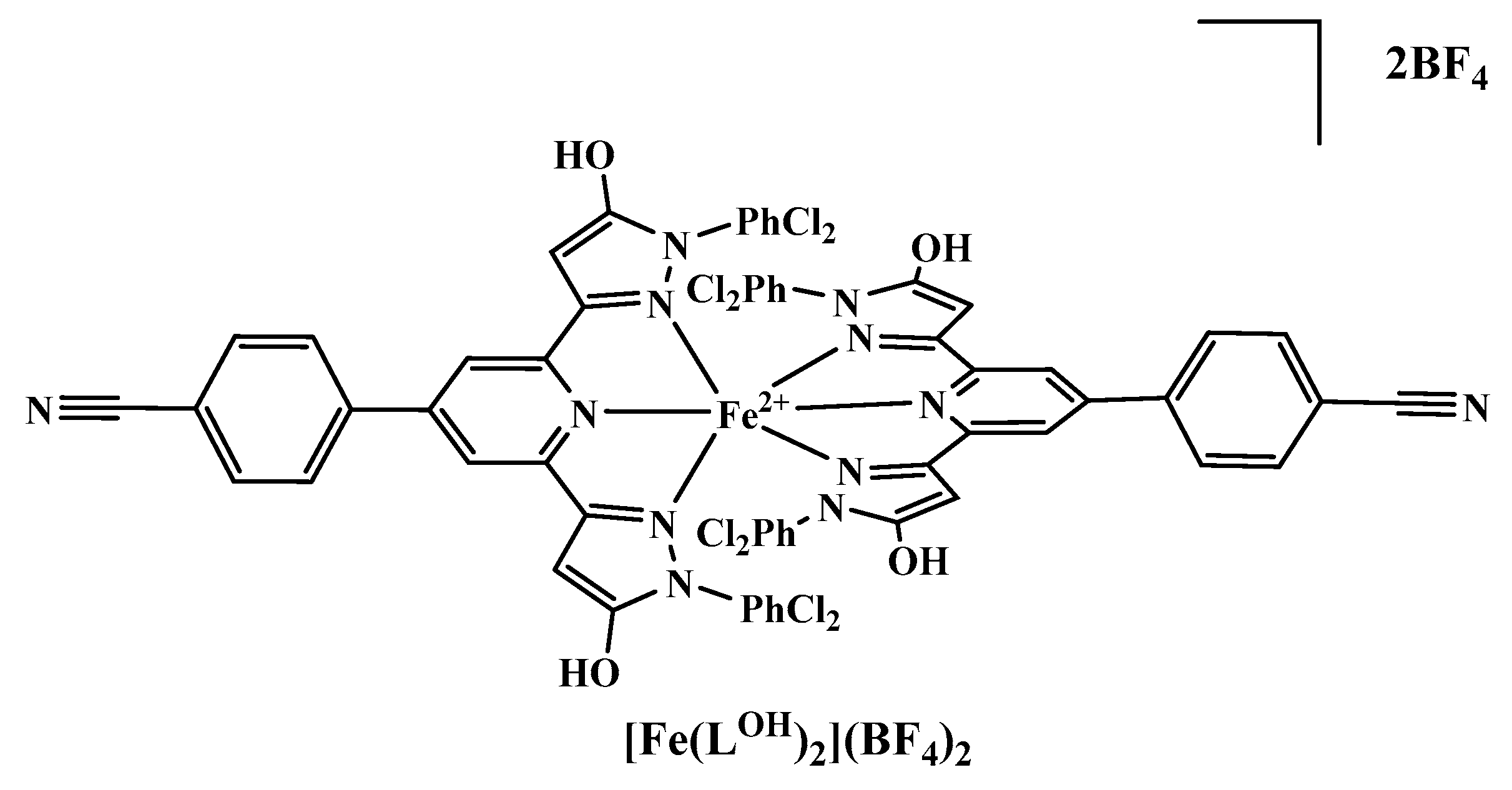
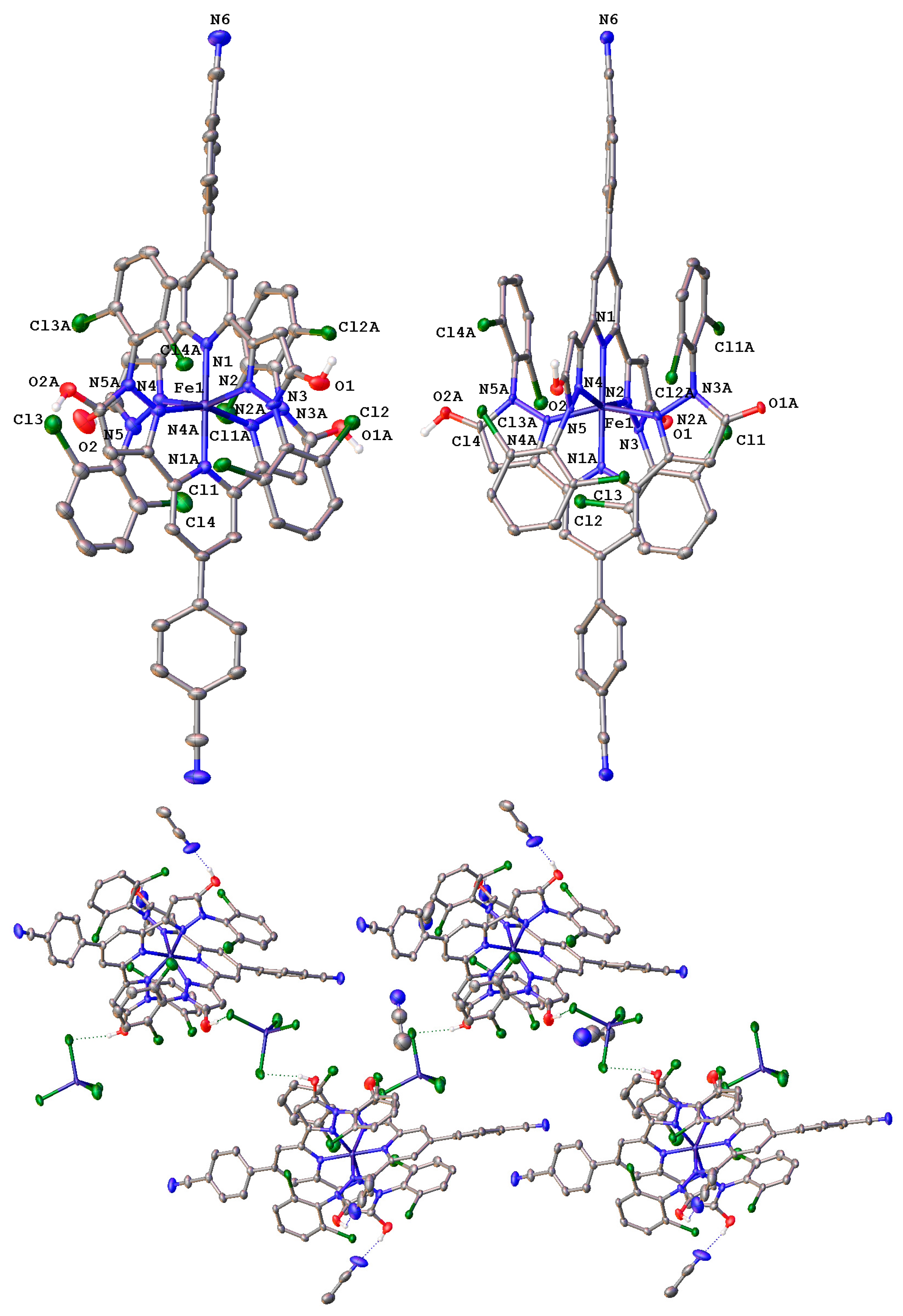
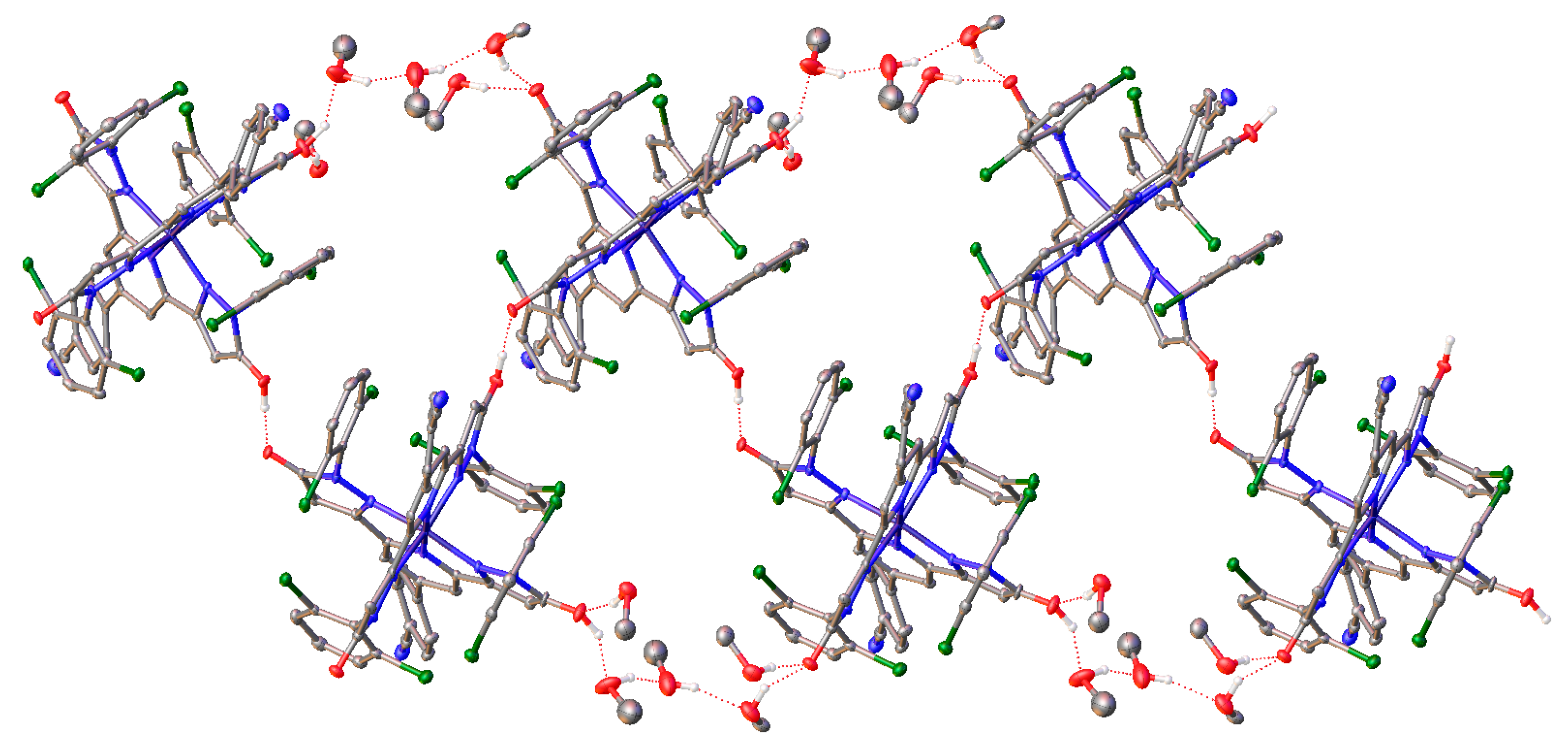

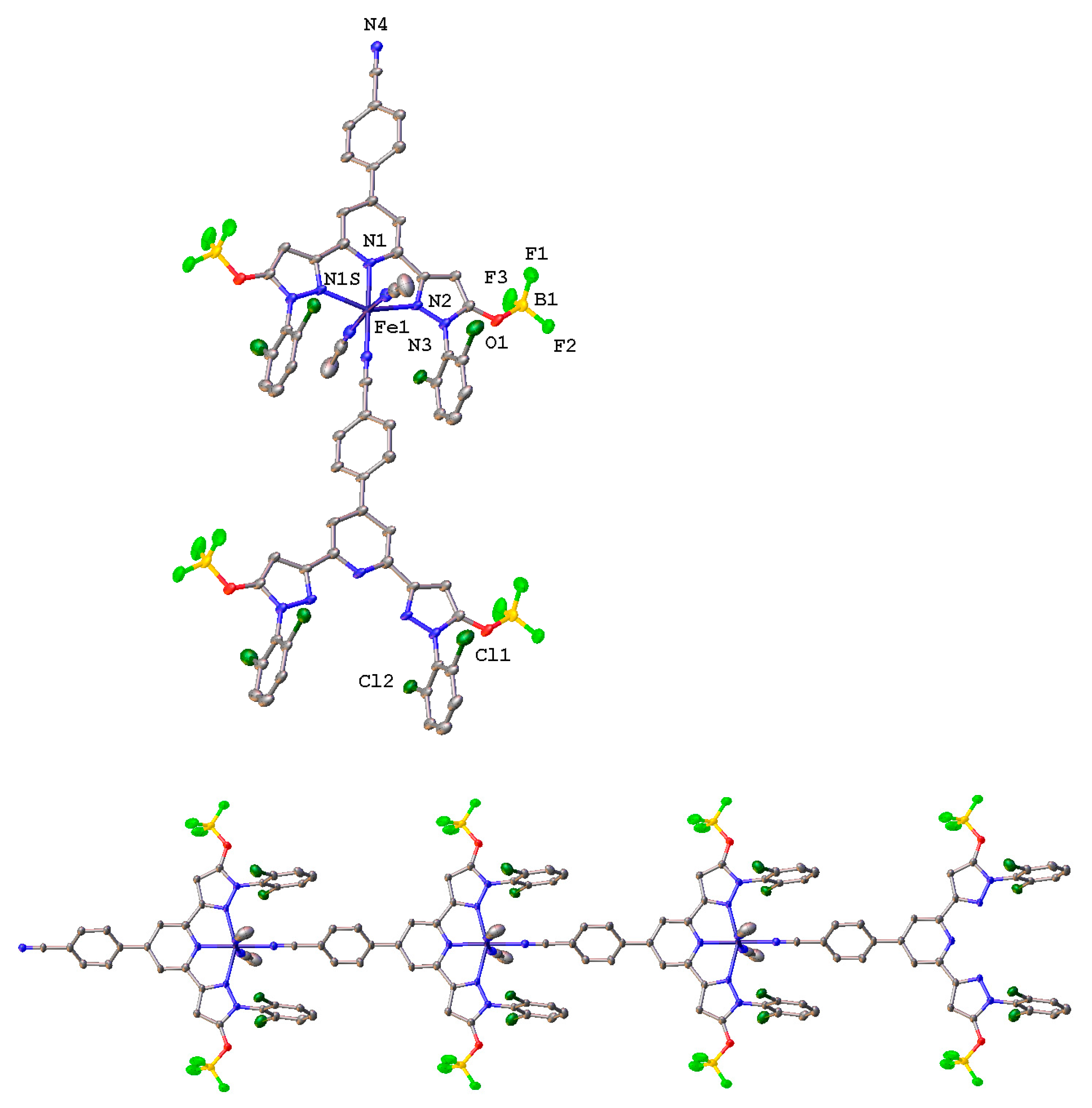
| Parameter | [Fe(LOH)2] [FeCl4]•5CH3CN | [Fe(LO−)2]• 5CH3OH | [Fe(LO−)2 AgNO3BF4•CH3OH]n•1.75nCH3OH•nH2O | [Fe(LOBF3)(CH3COO)(CH3CN)2]n• nCH3CN |
|---|---|---|---|---|
| Fe-NPy, Å | 1.920(6), 1.912(6) | 1.918(5), 1.914(5) | 1.915(6), 1.904(6) | 2.061(8) |
| Fe-NPz, Å | 1.999(5)–2.005(6) | 1.967(5)–1.998(5) | 1.914(7)–1.959(7) | 2.145(6) |
| Fe-NCN, Å | - | - | - | 2.079(8) [2.117(10)] [b] |
| θ, ° | 88.44(6) | 89.34(6) | 88.67(7) | 0.0 |
| ϕ, ° | 179.5(3) | 179.6(3) | 178.7(3) | 179.4(5) [c] |
| β, ° | 38.3(3), 36.0(3) | 21.4(2), 40.5(3) | 29.1(3), 39.9(4) [23.2(14)] [d] | 48.9(5) |
| γ, ° | 85.3(3)–89.3(3) | 82.5(3)–89.7(3) | 85.3(3)–88.2(3) | 82.8(4) |
| S(OC) | 2.486 | 2.280 | 1.895 | 2.011 |
| S(TPR) | 12.088 | 12.373 | 12.548 | 12.844 |
| S(ebcT) | 12.824 | 12.936 | 13.386 | 14.110 |
| S(SS) | - | - | 2.426 | - |
| S(T) | - | - | 4.975 |
| [Fe(LOH)2][FeCl4]•5CH3CN | [Fe(LO−)2]•5CH3OH | [Fe(LO−)2AgNO3BF4• CH3OH]n•1.75nCH3OH•nH2O | [Fe(LOBF3)(CH3COO) (CH3CN)2]n• nCH3CN | |
|---|---|---|---|---|
| Formula unit | C70H47Cl12Fe2 N17O4 | C65H50Cl8FeN12O9 | C62.75H43AgBCl8F4FeN13O10.75 | C76H52B4Cl8F12 Fe2N18O8 |
| Formula weight | 1727.34 | 1482.62 | 1685.23 | 2011.89 |
| Temperature, K | 120 | 120 | 120 | 100 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/n | P21/c | P21/c | C2/c |
| Z | 4 | 4 | 4 | 2 |
| a, Å | 19.095(4) | 20.951(4) | 15.792(5) | 17.488(4) |
| b, Å | 18.899(4) | 14.806(3) | 16.059(5) | 13.740(3) |
| c, Å | 22.565(5) | 23.122(4) | 26.900(8) | 21.498(4) |
| α, ° | 90 | 90 | 90 | 90 |
| β, ° | 91.163(4) | 110.525(4) | 106.675(6) | 104.66(3) |
| γ | 90 | 90 | 90 | 90 |
| V, Å3 | 8141(3) | 6717(2) | 6535(3) | 4997.6(18) |
| Dcalc (g cm−1) | 1.409 | 1.466 | 1.713 | 1.337 |
| Linear absorption, μ (cm−1) | 8.06 | 6.09 | 9.33 | 6.59 |
| F(000) | 3496 | 3032 | 3386 | 2028 |
| 2Θmax, ° | 52 | 52 | 54 | 52.6 |
| Reflections measured | 103,671 | 80,169 | 90,879 | 12,966 |
| Independent reflections | 15,995 | 13,197 | 14,264 | 4418 |
| Observed reflections [I > 2σ(I)] | 8258 | 6414 | 5673 | 2302 |
| Parameters | 955 | 861 | 940 | 328 |
| R1 | 0.0830 | 0.0729 | 0.0742 | 0.0944 |
| wR2 | 0.2441 | 0.2152 | 0.2071 | 0.3228 |
| GOF | 1.041 | 0.976 | 0.963 | 1.034 |
| Δρmax/Δρmin (e Å−3) | 1.539/−0.750 | 1.191/−0.879 | 1.064/−0.840 | 0.998/−0.527 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikovskiy, I.A.; Dorovatovskii, P.V.; Novikov, V.V.; Nelyubina, Y.V. Bis(2,6-pyrazolyl)pyridines as a New Scaffold for Coordination Polymers. Molecules 2023, 28, 4275. https://doi.org/10.3390/molecules28114275
Nikovskiy IA, Dorovatovskii PV, Novikov VV, Nelyubina YV. Bis(2,6-pyrazolyl)pyridines as a New Scaffold for Coordination Polymers. Molecules. 2023; 28(11):4275. https://doi.org/10.3390/molecules28114275
Chicago/Turabian StyleNikovskiy, Igor A., Pavel V. Dorovatovskii, Valentin V. Novikov, and Yulia V. Nelyubina. 2023. "Bis(2,6-pyrazolyl)pyridines as a New Scaffold for Coordination Polymers" Molecules 28, no. 11: 4275. https://doi.org/10.3390/molecules28114275
APA StyleNikovskiy, I. A., Dorovatovskii, P. V., Novikov, V. V., & Nelyubina, Y. V. (2023). Bis(2,6-pyrazolyl)pyridines as a New Scaffold for Coordination Polymers. Molecules, 28(11), 4275. https://doi.org/10.3390/molecules28114275