Abstract
The plants in the Sideritis genus are postulated to exhibit several important medicinal properties due to their unique chemical composition. To isolate the targeted phytochemical compounds, the selection of a suitable extraction method is of primary importance. In this work, a comparative study on the phytochemical profiles of various Sideritis raeseri and Sideritis scardica extracts has been carried out. An untargeted metabolomics approach based on ultra-high performance liquid chromatography coupled with high-resolution mass spectrometry was applied to investigate the metabolic differences between extracts obtained by conventional extraction and extractions assisted by microwaves, ultrasounds and high pressure. Additionally, the influence of extraction solvents on HPLC antioxidant profiles obtained following the derivatization of analytes with ABTS reagent was evaluated. A total of 102 metabolites have been putatively identified. The major secondary metabolites groups were classified as flavonoids, terpenoids, phenylethanoid glycosides and phenolic acids. The main antioxidants in the extracts were isoscutellarein and hypolaetin derivatives as well as verbascoside and chlorogenic acid. The results showed that 70% ethanol was the most effective extractant for different classes of phytochemicals including antioxidants. In addition, extraction supported with microwaves, ultrasounds or high pressure improved the overall recovery of metabolites by about 3 times compared to the conventional extraction method.
Keywords:
Sideritis raeseri; Sideritis scardica; extraction; UPLC-HRMS; antioxidants; MAE; USAE; HPE 1. Introduction
The Mediterranean region, the Balkan Peninsula and the Middle East possess suitable climate conditions for house plant communities rich in herbs and shrubs from the Lamiaceae family, including the Sideritis genus [1]. In traditional medicine, infusions or decoctions prepared with the flowering aerial parts of the Sideritis species were widely used for the treatment of the common cold, cough, gastrointestinal disorders and for the healing of wounds [2,3]. Sideritis scardica, endemic to the Balkan Peninsula [4], is known also as ironwort. Depending on the region of origin, in Bulgaria, infusions from this herb are known as “Mursalski tea”, “Pirinski tea” or “Alibotushki tea”. In the Republic of North Macedonia, it is commonly named “Sharplaninsi chaj”, whereas in Greece, it can be called “Greek Mountain tea” or “Greek Olympus Tea” [5,6,7]. Sideritis raeseri is another popular herb variety endemic to the Balkans and the Iberian Peninsula, also cultivated in Greece [8,9]. All these mentioned plants contain a variety of health-promoting phytochemical constituents, including phenolic acids, flavonoids, phenylethanoid glycosides and terpenoids [1]. Previous studies indicated miscellaneous biological properties of the Sideritis species: anti-inflammatory, gastroprotective, cytotoxic, antimicrobial and antioxidant [7,10]. These chemopreventive properties can be fully exploited in the pharmaceutical or food industry only with the condition that effective isolation of the bioactive phytochemicals is ensured.
In the case of a complex matrix such as herbs, which often contain bioactive substances of very different polarities, the extraction solvent should be carefully selected. When extracting specific compounds, the solvent should match the polarity of the target compound. The simultaneous recovery of multiple compounds requires a more versatile solvent. Generally, in the food industry, non-toxic and easy-to-use solvents are preferred for plant extraction. Water is the safest and cheapest green solvent; nonetheless, its application is limited to polar compounds. In the case of less polar molecules, a higher efficiency can be achieved with the aid of organic solvents. The most satisfactory results are usually obtained with binary solvent mixtures (e.g., water and an organic solvent), which also have been shown to be more efficient and environmentally friendly than pure organic solvents [11]. In order to improve the effectiveness of the bioactive substance extraction, aqueous/organic solvents must be combined at the appropriate ratio. For example, despite the low solubility of polyphenols in water, its addition to an organic solvent increases the diffusivity of the solvent within the matrix [12,13]. Flavonoid glycosides are more water-soluble than aglycones, but these two chemical forms can be extracted simultaneously with a mixture of water and alcohol or pure alcohols. It has been observed that less polar flavonoids, such as flavanones, flavanols, isoflavones and methylated flavones are more soluble in ethyl acetate, diethyl ether, chloroform and dichloromethane. However, due to the toxicity of these solvents, they must be handled with caution and need to be evaporated to a safe limit permissible for food [11].
Conventional solvent extractions such as Soxhlet extraction and maceration exhibit some fundamental disadvantages, being time-consuming, costly, and not ecological. These drawbacks have directed research towards more cost-effective and greener methods for the extraction of bioactive compounds from plant material. The proposed alternative techniques include the following: ultrasound-assisted extraction, microwave-assisted extraction, pressurized liquid extraction and pressurized hot water extraction. These extraction techniques oftentimes employ elevated temperature, which on one hand increases solubility and mass transfer due to the reduced viscosity of the solvent used, but on the other hand may be destructive for thermosensitive compounds. Another alternative green extraction technique is supercritical fluid extraction, typically with carbon dioxide, a solvent generally recognized as safe (GRAS). This type of extraction is conducted at low temperatures (<5 °C); however, the slow diffusion of the solute from the solid matrix makes this process time-consuming [14]. The remedy may be high-pressure extraction conducted in sub-zero temperatures, which on top of speeding up the process prevents the thermal degradation and loss of bioactivity of the extracted compounds. The advantages of using high-pressure extraction for the isolation of polyphenols, including flavonoids, have been noticed previously [15]. It was observed that a high-pressure extraction time of less than 20 min gave a similar recovery of solutes as 2 h boiling or 3 h supercritical CO2 extraction. Moreover, the mentioned alternative extraction methods are recognized as environmentally friendly.
This study was aimed at finding out the method of preparation of Sideritis extracts that would ensure the maximum recovery of health-beneficial compounds from these plants. The aerial parts of Sideritis raeseri and Sideritis scardica are traditionally brewed with hot water for the preparation of infusions. In the presented study, it was hypothesized that the addition of an appropriate amount of a non-toxic organic solvent such as ethanol to the water during extraction may improve the extraction efficiency of the desired phytochemicals. The untargeted metabolomics approach based on ultra-high performance liquid chromatography coupled with high-resolution mass spectrometry (UHPLC-HRMS) fingerprinting was applied to investigate the differences in the composition of metabolites isolated from S. raeseri and S. scardica using extractants of different polarities achieved by changing the proportions of water and ethanol. Additionally, the influence of extraction solvents on HPLC antioxidant profiles obtained by the derivatization of analytes with an ABTS radical was evaluated. The composition of the phytochemicals in the Sideritis genus was the subject of several publications [7,10]. However, the number of detected and monitored metabolites was generally limited to the main groups of Sideritis metabolites such as phenolics, flavonoids and phenylethanoid glycosides, which resulted in the identification of only about twenty or thirty metabolites [3,4,16,17,18,19]. The analytical approach presented here enabled the tracking of over 100 metabolites belonging to different classes of phytochemicals. In addition, for the first time, according to our best knowledge, antioxidant profiling was used for species from the Sideritis genus, which made it possible to identify the most important phytochemicals responsible for the antioxidant properties of these plants. Another research goal was to determine whether and to what extent the use of assisted extraction with microwaves, ultrasounds or high pressure would affect the extraction efficiency and profile of phytochemicals. Former studies on extraction methods used for the isolation of Sideritis metabolites usually were limited to determinations of the total flavonoid content, the total phenolic content, the total antioxidant activity of extracts and selected individual compounds [1,2,19,20,21,22]. In this study, consideration has been extended to the assessment of the impact of the extraction method on the detailed phytochemical composition in S. raeseri and S. scardica extracts.
2. Results and Discussion
2.1. Phytochemical Composition of Sideritis scardica and Sideritis raeseri
The phytochemical composition of the extracts from Sideritis scardica and Sideritis raeseri was determined by HR-LC-ESI-Orbitrap-MS analysis in negative ion mode. The negative ion mode was selected for final data processing due to the lower background noise and matrix interferences, more fragment ion patterns and a larger number of identifiable phytochemicals. During the MS/MS experiments, a “data dependent scan” mode was used in which MS software selects the precursor ions corresponding to the most intense peaks in the LC-MS spectrum. Some of the main peaks were tentatively attributed according to the accurate masses, characteristic fragmentation patterns and retention times in comparison with literature data on the Sideritis genus. Finally, 102 phytochemicals were successfully identified, including 31 flavonoids, 14 phenolic acids and 14 terpenoids—mostly iridoid glycosides, 13 phenylethanoid glycosides and other compounds such as sugar acids and saccharides, carboxylic acids, etc. Relevant information on all of the compounds identified is provided in Table 1.

Table 1.
Major compounds identified by LC-Q-Orbitrap HRMS in different extracts of Sideritis raeseri and Sideritis scardica.
Flavonoids and their derivatives are the dominant phytochemicals in the Sideritis species [18]. In this study, about one-third of identified compounds were classified as flavonoids. These compounds were mostly glycosides and acetyl glycosides of flavonoids and their methylated forms, which is characteristic of the Sideritis species [29]. The main flavonoid aglycones found in studied plants were hypolaetin (m/z 301), methylhypolaetin (m/z 315), isoscutellarein (m/z 285), methylisoscutellarein (m/z 299) and apigenin (m/z 269). The characteristic fragmentation pattern of the acetylated flavonoid glycosides is the loss of the acetyl residue (m/z 42), H2O (m/z 18) and hexose (m/z 162) units. The abundant [M−H−342]− ions indicate the presence of two hexose units. Diacetylated derivatives were characterized by another neutral loss of acetyl residue. Compounds 49, 61 and 78 with [M−H]− at m/z 625.14099, 667.15155 and 709.16180 have been characterized as hypolaetin allosyl glycoside, hypolaetin acetyl allosyl glucoside and hypolaetin diacetyl allosyl glucoside. The common fragment ion of m/z 301.035 represented deprotonated hypolaetin [27]. Methylhypolaetin glycosides shared the characteristic 315.051 fragment, which was observed in compounds: 60, 66 and 83 ([M−H]− at m/z 639.15674, 477.10394, 723.17773) and two isomers of methylhypolaetin acetyl glucoside—72 and 77 ([M−H]− at m/z 681.16711 and 681.16766). Compounds 56, 63, 69 and 82 showed pseudo-molecular ions [M−H]− at m/z 609.14581, 447.09323, 651.15662 and 693.16705, respectively. They all gave a common fragment ion of m/z 285.04025 which could be attributed to isoscutellarein (81, [M−H]−at m/z 285.04037). Compounds 70, 76, 80 and 88 with [M−H]− at m/z 665.17224, 623.16193, 665.17242 and 707.18292 produced characteristic fragments of m/z 299.056 that corresponded to the methylated form of isoscutellarein. Fragments of m/z 269.04568 originating from deprotonated apigenin were registered in compounds 41, 53, 67, 68, 86 and 92 that gave pseudo-molecular ions [M−H]− at m/z 593.15137, 593.15155, 431.09845, 635.16150, 577.13519 and 283.06125, respectively. Compound 86 also gave characteristic fragments of m/z 145.02838 which correspond to a loss of apigenin and hexose unit [M−H−270−162]− and 413.0878 was produced by the loss of coumaroyl moiety [M−H−164]−, hence it was identified as apigenin-7-O-(6″-O-4-coumaroyl)-beta-glucoside (echinacin). Additionally, this compound was previously reported in S. scardica and S. raeseri [23,24].
Another abundant class of compounds found in Sideritis were phenylethanoid glycosides [18]. An investigation of the fragmentation pattern allowed for the identification of the phenylethanoid glycosides group by their common loss of masses: 162 Da, 146 Da, 18 Da, 15 Da and 179 Da, corresponding to hexose, rhamnose, H2O, Me and caffeoyl units, respectively. Among all detected compounds, 13 were classified as phenylethanoid glycosides. Compounds 52 and 55 with precursor ions at m/z 623.19812 shared the typical fragmentation pattern for verbascoside and isoverbascoside with 461.16638/461.16678 m/z fragments [M−H−162]− arising from the loss of caffeoyl moiety, which also was observed as a separate 179 m/z ion. The fragment ion of m/z 315.10938 represented the further loss of deoxyhexose and the 135.04388 fragment resulted from the subsequent loss of hexose and water. Compound 45 with [M−H]− at m/z 639.19348 was identified as β-hydroxyverbascoside, as it showed a similar fragmentation pattern to verbascoside, with the additional characteristic ion at m/z 151.03902. Compound 27 ([M−H]− at m/z 461.16660) was identified as a decaffeoyl-verbascoside, also known as verbasoside [30]. Compound 58 with a precursor ion [M−H]− at 799.26605 showed the fragmentation pattern of a glycosidic derivative of a methylether of verbascoside with 637.21533 and 623.21814 fragments due to the loss of hexose unit and subsequent loss of methyl group. The proposed molecular formula (C14H20O7) for compound 30 ([M−H]− at m/z 299.11365) with an error of −0.25 ppm and its fragmentation pattern was consistent with these of salidroside. Compound 59, identified as allysonoside, showed a precursor ion at m/z 769.25604, the fragment ion observed at m/z 593.20935 corresponded to the loss of the feruloyl unit [M−H]−176]−, whereas the m/z 461.16599 fragment was produced by the subsequent loss of apiosyl unit [M−H−176−132]−. Other fragments commonly observed for allysonoside [25,28], except the fragment of m/z at 637.2131, did not occur under applied analytical conditions. Compound 48 gave a precursor ion [M−H]− at m/z 785.25116 which corresponded to the chemical formula C35H45O20− that could be attributed to the deprotonated form of echinacoside [28]. Compound 51 yielded the base peak at m/z 755.74200 which was accurate for either samioside or lavandulifolioside. The formed product ions at m/z 593.20941 and 623.19867 occur in both compounds, so fragmentation did not allow for distinguishing them. Compound 64 ([M−H]− at m/z 637.21381), with the characteristic fragment of m/z at 461.1666 [28], was tentatively identified as leucoseptoside A. Compound 75 ([M−H]− at m/z 651.22961) showed fragments produced by the loss of feruloyl unit [M–H−176]− and was identified as martynoside as it is one of the main phenolic glycosides of the Sideritis species [10,19].
In the case of phenolic acids, the major one was chlorogenic acid (compound 37) with the main characteristic ions originating from caffeic and quinic acid fragments (179 and 191 Da). Compound 20 ([M−H]− m/z at 169.01329) was identified as gallic acid as it gave a characteristic fragment of m/z 125.02325. Compound 21, with a parent ion at m/z 183.02913, was identified as methyl gallate. Compound 28 ([M−H]− m/z at 153.01819) was identified as gentistic acid, as its fragmentation pattern corresponded to that reported previously [26]. Compound 42, with base peaks at m/z 179.03415, was tentatively identified as caffeic acid. Compounds 33 and 34 ([M−H]− m/z at 315.07239 and 299.07736) were assigned as gentistic acid glucoside and salicylic acid glucoside, based on fragments produced by the loss of a hexose [M−162−H]−.
The loss of glucose [M−162]− and characteristic loss of 182 amu indicate the presence of iridoid glycosides. Compounds 19 and 22 with pseudo-molecular ions at m/z 361.11432 and 523.16693 were identified as monomelittoside and melittoside, respectively. Fragments of m/z 163.03896 corresponding to a deprotonated coumaric acid ion led to the identification of compound 39 ([M−H]− m/z at 669.20361) as 10-O-(E)-p-coumaroylmelittoside. Compound 43 was tentatively assigned as ajugoside due to the presence of the precursor ion at 389.14542 m/z and its prior presence in some other Sideritis species [3,18]. Compounds 29 and 44 with precursor ions at m/z 375.12970 and 417.14059 were identified as 8-epiloganic acid and 7-O-acetyl-8-epiloganic acid. Compound 23, with the pseudo-molecular ion [M−H]− at m/z 373.11420, generated fragments of m/z 123.04385, 149.05965 and 89.02296, which was consistent with the fragmentation path of geniposidic acid. Among registered substance peaks, several belonged to abietane diterpenoids, which produced characteristic fragment ions due to the loss of carbon dioxide (44 Da), carbon monoxide (−28 Da), water (−18 Da) and methyl radical (15 Da). Compounds 89 ([M−H]− m/z at 345.17105), 94 ([M−H]− m/z at 329.17609) and 97 ([M−H]− m/z at 331.20456) were identified as rosmanol, carnosol and carnosic acid, respectively.
2.2. Comparative Analyses of Phytochemical Variation between Extracts with Different Polarities from Sideritis raeseri and Sideritis scardica
Four parallel extractions were carried out for two species of Sideritis studied with extractants of different polarities, regulated by changes in the proportions of water and ethanol. These solvents were chosen as non-toxic and easy-to-handle for plant extraction preferred in the food and pharmaceutical industry. The phytochemical profiles of each extract accompanied by the corresponding heat map with the signal intensity of individual phytochemicals detected in four different S. raeseri and S. scardica extracts are presented in Figure 1 and Figure 2, respectively.
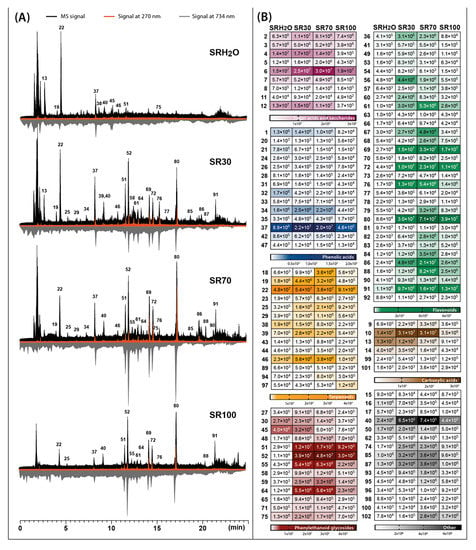
Figure 1.
Total ion chromatograms obtained by LC-Q-Orbitrap in negative mode (black) combined with chromatograms registered by UV-Vis detector at 270 nm (orange) and antioxidant profiles registered at 734 nm after post-column derivatization with ABTS (grey) (A), assembled with heat maps representing the mean MS peak area value of the identified compounds in four different Sideritis raeseri extracts: SRH2O—water extract; SR30—30% ethanol extract; SR70—70% ethanol extract; SR100—ethanol extract (B). For identity of peaks, see Table 1.
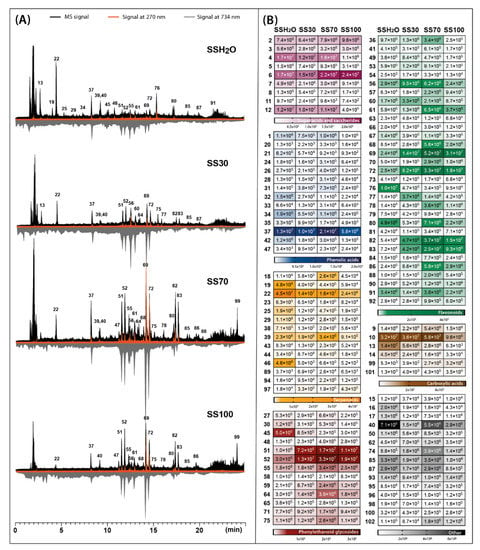
Figure 2.
Total ion chromatograms obtained by LC-Q-Orbitrap in negative mode (black) combined with chromatograms registered by UV-Vis detector at 270 nm (orange) and antioxidant profiles registered at 734 nm after post-column derivatization with ABTS (grey) (A), assembled with heat maps representing the mean MS peak area value of the identified compounds in four different Sideritis scardica extracts: SSH2O—water extract; SS30—30% ethanol extract; SS70—70% ethanol extract; SS100—ethanol extract (B). For identity of peaks, see Table 1.
The main phytochemicals detected in two Sideritis species were phenolic compounds such as phenylethanoid glycosides, flavonoid glycosides and phenolic acids. In the case of the class of phenylethanoid glycosides and phenolic acids, verbascoside (compound 52) and chlorogenic acid (compound 31) were predominant compounds. The class of flavonoids was mainly represented in S. raeseri by 4′-O-methylisoscutellarein 7-O-[6‴-O-acetyl]-allosyl(1→2)glucoside (compound 80) and in S. scardica by isoscutellarein 7-O-[6‴-acetyl]-allosyl(1→2)-glucoside (compound 69). Another important class of phytochemicals present in the Sideritis extracts studied were terpenoids represented mostly by melittoside (compound 22) belonging to the iridoid glycosides. Considering the recovery of compounds belonging to these main classes, calculated as the sum of peak areas retrieved from MS analysis, four different solvents were compared (Figure 3).
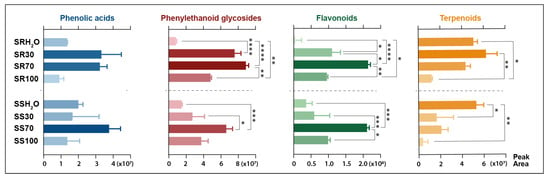
Figure 3.
Extraction efficiency of major classes of bioactive phytochemicals from two Sideritis species with four different solvents based on sum of peak areas of compounds assigned to the appropriate classes. SS—Sideritis scardica; SR—Sideritis raeseri; H2O—water extract; 30—30% ethanol extract, 70—70% ethanol extract; 100—ethanol extract. Asterisks representing p-value classification refer to: (*) p ≤ 0.05; (**) p ≤ 0.01; (***) p ≤ 0.001; (****) p ≤ 0.0001.
The highest total content of phenylethanoid glycosides was achieved with the use of 30% or 70% ethanol aqueous solution as a solvent. The best extractant for flavonoids was without a doubt 70% ethanol, as the total flavonoid area was twice greater than with the use of 30% ethanol or pure ethanol. The extraction of flavonoids with water was the least effective. The best solvent in the case of phenolic acids could not be clearly defined due to the lack of statistical differences between the tested samples. The optimal ethanol concentration for the high-rate extraction of total phenols from S. raeseri was established as 70% in a previous study [1]. Moreover, when targeting phenolic compounds from S. raeseri, it was proven that aqueous ethanolic extract could be further enriched by successive extraction with ethyl acetate [2]. In previous research reports, total phenolic content was significantly higher in S. raeseri, whereas total flavonoid content was higher in S. scardica [18]. In this study, the approximate content of flavonoids and phenolics based on the total peak area did not differ between these varieties. Terpenoid compounds showed the greatest affinity for water. The peak area of terpenoids from S. raeseri was on a similar level for all water-containing extracts. In the case of S. scardica, the extraction of terpenoids with water was significantly better than with any other studied extractant. Terpenoids are a wide class of compounds; in the studied plants, they were predominantly classified as iridoids, mostly in the form of glycosides, which explains the stronger affinity for polar solvents. The high total area of iridoid compounds suggested their presence in both studied varieties, although they were not reported previously in S. scardica [18]. Other representatives of this class found in studied extracts were abietane diterpenoids such as carnosol and carnosic acid. They are common in various plants from the Lamiaceae family, but in the Sideritis species, their concentration is rather low. Due to their non-polar nature, these compounds’ abietane diterpenoids tended to be better extracted with ethanolic solvents. Higher levels of these compounds observed in aqueous-ethanolic extracts of S. raeseri compared to S. scardica may be explained by the difference between the terpenoid profiles in these two varieties.
Differential analysis of Sideritis scardica and Sideritis raeseri extracts performed by a fold change analysis coupled with a t-test indicated that the largest number of differentiating compounds was present in 70% ethanolic extracts (Figure 4A). For these extracts, a total of 630 substance peaks were assigned at significantly higher levels in the S. raeseri variety, estimated based on the MS peak areas. Conversely, 591 substance peaks were assigned in favor of the S. scardica variety. Among them, 10 main compounds which can be the basis for distinction between these varieties were distinguished (Figure 4B). In the case of S. raeseri, the area of peaks attributed to 4′-O-methylisoscutellarein 7-O-[6‴-O-acetyl]-allosyl(1→2)-glucoside (80), verbascoside/isoverbascoside (52), melittoside (22), apigenin-7-O-(6″-O-4-coumaroyl)-glucoside (86) and (−)-usnic acid/eupatorin (91) were significantly greater compared to S. scardica. In contrast, the other five compounds’ areas were notably greater in S. scardica. These compounds are the following: isoscutellarein 7-O-[6‴-O-acetyl]-allosyl(1→2)-glucoside (69), quinic acid (10), isoscutellarein 7-O-[6‴-O-acetyl]-allosyl(1→2)-[6″-O-acetyl]-glucoside (82), 4′-O-methylhypolaetin 7-O-[6‴-O-acetyl]-allosyl(1→2)-glucoside (72) and 4′-O-methylhypolaetin 7-O-[6‴-O-acetyl]-allosyl-(1→2)[6′′-O-acetyl]-glucoside (83).
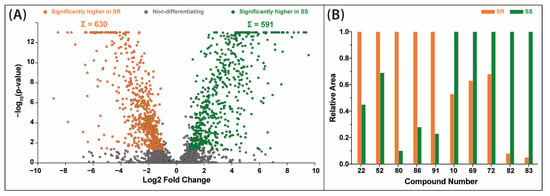
Figure 4.
Volcano plot combining the results of fold change (FC) analysis and t-tests comparing Sideritis raeseri (SR) and Sideritis scardica (SS) extracts prepared with 70% ethanol (A) juxtaposed with a bar graph showing the relative peak area of the 10 characteristic metabolites differentiating the SS and SR varieties (the contents of each higher one converted to 1.0) (B). For identity of peaks, see Table 1.
The presence of compounds exhibiting antioxidant activity in the plant material has become an important aspect in defining its health-promoting qualities. In the presented study, the antioxidant profiles were generated for S. raeseri and S. scardica extracts prepared with extractants of different polarities. Post-column addition of the ABTS reagent during HPLC analysis of the extracts causes the reduction of blue-green colored radicals by the analytes leaving the column, which is recorded at 734 nm as chromatograms with characteristic negative peaks (Figure 1A and Figure 2A—grey chromatograms). The obtained results indicated that antioxidant profiles also depended on the solvent used for extraction. In the case of 70% ethanolic extracts, the signals corresponding to antioxidants were the most intensive, while water extracts showed the least intensive signals. The dominant antioxidants in S. scardica were compounds 69 and 72, identified as isoscutellarein 7-O-[6‴-O-acetyl]-allosyl(1→2)-glucoside and 4′-O-methylhypolaetin 7-O-[6‴-O-acetyl]-allosyl(1→2)-glucoside (Figure 2A). These compounds also contributed to the antioxidant activity of S. raeseri, but not as strongly as compounds 80 (4′-O-methylisoscutellarein 7-O-[6‴-O-acetyl]-allosyl(1→2)glucoside) and 52 (verbascoside) (Figure 1A). According to Krgović et al. [2], the antioxidant activity of S. raeseri was the most positively correlated with the content of 4′-O-methyl-isoscutellarein 7-O-[6‴-O-acetyl-β-D-allopyranosyl-(1→2)]-β-D-glucopyranoside and 4′-O-methyl-hypolaetin 7-O-[6‴-O-acetyl-β-D-allopyranosyl(1→2)]-6‴-O-acetyl-β-D-glucopyranoside, identified also in our study as compounds 80 and 83, respectively. However, in our study, the antioxidant activity of compound 83 was not observed at all in S. raeseri extracts, whereas in S. scardica extracts, it was relatively weak compared to other antioxidants. In both varieties, the antioxidant activity of the chlorogenic acid assigned as compound 37 was also noticeable. Another important metabolite that showed relatively strong antioxidant activity was phenylethanoid glycoside, assigned as compound 51, and a peak originating from compound 61 (hypolaetin 7-O-[6‴-O-acetyl]-allosyl(1→2)-glucoside). The antioxidant activity of chlorogenic acid, phenylethanoid glycosides and flavonoids derived from isoscutellarein and hypolaetin have also been reported in other studies [10,31,32,33].
The significant influence that solvents with different polarities have on yield, composition profile and antioxidant activity has been clearly shown in this study for the extracts from S. raeseri and S. scardica. The obtained results on metabolite identification and antioxidant profiling indicated that 70% ethanol aqueous solution turned out to be the most effective extractant for the bioactives present in the studied Sideritis species. However, the aerial parts of S. scardica and S. raeseri are commonly used to prepare infusions (known as mountain tea). According to Irakli et al. [34], to prepare an S. scardica infusion with the highest level of phenolics, flavonoids and antioxidant activity, the temperature of water should be between 87.5 and 99.8 °C and the contact time of the dried plant material with water should be 10 min. However, the obtained results clearly indicate that the addition of ethanol to water significantly increases the extraction efficiency of various groups of bioactive phytochemicals. The water-ethanol solution is recognized as a low-toxicity and environmentally friendly extraction medium, therefore, as a GRAS (Generally Recognized as Safe) system, it can be used in the food, cosmetics and pharmaceutical industries. Therefore, a 70% aqueous ethanol solution was selected for further research aimed at increasing the efficiency of analytes’ solubilization by using assisted extraction methods.
2.3. Comparative Analyses of Phytochemical Variation between Extracts from Conventional and Assisted Solvent Extractions
For environmental, economic and safety reasons, there is a need to develop alternative, more ecological methods of extraction, enabling sustainable and selective recovery of valuable compounds and overcoming the limitations of conventional methods. For this reason, in the next stage of our research, the comparative analysis of phytochemical variability between extracts from conventional (CSE) and assisted solvent extractions such as microwave-assisted extraction (MAE), ultrasound-assisted extraction (USAE) and high-pressure extraction (HPE) was carried out using LC-Q-Orbitrap HRMS.
In this step, all extractions were conducted with the 70% aqueous ethanol solution. The MS data acquired from the analysis of extracts obtained by different methods were imported into Compound Discoverer 2.1 software for the identification of extracted metabolites. At this stage, a total of 3639 substance peaks were detected in both Sideritis varieties studied. Each extraction was conducted in triplicate, which allowed for the performance of a statistical analysis. For differential analysis purposes, each alternative solvent extraction (ASE) method was compared to conventional solvent extraction (CSE) by combined fold change analysis and t-tests, taking into account all of the substance peaks. Venn diagrams show the number and relationship of substance signals significantly differentiating the USAE, MAE, HPE-S and HPE-L extracts from CE extracts (Figure 5). Regardless of the alternative extraction method used, the significant increase of areas attributable to 1600 and 1755 individual metabolites was observed in S. scardica and S. raeseri, respectively. Thus, all of the alternative methods had a significant impact on the complexity of the extracts. Additionally, the impact of the extraction method on the recovery of the main groups of bioactive compounds characteristic of the Sideritis species and individual bioactives was investigated (Figure 6A,B). All of the alternative extraction methods studied have improved the overall recovery of metabolites compared to the conventional extraction method. The improvement was especially noticeable in the case of the extraction of phenylethanoid glycosides and flavonoids, which were the main bioactives in the Sideritis species. Considering these two classes, the total areas obtained with USAE and MAE were significantly greater than while using both types of HPE. In the case of MAE, the yields of phenolics and flavonoids may decrease as a result of prolonged exposure to high temperature [20,35]. Hence, the importance of introducing a cooling phase between each irradiation step when it is not possible to control the temperature of the process should be recognized. On the other hand, according to Šavikin et al. [1], the high temperature used during USAE had a positive influence on the total phenolics content in S. raeseri extracts, with an optimum level at 62.75 °C. No significant differences occurred in the total areas of phenolic acids and terpenoids. Moreover, the extraction of some terpenoid compounds, including rosmanol (89), carnosol (94) and carnosic acid (97), did not benefit from any of the studied alternative solvent extraction methods. As shown in Figure 6B, the relative peak areas determined for these compounds were lower than 0, which means that in this case, ASE was worse than CSE. Considering all ASE methods, HPE-S did not introduce any new substance signals (blue fields in Figure 5). However, prolonging the high-pressure extraction time to 18 h (HPE-L) resulted in the extracts with the greatest number of additional substances differentiating from CSE (134 in SR extracts and 100 in SS extracts). However, this may be a result of the presence of low-molecular-weight degradation products. However, considering individual compounds, longer exposure to high pressure led to higher recovery of scutellarein or its isomer isoscutellarein (81) compared to other extraction methods. In this study, the HPE turned out to be the least effective of the ASE methods. This may be due to the used parameters of the process. The temperature was −20 °C, while oftentimes the HPE is conducted at room temperature or higher (even up to 60 °C). Additionally, the pressure (193 MPa) was relatively low. As previously reported [36], the pressure in HPE can reach up to 600 MPa or even 1000 MPa [37].
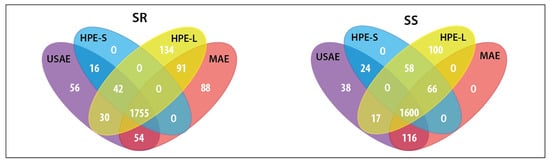
Figure 5.
Venn diagrams showing the number and relations between the significantly better-extracted metabolites, in terms of peaks areas obtained by using alternative solvent extraction (ASE) compared to the extracts obtained with conventional solvent extraction method. SR—Sideritis raeseri; SS—Sideritis scardica; CSE—conventional solvent extraction; USAE—ultrasound-assisted extraction; MAE—microwave-assisted extraction; HPE—high-pressure extraction (S—20 min of extraction; L—18 h of extraction).
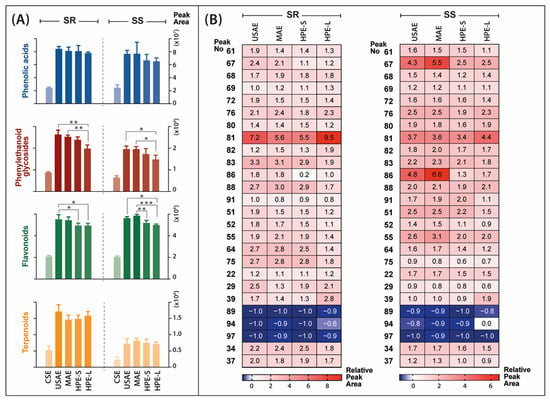
Figure 6.
Comparison of the extraction methods in terms of bioactive compound recovery. Bar graphs represent total peak areas of compounds isolated with different extraction methods and grouped into classes characteristic for Sideritis species (A). The heatmaps show the relative peak areas of main identified bioactive metabolites obtained by different extraction methods. The results are presented in relation to the conventional solvent extraction areas, which have been assumed to equal 0.0 (B). Red indicates an improvement in bioactive extraction, blue means deterioration. The abbreviations refer to: SR—Sideritis raeseri; SS—Sideritis scardica; CSE—conventional solvent extraction; USAE—ultrasound-assisted extraction; MAE—microwave-assisted extraction; HPE—high-pressure extraction (S—20 min of extraction; L—18 h of extraction). Asterisks representing p-value classification are as follows: (*) p ≤ 0.05; (**) p ≤ 0.01; (***) p ≤ 0.001. For identity of peaks, see Table 1.
3. Materials and Methods
3.1. Plant Material
The flowering aerial parts of Sideritis raeseri and Sideritis scardica used for this study were obtained from ecological producer (GRECO Bio Products, Thessaloniki, Greece). According to manufacturer’s information, the plant material was traditionally air-dried without exposure to sunlight.
3.2. Determination of Moisture Content
The moisture content was determined with the aid of a moisture analyzer RADWAG MAX 50/1 (Radom, Poland).
3.3. Preparation of Extracts
Before extraction, dried herbs were finely ground in a laboratory mill (2 min) and mixed thoroughly before weighing. Moisture content of the plant material was 5.46 and 5.12% in S. raeseri and S. scardica, respectively. Each extraction was performed with accurately weighed 5 g of plant material suspended in 110 mL of solvent (1:22, w:v).
3.3.1. Conventional Solvent Extraction (CSE)
For CSE, plant material was mixed with four extractants of different polarities, adjusted with varying proportions of water and ethanol. The solvents for extraction were water, ethanol, and 30% and 70% v/v aqueous ethanol solutions. Extractions were carried out at room temperature, except for the aqueous extract, which was prepared with boiling water to resemble typical infusions. The contact time of the plant material with the solvent was 10 min. After extraction, the samples were centrifuged at 13,000 rpm for 5 min and supernatants were collected. The extraction was conducted in triplicate with each extraction solvent.
3.3.2. Assisted Solvent Extractions (ASE)
For each assisted extraction, the plant material was mixed with 70% ethanol aqueous solution. For microwave-assisted extraction (MAE), the method described by Alipieva et al. [20] with slight modifications was used. The microwave extraction was performed in a Bartscher 610.835 microwave oven (Salzkotten, Germany) with a power of 360 W. To avoid super-boiling, the extraction process was carried out for a total of 2 min, with alternating 10 s of microwave irradiation and 10 s cooling periods. The ultrasound-assisted extraction (USAE) was performed in POLSONIC SONIC-3 ultrasonic bath (Warsaw, Poland) with an ultrasonic frequency of 40 kHz and power of 310 W. The samples were treated with ultrasound for 20 min at 25 °C. For high-pressure extraction (HPE), the samples were suspended in 70% ethanol aqueous solution and transferred to sterile plastic, flexible containers, which were deaerated and sealed. The samples were pressurized at 193 MPa and −20 °C. Compression step lasted 90 min and samples were kept in these conditions for either 20 min (HPE-S) or 18 h (HPE-L). Decompression was performed for 30 min. The extraction was carried out in high-pressure equipment designed at the Department of Food Chemistry, Technology and Biotechnology, Gdansk University of Technology, and built by DS-Technology Ltd. (Slupsk, Poland). The details of the procedure have been previously described by Malinowska-Pańczyk et al. [38]. All extractions were conducted in triplicate. After extraction, the samples were centrifuged at 13,000 rpm for 5 min and the supernatants were collected.
3.4. LC-Q-Orbitrap HRMS Analysis
Extracts of S. scardica and S. raeseri were analyzed by the UltiMate 3000 UPLC system (Thermo Scientific Dionex) consisting of a quaternary pump, well plate autosampler, column compartment equipped with Kinetex® column (150 × 4.6 mm, 5 μm, Phenomenex) and PDA detector, coupled with a high-resolution Thermo Q-ExactiveTM Focus quadrupole-Orbitrap mass spectrometer (Thermo, Bremen, Germany). Chromatographic system was controlled with Chromeleon 7.2.8 software (Thermo Fisher Scientific, Waltham, MA, USA). Mobile phases used for elution were as follows: A—water acidified with formic acid (0.1%) and B—acetonitrile acidified with formic acid (0.1%). The flow rate of 0.8 mL/min was used in all separations. The gradient started with 5% B and then increased to 40% B within 18 min, then reached 100% B in 20 min and was kept at this level up to 25 min. The column was conditioned with the initial mobile phase for 7 min period and the system was flushed with injection of MeOH:H2O (1:1, v:v) after each analysis. The injection volume was 2 μL. Ionization of the analytes in negative ion mode was performed with HESI. The flow rate of sheath gas, auxiliary gas and sweep gas was set at 35 arb, 15 arb and 3 arb, respectively. The spray voltage was 2.5 kV, and S-lens RF level was 50. Capillary temperature and heater temperature were 350 °C and 300 °C, respectively. The mass range for the full MS scan was 120–1200 m/z with resolution of 70,000 FWHM, and AGC target at 2 × 105 and max inject time of 100 ms. MS2 parameters were as follows: 17,500 FWHM (resolution), 3 m/z (isolation window, 30 eV (collision energy), 2 × 105 (AGC target) and 100 ms (max inject time). Data processing was done using Compound Discoverer 2.1 software and Freestyle 1.3 software.
3.5. Antioxidant Profiling by Post-Column Derivatization with ABTS
To obtain profiles of antioxidants present in S. scardica and S. raeseri extracts, HPLC-PAD system (1200 series, Agilent Technologies, Wilmington, DE, USA) coupled with Pinnacle PCX Derivatization Instrument (Pickering Laboratories Inc., Mountain View, CA, USA) was used. The detailed method has been described previously by Kusznierewicz et al. [39]. The chromatographic column and conditions of chromatographic separation were the same as in the case of LC-HRMS analysis. However, in this case, the eluate leaving the PAD detector was mixed with methanolic ABTS solution stream (1 mM, 0.1 mL/min) and directed to the reaction loop of derivatization instrument (1 mL, 130 °C). Then, the eluate stream was led further to the UV-Vis detector (Agilent Technologies, Wilmington, DE, USA) where reduction of ABTS radical by extract components was monitored at 734 nm.
3.6. Statistical Analysis
Microsoft Excel and GraphPad Prism 8 software were used for statistical analysis. Two-way analysis of variance (ANOVA) and Šídák’s multiple comparisons test were carried out while comparing the MS peak areas of individual classes of compounds between different extracts. All differences with p < 0.05 were considered statistically significant. Differential analysis of metabolites was performed with Compound Discoverer 2.1 software. Differential metabolites were defined as metabolites with log2fold change ≥ 1. In order to separate differential metabolites from not-significantly differential metabolites, a threshold of −log10(p) < 0.05 was used.
4. Conclusions
The overall aim of this study was to thoroughly investigate the total phytochemical profile of S. scardica and S. raeseri. The UHPLC-HRMS analysis led to the identification of 102 metabolites, which is twice as many as reported so far in the literature. Besides aqueous extracts similar to the traditional Sideritis infusions, also known as mountain tea, herein three other extracts prepared with non-toxic solvents had been studied. The most complex and rich in phytochemicals were the extracts obtained with the binary solvent, i.e., 70% ethanol aqueous solution. Additionally, the antioxidant profiles of aqueous-ethanolic extracts indicated the advantage of binary solvent extraction over single solvent extraction in terms of health-beneficial compound recovery. In addition, three different assisted solvent extraction techniques were used in an attempt to further improve the extraction of Sideritis metabolites. The same extractant for all methods was selected based on previous results. Methods used included well-established techniques in phytochemical recovery such as ultrasound-assisted extraction, microwave-assisted extraction and less commonly used high-pressure extraction. The proposed assisted solvent extraction methods for Sideritis gave promising results as the recovery of the metabolites was three times higher in comparison to conventional solvent extraction. The obtained results indicate the legitimacy of further research that will enable the industrial application of such Sideritis extracts.
Author Contributions
Conceptualization, M.M. and B.K.; methodology, M.M., E.M.-P. and B.K.; investigation, M.M., B.K. and E.M.-P.; writing—original draft preparation, M.M., B.K. and E.M.-P. writing—review and editing, A.B. and B.K.; visualization, B.K. and M.M.; supervision, A.B., B.K. and E.M.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Šavikin, K.; Živković, J.; Janković, T.; Ćujić-Nikolić, N.; Zdunić, G.; Menković, N.; Drinić, Z. Optimization of Ultrasound-Assisted Extraction of Phenolics from Sideritis raeseri Using Response Surface Methodology. Molecules 2021, 26, 3949. [Google Scholar] [CrossRef] [PubMed]
- Krgović, N.; Jovanović, M.; Aradski, A.A.; Jankovi´c, T.; Stević, T.; Zdunić, G.; Laušević, S.D.; Šavikin, K. Bioassay-Guided Skin-Beneficial Effects of Fractionated Sideritis raeseri subsp. raeseri Extract. Plants 2022, 11, 2677. [Google Scholar] [CrossRef] [PubMed]
- Bardakci, H.; Cevik, D.; Barak, T.H.; Gozet, T.; Kan, Y.; Kirmizibekmez, H. Secondary metabolites, phytochemical characterization and antioxidant activities of different extracts of Sideritis congesta P.H. Davis et Hub.-Mor. Biochem. Syst. Ecol. 2020, 92, 104120. [Google Scholar] [CrossRef]
- Zengin, G.; Uğurlu, A.; Baloglu, M.C.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Ceylan, R.; Aktumsek, A.; Picot-Allain, C.M.N.; Mahomoodally, M.F. Chemical fingerprints, antioxidant, enzyme inhibitory, and cell assays of three extracts obtained from Sideritis ozturkii Aytaç & Aksoy: An endemic plant from Turkey. J. Pharm. Biomed. Anal. 2019, 171, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, I. Sideritis scardica Griseb. (Mursalski Tea.; Pirinski Tea) from Bulgaria, which Is Growing in Zones with High Percent of Long Living People. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 141–153. [Google Scholar]
- Moussavi, N.; Azizullah, H.; Malterud, K.E.; Inngjerdingen, K.T.; Wangensteen, H. Immunomodulating polyphenols from Sideritis scardica. J. Funct. Foods 2022, 96, 105197. [Google Scholar] [CrossRef]
- Todorova, M.; Trendafilova, A. Sideritis scardica Griseb., an endemic species of Balkan peninsula: Traditional uses, cultivation, chemical composition, biological activity. J. Ethnopharmacol. 2014, 152, 256–265. [Google Scholar] [CrossRef]
- Krasniqi, B.; Thaçi, S.; Dërmaku-Sopjani, M.; Rifati-Nixha, A.; Abazi, S.; Sopjani, M. Insight into the Mechanisms Underlying the Tracheorelaxant Properties of the Sideritis raeseri Extract. Evid. Based Complementary Altern. Med. 2020, 2020, 6510708. [Google Scholar] [CrossRef]
- Patelou, E.; Chatzopoulou, P.; Polidoros, A.N.; Mylona, P.V. Genetic diversity and structure of Sideritis raeseri Boiss. & Heldr. (Lamiaceae) wild populations from Balkan Peninsula. J. Appl. Res. Med. Aromat. Plants 2020, 16, 100241. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Kulbat-Warycha, K.; Oracz, J.; Żyżelewicz, K. Polyphenols and Other Bioactive Compounds of Sideritis Plants and Their Potential Biological Activity. Molecules 2020, 25, 3763. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, Antioxidative action, bioavailability and Anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Shouqin, Z.; Junjie, Z.; Changzhen, W. Novel high pressure extraction technology. Int. J. Pharm. 2004, 278, 471–474. [Google Scholar] [CrossRef]
- Sklirou, A.D.; Angelopoulou, M.T.; Argyropoulou, A.; Chaita, E.; Boka, V.I.; Cheimonidi, C.; Niforou, K.; Mavrogonatou, E.; Pratsinis, H.; Kalpoutzakis, E.; et al. Phytochemical Study and In Vitro Screening Focusing on the Anti-Aging Features of Various Plants of the Greek Flora. Antioxidants 2021, 10, 1206. [Google Scholar] [CrossRef]
- Vasilopoulou, C.G.; Kontogianni, V.G.; Linardaki, Z.I.; Iatrou, G.; Lamari, F.N.; Nerantzaki, A.A.; Gerothanassis, I.P.; Tzakos, A.G.; Margarity, M. Phytochemical composition of “mountain tea” from Sideritis clandestina subsp. clandestina and evaluation of its behavioral and oxidant/antioxidant effects on adult mice. Eur. J. Nutr. 2013, 52, 107–116. [Google Scholar] [CrossRef]
- Dimaki, V.D.; Zeliou, K.; Nakka, F.; Stavreli, M.; Bakratsas, I.; Papaioannou, L.; Iatrou, G.; Lamari, F.N. Characterization of Sideritis clandestina subsp. peloponnesiaca Polar Glycosides and Phytochemical Comparison to Other Mountain Tea Populations. Molecules 2022, 27, 7613. [Google Scholar] [CrossRef]
- Tomou, E.-M.; Papaemmanouil, C.D.; Diamantis, D.A.; Kostagianni, A.D.; Chatzopoulou, P.; Mavromoustakos, T.; Tzakos, A.G.; Skaltsa, H. Anti-Ageing Potential of S. Euboea Heldr. Phenolics. Molecules 2021, 26, 3151. [Google Scholar] [CrossRef]
- Alipieva, K.; Petreska, J.; Gil-Izquierdo, Á.; Stefova, M.; Evstatieva, L.; Bankova, V. Influence of the Extraction Method on the Yield of Flavonoids and Phenolics from Sideritis spp. (Pirin Mountain tea). Nat. Prod. Commun. 2010, 5, 51–54. [Google Scholar] [CrossRef]
- Lakka, A.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Evaluation of Pulsed Electric Field Polyphenol Extraction from Vitis vinifera, Sideritis scardica and Crocus sativus. ChemEngineering 2021, 5, 25. [Google Scholar] [CrossRef]
- Samanidou, V.; Tsagiannidis, A.; Sarakatsianos, I. Simultaneous determination of polyphenols and major purine alkaloids in Greek Sideritis species, herbal extracts, green tea, black tea, and coffee by high-performance liquid chromatography-diode array detection. J. Sep. Sci. 2012, 35, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Dina, E.; Vontzalidou, A.; Cheilari, A.; Bagatzounis, P.; Agapidou, E.; Giannenas, I.; Grigoriadou, K.; Aligiannis, N. Sustainable Use of Greek Herbs By-Products, as an Alternative Source of Biologically Active Ingredients for Innovative Products. Front. Nutr. 2022, 9, 867666. [Google Scholar] [CrossRef] [PubMed]
- Pljevljakušić, D.; Šavikin, K.; Janković, T.; Zdunić, G.; Ristić, M.; Godjevac, D.; Konić-Ristić, A. Chemical properties of the cultivated Sideritis raeseri Boiss. & Heldr. subsp. raeseri. Food Chem. 2011, 124, 226–233. [Google Scholar] [CrossRef]
- Axiotis, E.; Petrakis, E.A.; Halabalaki, M.; Mitakou, S. Phytochemical Profile and Biological Activity of Endemic Sideritis sipylea Boiss. in North Aegean Greek Islands. Molecules 2020, 25, 2022. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Sinan, K.I.; Etienne, O.K.; Zengin, G.; Gevrenova, R.; Mahomoodally, M.F.; Lobine, D.; Mollica, A. Chemical composition and biological properties of Synedrella nodiflora (L.) Gaertn: A comparative investigation of different extraction methods. Process. Biochem. 2020, 96, 202–212. [Google Scholar] [CrossRef]
- Kassi, E.; Paliogianni, A.; Dontas, I.; Aligiannis, N.; Halabalaki, M.; Papoutsi, Z.; Skaltsounis, A.-L.; Moutsatsou, P. Effects of Sideritis euboea (Lamiaceae) Aqueous Extract on IL-6, OPG and RANKL Secretion by Osteoblasts. Nat. Prod. Commun. 2011, 6, 1689–1696. [Google Scholar] [CrossRef]
- Petreska, J.; Stefkov, G.; Kulevanova, S.; Alipieva, K.; Bankova, V.; Stefova, M. Phenolic Compounds of Mountain Tea from the Balkans: LC/DAD/ESI/MSn Profile and Content. Nat. Prod. Commun. 2011, 6, 21–30. [Google Scholar] [CrossRef]
- Petreska Stanoeva, J.; Stefova, M. Evaluation of the ion trap MS performance for quantification of flavonoids and comparison to UV detection. J. Mass Spectrom. 2012, 47, 1395–1406. [Google Scholar] [CrossRef]
- Abdel-Hady, H.; El-Sayed, M.M.; Abdel-Hady, A.A.; Hashash, M.M.; Abdel-Hady, A.M.; Aboushousha, T.; Abdel-Hameed, E.S.; Abdel-Lateef, E.E.; Morsi, E.A. Nephroprotective Activity of Methanolic Extract of Lantana camara and Squash (Cucurbita pepo) on Cisplatin-Induced Nephrotoxicity in Rats and Identification of Certain Chemical Constituents of Lantana camara by HPLC-ESI- MS. Pharmacogn J. 2018, 10, 136–147. [Google Scholar] [CrossRef]
- Rios, J.L.; Mañez, S.; Paya, M.; Alcaraz, M.J. Antioxidant activity of flavonoids from Sideritis javalambrensis. Phytochemistry 1992, 31, 1947–1950. [Google Scholar] [CrossRef] [PubMed]
- Pilipczuk, T.; Kusznierewicz, B.; Zielińska, D.; Bartoszek, A. The influence of roasting and additional processing on the content of bioactive components in special purpose coffees. J. Food Sci. Technol. 2015, 52, 5736–5744. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M.; Marino, T.; Prejanò, M.; Russo, N. Primary and secondary antioxidant properties of scutellarin and scutellarein in water and lipid-like environments: A theoretical investigation, J. Mol. Liq. 2022, 366, 120343. [Google Scholar] [CrossRef]
- Irakli, M.; Tsifodimou, K.; Sarrou, E.; Chatzopoulou, P. Optimization infusions conditions for improving phenolic content and antioxidant activity in Sideritis scardica tea using response surface methodology. J. Appl. Res. Med. Aromat. Plants. 2018, 8, 67–74. [Google Scholar] [CrossRef]
- Akbaba, E. Characterization of Bioactive and Antioxidant Composition of Mountain Tea (Sideritis montana ssp. montana): Microwave-Assisted Technology. Int. J. Second. Metab. 2021, 8, 159–171. [Google Scholar] [CrossRef]
- Khan, S.A.; Aslam, R.; Makroo, H.A. High pressure extraction and its application in the extraction of bio-active compounds: A review. J. Food Process. Eng. 2019, 42, 54–62. [Google Scholar] [CrossRef]
- Huang, H.-W.; Hsu, C.-P.; Yang, B.B.; Wang, C.-Y. Advances in the extraction of natural ingredients by high pressure extraction technology. Trends Food Sci. Technol. 2013, 33, 54–62. [Google Scholar] [CrossRef]
- Malinowska-Pańczyk, E.; Walecka, M.; Pawłowicz, R.; Tylingo, R.; Kołodziejska, I. The effect of high pressure at subzero temperature on proteins solubility, drip loss and texture of fish (cod and salmon) and mammal’s (pork and beef) meat. Food Sci. Technol. Int. 2014, 20, 383–395. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Mróz, M.; Koss-Mikołajczyk, I.; Namieśnik, J. Comparative evaluation of different methods for determining phytochemicals and antioxidant activity in products containing betalains—Verification of beetroot samples. Food Chem. 2021, 362, 130132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).