Determination of Phosphodiesterase Type-5 Inhibitors (PDE-5) in Dietary Supplements
Abstract
1. Introduction
2. Results
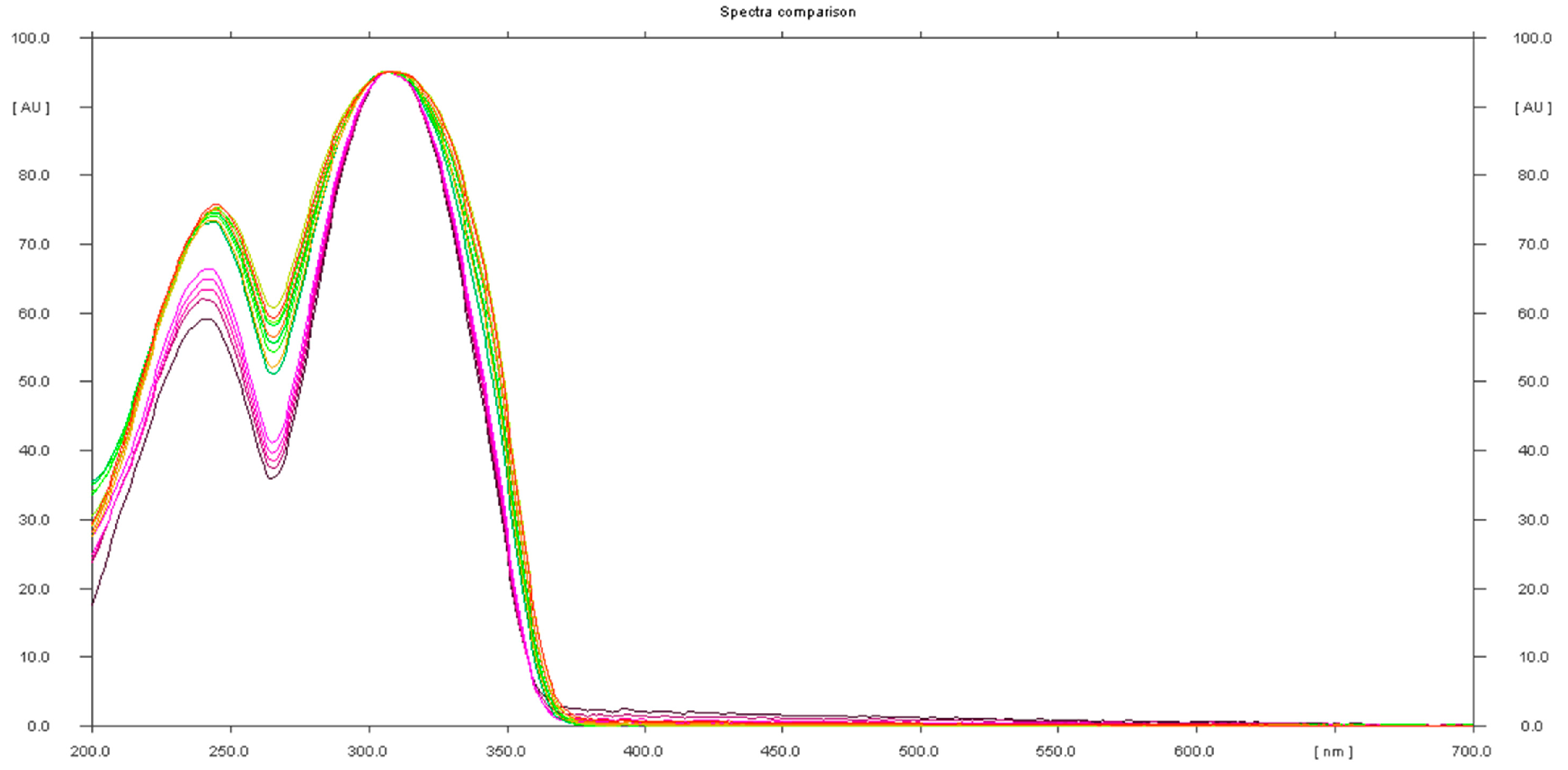
2.1. HPTLC Method Development
2.2. Analysis of Herbal Supplements
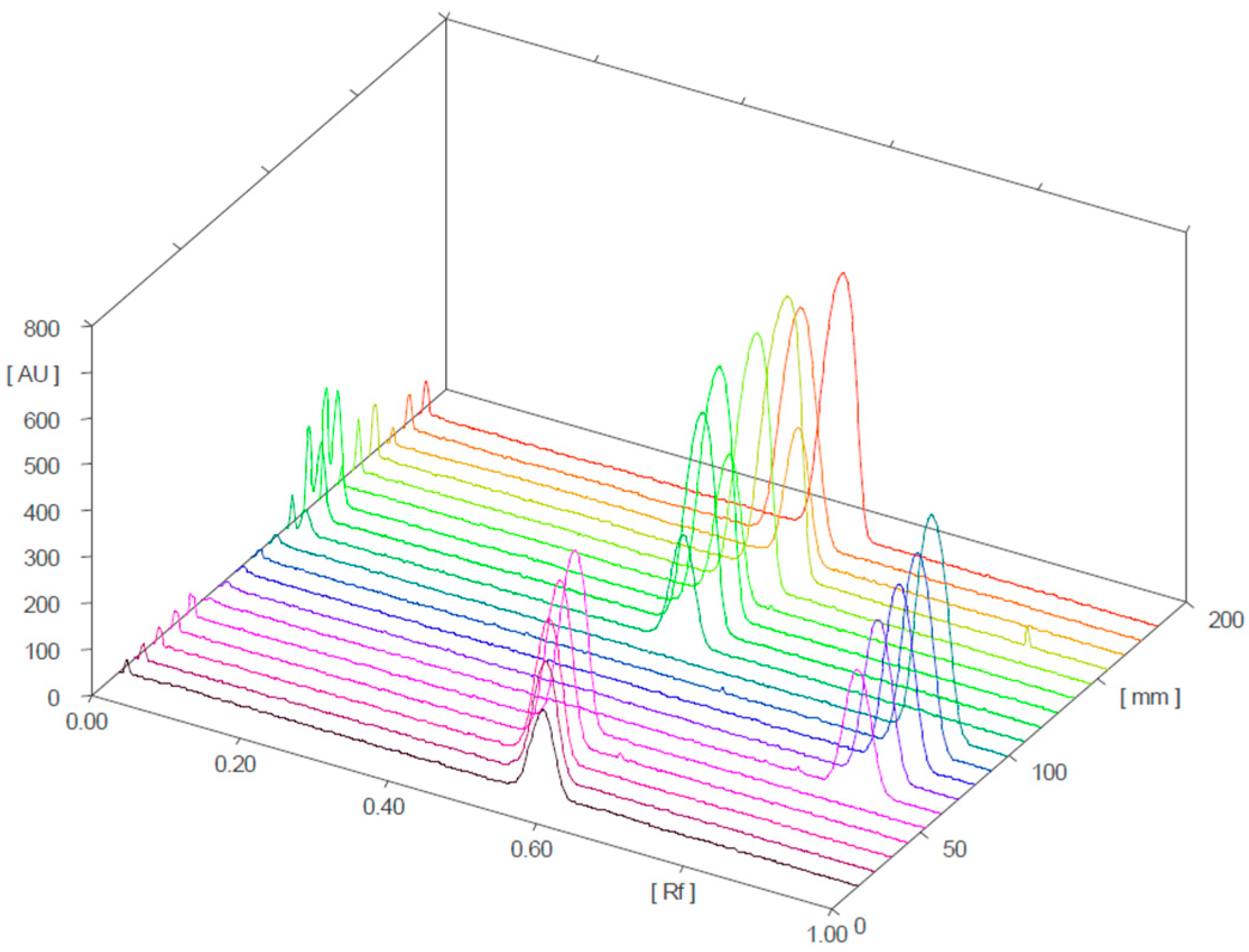
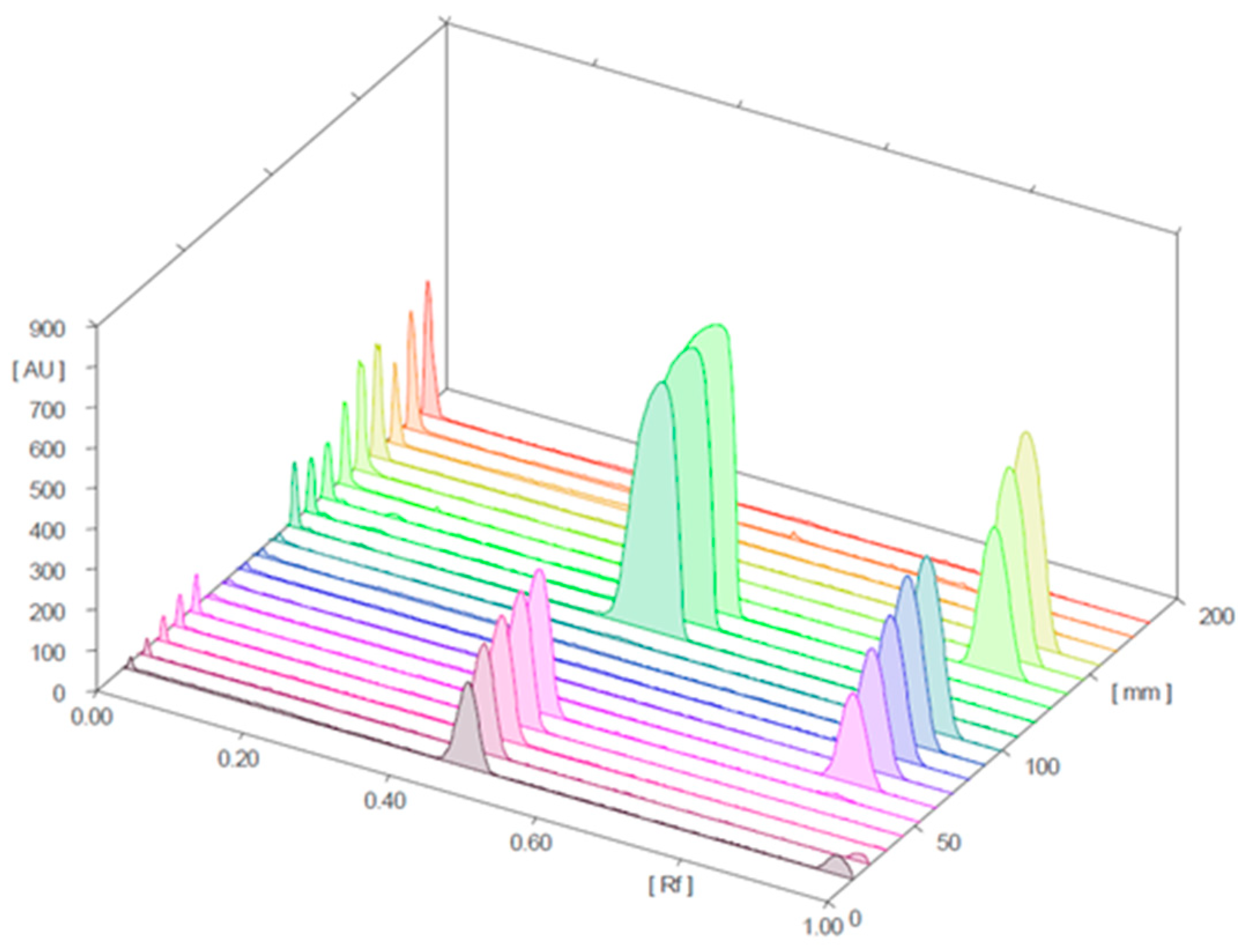
2.2.1. Characterization of Analyzed Products and HPTLC Analysis
2.2.2. Quantitative Determination of Sildenafil and Tadalafil in Herbal Samples Analyzed Via HPTLC
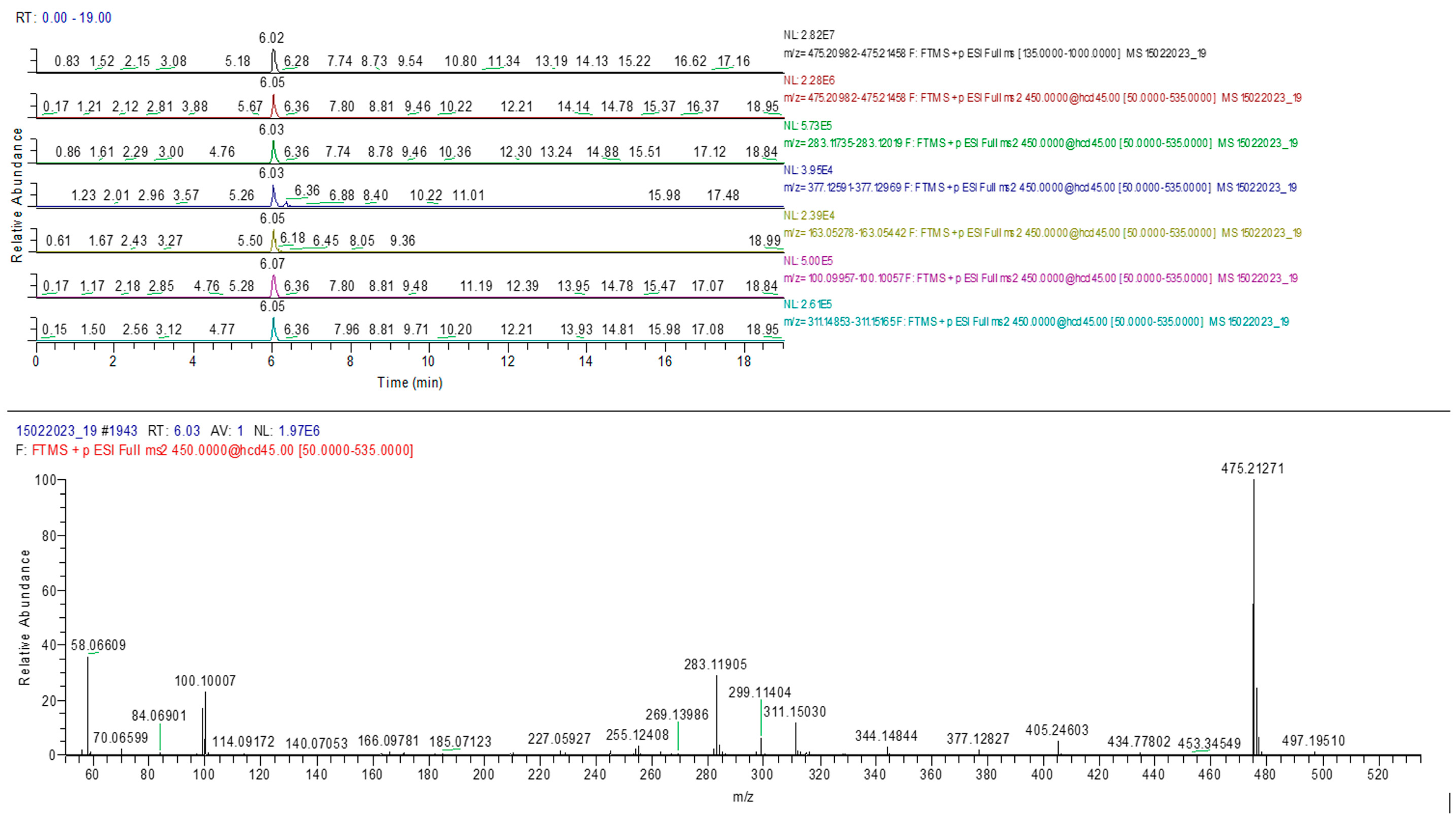
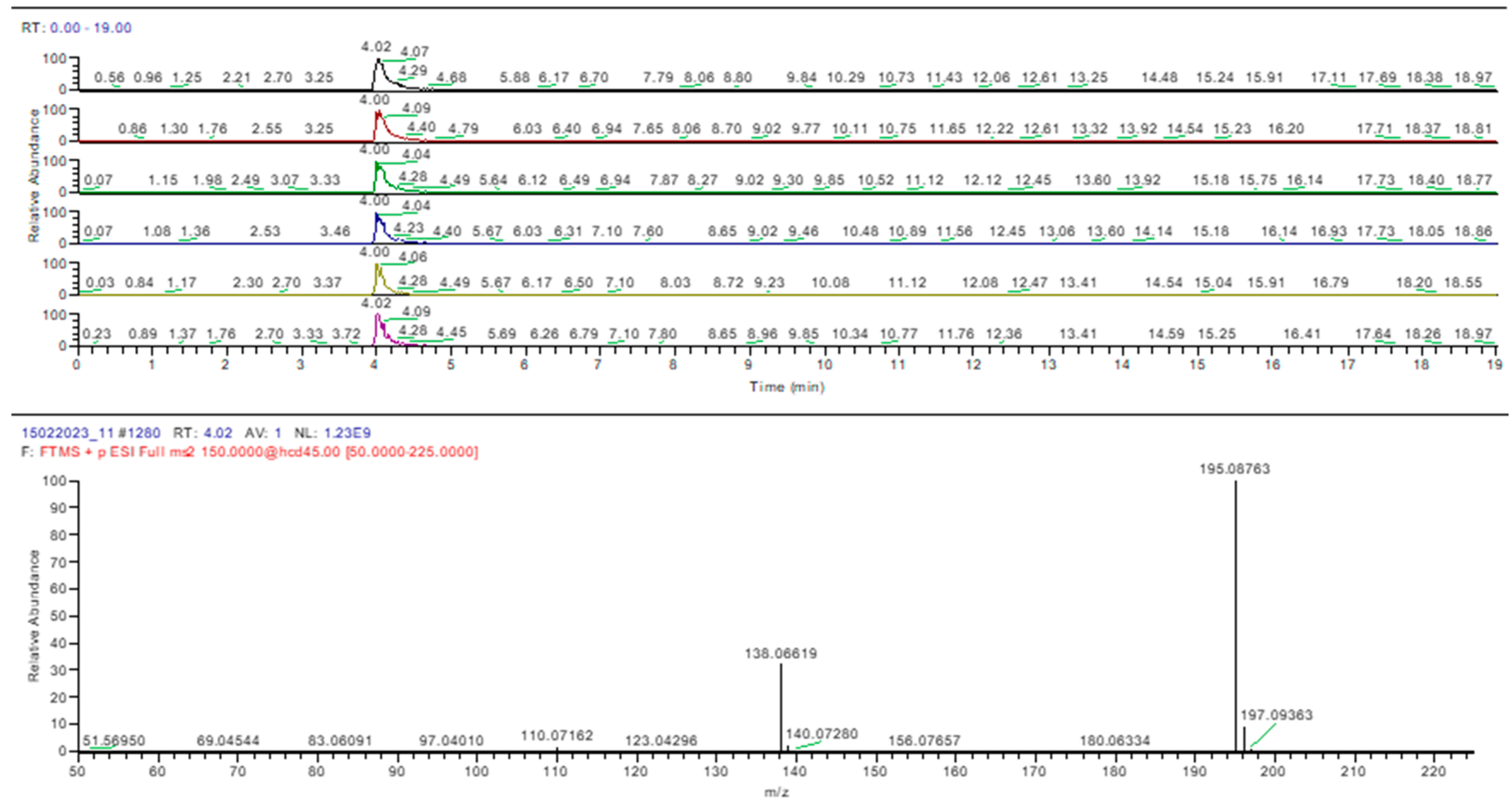
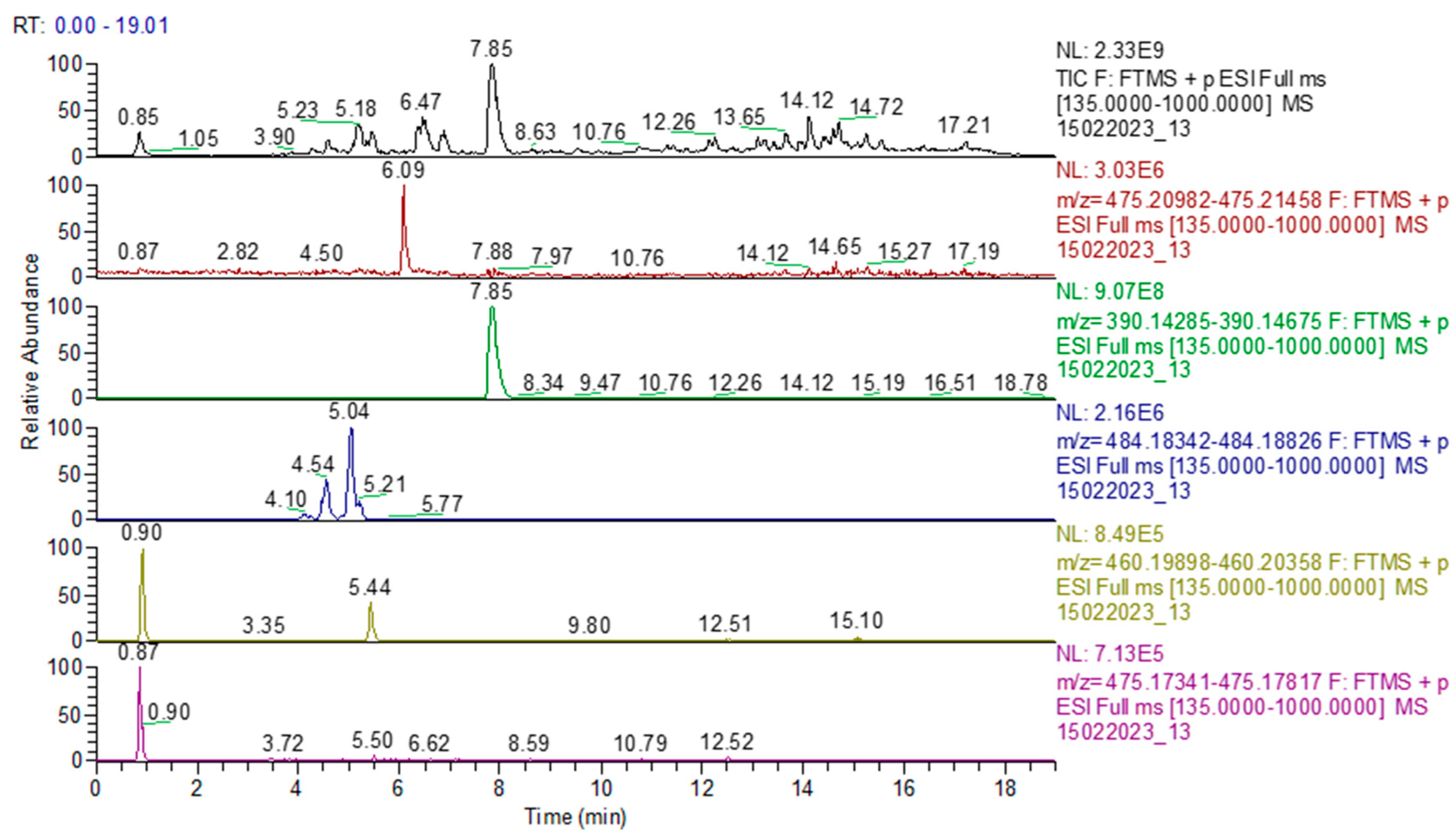
2.3. UHPLC-HRMS-MS Analysis
3. Discussion
4. Materials and Methods
4.1. HPTLC Analysis
4.1.1. Standards
4.1.2. Solvents
4.1.3. Chromatographic Parameters
4.1.4. Calibration Curve for HPTLC
4.2. Samples Preparation/Extraction
4.3. UHPLC-HRMS-MS Analysis
4.3.1. LC Parameters
4.3.2. MS Parameters
4.3.3. Calibration Curve for UHPLC-HRMS-MS
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Fode, M.; Wiborg, M.H.; Fojecki, G.; Joensen, U.N.; Jensen, C.F.S. Organic erectile dysfunction. Ugeskr. Laeger 2020, 182, V09190546. [Google Scholar] [PubMed]
- Patel, D.N.; Li, L.; Kee, C.-L.; Ge, X.; Low, M.-Y.; Koh, H.-L. Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: Analytical techniques and challenges. J. Pharm. Biomed. Anal. 2014, 87, 176–190. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 135398744, Sildenafil. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sildenafil (accessed on 17 February 2023).
- Žuntar, I.; Krivohlavek, A.; Kosić-Vukšić, J.; Granato, D.; Kovačević, D.B.; Putnik, P. Pharmacological and toxicological health risk of food (herbal) supplements adulterated with erectile dysfunction medications. Curr. Opin. Food Sci. 2018, 24, 9–15. [Google Scholar] [CrossRef]
- Sifuma, S.W.A.; Mwangi, J.W.; Mutai, P.C.; Ongarora, D.B.; Njuguna, N.M. Evaluation of adulteration of herbal medicine used for treatment of erectile dysfunction in Nairobi County, Kenya. IJPSR 2022, 13, 2095–2100. [Google Scholar]
- Redko, F.C.; Flor, S.A.; Lucangioli, S.E.; Ulloa, J.L.; Ricco, R.A.; Fernández, C.A.; Sambrotta, L.J.; Muschietti, L.V. Identification and Quantification of an Adulterant in a Dietary Supplement Marketed for Sexual Enhancement. J. Adv. Pharm. Sci. Technol. 2018, 1, 25–33. [Google Scholar] [CrossRef]
- Roessler, G.; Vobig, M.; Walter, P.; Mazinani, B.A. Ocular side effects of levitra® (vardenafil)—Results of a double-blind crossover study in healthy male subjects. Drug Des. Dev. Ther. 2018, 13, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Akuamoa, F.; Bovee, T.F.H.; van Dam, R.; Maro, L.; Wesseling, S.; Vervoort, J.; Rietjens, I.M.C.M.; Hoogenboom, R.L.A.P. Identification of phosphodiesterase type-5 (PDE-5) inhibitors in herbal supplements using a tiered approach and associated consumer risk. Food Addit. Contam. Part A 2022, 39, 1021–1032. [Google Scholar] [CrossRef]
- Graziano, S.; Montana, A.; Zaami, S.; Rotolo, M.C.; Minutillo, A.; Busardò, F.P.; Marinelli, E. Sildenafil-associated hepatoxicity: A review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2017, 21 (Suppl. S1), 17–22. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 110635, Tadalafil. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tadalafil (accessed on 17 February 2023).
- Dhaliwal, A.; Gupta, M. PDE5 Inhibitors. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 135400189, Vardenafil. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Vardenafil (accessed on 17 February 2023).
- Vardenafil. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548920/ (accessed on 16 February 2023).
- Akuamoa, F.; Hoogenboom, R.L.A.P.; Hamers, A.; Rietjens, I.M.C.M.; Bovee, T.F.H. PDE-5 inhibitors in selected herbal supplements from the Ghanaian market for better erectile function as tested by a bioassay. Toxicol. In Vitro 2021, 73, 105130. [Google Scholar] [CrossRef]
- Kotta, S.; Ansari, S.H.; Ali, J. Exploring Scientifically Proven Herbal Aphrodisiacs. Pharmacogn. Rev. 2013, 7, 1–10. [Google Scholar]
- Jang, D.J.; Lee, M.S.; Shin, B.C.; Lee, Y.C.; Ernst, E. Red ginseng for treating erectile dysfunction: A systematic review. Br. J. Clin. 2008, 66, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, F. Chemical composition and health effects of maca (Lepidium meyenii). Food Chem. 2019, 288, 422–443. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.F.; Ruiz, A.; Gonzales, C.; Villegas, L.; Cordova, A. Effect of Lepidium meyenii (maca) roots on spermatogenesis of male rats. Asian J. Androl. 2001, 3, 231–233. [Google Scholar]
- Zheng, B.L.; He, K.; Kim, C.H.; Rogers, L.; Shao, Y.; Huang, Z.Y.; Lu, Y.; Yan, S.J.; Qien, L.C.; Zheng, Q.Y. Effect of a lipidic extract from lepidium meyenii on sexual behavior in mice and rats. Urology 2000, 55, 598–602. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Cordova, A.; Gonzales, C.; Chung, A.; Vega, K.; Villena, A. Lepidium meyenii (Maca) improved semen parameters in adult men. Asian J. Androl. 2001, 3, 301–303. [Google Scholar] [PubMed]
- Gonzales, G.F.; Córdova, A.; Vega, K.; Chung, A.; Villena, A.; Góñez, C.; Castillo, S. Effect of Lepidium meyenii (MACA) on sexual desire and its absent relationship with serum testosterone levels in adult healthy men. Andrologia 2002, 34, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.; Stoddard, M.D.; Chughtai, B. Nutritional supplements and erectile dysfunction. In Molecular Mechanisms of Nutritional Interventions and Supplements for the Management of Sexual Dysfunction and Benign Prostatic Hyperplasia, 1st ed.; Chughtai, B., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 113–126. [Google Scholar]
- Zhang, Z.B.; Yang, Q.T. The testosterone mimetic properties of icariin. Asian J. Androl. 2006, 8, 601–605. [Google Scholar] [CrossRef]
- Chen, M.; Hao, J.; Yang, Q.; Li, G. Effects of Icariin on Reproductive Functions in Male Rats. Molecules 2014, 19, 9502–9514. [Google Scholar] [CrossRef]
- Guha, A. Therapeutic Use of Ginkgo Biloba. SoM Artic. 2005, 19. Available online: https://opencommons.uconn.edu/som_articles/19 (accessed on 26 February 2023).
- Wu, Y.N.; Liao, C.H.; Chen, K.C.; Liu, S.P.; Chiang, H.S. Effect of Ginkgo biloba Extract (EGb-761) on Recovery of Erectile Dysfunction in Bilateral Cavernous Nerve Injury Rat Model. Urology 2015, 85, 1214.e7–e15. [Google Scholar] [CrossRef]
- Mashhadi, Z.N.; Irani, M.; Mask, M.K.; Methie, C. A systematic review of clinical trials on Ginkgo (Ginkgo biloba) effectiveness on sexual function and its safety. Avicenna J. Phytomed. 2021, 11, 324–331. [Google Scholar]
- McKay, D. Nutrients and botanicals for erectile dysfunction: Examining the evidence. Altern. Med. Rev. 2004, 9, 4–16. [Google Scholar] [PubMed]
- Venhuis, B.J.; de Kaste, D. Towards a decade of detecting new analogues of sildenafil, tadalafil and vardenafil in food supplements: A history, analytical aspects and health risks. J. Pharm. Biomed. Anal. 2012, 69, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Fard, H.H.; Akhgari, M. Analytical perspectives of chemical adulterants in herbal sexual enhancer drugs. J. Pharm. Pharmacogn. Res. 2018, 6, 45–53. [Google Scholar]
- Do, T.T.; Theocharis, G.; Reich, E. Simultaneous detection of three Phosphodiesterase type 5 inhibitors and their analogs in lifestyle products and screening for adulteration by HPTLC. J. AOAC Int. 2015, 98, 1226–1233. [Google Scholar] [CrossRef]
- Wollein, U.; Eisenreich, W.; Schramek, N. Identification of novel sildenafil-analogues in an adulterated herbal food supplement. J. Pharm. Biomed. Anal. 2011, 56, 705–712. [Google Scholar] [CrossRef]
- Song, F.; El-Demerdash, A.; Lee, S.J. Screening for multiple phosphodiesterase type 5 inhibitor drugs in dietary supplement materials by flow injection mass spectrometry and their quantification by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012, 70, 40–46. [Google Scholar] [CrossRef]
- Ainiyah, E.Q.; Supandi, S.; Anggia, V. Review of Adulterated Herbal Products and Supplements and Methods of Analysis. Pharm. Biomed. Sci. J. 2020, 2, 53–64. [Google Scholar] [CrossRef]
- Moffat, A.C.; Osselton, M.D.; Widdop, B. Clarke’s Analysis of Drugs and Poisons, 4th ed.; Pharmaceutical Press: London, UK, 2011; p. 600. [Google Scholar]
- Dural, E. Investigation of the Presence of Sildenafil in Herbal Dietary Supplements by Validated HPLC Method. Turk. J. Pharm. Sci. 2020, 17, 56–62. [Google Scholar] [CrossRef]
- Komsta, L.; Waksmundzka-Hajnos, M.; Sherma, J. Thin Layer Chromatography in Drug Analysis, 1st ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 187–193. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2021/80 of 22 March 2021 on the Performance of Analytical Methods for Residues of Pharmacologically Active Substances Used in Food-Producing Animals and on the Interpretation of Results as Well as on the Methods to be Used for Sampling and Repealing Decisions 2002/657/EC and 98/179/EC L180/84. Available online: http://data.europa.eu/eli/reg_impl/2021/808/oj (accessed on 5 February 2023).
- Campbell, N.; Clark, J.P.; Stecher, V.J.; Thomas, J.W.; Callanan, A.C.; Donnelly, B.F.; Goldstein, I.; Kaminetsky, J.C. Adulteration of purported herbal and natural sexual performance enhancement dietary supplements with synthetic phosphodiesterase type 5 inhibitors. J. Sex. Med. 2013, 10, 1842–1849. [Google Scholar] [CrossRef]
- Assemat, G.; Balayssac, S.; Gilard, V.; Martins-Froment, N.; Fabing, I.; Rodriguez, F.; Génisson, Y.; Martino, R.; Malet-Martino, M. Isolation and identification of ten new sildenafil derivatives in an alleged herbal supplement for sexual enhancement. J. Pharm. Biomed. Anal. 2020, 191, 113482. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, S.U.; Chin, S.T.; Kee, C.L.; Low, M.Y.; Drummer, O.H.; Marriott, P.J. Rapid determination of sildenafil and its analogues in dietary supplements using gas chromatography-triple quadrupole mass spectrometry. J. Pharm. Biomed. Anal. 2016, 121, 188–196. [Google Scholar] [CrossRef]
- Ulloa, J.; Sambrotta, L.; Redko, F.; Mazza, O.N.; Garrido, G.; Becher, E.F.; Muschietti, L. Detection of a tadalafil analogue as an adulterant in a dietary supplement for erectile dysfunction. J. Sex. Med. 2015, 12, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Petkova-Gueorguieva, E.; Gueorguiev, S.; Lebanova, H.; Madzharov, V.; Mihaylova, A. Survey on Sildenafil, Tadalafil, and Vardenafil Concentrations in Food Supplements for Erectile Dysfunction. Int. J. Anal. Chem. 2022, 2022, 3950190. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.S.; Wang, R.; Tsilidis, K.K.; Zhu, H.; Daniel, C.R.; Sinha, A.; Canfield, S. Role of Caffeine Intake on Erectile Dysfunction in US Men: Results from NHANES 2001–2004. PLoS ONE 2015, 10, e0123547. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, J.; Chen, Y.; Sun, Z.; Wang, R.; Dai, Y. Effect of caffeine on erectile function via up-regulating cavernous cyclic guanosine monophosphate in diabetic rats. J. Androl. 2008, 29, 586–591. [Google Scholar] [CrossRef]
- Therapeutic Goods Administration (TGA) Safety Advisory Horny Little Devil Capsules. 2019. Available online: https://www.tga.gov.au/news/safety-alerts/horny-little-devil-capsules (accessed on 27 February 2023).
- European Pharmacopoeia, 11th ed.; The European Directorate for the Quality of Medicine & Health Care (EDQM), Council of Europe: Strasbourg, France, 2023; pp. 3957–3959.
- Jairoun, A.A.; Al-Hemyari, S.S.; Shahwan, M.; Zyoud, S.H.; Ibrahim, B.; Zyoud, S.H. Screening and Determination of Synthetic PDE-5 Inhibitors in Adulterated Sexual Enhancement Supplements. Molecules 2022, 27, 6737. [Google Scholar] [CrossRef]



| PGE-5 Inhibitor | PGE-5 Inhibitor Analogs |
|---|---|
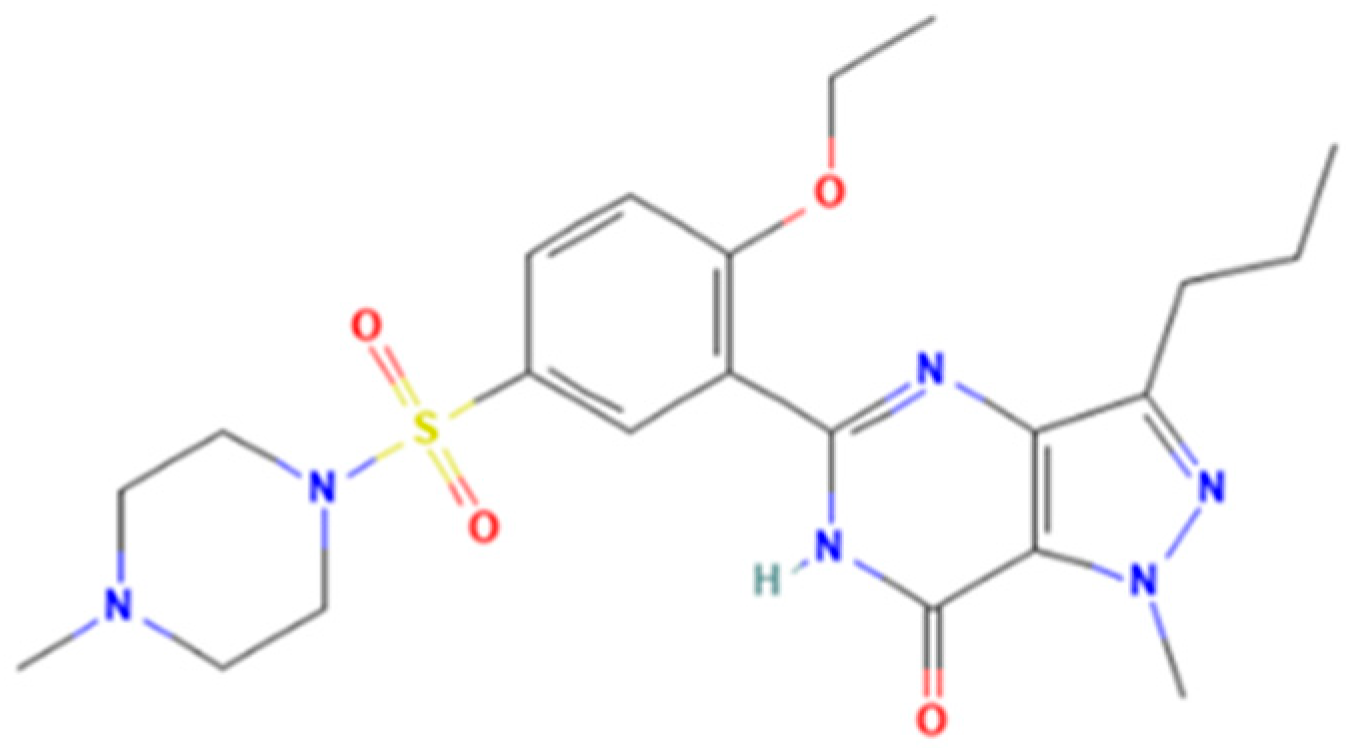
| Sildenafil | 1 N-desmethylsildenafil, 1 homosildenafil, 1 alidenafil, 1 benzylsildenafil, 1 hydroxyhomosildenafil, 1 norneosildenafil, 1 descarbonsildenafil, 1 cyclopentynafil, 2 propoxyphenyl sildenafil, 2 ropoxyphenyl hydroxyhomosildenafil, 2 propoxyphenyl alidenafil, 2 mirodenafil, 2 udenafil, 3 acetildenafil, 3 noracetidenafil, 3 hydroxyacetildenafil, 3 oxohongdenafil, 3 isopiperazinonafil, 3 nitrodenafil, 3 dimethylacetildenafil, 3 dioxiacetildenafil, 3 piperidino acetildenafil, 3 cinnamyldenafil, 3 hydroxychlorodenafil |
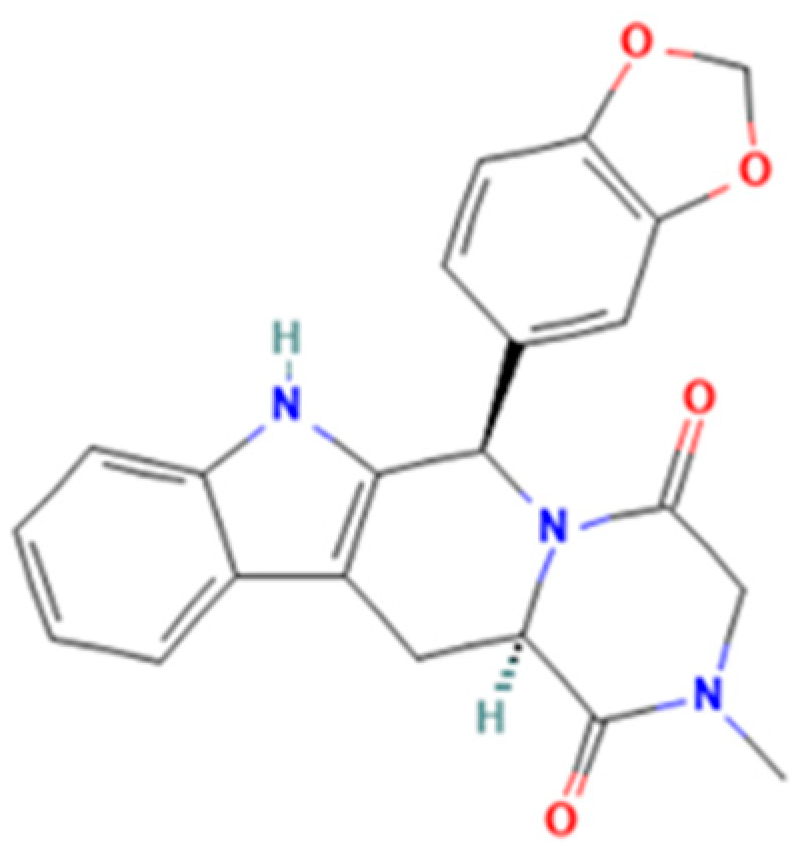
| Tadalafil | Nortadalafil, (−)-trans-tadalafil, aminotadalafil, SR-amino-tadalafil, (+)-trans-aminotadalafil, acetaminotadalafil, hydroxypropylnortadalafil, N-buthylnortadalafil, N-octylnortadalafil, chloropretadalafil |
| Vardenafil | 4 N-desmethylvardenafil, 4 hydroxyvardenafil, 4 pipeirinovardenafil, 4 pseudovardenafil, 4 piperidenafil, 5 hydroxythiovardenafil, 6 acethylvardenafil, 6 norneovardemafil, 6 imidazosagatriazinone, desulfovadenafil |
| Product Sample No./ Dosage Form | Manufacturer (Country) | Price (EUR/Unit Dose) | Description | Lot Expiry Date | Results: Labeling Inconsistencies/ Inadequacies | Presence of PDE-5 Inhibitor | Peak Purity Index |
|---|---|---|---|---|---|---|---|
| 1/ hard capsules | China | 1.00 | 100% natural product: Eleutherococcus senticosus, Poligonum multiflorum | 1 March 2026 | Not a 100% natural product | Sildenafil | 0.999687 |
| 2/ hard capsules | China | 3.5 | 100% natural product: Lycium barbarum, Cinnamomum cassia, Rhodiola rosea, Panax ginseng, Allium porum, Ginkgo biloba, vitamin D | October 2025 | Not a 100% natural product | Sildenafil | 0.999701 |
| 3/ hard capsules | China | 6.7 | 100% natural product: Epimedium brevicornum, Panax ginseng, Rhodiola rosea, Cinnamomum zeylancium | 16 March 2024 | Not a 100% natural product | Sildenafil | 0.999801 |
| 4/ hard capsules | Uncertain | 3.95 | 100% natural product: Panax ginseng, Tribulus terrestris, Serenoa repens, Zingiber officinale | September 2025 | Not a 100% natural product | Sildenafil Caffeine | 0.999630 0.999232 |
| 5/ hard capsules | Romania | 0.28 | “Active principles from natural sources”: l-arginine, royal jelly, Panax ginseng, Lepidium meyenii (Maca root extract), Tribulus terrestris, niacin | May 2024 | Possibly adulterated | Caffeine | 0.999369 |
| 6/ hard capsules | Uncertain | 6.33 | “100% Safe herbal alternative”: l-arginine, magnesium, vitamin C, Maca, ginseng root, saw palmetto (Serenoa repens), fenugreek (Trigonella foenum), l-selenomethionine, vitamin B5, zinc, vitamin B12 | - | Not a 100% natural product | Sildenafil, Tadalafil, Unidentified substance with Rf 0.4 | 0.999499 0.999623 |
| 7/ hard capsules | China | 8.66 | 100% natural product, plant extract: Panax Ginseng, Cinnamonum cassia, Apium graveolens, Brassica juncea | 9 May 2024 | Not a 100% natural product | Sildenafil | 0.999730 |
| 8/ hard capsules | China | 6.00 | 100% natural product: Epidemium brevicornum, Morinda officinalis, Cynomorium songoricum, Lycium barbarum, Panax ginseng, Poria cocos mushroom, Dioscorea opposite, Calendula officinalis | 1 May 2025 | Not a 100% natural product | Sildenafil | 0.999659 |
| 9/ hard capsules | China | 9.80 | 100% natural product: Epimedium brevicornum, Tribulus terrestris, Panax ginseng, Rhodiola rosea, Lycium chinense, Cinnamomum cassia | 17 April 2023 | Not a 100% natural product | Sildenafil | 0.999710 |
| 10/ hard capsules | China | 5.72 | 100% natural product: Rehmannia glutinosa, Panax ginseng, Dioscorea opposita, Lycium barbarum, Alpinia oxyphylla, Perenniporia fraxinea, Cinnamomum zeylanicum | 18 November 2024 | Not a 100% natural product | Sildenafil | 0.999373 |
| 11/ hard capsules | Uncertain | 8.28 | 100% Natural product: Gingko Biloba, Panax ginseng, Cinnamomum zeylancium, Poria Cocos mushroom, Codonopsis Pilosus, Ligusticum root, Angelica archangelica, Glycyrrhiza glabra | - | Not a 100% natural product | Tadalafil | 0.999669 |
| 12/ tablets | The Netherlands | 1.60 | 100% natural product: D-biotin, vitamin B6, vitamin B1, vitamin B12, magnesium | February 2025 | Clean | Negative | - |
| 13/ hard capsules | Uncertain | 4.40 | 100% Natural product: Lycium barbarum, Cinnamomum Cassia, Codonompsis pilosula, Angelica archangelica, Panax Ginseng, Poria Cocos mushroom, Lingusticum porteri, Glycyrrhiza Glabra | February 2025 | Clean | Negative | - |
| 14/ hard capsules | China | 3.70 | 100% natural product: Epimedium brevicornum, Tribulus terrestris, Panax Ginseng, Rhodiola Rosea roots, Lycium Chinese, Cinnamomum Cassia | 31 March 2025 | Not a 100% natural product | Sildenafil | 0.999679 |
| 15/ hard capsules | Romania | 0.34 | 100% natural product: Tribulus terrestris, Poligonum multiflorum, Lepidium meyenii (Maca extract), Heracleum sphondylium, Turnera difussa, Lycium barbarum (Goji extract), Ptychopetalum olacoides, Indian ginseng/Ashwagandha (Withania somnifera), Siberian ginseng (Eleutherococcus senticosus) | July 2025 | Clean | Negative | - |
| Adulterant | Number of Samples with Concentration (mg/Capsule) | ||
|---|---|---|---|
| Lower than Therapeutic Concentration | Similar to Therapeutic Concentration | Higher than Therapeutic Concentration | |
| Sildenafil | 1 (sample 6) | 8 (samples 1, 2, 3, 4, 7, 9, 10, and 14) | 1 (sample 8) |
| Tadalafil | - | - | 2 (samples 6 and 11) |
| Compound Name | R.T. (min) | Molecular Formula | Exact Mass (m/z) | Error (ppm) | Adduct Ion (m/z) | MS2 Fragments (m/z) |
|---|---|---|---|---|---|---|
| Sildenafil | 6.02 | C22H30N6O4S | 474.20492 | 0.19 | 475.21220 | 377.1278, 311.1500, 283.1187, 163.0536, 100.0995 |
| Tadalafil | 7.85 | C22H19N3O4 | 389.13756 | 0.40 | 390.14483 | 268.107, 262.0864, 240.1128, 162.0864, |
| Desmethylsildenafil | 5.17 | C21H28N6O4S | 460.18927 | 0.83 | 461.19655 | 197.0704, 135.0440 |
| Vardenafil | 5.20 | C23H32N6O4S | 488.22057 | −1.02 | 489.22783 | 377.1278, 283.1187, 87.0760 |
| Dimethylsildenafil | 6.70 | C23H32N6O4S | 488.22057 | 0.56 | 489.22783 | 376.1074, 312.1581, 169.0968, 72.0808 |
| Pseudovardenafil | 0.90 | C22H29N5O4S | 459.19403 | 0.58 | 460.20128 | 377.1278, 311.1503, 113.1073, 99.0917 |
| Vardenafil oxopiperazine | 0.87 | C21H26N6O5S | 474.16854 | −0.68 | 475.17579 | 245.0506, 169.0967 |
| Avanafil | 5.06 | C23H26ClN7O3 | 483.17856 | 1.11 | 484.18584 | 113.0084 |
| Caffeine | 4.02 | C8H10N4O2 | 194.08038 | −0.66 | 195.08765 | 344.1478, 299.1138, 169.0971 |
| Product Sample No. | Adulterant Identified | Specific Fragments (m/z) | Ion Ratio * |
|---|---|---|---|
| 1 | Sildenafil | 311.1500; 283.1187 | 42.195 |
| 2 | Sildenafil | 311.1500; 283.1187 | 43.841 |
| 3 | Sildenafil | 311.1500; 283.1187 | 44.911 |
| 4 | Sildenafil | 311.1500; 283.1187 | 44.197 |
| 6 | Sildenafil Tadalafil | 311.1500; 283.1187 268.107; 262.0864 | 40.943 82.265 |
| 7 | Sildenafil | 311.1500; 283.1187 | 43.057 |
| 8 | Sildenafil | 311.1500; 283.1187 | 42.857 |
| 9 | Sildenafil | 311.1500; 283.1187 | 42.944 |
| 10 | Sildenafil | 311.1500; 283.1187 | 43.723 |
| 11 | Tadalafil | 268.107; 262.0864 | 92.006 |
| 14 | Sildenafil | 311.1500; 283.1187 | 43.536 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghiu, O.R.C.; Ciobanu, A.M.; Guțu, C.M.; Chițescu, C.L.; Costea, G.V.; Anghel, D.M.; Vlasceanu, A.M.; Baconi, D.L. Determination of Phosphodiesterase Type-5 Inhibitors (PDE-5) in Dietary Supplements. Molecules 2023, 28, 4116. https://doi.org/10.3390/molecules28104116
Gheorghiu ORC, Ciobanu AM, Guțu CM, Chițescu CL, Costea GV, Anghel DM, Vlasceanu AM, Baconi DL. Determination of Phosphodiesterase Type-5 Inhibitors (PDE-5) in Dietary Supplements. Molecules. 2023; 28(10):4116. https://doi.org/10.3390/molecules28104116
Chicago/Turabian StyleGheorghiu, Oana Ramona Cătălina, Anne Marie Ciobanu, Claudia Maria Guțu, Carmen Lidia Chițescu, Giorgiana Valentina Costea, Daniela Mădălina Anghel, Ana Maria Vlasceanu, and Daniela Luiza Baconi. 2023. "Determination of Phosphodiesterase Type-5 Inhibitors (PDE-5) in Dietary Supplements" Molecules 28, no. 10: 4116. https://doi.org/10.3390/molecules28104116
APA StyleGheorghiu, O. R. C., Ciobanu, A. M., Guțu, C. M., Chițescu, C. L., Costea, G. V., Anghel, D. M., Vlasceanu, A. M., & Baconi, D. L. (2023). Determination of Phosphodiesterase Type-5 Inhibitors (PDE-5) in Dietary Supplements. Molecules, 28(10), 4116. https://doi.org/10.3390/molecules28104116












