Spectroscopic Insights of an Emissive Complex between 4′-N,N-Diethylaminoflavonol in Octa-Acid Deep-Cavity Cavitand and Rhodamine 6G
Abstract
1. Introduction
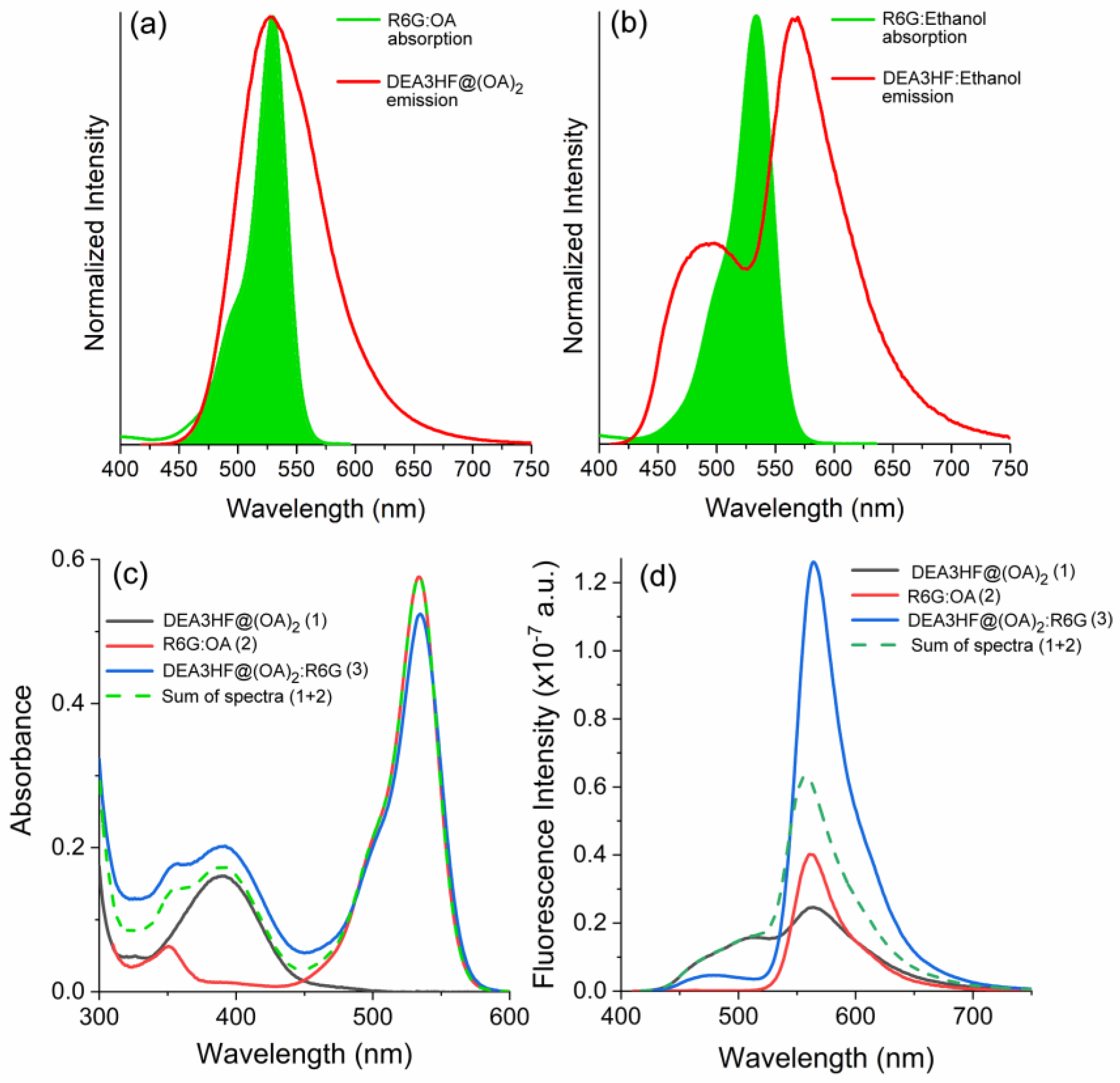
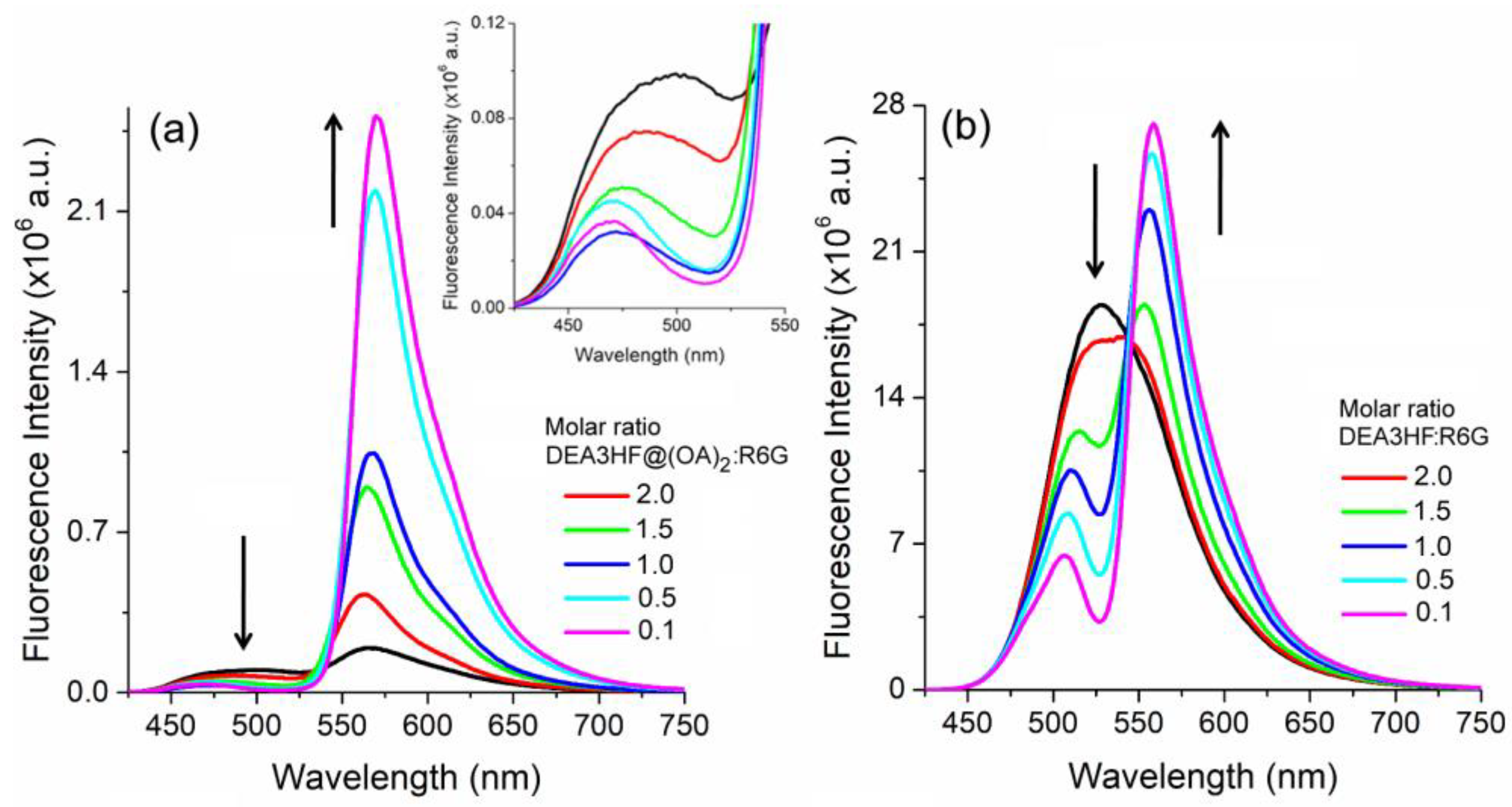
2. Results and Discussion
3. Experimental
3.1. Materials and Methods
3.2. Titration Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Descalzo, A.B.; Martínez-Máñez, R.; Sancenon, F.; Hoffmann, K.; Rurack, K. The supramolecular chemistry of organic–inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 5924–5948. [Google Scholar] [CrossRef] [PubMed]
- Cram, D.J. The design of molecular hosts, guests, and their complexes. Science 1988, 240, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Cram, D.J.; Cram, J.M. Host-guest chemistry: Complexes between organic compounds simulate the substrate selectivity of enzymes. Science 1974, 183, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.B.; Lee, S.L. Supramolecular chemistry: Host–guest molecular complexes. Molecules 2021, 26, 3995. [Google Scholar] [CrossRef]
- Yu, G.; Chen, X. Host–guest chemistry in supramolecular theranostics. Theranostics 2019, 9, 3041–3074. [Google Scholar] [CrossRef]
- Adeli, F.; Abbasi, F.; Babazadeh, M.; Davaran, S. Thermo/pH dual-responsive micelles based on the host–guest interaction between benzimidazole-terminated graft copolymer and β-cyclodextrin-functionalized star block copolymer for smart drug delivery. J. Nanobiotechnol. 2022, 20, 91. [Google Scholar] [CrossRef]
- Sayed, M.; Pal, H. Supramolecularly assisted modulations in chromophoric properties and their possible applications: An overview. J. Mater. Chem. C 2016, 4, 2685–2706. [Google Scholar] [CrossRef]
- Aree, T. How cyclodextrin encapsulation improves molecular stability of apple polyphenols phloretin, phlorizin, and ferulic acid: Atomistic insights through structural chemistry. Food Chem. 2023, 409, 135326. [Google Scholar] [CrossRef]
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; Barrio, J.; Scherman, O.A. Cucurbituril-based molecular recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef]
- Yi, W.Y.; Supian, F.L.; Musa, M.; Karim, N.F.N.A.; Naim, A.F. Calixarenes as host molecules for drug carriers in the cosmetic and medical field. Macromol. Res. 2022, 30, 853–862. [Google Scholar] [CrossRef]
- Xue, M.; Yang, Y.; Chi, X.; Zhang, Z.; Huang, F. Pillararenes, A new class of macrocycles for supramolecular chemistry. Acc. Chem. Res. 2012, 45, 1294–1308. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, V.; Jockusch, S.; Porel, M. Supramolecular photochemistry in solution and on surfaces: Encapsulation and dynamics of guest molecules and communication between encapsulated and free molecules. Langmuir 2015, 31, 5554–5570. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.T.; Bassani, D.M. Energy transfer in supramolecular materials for new applications in photonics and electronics. NPG Asia Mater. 2014, 6, e116. [Google Scholar] [CrossRef]
- Xi, H.; Gibb, C.L.D.; Stevens, E.D.; Gibb, B.C. Deep-cavity cavitands: Synthesis and solid state structure of host molecules possessing large bowl-shaped cavities. Chem. Commun. 1998, 16, 1743–1744. [Google Scholar] [CrossRef]
- Xi, H.; Gibb, C.L.D.; Gibb, B.C. Functionalized deep-cavity cavitands. J. Org. Chem. 1999, 64, 9286–9288. [Google Scholar] [CrossRef]
- Gupta, S.; Adhikari, A.; Mandal, A.K.; Bhattacharyya, K.; Ramamurthy, V. Ultrafast singlet/singlet energy transfer between an acceptor electrostatically attached to the walls of an organic capsule and the enclosed donor. J. Phys. Chem. C 2011, 115, 9593–9600. [Google Scholar] [CrossRef]
- Ishida, Y.; Kulasekharan, R.; Shimada, T.; Takagi, S.; Ramamurthy, V. Efficient singlet-singlet energy transfer in a novel host-guest assembly composed of an organic cavitand, aromatic molecules, and a clay nanosheet. Langmuir 2013, 29, 1748–1753. [Google Scholar] [CrossRef]
- Santos, F.S.; Ramasamy, E.; Ramamurthy, V.; Rodembusch, F.S. Confinement effect on the photophysics of ESIPT fluorophores. J. Mater. Chem. C 2016, 4, 2820–2827. [Google Scholar] [CrossRef]
- Chuang, C.H.; Porel, M.; Choudhury, R.; Burda, C.; Ramamurthy, V. Ultrafast electron transfer across a nanocapsular wall: Coumarins as donors, viologen as acceptor, and octa acid capsule as the mediator. J. Phys. Chem. B 2018, 122, 328–337. [Google Scholar] [CrossRef]
- Santos, F.S.; Ramasamy, E.; Ramamurthy, V.; Rodembusch, F.S. Excited state behaviour of benzoxazole derivatives in a confined environment afforded by a water soluble octaacid capsule. J. Photochem. Photobiol. A Chem. 2016, 317, 175–185. [Google Scholar] [CrossRef]
- Liu, S.; Whisenhunt-Ioup, S.E.; Gibb, C.L.D.; Gibb, B.C. An improved synthesis of ‘octa-acid’ deep-cavity cavitand. Supramol. Chem. 2011, 23, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.; Pal, H. An overview from simple host–guest systems to progressively complex supramolecular assemblies. Phys. Chem. Chem. Phys. 2021, 23, 26085–26107. [Google Scholar] [CrossRef] [PubMed]
- Jagadesan, P.; Mondal, B.; Parthasarathy, A.; Rao, V.J.; Ramamurthy, V. Photochemical reaction containers as energy and electron-transfer agents. Org. Lett. 2013, 15, 1326–1329. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Chattaraj, P.K. Host–guest interactions between octa acid and cations/nucleobases. J. Comput. Chem. 2018, 39, 161–175. [Google Scholar] [CrossRef]
- Porel, M.; Jayaraj, N.; Kaanumalle, L.S.; Maddipatla, M.V.S.N.; Parthasarathy, A.; Ramamurthy, V. cavitand octa acid forms a nonpolar capsuleplex dependent on the molecular size and hydrophobicity of the guest. Langmuir 2009, 25, 3473–3481. [Google Scholar] [CrossRef]
- Choudhury, R.; Ramamurthy, V. Understanding the complexation of aliphatic and aromatic acids guests with octa acid. J. Phys. Org. Chem. 2018, 31, e3795. [Google Scholar] [CrossRef]
- Gibb, C.L.D.; Gibb, B.C. Well-defined, organic nanoenvironments in water: The hydrophobic effect drives a capsular assembly. J. Am. Chem. Soc. 2004, 126, 11408–11409. [Google Scholar] [CrossRef]
- Cendejas, K.; Choudhury, R. Predicting conformations and orientations of guests within a water soluble host: A molecular docking approach. J. Incl. Phenom. Macrocycl. Chem. 2017, 87, 349–356. [Google Scholar] [CrossRef]
- Jayaraj, N.; Zhao, Y.; Parthasarathy, A.; Porel, M.; Liu, R.S.H.; Ramamurthy, V. Nature of supramolecular complexes controlled by the structure of the guest molecules: Formation of octa acid based capsuleplex and cavitandplex. Langmuir 2009, 25, 10575–10586. [Google Scholar] [CrossRef]
- Samanta, S.R.; Baldridge, A.; Tolbert, L.M.; Ramamurthy, V. Guest/host complexes of octa acid and amphiphilic benzylidene-3- methylimidazolidinones exchange hosts within the NMR time scale. ACS Omega 2020, 5, 8230–8241. [Google Scholar] [CrossRef]
- Varadharajan, R.; Kelley, S.A.; Jayasinghe-Arachchige, V.M.; Prabhakar, R.; Ramamurthy, V.; Blackstock, S.C. Organic host encapsulation effects on nitrosobenzene monomer-dimer distribution and C-NO bond rotation in an aqueous solution. ACS Org. Inorg. Au 2022, 2, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.S.; Ramasamy, E.; Ramamurthy, V.; Rodembusch, F.S. Excited state chemistry of flavone derivatives in a confined medium: ESIPT emission in aqueous media. Photochem. Photobiol. Sci. 2014, 13, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shang, C.; Cao, Y.; Cui, J.; Sun, C. A DFT/TD-DFT study on the ESIPT-type flavonoid derivatives with high emission intensity. Materials 2022, 15, 2896. [Google Scholar] [CrossRef]
- Dsouza, R.N.; Pischel, U.; Nau, W.M. Fluorescent dyes and their supramolecular host/guest complexes with macrocycles in aqueous solution. Chem. Rev. 2011, 111, 7941–7980. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, V. Photochemistry within a water-soluble organic capsule. Acc. Chem. Res. 2015, 48, 2904–2917. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Zheng, Z.; Maiti, B.; Chuang, C.H.; Porel, M.; You, Z.Q.; Ramamurthy, V.; Burda, C.; Herbert, J.M.; Dunietz, B.D. What is the optoelectronic effect of the capsule on the guest molecule in aqueous host/guest complexes? A combined computational and spectroscopic perspective. J. Phys. Chem. C 2017, 121, 15481–15488. [Google Scholar] [CrossRef]
- Micheau, J.C.; Zakharova, G.V.; Chibisov, A.K. Reversible aggregation, precipitation and re-dissolution of rhodamine 6G in aqueous sodium dodecyl sulfate. Phys. Chem. Chem. Phys. 2004, 6, 2420–2425. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, P.; Ahuja, S. Forster resonance energy transfer (FRET) and applications thereof. Anal. Methods 2020, 12, 5532–5550. [Google Scholar] [CrossRef]
- Algar, W.R.; Hildebrandt, N.; Vogel, S.S.; Medintz, I.L. FRET as a biomolecular research tool—Understanding its potential while avoiding pitfalls. Nat. Methods 2019, 16, 815–829. [Google Scholar] [CrossRef]
- Wu, L.; Huang, C.; Emery, B.P.; Sedgwick, A.C.; Bull, S.D.; He, X.P.; Tian, H.; Yoon, J.; Sessler, J.L.; James, T.D. Förster resonance energy transfer (FRET)-based small-molecule sensors and imaging agents. Chem. Soc. Rev. 2020, 49, 5110–5139. [Google Scholar] [CrossRef]
- Ramamurthy, V.; Sen, P.; Elles, C.G. Ultrafast excited state dynamics of spatially confined organic molecules. J. Phys. Chem. A 2022, 126, 4681–4699. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Dey, S.; Adhikari, A.; Mandal, U.; Bhattacharyya, K. Ultrafast fluorescence resonance energy transfer in the micelle and the gel phase of a PEO-PPO-PEO triblock copolymer: Excitation wavelength dependence. J. Phys. Chem. B 2007, 111, 7085–7091. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Ghosh, S.; Das, A.K.; Mondal, T.; Bhattacharyya, K. Effect of NaCl on ESPT-mediated FRET in a CTAC micelle: A femtosecond and FCS study. ChemPhysChem 2013, 14, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Chen, F.; Zhang, J.; Anpo, M. Manipulating energy transfer processes between Rhodamine 6G and Rhodamine B in different mesoporous hosts. J. Phys. Chem. C 2007, 111, 5541–5548. [Google Scholar] [CrossRef]
- Dhir, A.; Gogoi, H.; Datta, A. Modulation of FRET efficiency by donor-acceptor ratio in co-condensed fluorophore-silica nanoconjugates. J. Ind. Chem. Soc. 2021, 98, 100067. [Google Scholar] [CrossRef]
- Roth, D.J.; Nasir, M.E.; Ginzburg, P.; Wang, P.; Le Marois, A.; Suhling, K.; Richards, D.; Zayats, A.V. Förster Resonance Energy Transfer inside hyperbolic metamaterials. ACS Photonics 2018, 5, 4594–4603. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, R.; Tang, D.; Hou, X.; Wu, P. Optically-active nanocrystals for inner filter effect-based fluorescence sensing: Achieving better spectral overlap. TrAC-Trend Anal. Chem. 2019, 110, 183–190. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Taniguchi, M.; Du, H.; Lindsey, J.S. PhotochemCAD 3: Diverse modules for photophysical calculations with multiple spectral databases. Photochem. Photobiol. 2018, 94, 277–289. [Google Scholar] [CrossRef]
- Kimball, J.; Chavez, J.; Ceresa, L.; Kitchner, E.; Nurekeyev, Z.; Doan, H.; Szabelski, M.; Borejdo, J.; Gryczynski, I.; Gryczynski, Z. On the origin and correction for inner filter effects in fluorescence part I: Primary inner filter effect-the proper approach for sample absorbance correction. Methods Appl. Fluoresc. 2020, 8, 033002. [Google Scholar] [CrossRef]
- Weitner, T.; Friganović, T.; Šakić, D. Inner filter effect correction for fluorescence measurements in microplates using variable vertical axis focus. Anal. Chem. 2022, 94, 7107–7114. [Google Scholar] [CrossRef]
- Raza, S.A.; Naqvi, S.Q.; Usman, A.; Jennings, J.R.; Soon, Y.W. Spectroscopic study of the interaction between rhodamine B and graphene. J. Photochem. Photobiol. A Chem. 2021, 418, 113417. [Google Scholar] [CrossRef]
- Magde, D.; Rojas, G.E.; Seybold, P.G. Solvent dependence of the fluorescence lifetimes of xanthene dyes. Photochem. Photobiol. 1999, 75, 737–744. [Google Scholar] [CrossRef]
- Magde, D.; Wong, R.; Seybold, P.G. Fluorescence quantum yields and their relation to lifetimes of Rhodamine 6g and fluorescein in nine solvents: Improved absolute standards for quantum yields. Photochem. Photobiol. 2002, 75, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Gehlen, M. The centenary of the Stern-Volmer equation of fluorescence quenching: From the single line plot to the SV quenching map. J. Photochem. Photobiol. C Photochem. Rev. 2020, 42, 100338. [Google Scholar] [CrossRef]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- HORIBA Scientific. Fluorescence Spectroscopy—Applications and Principles. AZoM. Available online: https://www.azom.com/article.aspx?ArticleID=16086 (accessed on 4 May 2023).


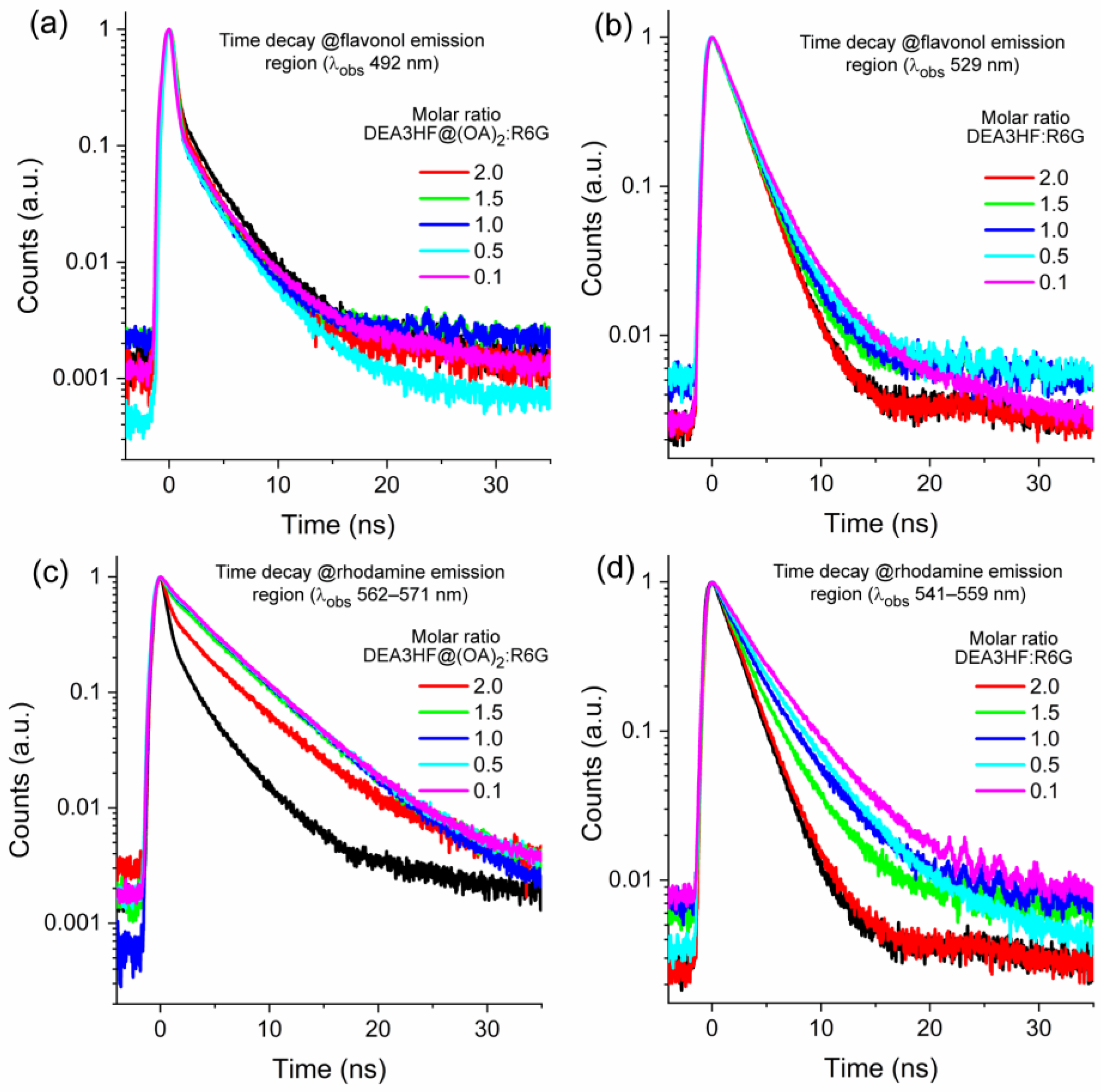

| Molar Ratio DEA3HF@(OA)2:R6G | λobs (nm) | τ1 (ns) | Rel. | τ2 (ns) | Rel. | χ2 | τav (ns) | τ0/τ b |
|---|---|---|---|---|---|---|---|---|
| 0 a | 492 | 1.179 | 16.94 | 3.320 | 83.06 | 1.075 | 2.96 | 1.00 |
| 2.0 | 492 | 1.327 | 19.20 | 3.254 | 80.80 | 1.080 | 2.88 | 1.03 |
| 1.5 | 492 | 1.292 | 19.07 | 3.122 | 80.93 | 1.029 | 2.77 | 1.07 |
| 1.0 | 492 | 1.443 | 22.80 | 3.367 | 77.20 | 1.032 | 2.93 | 1.01 |
| 0.5 | 492 | 1.725 | 29.71 | 3.808 | 70.29 | 1.015 | 3.19 | 0.93 |
| 0.1 | 492 | 1.570 | 24.37 | 3.768 | 75.63 | 1.052 | 3.23 | 0.92 |
| Molar Ratio DEA3HF@(OA)2:R6G | λobs (nm) | τ1 (ns) | Rel. | τ2 (ns) | Rel. | χ2 | τav (ns) | τ0/τ b |
|---|---|---|---|---|---|---|---|---|
| 0 a | 566 | 1.21 | 21 | 3.69 | 79 | 1.04 | 3.17 | 1.00 |
| 2.0 | 562 | 1.76 | 11 | 5.15 | 89 | 1.07 | 4.79 | 1.51 |
| 1.5 | 564 | 2.27 | 6 | 5.18 | 94 | 1.08 | 5.00 | 1.58 |
| 1.0 | 567 | 3.31 | 24 | 5.54 | 76 | 1.09 | 5.01 | 1.58 |
| 0.5 | 569 | 3.19 | 17 | 5.37 | 83 | 1.04 | 4.99 | 1.58 |
| 0.1 | 571 | 2.98 | 16 | 5.32 | 84 | 1.05 | 4.96 | 1.56 |
| Molar Ratio DEA3HF:R6G | λobs (nm) | τ1 (ns) | Rel. | τ2 (ns) | Rel. | χ2 | τav (ns) | τ0/τ b |
|---|---|---|---|---|---|---|---|---|
| 0 a | 529 | 2.087 | 100 | - | - | 1.101 | 2.09 | 1.00 |
| 2.0 | 526 | 2.030 | 100 | - | - | 1.105 | 2.03 | 1.03 |
| 1.5 | 529 | 2.071 | 100 | - | - | 1.101 | 2.07 | 1.01 |
| 1.0 | 529 | 2.195 | 100 | - | - | 1.118 | 2.20 | 0.95 |
| 0.5 | 529 | 2.097 | 85.15 | 5.645 | 14.85 | 1.033 | 2.62 | 0.80 |
| 0.1 | 529 | 2.131 | 76.92 | 5.789 | 23.08 | 1.083 | 2.98 | 0.70 |
| Molar Ratio DEA3HF:R6G | λobs (nm) | τ1 (ns) | Rel. | τ2 (ns) | Rel. | χ2 | τav (ns) | τ0/τ b |
|---|---|---|---|---|---|---|---|---|
| 0 a | 557 | 2.07 | 100 | - | - | 1.08 | 2.07 | 1.00 |
| 2.0 | 541 | 2.14 | 100 | - | - | 1.09 | 2.14 | 1.03 |
| 1.5 | 553 | 2.19 | 67 | 4.52 | 33 | 0.96 | 2.97 | 1.43 |
| 1.0 | 556 | 2.23 | 44 | 4.39 | 56 | 1.08 | 3.46 | 1.67 |
| 0.5 | 557 | 2.34 | 35 | 4.54 | 65 | 1.07 | 3.77 | 1.82 |
| 0.1 | 559 | 2.57 | 32 | 4.98 | 68 | 1.03 | 4.22 | 2.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, F.d.S.; Ramasamy, E.; da Luz, L.C.; Ramamurthy, V.; Rodembusch, F.S. Spectroscopic Insights of an Emissive Complex between 4′-N,N-Diethylaminoflavonol in Octa-Acid Deep-Cavity Cavitand and Rhodamine 6G. Molecules 2023, 28, 4260. https://doi.org/10.3390/molecules28114260
Santos FdS, Ramasamy E, da Luz LC, Ramamurthy V, Rodembusch FS. Spectroscopic Insights of an Emissive Complex between 4′-N,N-Diethylaminoflavonol in Octa-Acid Deep-Cavity Cavitand and Rhodamine 6G. Molecules. 2023; 28(11):4260. https://doi.org/10.3390/molecules28114260
Chicago/Turabian StyleSantos, Fabiano da Silveira, Elamparuthi Ramasamy, Lilian Camargo da Luz, Vaidhyanathan Ramamurthy, and Fabiano Severo Rodembusch. 2023. "Spectroscopic Insights of an Emissive Complex between 4′-N,N-Diethylaminoflavonol in Octa-Acid Deep-Cavity Cavitand and Rhodamine 6G" Molecules 28, no. 11: 4260. https://doi.org/10.3390/molecules28114260
APA StyleSantos, F. d. S., Ramasamy, E., da Luz, L. C., Ramamurthy, V., & Rodembusch, F. S. (2023). Spectroscopic Insights of an Emissive Complex between 4′-N,N-Diethylaminoflavonol in Octa-Acid Deep-Cavity Cavitand and Rhodamine 6G. Molecules, 28(11), 4260. https://doi.org/10.3390/molecules28114260








