Wheat Oxylipins in Response to Aphids, CO2 and Nitrogen Regimes
Abstract
1. Introduction
2. Results
2.1. Wheat Samples
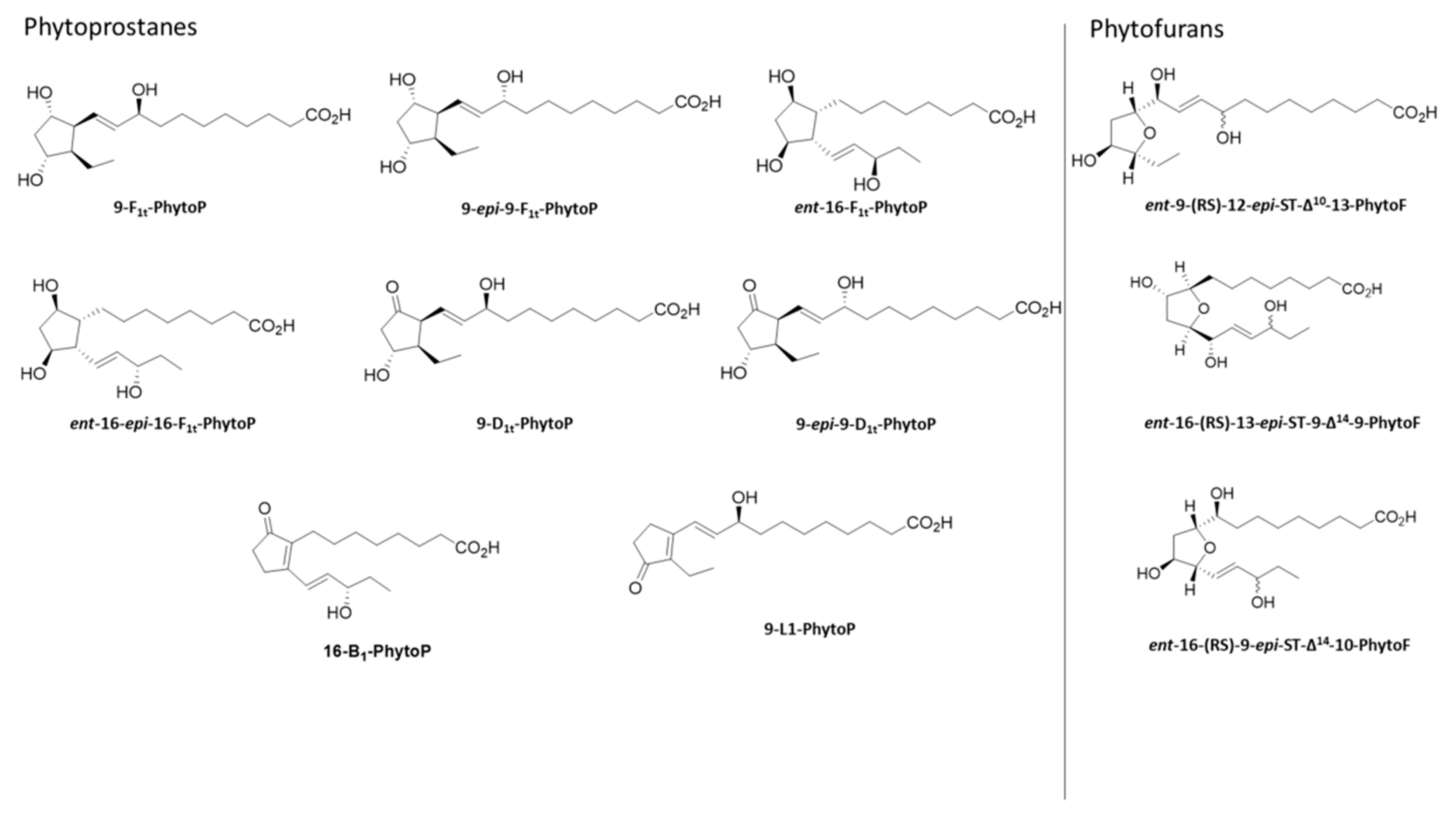
2.2. Qualitative Profile of Phytoprostanes and Phytofurans in Wheat Leaves
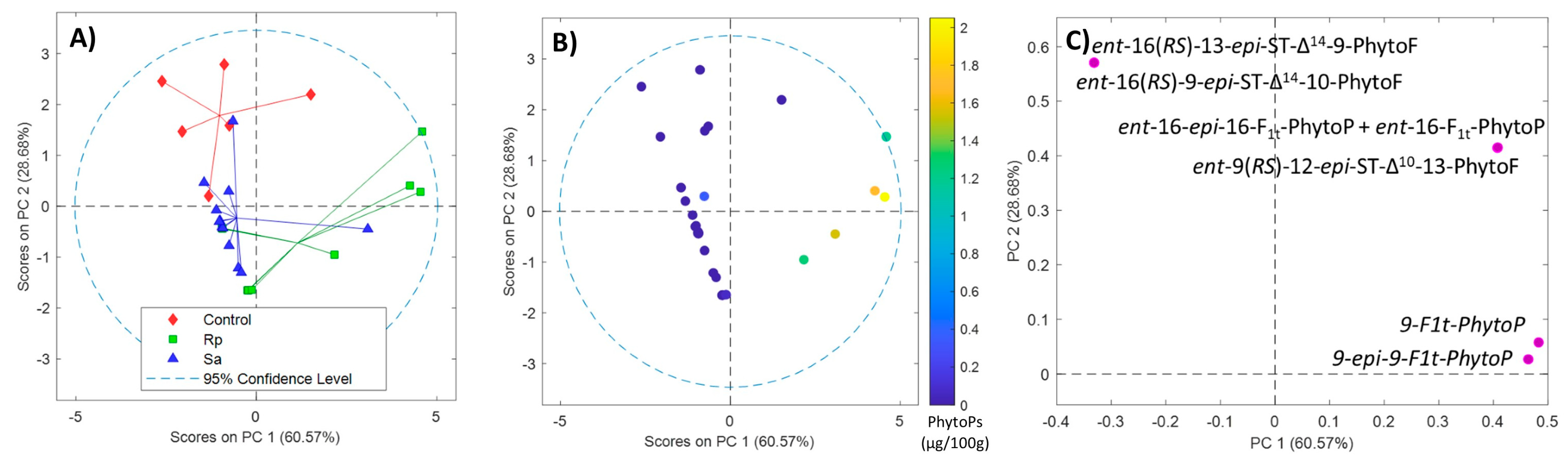
2.3. Phytoprostane and Phytofuran Content in Wheat before and after Aphid Treatment
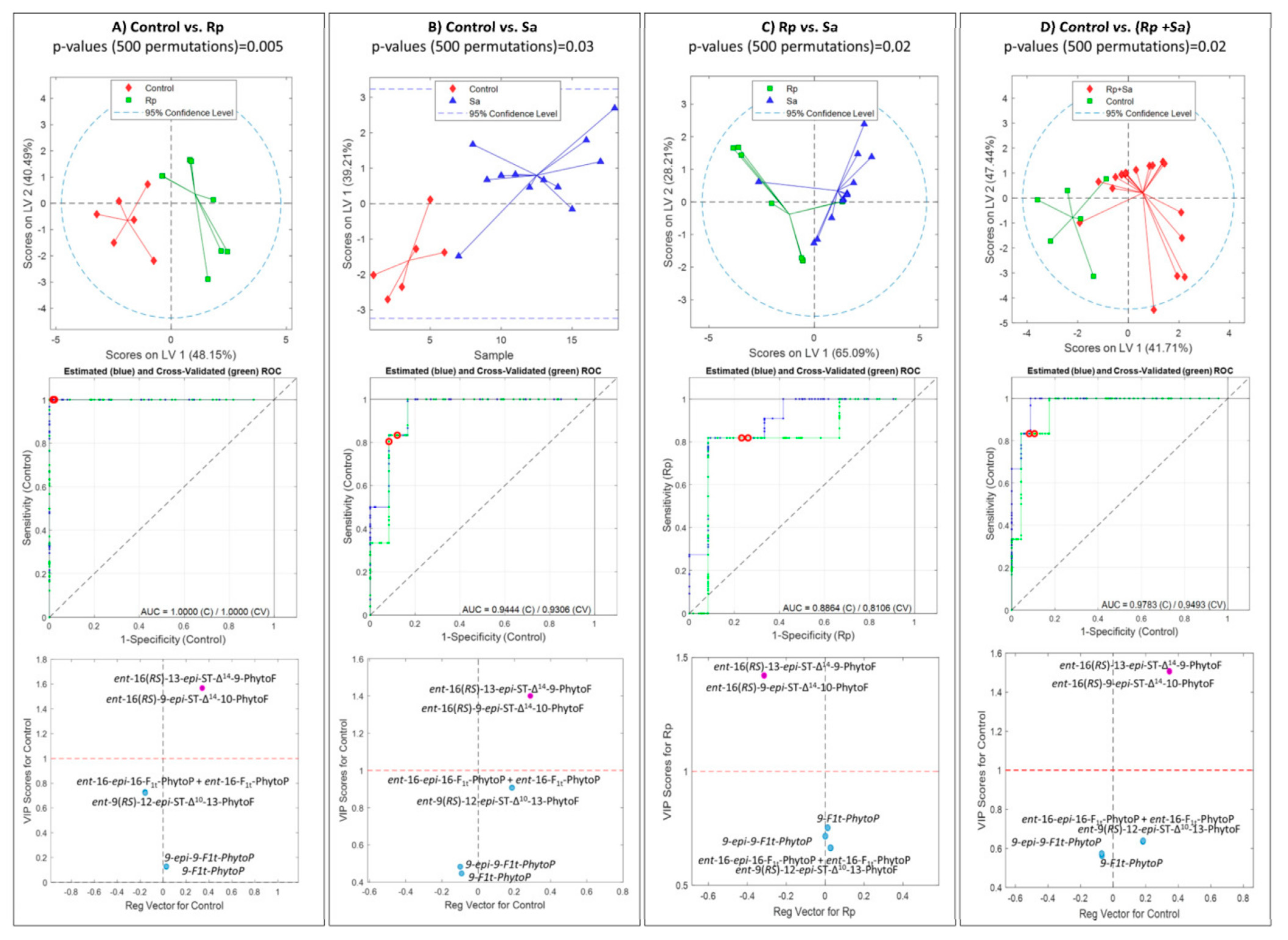
2.4. Effect of Aphids on Phytoprostane and Phytofuran Levels in Wheat Leaves
3. Discussion
Strengths and Limitations of the Study
4. Materials and Methods
4.1. Wheat Samples
4.2. Standards and Reagents
4.3. Stock, Working and Standard Solutions
4.4. Phytoprostanes and Phytofurans Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Madani, B.; Mirshekari, A.; Imahori, Y. Physiological Responses to Stress. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2019; pp. 405–423. [Google Scholar] [CrossRef]
- Pyl, E.; Tschoep, H.; Steinhauser, C.; Guenther, M.; Hoehne, M.; Rohwer, J.M.; Altmann, T.; Fernie, A.R.; Stitt, M. Network Analysis of Enzyme Activities and Metabolite Levels and Their Relationship to Biomass in a Large Panel of Arabidopsis Accessions. Plant Cell 2010, 22, 2872–2893. [Google Scholar] [CrossRef]
- Ali, M.S.; Baek, K.-H. Jasmonic Acid Signaling Pathway in Response to Abiotic Stresses in Plants. Int. J. Mol. Sci. 2020, 21, E621. [Google Scholar] [CrossRef]
- Sadras, V.; Vázquez, C.; Garzo, E.; Moreno, A.; Medina, S.; Taylor, J.; Fereres, A. The role of plant labile carbohydrates and nitrogen on wheat-aphid relations. Sci. Rep. 2021, 11, 12529. [Google Scholar] [CrossRef] [PubMed]
- Pyo, Y.; Moon, H.; Nugroho, A.B.D.; Yang, S.W.; Jung, I.L.; Kim, D.-H. Transcriptome analysis revealed that jasmonic acid biosynthesis/signaling is involved in plant response to Strontium stress. Ecotoxicol. Environ. Saf. 2022, 237, 113552. [Google Scholar] [CrossRef]
- Wasternack, C.; Feussner, I. The Oxylipin Pathways: Biochemistry and Function. Annu. Rev. Plant. Biol. 2018, 69, 363–386. [Google Scholar] [CrossRef]
- Ballhorn, D.; Reisdorff, C.; Pfanz, H. Quantitative effects of enhanced CO2 on jasmonic acid induced plant volatiles of lima bean (Phaseolus lunatus L.). J. Appl. Bot. Food Qual. 2011, 84, 65–71. [Google Scholar]
- Stumpe, M.; Göbel, C.; Faltin, B.; Beike, A.K.; Hause, B.; Himmelsbach, K.; Bode, J.; Kramell, R.; Wasternack, C.; Frank, W.; et al. The moss Physcomitrella patens contains cyclopentenones but no jasmonates: Mutations in allene oxide cyclase lead to reduced fertility and altered sporophyte morphology. New Phytol. 2010, 188, 740–749. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, J.; Cao, H.; Li, C.; Kang, L.; Ge, F. Elevated CO2 Influences Nematode-Induced Defense Responses of Tomato Genotypes Differing in the JA Pathway. PLoS ONE 2011, 6, e19751. [Google Scholar] [CrossRef]
- Thoma, I.; Loeffler, C.; Sinha, A.K.; Gupta, M.; Krischke, M.; Steffan, B.; Roitsch, T.; Mueller, M.J. Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant J. 2003, 34, 363–375. [Google Scholar] [CrossRef]
- Collado-González, J.; Grosso, C.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Durand, T.; Guy, A.; Galano, J.-M.; Torrecillas, A.; Gil-Izquierdo, Á. Inhibition of α-glucosidase and α-amylase by Spanish extra virgin olive oils: The involvement of bioactive compounds other than oleuropein and hydroxytyrosol. Food Chem. 2017, 235, 298–307. [Google Scholar] [CrossRef]
- Collado-González, J.; Durand, T.; Ferreres, F.; Medina, S.; Torrecillas, A.; Gil-Izquierdo, Á. Phytoprostanes. Lipid Technol. 2015, 27, 127–130. [Google Scholar] [CrossRef]
- Imbusch, R.; Mueller, M.J. Analysis of Oxidative Stress and Wound-Inducible Dinor Isoprostanes F1 (Phytoprostanes F1) in Plants1. Plant Physiol. 2000, 124, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Jahn, U.; Galano, J.-M.; Durand, T. A cautionary note on the correct structure assignment of phytoprostanes and the emergence of a new prostane ring system. Prostaglandins Leukot Essent Fat. Acids 2010, 82, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.S.; Oger, C.; Guy, A.; Bultel-Poncé, V.; Vigor, C.; Durand, T.; Gil-Izquierdo, A.; Medina, S.; Galano, J.-M.; Lee, J.C.-Y. Chapter Eleven-Alpha-linolenic acid, phytoprostanes and phytofurans in plant, algae and food. In Advances in Botanical Research; Rébeillé, F., Maréchal, E., Eds.; Academic Press: Cambridge, MA, USA, 2022; Volume 101, pp. 437–468. [Google Scholar] [CrossRef]
- Medina, S.; Gil-Izquierdo, Á.; Durand, T.; Ferreres, F.; Domínguez-Perles, R. Structural/Functional Matches and Divergences of Phytoprostanes and Phytofurans with Bioactive Human Oxylipins. Antioxidants 2018, 7, E165. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.; Collado-González, J.; Ferreres, F.; Londoño-Londoño, J.; Jiménez-Cartagena, C.; Guy, A.; Durand, T.; Galano, J.-M.; Gil-Izquierdo, A. Quantification of phytoprostanes-bioactive oxylipins- and phenolic compounds of Passiflora edulis Sims shell using UHPLC-QqQ-MS/MS and LC-IT-DAD-MS/MS. Food Chem. 2017, 229, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cuyamendous, C.; Leung, K.S.; Durand, T.; Lee, J.C.-Y.; Oger, C.; Galano, J.-M. Synthesis and discovery of phytofurans: Metabolites of α-linolenic acid peroxidation. Chem. Commun. 2015, 51, 15696–15699. [Google Scholar] [CrossRef]
- Collado-González, J.; Cano-Lamadrid, M.; Pérez-López, D.; Carbonell-Barrachina, Á.A.; Centeno, A.; Medina, S.; Griñán, I.; Guy, A.; Galano, J.-M.; Durand, T.; et al. Effects of Deficit Irrigation, Rootstock, and Roasting on the Contents of Fatty Acids, Phytoprostanes, and Phytofurans in Pistachio Kernels. J. Agric. Food Chem. 2020, 68, 8915–8924. [Google Scholar] [CrossRef]
- Collado-González, J.; Pérez-López, D.; Memmi, H.; Gijón, M.C.; Medina, S.; Durand, T.; Guy, A.; Galano, J.-M.; Fernández, D.J.; Carro, F.; et al. Effect of the season on the free phytoprostane content in Cornicabra extra virgin olive oil from deficit-irrigated olive trees. J. Sci. Food Agric. 2016, 96, 1585–1592. [Google Scholar] [CrossRef]
- Lipan, L.; Collado-González, J.; Domínguez-Perles, R.; Corell, M.; Bultel-Poncé, V.; Galano, J.-M.; Durand, T.; Medina, S.; Gil-Izquierdo, Á.; Carbonell-Barrachina, Á. Phytoprostanes and Phytofurans-Oxidative Stress and Bioactive Compounds-in Almonds are Affected by Deficit Irrigation in Almond Trees. J. Agric. Food Chem. 2020, 68, 7214–7225. [Google Scholar] [CrossRef]
- Pinciroli, M.; Domínguez-Perles, R.; Abellán, Á.; Bultel-Poncé, V.; Durand, T.; Galano, J.M.; Ferreres, F.; Gil-Izquierdo, Á. Statement of Foliar Fertilization Impact on Yield, Composition, and Oxidative Biomarkers in Rice. J. Agric. Food Chem. 2019, 67, 597–605. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, E681. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- González Roldán, N.; Engel, R.; Düpow, S.; Jakob, K.; Koops, F.; Orinska, Z.; Vigor, C.; Oger, C.; Galano, J.-M.; Durand, T.; et al. Lipid Mediators from Timothy Grass Pollen Contribute to the Effector Phase of Allergy and Prime Dendritic Cells for Glycolipid Presentation. Front. Immunol. 2019, 10, 974. [Google Scholar] [CrossRef]
- Rac, M.; Shumbe, L.; Oger, C.; Guy, A.; Vigor, C.; Ksas, B.; Durand, T.; Havaux, M. Luminescence imaging of leaf damage induced by lipid peroxidation products and its modulation by β-cyclocitral. Physiol. Plant 2021, 171, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Del Amor, A.M.; Aguayo, E.; Collado-González, J.; Guy, A.; Galano, J.-M.; Durand, T.; Gil-Izquierdo, Á. Impact of processing conditions on the phytoprostanes profile of three types of nut kernels. Free. Radic. Res. 2017, 51, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Del Amor, A.M.; Aguayo, E.; Collado-González, J.; Guy, A.; Galano, J.-M.; Durand, T.; Gil-Izquierdo, Á. Impact of packaging atmosphere, storage and processing conditions on the generation of phytoprostanes as quality processing compounds in almond kernels. Food Chem. 2016, 211, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Del Amor, A.M.; Collado-González, J.; Aguayo, E.; Guy, A.; Galano, J.M.; Durand, T.; Gil-Izquierdo, A. Phytoprostanes in almonds: Identification, quantification, and impact of cultivar and type of cultivation. RSC Adv. 2015, 5, 51233–51241. [Google Scholar] [CrossRef]
- Collado-González, J.; Medina, S.; Durand, T.; Guy, A.; Galano, J.-M.; Torrecillas, A.; Ferreres, F.; Gil-Izquierdo, A. New UHPLC–QqQ-MS/MS method for quantitative and qualitative determination of free phytoprostanes in foodstuffs of commercial olive and sunflower oils. Food Chem. 2015, 178, 212–220. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Abellán, Á.; León, D.; Ferreres, F.; Guy, A.; Oger, C.; Galano, J.M.; Durand, T.; Gil-Izquierdo, Á. Sorting out the phytoprostane and phytofuran profile in vegetable oils. Food Res. Int. 2018, 107, 619–628. [Google Scholar] [CrossRef]
- Marhuenda, J.; Medina, S.; Díaz-Castro, A.; Martínez-Hernández, P.; Arina, S.; Zafrilla, P.; Mulero, J.; Oger, C.; Galano, J.-M.; Durand, T.; et al. Dependency of Phytoprostane Fingerprints of Must and Wine on Viticulture and Enological Processes. J. Agric. Food Chem. 2015, 63, 9022–9028. [Google Scholar] [CrossRef] [PubMed]
- Tkacz, K.; Gil-Izquierdo, Á.; Medina, S.; Turkiewicz, I.P.; Domínguez-Perles, R.; Nowicka, P.; Wojdyło, A. Phytoprostanes, phytofurans, tocopherols, tocotrienols, carotenoids and free amino acids and biological potential of sea buckthorn juices. J. Sci. Food Agric. 2022, 102, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, J.; Pérez-López, D.; Memmi, H.; Gijón, M.C.; Medina, S.; Durand, T.; Guy, A.; Galano, J.-M.; Ferreres, F.; Torrecillas, A.; et al. Water Deficit during Pit Hardening Enhances Phytoprostanes Content, a Plant Biomarker of Oxidative Stress, in Extra Virgin Olive Oil. J. Agric. Food Chem. 2015, 63, 3784–3792. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, J.; Moriana, A.; Girón, I.F.; Corell, M.; Medina, S.; Durand, T.; Guy, A.; Galano, J.-M.; Valero, E.; Garrigues, T.; et al. The phytoprostane content in green table olives is influenced by Spanish-style processing and regulated deficit irrigation. LWT-Food Sci. Technol. 2015, 64, 997–1003. [Google Scholar] [CrossRef]
- Pinciroli, M.; Domínguez-Perles, R.; Garbi, M.; Abellán, A.; Oger, C.; Durand, T.; Galano, J.M.; Ferreres, F.; Gil-Izquierdo, A. Impact of Salicylic Acid Content and Growing Environment on Phytoprostane and Phytofuran (Stress Biomarkers) in Oryza sativa L. J. Agric. Food Chem. 2018, 66, 12561–12570. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Perles, R.; Sánchez-Martínez, I.; Rodríguez-Hernández, M.D.; López-González, I.; Oger, C.; Guy, A.; Durand, T.; Galano, J.M.; Ferreres, F.; Gil-Izquierdo, A. Optimization of Free Phytoprostane and Phytofuran Production by Enzymatic Hydrolysis of Pea Extracts Using Esterases. J. Agric. Food Chem. 2020, 68, 3445–3455. [Google Scholar] [CrossRef]
- Xiao, R.; Zou, Y.; Guo, X.; Li, H.; Lu, H. Fatty acid desaturases (FADs) modulate multiple lipid metabolism pathways to improve plant resistance. Mol. Biol. Rep. 2022, 49, 9997–10011. [Google Scholar] [CrossRef]
- Li, J.; Galla, A.L.; Avila, C.A.; Flattmann, K.; Vaughn, K.L.; Goggin, F.L. Fatty Acid Desaturases in the Chloroplast and Endoplasmic Reticulum Promote Susceptibility to the Green Peach Aphid Myzus persicae in Arabidopsis thaliana. Mol. Plant-Microbe Interact. MPMI 2021, 34, 691–702. [Google Scholar] [CrossRef]
- Yonny, M.E.; Torresi, A.R.; Cuyamendous, C.; Réversat, G.; Oger, C.; Galano, J.-M.; Durand, T.; Vigor, C.; Nazareno, M.A. Thermal Stress in Melon Plants: Phytoprostanes and Phytofurans as Oxidative Stress Biomarkers and the Effect of Antioxidant Supplementation. J. Agric. Food Chem. 2016, 64, 8296–8304. [Google Scholar] [CrossRef]
- Medina, S.; Gil-Izquierdo, Á.; Abu-Reidah, I.M.; Durand, T.; Bultel-Poncé, V.; Galano, J.-M.; Domínguez-Perles, R. Evaluation of Phoenix dactylifera Edible Parts and Byproducts as Sources of Phytoprostanes and Phytofurans. J. Agric. Food Chem. 2020, 68, 8942–8950. [Google Scholar] [CrossRef]
- Ahmed, O.S.; Sedraoui, S.; Zhou, B.; Reversat, G.; Rocher, A.; Bultel-Poncé, V.; Guy, A.; Vercauteren, J.; Selim, S.; Galano, J.-M.; et al. Phytoprostanes from Date Palm Fruit and Byproducts: Five Different Varieties Grown in Two Different Locations As Potential sources. J. Agric. Food Chem. 2021, 69, 13754–13761. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Collado-González, J.; Andrade, P.B.; Ferreres, F.; Valentão, P.; Galano, J.-M.; Durand, T.; Gil-Izquierdo, Á. Nonenzymatic α-Linolenic Acid Derivatives from the Sea: Macroalgae as Novel Sources of Phytoprostanes. J. Agric. Food Chem. 2015, 63, 6466–6474. [Google Scholar] [CrossRef]
- Vigor, C.; Reversat, G.; Rocher, A.; Oger, C.; Galano, J.-M.; Vercauteren, J.; Durand, T.; Tonon, T.; Leblanc, C.; Potin, P. Isoprostanoids quantitative profiling of marine red and brown macroalgae. Food Chem. 2018, 268, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Pino Ramos, L.L.; Jiménez-Aspee, F.; Theoduloz, C.; Burgos-Edwards, A.; Domínguez-Perles, R.; Oger, C.; Durand, T.; Gil-Izquierdo, Á.; Bustamante, L.; Mardones, C.; et al. Phenolic, oxylipin and fatty acid profiles of the Chilean hazelnut (Gevuina avellana): Antioxidant activity and inhibition of pro-inflammatory and metabolic syndrome-associated enzymes. Food Chem. 2019, 298, 125026. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.; Collado-González, J.; Ferreres, F.; Londoño-Londoño, J.; Jiménez-Cartagena, C.; Guy, A.; Durand, T.; Galano, J.-M.; Gil-Izquierdo, A. Valorization Strategy of Banana Passion Fruit Shell Wastes: An Innovative Source of Phytoprostanes and Phenolic Compounds and Their Potential Use in Pharmaceutical and Cosmetic Industries. J. Food Nutr. Res. 2017, 5, 801–808. [Google Scholar] [CrossRef]
- Medina, S.; Collado-González, J.; Ferreres, F.; Londoño-Londoño, J.; Jiménez-Cartagena, C.; Guy, A.; Durand, T.; Galano, J.-M.; Gil-Izquierdo, Á. Potential of Physalis peruviana calyces as a low-cost valuable resource of phytoprostanes and phenolic compounds. J. Sci. Food Agric. 2018, 99, 2194–2204. [Google Scholar] [CrossRef]
- Pinciroli, M.; Domínguez-Perles, R.; Abellán, A.; Guy, A.; Durand, T.; Oger, C.; Galano, J.M.; Ferreres, F.; Gil-Izquierdo, A. Comparative Study of the Phytoprostane and Phytofuran Content of indica and japonica Rice (Oryza sativa L.) Flours. J. Agric. Food Chem. 2017, 65, 8938–8947. [Google Scholar] [CrossRef]
- García-García, M.C.; Celestino, M.D.R.; Gil-Izquierdo, Á.; Egea-Gilabert, C.; Galano, J.M.; Durand, T.; Oger, C.; Fernández, J.A.; Ferreres, F.; Domínguez-Perles, R. The Value of Legume Foods as a Dietary Source of Phytoprostanes and Phytofurans Is Dependent on Species, Variety, and Growing Conditions. Eur. J. Lipid Sci. Technol. 2019, 121, 1800484. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E.; Suárez-Quiroz, M.L.; González-Ríos, O.; Rocher, A.; Reversat, G.; Vercauteren, J.; Oger, C.; Galano, J.-M.; et al. Identification and quantification of phytoprostanes and phytofurans of coffee and cocoa by- and co-products. Food Funct. 2019, 10, 6882–6891. [Google Scholar] [CrossRef]
- León-Perez, D.; Medina, S.; Londoño-Londoño, J.; Cano-Lamadrid, M.; Carbonell-Barrachina, Á.; Durand, T.; Guy, A.; Oger, C.; Galano, J.-M.; Ferreres, F.; et al. Comparative study of different cocoa (Theobroma cacao L.) clones in terms of their phytoprostanes and phytofurans contents. Food Chem. 2019, 280, 231–239. [Google Scholar] [CrossRef]
- León-Perez, D.; Domínguez-Perles, R.; Collado-González, J.; Cano-Lamadrid, M.; Durand, T.; Guy, A.; Galano, J.-M.; Carbonell-Barrachina, Á.; Londoño-Londoño, J.; Ferreres, F.; et al. Bioactive plant oxylipins-based lipidomics in eighty worldwide commercial dark chocolates: Effect of cocoa and fatty acid composition on their dietary burden. Microchem. J. 2020, 157, 105083. [Google Scholar] [CrossRef]
- Cuyamendous, C.; Leung, K.S.; Bultel-Poncé, V.; Guy, A.; Durand, T.; Galano, J.-M.; Lee, J.C.-Y.; Oger, C. Total Synthesis and in Vivo Quantitation of Phytofurans Derived from α-Linolenic Acid. Eur. J. Org. Chem. 2017, 2017, 2486–2490. [Google Scholar] [CrossRef]
- Pretorius, C.J.; Zeiss, D.R.; Dubery, I.A. The presence of oxygenated lipids in plant defense in response to biotic stress: A metabolomics appraisal. Plant Signal. Behav. 2021, 16, 1989215. [Google Scholar] [CrossRef] [PubMed]
- Kanobe, C.; McCarville, M.T.; O’neal, M.E.; Tylka, G.L.; MacIntosh, G.C. Soybean Aphid Infestation Induces Changes in Fatty Acid Metabolism in Soybean. PLoS ONE 2015, 10, e0145660. [Google Scholar] [CrossRef]
- Batsale, M.; Bahammou, D.; Fouillen, L.; Mongrand, S.; Joubès, J.; Domergue, F. Biosynthesis and Functions of Very-Long-Chain Fatty Acids in the Responses of Plants to Abiotic and Biotic Stresses. Cells 2021, 10, 1284. [Google Scholar] [CrossRef]
- Cano-Ramirez, D.L.; Carmona-Salazar, L.; Morales-Cedillo, F.; Ramírez-Salcedo, J.; Cahoon, E.B.; Gavilanes-Ruíz, M. Plasma Membrane Fluidity: An Environment Thermal Detector in Plants. Cells 2021, 10, 2778. [Google Scholar] [CrossRef]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Islam, Z.; Paul, K.; Bhattacharjee, Y.S. Lipid chemistry in stress tolerance of plants. Food Sci. Rep. 2020, 1, 22. [Google Scholar]
- Chi, X.; Yang, Q.; Lu, Y.; Wang, J.; Zhang, Q.; Pan, L.; Chen, M.; He, Y.; Yu, S. Genome-Wide Analysis of Fatty Acid Desaturases in Soybean (Glycine max). Plant Mol. Biol. Rep. 2011, 29, 769–783. [Google Scholar] [CrossRef]
- Taber, D.F.; Morrow, J.D.; Roberts, L.J. A nomenclature system for the isoprostanes. Prostaglandins 1997, 53, 63–67. [Google Scholar] [CrossRef]
| Control | Rhopalosiphum padi | Sitobion avenae | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Median (µg/100 g) | IQR (µg/100 g) | Median (µg/100 g) | IQR (µg/100 g) | Median (µg/100 g) | IQR (µg/100 g) | Control vs. Rp | Control vs. Sa | Rp vs. Sa | Control vs. Rp + Sa | |
| PhytoP | 9-F1t-PhytoP | 0.0002 | 0.0002–0.005 | 0.01 | 0.0011–0.4 | 0.0002 | 0.0002–0.012 | 0.05 | 0.4 | 0.16 | 0.13 |
| 9-epi-9-F1t-PhytoP | 0.0002 | 0.0002–0.0002 | 0.012 | 0.0002–0.9 | 0.009 | 0.0002–0.02 | 0.12 | 0.10 | 0.5 | 0.09 | |
| ent-16-epi-16-F1t-PhytoP + ent-16-F1t-PhytoP | 0.003 | 0.0002–0.007 | 0.0002 | 0.0002–0.008 | 0.0002 | 0.0002–0.002 | 0.80 | 0.20 | 0.20 | 0.30 | |
| PhytoF | ent-9(RS)-12-epi-ST-Δ10-13-PhytoF | 4 | 0.0002–11 | 0.0002 | 0.0002–12 | 0.0002 | 0.0002–0.002 | 0.80 | 0.20 | 0.20 | 0.30 |
| ent-16(RS)-13-epi-ST-Δ14-9-PhytoF | 3 | 2–4 | 0.0002 | 0.0002–0.0002 | 1 | 0.7–2 | 0.0002 | 0.010 | 0.0008 | 0.0011 | |
| ent-16(RS)-9-epi-ST-Δ14-10-PhytoF | 7 | 5–8 | 0.0002 | 0.0002–0.0002 | 2 | 1.7–4 | 0.0002 | 0.010 | 0.0008 | 0.0011 | |
| Total | PhytoP | 0.007 | 0.005–0.02 | 0.01 | 0.005–1.2 | 0.02 | 0.004–0.03 | 0.40 | 0.50 | 0.50 | 0.40 |
| PhytoF | 16 | 11–23 | 4 | 0.0002–12 | 5 | 4–7 | 0.02 | 0.005 | 0.2 | 0.005 | |
| Plant/Sample | 9-F1t-PhytoP | 9-epi-9-F1t-PhytoP | 9-D1t-PhytoP | 9-epi-9-D1t-PhytoP | 9-L1-PhytoP | 16-B1-PhytoP | ent-16-F1t-PhytoP + ent-16-epi-16-F1t-PhytoP | Reference |
|---|---|---|---|---|---|---|---|---|
| Wheat Leaves | ✓ | ✓ | ✓ | This Study | ||||
| Cucumis melo L. leaves | ✓ | ✓ | ✓ | ✓ | ✓ | [41] | ||
| Date tree leaves | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | [42,43] | |
| Chilean hazelnut (Gevuina avellana Mol., Proteaceae) cotyledons | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | [46] | |
| Macroalgae | ✓ | ✓ | ✓ | ✓ | [44] | |||
| Brown macroalgae (Ectocarpus siliculosus) | ✓ | ✓ | ✓ | ✓ | ✓ | [45] | ||
| Brown macroalgae (Laminaria digitate) | ✓ | ✓ | ✓ | ✓ | ✓ | [45] | ||
| Brown macroalgae (Fucus spiralis) | ✓ | ✓ | ✓ | ✓ | ✓ | [45] | ||
| Red macroalgae (Osmundea pinnatifida) | ✓ | ✓ | ✓ | [45] | ||||
| Red macroalgae (Grateloupia turuturu) | ✓ | ✓ | ✓ | [45] | ||||
| Brown macroalgae (Pelvetia canaliculata) | ✓ | ✓ | ✓ | ✓ | ✓ | [45] | ||
| Passiflora edulis Sims shell | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | [18] | |
| Passiflora tripartita var.mollisima shell | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | [47] |
| Physalis peruviana calyx | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | [48] |
| Plant/Food Sample | ent-16(RS)-9-epi-ST-Δ14-10-PhytoF | ent-9(RS)-12-epi-ST-Δ10-13-PhytoF | ent-16(RS)-13-epi-ST-Δ14-9-PhytoF | Reference |
|---|---|---|---|---|
| Wheat Leaves | ✓ | ✓ | ✓ | This Study |
| Cucumis melo L. leaves | ✓ | ✓ | [41] | |
| Date tree leaves | ✓ | ✓ | ✓ | [42,43] |
| Chilean hazelnut (Gevuina avellana Mol., Proteaceae) cotyledons | ✓ | ✓ | ✓ | [46] |
| Brown macroalgae (Ectocarpus siliculosus) | ✓ | ✓ | ✓ | [45] |
| Brown macroalgae (Laminaria digitate) | ✓ | ✓ | ✓ | [45] |
| Brown macroalgae (Pelvetia canaliculata) | ✓ | [45] | ||
| Red macroalgae (Osmundea pinnatifida) | ✓ | [45] | ||
| Red macroalgae (Grateloupia turuturu) | ✓ | ✓ | [45] | |
| Brown macroalage (Fucus spiralis) | ✓ | ✓ | ✓ | [45] |
| Compound | RT (min) | MRM Transition (m/z) | Fragmentor (V) | CE (V) | |
|---|---|---|---|---|---|
| Phytoprostanes | ent-16-epi-16-F1t-PhytoP | 1.583 | 327.1 > 283.2 | 80 | 15 |
| 327.1 > 225.1 | 80 | 15 | |||
| 9-F1t-PhytoP | 1.631 | 327.2 > 273.1 | 110 | 15 | |
| 327.2 > 171.0 | 110 | 15 | |||
| ent-16-F1t-PhytoP | 1.712 | 327.2 > 283.2 | 80 | 10 | |
| 327.2 > 225.1 | 80 | 10 | |||
| 9-epi-9-F1t-PhytoP | 1.785 | 327.2 > 272.8 | 110 | 10 | |
| 327.2 > 171.0 | 110 | 10 | |||
| 9-D1t-PhytoP | 1.791 | 325.2 > 307.3 | 100 | 4 | |
| 325.2 > 134.7 | 100 | 4 | |||
| 9-epi-9-D1t-PhytoP | 2.022 | 325.2 > 307.2 | 100 | 7 | |
| 325.2 > 134.9 | 100 | 7 | |||
| 16-B1-PhytoP | 2.62 | 307.2 > 223.2 | 100 | 10 | |
| 307.2 > 235.1 | 100 | 10 | |||
| 9-L1-PhytoP | 3.079 | 307.2 > 185.1 | 110 | 7 | |
| 307.2 > 185.2 | 110 | 7 | |||
| Phytofurans | ent-9-(RS)-12-epi-ST-Δ10-13-PhtoF | 0.906 | 344.0 > 300.0 | 110 | 10 |
| 344.0 > 255.9 | 110 | 10 | |||
| ent-16-(RS)-9-epi-ST-Δ14-10-PhytoF | 1.501 | 343.9 > 209.9 | 90 | 12 | |
| 343.9 > 201.1 | 90 | 12 | |||
| ent-16-(RS)-13-epi-ST-Δ14-9-PhytoF | 1.523 | 343.0 > 171.1 | 90 | 22 | |
| 343.0 > 97.2 | 90 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascant-Vilaplana, M.M.; Viteritti, E.; Sadras, V.; Medina, S.; Sánchez-Iglesias, M.P.; Oger, C.; Galano, J.-M.; Durand, T.; Gabaldón, J.A.; Taylor, J.; et al. Wheat Oxylipins in Response to Aphids, CO2 and Nitrogen Regimes. Molecules 2023, 28, 4133. https://doi.org/10.3390/molecules28104133
Cascant-Vilaplana MM, Viteritti E, Sadras V, Medina S, Sánchez-Iglesias MP, Oger C, Galano J-M, Durand T, Gabaldón JA, Taylor J, et al. Wheat Oxylipins in Response to Aphids, CO2 and Nitrogen Regimes. Molecules. 2023; 28(10):4133. https://doi.org/10.3390/molecules28104133
Chicago/Turabian StyleCascant-Vilaplana, Mari Merce, Eduardo Viteritti, Víctor Sadras, Sonia Medina, María Puerto Sánchez-Iglesias, Camille Oger, Jean-Marie Galano, Thierry Durand, José Antonio Gabaldón, Julian Taylor, and et al. 2023. "Wheat Oxylipins in Response to Aphids, CO2 and Nitrogen Regimes" Molecules 28, no. 10: 4133. https://doi.org/10.3390/molecules28104133
APA StyleCascant-Vilaplana, M. M., Viteritti, E., Sadras, V., Medina, S., Sánchez-Iglesias, M. P., Oger, C., Galano, J.-M., Durand, T., Gabaldón, J. A., Taylor, J., Ferreres, F., Sergi, M., & Gil-Izquierdo, A. (2023). Wheat Oxylipins in Response to Aphids, CO2 and Nitrogen Regimes. Molecules, 28(10), 4133. https://doi.org/10.3390/molecules28104133