Adsorption of Brilliant Green Dye onto a Mercerized Biosorbent: Kinetic, Thermodynamic, and Molecular Docking Studies
Abstract
1. Introduction
2. Results and Discussion
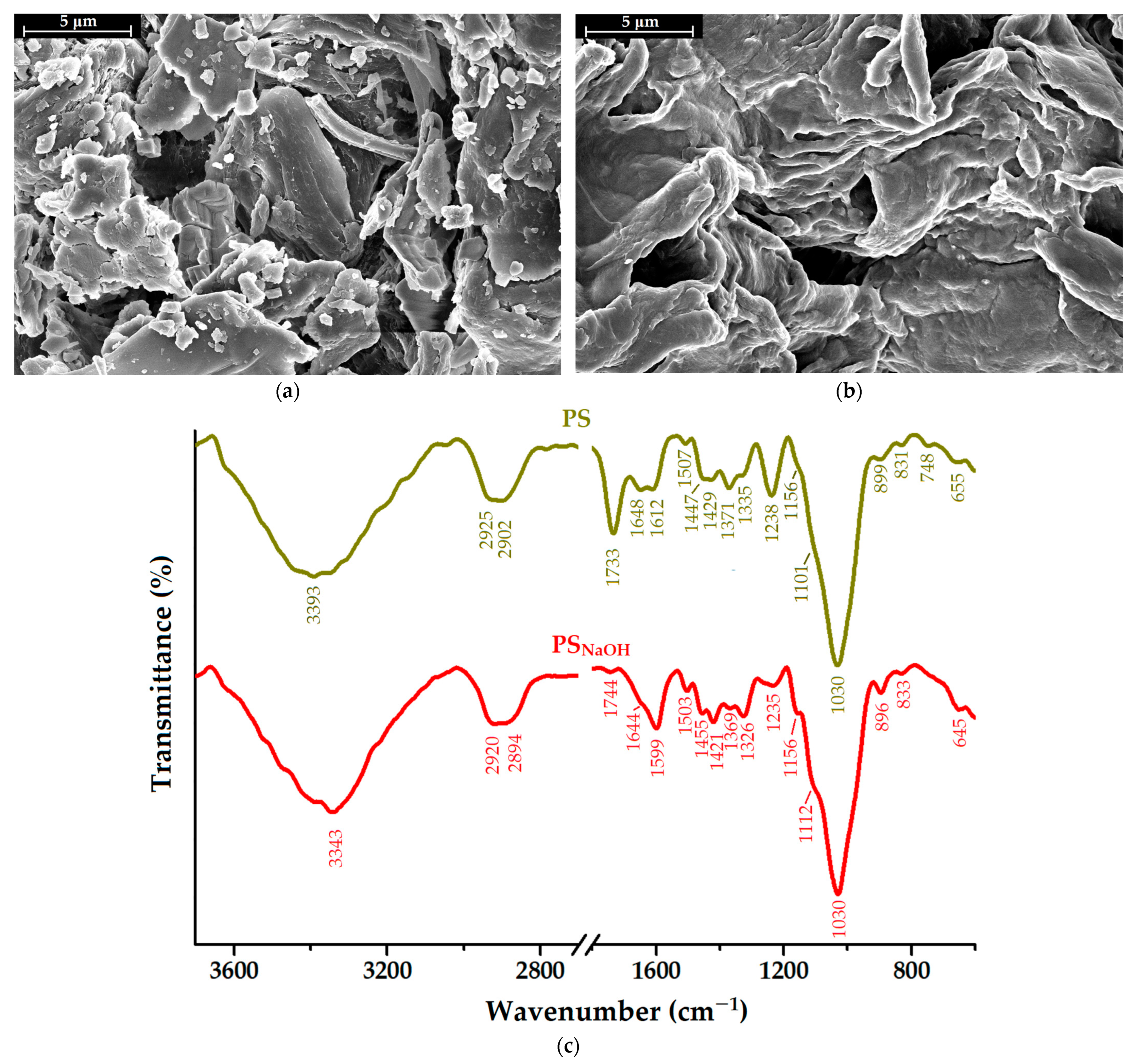
2.1. Modification of Pistachio Shells by Mercerization (PSNaOH)
2.2. Characterization of PSNaOH Biosorbent
2.3. Adsorption Studies of Brilliant Green onto PSNaOH Biosorbent
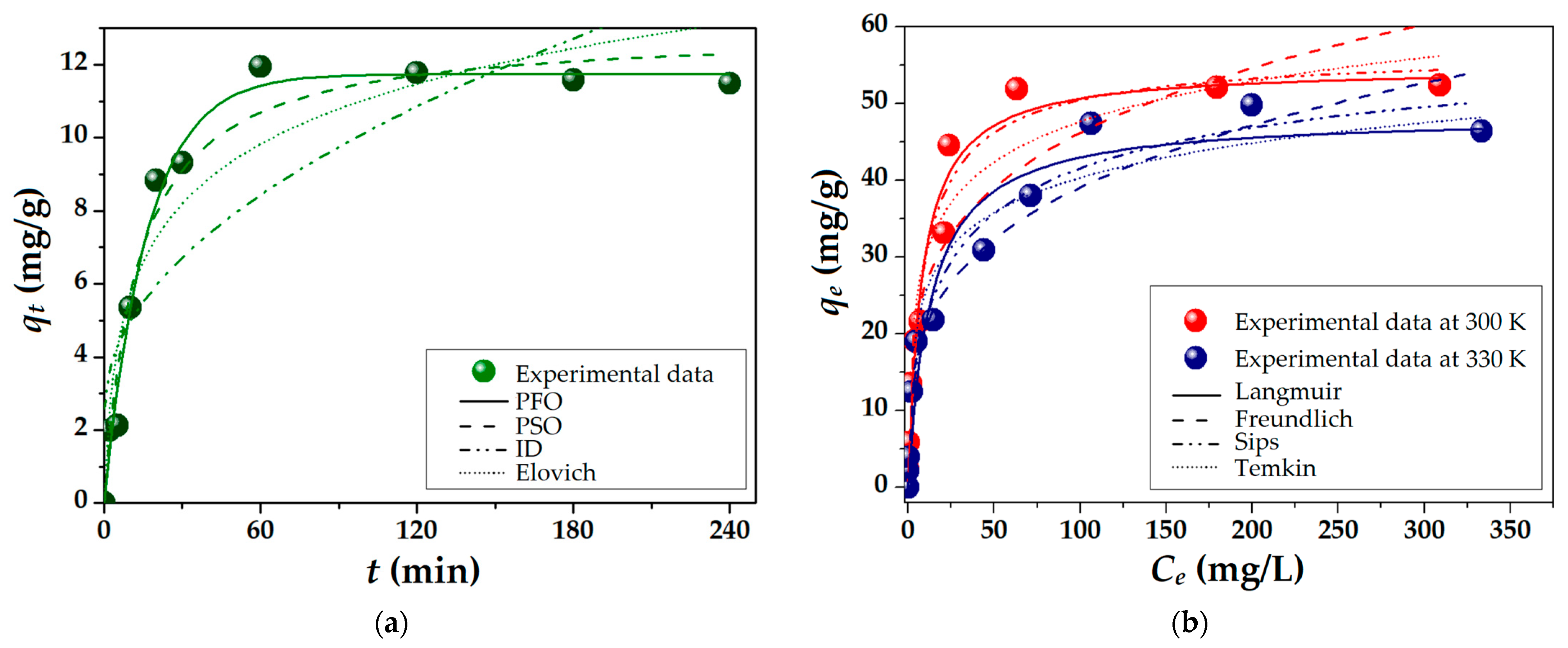
2.3.1. Adsorption Kinetics and Isotherms
2.3.2. Thermodynamic Parameters
2.3.3. Design of Experiments (DoE) for Data-Driven Modeling and Optimization
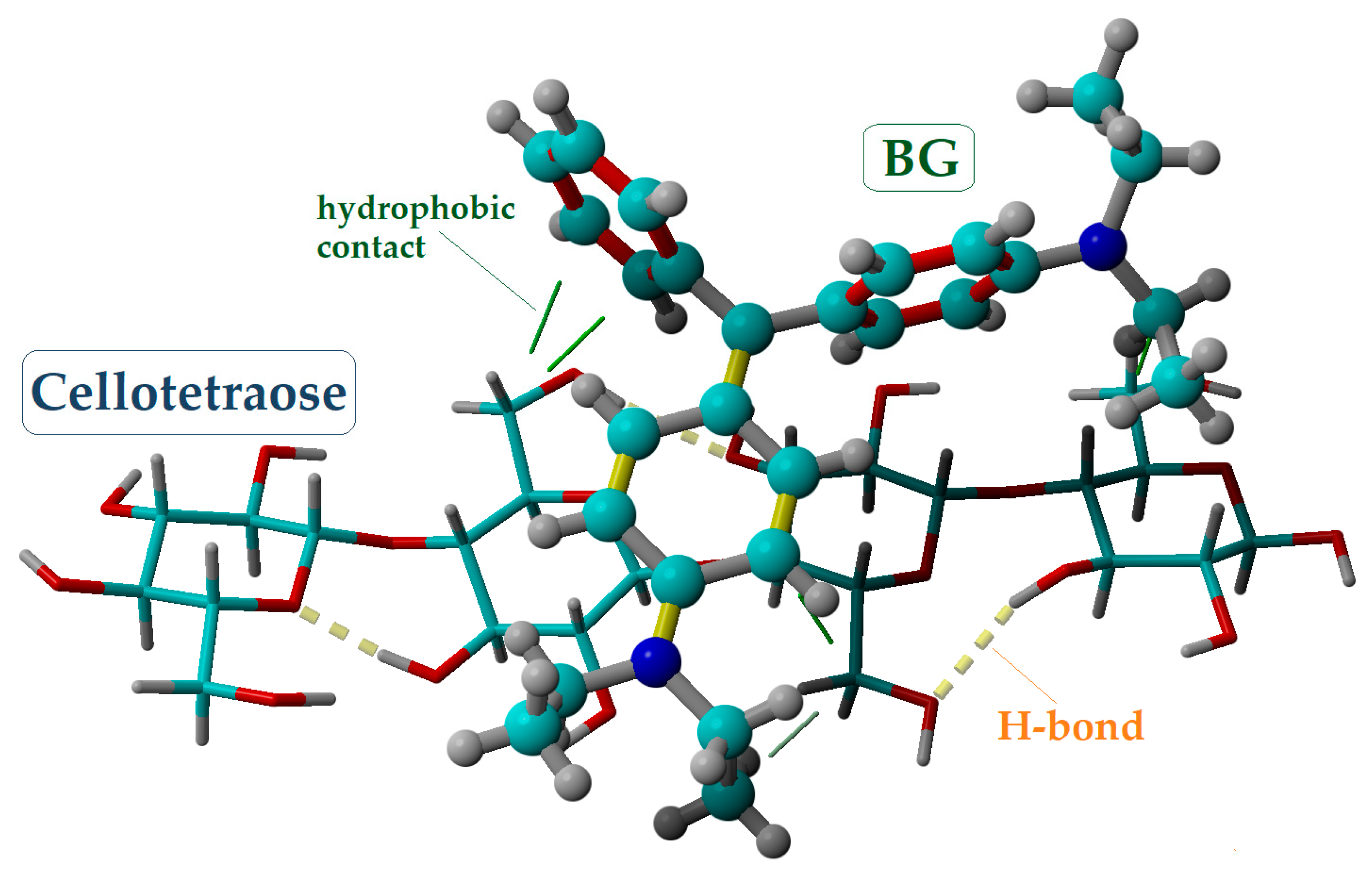
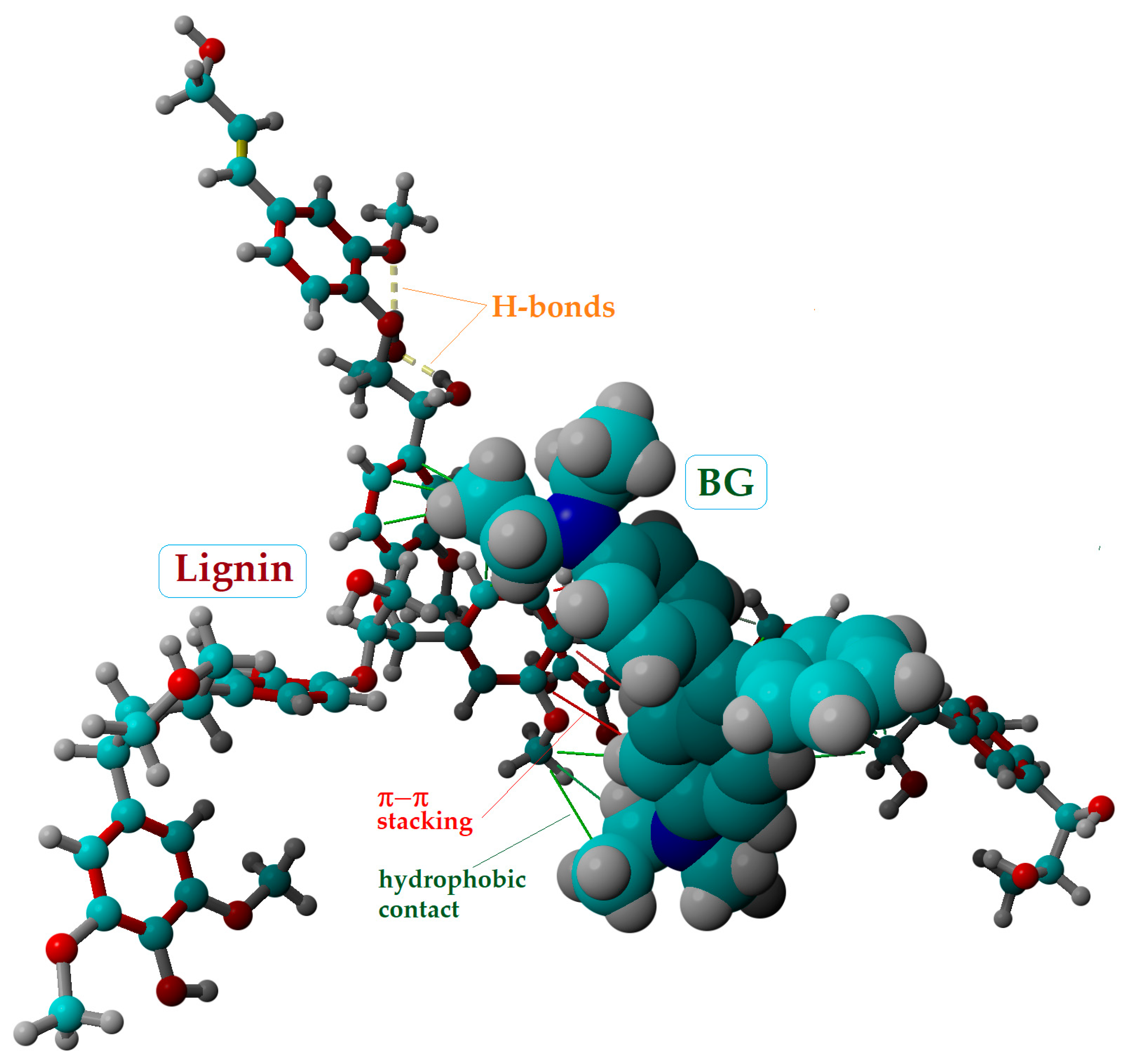
2.4. Molecular Modeling and Docking
3. Materials and Methods
3.1. Materials
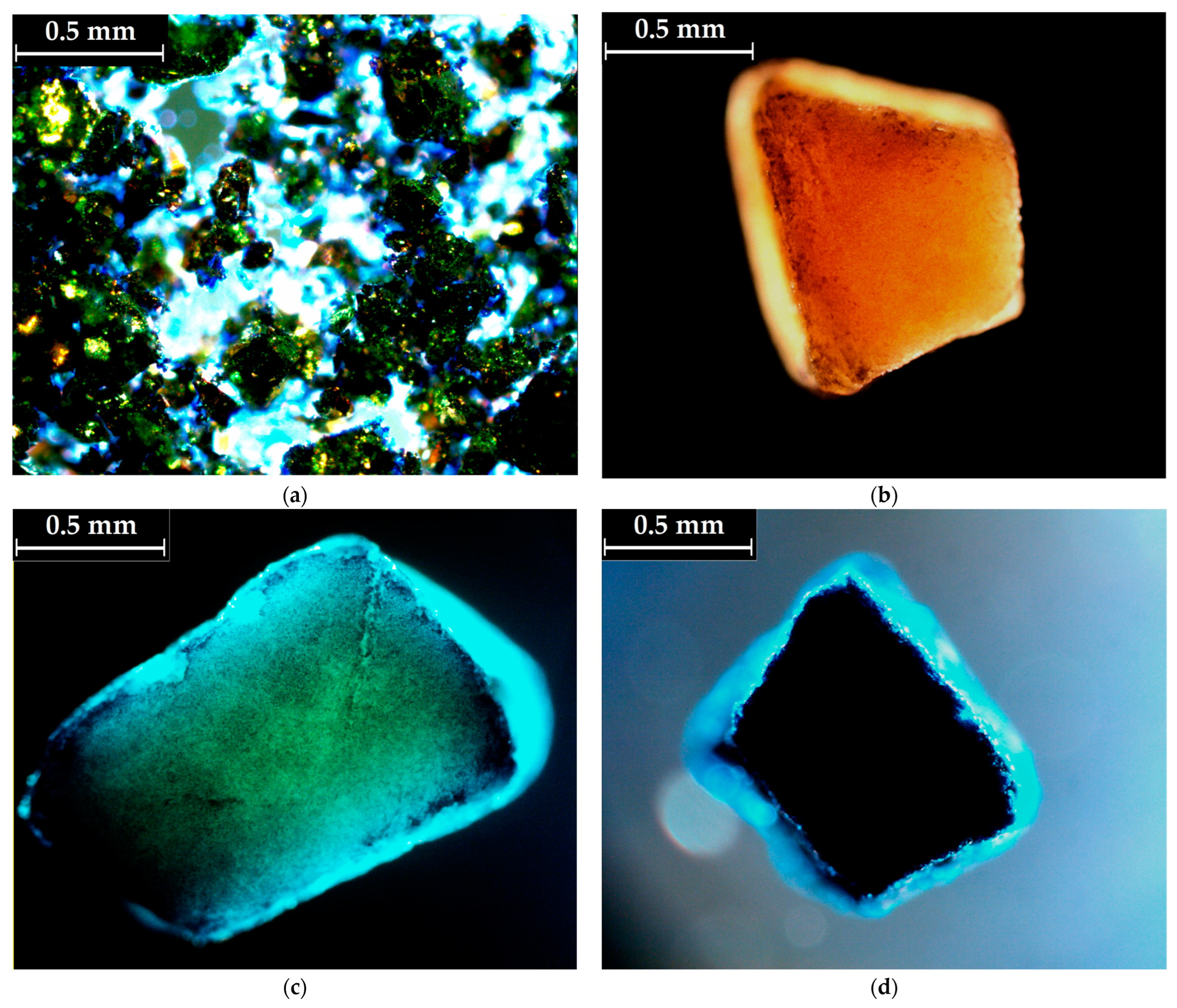
3.2. PSNaOH Biosorbent Characterization
3.3. Adsorption of Brilliant Green onto PSNaOH Biosorbent
3.4. Optimization of the Adsorption Process
3.5. Molecular Modeling of Brilliant Green Cationic Dye
3.6. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pratap, B.; Kumar, S.; Nand, S.; Azad, I.; Bharagava, R.N.; Ferreira, L.F.R.; Dutta, V. Wastewater generation and treatment by various eco-friendly technologies: Possible health hazards and further reuse for environmental safety. Chemosphere 2023, 313, Z37547. [Google Scholar] [CrossRef] [PubMed]
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Teo, S.H.; Ng, C.H.; Islam, A.; Abdulkareem-Alsultan, G.; Joseph, C.G.; Janaun, J.; Taufiq-Yap, Y.H.; Khandaker, S.; Islam, G.J.; Znad, H.; et al. Sustainable toxic dyes removal with advanced materials for clean water production: A comprehensive review. J. Clean. Prod. 2021, 332, 130039. [Google Scholar] [CrossRef]
- Adnan, A.; Omer, M.; Khan, B.; Khan, I.; Alamzeb, M.; Zada, F.M.; Ullah, I.; Shah, R.; Alqarni, M.; Simal-Gandara, J. Equilibrium, Kinetic and Thermodynamic Studies for the Adsorption of Metanil Yellow Using Carbonized Pistachio Shell-Magnetic Nanoparticles. Water 2022, 14, 4139. [Google Scholar] [CrossRef]
- Mansour, R.; Simeda, M.G.; Zaatout, A. Adsorption studies on brilliant green dye in aqueous solutions using activated carbon derived from guava seeds by chemical activation with phosphoric acid. Desalination Water Treat. 2020, 202, 396–409. [Google Scholar] [CrossRef]
- Karanfil, D.Y.; Coşkun, R.; Delibaş, A. Aminated magnetic polymeric resin for removal of anthraquinone and azo dyes from aqueous solutions. J. Polym. Res. 2022, 29, 87. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, H.; Dhiman, S.; Sahu, N.K. Decontamination of cationic dye brilliant green from the aqueous media. Appl. Water Sci. 2022, 12, 61. [Google Scholar] [CrossRef]
- Şentürk, I.; Alzein, M. Adsorptive removal of basic blue 41 using pistachio shell adsorbent—Performance in batch and column system. Sustain. Chem. Pharm. 2020, 16, 100254. [Google Scholar] [CrossRef]
- Ghoniem, M.G.; Ali, F.A.M.; Abdulkhair, B.Y.; Elamin, M.R.A.; Alqahtani, A.M.; Rahali, S.; Ben Aissa, M.A. Highly Selective Removal of Cationic Dyes from Wastewater by MgO Nanorods. Nanomaterials 2022, 12, 1023. [Google Scholar] [CrossRef] [PubMed]
- Nandi, B.K.; Goswami, A.; Purkait, M.K. Adsorption characteristics of brilliant green dye on kaolin. J. Hazard. Mater. 2009, 161, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Babalska, Z.Ł.; Korbecka-Paczkowska, M.; Karpiński, T.M. Wound Antiseptics and European Guidelines for Antiseptic Application in Wound Treatment. Pharmaceuticals 2021, 14, 1253. [Google Scholar] [CrossRef]
- Mane, V.S.; Mall, I.D.; Srivastava, V.C. Use of bagasse fly ash as an adsorbent for the removal of brilliant green dye from aqueous solution. Dyes. Pigm. 2007, 73, 269–278. [Google Scholar] [CrossRef]
- Mane, V.S.; Babu, P.V.V. Studies on the adsorption of Brilliant Green dye from aqueous solution onto low-cost NaOH treated saw dust. Desalination 2011, 273, 321–329. [Google Scholar] [CrossRef]
- Pohanish, R.P. Sittig’s Handbook of Toxic and Hazardous Chemicals and Carcinogens, 7th ed.; Elsevier Inc.: Oxford, UK, 2017; pp. 2791–2792. [Google Scholar]
- El-Chaghaby, G.A.; Ramis, E.S.; Ahmad, A.F. Rice straw and rice straw ash for the removal of brilliant green dye from wastewater. Asian J. Appl. Chem. 2018, 15, 1–9. [Google Scholar] [CrossRef]
- Oplatowska, M.; Donnelly, R.F.; Majithiya, R.J.; Kennedy, D.G.; Elliott, C.T. The potential for human exposure, direct and indirect, to the suspected carcinogenic triphenylmethane dye Brilliant Green from green paper towels. Food Chem. Toxicol. 2011, 49, 1870–1876. [Google Scholar] [CrossRef]
- Abbas, M. Removal of brilliant green (BG) by activated carbon derived from medlar nucleus (ACMN)—Kinetic, isotherms and thermodynamic aspects of adsorption. Adsorp. Sci. Technol. 2020, 38, 464–482. [Google Scholar] [CrossRef]
- Kismir, Y.; Aroguz, A.Z. Adsorption characteristics of the hazardous dye Brilliant Green on Saklıkent mud. J. Chem. Eng. 2011, 172, 199–206. [Google Scholar] [CrossRef]
- Przystaś, W.; Zabłocka-Godlewska, E.; Grabińska-Sota, E. Biological Removal of Azo and Triphenylmethane Dyes and Toxicity of Process By-Products. Water Air Soil Pollut. 2012, 223, 1581–1592. [Google Scholar] [CrossRef]
- Rehman, F.; Sayed, M.; Khan, J.A.; Shah, N.S.; Khan, H.M.; Dionysiou, D.D. Oxidative removal of brilliant green by UV/S2O82−, UV/HSO5− and UV/H2O2 processes in aqueous media: A comparative study. J. Hazard. Mater. 2018, 357, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Mariah, G.K.; Pak, K.S. Removal of brilliant green dye from aqueous solution by electrocoagulation using response surface methodology. Mater. Today Proc. 2020, 20, 488–492. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Tripathy, B.K.; Debnath, A.; Kumar, M. Enhanced persulfate activated sono-catalytic degradation of brilliant green dye by magnetic CaFe2O4 nanoparticles: Degradation pathway study, assessment of bio-toxicity and cost analysis. Surf. Interfaces 2021, 26, 101412. [Google Scholar] [CrossRef]
- Sharma, N.; Purkait, M.K. Enantiomeric and racemic effect of tartaric acid on polysulfone membrane during crystal violet dye removal by MEUF process. J. Water Process. Eng. 2016, 10, 104–112. [Google Scholar] [CrossRef]
- Samiyammal, P.; Kokila, A.; Pragasan, L.A.; Rajagopal, R.; Sathya, R.; Ragupathy, S.; Krishnakumar, M.; Minnam Reddy, V.R. Adsorption of brilliant green dye onto activated carbon prepared from cashew nut shell by KOH activation: Studies on equilibrium isotherm. Environ. Res. 2022, 212, 113497. [Google Scholar] [CrossRef]
- Ghaedi, M.; Hossainian, H.; Montazerozohori, M.; Shokrollahi, A.; Shojaipour, F.; Soylak, M.; Purkait, M.K. A novel acorn based adsorbent for the removal of brilliant green. Desalination 2011, 281, 226–233. [Google Scholar] [CrossRef]
- Enache, A.-C.; Samoila, P.; Cojocaru, C.; Apolzan, R.; Predeanu, G.; Harabagiu, V. An Eco-Friendly Modification of a Walnut Shell Biosorbent for Increased Efficiency in Wastewater Treatment. Sustainability 2023, 15, 2704. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, T.; Han, C.; Bai, L.; Sun, X. One-step preparation of lignin-based magnetic biochar as bifunctional material for the efficient removal of Cr(VI) and Congo red: Performance and practical application. Bioresour. Technol. 2023, 369, 128373. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, T.; Han, C.; Lv, X.; Bai, L.; Sun, X.; Zhang, P. Facile synthesis of Fe-modified lignin-based biochar for ultra-fast adsorption of methylene blue: Selective adsorption and mechanism studies. Bioresour. Technol. 2022, 344, 126186. [Google Scholar] [CrossRef] [PubMed]
- Bushra, R.; Mohamad, S.; Alias, Y.; Jin, Y.; Ahmad, M. Current approaches and methodologies to explore the perceptive adsorption mechanism of dyes on low-cost agricultural waste: A review. Microporous Mesoporous Mater. 2021, 319, 111040. [Google Scholar] [CrossRef]
- Khezri, M.; Heerema, R.; Brar, G.; Ferguson, L. Alternate bearing in pistachio (Pistacia vera L.): A review. Trees 2020, 34, 855–868. [Google Scholar] [CrossRef]
- Taghizadeh, A.; Rad-Moghadam, K. Green fabrication of Cu/pistachio shell nanocomposite using Pistacia Vera L. hull: An efficient catalyst for expedient reduction of 4-nitrophenol and organic dyes. J. Clean. Prod. 2018, 198, 1105–1119. [Google Scholar] [CrossRef]
- Mandalari, G.; Barreca, D.; Gervasi, T.; Roussell, M.A.; Klein, B.; Feeney, M.J.; Carughi, A. Pistachio Nuts (Pistacia vera L.): Production, Nutrients, Bioactives and Novel Health Effects. Plants 2022, 11, 18. [Google Scholar] [CrossRef]
- Pumilia, G.; Cichon, M.J.; Cooperstone, J.L.; Giuffrida, D.; Dugo, G.; Schwartz, S.J. Changes in chlorophylls, chlorophyll degradation products and lutein in pistachio kernels (Pistacia vera L.) during roasting. Int. Food Res. J. 2014, 65, 193–198. [Google Scholar] [CrossRef]
- Huang, J.; Fu, S.; Gan, L. Lignin Chemistry and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 25–37. [Google Scholar]
- Aki, M.A.; Mostafa, M.M.; Bashanaini, M.S.A. Enhanced Removal of Some Cationic Dyes from Environmental Samples Using Sulphuric Acid Modified Pistachio Shells Derived Activated Carbon. J. Chromatogr. Sep. Tech. 2016, 7, 329. [Google Scholar] [CrossRef]
- Azizian, S.; Eris, S.; Wilson, L.D. Re-evaluation of the century-old Langmuir isotherm for modeling adsorption phenomena in solution. Chem. Phys. 2018, 513, 99–104. [Google Scholar] [CrossRef]
- Akbarpour, R.; Rasooli, A.; Salimi, H.; Mahroudi, A. Using Pistachio Agricultural Waste to Remove Environmental Pollutants: A Review Study. Pistachio Health J. 2022, 5, 40–48. [Google Scholar] [CrossRef]
- Zafeiropoulos, N.E. Engineering the fibre—Matrix interface in natural-fibre composites. In Properties and Performance of Natural-Fibre Composites; Pickering, K.L., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2008; pp. 127–162. [Google Scholar] [CrossRef]
- Ferro, M.; Mannu, A.; Panzeri, W.; Theeuwen, C.H.J.; Mele, A. An Integrated Approach to Optimizing Cellulose Mercerization. Polymers 2020, 12, 1559. [Google Scholar] [CrossRef]
- Khan, M.A.; Al Othman, Z.A.; Kumar, M.; Ola, M.S.; Siddique, M.R. Biosorption potential assessment of modified pistachio shell waste for methylene blue: Thermodynamics and kinetics study. Desalin. Water Treat. 2014, 56, 146–160. [Google Scholar] [CrossRef]
- Popescu, M.-C.; Popescu, C.-M.; Lisa, G.; Sakata, Y. Evaluation of morphological and chemical aspects of different wood species by spectroscopy and thermal methods. J. Mol. Struct. 2011, 988, 65–72. [Google Scholar] [CrossRef]
- Javier-Astete, R.; Jimenez-Davalos, J.; Zolla, G. Determination of hemicellulose, cellulose, holocellulose and lignin content using FTIR in Calycophyllum spruceanum (Benth.) K. Schum. and Guazuma crinita Lam. PLoS ONE 2021, 16, e0256559. [Google Scholar] [CrossRef] [PubMed]
- Mustikaningrum, M.; Cahyono, R.B.; Yuliansyah, A.T. Effect of NaOH Concentration in Alkaline Treatment Process for Producing Nano Crystal Cellulose-Based Biosorbent for Methylene Blue. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 012005. [Google Scholar] [CrossRef]
- Chambre, D.R.; Dochia, M. FT-IR characterization of cellulose crystallinity from raw bast fibers. Scien. Tech. Bull-Chem. Food Sci. Eng. 2021, 18, 10–17. [Google Scholar]
- Agwuncha, S.C.; Owonubi, S.; Fapojuwo, D.P.; Abdulkarim, A.; Okonkwo, T.P.; Makhatha, E.M. Evaluation of mercerization treatment conditions on extracted cellulose from shea nut shell using FTIR and thermogravimetric analysis. Mater. Today Proc. 2020, 38, 958–963. [Google Scholar] [CrossRef]
- Humelnicu, A.-C.; Samoila, P.; Cojocaru, C.; Dumitriu, R.; Bostanaru, A.-C.; Mares, M.; Harabagiu, V.; Simionescu, B.C. Chitosan-Based Therapeutic Systems for Superficial Candidiasis Treatment. Synergetic Activity of Nystatin and Propolis. Polymers 2022, 14, 689. [Google Scholar] [CrossRef]
- Bouziane, N.; Aloui, A.; Behloul, S.; Zertal, A. Kinetic models of aqueous 2-mercaptobenzothiazole adsorption on local clay and activated carbon. Rev. Roum. Chim. 2021, 66, 479–491. [Google Scholar]
- Baidya, K.S.; Kumar, U. Adsorption of Brilliant green dye from aqueous solution onto chemically modified areca nut husk. S. Afr. J. Chem. Eng. 2020, 35, 33–43. [Google Scholar] [CrossRef]
- Nguyen, T.H.A.; Pham, T.T. Brilliant Green Biosorption from Aqueous Solutions on Okara: Equilibrium, Kinetic and Thermodynamic Studies. J. Water Environ. Technol. 2023, 21, 30–40. [Google Scholar] [CrossRef]
- Ebelegi, A.; Ayawei, N.; Wankasi, D. Interpretation of Adsorption Thermodynamics and Kinetics. Open J. Phys. Chem. 2020, 10, 166–182. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, F.; Sang, Q.; Zhang, Y.; Chang, L.; Huang, D.; Mu, B. Investigation of 3-aminopropyltriethoxysilane modifying attapulgite for Congo red removal: Mechanisms and site energy distribution. Powder Technol. 2021, 383, 74–83. [Google Scholar] [CrossRef]
- Cojocaru, C.; Samoila, P.; Pascariu, P. Chitosan-based magnetic adsorbent for removal of water-soluble anionic dye: Artificial neural network modeling and molecular docking insights. Int. J. Biol. Macromol. 2018, 123, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Karam, T.E.; Siraj, N.; Zhang, Z.; Ezzir, A.F.; Warner, I.M.; Haber, L.H. Ultrafast and nonlinear spectroscopy of brilliant green-based nanoGUMBOS with enhanced near-infrared emission. J. Chem. Phys. 2017, 147, 144701. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Murtaza, S.; Shahid, K.; Munir, M.; Ayub, R.; Akber, S. Comparative study of Adsorptive removal of congo red and brilliant green dyes from water using peanut shell. J. Sci. Res. 2012, 11, 828–832. [Google Scholar]
- Badr, S.M.; Samaka, S. Using agricultural waste as biosorbent for hazardous brilliant green dye removal from aqueous solutions. J. Eng. Sci. Technol. 2021, 16, 3435–3454. [Google Scholar]
- Laskar, N.; Kumar, U. Removal of Brilliant Green dye from water by modified Bambusa Tulda: Adsorption isotherm, kinetics and thermodynamics study. Int. J. Environ. Sci. Technol. 2019, 16, 1649–1662. [Google Scholar] [CrossRef]
- Qu, L.; Han, T.; Luo, Z.; Liu, C.; Mei, Y.; Zhu, T. One-step fabricated Fe3O4@C core–shell composites for dye removal: Kinetics, equilibrium and thermodynamics. J. Phys. Chem. Solids 2015, 78, 20–27. [Google Scholar] [CrossRef]
- Ghosal, P.S.; Gupta, A.K. Determination of thermodynamic parameters from Langmuir isotherm constant-revisited. J. Mol. Liq. 2017, 225, 137–146. [Google Scholar] [CrossRef]
- Liu, Y. Is the free energy change of adsorption correctly calculated? J. Chem. Eng. 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- Khan, A.A.; Singh, R.P. Adsorption thermodynamics of carbofuran on Sn (IV) arsenosilicate in H+, Na+ and Ca2+ forms. Colloids Surf. 1987, 24, 33–42. [Google Scholar] [CrossRef]
- Cojocaru, C.; Humelnicu, A.C.; Pascariu, P.; Samoila, P. Artificial neural network and molecular modeling for assessing the adsorption performance of a hybrid alginate-based magsorbent. J. Mol. Liq. 2021, 337, 116406. [Google Scholar] [CrossRef]
- Soldatkina, L.; Yanar, M. Optimization of Adsorption Parameters for Removal of Cationic Dyes on Lignocellulosic Agricultural Waste Modified by Citric Acid: Central Composite Design. ChemEngineering 2023, 7, 6. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistr. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Mäkelä, M. Experimental design and response surface methodology in energy applications: A tutorial review. Energy Convers. Manag. 2017, 151, 630–640. [Google Scholar] [CrossRef]
- PubChem Database, National Library of Medicine, National Center for Biotechnology. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 9 February 2023).
- Cojocaru, C.; Humelnicu, A.-C.; Samoila, P.; Pascariu, P.; Harabagiu, V. Optimized formulation of NiFe2O4@Ca-alginate composite as a selective and magnetic adsorbent for cationic dyes: Experimental and modeling study. React. Funct. Polym. 2018, 125, 57–69. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2009; Available online: http://www.gaussian.com/ (accessed on 10 February 2023).
- Austin, A.; Petersson, G.A.; Frisch, M.J.; Dobek, F.J.; Scalmani, G.; Throssell, K. A density functional with spherical atom dispersion terms. J. Chem. Theory Comput. 2012, 8, 4989–5007. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.1; Semichem Inc.: Shawnee Mission, KS, USA, 2016; Available online: https://gaussian.com/gaussview6/ (accessed on 10 February 2023).
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Krieger, E.; Koraimann, G.; Vriend, G. Increasing the precision of comparative models with YASARA NOVA—A selfparameterizing force field. Proteins 2002, 47, 393–402. [Google Scholar] [CrossRef]
- Krieger, E.; Vriend, G. YASARA View—molecular graphics for all devices—from smartphones to workstations. Bioinformatics 2014, 30, 2981–2982. [Google Scholar] [CrossRef] [PubMed]
- Gessler, K.; Krauss, N.; Steiner, T.; Betzel, C.; Sarko, A.; Saenger, W. β-D-Cellotetraose Hemihydrate as a Structural Model for Cellulose II. An X-ray Diffraction Study. J. Am. Chem. Soc. 1995, 117, 11397–11406. [Google Scholar] [CrossRef]

| Sorbents Derived from Lignocellulosic-Based Biosorbents | Modifications | Maximum Adsorption Capacity (qm, mg/g) | Ref. |
|---|---|---|---|
| Activated carbon (AC) from acorn | Carbonization in argon atmosphere | 2.01 | [25] |
| Activated carbon (AC) from guava seeds | Chemical activation with phosphoric acid | 80.50 | [5] |
| Activated carbon (AC) from pistachio shells | Chemical activation with sulfuric acid | 151.52 | [35] |
| Activated carbon (AC) from cashew nut shells | Chemical activation with potassium hydroxide | 243.90 | [24] |
| Peanut shells | Washing with water | 19.92 | [54] |
| Watermelon peels | Hot water treatment | 25.00 | [55] |
| Rice straw | Washed with water | 30.68 | [15] |
| Areca nut husk | NaOH treatment | 18.21 | [47] |
| Sawdust from Indian Eucalyptus wood | NaOH treatment | 58.48 | [13] |
| Bambusa Tulda | Na2CO3 treatment HCl treatment Distilled water washing | 41.67 31.25 32.25 | [56] |
| Pistachio shells | NaOH treatment | 54.74 | This work |
| Approach 1 | T (K) | Kad | ΔGad (kJ/mol) | ΔHad (kJ/mol) | ΔSad (J/K∙mol) |
|---|---|---|---|---|---|
| (1) Kad = KL × CS × | 300 K | 1.779 × 104 | −24.411 | −12.602 | 39.363 |
| 330 K | 1.124 × 104 | −25.592 | 39.364 | ||
| (2) Kad = KML, | 300 K | 1.236 × 104 | −23.502 | −12.681 | 36.070 |
| 330 K | 7.786 × 103 | −24.584 | 36.069 |
| Run | Sorbent Dose | Initial Concentration of BG Dye | Removal Efficiency (Response), Determined after 120 Min of Contact Time | ||
|---|---|---|---|---|---|
| Coded x1 | Actual SD, g/L | Coded x2 | Actual C0, mg/L | ||
| 1 | −1 | 1.00 | −1 | 50.0 | 91.40 |
| 2 | +1 | 6.00 | −1 | 50.0 | 96.12 |
| 3 | −1 | 1.00 | +1 | 250.0 | 25.57 |
| 4 | +1 | 6.00 | +1 | 250.0 | 84.42 |
| 5 | −1.414 | 1.17 | 0 | 150.0 | 23.83 |
| 6 | +1.414 | 6.83 | 0 | 150.0 | 93.88 |
| 7 | 0 | 4.00 | −1.414 | 8.6 | 98.02 |
| 8 | 0 | 4.00 | +1.414 | 292.4 | 60.25 |
| 9 | 0 | 4.00 | 0 | 150.0 | 93.15 |
| 10 | 0 | 4.00 | 0 | 150.0 | 93.84 |
| 11 | 0 | 4.00 | 0 | 150.0 | 94.06 |
| Docking System (Ligand–Receptor) | Total Intermolecular: ΔE = ΔEvdW + ΔECL (kcal/mol) | van-der-Waals: ΔEvdW (kcal/mol) | Coulomb: ΔECL (kcal/mol) |
|---|---|---|---|
| Cellotetraose–BG | −9.97 | −9.00 | −0.97 |
| Lignin–BG | −29.51 | −8.21 | −21.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enache, A.-C.; Cojocaru, C.; Samoila, P.; Ciornea, V.; Apolzan, R.; Predeanu, G.; Harabagiu, V. Adsorption of Brilliant Green Dye onto a Mercerized Biosorbent: Kinetic, Thermodynamic, and Molecular Docking Studies. Molecules 2023, 28, 4129. https://doi.org/10.3390/molecules28104129
Enache A-C, Cojocaru C, Samoila P, Ciornea V, Apolzan R, Predeanu G, Harabagiu V. Adsorption of Brilliant Green Dye onto a Mercerized Biosorbent: Kinetic, Thermodynamic, and Molecular Docking Studies. Molecules. 2023; 28(10):4129. https://doi.org/10.3390/molecules28104129
Chicago/Turabian StyleEnache, Andra-Cristina, Corneliu Cojocaru, Petrisor Samoila, Victor Ciornea, Roxana Apolzan, Georgeta Predeanu, and Valeria Harabagiu. 2023. "Adsorption of Brilliant Green Dye onto a Mercerized Biosorbent: Kinetic, Thermodynamic, and Molecular Docking Studies" Molecules 28, no. 10: 4129. https://doi.org/10.3390/molecules28104129
APA StyleEnache, A.-C., Cojocaru, C., Samoila, P., Ciornea, V., Apolzan, R., Predeanu, G., & Harabagiu, V. (2023). Adsorption of Brilliant Green Dye onto a Mercerized Biosorbent: Kinetic, Thermodynamic, and Molecular Docking Studies. Molecules, 28(10), 4129. https://doi.org/10.3390/molecules28104129







