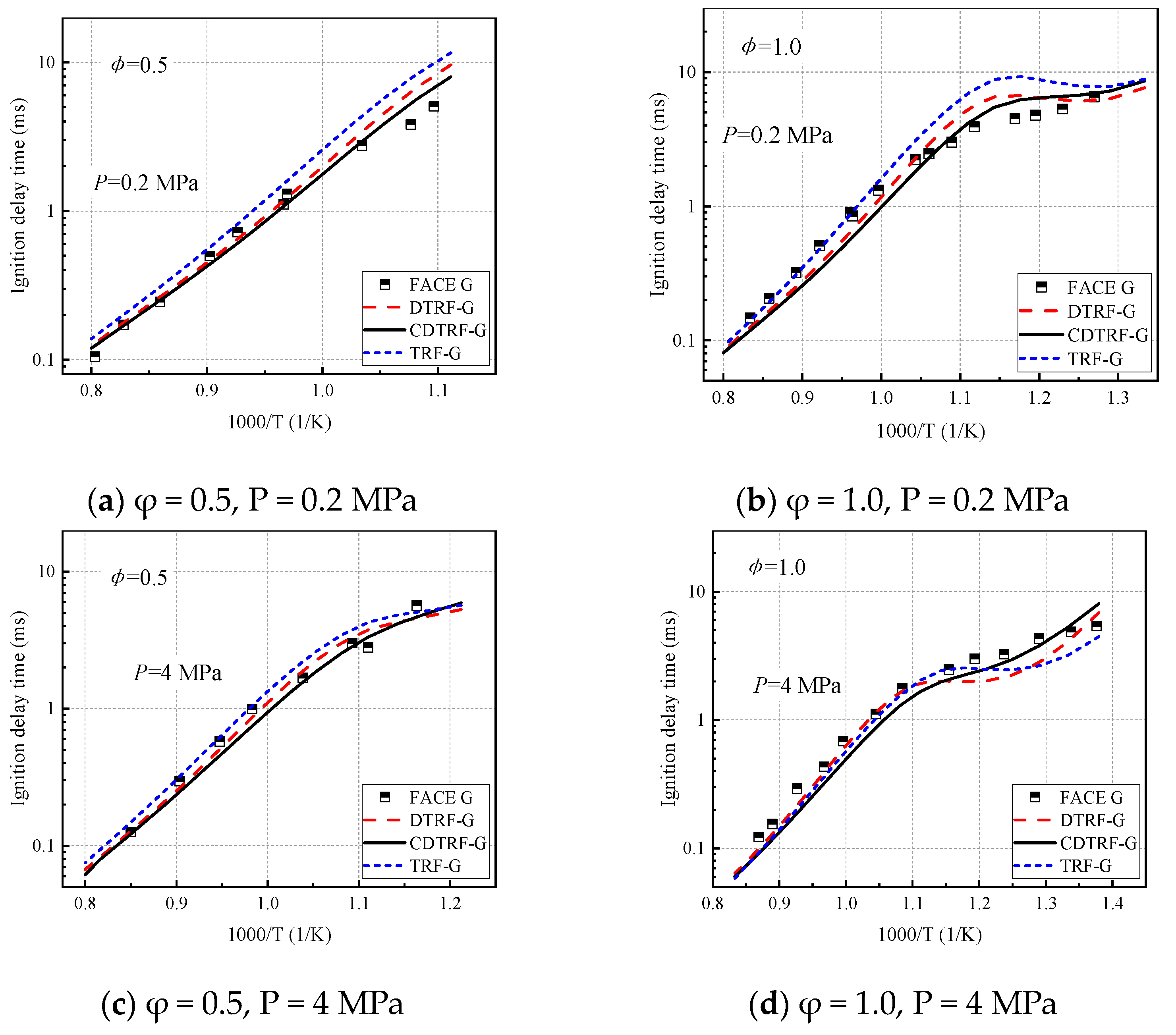
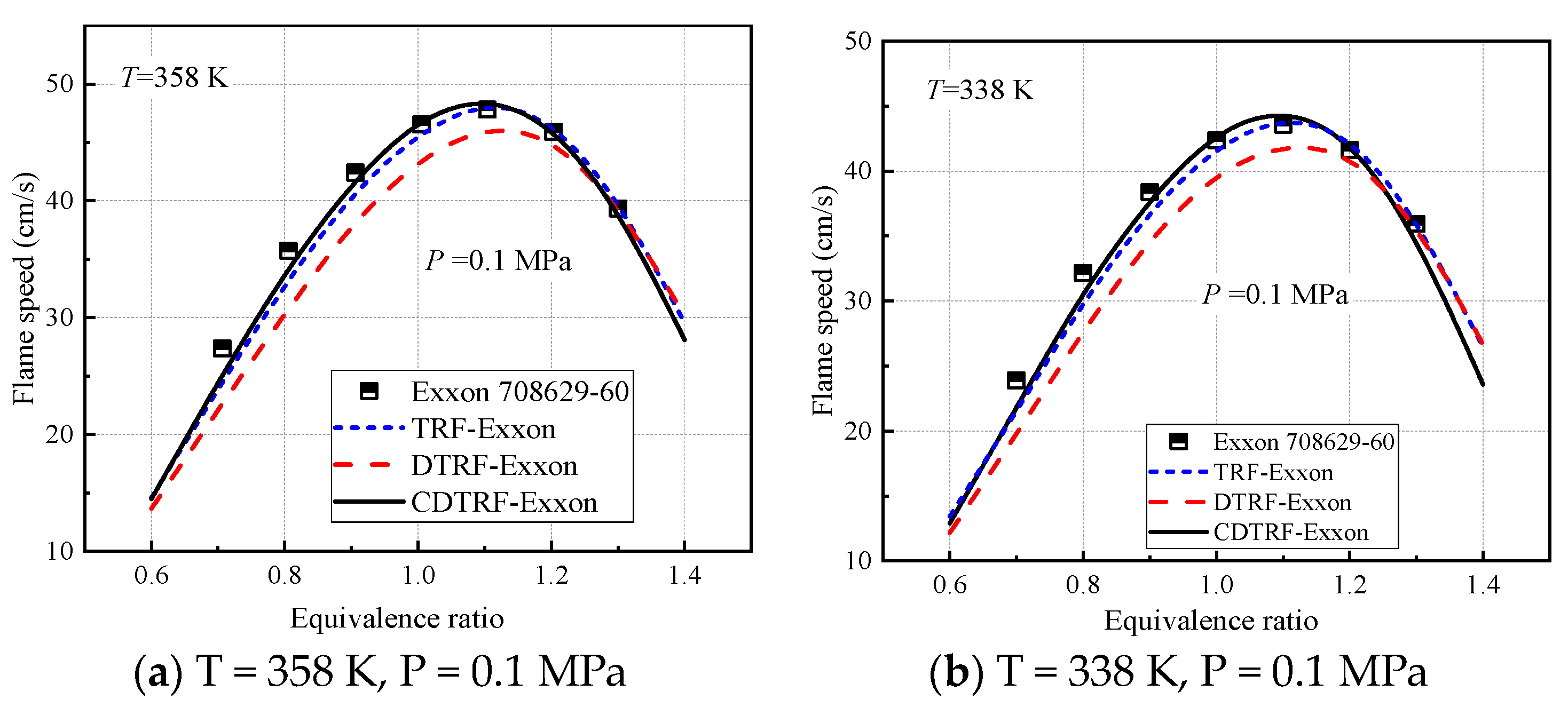
3.1. Effect of Cyclohexane on Ignition-Delay Time
The ignition characteristics of the fuel are controlled by the chemical-reaction kinetic process of the fuel itself and the input of external boundary conditions. Therefore, in this paper, the effect of cyclohexane addition on the ignition-delay time of gasoline–surrogate fuel mixtures are first investigated under lean combustion conditions.
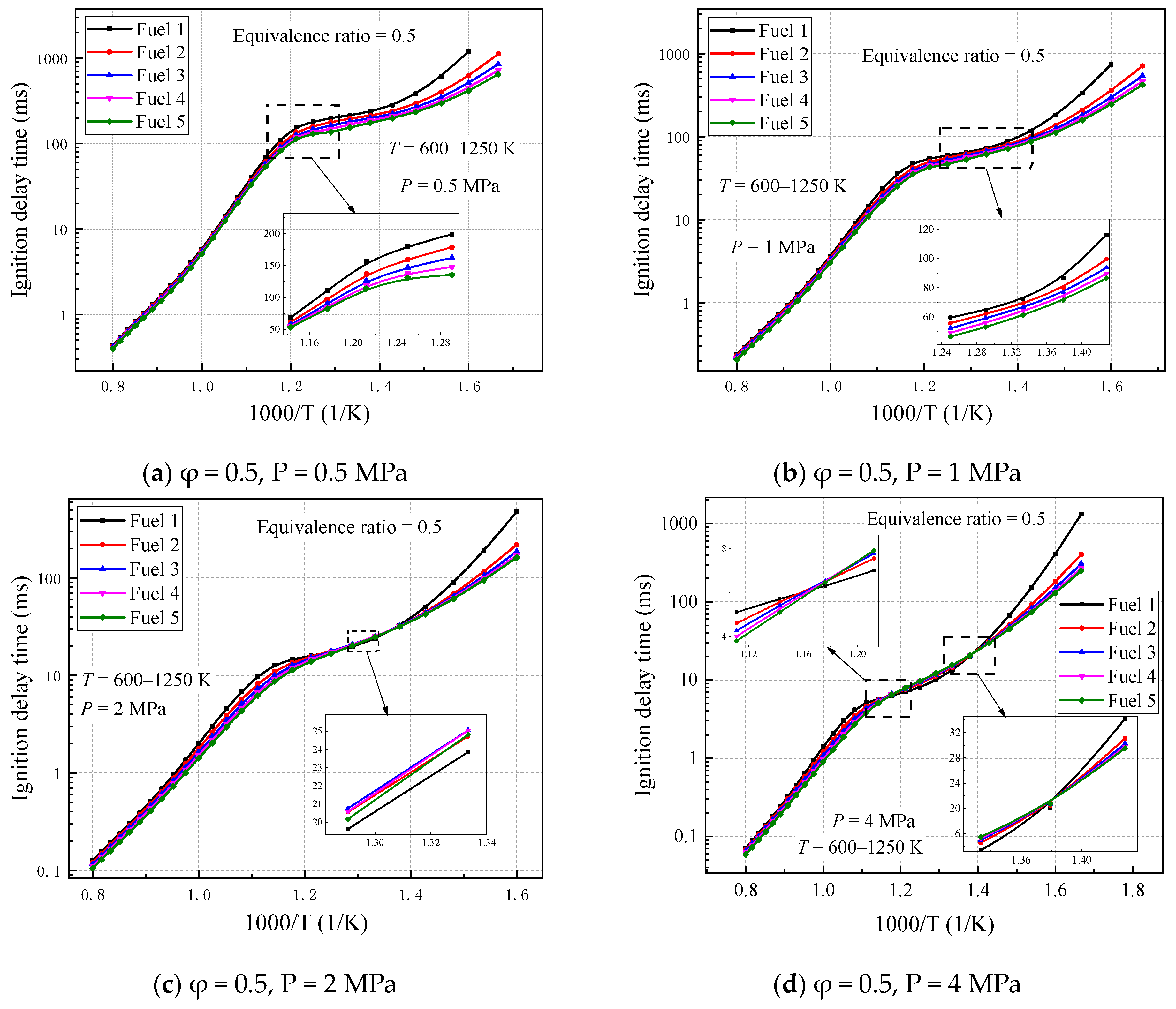
The ignition-delay times are calculated for five gasoline–surrogate fuel mixtures (Fuel 1–5) under the lean combustion condition (equivalent ratio of 0.5), temperature range of 600–1250 K, and pressure range of 0.5–4 MPa. The ignition-delay times of the five surrogate-fuel models calculated using the selected mechanism are given in
Figure 4.
As can be seen in
Figure 4, at higher initial temperatures (T > 1000 K), the differences in ignition-delay times of the five fuels are not significant and maintained a consistent trend with temperature changes. However, at higher pressures (2 MPa or 4 MPa), the differences in ignition-delay times of the five fuels increase, and the more surrogate fuels containing cyclohexane content, the smaller the ignition-delay times. When the initial temperature is small (T < 715 K), the ignition-delay times of the five surrogate fuels are gradually separated, with fuel 1, which does not contain cyclohexane, being the most affected by the temperature, indicating that the addition of cyclohexane reduces the ignition delays of the surrogate fuels in the low-temperature reaction stage. The largest difference occurs in the initial temperature range of 715–1000 K. At lower pressures, fuel 1 without cyclohexane still lags behind the other fuels. As the pressure increases, the ignition-delay times of fuels 2–5 are relatively higher than those of fuel 1, with fuel 5, which contains the largest volume fraction of cyclohexane, exhibiting the least pronounced NTC (
Figure 4d), suggesting that the addition of cyclohexane suppresses the negative temperature behavior of the surrogate fuels in the midtemperature region and becomes more pronounced as the pressure increases.
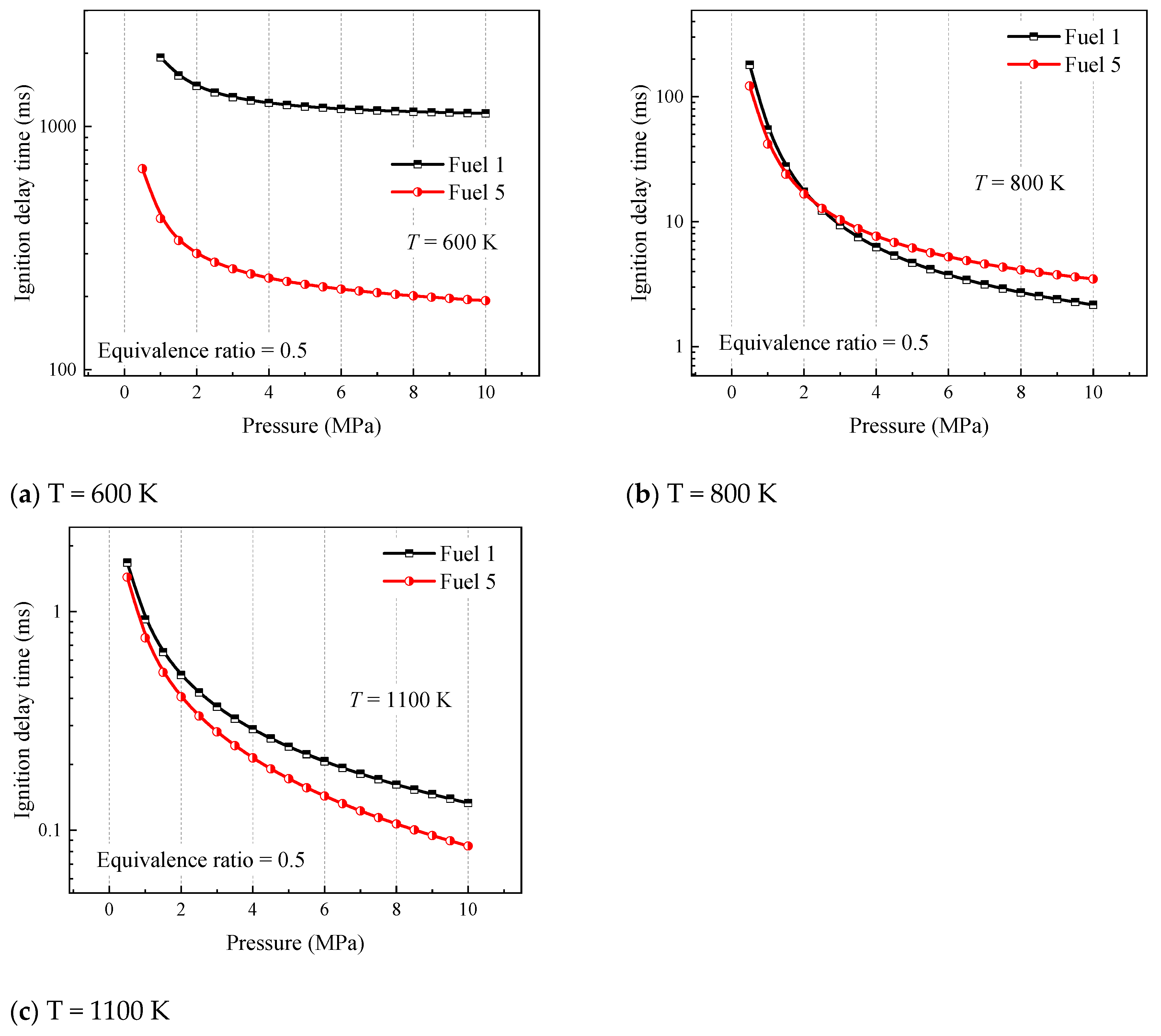
In order to further study the effect of initial pressure on the ignition laws of surrogate fuels, the ignition laws are investigated at different temperatures with different cyclohexane concentrations.
Figure 5 analyzes the ignition of fuel 1 and fuel 5 at initial pressures of 1–10 MPa, where fuel 1 is a four-component surrogate fuel without cyclohexane and fuel 5 is the surrogate fuel with the largest volume fraction of cyclohexane constructed in this paper. Three representative temperatures are selected according to
Figure 5, 600 K for the low-temperature region, 800 K for the medium-temperature region, and 1100 K for the high-temperature region.
The results shown in
Figure 5 indicate that the difference in ignition-delay time between fuel 1 and fuel 5 at 600 K and 1100 K increases with increasing pressure, which explains the phenomenon in
Figure 4 that the difference in ignition-delay time between different fuels at low and high temperatures increases with increasing pressure. In addition, the gap in ignition-delay time between the two surrogate mixtures is the largest at 600 K. Based on the previous analysis, this is because the chemical reaction rate in the low-temperature phase is more dependent on the oxidation of the fuel macromolecules. At 800 K, it can be seen that the ignition-delay time of fuel 1 is not always higher than that of fuel 5, and both shift at a pressure of 2.25 MPa, as shown in
Figure 5b. It can be seen that the addition of cyclohexane changes the ignition pattern of the four-component surrogate fuels in the midtemperature region. The ignition delay of the fuel containing cyclohexane in the midtemperature phase is less dependent on pressure, which affects the NTC behavior of the surrogate fuel in this region.
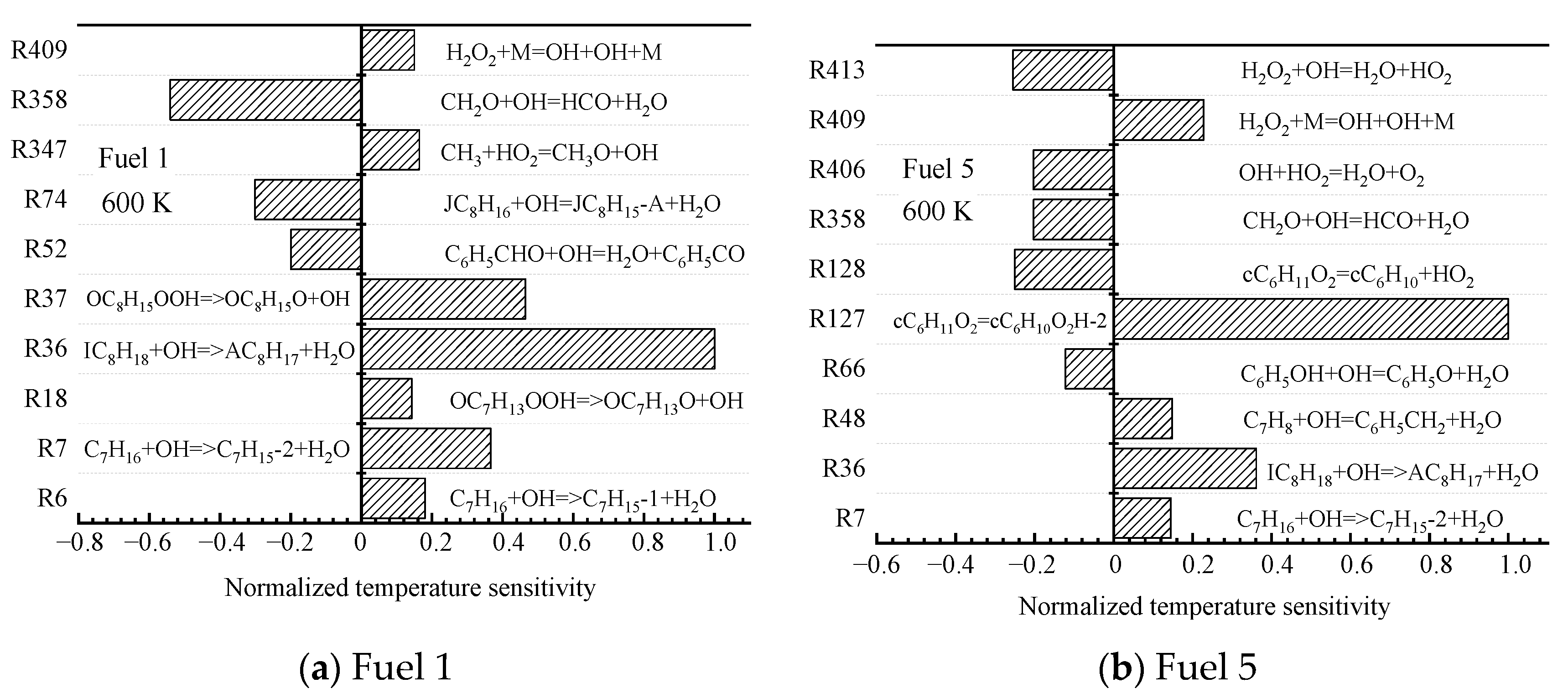
The root causes of the different surrogate fuels showing different ignition patterns are further analyzed at the microscopic level by chemical kinetics. For the two fuels (fuel 1 and fuel 5) with the largest difference in cyclohexane content, sensitivity analyses are performed at low (690 K), medium (800 K), and high (1200 K) temperatures at an initial pressure of 2 MPa and an equivalence ratio of 0.5, and the top ten most-sensitive reactions are selected.
The sensitivity reactions of the fuel ignition process for fuel 1 and fuel 5 at an initial temperature of 690 K are shown in
Figure 6. At low temperatures, the sensitivity of the fuel ignition process is dominated by macromolecular reactions.
Among them, for fuel 1, R7 and R36 are the reactions of straight and heterochain alkanes with OH radicals to remove a hydrogen atom to form the corresponding alkyl radical, and R37 is the further decomposition of hydroperoxyalkyl radicals (QOOH); for fuel 5, R36 is the same as the dehydrogenation of chain alkanes, and R127 is the isomerization of cyclohexane oxide cC6H12O2, which in fuel 5, the surrogate-fuel ignition process showed higher sensitivity; in addition, the small molecule reaction R358. CH2O + OH = HCO + H2O exhibits a greater sensitivity during fuel 1 ignition, which indicates that the depletion of OH radicals in the R358 reaction reduces the reactivity of fuel 1 fuel. The above analysis illustrates that the fuel ignition process in the low-temperature region is controlled to a great extent by the initial oxidation and dehydrogenation reactions of fuel macromolecules, and the cyclohexane molecules of fuel 5 are the first to react, and the accumulation of OH radicals drives the early ignition of fuel 5.
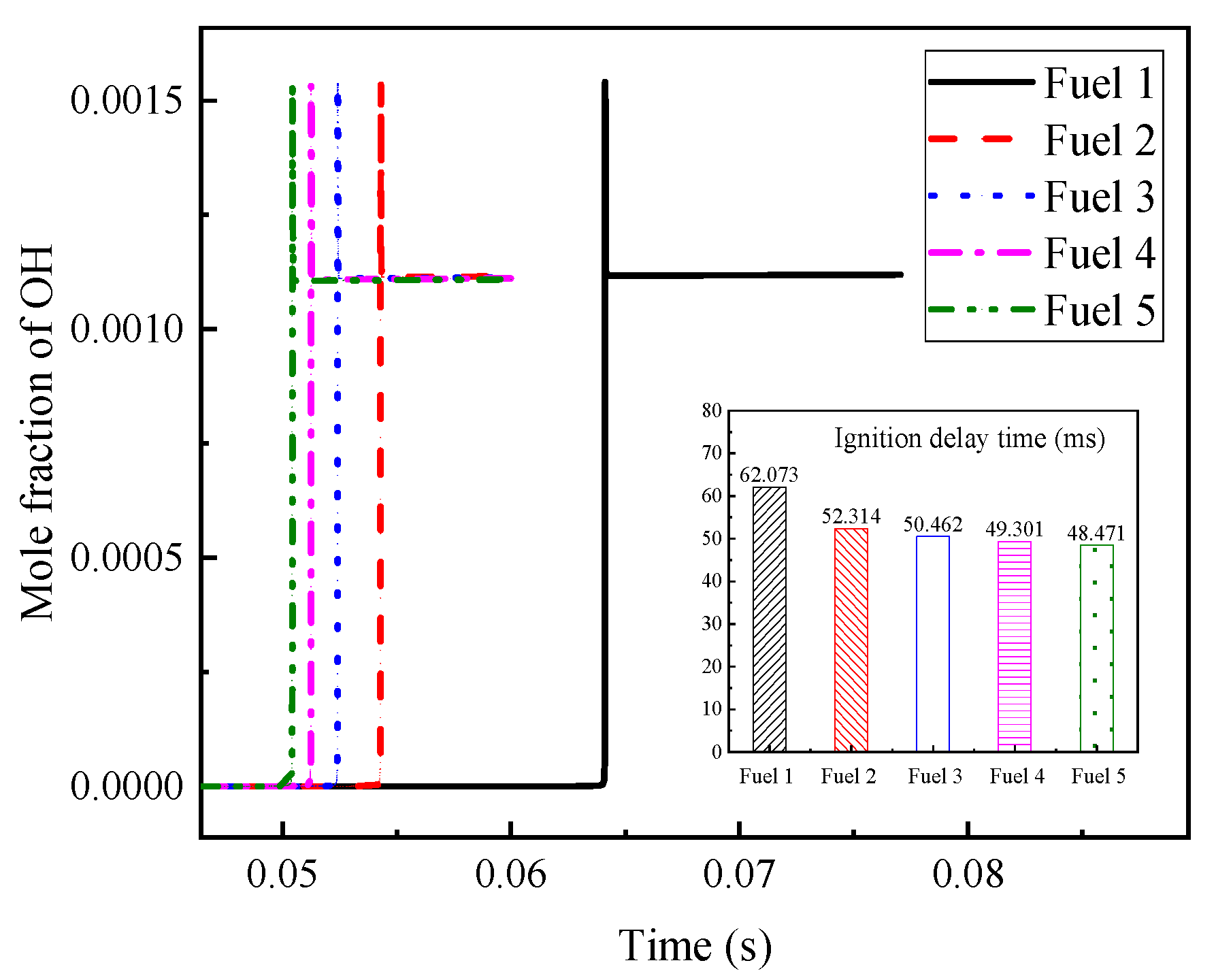
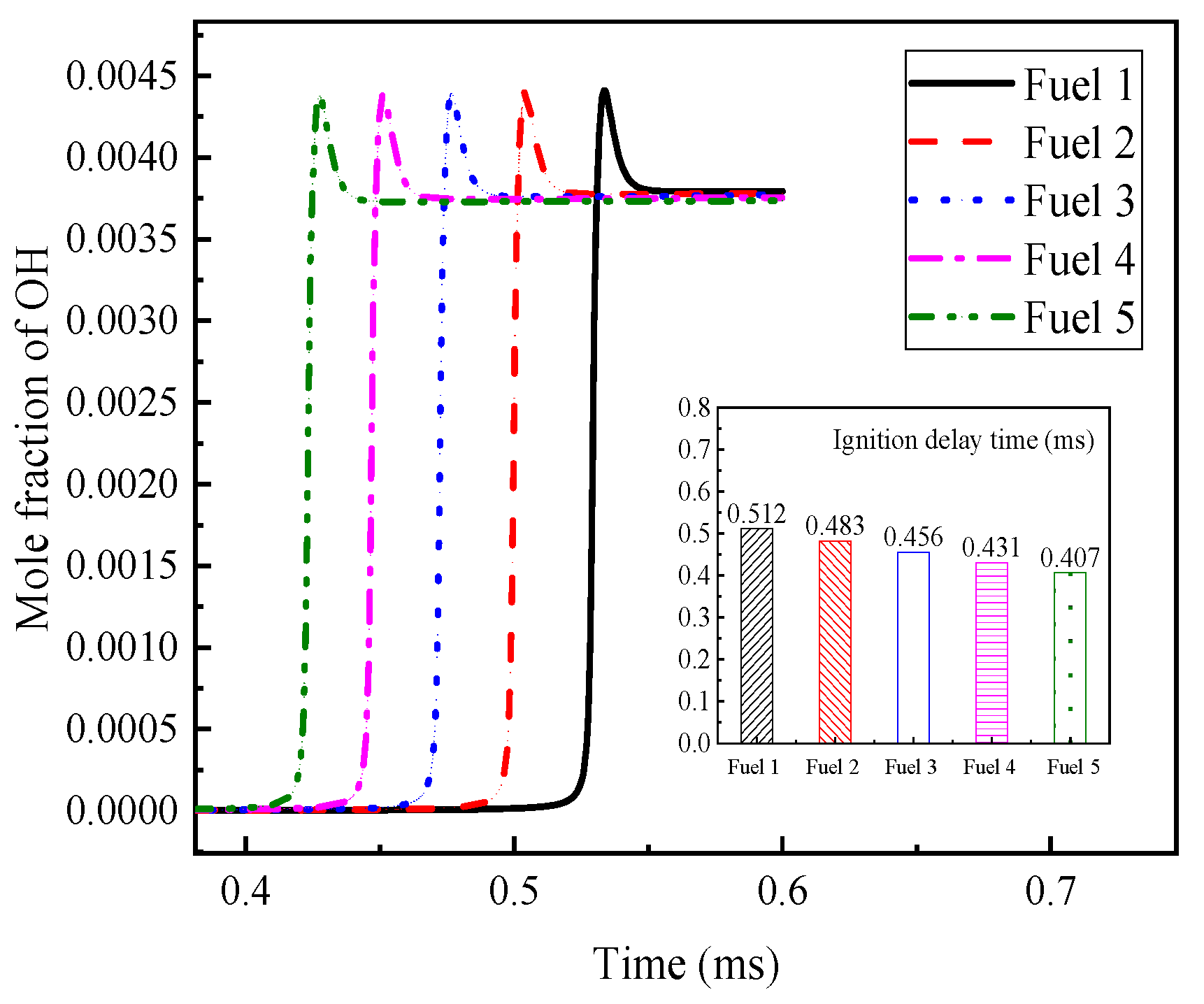
Among the above reactions with large, normalized sensitivity coefficients, OH radicals are found to appear more often, which indicates that OH radicals have a greater influence on the ignition and combustion of the system. The OH radical molar fraction curves for the fuels in
Table 7 are plotted in
Figure 7 at an initial temperature of 690 K, an initial pressure of 2 MPa, and an equivalence ratio of 0.5, and the corresponding ignition-delay times of the fuels are compared in the figure. The peak appearance of OH radicals for fuel 1 fuel is different from the other four fuels by about 10 ms, and the final OH radical production for the five fuels remained at a consistent level. The corresponding ignition-delay times indicate that the peak moments of OH radicals can correspond to the ignition-delay times of their fuels one by one.
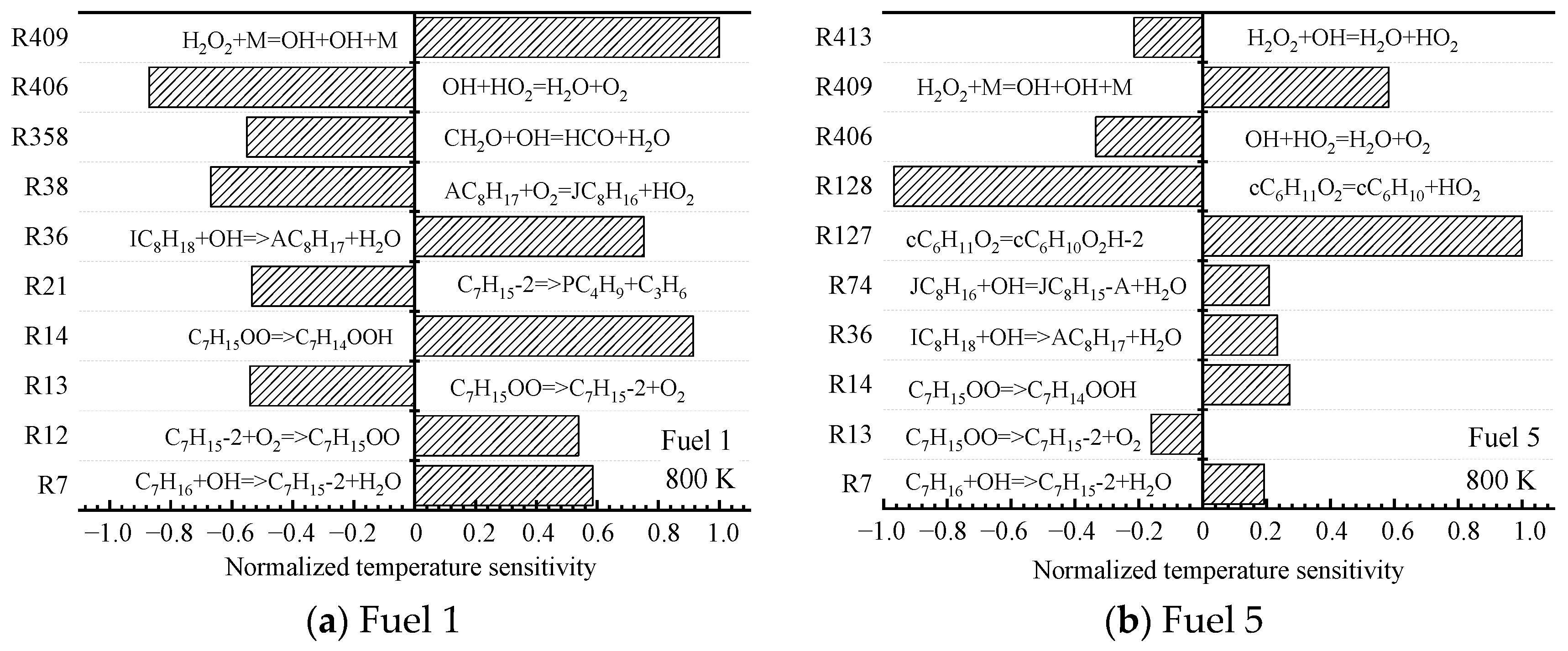
Next, the sensitivity of the ignition characteristics of fuel 1 and fuel 5 at a medium temperature of 800 K is analyzed. Unlike the low-temperature region, the temperature sensitivities calculated for the two surrogate-fuel ratios in the medium-temperature region appear significantly different. This is shown in
Figure 8.
For fuel 1, the reactions with larger normalized sensitivity coefficients shift from macromolecular reactions to small molecular reactions (R358, R406, and R409), and the small molecular reaction R409 has the largest normalized sensitivity coefficient. H
2O
2 is decomposed into two OH radicals under the action of the third body, which increases the system temperature and reduces the ignition-delay time, leading to the formation of the NTC region of the fuel. However, the reactivity of fuel 1 in the middle-temperature region is still lower than that in the high-temperature region, because some macromolecular reactions (R14, R36, and R38) have large, normalized sensitivity coefficients. From
Figure 8b, it can be seen that the fuel sensitivity of fuel 5 is still dominated by macromolecular reactions (R127 and R128), which are isomerization and decomposition reactions of cyclohexane oxide cC
6H
12O
2, respectively. In contrast, the small molecular reaction R409 only occupies a small proportion. This explains the phenomenon that fuel 2~5 inhibit the NTC effect in
Figure 4d.
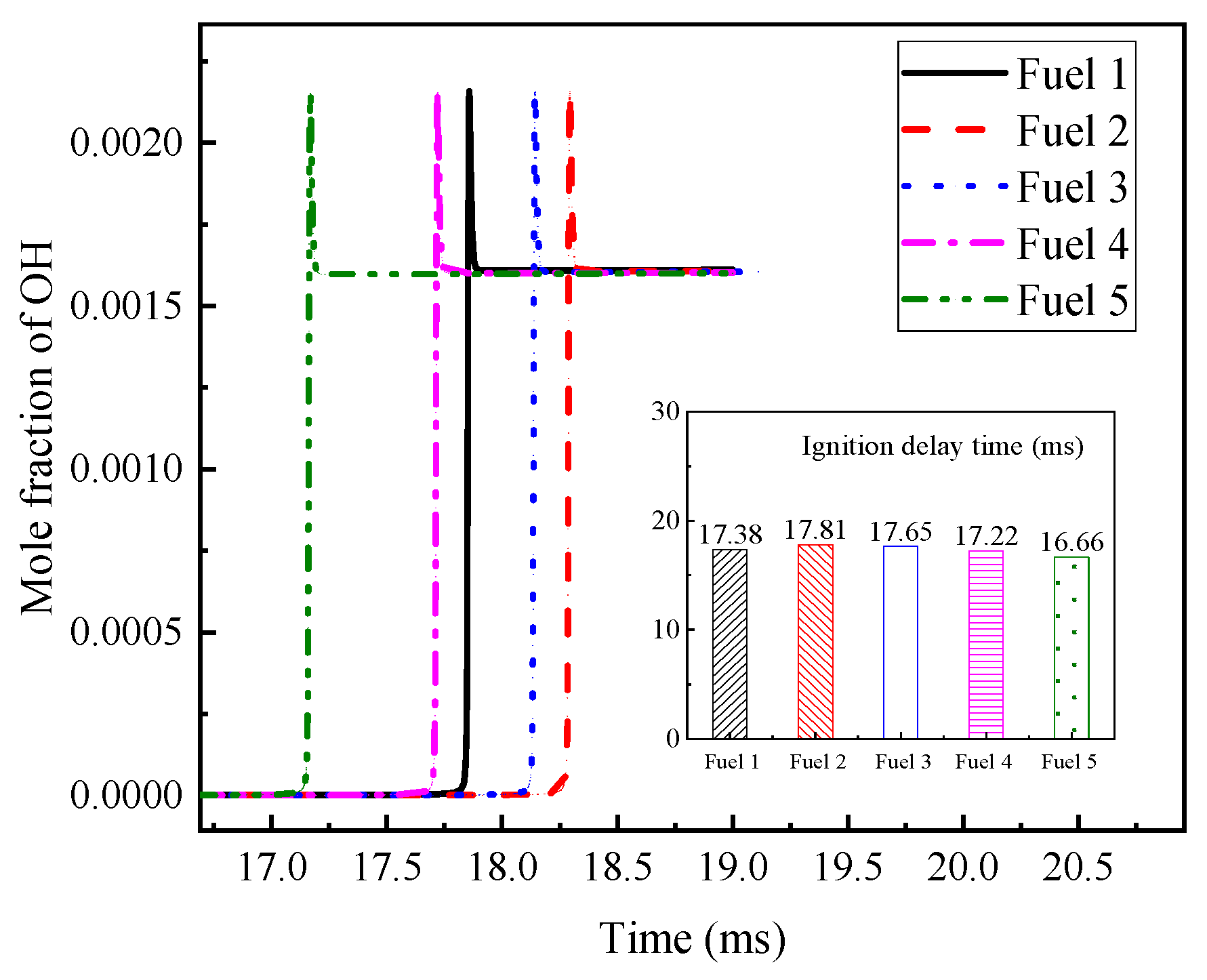
The OH radical generation curves for the five surrogate fuels in
Table 7 are calculated. The calculation conditions are 800 K initial temperature, 2 MPa pressure, and 0.5 equivalence ratio.
Figure 9 gives the OH radical generation curves and the corresponding ignition-delay times for the five surrogate fuels. It can be seen that the OH radical molar fraction of the five surrogate fuels peaks at 17–18.5 ms, and the OH radical peak of fuel 1 peaks earlier than that of fuel 2 and fuel 3, while the ignition-delay time of fuel 1 decreases correspondingly. This fully illustrates that the addition of cyclohexane has a great influence on the fuel NTC effect.
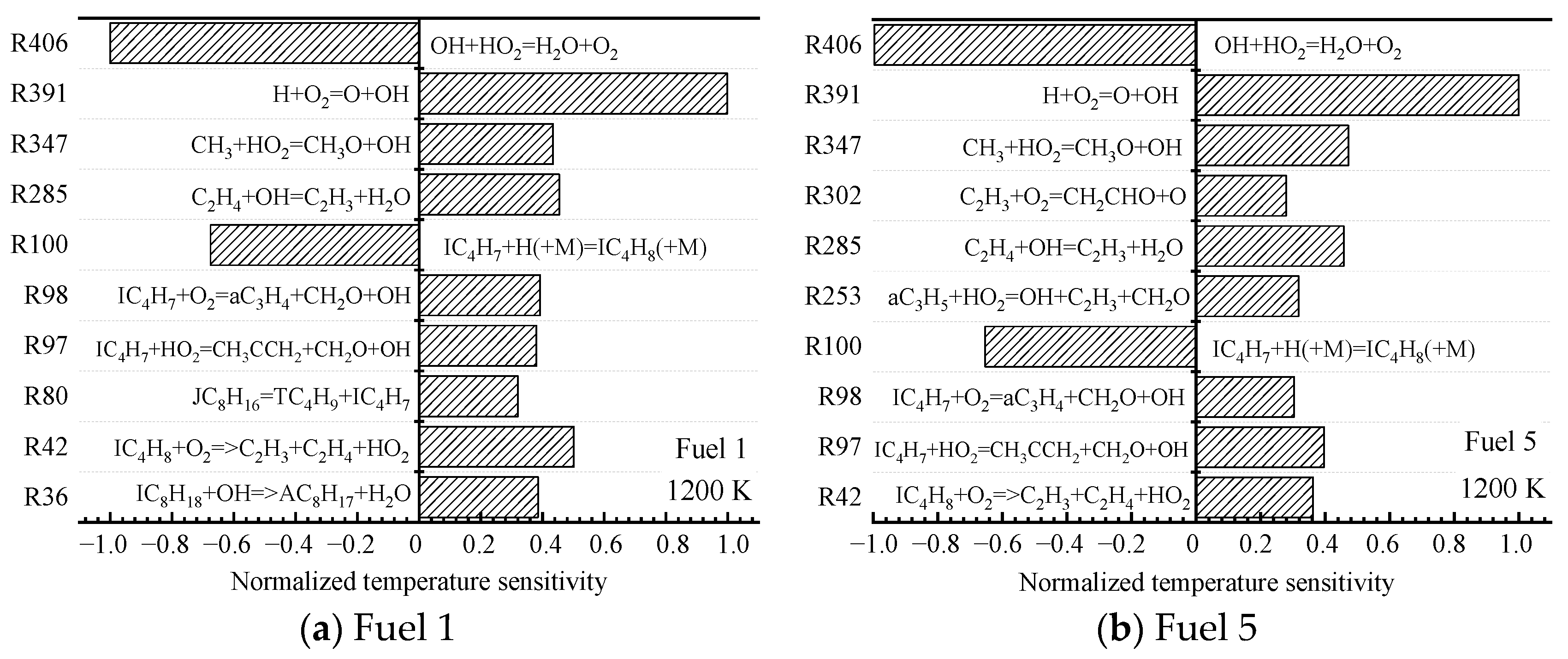
Finally, the sensitivities of the ignition characteristics of fuel 1 and fuel 5 at high temperatures of 1200 K are analyzed. Comparing the high-temperature normalized sensitivity coefficients in
Figure 10a,b, it can be seen that fuel 1 and fuel 5 basically contain the same high-temperature sensitivity responses, and although the top ten responses of sensitivity are not identical, several important primitive responses of greater sensitivity are consistent.
Among them, the isobutylene radical hydrogenation reaction represented by the radical reaction R100 has a large, negative temperature-sensitivity coefficient, and the consumption of H radicals suppresses the elevation of the system temperature. In addition to exhibiting a large negative temperature sensitivity coefficient is the radical reaction R406, and the absolute value of the sensitivity coefficient is the largest for this radical reaction, which is a depletion reaction of OH radicals, and the unstable intermediate product, peroxyhydroxyl radical HO2, generates water and oxygen molecules with OH radicals. Although the depletion of the active molecule OH radical has an inhibitory effect on the system ignition, the oxygen molecules generated by the reaction provide the oxidant for the subsequent fuel reaction. The radical reaction R391 shows the greatest positive sensitivity, which is a branching reaction of the chain, consisting of H radicals colliding with O2 molecules to generate active OH radicals and O radicals, while O radicals continue to collide with H2 molecules parametrically OH radicals, increasing the concentration of OH radicals in the system, raising the system temperature and prompting the fuel to reach ignition in a short time.
The small difference in ignition-delay time between the two fuels at high temperatures is mainly attributed to the same reactivity of the small molecule reactions controlling the reaction progression. It can also be seen from
Figure 10 that the earlier ignition of fuel 5 compared to fuel 1 is due to the inclusion of more small molecule reactions in the high-temperature positive sensitivity of Fuel 5.
The OH radical generation curves at high temperatures in
Figure 11 again explain the above point, as the OH radical peaks of the five surrogate fuels do not differ by more than 0.1 ms, and the reaction proceeds quite rapidly. This shows that the ignition characteristics of the five surrogate fuels remain the same at high temperatures, with a small difference due to the role of cyclohexane, which is more prevalent at higher concentrations.
The effect of cyclohexane on the ignition-delay time of surrogate fuels was analyzed in the temperature range of 600–1250 K, pressure of 0.1–4 MPa, and lean conditions with an equivalence ratio of 0.5. The addition of cyclohexane inhibits the occurrence of fuel NTC, especially at higher pressures. At low and high temperatures, the addition of cyclohexane shortens the ignition time of the fuel, and this effect is most obvious in the low-temperature region. Based on this influence pattern, the ignition pattern of gasoline containing naphthenic hydrocarbons can be analyzed for the subsequent gasoline containing naphthenic hydrocarbons.
3.2. Effect of Cyclohexane on Laminar Flame Speed
As mentioned in the previous validation of the mechanism, applying the chemical kinetic model to the numerical simulation of in-cylinder combustion, besides focusing on the ignition characteristics of the fuel, flame propagation is also an extremely important combustion characteristic, which is related to the normal propagation of the fuel flame during in-cylinder combustion and the study of in-cylinder detonation problems. Therefore, this paper investigates the effect of adding cyclohexane to gasoline surrogate fuels on the laminar flame speed of the overall mixture.
Atmospheric pressure and lower initial-temperature conditions are commonly used to study the laminar flame speed of fuels, and an initial temperature of 298 K and an initial pressure of 0.1 MPa are the general conditions under which the laminar flame speed of fuels is studied into the burner, and the experimental data obtained from different laboratories using different experimental methods can be easily compared at this condition. They can also be used as reference values for analyzing the pressure and temperature dependence of laminar and turbulent flames [
40]. In addition, the laminar flame speed of the fuel obtained at higher initial temperature and pressure conditions can carry out the combustion of the gasoline when it is ignited before the top dead center of in-cylinder compression, and this initial temperature and pressure will continue to increase for gasoline compression-ignition engines. In this study, the corresponding laminar flame speeds were also calculated in the premixed laminar flame-speed calculation model [
41] using surrogate fuels consisting of five different proportions of components, as shown in
Table 7. Two calculation conditions were chosen to represent the above two cases: an initial temperature of 298 K and an initial pressure of 0.1 MPa; and an initial temperature of 500 K and an initial pressure of 2 MPa. In addition, two comparison conditions were also used to better compare the effects of temperature and pressure: an initial temperature of 298 K and an initial pressure of 2 MPa; and an initial temperature of 500 K and an initial pressure of 0.1 MPa. The composition of the air is 79% N
2 and 21% O
2.
Figure 12 shows the calculated laminar flame speed for the five surrogate fuel mixtures at four operating conditions. It can be seen that the laminar flame speed of the fuels shows a trend of increasing and then decreasing in covering all the range of equivalent ratios, with the peak occurring near an equivalent ratio of 1.1. This is the same as most of the literature studies [
14,
38,
40,
42,
43,
44], where the chemical-reaction rate is influenced by the fuel-vapor concentration under lean conditions; the higher the equivalent ratio, the higher the laminar flame speed. Under fuel-rich conditions, the chemical reaction rate is mainly affected by the air concentration, and the effect of the equivalent ratio on the laminar flame speed is smaller than that of the specific heat capacity and density of the gas mixture, the larger the equivalent ratio, the smaller the laminar flame speed. The equilibrium between the two is reached near the equivalence ratio of 1.1 to obtain the maximum laminar flame speed.
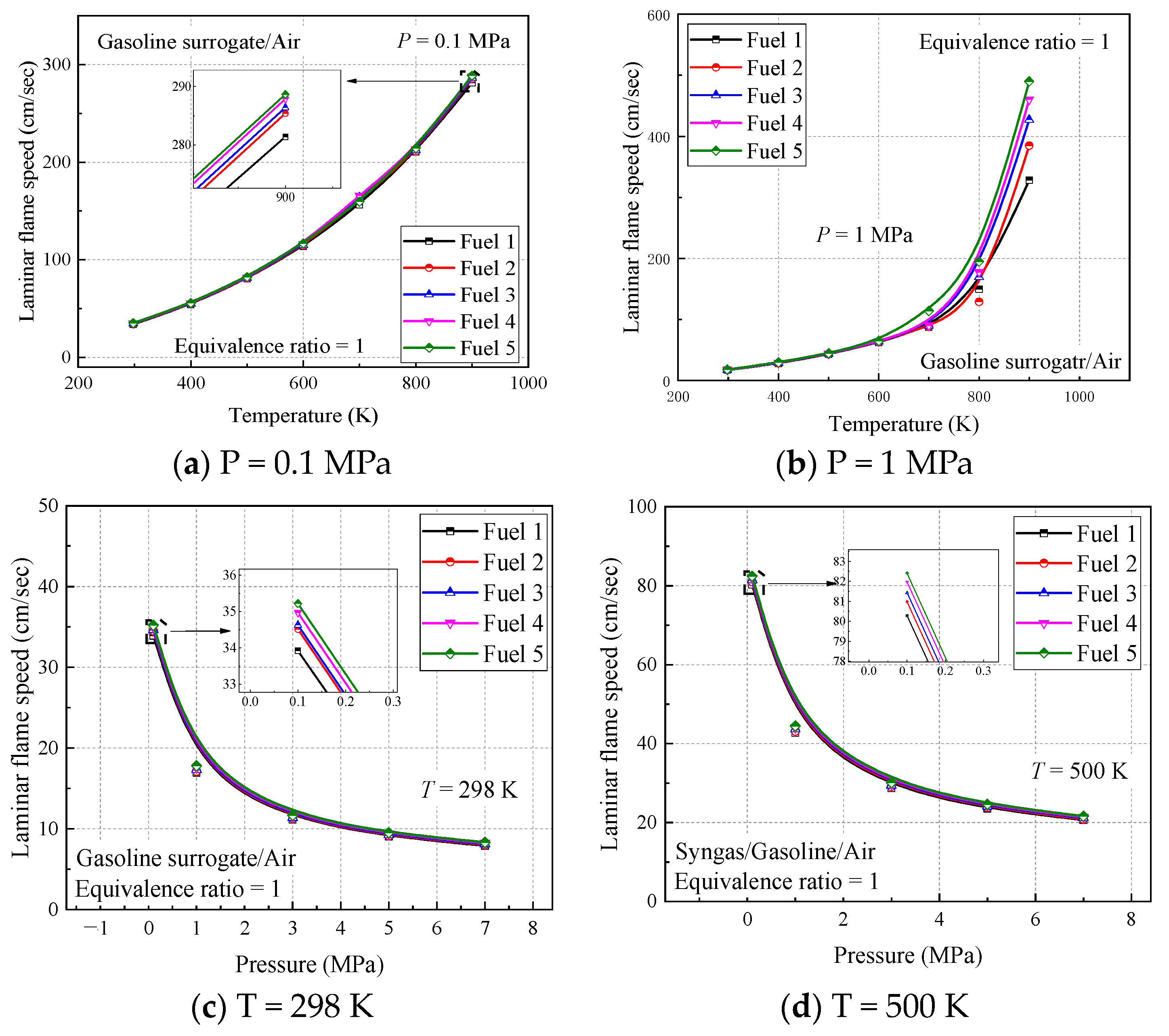
From the calculated results of the four working conditions, the trend and magnitude of the laminar flame speed changes are similar for the five surrogate fuels, but by the local enlargement in
Figure 12, fuel 5 has the largest calculated result while fuel 1 has the smallest, and this change increases with the comparative increase of cyclohexane in the fuel. This difference can be explained by the fact that the addition of cyclohexane dilutes the proportion of chain alkanes and aromatic hydrocarbons in the mixture. According to the laminar flame speed verification of pure component fuels in previous work [
30], the approximate order of magnitude of the laminar flame speed of several components is n-heptane > cyclohexane > isooctane > diisobutene > toluene, which, from a microscopic point of view, is the difference in the concentration of reactive radicals such as H and OH generated by each component. The C-C and C-H bonds in toluene are more stable than the other hydrocarbons, so the addition of cyclohexane increases the laminar flame speed of the fuel mixture, and the small difference is attributed to the proportion of n-heptane, which has a larger flame speed, being reduced accordingly.
The results of the initial temperature and pressure effects on the laminar flame speed can be observed in the calculated results of the four working conditions. From
Figure 12a,b, it can be seen that the initial temperature increases from 298 K to 500 K, the laminar flame speed increases significantly, and the location where the peak laminar flame speed appears moves toward the fuel-rich direction, indicating that the laminar flame vs. speed of the fuel with increasing temperature is more dependent on the fuel concentration; while from
Figure 12a,c, it can be seen that the initial pressure increases from 0.1 MPa to 2 MPa, the laminar flame speed decreases significantly and the peak laminar flame speed appears in the air-rich direction, indicating that the laminar flame speed of the fuel with increasing pressure is more dependent on the concentration of air.
In order to better understand the above phenomenon, the laws of laminar flame speed with temperature and pressure are analyzed in
Figure 13, respectively, when the ratio of surrogate-fuel components is varied. The calculated temperature range is 298–900 K and the pressure range is 0.1–7 MPa. From the figures, it can be seen that the laminar flame speed increases with increasing temperature at constant pressure (
Figure 13a,b). At a pressure of 1 MPa, the laminar flame speed decreases significantly, but the effect of temperature is also more obvious, and the laminar flame speed increases rapidly after the temperature exceeds 800 K. The effect of fuel components is mainly reflected in the higher-pressure condition (1 MPa), where the simulated flame speed of fuel 1 is the smallest and fuel 5 is the largest. The laminar flame speeds calculated for the five surrogate fuels at an equivalence ratio of one at a constant temperature maintained a consistent trend and decreased with increasing pressure.
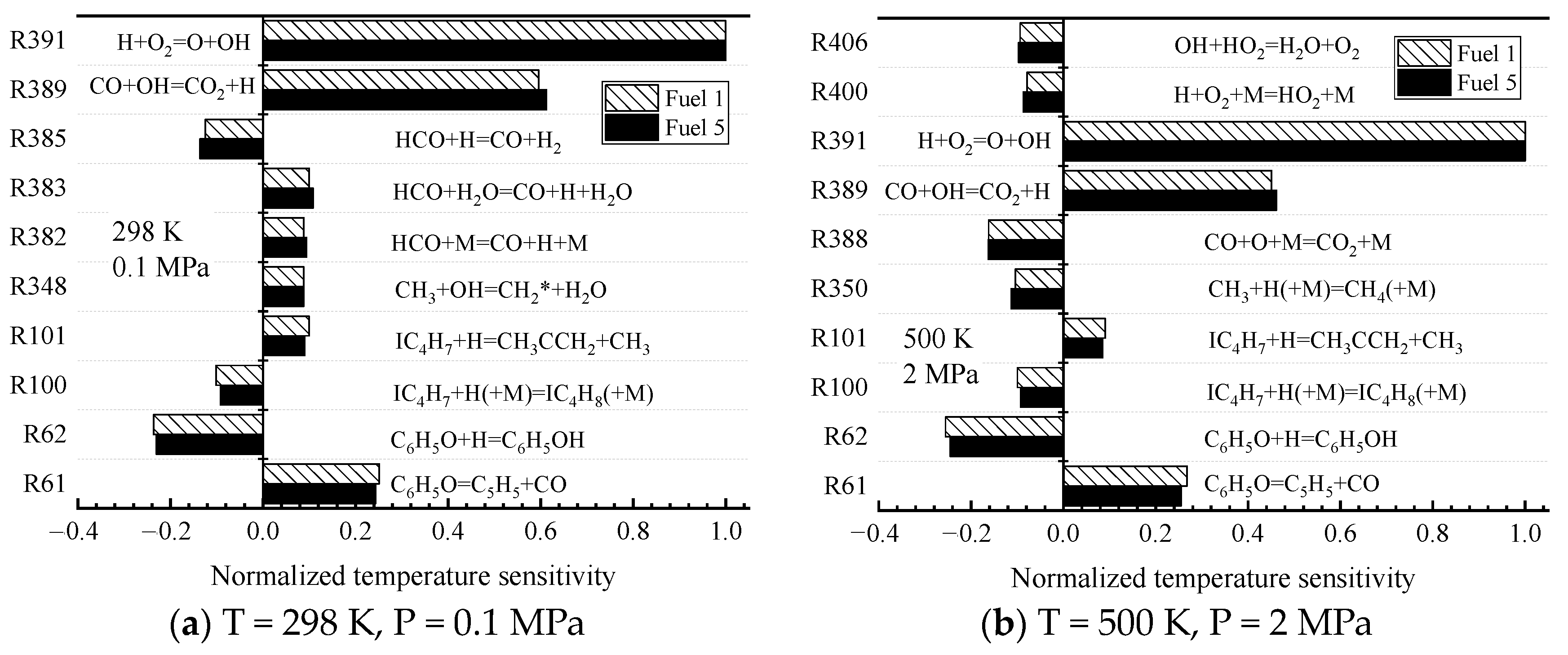
The variability of the surrogate-fuel laminar flame speeds was further analyzed in chemical kinetics. The sensitivity of fuel 1 and fuel 5 was calculated in
Figure 14 at two operating conditions. Ambient temperature and pressure (298 K, 0.1 MPa) and higher temperature and pressure (500 K, 2 MPa), respectively. The results show that fuel 1 and fuel 5 exhibit the same sensitivity at both operating conditions. The reactions with larger sensitivity coefficients are mainly small molecule reactions.
Among them, R62 exhibits the largest negative sensitivity because the reaction drives the consumption of H radicals. The reaction with the largest positive sensitivity, R391, which was introduced in the previous kinetic analysis of ignition characteristics, generates a large number of active OH radicals and O radicals that initiate the next chain reaction, which greatly increases the chemical reaction rate. R389, which first appeared in the sensitivity analysis, is an oxidation reaction of CO The reaction of CO and OH radicals produces CO2 and one H radical, which releases heat and raises the system temperature, and the generated H radicals promote the reaction R391. Therefore, the same sensitivity reaction and sensitivity coefficients of Fuel 1 and Fuel 5 under two working conditions can explain the reason why the laminar flame speed shows the same trend.
Furthermore, all the radical reactions with large sensitivity coefficients in
Figure 14 involve the production and consumption of OH and H radicals, suggesting a close association between OH, H radicals, and the laminar flame speed of the fuel. This phenomenon was also reported in a study by Kelley [
45], and the similarity of OH and H radicals provides support for the fuel similarity observed in the laminar flame speed. In this study, the similarity between OH and H radical concentrations and laminar flame speed were compared to investigate the relationship between laminar flame speed and OH and H radicals for different component fuels.
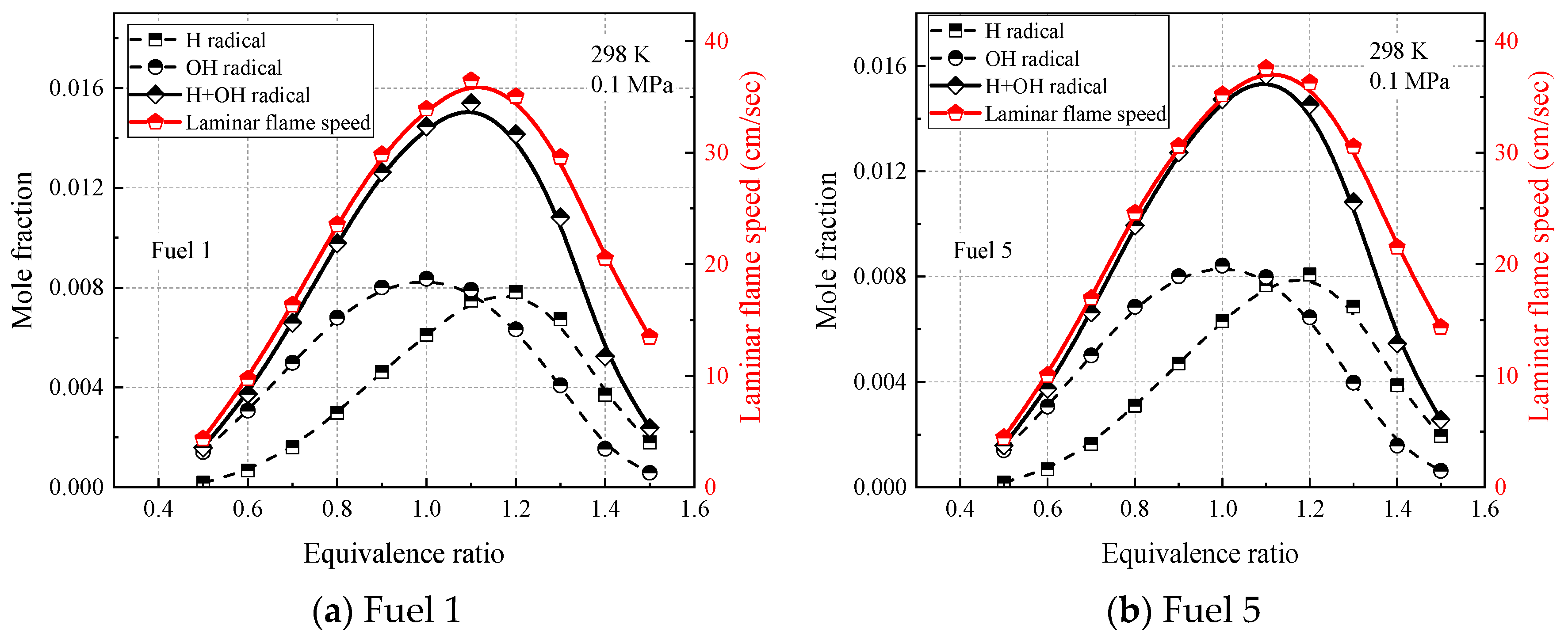
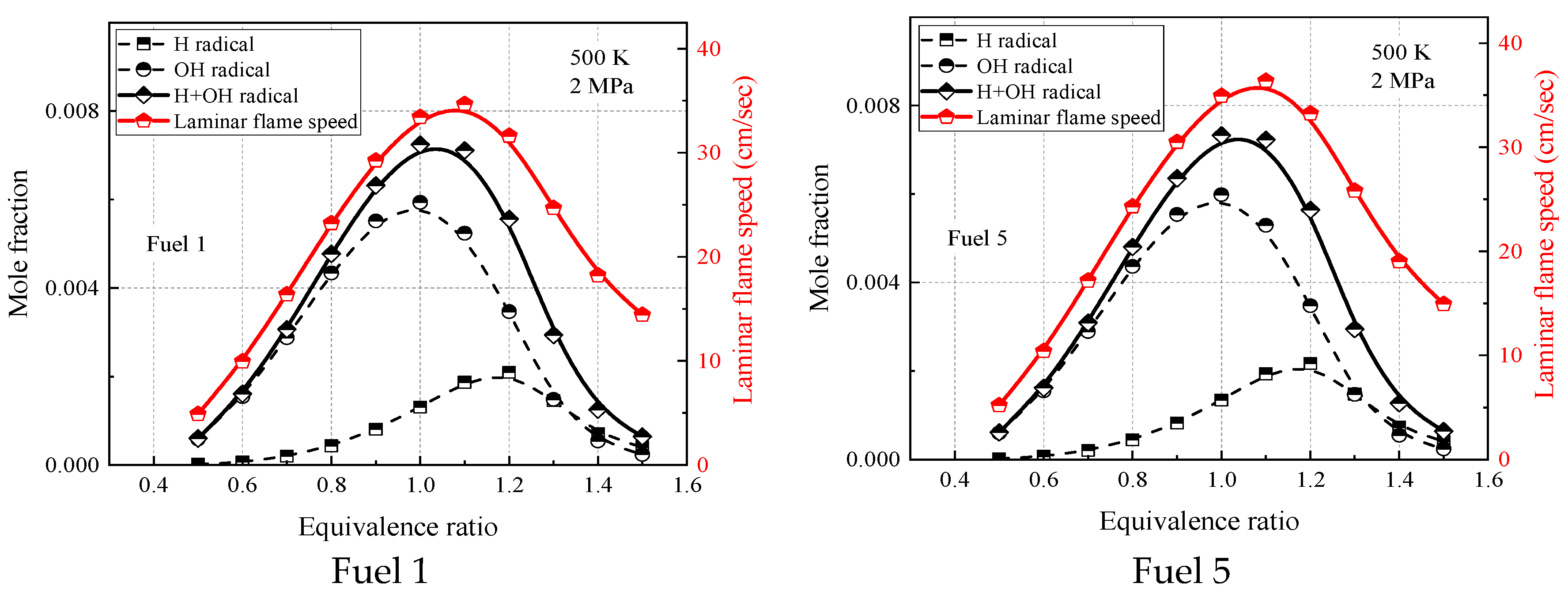
Figure 15 and
Figure 16 show the laminar flame-speed curves and the corresponding OH, H, and OH + H molar fraction curves for fuel 1 and fuel 5 surrogate fuels at two different operating conditions in the range of 0.5–1.5 equivalence ratios, respectively. It is clear that the molar fraction curves of OH radicals or H radicals alone do not show any similarity with the laminar flame speed, while the sum of molar fractions of OH and H radicals and OH + H show consistency with the laminar flame speed in terms of peak equivalence ratio and curve shape. At room temperature and pressure (298 K, 0.1 MPa), the difference between the peak molar fraction of OH radicals and H radicals is not significant, while at higher temperature and pressure (500 K, 2 MPa), the peak molar fraction of OH radicals is more than twice that of H radicals, indicating that the laminar flame speed is more dependent on OH radicals at higher temperature and pressure. The effect of cyclohexane addition on the surrogate-fuel laminar flame speed and for OH radicals and H radical molar fraction was smaller for both operating conditions.
3.3. Effect of Cyclohexane on the Combustion Characteristics of HCCI
In addition to ignition and flame propagation characteristics, fuel combustion in the cylinder is also a major characteristic. In HCCI engines, the mixture reaches the autoignition temperature almost simultaneously in the cylinder while exothermic reactions occur, which can be carried out in a thinner mixture, significantly reducing the generation of NOX and carbon smoke. To investigate the effect of cyclohexane addition on the in-cylinder combustion of surrogate fuels, the phase combustion of four- and five-component surrogate fuels in HCCI engines under lean conditions was investigated. The effect of cyclohexane on fuel autoignition was studied and explained from the perspective of fuel chemistry kinetics. In addition, the effects of engine speed, fuel/air ratio, and intake air temperature are analyzed.
Sjöberg [
46] investigated the intake air temperature required for the combustion timing of gasoline (RON = 90.8, MON = 83.4) in a Cummins B-series engine with 102 mm bore, 120 mm stroke, and 192 mm connecting rod length, with fuel ignition by compression. The HCCI model with a zero-dimensional single-zone homogeneous compression ignition was used for the calculations, starting at −180 °CA. Four-component fuel (fuel 1) and five-component fuel (fuel 5) from
Table 7 were chosen as surrogate fuels. The combustion phase was defined as the moment when the CO molar fraction reached its peak. The three engine speeds were 600 rpm, 1200 rpm, and 1800 rpm, respectively, and the compression ratio increased with increasing speed, due to the consideration of heat loss during the calculation [
27]. It was performed with natural intake and an intake pressure of 0.1 MPa.
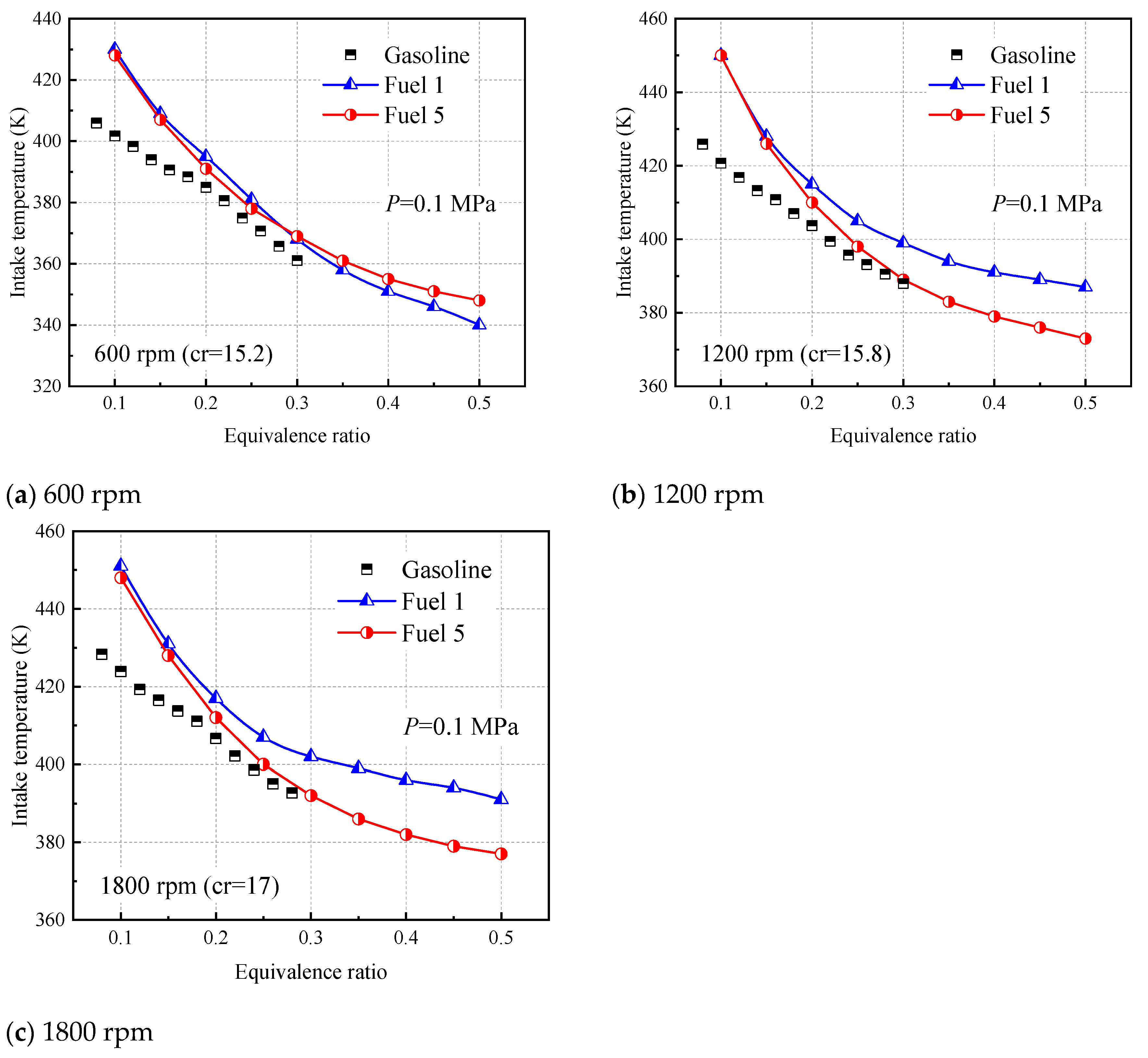
Figure 17 shows the inlet gas temperatures required for fuel 1 and fuel 5 to burn at positive ignition times in HCCI with equivalent ratios ranging from 0.1 to 0.5, with gasoline experimental data from Sjöberg [
46]. The results of the model calculations are recorded in
Table 8.
As can be seen from
Figure 17, the predictions for both fuel 1 and fuel 5 are higher than the experimental values for very lean conditions with equivalence ratios less than 0.2. This indicates that the model selected in this paper is applicable in the region where the lean limit is above the equivalent ratio of 0.2, which is obviously sufficient for the gasoline engine developed so far. In
Figure 17a, there is little difference between the inlet temperatures required for fuel 1 and fuel 5 to reach ignition at the top stop of compression (TDC) at 600 rpm, and fuel 5 predicts higher inlet temperatures than fuel 1 after the equivalence ratio is greater than 0.3. However, at higher speeds of 1200 rpm and 1800 rpm (
Figure 17b,c), fuel 5, a five-component surrogate fuel containing cyclohexane, shows a better predictive trend, while fuel 1 requires a higher intake temperature to achieve positive ignition at higher engine speeds. This can be explained in the sensitivity analysis of the two fuels in
Figure 6. At lower temperatures, the macromolecular reactions of the fuel dominate, while cyclohexane molecules are more likely to shed H atoms in the low-temperature region, and H radicals collide with O
2 molecules to produce a large number of OH radicals, making fuel 5 the first to ignite at the same intake temperature. In other words, fuel 5 requires a lower intake temperature than fuel 1 for ignition timing. In addition, the easier ignition of fuel 1 at low rpm (600 rpm) is attributed to the accelerated conversion of fuel molecules during the low-temperature stall phase (cold-flame phase) of the four-component fuel, which releases heat and leads to system temperature rise, as will be further discussed in the later analysis.
Figure 18 and
Figure 19 show the variation in temperature, CO molar fraction, and conversion of various surrogate-fuel components calculated for fuel 1 and fuel 5 surrogate fuels at two different engine speeds. Considering the maximum observation of the differences exhibited by the two fuels, two equivalent ratios (0.25, 0.5) were chosen for the calculations, and the intake air temperature was chosen to correspond to the intake air temperature at the fuel 5 ignition timing.
In
Figure 18, the temperatures of fuel 1 and fuel 5 increase with an increasing crankshaft rotation angle at an engine speed of 600 rpm, reaching a maximum at the compression upper stop, followed by a slow decrease in temperature as the mixture expands and does work by combustion, and the peak CO molar fraction also occurs near the compression upper stop and then decreases rapidly (
Figure 18a,b). The fuel conversion rate shows that cyclohexane is the fastest, n-heptane is faster than isooctane, and toluene is the smallest (
Figure 18c,d). At smaller equivalent ratios (φ = 0.25) the peak CO molar fraction and the moment of maximum temperature gradient for fuel 1 and fuel 5 are not very different (2 °CA), and the inlet temperature of the fuel 5 ignition timing combustion makes the fuel 1 delayed ignition. The reason for this can be found in
Figure 18c, although fuel 1 has cold-flame combustion in the crankshaft rotation angle range of −22.5~20 °CA, the conversion of n-heptane and isooctane fuel molecules is not driven at this small equivalent ratio, and the rapid conversion of fuel 5 fuel molecules leads to a delayed ignition of fuel 1 relative to fuel 5. Negative conversion of DIB fuel is also observed in
Figure 18c in the −20–8 °CA cranking angle range, which is attributed to the crossover reaction R38. AC
8H
17 + O
2 = JC
8H
16 + HO
2 generated by the isooctane submechanism and the diisobutene submechanism. The conversion of isooctane in the cold-flame stage results in a net production of DIB. The negative conversion of DIB is more pronounced for fuel 5 because the conversion of isooctane in fuel 5 increases rapidly after −15 °CA. When the volume ratio increases to 0.5, a shift in the ignition sequence of fuel 1 and fuel 5 can be observed, and the inlet air temperature of fuel 5 burning at the right time of ignition causes fuel 5 fuel to ignite earlier. The n-heptane and isooctane conversion rates of fuel 1 in
Figure 18d explain this change. After the equivalent ratio increases to 0.5, fuel 1 has a large cold-flame combustion phase, which leads to the conversion of a large amount of n-heptane and isooctane, and the OH and H radicals generated drive the system temperature up and, eventually, fuel 1 fuel ignites early. In addition, it can be clearly found that the negative conversion of DIB is suppressed at an equivalence ratio of 0.5, which is due to the sufficient oxidant to promote the conversion of DIB.
In
Figure 19, the temperature curves and molar fraction curves for fuel 1 and fuel 5 at 1200 rpm engine speed follow similar trends to the 600 rpm engine speed, with the same moments of peak temperature and peak CO molar fraction, and the fuel-conversion rates show that cyclohexane converts the fastest, n-heptane converts faster than isooctane, and toluene converts the least. Comparing the calculated results for the two surrogate fuels at different equivalence ratios, there are significant differences in the fuel ignition timing and conversion curves. The conversion curves of fuel components showed that the conversion rate of each component in fuel 5 fuel was higher than that of fuel 1 until the compression stop, and the addition of cyclohexane reduced the amount of TRF in the surrogate fuel, especially toluene, which had the lowest conversion rate, making fuel 5 to burn at the right time of ignition. No cold flame combustion region was found for fuel 5 in all operating conditions, as also observed in
Figure 18, due to the increased reactivity of the fuel by the addition of cyclohexane. These phenomena indicate that the addition of the cyclohexane component has a great influence on the combustion characteristics of the surrogate fuel in HCCI.
In addition to the effect of cyclohexane, the effects of engine speed, equivalence ratio, and intake temperature were analyzed by comparing
Figure 18 and
Figure 19. For different engine speeds, it is clearly observed that higher intake air temperature is required for the fuel mixture to achieve ignition timing combustion at higher engine speeds. In addition, increasing engine speed inhibited the cold flame behavior of the fuel 1 four-component surrogate fuel because the fuel residence time was reduced at higher speeds and the response in the low-temperature region could not be maintained. For the equivalence ratio, increasing the equivalence ratio is more favorable for the ignition timing combustion of the fuel, which can be compared with
Figure 17. The required intake-gas temperature for the ignition timing of the fuel decreases with increasing the equivalence ratio, and the increase of the oxidizer can promote the oxidation of the fuel in the lean condition. For the effect of inlet gas temperature, it is mainly reflected in the ignition timing of fuel 1 and fuel 5; the higher the inlet gas temperature, the easier the fuel ignites.
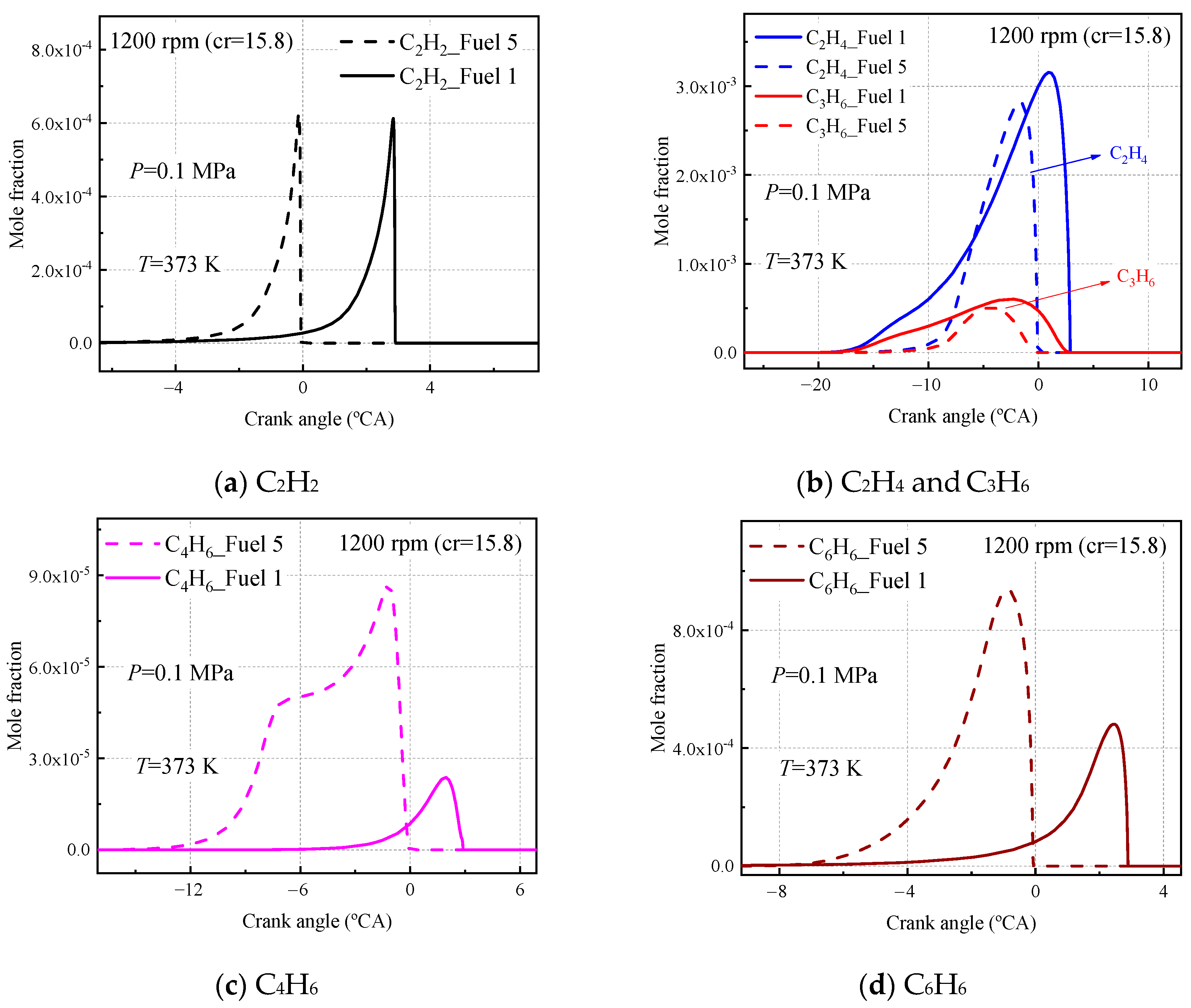
Finally, to investigate the emissions of both fuel 1 and fuel 5 surrogate fuels in the HCCI engine, the molar fraction production of some important carbon-soot precursors was analyzed at an inlet temperature of 373 K, an inlet pressure of 0.1 MPa, an equivalent ratio of 0.5, and an engine speed of 1200 rpm, as shown in
Figure 20. The production of acetylene (C
2H
2), ethylene (C
2H
4), propylene (C
3H
6), 1, 3-butadiene (C
4H
6), and benzene (C
6H
6) were included.
As seen in
Figure 20, the fuel 1 and fuel 5 calculations for ethylene, acetylene, and propylene are the same, and the difference in the corresponding peaks is due to the different moments of ignition for the two fuels at 373 inlet temperatures. In
Figure 20b, the C
2H
4 and C
3H
6 curves calculated for the two fuels differ, with the molar fraction curves for ethylene and propylene calculated by fuel 5 being more concentrated in front of the compression upper stop, while the curves calculated by fuel 1 are more dispersed, due to the cold-flame behavior of fuel 1. The molar fraction curves for C
4H
6 and C
6H
6 calculated by fuel 5 are clearly different from those of fuel 1 (
Figure 20c,d), the 1,3 butadiene molar fraction experienced a rapid increase and then leveled off before growing to a peak, which was not observed in fuel 1. In addition, the peak molar fraction of C
6H
6 calculated in fuel 5 was twice as high as that calculated in fuel 1.
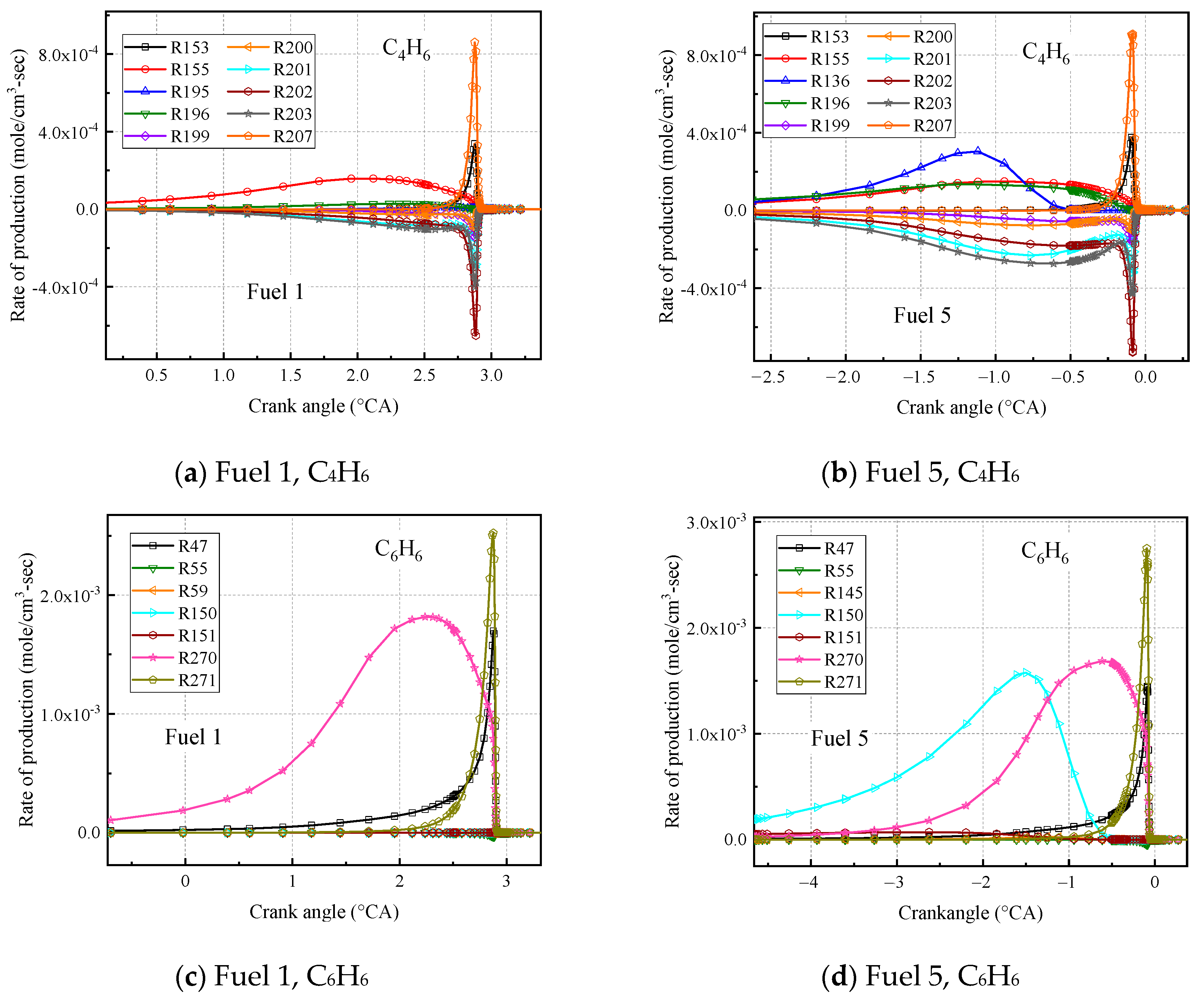
The analysis of the rate of production (ROP) was further analyzed for C
4H
6 and C
6H
6 at this working condition, as shown in
Figure 21.
Figure 21a,b shows the first ten reactions with faster chemical reaction rates for C
4H
6 calculated for the surrogate fuels fuel 1 and fuel 5, where a positive reaction rate indicates fuel production and vice versa for consumption. It can be seen that among the first ten radical reactions calculated for the latter, R136; cC
6H
10 = C
4H
6 + C
2H
4 is the added reaction, which produces a 1, 3 butadiene molecule and an ethylene molecule from the decomposition reaction of cyclohexene C-C bond breakage. In addition, the reaction rate of the radical reaction R196. C
4H
7 + O
2 = C
4H
6 + HO
2 was increased in the fuel 5 calculations, and the R136 and R196 reactions together contributed to the higher molar fraction of C
4H
6 calculated for fuel 5.
Figure 21c,d shows the first ten reactions with faster chemical reaction rates for the surrogate fuels fuel 1 and fuel 5 calculated for C
6H
6. It is observed that the addition of cyclohexane greatly increases the chemical reaction rate for the radical reaction R150. SAXcC
6H
7 = C
6H
6 + H. The removal of an H atom from SAXcC
6H
7 to form C
6H
6 is what leads to the increase in the molar fraction of C
6H
6 in fuel 5.
From the above analysis, it is evident that the addition of cyclohexane has different effects on the surrogate-fuel ignition, flame propagation, and combustion characteristics in the HCCI cylinder. In the low-temperature reaction stage, the ignition-delay time of the fuel decreased gradually as the proportion of cyclohexane in the surrogate fuel increased, which was caused by the early oxidation and decomposition of cyclohexane molecules to produce more OH-active molecules. In the medium-temperature reaction stage, the addition of cyclohexane suppressed the negative temperature behavior of the surrogate fuels and became more pronounced with increasing pressure because the fuels containing cyclohexane were still dominated by macromolecular reactions in the medium-temperature stage and their ignition delay was less dependent on pressure, which affected the NTC behavior of the surrogate fuels in this region. The role of cyclohexane becomes less pronounced as the combustion characteristics in the high-temperature reaction phase are controlled by the small molecule reactions.
The coupling mechanism considers H2/CO/C1 as the core mechanism, which responds to the laminar flame speed of the fuel so that the laminar flame speeds of surrogate fuels with different cyclohexane ratios are similar. The small difference is attributed to the fact that the proportion of n-heptane, which has a larger flame speed, is reduced with the addition of cyclohexane.
HCCI engine tests showed that the five-component surrogate fuel containing cyclohexane required lower intake-gas temperature to achieve ignition timing at higher engine speed, and was closer to the in-cylinder ignition of real gasoline. In addition, the addition of cyclohexane results in higher concentrations of 1, 3-butadiene and benzene at ignition timing than in the four-component fuel.