Removal of p-Nitrophenol by Adsorption with 2-Phenylimidazole-Modified ZIF-8
Abstract
1. Introduction
2. Results and Discussion
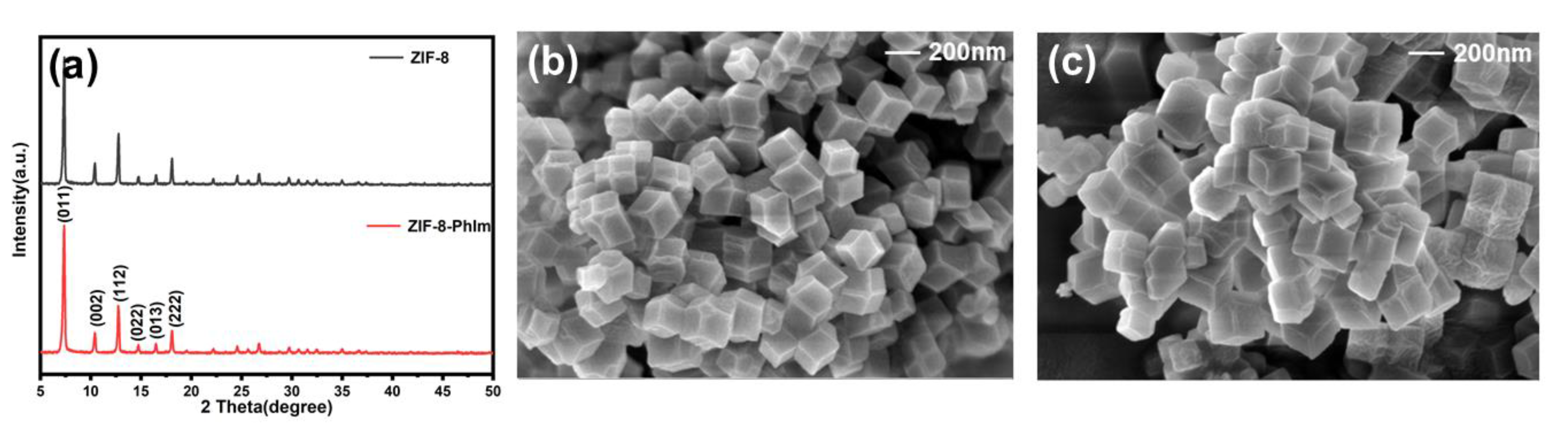
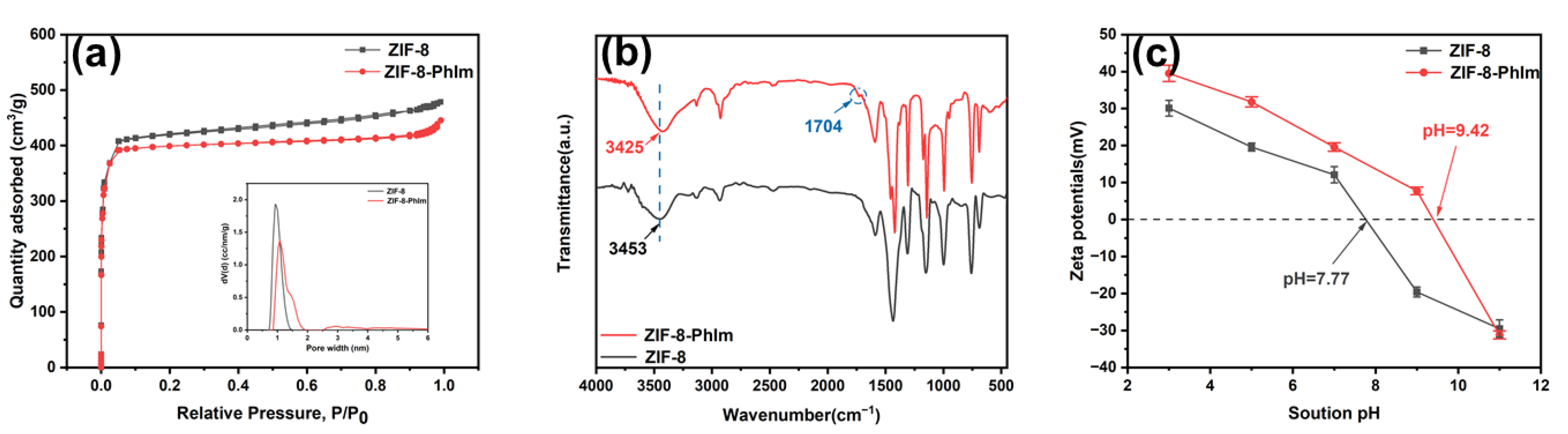
2.1. Characterisation of ZIF-8-PhIm and ZIF-8
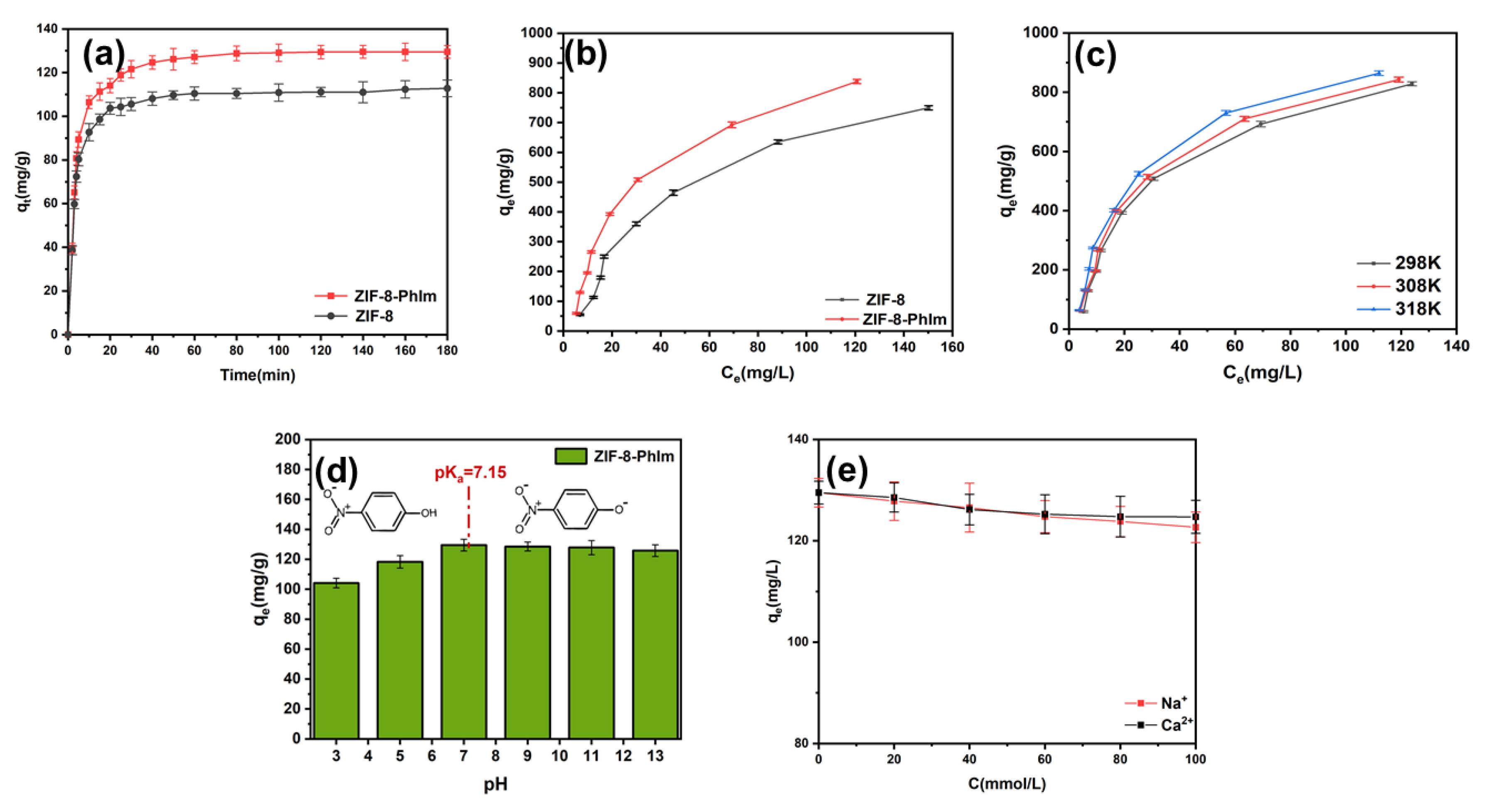
2.2. p-Nitrophenol Adsorption by ZIF-8-PhIm
2.2.1. Effect of Time
2.2.2. Effect of Initial Concentration
2.2.3. Effect of Temperature
2.2.4. Effect of pH
2.2.5. Effect of Ion Concentration
| Material | Optimised Adsorption Conditions | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|
| HKUST-1 | C0 200 mg/L; T 293 K | 371 | [16] |
| MOF-AgTz-1 | C0 50 mg/L; T 298 K | 143.5 | [17] |
| NH2-MIL-53 | C0 800 mg/L; T 298 K | 297.85 | [18] |
| Platanus leaves | C0 300 mg/L; T 298 K | 622.73 | [32] |
| AC-NH2-MIL-101(Cr) | C0 200 mg/L; T 298 K | 182.3 | [33] |
| PS-CH2-[C2NH2MIm][Br] | C0 10,000 mg/L; T 298 K | 1269.8 | [34] |
| ZnAl-layered double hydroxides | C0 120 mg/L; T 298 K | 101.6 | [35] |
| MgCo-3D hydrotalcite nanospheres | C0 300 mg/L; T 298 K | 625.2 | [36] |
| ZIF-8-PhIm | C0 400 mg/L; T 298 K | 828.29 ± 6.95 | this work |
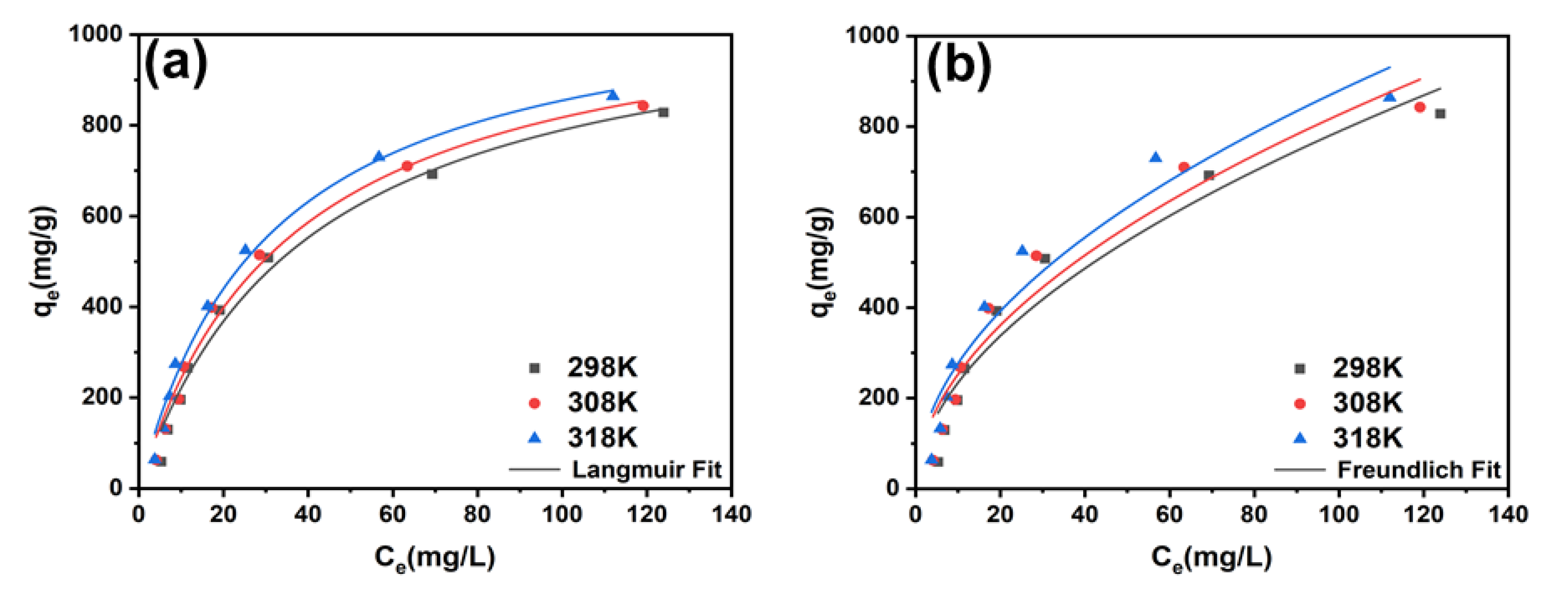
2.3. Isotherms and Thermodynamics
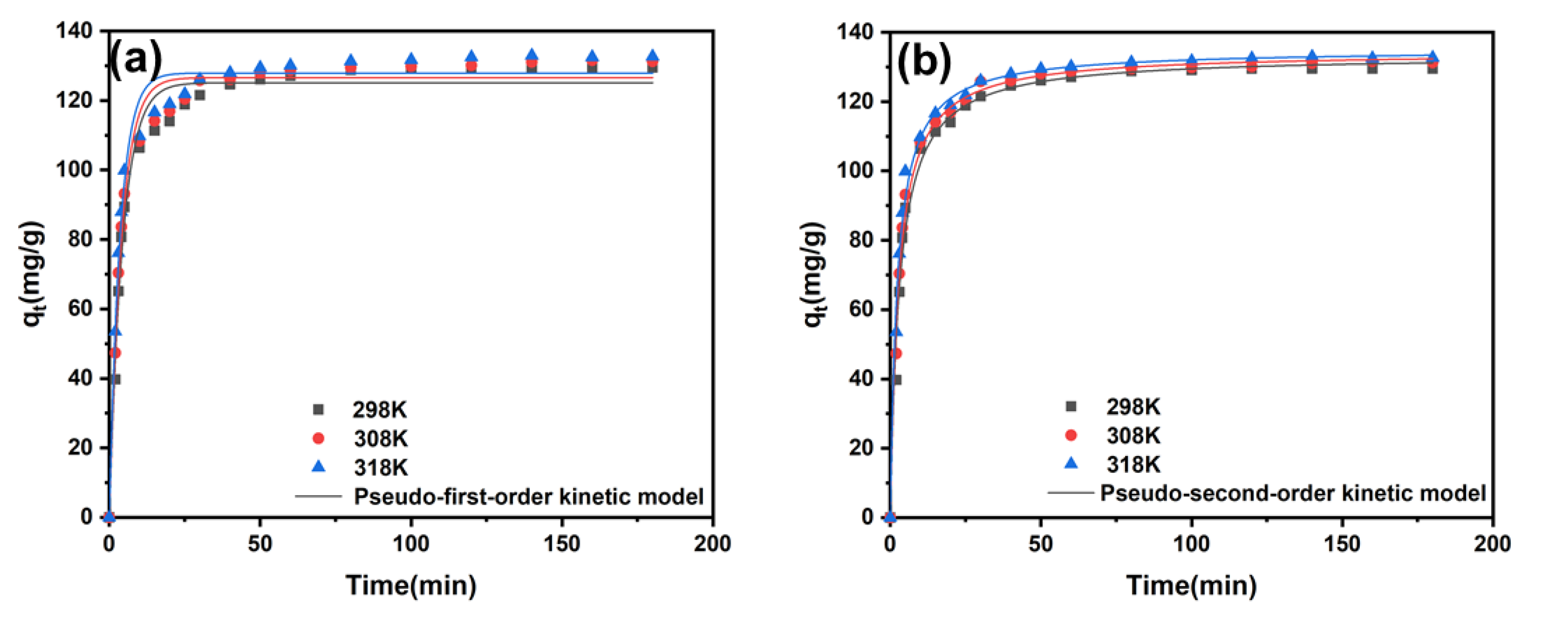
2.4. Adsorption Kinetics
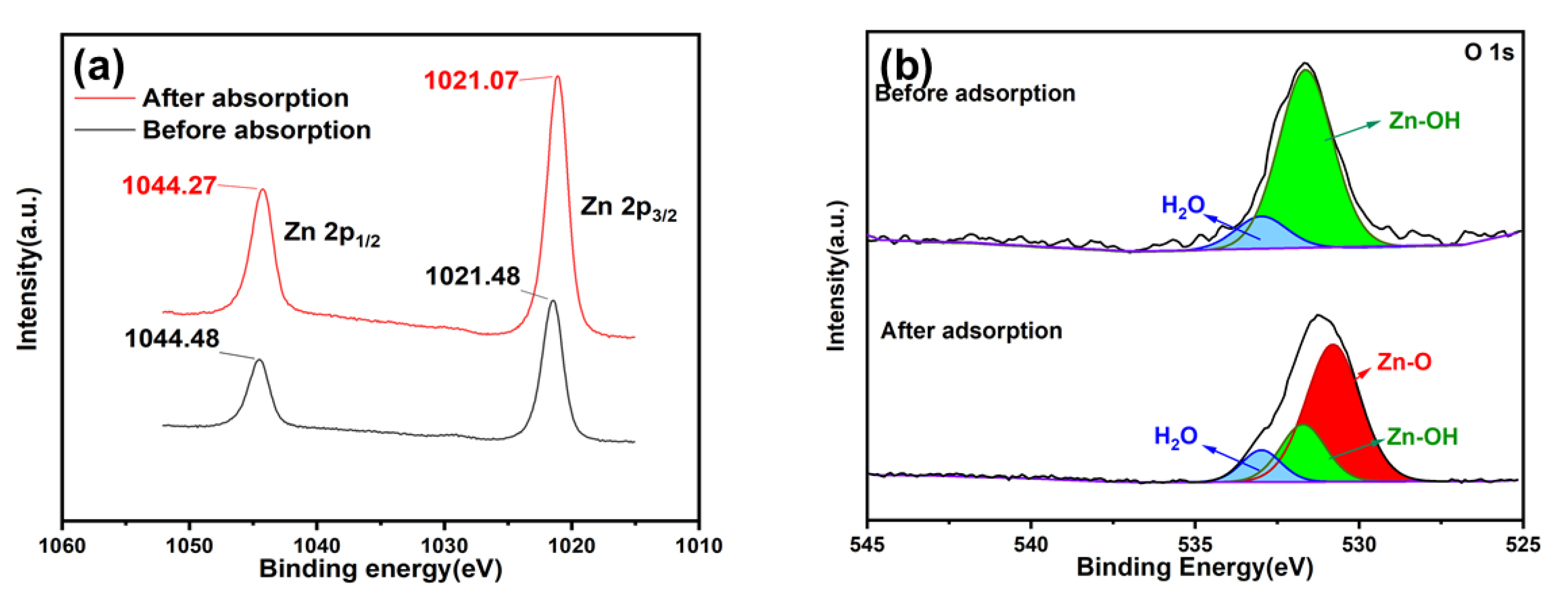
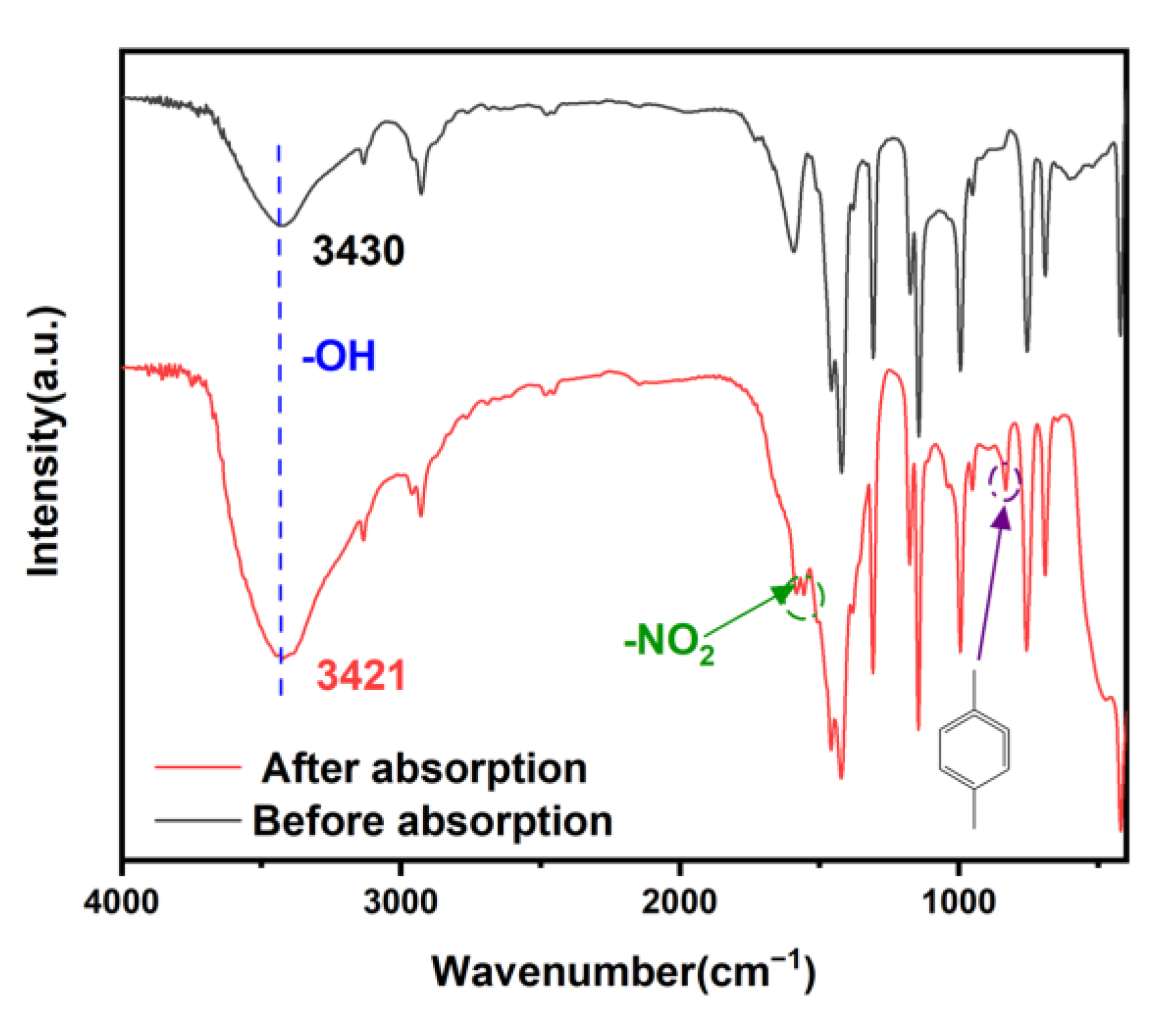
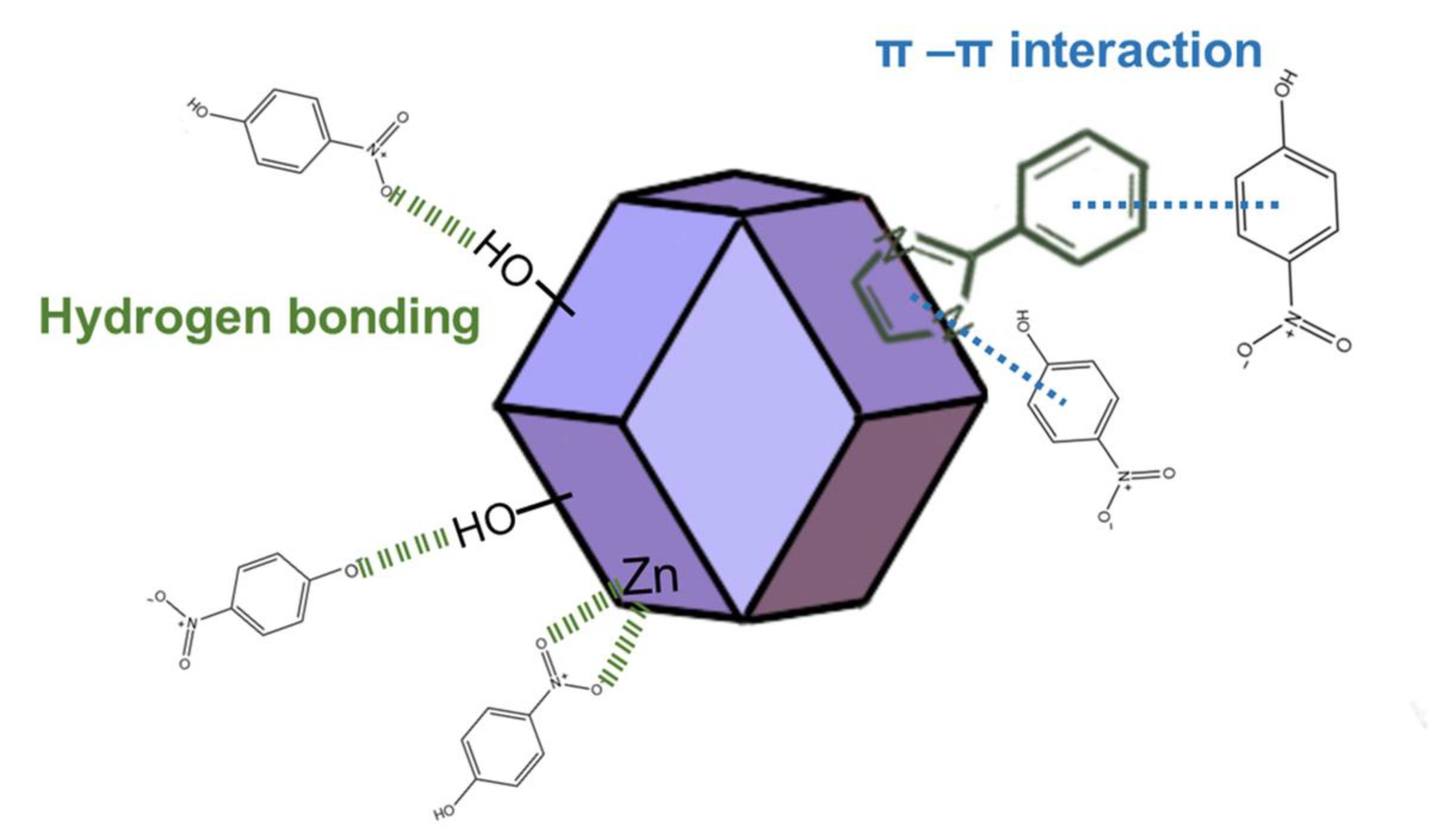
2.5. Adsorption Mechanism
2.6. Regeneration Experiment
3. Materials and Methods
3.1. Chemicals
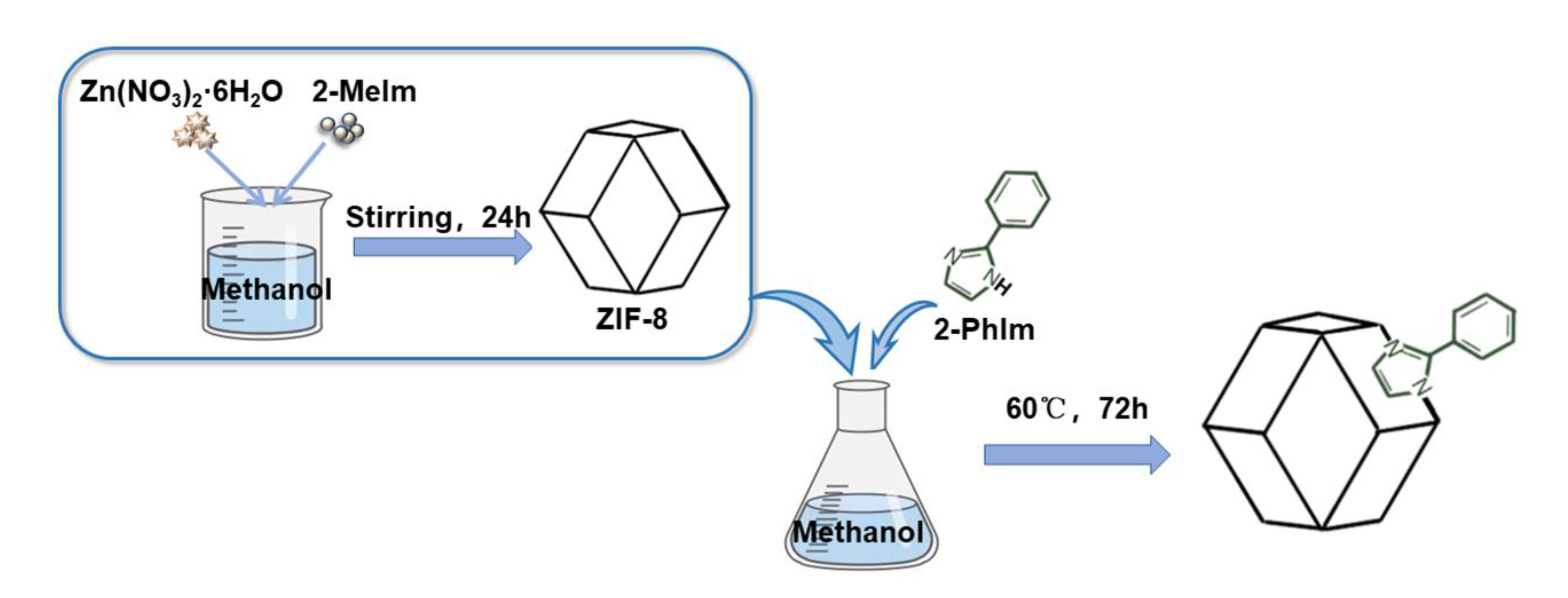
3.2. Synthesis of Adsorbent
3.3. Characterisation
3.4. Static Adsorption
3.5. Regeneration Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhu, S.Y.; Niu, W.X.; Li, H.J.; Han, S.; Xu, G.B. Single-walled carbon nanohorn as new solid-phase extraction adsorbent for determination of 4-nitrophenol in water sample. Talanta 2009, 79, 1441–1445. [Google Scholar] [CrossRef] [PubMed]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Tchieno, F.M.M.; Tonle, I.K. P-Nitrophenol determination and remediation: An overview. Rev. Anal. Chem. 2018, 37, 1–26. [Google Scholar] [CrossRef]
- Lazo-Cannata, J.C.; Nieto-Márquez, A.; Jacoby, A.; Paredes-Doig, A.L.; Romero, A.; Sun-Kou, M.R.; Valverde, J.L. Adsorption of phenol and nitrophenols by carbon nanospheres: Effect of pH and ionic strength. Sep. Purif. Technol. 2011, 80, 217–224. [Google Scholar] [CrossRef]
- Extremera, R.; Pavlovic, I.; Pérez, M.R.; Barriga, C. Removal of acid orange 10 by calcined Mg/Al layered double hydroxides from water and recovery of the adsorbed dye. Chem. Eng. J. 2012, 213, 392–400. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Zhou, J.B.; Cai, W.Q.; Zhao, R.S.; Yuan, J.P. Hierarchically porous NiAl-LDH nanoparticles as highly efficient adsorbent for p-nitrophenol from water. Appl. Surf. Sci. 2015, 349, 897–903. [Google Scholar] [CrossRef]
- Ania, C.O.; Cabal, B.; Pevida, C.; Arenillas, A.; Parra, J.B.; Rubiera, F. Removal of naphthalene from aqueous solution on chemically modified activated carbons. Water Res. 2007, 41, 333–340. [Google Scholar] [CrossRef]
- Kumar, N.; Reddy, L.; Parashar, V.; Ngila, J.C. Controlled synthesis of microsheets of ZnAl layered double hydroxides hexagonal nanoplates for efficient removal of Cr(VI) ions and anionic dye from water. J. Environ. Chem. Eng. 2017, 5, 1718–1731. [Google Scholar] [CrossRef]
- Ahmadi, S.A.R.; Kalaee, M.R.; Moradi, O.; Nosratinia, F.; Abdouss, M. Synthesis of novel zeolitic imidazolate framework (ZIF-67)-zinc oxide (ZnO) nanocomposite (ZnO@ ZIF-67) and potential adsorption of pharmaceutical (tetracycline (TCC)) from water. J. Mol. Struct. 2022, 1251, 132013. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Wang, L.; Zhu, L.C.; Gao, F.; Xu, X.R.; Yang, J.Y. Removal of p-nitrophenol from simulated sewage using steel slag: Capability and mechanism. Environ. Res. 2022, 212, 113450. [Google Scholar] [CrossRef]
- Santos, A.D.; Viante, M.F.; Pochapski, D.J.; Downs, A.J.; Almeida, C.A.P. Enhanced removal of p-nitrophenol from aqueous media by montmorillonite clay modified with a cationic surfactant. J. Hazard. Mater. 2018, 355, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Torrellas, S.; Martin-Martinez, M.; Gomes, H.T.; Ovejero, G.; García, J. Enhancement of p-nitrophenol adsorption capacity through N2-thermal-based treatment of activated carbons. Appl. Surf. Sci. 2017, 414, 424–434. [Google Scholar] [CrossRef]
- Lgaz, H.; Lee, H.S. Computational investigation on interaction mechanism of sulfur mustard adsorption by zeolitic imidazolate frameworks ZIF-8 and ZIF-67: Insights from periodic and cluster DFT calculations. J. Mol. Liq. 2021, 344, 117705. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. Exploring the coordination chemistry of MOF-graphite oxide composites and their applications as adsorbents. Dalton Trans. 2012, 41, 4027–4035. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.J.; Xia, Q.B.; Zhao, Z.X.; Li, Y.W.; Li, Z. Synthesis and adsorption performance of MIL-101(Cr)/graphite oxide composites with high capacities of n-hexane. Chem. Eng. J. 2014, 239, 226–232. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Hsieh, Y.T. Copper-based metal organic framework (MOF), HKUST-1, as an efficient adsorbent to remove p-nitrophenol from water. J. Taiwan Inst. Chem. Eng. 2015, 50, 223–228. [Google Scholar] [CrossRef]
- Miao, H.X.; Song, S.Y.; Chen, H.; Zhang, W.H.; Han, R.P.; Yang, G. Adsorption study of p-nitrophenol on a silver (I) triazolate MOF. J. Porous Mater. 2020, 27, 1409–1417. [Google Scholar] [CrossRef]
- Jia, Z.Q.; Jiang, M.C.; Wu, G.R. Amino-MIL-53(Al) sandwich-structure membranes for adsorption of p-nitrophenol from aqueous solutions. Chem. Eng. J. 2017, 307, 283–290. [Google Scholar] [CrossRef]
- Stephenson, C.J.; Hupp, J.T.; Farha, O.K. Postassembly transformation of a catalytically active composite material, Pt@ ZIF-8, via solvent-assisted linker exchange. Inorg. Chem. 2016, 55, 1361–1363. [Google Scholar] [CrossRef]
- Pullen, S.; Fei, H.; Orthaber, A.; Cohen, S.M.; Ott, S. Enhanced photochemical hydrogen production by a molecular diiron catalyst incorporated into a metal-organic framework. J. Am. Chem. Soc. 2013, 135, 16997–17003. [Google Scholar] [CrossRef]
- Bury, W.; Fairen-Jimenez, D.; Lalonde, M.B.; Snurr, R.Q.; Farha, O.K.; Hupp, J.T. Control over catenation in pillared paddlewheel metal–organic framework materials via solvent-assisted linker exchange. Chem. Mater. 2013, 25, 739–744. [Google Scholar] [CrossRef]
- Kenyotha, K.; Chanapattharapol, K.; Mccloskey, S.; Jantaharn, P. Water based synthesis of ZIF-8 assisted by hydrogen bond acceptors and enhancement of CO2 uptake by solvent assisted ligand exchange. Crystals 2020, 10, 599. [Google Scholar] [CrossRef]
- Li, M.H.; Liu, Y.B.; Li, F.; Shen, C.S.; Kaneti, Y.V.; Yamauchi, Y.; Yuliarto, B.; Chen, B.; Wang, C.C. Defect-Rich hierarchical porous UiO-66(Zr) for tunable phosphate removal. Environ. Sci. Technol. 2021, 55, 13209–13218. [Google Scholar] [CrossRef]
- Chen, Z.C.; Zhang, H.W.; Fan, G.Y.; He, X.Y.; He, Z.B.; Zhang, L.H. Diatomite composited with a zeolitic imidazolate framework for removing phosphate from water. ACS Omega 2022, 7, 26154–26164. [Google Scholar] [CrossRef]
- Yu, D.B.; Shao, Q.; Song, Q.J.; Cui, J.W.; Zhang, Y.L.; Wu, B.; Ge, L.; Wang, Y.; Zhang, Y.; Qin, Y.Q.; et al. A solvent-assisted ligand exchange approach enables metal-organic frameworks with diverse and complex architectures. Nat. Commun. 2020, 11, 927. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Niemantsverdriet, J.W.; Langner, E.H.G. Enhanced CO2 adsorption in nano-ZIF-8 modified by solvent assisted ligand exchange. Microporous Mesoporous Mater. 2018, 262, 98–105. [Google Scholar] [CrossRef]
- Amrhar, O.; Gana, L.E.; Mobarak, M. Calculation of adsorption isotherms by statistical physics models: A review. Environ. Chem. Lett. 2021, 19, 4519–4547. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Moshtkob, A.; Shahabadi, N.; Masoomi, M.Y.; Morsali, A. Synthesis of nano zinc-based metal–organic frameworks under ultrasound irradiation in comparison with solvent-assisted linker exchange: Increased storage of N2 and CO2. Ultrason. Sonochem. 2019, 59, 104729. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.N.; Zhang, X.F.; Liu, H.T.; Han, J.Y.; Yang, Z.J.; Gu, L.; Tang, Z.Y. Metalation of catechol-functionalized defective covalent organic frameworks for lewis acid catalysis. Small 2020, 16, 2001998. [Google Scholar] [CrossRef] [PubMed]
- Massahud, E.; Ahmed, H.; Babarao, R.; Ehrnst, Y.; Alijani, H.; Darmanin, C.; Murdoch, B.J.; Rezk, A.; Yeo, L.Y. Acoustomicrofluidic defect engineering and ligand exchange in ZIF-8 metal–organic frameworks. Small Methods 2023, 2201170. [Google Scholar] [CrossRef]
- Gao, S.; Hou, J.W.; Deng, Z.Y.; Wang, T.S.; Beyer, S.; Buzanich, A.G.; Richardson, J.J.; Rawal, A.; Seidel, R.; Zulkifli, M.Y.; et al. Improving the acidic stability of zeolitic imidazolate frameworks by biofunctional molecules. Chem 2019, 5, 1597–1608. [Google Scholar] [CrossRef]
- Ma, H.F.; Xu, Z.G.; Wang, W.Y.; Gao, X.; Ma, H.F. Adsorption and regeneration of leaf-based biochar for p-nitrophenol adsorption from aqueous solution. RSC Adv. 2019, 9, 39282–39293. [Google Scholar] [CrossRef] [PubMed]
- Aldawsari, A.M.; Alsohaimi, I.H.; Hassan, H.M.A.; Berber, M.R.; Abdalla, Z.E.A.; Hassan, I.; Saleh, E.A.M.; Hameed, B.H. Activated carbon/MOFs composite: AC/NH2-MIL-101(Cr), synthesis and application in high performance adsorption of p-nitrophenol. J. Saudi Chem. Soc. 2020, 24, 693–703. [Google Scholar] [CrossRef]
- Cheng, M.; Jiang, J.; Wang, J.J.; Fan, J. Highly salt resistant polymer supported ionic liquid adsorbent for ultrahigh capacity removal of p-nitrophenol from water. ACS Sustainable Chem. Eng. 2019, 7, 8195–8205. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Zhou, J.B.; Cheng, Y.; Yu, J.G.; Cai, W.Q. Hydrothermal synthesis of modified hydrophobic Zn-Al-layered double hydroxides using structure-directing agents and their enhanced adsorption capacity for p-nitrophenol. Adsorpt. Sci. Technol. 2014, 32, 351–364. [Google Scholar] [CrossRef]
- Gao, F.; Xu, X.R.; Yang, J.Y. Removal of p-nitrophenol from simulated sewage using MgCo-3D hydrotalcite nanospheres: Capability and mechanism. RSC Adv. 2022, 12, 27044–27054. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Duranoğlu, D.; Trochimczuk, A.W.; Beker, U. Kinetics and thermodynamics of hexavalent chromium adsorption onto activated carbon derived from acrylonitrile-divinylbenzene copolymer. Chem. Eng. J. 2012, 187, 193–202. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Milmile, S.N.; Pande, J.V.; Karmakar, S.; Bansiwal, A.; Chakrabarti, T.; Biniwale, R.B. Equilibrium isotherm and kinetic modeling of the adsorption of nitrates by anion exchange Indion NSSR resin. Desalination 2011, 276, 38–44. [Google Scholar] [CrossRef]
- Wang, J.; Xiang, L. Formation of ZnO rods with varying diameters from ε-Zn (OH)2. J. Cryst. Growth 2014, 401, 279–284. [Google Scholar] [CrossRef]
- Ge, X.; Song, X.Y.; Ma, Y.; Zhou, H.J.; Wang, G.Z.; Zhang, H.M.; Zhang, Y.X.; Zhao, H.J.; Wong, P.K. Fabrication of hierarchical iron-containing MnO2 hollow microspheres assembled by thickness-tunable nanosheets for efficient phosphate removal. J. Mater. Chem. A 2016, 4, 14814–14826. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Li, Z.; Zhang, B.; Zhu, M.; Hu, X.; Zhang, Y.M.; Li, F.T. Zeolitic imidazolate framework-8 with high efficiency in trace arsenate adsorption and removal from water. J. Phys. Chem. C 2014, 118, 27382–27387. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Hu, C.Y.; Zuo, C.; Wang, P.; Chen, W.Q.; Ao, T.Q. Efficient removal of tetracycline by a hierarchically porous ZIF-8 metal organic framework. Environ. Res. 2021, 198, 111254. [Google Scholar] [CrossRef]
- Li, C.H.; Wang, F.L.; Xu, X.; Shi, Y.B.; Liang, J.S.; Yang, R.S.; Liu, J.; Zhao, Z.L. A high-capacity malleable cellulose aerogel with layered double hydroxide decorating ZIF-8 for efficient adsorption of ciprofloxacin. Chem. Eng. J. 2023, 455, 140841. [Google Scholar] [CrossRef]
- Jung, B.K.; Hasan, Z.; Jhung, S.H. Adsorptive removal of 2, 4- dichlorophenoxyacetic acid (2, 4-D) from water with a metal–organic framework. Chem. Eng. J. 2013, 234, 99–105. [Google Scholar] [CrossRef]
- Fraga, T.J.M.; Ghislandi, M.G.; Carvalho, M.N.; Sobrinho, M.A.D.M. One step forward: How can functionalization enhance the adsorptive properties of graphene towards metallic ions and dyes? Environ. Res. 2020, 184, 109362. [Google Scholar] [CrossRef]
- Fraga, T.J.M.; Carvalho, M.N.; Ghislandi, M.G.; Sobrinho, M.A.D.M. Functionalized graphene-based materials as innovative adsorbents of organic pollutants: A concise overview. Braz. J. Chem. Eng. 2019, 36, 1–31. [Google Scholar] [CrossRef]
- Fraga, T.J.M.; Carvalho, M.N.; Fraga, D.M.S.M.; Silva, M.D.C.L.D.; Ferreira, J.M.; Sobrinho, M.A.D.M. Treated residue from aluminium lamination as adsorbent of toxic reactive dyes-a kinetic, equilibrium and thermodynamic study. Environ. Technol. 2020, 41, 669–681. [Google Scholar] [CrossRef]

| Sample | Surface Area (m2/g) | Average Pore Diameter (nm) | Pore Volume (cm3/g) | Micropore Volume (cm3/g) | Mesopore Volume (cm3/g) | Mesopore Volume Percentage (%) |
|---|---|---|---|---|---|---|
| ZIF-8-PhIm | 1663.79 | 2.08 | 0.85 | 0.54 | 0.31 | 36.47 |
| ZIF-8 | 1700.39 | 1.85 | 0.79 | 0.60 | 0.19 | 24.05 |
| Model | 298 K | 308 K | 318 K | |
|---|---|---|---|---|
| Langmuir | qm (mg/g) | 1105.04 | 1108.91 | 1117.72 |
| KL (L/mg) | 0.03 | 0.03 | 0.03 | |
| R2 | 0.981 | 0.985 | 0.987 | |
| Freundlich | 1/n | 0.53 | 0.52 | 0.50 |
| KF ((mg/g)(mg/L)1/n) | 69.54 | 77.37 | 87.26 | |
| R2 | 0.926 | 0.928 | 0.925 | |
| T (K) | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (J/(K∙mol)) |
|---|---|---|---|
| 298.15 | −1.98 | 27.85 | 95.45 |
| 318.15 | −3.89 |
| Model | 298 K | 308 K | 318 K | |
|---|---|---|---|---|
| Pseudo-first-order kinetic model | k1 (min−1) | 0.23 | 0.25 | 0.29 |
| qe1, cal (mg/g) | 125.12 | 126.61 | 127.28 | |
| qe1, exp (mg/g) | 129.51 | 131.27 | 128.27 | |
| R2 | 0.969 | 0.971 | 0.973 | |
| Pseudo-second-order kinetic model | k2 (g/(mg.min)) | 2.4 × 10−3 | 2.7 × 10−3 | 3.3 × 10−3 |
| qe2, cal (mg/g) | 133.41 | 134.32 | 134.35 | |
| qe2, exp (mg/g) | 129.51 | 131.27 | 132.70 | |
| R2 | 0.989 | 0.992 | 0.991 | |
| Species | Before Adsorption | After Adsorption | |||
|---|---|---|---|---|---|
| Binding Energy (eV) | Atom (%) | Binding Energy (eV) | Atom (%) | ||
| O 1s | Zn-O | 530.80 | 530.80 | 65.83 | |
| Zn-OH | 531.71 | 87.08 | 531.71 | 22.94 | |
| H2O | 532.98 | 12.92 | 532.99 | 11.23 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Yuan, P.; Xu, X.; Yang, J. Removal of p-Nitrophenol by Adsorption with 2-Phenylimidazole-Modified ZIF-8. Molecules 2023, 28, 4195. https://doi.org/10.3390/molecules28104195
Zhao Y, Yuan P, Xu X, Yang J. Removal of p-Nitrophenol by Adsorption with 2-Phenylimidazole-Modified ZIF-8. Molecules. 2023; 28(10):4195. https://doi.org/10.3390/molecules28104195
Chicago/Turabian StyleZhao, Yu, Peiqing Yuan, Xinru Xu, and Jingyi Yang. 2023. "Removal of p-Nitrophenol by Adsorption with 2-Phenylimidazole-Modified ZIF-8" Molecules 28, no. 10: 4195. https://doi.org/10.3390/molecules28104195
APA StyleZhao, Y., Yuan, P., Xu, X., & Yang, J. (2023). Removal of p-Nitrophenol by Adsorption with 2-Phenylimidazole-Modified ZIF-8. Molecules, 28(10), 4195. https://doi.org/10.3390/molecules28104195






