Synthesis and Molecular Docking Studies of Alkoxy- and Imidazole-Substituted Xanthones as α-Amylase and α-Glucosidase Inhibitors
Abstract
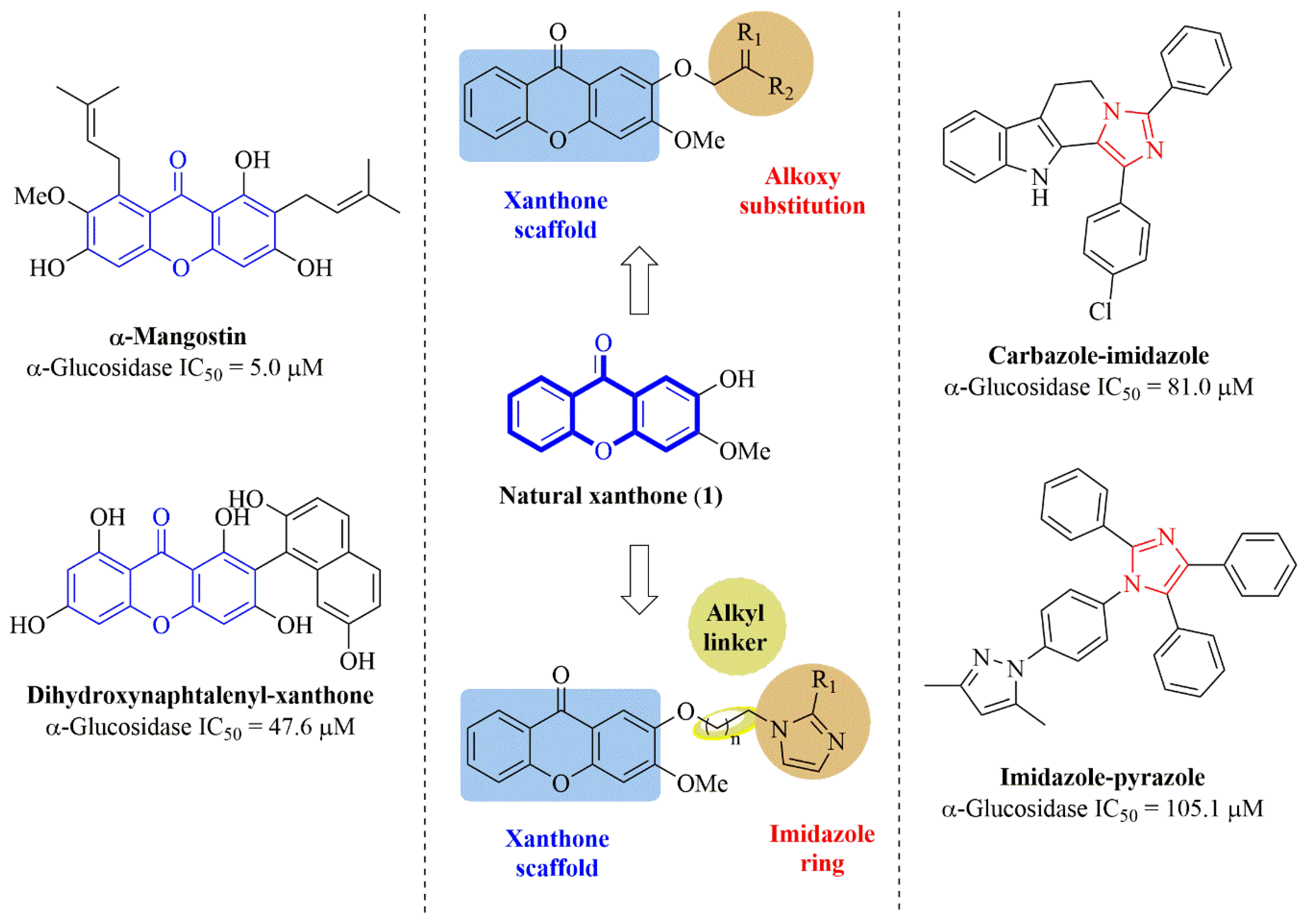
:1. Introduction
2. Results and Discussion
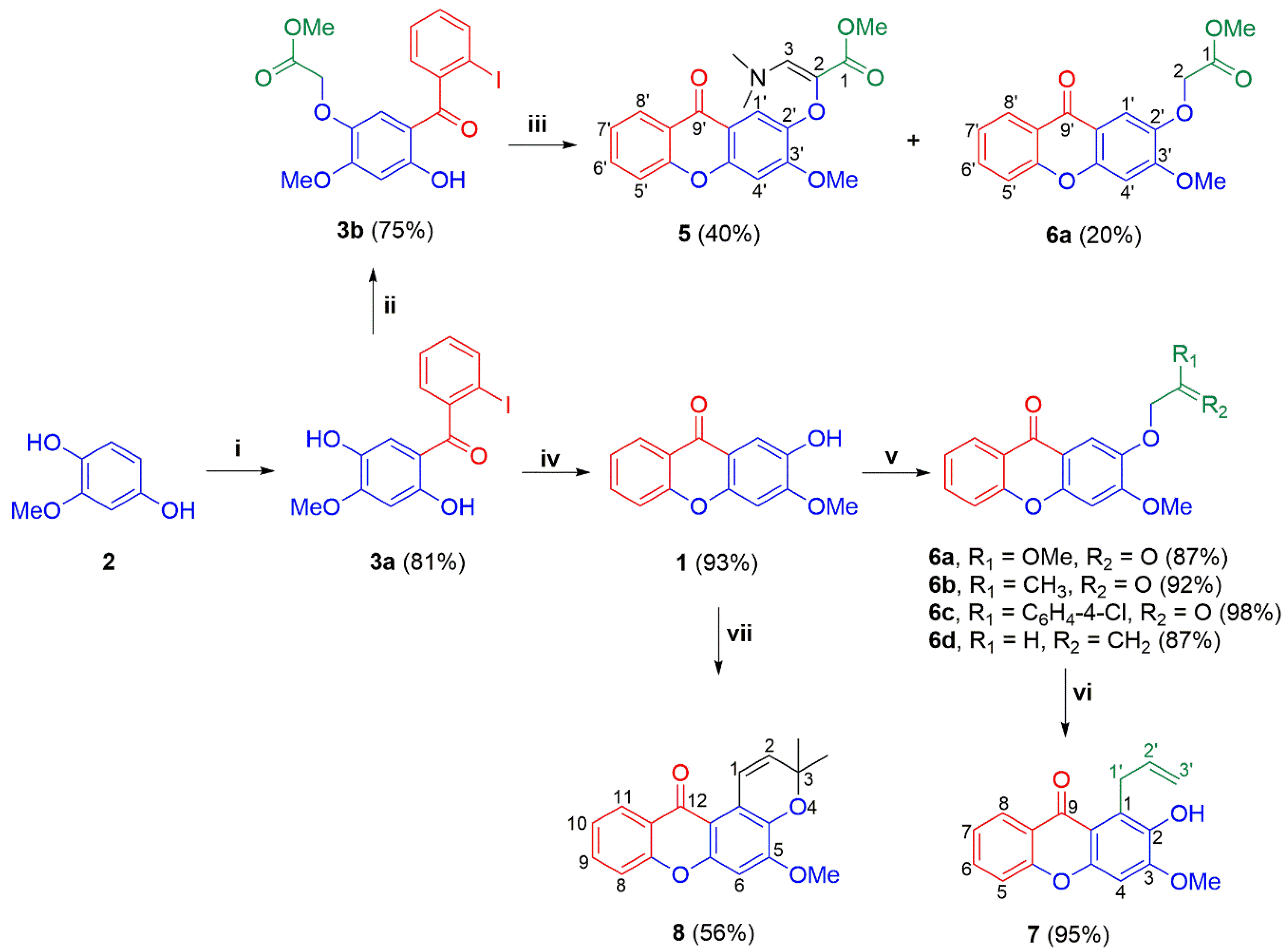
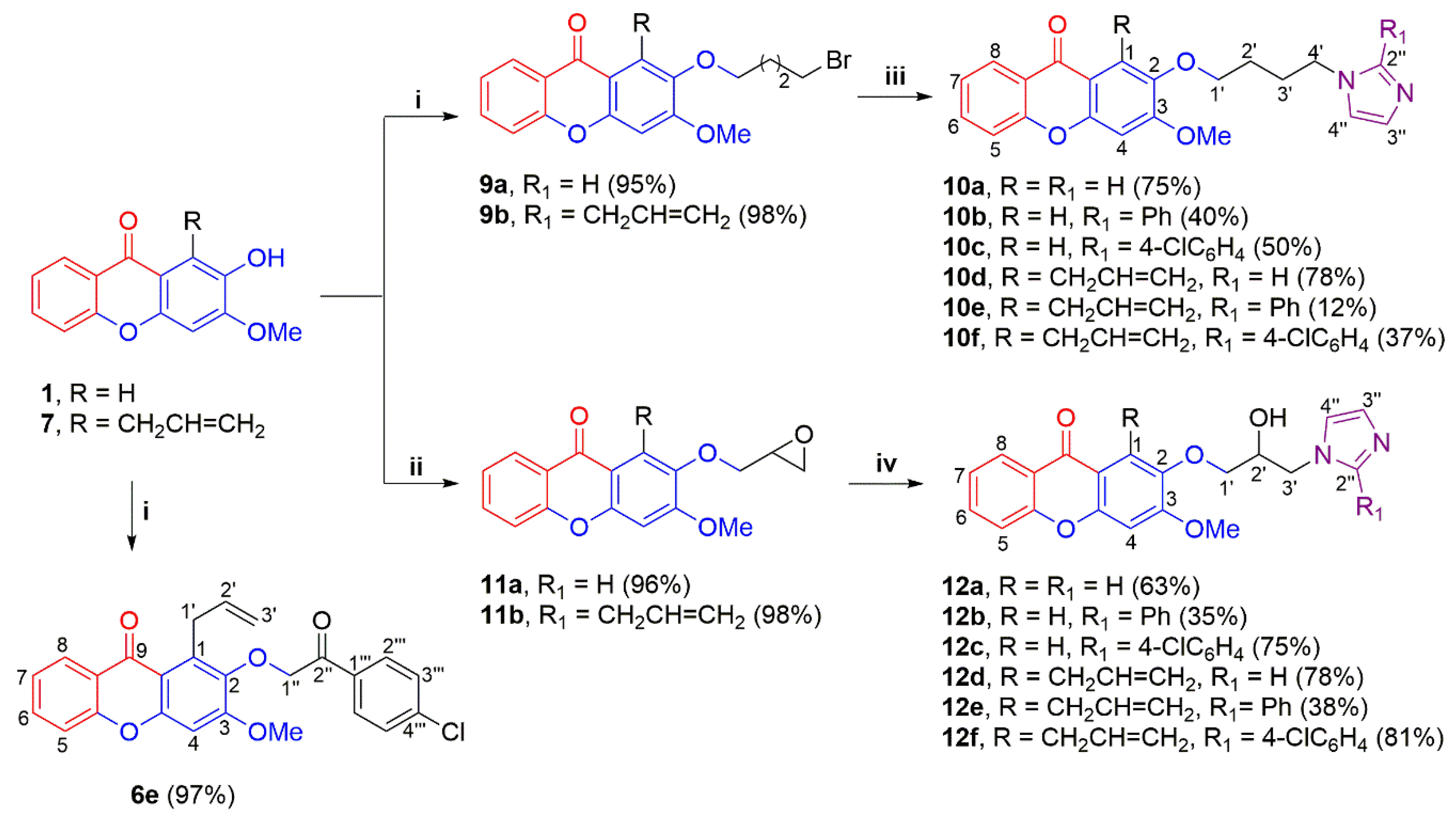
2.1. Chemistry
2.2. In Vitro α-Glucosidase Inhibition
2.3. In Vitro α-Amylase Inhibition
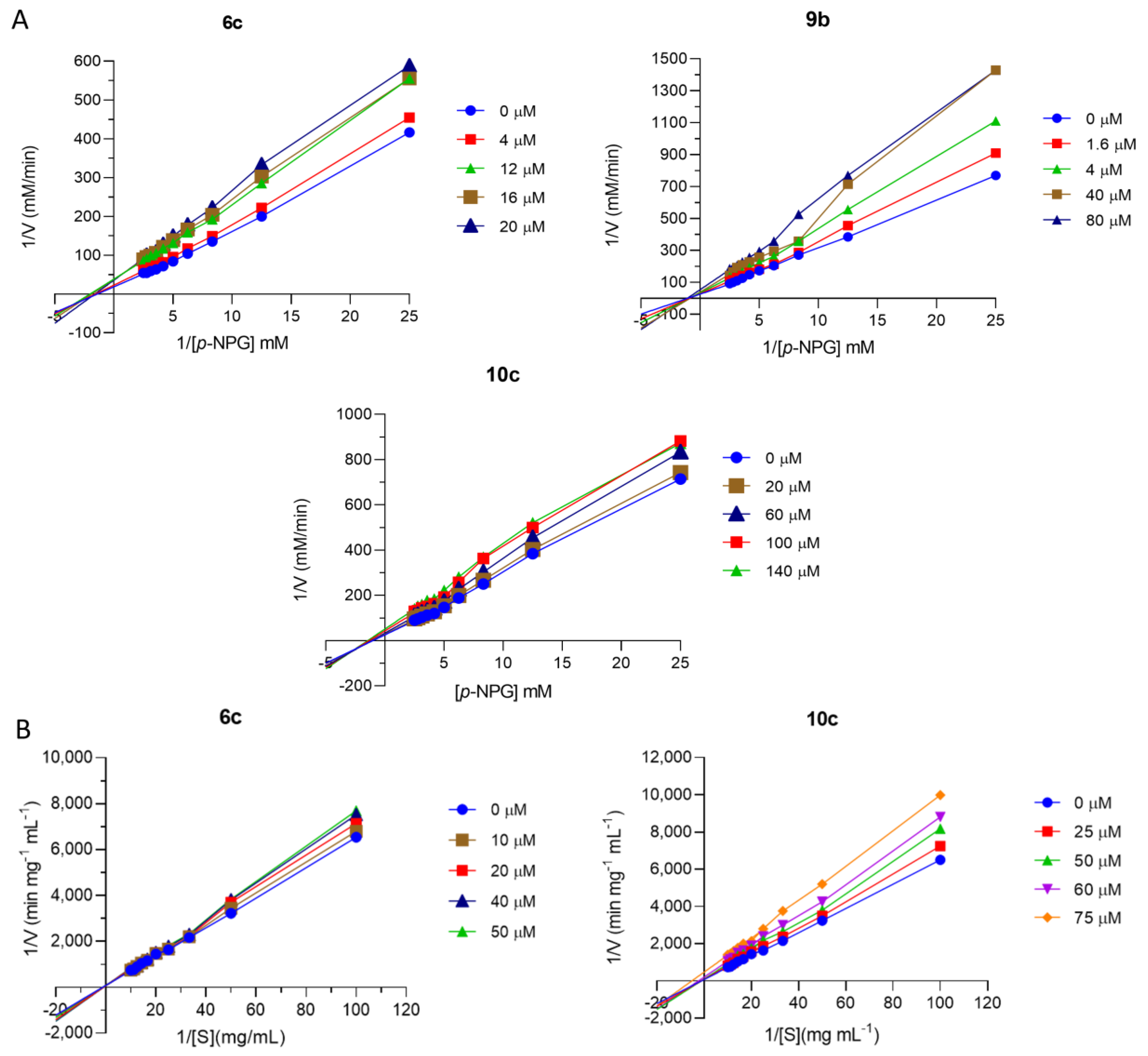
2.4. Enzymatic Kinetic Study
2.5. Evaluation of Antioxidant Activity
2.6. Molecular Docking Analysis
2.7. Prediction of Drug-like Properties
2.8. Prediction of Druglikeness, ADME Properties, and Toxicity
3. Materials and Methods
3.1. General Information
3.2. Chemistry
- General method for preparing 2-alkoxy-substituted xanthones 6a–d
- General method for preparing imidazole-substituted xanthones 10a–f
- General procedure for preparing 2-alkoxy-substituted xanthones 11a–b
- General procedure for preparing imidazole-substituted xanthones 12a–f
3.3. Biological Evaluation
3.3.1. α-Glucosidase Inhibition Assay
3.3.2. α-Amylase Inhibition Assay
3.3.3. Kinetic Study
3.4. DPPH Radical Scavenging Assay
3.5. Docking Studies
3.6. Physicochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. Diabetes Atlas Reports. Available online: https://www.idf.org (accessed on 7 February 2023).
- Kerru, N.; Singh-Pillay, A.; Awolade, P.; Singh, P. Current anti-diabetic agents and their molecular targets: A review. Eur. J. Med. Chem. 2018, 152, 436–486. [Google Scholar] [CrossRef]
- Kalita, D.; Holm, D.G.; LaBarbera, D.V.; Petrash, J.M.; Jayanty, S.S. Inhibition of α-glucosidase, α-amylase, and aldose reductase by potato polyphenolic compounds. PLoS ONE 2018, 13, e0191025. [Google Scholar] [CrossRef]
- Hakamata, W.; Kurihara, M.; Okuda, H.; Nishio, T.; Oku, T. Design and screening strategies for α-glucosidase inhibitors based on enzymological information. Curr. Top. Med. Chem. 2009, 9, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Umpierrez, G.E.; Bailey, T.S.; Carcia, D.; Shaefer, C.; Shubrook, J.H.; Skolnik, N. Improving postprandial hyperglycemia in patients with type 2 diabetes already on basal insulin therapy: Review of current strategies. J. Diabetes 2018, 10, 94–111. [Google Scholar] [CrossRef]
- Upadhyay, J.; Polyzos, S.A.; Perakis, B.T.; Paschou, S.A.; Katsiki, N.; Underwood, P.; Park, K.-H.; Seufert, J.; Kang, E.S.; Sternthal, E.; et al. Pharmacotherapy of type 2 diabetes: An update. Metabolism 2018, 78, 13–42. [Google Scholar] [CrossRef] [PubMed]
- Santoso, M.; Ong, L.L.; Aijijiyah, N.P.; Wati, F.A.; Azminah, A.; Annuur, R.M.; Fadlan, A.; Judeh, Z.M.A. Synthesis, α-glucosidase inhibition, α-amylase inhibition, and molecular docking studies of 3,3-di(indolyl)indolin-2-ones. Heliyon 2022, 8, e09045. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kaur, N.; Sharma, S.; Mohinder, P.; Bedi, S. Recent progress in biologically active xanthones. J. Chem. Pharm. Res. 2016, 8, 75–131. [Google Scholar]
- Zhang, X.; Li, X.; Ye, S.; Zhang, Y.; Tao, L.; Gao, Y.; Gong, D.; Xi, M.; Meng, H.; Zhang, M.; et al. Synthesis, SAR and biological evaluation of natural and non-natural hydroxylated and prenylated xanthones as antitumor agents. Med. Chem. 2012, 8, 1012–1025. [Google Scholar] [CrossRef]
- Gobbi, S.; Hu, Q.; Negri, M.; Zimmer, C.; Belluti, F.; Rampa, A.; Hartmann, R.W.; Bisi, A. Modulation of cytochromes P450 with xanthone-based molecules: From aromatase to aldosterone synthase and steroid 11β-hydroxylase inhibition. J. Med. Chem. 2013, 56, 1723–1729. [Google Scholar] [CrossRef]
- Uvarani, C.; Arumugasamy, K.; Chandraprakash, K.; Sankaran, M.; Ata, A.; Subramaniam, M.P. A new DNA-intercalative cytotoxic allylic xanthone from Swertia corymbose. Chem. Biodivers. 2015, 12, 358–370. [Google Scholar] [CrossRef]
- Dharmaratne, H.R.W.; Sakagami, Y.; Piyasena, K.G.P.; Thevanesam, V. Antibacterial activity of xanthones from Garcinia mangostana (L.) and their structure-activity relationship studies. Nat. Prod. Res. 2013, 27, 938–941. [Google Scholar] [CrossRef]
- Sriyatep, T.; Siridechakorn, I.; Maneerat, W.; Pansanit, A.; Ritthiwigrom, T.; Andersen, J.R.; Laphookhieo, S. Bioactive prenylated xanthones from the young fruits and flowers of Garcinia cowa. J. Nat. Prod. 2015, 78, 265–271. [Google Scholar] [CrossRef]
- Thong, N.M.; Quang, D.T.; Bui, N.H.T.; Dao, D.Q.; Nam, P.C. Antioxidant properties of xanthones extracted from the pericarp of Garcinia mangostana (Mangosteen): A theorical study. Chem. Phys. Lett. 2015, 625, 30–35. [Google Scholar] [CrossRef]
- Fei, X.; Jo, M.; Lee, B.; Han, S.-B.; Lee, K.; Jung, J.-K.; Seo, S.-Y.; Kwak, Y.-S. Synthesis of xanthone derivatives based on α-mangostin and their biological evaluation for anti-cancer agents. Bioorg. Med. Chem. Lett. 2014, 24, 2062–2065. [Google Scholar] [CrossRef]
- Klein-Júnior, L.C.; Campos, A.; Niero, R.; Corrêa, R.; Heyden, Y.V.; Filho, V.C. Xanthones and cancer: From natural sources to mechanism of action. Chem. Biodivers. 2020, 17, e1900499. [Google Scholar] [CrossRef] [PubMed]
- Soda, M.; Endo, S.; Matsunaga, T.; Zhao, H.-T.; El-Kabbani, O.; Linuma, M.; Yamamura, K.; Hara, A. Inhibition of human aldose reductase-like protein (AKR1B10) by α- and γ-mangostins, major components of pericarps of mangosteen. Biol. Pharm. Bull. 2012, 35, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, D.; Li, J.-B.; Zhang, X.; Zhou, N.; Zhang, W.-Y.; Lu, H. Prenylated xanthones with α-glucosidase and α-amylase inhibitory effects from the pericarp of Garcinia mangostana. J. Asian Nat. Prod. Res. 2022, 24, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Shagufta; Ahmad, I. Recent insight into the biological activities of synthetic xanthone derivatives. Eur. J. Med. Chem. 2016, 116, 267–280. [Google Scholar] [CrossRef]
- Tousian, S.H.; Razavi, H.; Hosseinzadeh, B.M. Review of Garcinia mangostana and its xanthones in metabolic syndrome and related complications. Phytother. Res. 2017, 31, 1173–1182. [Google Scholar] [CrossRef]
- Santos, C.M.M.; Freitas, M.; Fernandes, E. A comprehensive review on xanthone derivatives as α-glucosidase inhibitors. Eur. J. Med. Chem. 2018, 157, 1460–1479. [Google Scholar] [CrossRef]
- Zou, H.; Koh, J.-J.; Li, J.; Qiu, S.; Aung, T.T.; Lin, H.; Lakshminarayanan, R.; Dai, X.; Tang, C.; Lim, F.H.; et al. Design and synthesis of amphiphilic xanthone-based, membrane-targeting antimicrobials with improved membrane selectivity. J. Med. Chem. 2013, 56, 2359–2373. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.-J.; Zou, H.; Mukherjee, D.; Lin, S.; Lim, F.; Tan, J.K.; Tan, D.-Z.; Stocker, B.L.; Timmer, M.S.M.; Corkran, H.M.; et al. Amphiphilic xanthones as a potent chemical entity of anti-mycobacterial agents with membrane-targeting properties. Eur. J. Med. Chem. 2016, 123, 684–703. [Google Scholar] [CrossRef]
- Lin, S.; Sin, W.L.W.; Koh, J.-J.; Lim, F.; Wang, L.; Cao, D.; Beuerman, R.W.; Ren, L.; Liu, S. Semisynthesis and biological evaluation of xanthones amphiphilics as selective, highly antifungal agents to combat fungal resistance. J. Med. Chem. 2017, 60, 10135–10150. [Google Scholar] [CrossRef]
- Chi, X.-Q.; Zi, C.-T.; Li, H.-M.; Yang, L.; Lv, Y.-F.; Li, J.-Y.; Hou, B.; Ren, F.-C.; Hu, J.-M.; Zhou, J. Design, synthesis and structure-activity relationships of mangostin analogs as cytotoxic agents. RSC Adv. 2018, 8, 41377–41388. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, S.; Zhang, C.; Wu, D.; Lou, H.-B.; Wu, Y. Docking-assisted 3D-QSAR studies on xanthones as α-glucosidase inhibitors. J. Mol. Model. 2017, 23, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Gunatilaka, L.A.A.; Jasmin De Silva, A.M.Y.; Subramaniam, S. Minor xanthones of Hypericum mysorense. Phytochemistry 1982, 21, 1751–1753. [Google Scholar] [CrossRef]
- Wilairat, R.; Manosroi, J.; Manosroi, A.; Kijjoa, A.; Nascimiento, M.S.J.; Pinto, M.; Silva, A.M.S.; Eaton, G.; Herz, W. Cytotoxicities of xanthones and cinnamate esters from Hypericum hookerianum. Planta Med. 2005, 71, 680–682. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, R.; Zhang, C.; Li, X.; Negrin, A.; Yuan, C.; Kennelly, E.E.; Long, C. Cytotoxic xanthones from Hypericum stellatum, an ethnomedicine in Southwest China. Molecules 2019, 24, 3568. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ashtari, A.; Khoshneviszadeh, M.; Ranjbar, S.; Dehghani, A.; Akbarzadeh, T.; Larijani, B.; Khoshneviszadeh, M.; Saeedi, M. Synthesis of new benzimidazole-1,2,3-triazole hybrids as tyrosinase inhibitors. Chem. Biodivers. 2018, 15, e1800120. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Sobel, J.D. Miconazole mucoadhesive tablets: A novel delivery system. Clin. Infect. Dis. 2012, 54, 1480–1484. [Google Scholar] [CrossRef]
- Shukla, P.K.; Singh, P.; Yadav, R.K.; Pandey, S.; Bhunia, S.S. Past, present, and future of antifungal drug development. In Communicable Diseases of the Developing World; Topics in Medicinal Chemistry; Saxena, A.K., Ed.; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany, 2016; Volume 29, pp. 125–167. [Google Scholar] [CrossRef]
- Shojaei, P.; Mokhtari, B.; Ghorbanpoor, M. Synthesis, in vitro antifungal evaluation and docking studies of novel derivatives of imidazoles and benzimidazoles. Med. Chem. Res. 2019, 28, 1359–1367. [Google Scholar] [CrossRef]
- Tehrani, M.B.; Emami, S.; Asadi, M.; Saeedi, M.; Mirzahekmati, M.; Ebrahimi, S.M.; Mahdavi, M.; Nadri, H.; Moradi, A.; Moghadam, F.H.; et al. Imidazo[2,1-b]thiazole derivatives as new inhibitors of 15-lipoxygenase. Eur. J. Med. Chem. 2014, 87, 759–764. [Google Scholar] [CrossRef]
- Lohitha, N.; Vijayakumar, V. Imidazole appended novel phenoxyquinolines as new inhibitors of α-amylase and α-glucosidase evidence with molecular docking studies. Polycycl. Aromat. Compd. 2022, 42, 5521–5533. [Google Scholar] [CrossRef]
- Chaudhry, F.; Naureen, S.; Huma, R.; Shaukat, A.; Al-Rashida, M.; Asif, N.; Ashraf, M.; Munawar, M.A.; Khan, M.A. In search of new α-glucosidase inhibitors: Imidazolylpyrazole derivatives. Bioorg. Chem. 2017, 71, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Naureen, S.; Chaudhry, F.; Munawar, M.A.; Ashraf, M.; Hamid, S.; Khan, M.A. Biological evaluation of new imidazole derivatives tethered with indole moiety as potent α-glucosidase inhibitors. Bioorg. Chem. 2018, 76, 365–369. [Google Scholar] [CrossRef]
- Adib, M.; Peytam, F.; Shourgeshty, R.; Mohammadi-Khanaposhtani, M.; Jahani, M.; Imanparast, S.; Faramarzi, M.A.; Larijani, B.; Moghadamnia, A.A.; Esfahani, E.N.; et al. Design and synthesis of new fused carbazole-imidazole derivatives as anti-diabetic agents: In vitro α-glucosidase inhibition, kinetic, and in silico studies. Bioorg. Med. Chem. Lett. 2019, 29, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Dhameja, M.; Gupta, P. Synthetic heterocyclic candidates as promising α-glucosidase inhibitors: An overview. Eur. J. Med. Chem. 2019, 176, 343–377. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, F.; Naureen, S.; Ashraf, M.; Al-Rashida, M.; Jahan, B.; Ali, M.M.; Ain, K.M. Imidazole-pyrazole hybrids: Synthesis, characterization and in-vitro bioevaluation against α-glucosidase enzyme with molecular docking studies. Bioorg. Chem. 2019, 82, 267–273. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.-H.; Xie, H.-X.; Ge, Y.-X.; Wang, K.-M.; Song, Z.-L.; Zhu, K.-K.; Zhang, J.; Jiang, C.-S. Discovery of new 2-phenyl-1H-benzo[d]imidazole core-based potent α-glucosidase inhibitors: Synthesis, kinetic study, molecular docking, and in vivo anti-hyperglycemic evaluation. Bioorg. Chem. 2021, 117, 105423. [Google Scholar] [CrossRef]
- Chaudhry, F.; Shahid, W.; Al-Rashida, M.; Ashraf, M.; Ali, M.M.; Ain, K.M. Synthesis of imidazole-pyrazole conjugates bearing aryl spacer and exploring their enzyme inhibition potentials. Bioorg. Chem. 2021, 108, 104886. [Google Scholar] [CrossRef]
- Noori, M.; Davoodi, A.; Iraji, A.; Dastyafteh, N.; Khalili, M.; Asadi, M.; Mohammadi, K.M.; Mojtabavi, S.; Dianatpour, M.; Ali, F.M.; et al. Design, synthesis, and in silico studies of quinoline-based[d]imidazole bearing different acetamide derivatives as potent α-glucosidase inhibitors. Sci. Rep. 2022, 12, 14019. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Azam, U.; Mehreen, S.; Nasser, M. Synthetic α-glucosidase inhibitors as promising anti-diabetic agents: Recent development and future challenges. Eur. J. Med. Chem. 2023, 249, 115119. [Google Scholar] [CrossRef]
- Barbero, N.; SanMartin, R.; Domínguez, E.A. A convenient approach to the xanthone scaffold by an aqueous aromatic substitution of bromo- and iodoarenes. Tetrahedron 2009, 65, 5729–5732. [Google Scholar] [CrossRef]
- Mendieta-Moctezuma, A.; Rugerio-Escalona, C.; Villa-Ruano, N.; Gutierrez, R.U.; Jiménez-Montejo, E.F.; Fragoso-Vázquez, M.J.; Correa-Basurto, J.; Cruz-López, M.C.; Delgado, F.; Tamariz, J. Synthesis and biological evaluation of novel chromonyl enaminones as α-glucosidase inhibitors. Med. Chem. Res. 2019, 28, 831–848. [Google Scholar] [CrossRef]
- Ding, S.-M.; Lan, T.; Ye, G.-J.; Huang, J.-J.; Hu, Y.; Zhu, Y.-R.; Wang, B. Novel oxazolxanthone derivatives as a new type of α-glucosidase inhibitor: Synthesis, activities, inhibitory modes and synergetic effect. Bioorg. Med. Chem. 2018, 26, 3370–3378. [Google Scholar] [CrossRef]
- Fan, M.; Yang, W.; Peng, Z.; He, Y.; Wang, G. Chromone-based benzohydrazide derivatives as potential α-glucosidase inhibitor: Synthesis, biological evaluation and molecular docking study. Bioorg. Chem. 2023, 131, 106276. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Miyake, H.; Kusunoki, M.; Osaki, S. Crystal structures of isomaltase from Saccharomyces cerevisiae and in complex with its competitive inhibitor maltose. FEBS J. 2010, 277, 4205–4214. [Google Scholar] [CrossRef]
- Murugesu, S.; Ibrahim, Z.; Ahmed, Q.U.; Uzir, B.F.; Yusoff, N.I.N.; Perumal, V.; Abas, F.; Shaari, K.; Khatib, A. Identification of α-glucosidase inhibitors from Clinacanthus nutans leaf extract using liquid chromatography-mass spectrometry-based metabolomics and protein-ligand interaction with molecular docking. J. Pharm. Anal. 2019, 9, 91–99. [Google Scholar] [CrossRef]
- Nokhala, A.; Siddiqui, M.J.; Ahmed, Q.U.; Ahamad Bustamam, M.S.; Zakaria, Z.A. Investigation of α-glucosidase inhibitory metabolites from Tetracera scandens leaves by GC–MS metabolite profiling and docking studies. Biomolecules 2020, 10, 287. [Google Scholar] [CrossRef]
- Nipun, T.S.; Khatib, A.; Ibrahim, Z.; Ahmed, Q.U.; Redzwan, I.E.; Saiman, M.Z.; Supandi, F.; Primaharinastiti, R.; El-Seedi, H.R. Characterization of α-glucosidase inhibitors from Psychotria malayana Jack leaves extract using LC-MS-based multivariate data analysis and in-silico molecular docking. Molecules 2020, 25, 5885. [Google Scholar] [CrossRef] [PubMed]
- Aguila-Muñoz, D.G.; Cervantes-Espinoza, E.; Escalante, C.H.; Gutiérrez, R.U.; Cruz-López, M.C.; Jiménez-Montejo, F.E.; Villa-Ruano, N.; Gómez-García, O.; Tamariz, J.; Mendieta-Moctezuma, A. Synthesis of alkoxy-isoflavones as potential α-glucosidase inhibitors. Med. Chem. Res. 2022, 31, 1298–1312. [Google Scholar] [CrossRef]
- Cardozo-Muñoz, J.; Cuca-Suárez, L.E.; Prieto-Rodríguez, J.A.; Lopez-Vallejo, F.; Patiño-Ladino, O.J. Multitarget action of xanthones from Garcinia mangostana against α-amylase, α-glucosidase and pancreatic lipase. Molecules 2022, 27, 3283. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Taha, M.; Qureshi, F.; Ullah, N.; Selvaraj, M.; Shahzad, S.; Chigurupati, S.; Abubshait, S.A.; Ahmad, T.; Chinnam, S.; et al. Synthesis, α-amylase and α-glucosidase inhibition and molecular docking studies of indazole derivatives. J. Biomol. Struct. Dyn. 2021, 40, 10730–10740. [Google Scholar] [CrossRef] [PubMed]
- Saleem, F.; Khan, K.M.; Chigurupati, S.; Solangi, M.; Nemala, A.R.; Mushtaq, M.; Ul-Haq, Z.; Taha, M.; Perveen, S. Synthesis of azachalcones, their α-amylase, α-glucosidase inhibitory activities, kinetics, and molecular docking studies. Bioorg. Chem. 2021, 106, 104489. [Google Scholar] [CrossRef] [PubMed]
- Akshatha, J.V.; SantoshKumar, H.S.; Prakash, H.S.; Nalini, M.S. In silico docking studies of α-amylase inhibitors from the anti-diabetic plant Leucas ciliata Benth. and an endophyte, Streptomyces longisporoflavus. 3 Biotech 2021, 11, 11–51. [Google Scholar] [CrossRef] [PubMed]
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. Data Warrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Miller, R.R.; Madeira, M.; Wood, H.B.; Geissler, W.M.; Raab, C.E.; Martin, I.J. Integrating the impact of lipophilicity on potency and pharmacokinetic parameters enables the use of diverse chemical space during small molecule drug optimization. J. Med. Chem. 2020, 63, 12156–12170. [Google Scholar] [CrossRef]
- Hou, T.J.; Xia, K.; Zhang, W.; Xu, X.J. ADME evaluation in drug discovery. 4. Prediction of aqueous solubility based on atom contribution approach. J. Chem. Inf. Comput. Sci. 2004, 44, 266–275. [Google Scholar] [CrossRef]
- Clark, D.E. What has polar surface area ever done for drug discovery? Future Med. Chem. 2011, 3, 469–484. [Google Scholar] [CrossRef]
- Seul, South Corea: Bioinformatics and Molecular Design Research Center. 2004. Available online: https://preadmet.bmdrc.org (accessed on 15 January 2023).
- Salehi, P.; Asghari, B.; Esmaeili, M.A.; Dehghan, H.; Ghazi, I. α-Glucosidase and α-amylase inhibitory effect and antioxidant activity of ten plant extracts traditionally used in Iran for diabetes. J. Med. Plant Res. 2013, 7, 257–266. [Google Scholar] [CrossRef]
- Chokki, M.; Cudalbeanu, M.; Zongo, C.; Dah-Nouvlessounon, D.; Ghinea, I.O.; Furdui, B.; Raclea, R.; Savadogo, A.; Baba-Moussa, L.; Avamescu, S.M.; et al. Exploring antioxidant and enzymes (A-amylase and B-glucosidase) inhibitory activity of Morinda lucida and Momordica charantia leaves from Benin. Foods 2020, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Cevallos-Casals, B.; Cisneros-Zevallos, L. Stoichiometric and kinetics studies of phenolic antioxidants from Andean purple corn and red-fleshed sweetpotato. J. Agric. Food. Chem. 2003, 51, 3313–3319. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 2012, 46, 3–26. [Google Scholar] [CrossRef]


| Compounds | % DPPH Scavenging Activity (2.5 mM) | α-Glucosidase | α-Amylase | ||
|---|---|---|---|---|---|
| Inhibition (%) at 400 µM | IC50 (µM) | Inhibition (%) at 100 µM | IC50 (µM) | ||
| 1 | 32.6 ± 2.03 C | 26.5 ± 0.81 | >400 A | 98.7 ± 0.11 | 26.5 ± 0.01 E |
| 5 | 13.9 ± 1.14 E,F | 15.2 ± 0.16 | >400 A | NI | - |
| 6a | 1.7 ± 0.91 H,I | 99.5 ± 0.2 | 143.6 ± 0.17 H | 8.7 ± 0.2 | >200 A* |
| 6b | 11.8 ± 1.07 F,G | 9.3 ± 0.1 | >400 A | NA | - |
| 6c | 8.7 ± 0.67 G | 92.6 ± 0.11 | 16.0 ± 0.03 N | 64.8 ± 0.15 | 76.7 ± 0.03 B |
| 6d | 3.9 ± 0.58 H,I | 22.1 ± 1.04 | >400 A | NI | - |
| 6e | 10.4 ± 0.83 F,G | 98.6 ± 0.90 | 12.8 ± 0.01 O | 60.3 ± 0.1 | 68.1 ± 0.04 D |
| 7 | 45.9 ± 2.89 B† | 75.5 ± 0.50 | 196.4 ± 0.07 F | 7.8 ± 0.1 | >200 A |
| 8 | 1.9 ± 0.73 H,I | 38.3 ± 0.04 | >400 A | NI | - |
| 9a | 19.5 ± 1.22 D | 97.8 ± 0.3 | 40.0 ± 0.018 M | 62.9 ± 0.2 | 73.5 ± 0.04 C |
| 9b | 17.6 ± 1.17 D | 96.3 ± 0.2 | 4.00 ± 0.007 P | 3.87 ± 0.11 | >200 A |
| 10a | 0.8 ± 0.01 I | 1.3 ± 0.01 | >400 A | NI | - |
| 10b | 12.2 ± 0.08 F,G | 90.2 ± 0.21 | 212.4 ± 0.1 D | 9.7 ± 0.21 | >200 A |
| 10c | 11.6 ± 1.54 F,G | 95.1 ± 0.15 | 232.7 ± 0.11 C | 99.5 ± 0.10 | 5.4 ± 0.07 H |
| 10d | 5.1 ± 0.08 H | 2.5 ± 0.01 | >400 A | NI | - |
| 10f | 11.2 ± 1.02 F,G | 99.4 ± 0.51 | 145.2 ± 0.17 G | 93.3 ± 0.12 | 8.7 ± 0.06 G |
| 11a | 19.8 ± 1.04 D | 0.48 ± 0.02 | >400 A | NI | - |
| 11b | 18.4 ± 1.43 D | 99.7 ± 0.29 | 120.0 ± 0.03 I | 7.8 ± 0.5 | >200 A |
| 12a | 1.1 ± 0.01 I | 1.87 ± 0.47 | >400 A | NI | - |
| 12b | 16.3 ± 1.45 D,E | 84.9 ± 0.57 | 112.8 ± 0.12 J | 8.7 ± 0.81 | >200 A |
| 12c | 19.0 ± 0.22 D | 97.5 ± 1.78 | 210.9 ± 0.33 E | 3.9 ± 0.55 | >200 A |
| 12d | 10.1 ± 0.87 G | 52.2 ± 0.87 | 400.2 ± 0.24 A | 1.5 ± 0.10 | >200 A |
| 12e | 17.6 ± 1.14 D | 96.6 ± 1.45 | 104.9 ± 0.01 K | 2.4 ± 0.15 | >200 A |
| 12f | 18.1 ± 1.57 D | 99.1 ± 1.31 | 71.9 ± 0.08 L | 4.7 ± 0.32 | >200 A |
| BHT | 85.2 ± 3.33 A‡ | ND | ND | ND | ND |
| Acarbose | ND | 63.18 ± 0.7 | 306.7 ± 0.9 B | 99.3 ± 0.06 | 20.0 ± 0.07 F |
| Compound | Binding Energy ΔG (kcal/mol) | Interacting Residues | Polar Interactions | Hydrophobic Interactions |
|---|---|---|---|---|
| 14 | −7.78 | Asp69, Tyr72, His112, Tyr158, Phe159, Phe178, Arg213, Asp215, Val216, Glu277, Gln279, His280, Phe303, Asp307, Arg315, Tyr316, His351, Asp352, Gln353, Glu411, Arg442, Arg446 | C-H…..O (Asp69) O-H….O (Aps215) O…..H-N (Gln279) O-H…..O (Asp307) O…..H-N (His351) O-H…..O (Glu411) O…..H-N (Arg446) | - |
| 6c | −9.17 | Tyr72, His112, Val216, Gln279, Phe303, Arg315, Asp352, Glu411, Arg442 | O…..H-N (Gln279) C-H…..O (Asp352) O…..C-H (Arg442) | π-alkyl-Tyr72, His112, Phe178, Val216 π-sigma-Arg315 π-cation-Arg315 π-anion-Asp352 |
| 6e | −9.41 | Tyr72, His112, Val216, Gln279, Arg315, Asp352, Arg442 | O…..H-N (Gln279) C-H…..O (Asp352) | π-alkyl-Tyr72, His112, Val216, Arg315 π-π T-shaped-Tyr72 π-π stacked-Phe303 π-cation-Arg442 π-anion-Asp215, Glu277, Glu411 |
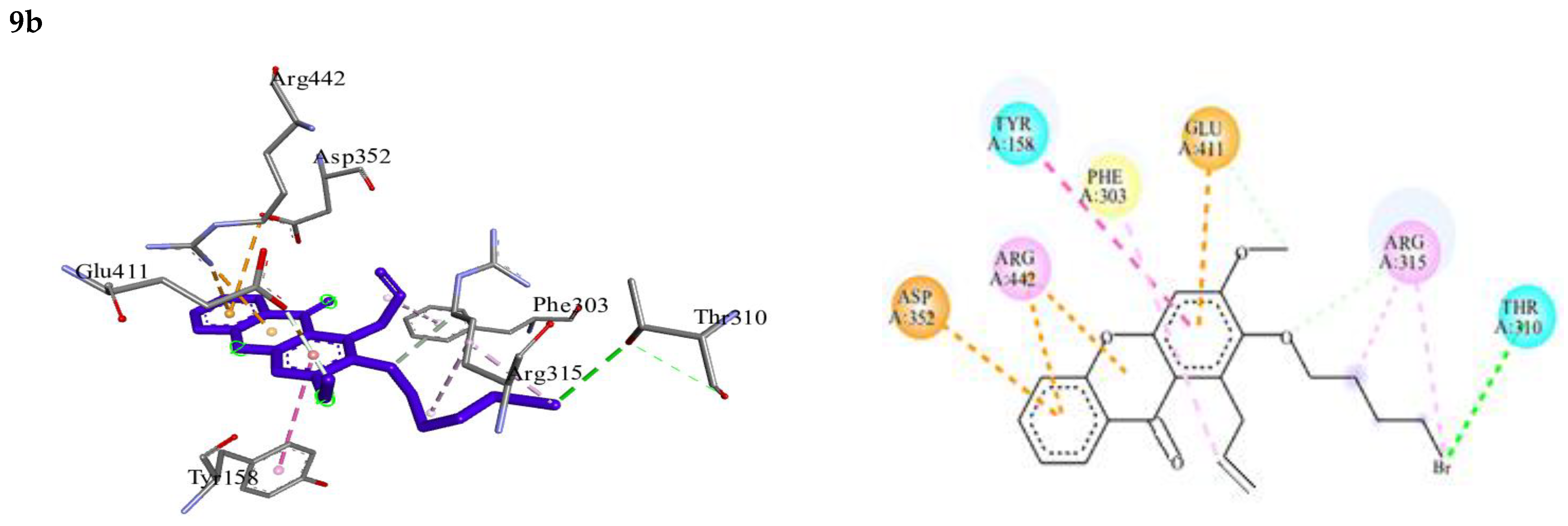
| 9b | −7.89 | Tyr158, Phe303, Thr310, Arg315, Asp352, Glu411, Arg442 | Halogen (Thr310) O…..C-H (Arg315) C-H…..O (Glu411) | π-π T-shaped-Tyr158 π-alkyl-Phe303 alkyl-Arg315 π-cation-Arg442 π-anion-Asp352, Glu411 |
| Compound | Binding Energy ΔG (kcal/mol) | Interacting Residues | Polar Interactions | Hydrophobic Interactions |
|---|---|---|---|---|
| 14 | −2.92 | Asp197, Glu233, Asp300, His305 | O-H……O (Asp197) O-H……O (Glu233) C-H……O (Glu233) O-H……O (Asp300) C-H……O (Asp300) O……H-N (His305) | - |
| 1 | −6.08 | Trp59, Tyr62, Asp197, Glu233, His299, Asp300 | O-H……O (Asp197) C-H……O (Glu233) C-H……O (Asp300) | π-π stacked-Trp59, Tyr62 π-alkyl-His299 |
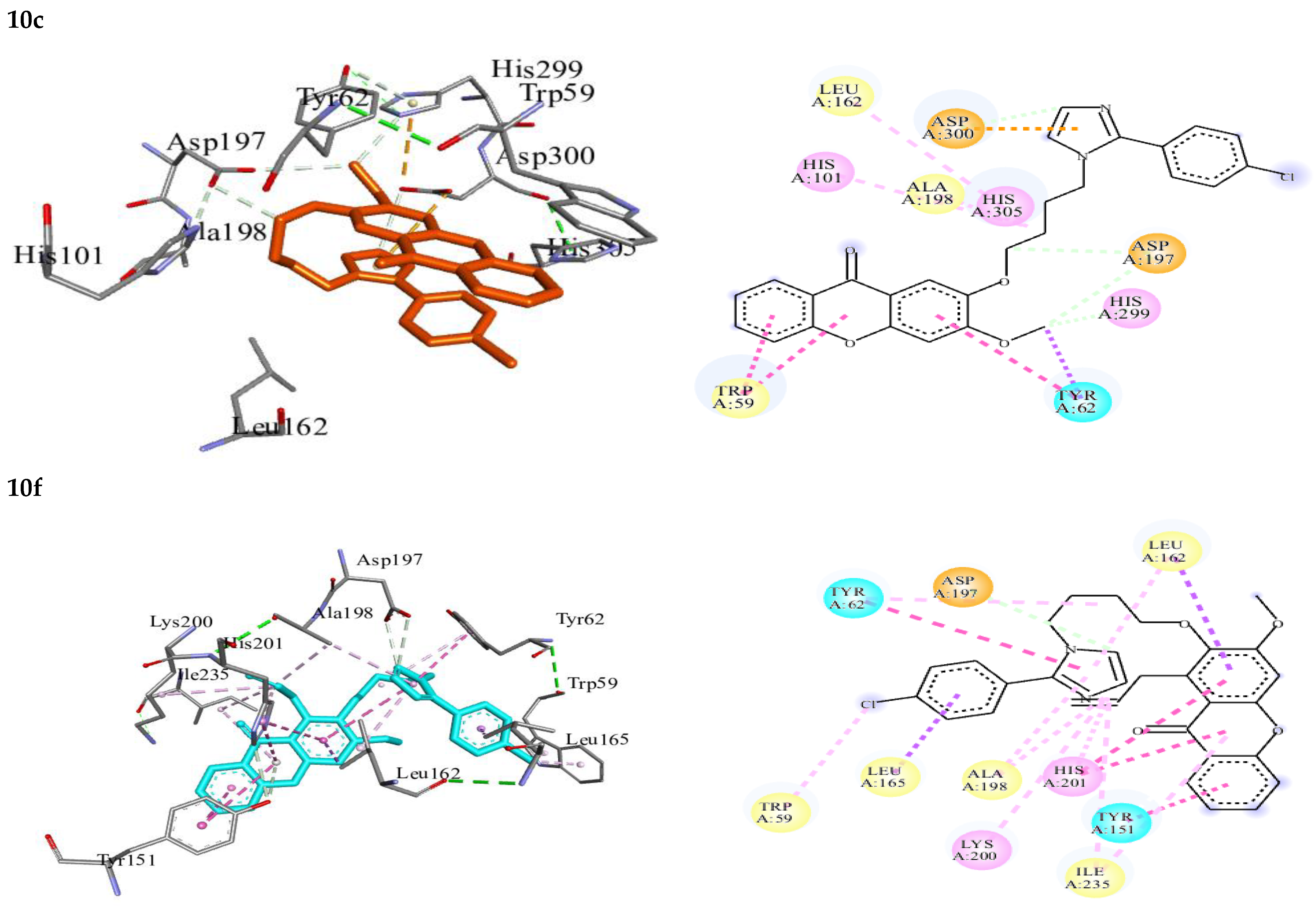
| 10c | −9.82 | Trp59, Tyr62, His101, Leu162, Asp197, Ala198, His299, Asp300, His305 | C-H……O (Asp197) C-H……O (His299) C-H……O (Asp300) | π-π stacked-Trp59, Tyr62 π-alkyl-His101 alkyl-Leu162 π-anion-Asp300 |
| 10f | −9.66 | Trp59, Tyr62, Tyr151, Leu162, Leu165, Asp197, Ala198, Lys200, His201, Ile235 | C-H……O (Asp197) | π-π stacked-Tyr62, Tyr151 π-π T-shaped-His201 π-alkyl-Trp59, Tyr62, Leu162, Ala198, His201, Ile235 alkyl-Ala198, Lys200, Ile235 π-sigma-Leu162, Leu165 |
| Druglikeness/ ADMET a | Compound | |||||
|---|---|---|---|---|---|---|
| 6c | 6e | 9a | 9b | 10c | 10f | |
| Rule of five | Suitable | Suitable | Suitable | Suitable | Suitable | Violated |
| Caco-2 | 35.309 | 42.1566 | 24.2146 | 25.8108 | 53.5637 | 54.9387 |
| HIA | 97.407182 | 97.510585 | 97.571763 | 97.757187 | 97.688197 | 97.911873 |
| BBB | 0.924897 | 0.355946 | 0.092018 | 0.282953 | 0.077422 | 0.105351 |
| Skin permeability | −2.79698 | −2.25319 | −2.84173 | −2.30488 | −2.76965 | −2.19626 |
| Ames test | Mutagen | Non-mutagen | Mutagen | Non-mutagen | Non-mutagen | Non-mutagen |
| Carcino mouse | Negative | Negative | Negative | Negative | Negative | Negative |
| Carcino rat | Negative | Negative | Positive | Positive | Negative | Negative |
| hERG inhibition | Medium risk | Medium risk | Medium risk | Medium risk | Medium risk | Medium risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguila-Muñoz, D.G.; Vázquez-Lira, G.; Sarmiento-Tlale, E.; Cruz-López, M.C.; Jiménez-Montejo, F.E.; López y López, V.E.; Escalante, C.H.; Andrade-Pavón, D.; Gómez-García, O.; Tamariz, J.; et al. Synthesis and Molecular Docking Studies of Alkoxy- and Imidazole-Substituted Xanthones as α-Amylase and α-Glucosidase Inhibitors. Molecules 2023, 28, 4180. https://doi.org/10.3390/molecules28104180
Aguila-Muñoz DG, Vázquez-Lira G, Sarmiento-Tlale E, Cruz-López MC, Jiménez-Montejo FE, López y López VE, Escalante CH, Andrade-Pavón D, Gómez-García O, Tamariz J, et al. Synthesis and Molecular Docking Studies of Alkoxy- and Imidazole-Substituted Xanthones as α-Amylase and α-Glucosidase Inhibitors. Molecules. 2023; 28(10):4180. https://doi.org/10.3390/molecules28104180
Chicago/Turabian StyleAguila-Muñoz, Dolores G., Gabriel Vázquez-Lira, Erika Sarmiento-Tlale, María C. Cruz-López, Fabiola E. Jiménez-Montejo, Víctor E. López y López, Carlos H. Escalante, Dulce Andrade-Pavón, Omar Gómez-García, Joaquín Tamariz, and et al. 2023. "Synthesis and Molecular Docking Studies of Alkoxy- and Imidazole-Substituted Xanthones as α-Amylase and α-Glucosidase Inhibitors" Molecules 28, no. 10: 4180. https://doi.org/10.3390/molecules28104180
APA StyleAguila-Muñoz, D. G., Vázquez-Lira, G., Sarmiento-Tlale, E., Cruz-López, M. C., Jiménez-Montejo, F. E., López y López, V. E., Escalante, C. H., Andrade-Pavón, D., Gómez-García, O., Tamariz, J., & Mendieta-Moctezuma, A. (2023). Synthesis and Molecular Docking Studies of Alkoxy- and Imidazole-Substituted Xanthones as α-Amylase and α-Glucosidase Inhibitors. Molecules, 28(10), 4180. https://doi.org/10.3390/molecules28104180







