Metal Complexes of Omadine (N-Hydroxypyridine-2-thione): Differences of Antioxidant and Pro-Oxidant Behavior in Light and Dark Conditions with Possible Toxicity Implications
Abstract
1. Introduction
2. Results
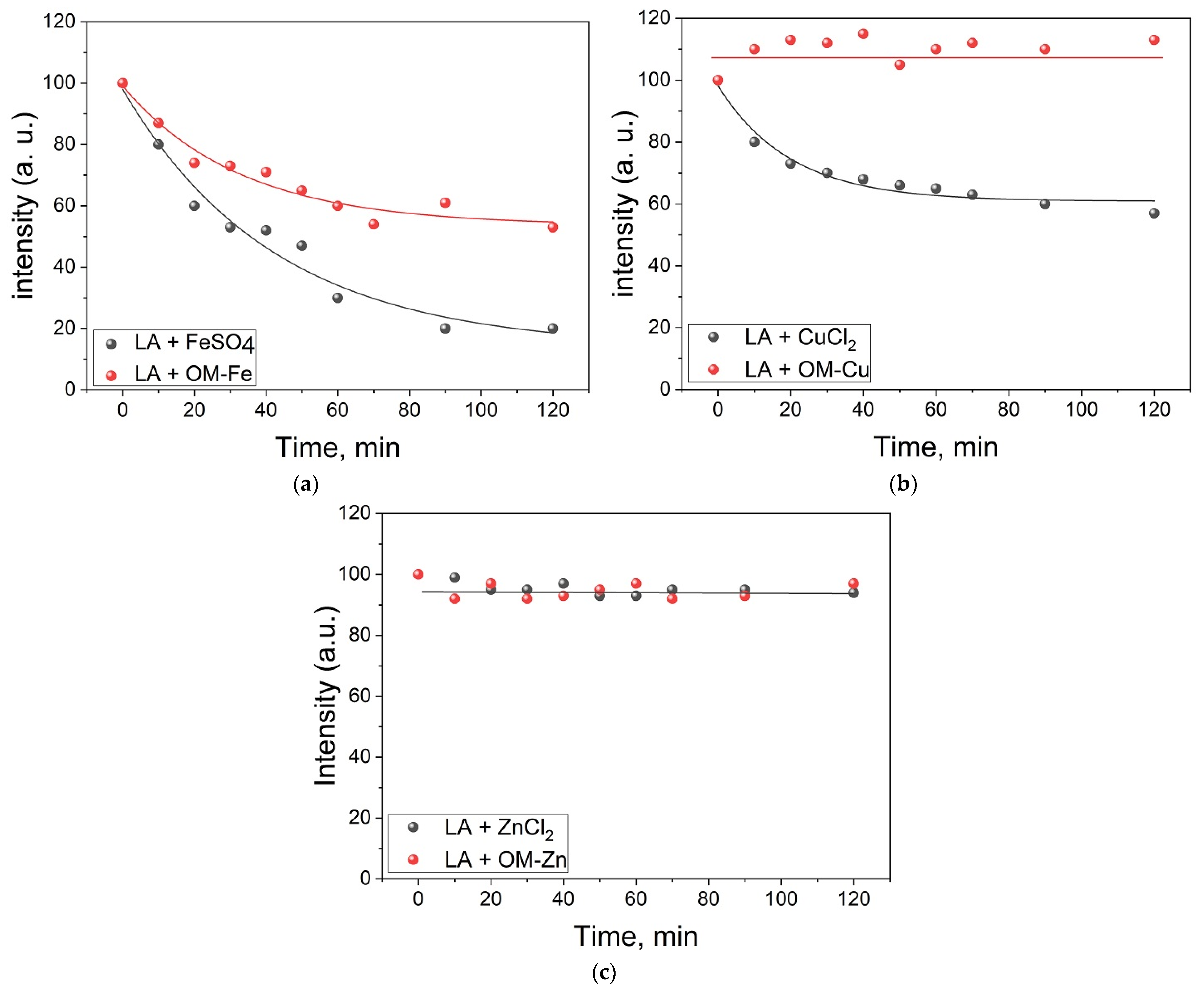
2.1. Photo-Induced Oxidation of Linoleic Acid Micelles in the Presence of Omadine and Its Metal Complexes
2.2. Peroxidation of Linoleic Acid Micelles in the Presence of Omadine and Its Metal Complexes in Dark Conditions
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. The 1H-NMR Study of Lipid Peroxidation
3.2.2. The 1H-NMR Study of the Photo-Oxidation of Linoleic Acid
3.2.3. The 1H-NMR Study of the Penetration of Omadine and Its Metal Complexes into Micelles
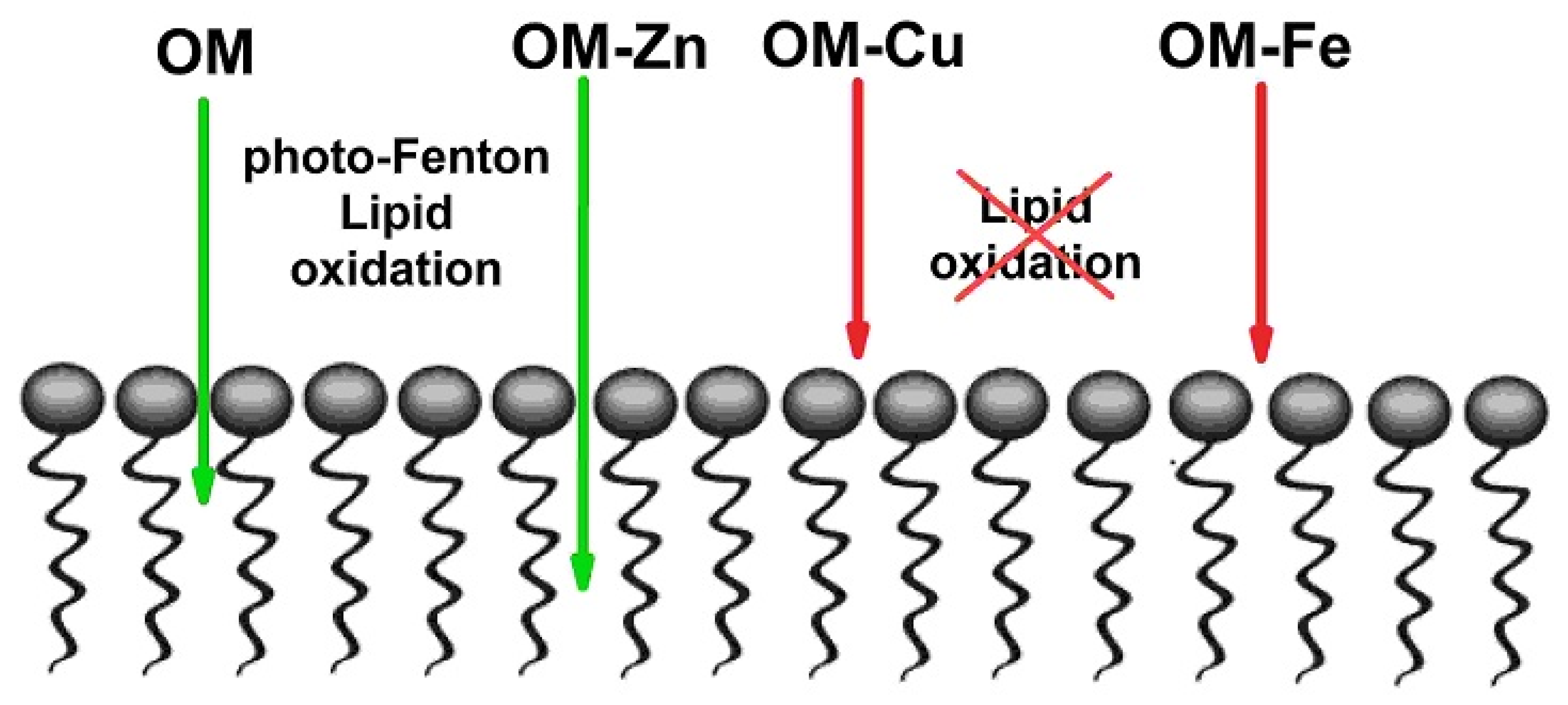
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| NOESY | Nuclear Overhauser Effect Spectroscopy |
| NMR | Nuclear magnetic Resonance |
| OM | Omadine |
| LA | Linoleic Acid |
| ROS | Reactive Oxygen Species |
References
- Pantopoulos, K.; Porwal, S.K.; Tartakoff, A.; Devireddy, L. Mechanisms of Mammalian Iron Homeostasis. Biochemistry 2012, 51, 5705–5724. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Iron and Chelation in Biochemistry and Medicine: New Approaches to Controlling Iron Metabolism and Treating Related Diseases. Cells 2020, 9, E1456. [Google Scholar] [CrossRef]
- Gozzelino, R.; Arosio, P. Iron Homeostasis in Health and Disease. Int. J. Mol. Sci. 2016, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Frazer, D.M. Current Understanding of Iron Homeostasis. Am. J. Clin. Nutr. 2017, 106 (Suppl. 6), 1559S–1566S. [Google Scholar] [CrossRef]
- Holm, R.H.; Kennepohl, P.; Solomon, E.I. Structural and Functional Aspects of Metal Sites in Biology. Chem. Rev. 1996, 2123, 2239–2314. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, I.; Dringen, R.; Mercer, J.F.B. Copper: Effects of Deficiency and Overload. Met. Ions Life Sci. 2013, 13, 359–387. [Google Scholar] [CrossRef]
- Weatherall, D.J.; Clegg, J.B. Inherited Haemoglobin Disorders: An Increasing Global Health Problem. Bull. World Health Organ. 2001, 79, 704–712. [Google Scholar] [CrossRef]
- Rees, D.C.; Williams, T.N.; Gladwin, M.T. Sickle-Cell Disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. How to Manage Iron Toxicity in Post-Allogeneic Hematopoietic Stem Cell Transplantation? Expert Rev. Hematol. 2020, 13, 299–302. [Google Scholar] [CrossRef]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; Taylor & Francis: Abingdon, UK, 2005; ISBN 9780824753566. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.; Cross, C.E. Free Radicals, Antioxidants, and Human Disease: Where Are We Now? J. Lab. Clin. Med. 1992, 119, 598–620. [Google Scholar]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Y.; Dixon, S.J. Mechanisms of Ferroptosis. Cell. Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, X.; Zhou, Y.; Wang, X.; Yu, Y. Autophagy, Ferroptosis, Pyroptosis, and Necroptosis in Tumor Immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 196. [Google Scholar] [CrossRef]
- Lei, G.; Zhuang, L.; Gan, B. Targeting Ferroptosis as a Vulnerability in Cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Carvalho, A.F.; Maes, M.; Walker, A.J.; Puri, B.K. Why Should Neuroscientists Worry about Iron? The Emerging Role of Ferroptosis in the Pathophysiology of Neuroprogressive Diseases. Behav. Brain Res. 2018, 341, 154–175. [Google Scholar] [CrossRef]
- Qiu, Y.; Cao, Y.; Cao, W.; Jia, Y.; Lu, N. The Application of Ferroptosis in Diseases. Pharmacol. Res. 2020, 159, 104919. [Google Scholar] [CrossRef] [PubMed]
- Manabe, E.; Ito, S.; Ohno, Y.; Tanaka, T.; Naito, Y.; Sasaki, N.; Asakura, M.; Masuyama, T.; Ishihara, M.; Tsujino, T. Reduced Lifespan of Erythrocytes in Dahl/Salt Sensitive Rats Is the Cause of the Renal Proximal Tubule Damage. Sci. Rep. 2020, 10, E22023. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Selyutina, O.Y.; Polyakov, N.E.; Didichenko, V.; Kontoghiorghes, G.J. Mechanistic Insights of Chelator Complexes with Essential Transition Metals: Antioxidant/Pro-Oxidant Activity and Applications in Medicine. Int. J. Mol. Sci. 2022, 23, 1247. [Google Scholar] [CrossRef]
- Wang, D.; Tang, L.; Zhang, Y.; Ge, G.; Jiang, X.; Mo, Y.; Wu, P.; Deng, X.; Li, L.; Zuo, S.; et al. Regulatory Pathways and Drugs Associated with Ferroptosis in Tumors. Cell Death Dis. 2022, 13, 544. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Q.; Song, H.; Zhang, Y.; Chen, H.; Liu, P.; Sun, T.; Jiang, C. A Triple Therapeutic Strategy with Antiexosomal Iron Efflux for Enhanced Ferroptosis Therapy and Immunotherapy. Small 2022, 18, 2201704. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. New Iron Metabolic Pathways and Chelation Targeting Strategies Affecting the Treatment of All Types and Stages of Cancer. Int. J. Mol. Sci. 2022, 23, 13990. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. Advances on Chelation and Chelator Metal Complexes in Medicine. Int. J. Mol. Sci. 2020, 21, E2499. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Jackson, M.J.; Lunec, J. In Vitro Screening of Iron Chelators Using Models of Free Radical Damage. Free Radic. Res. Commun. 1986, 2, 115–124. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Inhibition of Fe2+- and Fe3+- Induced Hydroxyl Radical Production by the Iron-Chelating Drug Deferiprone. Free Radic. Biol. Med. 2015, 78, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Timoshnikov, V.A.; Kobzeva, T.; Selyutina, O.Y.; Polyakov, N.E.; Kontoghiorghes, G.J. Effective Inhibition of Copper-Catalyzed Production of Hydroxyl Radicals by Deferiprone. J. Biol. Inorg. Chem. 2019, 24, 331–341. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Kichigina, L.A.; Selyutina, O.Y.; Polyakov, N.E.; Kontoghiorghes, G.J. Antioxidant Activity of Deferasirox and Its Metal Complexes in Model Systems of Oxidative Damage: Comparison with Deferiprone. Molecules. 2021, 26, 5064. [Google Scholar] [CrossRef]
- Sheppard, L.N.; Kontoghiorghes, G.J. Competition between Deferiprone, Desferrioxamine and Other Chelators for Iron and the Effect of Other Metals. Arzneimittelforschung 1993, 43, 659–663. [Google Scholar]
- Tang, H.M.; Fan, W.Y. Transition Metal Pyrithione Complexes (Ni, Mn, Fe, and Co) as Electrocatalysts for Proton Reduction of Acetic Acid. ACS Omega 2023, 8, 7234–7241. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Piga, A.; Hoffbrand, A.V. Cytotoxic and DNA-inhibitory Effects of Iron Chelators on Human Leukaemic Cell Lines. Hematol. Oncol. 1986, 4, 195–204. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Piga, A.; Hoffbrand, A.V. Cytotoxic Effects of the Lipophilic Iron Chelator Omadine. FEBS Lett. 1986, 204, 208–212. [Google Scholar] [CrossRef]
- Blatt, J.; Taylor, S.R.; Kontoghiorghes, G.J. Comparison of Activity of Deferoxamine with That of Oral Iron Chelators against Human Neuroblastoma Cell Lines—PubMed. Cancer Res. 1989, 49, 2925–2927. [Google Scholar]
- Forsbeck, K.; Nilsson, K.; Kontoghiorghes, G.J. Variation in Iron Accumulation, Transferrin Membrane Binding and DNA Synthesis in the K-562 and U-937 Cell Lines Induced by Chelators and Their Iron Complexes. Eur. J. Haematol. 1987, 39, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, N.; Liao, Y.; Liu, N.; Cai, J.; Xia, X.; Guo, Z.; Li, Y.; Wen, Q.; Yin, Q.; et al. Platinum-Containing Compound Platinum Pyrithione Suppresses Ovarian Tumor Proliferation through Proteasome Inhibition. J. Exp. Clin. Cancer Res. 2017, 36, 79. [Google Scholar] [CrossRef] [PubMed]
- Muthyala, R.; Shin, W.S.; Xie, J.; Sham, Y.Y. Discovery of 1-Hydroxypyridine-2-Thiones as Selective Histone Deacetylase Inhibitors and Their Potential Application for Treating Leukemia. Bioorg. Med. Chem. Lett. 2015, 25, 4320–4324. [Google Scholar] [CrossRef]
- Lan, X.; Zhao, C.; Chen, X.; Zhang, P.; Zang, D.; Wu, J.; Chen, J.; Long, H.; Yang, L.; Huang, H.; et al. Platinum Pyrithione Induces Apoptosis in Chronic Myeloid Leukemia Cells Resistant to Imatinib via DUB Inhibition-Dependent Caspase Activation and Bcr-Abl Downregulation. Cell Death Dis. 2017, 8, 2913. [Google Scholar] [CrossRef] [PubMed]
- Chaulk, S.G.; Pezacki, J.P.; MacMillan, A.M. Studies of RNA Cleavage by Photolysis of N-Hydroxypyridine-2(1H)-Thione. A New Photochemical Footprinting Method. Biochemistry 2000, 39, 10448–10453. [Google Scholar] [CrossRef]
- Chen, C.H.; Han, R.M.; Liang, R.; Fu, L.M.; Wang, P.; Ai, X.C.; Zhang, J.P.; Skibsted, L.H. Direct Observation of the β-Carotene Reaction with Hydroxyl Radical. J. Phys. Chem. B 2011, 115, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Aveline, B.M.; Kochevar, I.E.; Redmond, R.W. Photochemistry of the Nonspecific Hydroxyl Radical Generator, N-Hydroxypyridine-2(1H)-Thione. J. Am. Chem. Soc. 1996, 118, 10113–10123. [Google Scholar] [CrossRef]
- Aveline, B.M.; Kochevar, I.E.; Redmond, R.W. Photochemistry of N-Hydroxy-2(1H)-Pyridone, a More Selective Source of Hydroxyl Radicals than N-Hydroxypyridine-2(1H)-Thione. J. Am. Chem. Soc. 1996, 118, 10124–10133. [Google Scholar] [CrossRef]
- Adam, W.; Ballmaier, D.; Epe, B.; Grimm, G.N.; Saha-Möller, C.R. N-Hydroxypyridinethiones as Photochemical Hydroxyl Radical Sources for Oxidative DNA Damage. Angew. Chem. Int. Ed. Engl. 1995, 34, 2156–2158. [Google Scholar] [CrossRef]
- Reeder, N.L.; Xu, J.; Youngquist, R.S.; Schwartz, J.R.; Rust, R.C.; Saunders, C.W. The Antifungal Mechanism of Action of Zinc Pyrithione. Br. J. Dermatol. 2011, 165, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.R. Zinc Pyrithione: A Topical Antimicrobial with Complex Pharmaceutics. J. Drugs Dermatol. 2016, 15, 140–144. [Google Scholar] [PubMed]
- Srivastava, G.; Matta, A.; Fu, G.; Somasundaram, R.T.; Datti, A.; Walfish, P.G.; Ralhan, R. Anticancer Activity of Pyrithione Zinc in Oral Cancer Cells Identified in Small Molecule Screens and Xenograft Model: Implications for Oral Cancer Therapy. Mol. Oncol. 2015, 9, 1720–1735. [Google Scholar] [CrossRef]
- Reeder, N.L.; Kaplan, J.; Xu, J.; Youngquist, R.S.; Wallace, J.; Hu, P.; Juhlin, K.D.; Schwartz, J.R.; Grant, R.A.; Fieno, A.; et al. Zinc Pyrithione Inhibits Yeast Growth through Copper Influx and Inactivation of Iron-Sulfur Proteins. Antimicrob. Agents Chemother. 2011, 55, 5753–5760. [Google Scholar] [CrossRef]
- Santoro, A.; Vileno, B.; Palacios, Ò.; Peris-Díaz, M.D.; Riegel, G.; Gaiddon, C.; Krȩzel, A.; Faller, P. Reactivity of Cu(II)–, Zn(II)– and Fe(II)–Thiosemicarbazone Complexes with Glutathione and Metallothionein: From Stability to Dissociation to Transmetallation. Metallomics 2019, 11, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Stacy, A.E.; Palanimuthu, D.; Bernhardt, P.V.; Kalinowski, D.S.; Jansson, P.J.; Richardson, D.R. Zinc(II)-Thiosemicarbazone Complexes Are Localized to the Lysosomal Compartment Where They Transmetallate with Copper Ions to Induce Cytotoxicity. J. Med. Chem. 2016, 59, 4965–4984. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc Is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health. Front. Nutr. 2014, 1, 14. [Google Scholar] [CrossRef]
- Marreiro, D.d.N.; Cruz, K.J.C.; Morais, J.B.S.; Beserra, J.B.; Severo, J.S.; Soares de Oliveira, A.R. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef]
- Powell, S.R. The Antioxidant Properties of Zinc. J. Nutr. 2000, 130, 1447–1454. [Google Scholar] [CrossRef]
- Doose, C.A.; Szaleniec, M.; Behrend, P.; Müller, A.; Jastorff, B. Chromatographic Behavior of Pyrithiones. J. Chromatogr. A 2004, 1052, 103–110. [Google Scholar] [CrossRef]
- Doose, C.A.; Ranke, J.; Stock, F.; Bottin-Weber, U.; Jastorff, B. Structure–Activity Relationships of Pyrithiones—IPC-81 Toxicity Tests with the Antifouling Biocide Zinc Pyrithione and Structural Analogs. Green Chem. 2004, 6, 259–266. [Google Scholar] [CrossRef]
- Mihaljević, B.; Tartaro, I.; Ferreri, C.; Chatgilialoglu, C. Linoleic Acid Peroxidation vs. Isomerization: A Biomimetic Model of Free Radical Reactivity in the Presence of Thiols. Org. Biomol. Chem. 2011, 9, 3541–3548. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, M.; Tenon, M.; Bily, A.; Birti, S.; Laguerre, M.; Tenon, M.; Bily, A.; Birti, S. Toward a Spatiotemporal Model of Oxidation in Lipid Dispersions: A Hypothesis-Driven Review. Eur. J. Lipid Sci. Technol. 2020, 122, 1900209. [Google Scholar] [CrossRef]
- Schneider, C. An Update on Products and Mechanisms of Lipid Peroxidation. Mol. Nutr. Food Res. 2009, 53, 315–321. [Google Scholar] [CrossRef]
- Spiteller, G. Linoleic Acid Peroxidation—The Dominant Lipid Peroxidation Process in Low Density Lipoprotein-and Its Relationship to Chronic Diseases. Chem. Phys. Lipids 1998, 95, 105–162. [Google Scholar] [CrossRef]
- Cotticelli, M.G.; Crabbe, A.M.; Wilson, R.B.; Shchepinov, M.S. Insights into the Role of Oxidative Stress in the Pathology of Friedreich Ataxia Using Peroxidation Resistant Polyunsaturated Fatty Acids. Redox Biol. 2013, 1, 398–404. [Google Scholar] [CrossRef]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like Copper Redox Chemistry Revisited: Hydrogen Peroxide and Superoxide Mediation of Copper-Catalyzed Oxidant Production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Zhu, T.F.; Budin, I.; Szostak, J.W. Preparation of Fatty Acid Micelles. Methods Enzymol. 2013, 533, 283–288. [Google Scholar] [CrossRef]
- Barrera, G. Oxidative Stress and Lipid Peroxidation Products in Cancer Progression and Therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef]
- Zhu, H.; Sarkar, S.; Scott, L.; Danelisen, I.; Trush, M.A.; Jia, Z.; Li, Y.R. Doxorubicin Redox Biology: Redox Cycling, Topoisomerase Inhibition, and Oxidative Stress HHS Public Access. React. Oxigen Species 2016, 1, 189–198. [Google Scholar] [CrossRef]
- Li, Z.; Yang, X.; Dong, S.; Li, X. DNa Breakage Induced by Piceatannol and Copper(II): Mechanism and Anticancer Properties. Oncol. Lett. 2012, 3, 1087–1094. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA Damage Response in Cancer. Redox Biol. 2019, 25, 10084. [Google Scholar] [CrossRef] [PubMed]
- Selyutina, O.Y.; Mastova, A.V.; Polyakov, N.E. The Interaction of Anthracycline Based Quinone-Chelators with Model Lipid Membranes: 1H NMR and MD Study. Membranes 2023, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Mastova, A.V.; Selyutina, O.Y.; Evseenko, V.I.; Polyakov, N.E. Photoinduced Oxidation of Lipid Membranes in the Presence of the Nonsteroidal Anti-Inflammatory Drug Ketoprofen. Membranes 2022, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Selyutina, O.Y.; Kononova, P.A.; Koshman, V.E.; Fedenok, L.G.; Polyakov, N.E. The Interplay of Ascorbic Acid with Quinones-Chelators-Influence on Lipid Peroxidation: Insight into Anticancer Activity. Antioxidants 2022, 11, 376. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and Function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Feng, H.; Stockwell, B.R. Unsolved Mysteries: How Does Lipid Peroxidation Cause Ferroptosis? PLoS Biol. 2018, 16, e2006203. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular Mechanisms and Health Implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Salcedo-Sora, J.E.; Robison, A.T.R.; Zaengle-Barone, J.; Franz, K.J.; Kell, D.B. Membrane Transporters Involved in the Antimicrobial Activities of Pyrithione in Escherichia Coli. Molecules 2021, 26, 5826. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, S.; Liang, Z.; Li, L.; Guo, H. Effects of Copper Pyrithione (CuPT) on Apoptosis, ROS Production, and Gene Expression in Hemocytes of White Shrimp Litopenaeus Vannamei. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 256, 109232. [Google Scholar] [CrossRef] [PubMed]
- Lapinski, L.; Gerega, A.; Sobolewski, A.L.; Nowak, M.J. Thioperoxy Derivative Generated by UV-Induced Transformation of N-Hydroxypyridine-2(1H)-Thione Isolated in Low-Temperature Matrixes. J. Phys. Chem. A 2008, 112, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Adam, W.; Saha-Möller, C.R.; Stopper, H. Studies on Cytotoxic and Genotoxic Effects of N-Hydroxypyridine-2-Thione (Omadine) in L5178Y Mouse Lymphoma Cells. Toxicol. Lett. 2002, 136, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, K. Photochemistry of Organic Iron(III) Complexing Ligands in Oceanic Systems. Photochem. Photobiol. 2006, 82, 1505–1516. [Google Scholar] [CrossRef]
- Glebov, E.M.; Pozdnyakov, I.P.; Grivin, V.P.; Plyusnin, V.F.; Zhang, X.; Wu, F.; Deng, N. Intermediates in Photochemistry of Fe(Iii) Complexes with Carboxylic Acids in Aqueous Solutions. Photochem. Photobiol. Sci. 2011, 10, 425–430. [Google Scholar] [CrossRef]
- Benkelberg, H.J.; Warneck, P. Photodecomposition of Iron(III) Hydroxo and Sulfato Complexes in Aqueous Solution: Wavelength Dependence of OH and SO4- Quantum Yields. J. Phys. Chem. 1995, 99, 5214–5221. [Google Scholar] [CrossRef]
- Dinning, A.J.; Al-Adham, I.S.I.; Austin, P.; Charlton, M.; Collier, P.J. Pyrithione Biocide Interactions with Bacterial Phospholipid Head Groups. J. Appl. Microbiol. 1998, 85, 132–140. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; May, A. Uptake and Intracellular Distribution of Iron from Transferrin and Chelators in Erythroid Cells. Biol. Met. 1990, 3, 183–187. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Structure/Red Blood Cell Permeability. Activity of Iron(III) Chelator Complexes. Inorg. Chim. Acta 1988, 151, 101–106. [Google Scholar] [CrossRef]
- Wrona, M.; Lours, J.; Salafranca, J.; Joly, C.; Nerín, C. Innovative Surface-Enhanced Raman Spectroscopy Method as a Fast Tool to Assess the Oxidation of Lipids in Ground Pork. Appl. Sci. 2023, 13, 5533. [Google Scholar] [CrossRef]
- Schmedes, A.; Hølmer, G. A New Thiobarbituric Acid (TBA) Method for Determining Free Malondialdehyde (MDA) and Hydroperoxides Selectively as a Measure of Lipid Peroxidation. J. Am. Oil Chem. Soc. 1989, 66, 813–817. [Google Scholar] [CrossRef]
- Muik, B.; Lendl, B.; Molina-Díaz, A.; Ayora-Cañada, M.J. Direct Monitoring of Lipid Oxidation in Edible Oils by Fourier Transform Raman Spectroscopy. Chem. Phys. Lipids 2005, 134, 173–182. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selyutina, O.Y.; Timoshnikov, V.A.; Polyakov, N.E.; Kontoghiorghes, G.J. Metal Complexes of Omadine (N-Hydroxypyridine-2-thione): Differences of Antioxidant and Pro-Oxidant Behavior in Light and Dark Conditions with Possible Toxicity Implications. Molecules 2023, 28, 4210. https://doi.org/10.3390/molecules28104210
Selyutina OY, Timoshnikov VA, Polyakov NE, Kontoghiorghes GJ. Metal Complexes of Omadine (N-Hydroxypyridine-2-thione): Differences of Antioxidant and Pro-Oxidant Behavior in Light and Dark Conditions with Possible Toxicity Implications. Molecules. 2023; 28(10):4210. https://doi.org/10.3390/molecules28104210
Chicago/Turabian StyleSelyutina, Olga Yu., Viktor A. Timoshnikov, Nikolay E. Polyakov, and George J. Kontoghiorghes. 2023. "Metal Complexes of Omadine (N-Hydroxypyridine-2-thione): Differences of Antioxidant and Pro-Oxidant Behavior in Light and Dark Conditions with Possible Toxicity Implications" Molecules 28, no. 10: 4210. https://doi.org/10.3390/molecules28104210
APA StyleSelyutina, O. Y., Timoshnikov, V. A., Polyakov, N. E., & Kontoghiorghes, G. J. (2023). Metal Complexes of Omadine (N-Hydroxypyridine-2-thione): Differences of Antioxidant and Pro-Oxidant Behavior in Light and Dark Conditions with Possible Toxicity Implications. Molecules, 28(10), 4210. https://doi.org/10.3390/molecules28104210







