The First Phytochemical Investigation of Artemisia divaricate: Sesquiterpenes and Their Anti-Inflammatory Activity
Abstract
1. Introduction
2. Results and Discussion
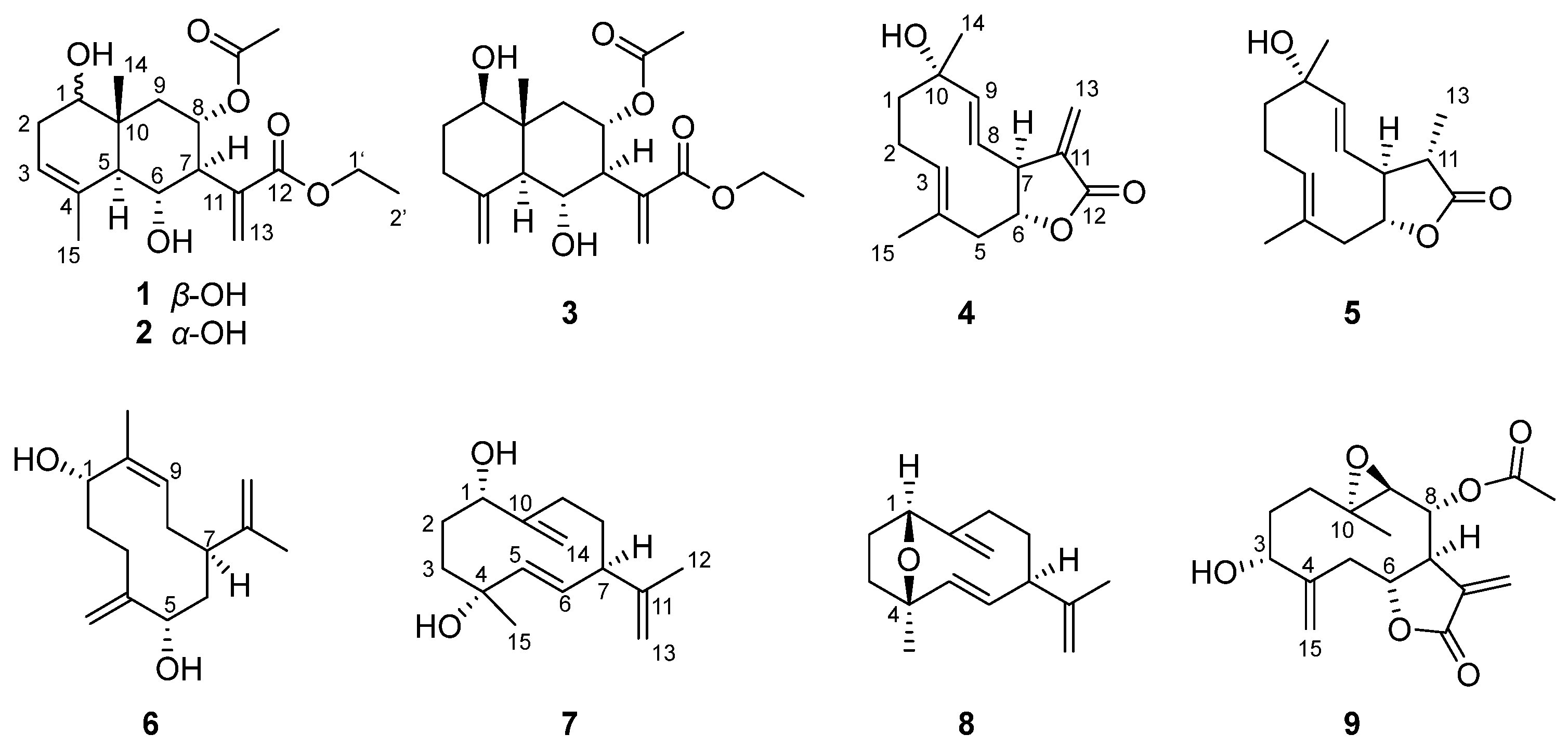
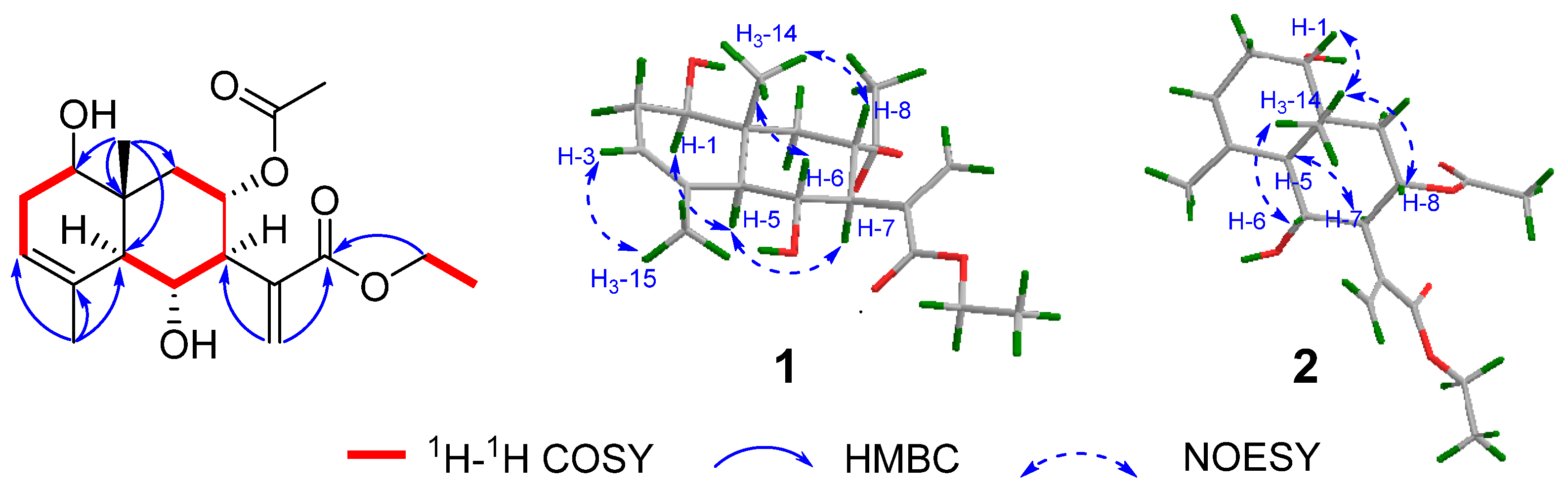
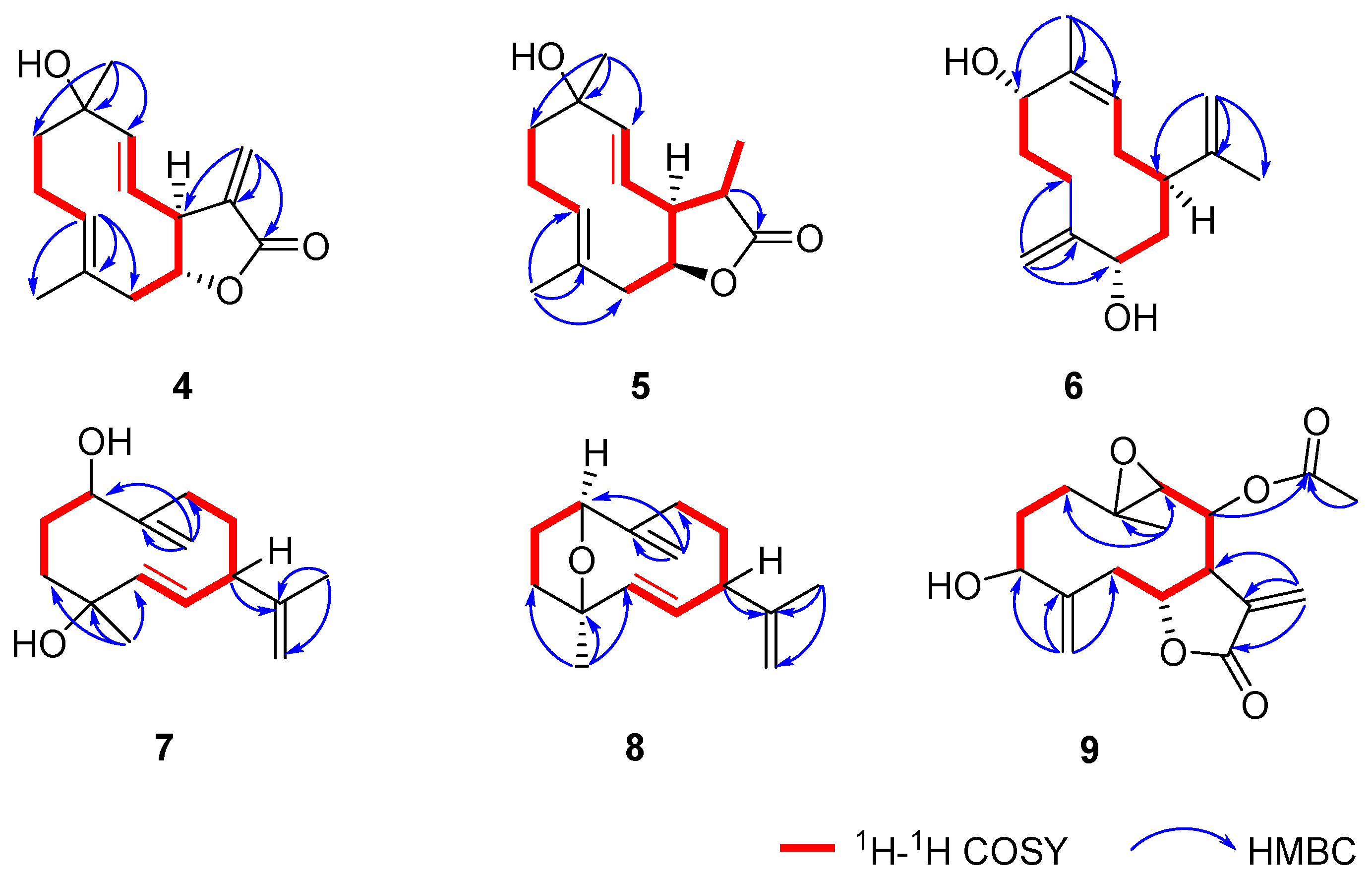
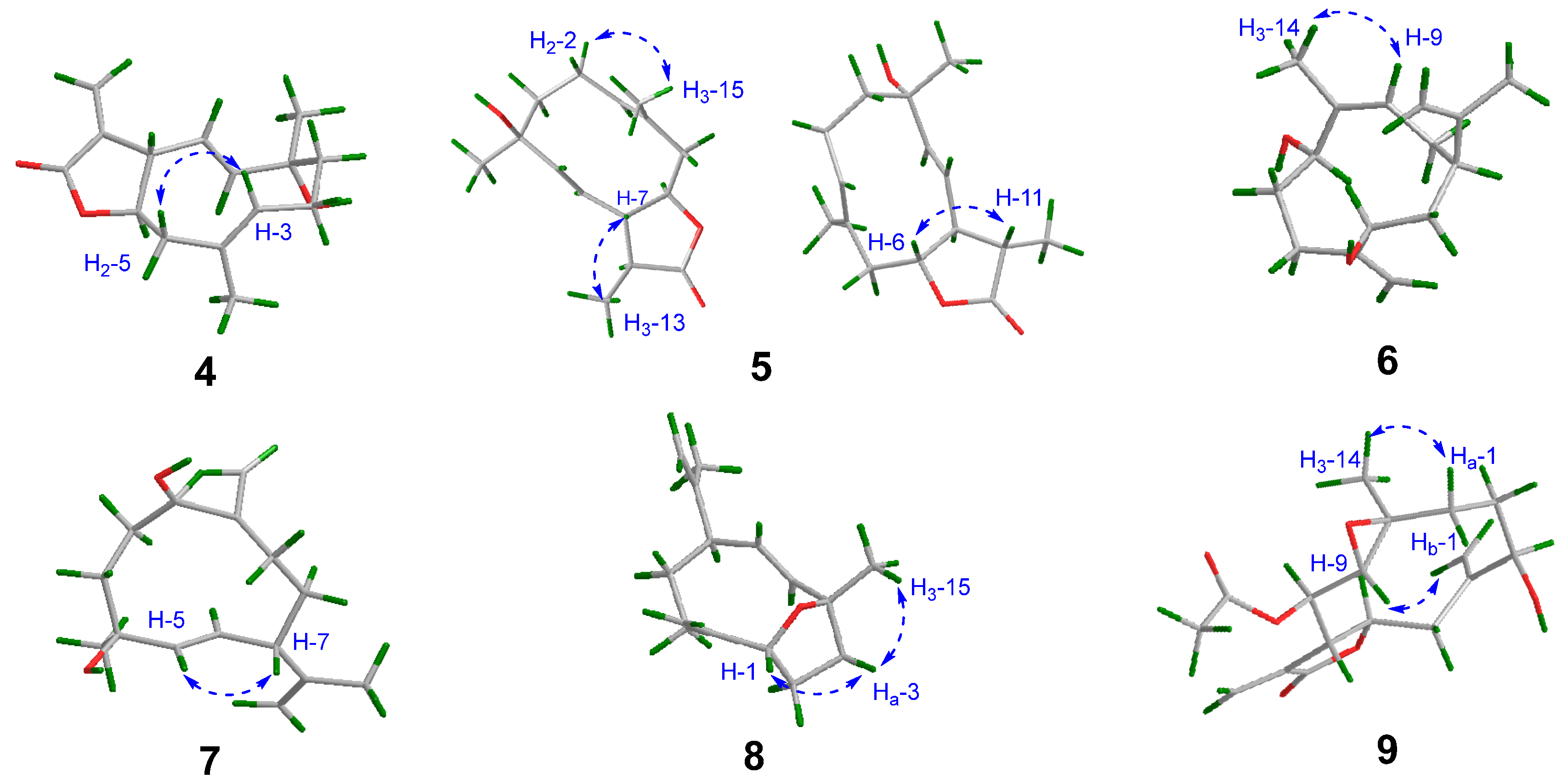
2.1. Structural Elucidation
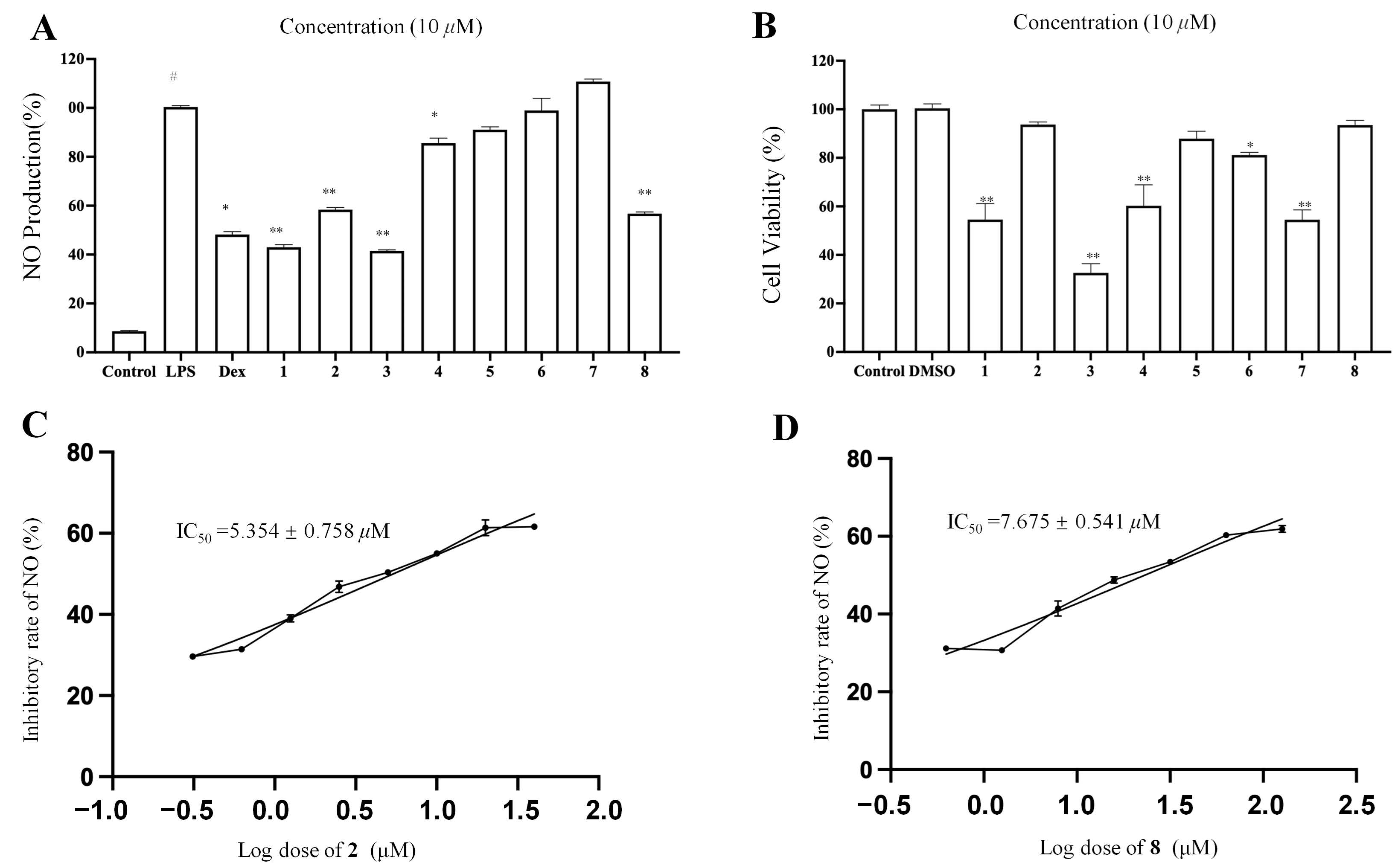
2.2. Anti-Inflammatory Activity Assay
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Computational Section
3.5. Cell Culture
3.6. Cell Viability
3.7. Measurement of Nitric Oxide (NO) Production
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene Lactones from Artemisia Genus: Biological Activities and Methods of Analysis. J. Anal. Methods Chem. 2015, 2015, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.J.A.; Olmo, L.; Ticona, L.A.; Benito, P.B. The Artemisia L. Genus: A Review of Bioactive Sesquiterpene Lactones. Stud. Nat. Prod. Chem. 2012, 37, 43–65. [Google Scholar]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.A.; Biavatti, M.W.; Brun, R.; Da, C.F.B.; De Castro, S.L.; Ferreira, V.F.; De Lacerda, M.V.G.; et al. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases—Part I. Curr. Med. Chem. 2012, 19, 2128–2175. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Chen, X.Q.; Zheng, M.B.; Jian, Y.; Tang, P.F. Cytotoxic sesquiterpene lactones from Artemisia myriantha. Phytochem. Lett. 2020, 37, 33–36. [Google Scholar]
- Shu, W.; Jian, S.; Ke, Z.; Chen, X.Q.; Zheng, M.B.; Jian, Y.; Tang, P.F. Sesquiter-penes from Artemisia argyi: Absolute Configurations and Biological Activities. Eur. J. Org. Chem. 2014, 5, 973–983. [Google Scholar]
- Reinhardt, J.K.; Klemd, A.M.; Danton, O.; De Mieri, M.; Smieško, M.; Huber, R.; Bürgi, T.; Gründemann, C.; Hamburger, M. Sesquiterpene Lactones from Artemisia argyi: Absolute Configuration and Immunosuppressant Activity. J. Nat. Prod. 2019, 82, 1424–1433. [Google Scholar] [CrossRef]
- Bai, M.; Chen, J.; Xu, W.; Dong, S.; Liu, Q.; Lin, B.; Huang, X.; Yao, G.; Song, S. Elephantopinolide A-P, germacrane-type sesquiter-pene lactones from Elephantopus scaber induce apoptosis, autophagy and G2/M phase arrest in hepatocellular carcinoma cells. Eur. J. Med. Chem. 2020, 198, 112362–112375. [Google Scholar] [CrossRef]
- Xu, W.; Bai, M.; Liu, D.; Qin, S.; Lv, T.; Li, Q.; Lin, B.; Song, S.; Huang, X. MS/MS-based molecular networking accelerated discovery of germacrane-type sesquiterpene lactones from Elephantopus scaber L. Phytochemistry 2022, 198, 113136–113146. [Google Scholar] [CrossRef]
- Perveen, S.; Alqahtani, J.; Orfali, R.; Aati, H.Y.; Al-Taweel, A.M.; Ibrahim, T.A.; Khan, A.; Yusufoglu, H.S.; Abdel-Kader, M.S.; Taglialatela-Scafati, O. Antibacterial and Antifungal Sesquiterpenoids from Aerial Parts of Anvillea garcinii. Molecules 2020, 25, 1730. [Google Scholar] [CrossRef]
- Zhu, N.; Tang, C.; Xu, C.; Ke, C.; Lin, G.; Jenis, J.; Yao, S.; Liu, H.; Ye, Y. Cytotoxic Germacrane-Type Sesquiterpene Lactones from the Whole Plant of Carpesium lipskyi. J. Nat. Prod. 2019, 82, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Long, Q.; Zhang, Y.; Babu, G.; Krishnapriya, M.V.; Qiu, J.; Song, J.; Rao, Q.; Yi, P.; Sun, M.; et al. Germacranolide sesquiterpenes from Carpesium cernuum and their anti-leukemia activity. Chin. J. Nat. Med. 2021, 19, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Raven, P.H.; Hong, D.Y. Flora of China; Science Press (Beijing) & Missouri Botanical Garden Press: Beijing, China, 2011. [Google Scholar]
- Gaussian 09; Computer Program. Gaussian, Inc.: Wallingford, CT, USA, 2013.
- CONFLEX 8; Computer Program. CONFLEX Corporation: Tokyo, Japan, 2017.
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An Improved Probability for the Stereochemical Assignment of Isomeric Compounds using Quantum Chemical Calculations of NMR Shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Li, B.; Dai, W.; Liu, L.; Zhang, M. Highly oxidized sesquiterpenes from Artemisia austroyunnanensis. Fitoterapia 2016, 115, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Lee, S.; Kim, K.H.; Oh, K.; Shin, J.; Mar, W. Effects of magnolialide isolated from the leaves of Laurus nobilis L. (Laura-ceae) on immunoglobulin E-mediated type I hypersensitivity in vitro. J. Ethnopharmacol. 2013, 149, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, J.; Li, Z.; Li, Y.; Qiu, M. New eudesmenoic acid methyl esters from the seed oil of Jatropha curcas. Fitoterapia 2013, 89, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Galal, A.M. Microbial Transformation of Pyrethrosin. J. Nat. Prod. 2001, 64, 1098–1099. [Google Scholar] [CrossRef]
- Zhen, X.; Wang, X.; Wang, C.P.; Li, G.H. Chemical constituents and biological activities of Pyrethrum cinerariifolium. Guihaia 2016, 36, 747–751. [Google Scholar]
- Xue, G.M.; Zhao, C.G.; Xue, J.F.; Xing, G.F.; Zhao, Z.Z.; Du, K.; Si, Y.Y.; Sun, Y.J.; Feng, W.S. Chemical constituents from seeds of Artemisia argyi. Chin. Tradit. Herbal Drugs 2022, 53, 2605–2611. [Google Scholar]
- Zhu, Y.; Zhang, L.; Zhao, Y.; Huang, G. Unusual sesquiterpene lactones with a new carbon skeleton and new acetylenes from Ajania przewalskii. Food Chem. 2010, 118, 228–238. [Google Scholar] [CrossRef]
- Yang, H.; Xie, J.L.; Sun, H.D. Study on chemical constituents from the roots of Saussurea lappa. Chin. J. Chin. Mater. Med. 1997, 12, 94–98. [Google Scholar]
- Konstantinopoulou, M.; Karioti, A.; Skaltsas, S.; Skaltsa, H. Sesquiterpene Lactones from Anthemisa ltissima and Their Anti-Helicobacter pylori Activity. J. Nat. Prod. 2003, 66, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Bethencourt-Estrella, C.J.; Nocchi, N.; López-Arencibia, A.; San Nicolás-Hernández, D.; Souto, M.L.; Suárez-Gómez, B.; Díaz-Marrero, A.R.; Fernández, J.J.; Lorenzo-Morales, J.; Piñero, J.E. Antikinetoplastid Activity of Sesquiterpenes Isolated from the Zoanthid Palythoa aff. clavata. Pharmaceuticals 2021, 14, 1095. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.D.; Yang, M.S.; Ha, T.J.; Park, K.M.; Park, K.H. Isolation and Identification of Dihydrochrysanolide and Its 1-Epimer from Chrysanthemum coro-narium L. Biosci. Biotechnol. Biochem. 2002, 4, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Izbosarov, M.B.; Abduazimov, B.K.; Yusupova, I.M.; Tashkhodzhaev, B.; Abdullaev, A.D.V.D. Germacranolide from Tanacetopis mucronata. Chem. Nat. Commun. 1998, 34, 456–461. [Google Scholar] [CrossRef]
- Fraga, B.M.; Terrero, D.; Cabrera, I.; Reina, M. Studies on the sesquiterpene lactones from Laurus novocanariensis lead to the clarification of the structures of 1-epi-tatridin B and its epimer tatridin B. Phytochemistry 2018, 153, 48–52. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Q.; Cen, Y.; Huang, J.; Zhang, X.; Guo, S.; Zhang, L.; Guo, T.; Ho, C.T.; Bai, N. A new sesquiterpene lactone glucoside and other constituents from Inula salsoloides with insecticidal activities on striped flea beetle (Phyllotreta striolata Fabricius). Nat. Prod. Res. 2018, 32, 552–557. [Google Scholar] [CrossRef]
- Ito, M.H.A.K. Regio- and Stereo-specific Allylic Oxidation of Germacrane-type Sesquiterpene Lactones with Selenium. Diox-ide and t-Butyl Hydroperoxide. J. Chem. Soc. Chemi. Commun. 1981, 10, 483–485. [Google Scholar]
- Cimmino, A.; Roscetto, E.; Masi, M.; Tuzi, A.; Radjai, I.; Gahdab, C.; Paolillo, R.; Guarino, A.; Catania, M.R.; Evidente, A. Sesquiterpene Lactones from Cotula cinerea with Antibiotic Activity against Clinical Isolates of Enterococcus faecalis. Antibiotics 2021, 10, 819. [Google Scholar] [CrossRef]
- Triana, J.; Eiroa, J.L.; Morales, M.; Perez, F.J.; Brouard, I.; Marrero, M.T.; Estevez, S.; Quintana, J.; Estevez, F.; Castillo, Q.A.; et al. A chemo-taxonomic study of endemic species of genus Tanacetum from the Canary Islands. Phytochemistry 2013, 92, 87–104. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, X.; Wang, Y.; Huang, M.; Duan, J.A.; Godecke, T.; Szymulanska-Ramamurthy, K.M.; Yin, Z.; Che, C.T. Germacranes and m-menthane from Illicium lanceolatum. Molecules 2014, 19, 4326–4337. [Google Scholar] [CrossRef] [PubMed]
- Triana, J.; Lopez, M.; Rico, M.; Gonzalez-Platas, J.; Quintana, J.; Estevez, F.; Leon, F.; Bermejo, J. Sesquiterpenoid derivatives from Gonospermum elegans and their cytotoxic activity for HL-60 human promyelocytic cells. J. Nat. Prod. 2003, 66, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.F.; García-Sarrión, A.; Marco, J.A. Germacrane derivatives from Santolina chamaecyparissus. Phytochemistry 1991, 30, 9–10. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; He, F.; Fu, W.; Tang, B.; Bin, Y.; Fang, M.; Wu, Z.; Qiu, Y. Anti-inflammatory sesquiterpenoids from the heartwood of Juniperus formosana Hayata. Fitoterapia 2022, 157, 105105–105123. [Google Scholar] [CrossRef]
- D’Abrosca, B.; De Maria, P.; DellaGreca, M.; Fiorentino, A.; Golino, A.; Izzo, A.; Monaco, P. Amarantholidols and amarantholidosides: New nerolidol derivatives from the weed Amaranthus retroflexus. Tetrahedron 2006, 62, 640–646. [Google Scholar] [CrossRef]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.R.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 130, 2619–2628. [Google Scholar] [CrossRef]
- Li, D.; Zhang, T.; Lu, J.; Peng, C.; Lin, L. Natural constituents from food sources as therapeutic agents for obesity and metabolic diseases targeting adipose tissue inflammation. Crit. Rev. Food Sci. 2020, 61, 1947–1965. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, L.; Zheng, Y.; Liu, Q.; Liu, J.; Feng, L.; Huang, L.; Zhang, Q.; Lu, J.; Lin, L. Norditerpenoids and Dinorditerpenoids from the Seeds of Podocarpus nagi as Cytotoxic Agents and Autophagy Inducers. J. Nat. Prod. 2017, 80, 2110–2117. [Google Scholar] [CrossRef]
- Feng, Z.; Chen, J.; Feng, L.; Chen, C.; Ye, Y.; Lin, L. Polyisoprenylated benzophenone derivatives from Garcinia cambogia and their anti-inflammatory activities. Food Funct. 2021, 12, 6432–6441. [Google Scholar] [CrossRef]

| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 75.6 | 73.3 | 78.5 | 41.2 | 40.9 | 67.7 | 76.2 | 76.2 | 37.3 |
| 2 | 32.8 | 32.3 | 31.8 | 24.8 | 24.8 | 29.8 | 27.8 | 27.8 | 23.7 |
| 3 | 122.1 | 120.1 | 34.9 | 123.4 | 132.3 | 22.7 | 38.0 | 38.0 | 77.7 |
| 4 | 134.5 | 134.7 | 144.6 | 127.2 | 127.3 | 150.0 | 74.0 | 73.7 | 144.5 |
| 5 | 52.0 | 45.3 | 54.8 | 45.4 | 45.3 | 74.6 | 138.0 | 138.0 | 41.9 |
| 6 | 71.3 | 71.5 | 68.5 | 77.4 | 76.7 | 31.2 | 130.9 | 130.9 | 81.8 |
| 7 | 57.2 | 57.6 | 54.8 | 55.8 | 58.8 | 42.2 | 52.0 | 52.0 | 49.7 |
| 8 | 70.0 | 70.3 | 69.6 | 132.5 | 125.0 | 28.7 | 31.6 | 31.6 | 73.9 |
| 9 | 40.2 | 39.3 | 41.9 | 147.1 | 145.1 | 128.4 | 30.5 | 30.5 | 77.1 |
| 10 | 38.9 | 38.7 | 41.1 | 73.0 | 72.9 | 136.0 | 150.3 | 151.4 | 69.8 |
| 11 | 138.6 | 138.6 | 138.5 | 139.6 | 42.2 | 147.1 | 148.0 | 148.0 | 138.7 |
| 12 | 167.0 | 166.9 | 166.9 | 170.6 | 178.1 | 23.5 | 21.5 | 21.5 | 168.8 |
| 13 | 128.9 | 128.8 | 128.2 | 121.1 | 12.6 | 110.5 | 109.3 | 109.3 | 120.6 |
| 14 | 24.2 | 24.8 | 12.4 | 23.3 | 23.3 | 16.5 | 110.6 | 110.5 | 20.6 |
| 15 | 11.5 | 14.3 | 109.1 | 17.8 | 17.8 | 113.0 | 29.3 | 29.3 | 118.2 |
| OAc | 170.3 21.2 | 170.5 21.2 | 170.3 21.2 | 173.0 20.6 | |||||
| 1′ | 61.2 | 61.3 | 61.1 | ||||||
| 2′ | 14.3 | 17.6 | 14.3 |
| No | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 3.61 (dd, 10.1, 7.1) | 3.40–3.36 (m) | 3.47 (dd, 11.6, 4.7) | 1.72 (2H, m) | 1.74–1.68 (2H, m) |
| 2 | 2.36–2.45 (m) | 2.46 (overlapped) | 1.88 (m) | 2.27 (m) | 2.25 (m) |
| 1.90 (overlapped) | 2.02 (ddd, 10.5, 4.5, 2.2) | 1.58 (overlapped) | 2.17 (m) | 2.17–2.11 (m) | |
| 3 | 5.34 (dd, 4.2, 2.3) | 5.30 (dd, 4.5, 2.2) | 2.36 (ddd, 13.6, 6.4, 3.7) 2.09 (ddd, 14.9, 13.6, 5.3) | 5.18 (dd, 8.2, 8.1) | 5.10 (dd, 11.1, 2.1) |
| 5 | 1.97 (overlapped) | 2.26 (d, 10.9) | 1.83 (d, 10.4) | 2.77 (dd, 12.2, 2.9) 2.61 (dd, 12.2, 12.2) | 2.73 (dd, 12.1, 2.9) 2.49 (dd, 12.1, 9.9) |
| 6 | 4.03 (dd, 11.4, 10.4) | 4.07 (dd, 10.9, 10.5) | 4.23 (dd, 10.4, 10.4) | 3.97 (ddd, 12.2, 9.8, 2.9) | 3.99 (ddd, 12.1, 9.9, 2.9) |
| 7 | 2.50 (dd, 11.7, 10.4) | 2.50 (dd, 11.4, 10.5) | 2.62 (dd, 11.3, 10.4) | 3.57 (dd, 9.8, 8.8) | 2.62 (dd, 12.1, 9.5) |
| 8 | 5.41 (ddd, 11.7, 11.1, 4.8) | 5.43 (ddd, 11.8, 11.4, 5.2) | 5.33 (ddd, 11.3, 11.2, 4.6) | 5.15 (dd, 16.1, 8.8) | 5.16 (dd, 16.0, 9.5) |
| 9 | 2.32 (dd, 12.2, 4.8) 1.19 (dd, 12.2, 11.1) | 1.88 (dd, 12.2, 11.8) 1.65 (dd, 12.2, 5.2) | 2.34–2.31 (m) 1.29 (dd, 11.2, 4.9) | 5.71 (d, 16.1) | 5.60 (d, 16.0) |
| 11 | 2.43 (dq, 12.1, 6.9) | ||||
| 12 | |||||
| 13 | 6.35 (s) 5.73 (s) | 6.34 (d, 1.2) 5.72 (d, 1.2) | 6.33 (d, 1.1) 5.71 (d, 1.1) | 6.17 (d, 3.3) 5.46 (d, 3.3) | 1.19 (3H, d, 6.9) |
| 14 | 0.94 (3H. s) | 0.94 (3H, s) | 0.88 (3H, s) | 1.39 (3H, s) | 1.37 (3H, s) |
| 15 | 1.86 (3H, s) | 1.91 (3H, br s) | 5.03 (d, 1.6) 4.76 (d, 1.6) | 1.50 (3H, overlapped) | 1.60 (3H, s) |
| OAc | 1.95 (3H, s) | 1.95 (3H, s) | 1.95 (3H, s) | ||
| 1′ | 4.25 (2H, q, 7.1) | 4.24 (2H, q, 7.1) | 4.25 (2H, q, 7.1) | ||
| 2′ | 1.33 (3H, t, 7.1) | 1.32 (3H, t, 7.1) | 1.32 (3H, t, 7.1) |
| No. | 6 | 7 | 8 | 9 |
|---|---|---|---|---|
| 1 | 4.91 (dd, 11.5, 5.3) | 4.10 (dd, 7.9, 3.3) | 4.11 (dd, 7.8, 3.2) | 1.73–1.67 (m) 1.66 (ddd, 10.8, 5.3, 2.9) |
| 2 | 2.16 (m) | 1.91 (m) | 1.91 (dd, 7.5, 3.2) | 2.06 (2H, m) |
| 1.81–1.75 (m) | 1.78 (overlapped) | 1.80–1.76 (m) | ||
| 3 | 2.09 (m) 1.90 (ddd, 17.3, 13.6, 4.0) | 1.69 (overlapped) 1.59 (ddd, 12.0, 7.7, 3.8) | 1.60 (m) 1.57 (m) | 4.47 (dd, 4.8, 2.3) |
| 5 | 4.10 (dd, 11.0, 3.1) | 5.51 (d, 16.0) | 5.51 (d, 16, 0) | 2.97 (br s) 2.51 (dd, 12.8, 10.8) |
| 6 | 2.04 (ddd, 14.3, 11.0, 3.3) 1.74 (m) | 5.10 (dd, 16.0, 10.1) | 5.11 (dd, 16.0, 10.1) | 4.16 (ddd, 10.8, 9.2, 1.7) |
| 7 | 2.26 (m) | 2.59 (ddd, 11.8, 10.1, 5.0) | 2.60 (ddd, 11.3, 10.1, 5.0) | 3.59 (dd, 9.2, 8.9) |
| 8 | 2.66 (ddd, 12.8, 12.6, 11.8) 1.83 (overlap) | 1.96 (m) 1.80 (m) | 2.00–1.93 (m) 1.84–1.80 (m) | 5.32 (dd, 8.9, 2.2) |
| 9 | 5.25 (d, 11.8) | 2.38–2.28 (m) 1.70 (overlapped) | 2.34 (m) 1.70 (overlapped) | 3.00 (d, 2.2) |
| 11 | ||||
| 12 | 1.85 (3H, s) | 1.72 (3H, s) | 1.73 (3H, s) | |
| 13 | 4.89 (s) 4.64 (s) | 4.72 (2H, s) | 4.73 (2H, s) | 6.18 (d, 3.2) 5.40 (d, 3.2) |
| 14 | 1.71 (3H, s) | 5.16 (s) 4.99 (s) | 5.00 (d, 1.9) 5.17 (d, 1.9) | 1.23 (3H, s) |
| 15 | 5.17 (s) 5.01 (s) | 1.30 (3H, s) | 1.31 (3H, s) | 5.39 (d, 2.0) 5.16 (d, 2.0) |
| OAc | 2.21 (3H, s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Ke, C.; Feng, Z.; Tang, C.; Ye, Y. The First Phytochemical Investigation of Artemisia divaricate: Sesquiterpenes and Their Anti-Inflammatory Activity. Molecules 2023, 28, 4254. https://doi.org/10.3390/molecules28104254
Yan S, Ke C, Feng Z, Tang C, Ye Y. The First Phytochemical Investigation of Artemisia divaricate: Sesquiterpenes and Their Anti-Inflammatory Activity. Molecules. 2023; 28(10):4254. https://doi.org/10.3390/molecules28104254
Chicago/Turabian StyleYan, Siqi, Changqiang Ke, Zheling Feng, Chunping Tang, and Yang Ye. 2023. "The First Phytochemical Investigation of Artemisia divaricate: Sesquiterpenes and Their Anti-Inflammatory Activity" Molecules 28, no. 10: 4254. https://doi.org/10.3390/molecules28104254
APA StyleYan, S., Ke, C., Feng, Z., Tang, C., & Ye, Y. (2023). The First Phytochemical Investigation of Artemisia divaricate: Sesquiterpenes and Their Anti-Inflammatory Activity. Molecules, 28(10), 4254. https://doi.org/10.3390/molecules28104254







