Molecular Design of Luminescent Complexes of Eu(III): What Can We Learn from the Ligands
Abstract
1. Introduction
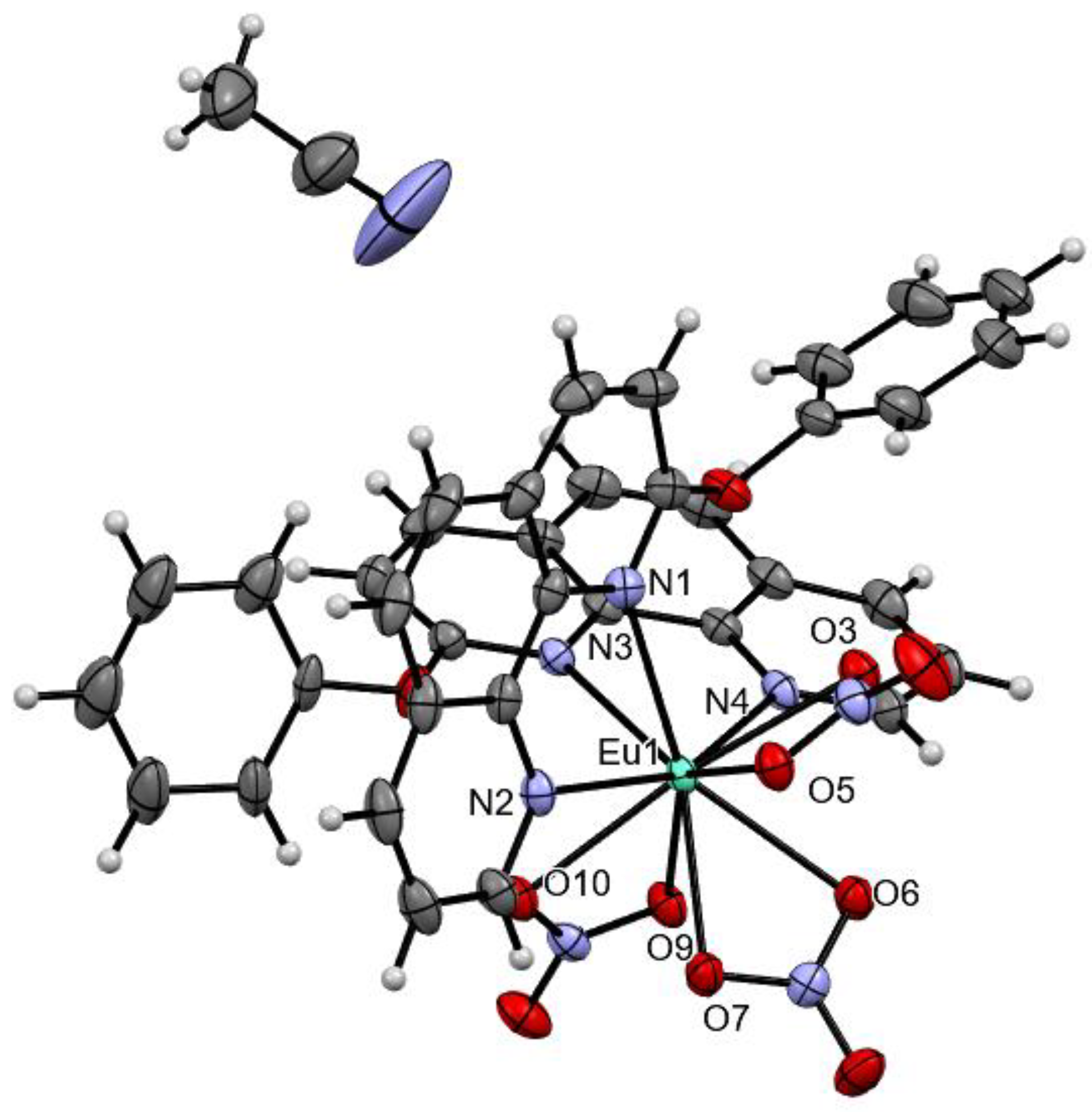
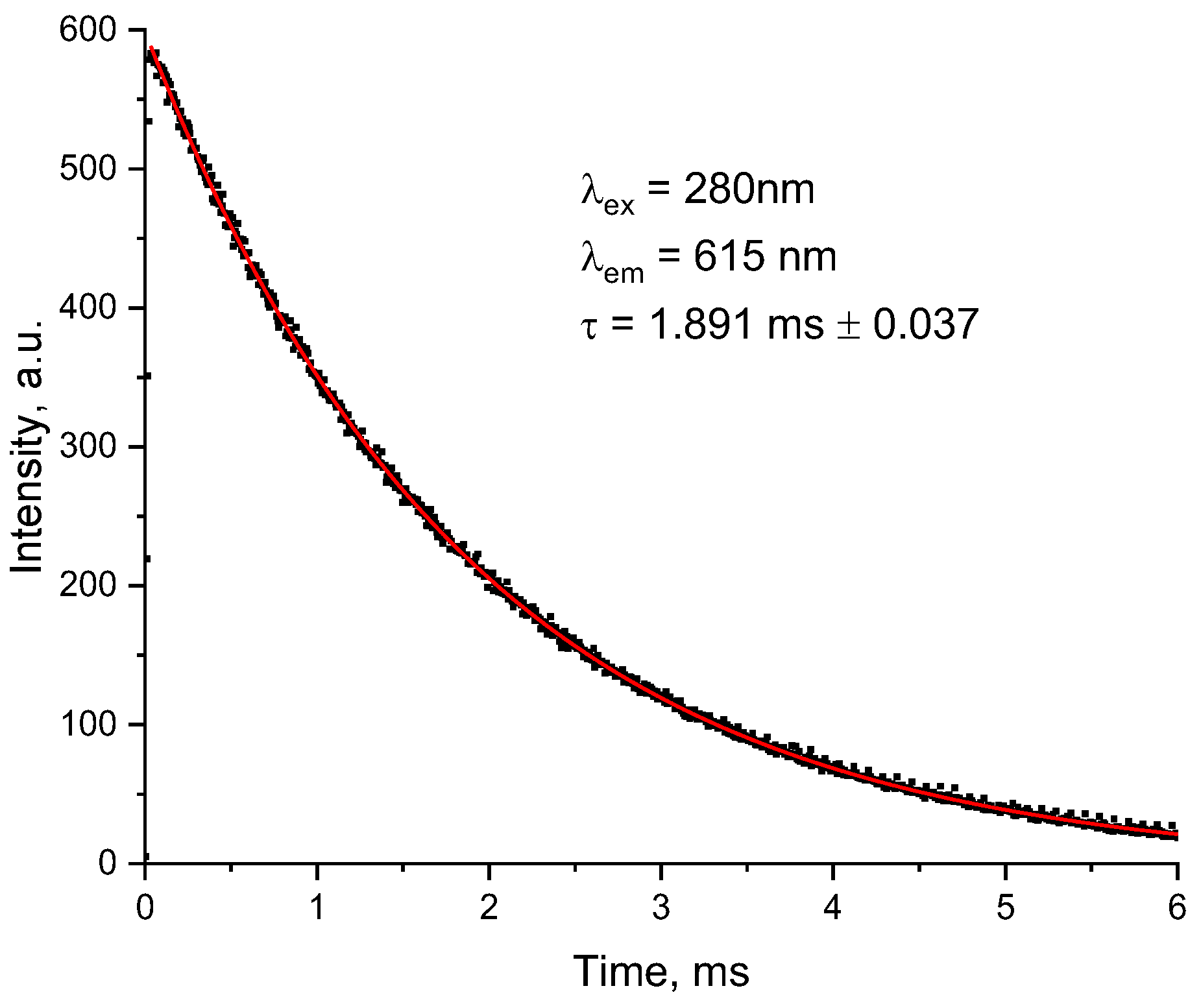
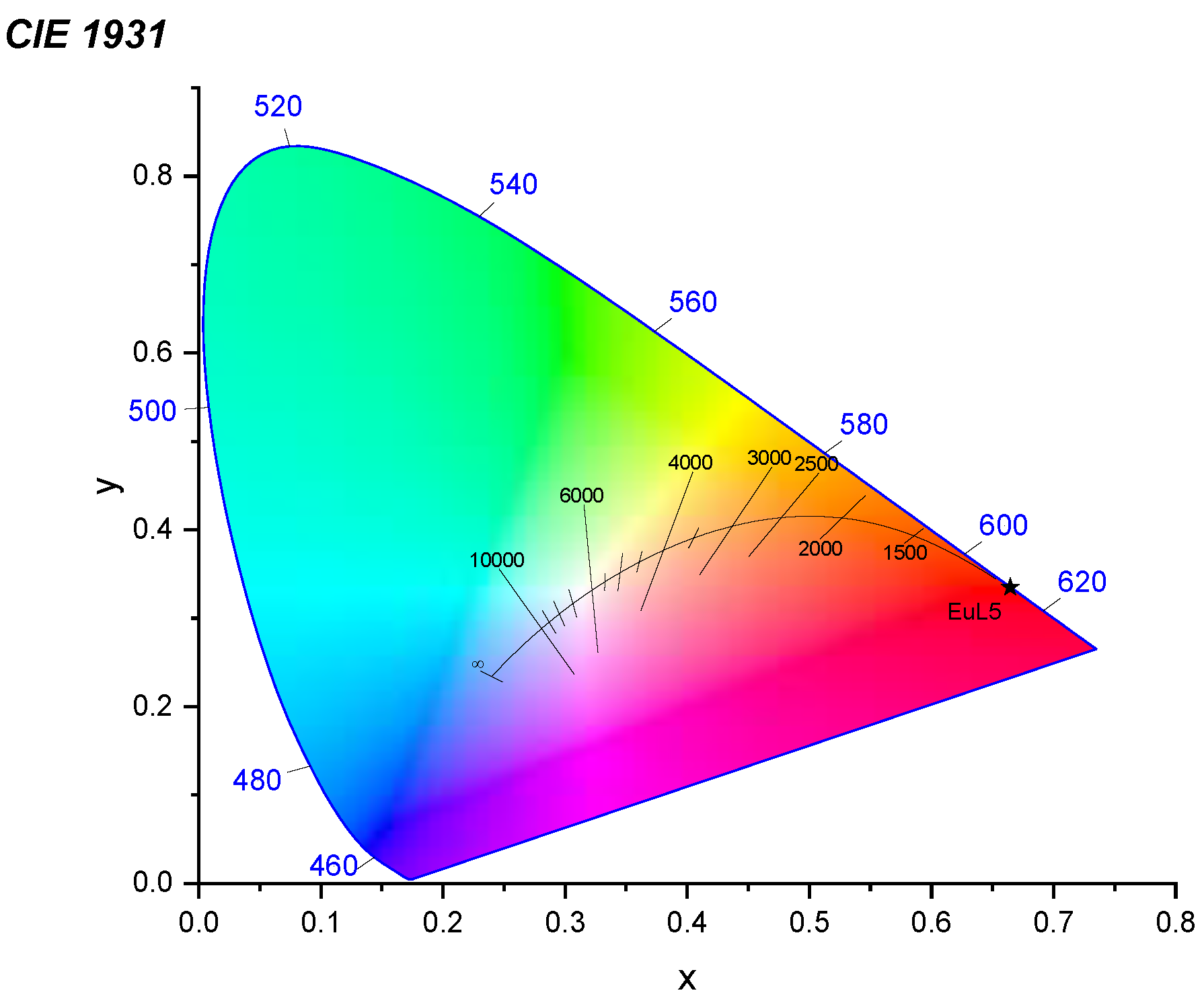
2. Results and Discussion
3. Materials and Methods
3.1. Computational Protocol
3.2. Preparation of the L5 Ligand
3.3. Synthesis of the Eu(L5)2(NO3)3 Complexes
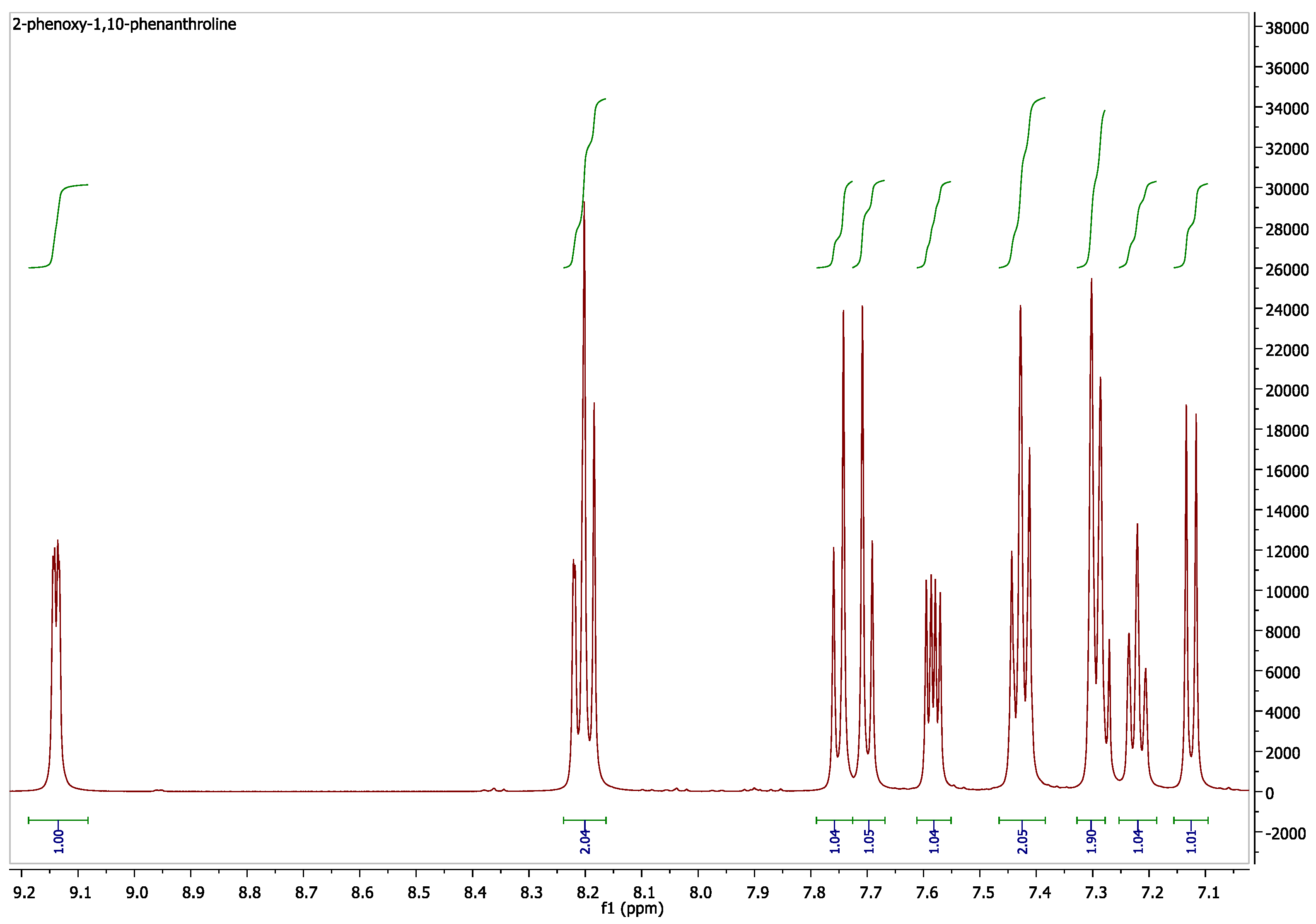
3.4. Characterization of L5 and Eu(L5)2(NO3)3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cohen, M.H. The Molecular Designing of Materials and Devices. MRS Bull. 2005, 30, 859–863. [Google Scholar] [CrossRef]
- Grimme, S.; Schreiner, P.R. Computational Chemistry: The Fate of Current Methods and Future Challenges. Angew. Chem. Int. Ed. 2018, 57, 4170–4176. [Google Scholar] [CrossRef] [PubMed]
- Tkatchenko, A. Machine learning for chemical discovery. Nat. Commun. 2020, 11, 4125. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Kitagawa, Y.; Nakanishi, T. Effective photosensitized, electrosensitized, and mechanosensitized luminescence of lanthanide complexes. NPG Asia Mater. 2018, 10, 52–70. [Google Scholar] [CrossRef]
- Qin, T.; Shi, Z.; Zhang, W.; Dong, X.; An, N.; Sakiyama, H.; Muddassir, M.; Srivastava, D.; Kumar, A. 2D isostructural Ln(III)-based coordination polymer derived from Imidazole carboxylic acid: Synthesis, structure and magnetic behavior. J. Mol. Struct. 2023, 1282, 135220. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Kirillov, A.M.; Dou, W.; Xu, C.; Xu, C.; Yang, L.; Fang, R.; Liu, W. Multifunctional Ln-MOF Luminescent Probe for Efficient Sensing of Fe3+, Ce3+, and Acetone. ACS Appl. Mater. Interfaces 2018, 10, 23976–23986. [Google Scholar] [CrossRef]
- Yu, H.; Fan, M.; Liu, Q.; Su, Z.; Li, X.; Pan, Q.; Hu, X. Two Highly Water-Stable Imidazole-Based Ln-MOFs for Sensing Fe3+, Cr2O72−/CrO42− in a Water Environment. Inorg. Chem. 2020, 59, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Utochnikova, V.V. Chapter 318—Lanthanide complexes as OLED emitters. In Including Actinides; Bünzli, J.-C.G., Pecharsky, V.K., Eds.; Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2021; Volume 59, pp. 1–91. [Google Scholar]
- Yang, Q.-Y.; Cao, Q.-Q.; Zhang, Y.-L.; Xu, X.-F.; Deng, C.-X.; Kumar, R.; Zhu, X.-M.; Wang, X.-J.; Liang, H.; Chen, Z.-F. Synthesis, structural characterization and antitumor activity of six rare earth metal complexes with 8-hydroxyquinoline derivatives. J. Inorg. Biochem. 2020, 211, 111175. [Google Scholar] [CrossRef]
- Bünzli, J.C.G. Lanthanide luminescent bioprobes (LLBs). Chem. Lett. 2009, 38, 104–109. [Google Scholar] [CrossRef]
- Hamon, N.; Roux, A.; Beyler, M.; Mulatier, J.-C.; Andraud, C.; Nguyen, C.; Maynadier, M.; Bettache, N.; Duperray, A.; Grichine, A.; et al. Pyclen-Based Ln(III) Complexes as Highly Luminescent Bioprobes for In Vitro and In Vivo One- and Two-Photon Bioimaging Applications. J. Am. Chem. Soc. 2020, 142, 10184–10197. [Google Scholar] [CrossRef]
- Weissman, S.I. Intramolecular Energy Transfer the Fluorescence of Complexes of Europium. J. Chem. Phys. 1942, 10, 214–217. [Google Scholar] [CrossRef]
- Latva, M.; Takalo, H.; Mukkala, V.-M.; Matachescu, C.; Rodríguez-Ubis, J.C.; Kankare, J. Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield. J. Lumin. 1997, 75, 149–169. [Google Scholar] [CrossRef]
- Babetto, L.; Carlotto, S.; Carlotto, A.; Rancan, M.; Bottaro, G.; Armelao, L.; Casarin, M. Antenna triplet DFT calculations to drive the design of luminescent Ln3+ complexes. Dalton Trans. 2020, 49, 14556–14563. [Google Scholar] [CrossRef] [PubMed]
- Arrué, L.; Santoyo-Flores, J.; Pizarro, N.; Zarate, X.; Páez-Hernández, D.; Schott, E. The role played by structural and energy parameters of β-Diketones derivatives as antenna ligands in Eu(III) complexes. Chem. Phys. Lett. 2021, 773, 138600. [Google Scholar] [CrossRef]
- Cosby, A.G.; Woods, J.J.; Nawrocki, P.; Sørensen, T.J.; Wilson, J.J.; Boros, E. Accessing lanthanide-based, in situ illuminated optical turn-on probes by modulation of the antenna triplet state energy. Chem. Sci. 2021, 12, 9442–9451. [Google Scholar] [CrossRef]
- Gross, E.K.U.; Kohn, W. Time-Dependent Density-Functional Theory. Adv. Quantum Chem. 1990, 21, 255–291. [Google Scholar] [CrossRef]
- Peach, M.J.G.; Williamson, M.J.; Tozer, D.J. Influence of Triplet Instabilities in TDDFT. J. Chem. Theory Comput. 2011, 7, 3578–3585. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ohmagari, H.; Tanaka, H.; Machida, K. Luminescence of lanthanide complexes: From fundamental to prospective approaches related to water- and molecular-stimuli. J. Photochem. Photobiol. C Photochem. Rev. 2022, 50, 100484. [Google Scholar] [CrossRef]
- Georgieva, I.; Trendafilova, N.; Zahariev, T.; Danchova, N.; Gutzov, S. Theoretical insight in highly luminescent properties of Eu(III) complex with phenanthroline. J. Lumin. 2018, 202, 192–205. [Google Scholar] [CrossRef]
- Accorsi, G.; Listorti, A.; Yoosaf, K.; Armaroli, N. 1,10-Phenanthrolines: Versatile building blocks for luminescent molecules, materials and metal complexes. Chem. Soc. Rev. 2009, 38, 1690–1700. [Google Scholar] [CrossRef]
- Akerboom, S.; Van Den Elshout, J.J.M.H.; Mutikainen, I.; Siegler, M.A.; Fu, W.T.; Bouwman, E. Substituted phenanthrolines as antennae in luminescent EuIII complexes. Eur. J. Inorg. Chem. 2013, 2013, 6137–6146. [Google Scholar] [CrossRef]
- Hirata, S.; Head-Gordon, M. Time-dependent density functional theory within the Tamm-Dancoff approximation. Chem. Phys. Lett. 1999, 314, 291–299. [Google Scholar] [CrossRef]
- Da Chai, J.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Elenkova, D.; Lyapchev, R.; Romanova, J.; Morgenstern, B.; Dimitrova, Y.; Dimov, D.; Tsvetkov, M.; Zaharieva, J. Luminescent Complexes of Europium (III) with 2-(Phenylethynyl)-1,10-phenanthroline: The Role of the Counterions. Molecules 2021, 26, 7272. [Google Scholar] [CrossRef]
- Zahariev, T.; Shandurkov, D.; Gutzov, S.; Trendafilova, N.; Enseling, D.; Jüstel, T.; Georgieva, I. Phenanthroline chromophore as efficient antenna for Tb3+ green luminescence: A theoretical study. Dye. Pigment. 2021, 185, 108890. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Brinen, J.S.; Rosebrook, D.D.; Hirt, R.C. Phosphorescence of o-phenanthroline. J. Phys. Chem. 1963, 67, 2651–2655. [Google Scholar] [CrossRef]
- Monguzzi, A.; Milani, A.; Mech, A.; Brambilla, L.; Tubino, R.; Castellano, C.; Demartin, F.; Meinardi, F.; Castiglioni, C. Predictive modeling of the vibrational quenching in emitting lanthanides complexes. Synth. Met. 2012, 161, 2693–2699. [Google Scholar] [CrossRef]
- Artizzu, F.; Mara, D.; Smet, P.F.; Kaczmarek, A.M.; Van Hecke, K.; Van Deun, R. Vibrational Quenching in Near-Infrared Emitting Lanthanide Complexes: A Quantitative Experimental Study and Novel Insights. Chem. Eur. J. 2019, 25, 15944–15956. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Pleshkov, D.N.; Lyssenko, K.A.; Lepnev, L.S.; Bünzli, J.-C.G.; Kuzmina, N.P. Highly Luminescent and Triboluminescent Coordination Polymers Assembled from Lanthanide β-Diketonates and Aromatic Bidentate O-Donor Ligands. Inorg. Chem. 2010, 49, 9300–9311. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Bandyopadhyay, B.N.; Harriman, A. Photoreduction of 1,10-phenanthroline. J. Chem. Soc. Faraday Trans. 1 1977, 73, 663–674. [Google Scholar] [CrossRef]
- Pan, Z.; Jia, G.; Duan, C.K.; Wong, W.Y.; Wong, W.T.; Tanner, P.A. Crystal structure, spectroscopy and crystal field analysis of substituted 1,10-phenanthroline-europium complexes. Eur. J. Inorg. Chem. 2011, 2011, 637–646. [Google Scholar] [CrossRef]
- Biju, S.; Gopakumar, N.; Bünzli, J.-C.G.; Scopelliti, R.; Kim, H.K.; Reddy, M.L.P. Brilliant Photoluminescence and Triboluminescence from Ternary Complexes of DyIII and TbIII with 3-Phenyl-4-propanoyl-5-isoxazolonate and a Bidentate Phosphine Oxide Coligand. Inorg. Chem. 2013, 52, 8750–8758. [Google Scholar] [CrossRef]
- Dolg, M.; Stoll, H.; Savin, A.; Preuss, H. Energy-adjusted pseudopotentials for the rare earth elements. Theor. Chim. Acta 1989, 75, 173–194. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A.02 2016; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Krapcho, A.P.; Lanza, J.B. Improved synthesis of 2-chloro- and 2, 9-dichloro-1,10-phenanthrolines. OPPI 2007, 39, 603–608. [Google Scholar] [CrossRef]
- Conroy, H.; Firestone, R.A. The Intermediate Dienone in the para-Claisen Rearrangement. J. Am. Chem. Soc. 1956, 78, 2290–2297. [Google Scholar] [CrossRef]
- Takamatsu, K.; Hayashi, Y.; Kawauchi, S.; Hirano, K.; Miura, M. Copper-Catalyzed Regioselective C–H Amination of Phenol Derivatives with Assistance of Phenanthroline-Based Bidentate Auxiliary. ACS Catal. 2019, 9, 5336–5344. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- The ColorCalculator Software; Osram Sylvania Inc.: Beverly, MA, USA; Available online: https://www.osram.us/cb/tools-and-resources/applications/led-colorcalculator/index.jsp (accessed on 12 February 2023).
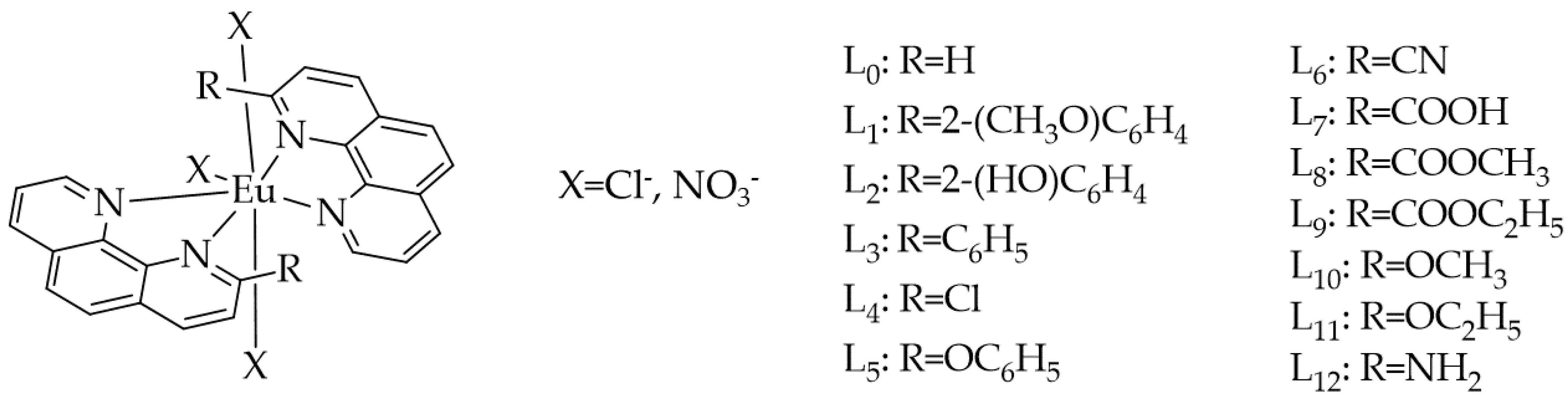



| ∆ET1→S0adiab. [eV] (λ T1→S0adiab. [nm]) and ∆ET1→S0vert. [eV] (λ T1→S0vert [nm]) | |||
|---|---|---|---|
| B97XD | |||
| Complex | Ligand | ||
| Eu(L0)2Cl3 | 2.80adiab.(443) and 2.48 vert.(499) | L0 | 2.93 adiab.(423) and 2.46vert.(505) 2.75–2.74 exp. [28] |
| Eu(L0)2(NO3)3 | 2.80adiab.(444) and 2.48 vert.(500) 2.58exp. [20] | ||
| Eu(L1)2Cl3 | 2.76adiab.(449) and 2.44 vert.(508) | L1 | 2.85adiab.(435) and 2.40 vert.(517) |
| Eu(L2)2Cl3 | 2.79adiab.(444) and 2.48 vert.(500) | L2 | 2.86adiab.(434) and 2.40 vert.(516) |
| Eu(L3)2Cl3 | 2.73adiab.(454) and 2.43 vert.(511) | L3 | 2.77adiab.(447) and 2.36 vert.(526) |
| Eu(L4)2(NO3)3 | 2.81adiab.(442) and 2.50 vert.(497) | L4 | 2.93adiab.(423) and 2.46 vert.(504) |
| Eu(L5)2(NO3)3 | 2.83adiab.(438) and 2.52 vert.(493) | L5 | 2.92adiab.(425) and 2.46 vert.(503) |
| EHOMO [eV] | ELUMO [eV] | HOMO-LUMO Gap [eV] | |
|---|---|---|---|
| L0 | −8.17 | 0.39 | 8.56 |
| L1 | −7.56 | 0.41 | 7.96 |
| L2 | −7.65 | 0.41 | 8.06 |
| L3 | −7.74 | 0.17 | 7.91 |
| L4 | −8.31 | 0.15 | 8.46 |
| L5 | −7.82 | 0.46 | 8.28 |
| Ligand | HOMO-LUMO Gap [eV] | Predicted ∆ET1→S0adiab. [eV] | Predicted and Corrected ∆ET1→S0adiab. [eV] | Luminescent Behavior |
|---|---|---|---|---|
| L6 | 8.17 | 2.77 | 2.55 | yes |
| L7 | 8.20 | 2.78 | 2.56 | yes |
| L8 | 8.23 | 2.79 | 2.57 | yes |
| L9 | 8.24 | 2.80 | 2.58 | yes |
| L10 | 8.24 | 2.80 | 2.58 | yes |
| L11 | 8.24 | 2.80 | 2.58 | yes |
| L12 | 8.24 | 2.80 | 2.58 | no * |
| Eu(L5)2(NO3)3 | |
| Crystal data | |
| Empirical formula | C38H27N8O11Eu |
| Molecular weight (g/mol) | 923.63 |
| Crystal system, space group | Triclinic, P |
| Temperature (K) | 133(2) |
| a, b, c (Å) | 10.3835(4), 11.4085(5), 16.0632(6) |
| α, β, γ (°) | 92.860(2), 103.351(2), 95.062(2) |
| V, (Å3) | 1839.35(13) |
| Z | 2 |
| Radiation type | Mo Kα |
| μ, (mm−1) | 1.780 |
| Crystal size (mm) | 0.220 × 0.120 × 0.040 |
| Data collection | |
| Diffractometer | Bruker D8 Venture |
| Absorption correction | Multi-scan (SADABS) [32] |
| Tmin, Tmax | 0.6838, 0.7456 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 50299, 8799, 8306 |
| Rint | 0.0456 |
| (sin θ/λ)max, (Å−1) | 0.639 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.0196, 0.0487, 1.066 |
| No. of reflections | 8799 |
| No. of restrains | 282 |
| No. of parameters | 579 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.393, −0.458 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanova, J.; Lyapchev, R.; Kolarski, M.; Tsvetkov, M.; Elenkova, D.; Morgenstern, B.; Zaharieva, J. Molecular Design of Luminescent Complexes of Eu(III): What Can We Learn from the Ligands. Molecules 2023, 28, 4113. https://doi.org/10.3390/molecules28104113
Romanova J, Lyapchev R, Kolarski M, Tsvetkov M, Elenkova D, Morgenstern B, Zaharieva J. Molecular Design of Luminescent Complexes of Eu(III): What Can We Learn from the Ligands. Molecules. 2023; 28(10):4113. https://doi.org/10.3390/molecules28104113
Chicago/Turabian StyleRomanova, Julia, Rumen Lyapchev, Mihail Kolarski, Martin Tsvetkov, Denitsa Elenkova, Bernd Morgenstern, and Joana Zaharieva. 2023. "Molecular Design of Luminescent Complexes of Eu(III): What Can We Learn from the Ligands" Molecules 28, no. 10: 4113. https://doi.org/10.3390/molecules28104113
APA StyleRomanova, J., Lyapchev, R., Kolarski, M., Tsvetkov, M., Elenkova, D., Morgenstern, B., & Zaharieva, J. (2023). Molecular Design of Luminescent Complexes of Eu(III): What Can We Learn from the Ligands. Molecules, 28(10), 4113. https://doi.org/10.3390/molecules28104113











