Investigation of Polyphenolic Compounds in Different Varieties of Black Chokeberry Aronia melanocarpa
Abstract
1. Introduction
2. Results
2.1. Anthocyanins
2.2. Proanthocyanidins
2.3. Flavonols
2.4. Hydroxycinnamic Acids (HCAs)
2.5. Total Polyphenols and Antiradical Activity
2.6. Organic Acids
2.7. Sugars and Sorbitol
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Reagents and Solvents
4.3. Extraction Procedure
4.4. UV Measurements
4.4.1. Total Anthocyanins
4.4.2. Total Proanthocyanidins
4.4.3. Total Polyphenols
4.4.4. Free Radical Scavenging Activity towards DPPH Radicals
4.5. Determination of Anthocyanin, Flavonol, Catechin, and HCA Profiles Using HPLC–DAD–MS
4.6. Determination of Monosaccharides, Disaccharides, and Sorbitol via Capillary Electrophoresis with DAD
4.7. Determination of Organic Acids Using HPLC–VWD
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Engels, G.; Brinckmann, J. Black chokeberry, Aronia melanocarpa. Am. Bot. Counc. 2014, 101, 1–5. [Google Scholar]
- Ćujić, N.; Kardum, N.; Šavikin, K.; Zdunić, G.; Janković, T.; Menković, N. Potential of Chokeberry (Aronia melanocarpa L.) as a Therapeutic Food. In Handbook of Food Bioengieneering; Holban, A.M., Grumezescu, A.M., Eds.; Andre Gerhard Wolff: London, UK, 2018; Volume 8, pp. 209–237. [Google Scholar]
- State Pharmacopoeia of the Russian Federation. XIV Edition. Moscow, 2018//Federal Electronic Medical Library/Ministry of Health of the Russian Federation: Official Website. Available online: https://docs.rucml.ru/feml/pharma/v14/vol4/671/ (accessed on 1 March 2023).
- Asănică, A.C.; Catană, L.; Catană, M.; Burnete, A.G.; Lazăr, M.A.; Belc, N.; Sanmartin, A.M. Internal Validation of the Methods for Determination of Water-Soluble Vitamins from Frozen Fruits by HPLC-HRMS. Rom. Biotechnol. Lett. 2019, 24, 1000–1007. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Borowska, S.; Brzóska, M.M. Chokeberries (Aronia melanocarpa) and their products as a possible means for the prevention and treatment of noncommunicable diseases and unfavorable health effects due to exposure to xenobiotics. Compr. Rev. Food Sci. Food Saf. 2016, 15, 982–1017. [Google Scholar] [CrossRef] [PubMed]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Kubica, P.; Banaszczak, P.; Wojtanowska-Krośniak, A.; Krośniak, M.; Ekiert, H. Comparative analysis of different groups of phenolic compounds in fruit and leaf extracts of Aronia sp.: A. melanocarpa, A. arbutifolia, and A.× prunifolia and their antioxidant activities. Eur. Food Res. Technol. 2017, 243, 1645–1657. [Google Scholar] [CrossRef]
- Lee, K.H.; Chun, Y.; Jang, Y.W.; Lee, S.K.; Kim, H.R.; Lee, J.H.; Kim, S.W.; Park, C.; Yoo, H.Y. Fabrication of Functional Bioelastomer for Food Packaging from Aronia (Aronia melanocarpa) Juice Processing By-Products. Foods 2020, 9, 1565. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Lachowicz, S. Effect of the Production of Dried Fruits and Juice from Chokeberry (Aronia melanocarpa L.) on the Content and Antioxidative Activity of Bioactive Compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Black Chokeberry Aronia melanocarpa L.—A Qualitative Composition, Phenolic Profile and Antioxidant Potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef] [PubMed]
- Kaloudi, T.; Tsimogiannis, D.; Oreopoulou, V. Aronia melanocarpa: Identification and Exploitation of Its Phenolic Components. Molecules 2022, 27, 4375. [Google Scholar] [CrossRef]
- Denev, P.; Kratchanova, M.; Petrova, I.; Klisurova, D.; Georgiev, Y.; Ognyanov, M.; Yanakieva, I. Black Chokeberry (Aronia melanocarpa (Michx.) Elliot) Fruits and Functional Drinks Differ Significantly in Their Chemical Composition and Antioxidant Activity. J. Chem. 2018, 2018, 9574587. [Google Scholar] [CrossRef]
- Bräunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.E.; Barsett, H. Extracts, anthocyanins and procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutrients 2013, 5, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Skupień, K.; Kostrzewa-Nowak, D.; Oszmiański, J.; Tarasiuk, J. In vitro antileukaemic activity of extracts from chokeberry (Aronia melanocarpa [Michx] Elliott) and mulberry (Morus alba L.) leaves against sensitive and multidrug resistant HL60 cells. Phytother. Res. 2008, 22, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Cui, H.; Tian, H.; Zhang, X.; Ma, L.; Ramassamy, C.; Li, J. Isolation of Neuroprotective Anthocyanins from Black Chokeberry (Aronia melanocarpa) against Amyloid-β-Induced Cognitive Impairment. Foods 2021, 10, 63. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P.; Ištuk, J.; Barron, A.R. Study of Interactions between Individual Phenolics of Aronia with Barley Beta-Glucan. Pol. J. Food Nutr. Sci. 2021, 71, 187–196. [Google Scholar] [CrossRef]
- Rop, O.; Mlček, J.; Juríková, T.; Valšíková, M.; Sochor, J.; Řezníček, V.; Kramářová, D. Phenolic content, antioxidant capacity, radical oxygen species scavenging and lipid peroxidation inhibiting activities of extracts of five black chokeberry (Aronia melanocarpa (Michx.) Elliot) cultivars. J. Med. Plants Res. 2010, 4, 2431–2437. [Google Scholar]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Miebach, E.; Adamiuk, M.; Behsnilian, D. Stability of Chokeberry Bioactive Polyphenols during Juice Processing and Stabilization of a Polyphenol-Rich Material from the By-Product. Agriculture 2012, 2, 244–258. [Google Scholar] [CrossRef]
- Slimestad, R.; Torskangerpoll, K.; Nateland, H.S.; Johannessen, T.; Giske, N.H. Flavonoids from black chokeberries, Aronia melanocarpa. J. Food Compos. Anal. 2005, 18, 61–68. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Satora, P.; Michalik, J. Transformations of Phenolic Compounds in an in vitro Model Simulating the Human Alimentary Tract. Food Technol. Biotechnol. 2009, 47, 456–463. Available online: https://hrcak.srce.hr/43906 (accessed on 23 January 2023).
- Zielińska, A.; Siudem, P.; Paradowska, K.; Gralec, M.; Kaźmierski, S.; Wawer, I. Aronia melanocarpa Fruits as a Rich Dietary Source of Chlorogenic Acids and Anthocyanins: 1H-NMR, HPLC-DAD, and Chemometric Studies. Molecules 2020, 25, 3234. [Google Scholar] [CrossRef] [PubMed]
- Bate-Smith, E.C. The phenolic constituents of plants and their taxonomic significance. I. Dicotyledons. J. Linn. Soc. Lond. Bot. 1962, 58, 95–173. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Soga, T.; Serwe, M. Rapid Monitoring of Carbohydrates in Food with Capillary Electrophoresis; Application Note; Agilent Technologies: Santa Clara, CA, USA, 2008. [Google Scholar]

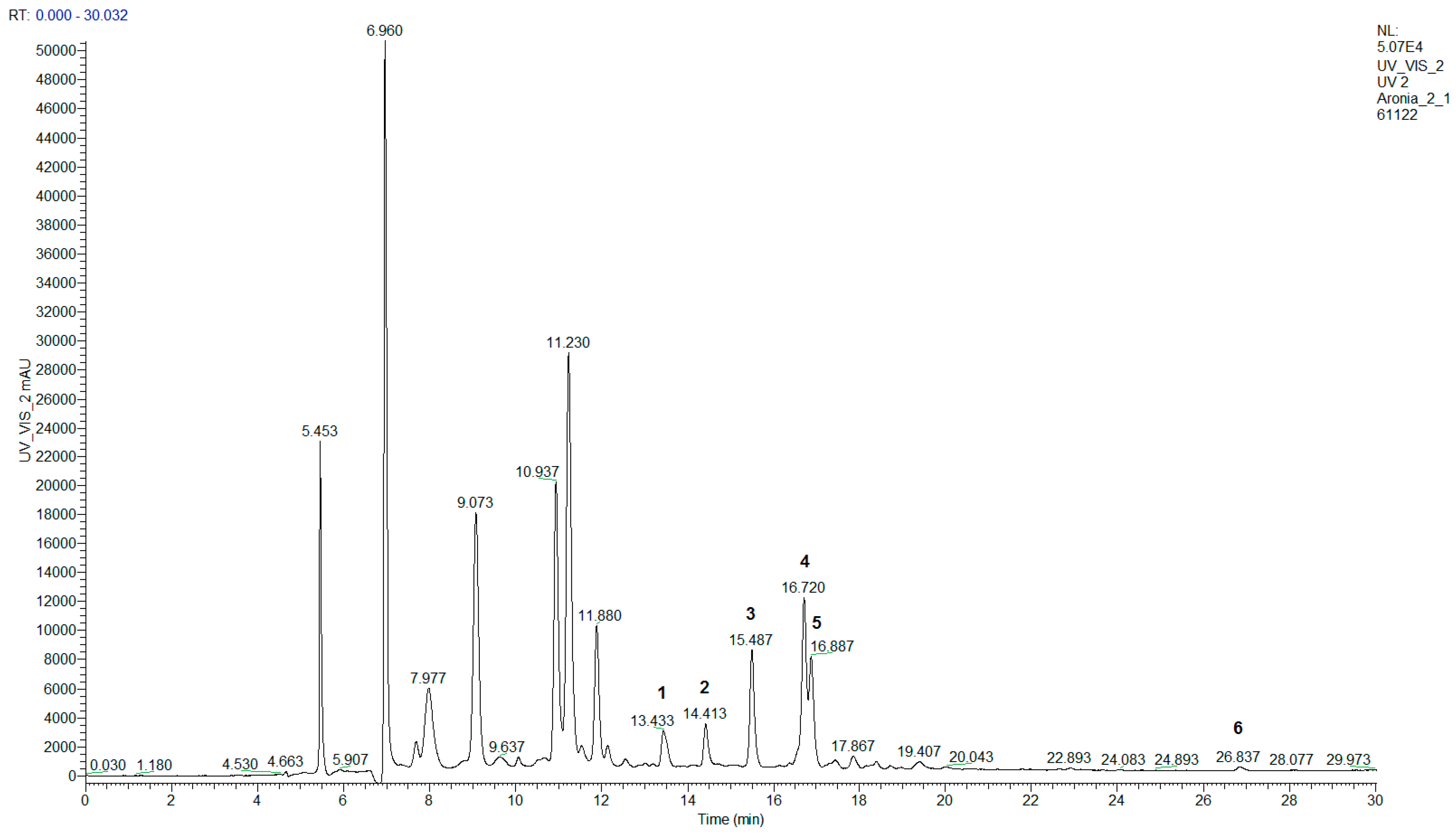
| No. | Anthocyanin | Rt, min | λmax, nm | HESI–MS+ |
|---|---|---|---|---|
| 1 | Cyanidin-3-hexoside-epicatechin | 8.1 | 524, 280 | 737.30 [M]+, 287.21 [M − hexose * − epicatechin]+ |
| 2 | Cyanidin-3-pentoside-epicatechin | 9.9 | 520, 280 | 707.31 [M]+, 287.20 [M − pentose − epicatechin]+ |
| 3 | Cyanidin-3-hexoside-epicatechin- epicatechin | 10.7 | 520, 280 | 1025.49 [M]+, 287.21 [M − hexose − epicatechin − epicatechin]+ |
| 4 | Cyanidin-3-galactoside | 15.0 | 516, 280 | 449.20 [M]+, 287.14 [M − galactose]+ |
| 5 | Cyanidin-3-glucoside | 16.9 | 516, 280 | 449.20 [M]+, 287.14 [M − glucose]+ |
| 6 | Cyanidin-3-arabinoside | 19.5 | 516, 280 | 419.18 [M]+, 287.14 [M − arabinose]+ |
| 7 | 5-carboxypyranocyanidin-3-hexoside | 20.7 | 504, 280 | 517.25 [M]+ |
| 8 | 5-carboxypyranocyanidin-3-pentoside | 24.4 | 508, 280 | 487.21 [M]+ |
| 9 | Cyanidin-3-xyloside | 25.2 | 516, 280 | 419.18 [M]+, 287.16 [M − xylose]+ |
| No. | Anthocyanin | “Nadzeya” | “Aron” | “Cherno- Plodnaya” | “Venisa” | “Mulatka” |
|---|---|---|---|---|---|---|
| Anthocyanin profile, % from TAC | ||||||
| 1 | Cyanidin-3-hexoside-epicatechin | 0.3 ± 0.02 | 0.3 ± 0.01 | 0.2 ± 0.01 | 0.2 ± 0.01 | 0.2 ± 0.02 |
| 2 | Cyanidin-3-pentoside-epicatechin | 0.2 ± 0.01 | 0.1 ± 0.02 | 0.2 ± 0.02 | 0.1 ± 0.01 | 0.1 ± 0.01 |
| 3 | Cyanidin-3-hexoside-epicatechin- epicatechin | 0.1 ± 0.01 | 0.1 ± 0.01 | ND | 0.1 ± 0.01 | 0.1 ± 0.02 |
| 4 | Cyanidin-3-galactoside | 68.7 ± 0.5 | 69.3 ± 0.6 | 65.6 ± 0.5 | 68.9 ± 0.4 | 69.3 ± 0.6 |
| 5 | Cyanidin-3-glucoside | 2.6 ± 0.1 | 2.5 ± 0.1 | 2.6 ± 0.1 | 2.5 ± 0.1 | 2.6 ± 0.2 |
| 6 | Cyanidin-3-arabinoside | 25.0 ± 0.2 | 24.2 ± 0.2 | 26.9 ± 0.3 | 24.6 ± 0.2 | 24.2 ± 0.3 |
| 7 | 5-carboxypyranocyanidin-3-hexoside | 0.3 ± 0.02 | 0.3 ± 0.01 | 0.3 ± 0.01 | 0.3 ± 0.02 | 0.2 ± 0.01 |
| 8 | 5-carboxypyranocyanidin-3-pentoside | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.2 ± 0.02 | 0.1 ± 0.01 | 0.1 ± 0.01 |
| 9 | Cyanidin-3-xyloside | 2.7 ± 0.1 | 3.1 ± 0.1 | 4.0 ± 0.2 | 3.2 ± 0.1 | 3.2 ± 0.1 |
| Total anthocyanins, mg C3GlE */100 g FW | 590.0 ± 12.4 | 620.5 ± 16.5 | 388.1 ± 8.9 | 614.7 ± 13.3 | 615.8 ± 13.7 | |
| Compound | “Nadzeya” | “Aron” | “Cherno-Plodnaya” | “Venisa” | “Mulatka” |
|---|---|---|---|---|---|
| Catechin, mg/100 g FW | ND 1 | ND | Traces 2 | ND | Traces |
| Epicatechin, mg/100 g FW | 8.3 ± 0.2 | 6.3 ± 0.2 | 1.7 ± 0.1 | 2.5 ± 0.1 | 1.7 ± 0.1 |
| Total proanthocyanidins, mg PCB2E/100 g FW | 2524.1 ± 37.7 | 2198.9 ± 35.6 | 1395.8 ± 24.0 | 2212.4 ± 31.3 | 2476.0 ± 35.5 |
| No. | Flavonol | Rt, min | λmax, nm | HESI–MS+ |
|---|---|---|---|---|
| 1 | Quercetin-dihexoside | 13.4 | 255, 265, 354 | 627.25 [M + H]+, 465.39 [M − hexose + H]+, 303.10 [M − 2 hexoses + H]+ |
| 2 | Quercetin-3-vicianoside | 14.4 | 256, 266, 355 | 597.25 [M + H]+, 303.09 [M − vicianose + H]+ |
| 3 | Rutin | 15.5 | 256, 266, 355 | 611.25 [M + H]+, 465.17 [M − rhamnose + H]+, 303.12 [M − rutinose + H]+ |
| 4 | Hyperoside | 16.7 | 256, 266, 356 | 465.22 [M + H]+, 303.13 [M − galactose + H]+ |
| 5 | Isoquercitrin | 16.9 | 256, 266, 356 | 465.20 [M + H]+, 303.08 [M − glucose + H]+ |
| 6 | Quercetin | 26.8 | 255, 267, 372 | 303.10 [M + H]+ |
| No. | Flavonol | “Nadzeya” | “Aron” | “Cherno- Plodnaya” | “Venisa” | “Mulatka” |
|---|---|---|---|---|---|---|
| 1 | Quercetin-dihexoside | 5.8 ± 0.1 | 6.3 ± 0.2 | 2.2 ± 0.1 | 3.8 ± 0.1 | 3.8 ± 0.1 |
| 2 | Quercetin-3-vicianoside | 5.7 ± 0.1 | 5.7 ± 0.1 | 3.0 ± 0.1 | 3.5 ± 0.1 | 3.4 ± 0.1 |
| 3 | Rutin | 12.3 ± 0.3 | 15.8 ± 0.3 | 7.0 ± 0.2 | 10.2 ± 0.2 | 9.8 ± 0.2 |
| 4 | Hyperoside | 16.7 ± 0.3 | 19.5 ± 0.3 | 5.7 ± 0.1 | 11.2 ± 0.2 | 10.9 ± 0.2 |
| 5 | Isoquercitrin | 10.0 ± 0.2 | 10.5 ± 0.2 | 3.1 ± 0.1 | 6.4 ± 0.1 | 6.1 ± 0.1 |
| 6 | Quercetin | 0.4 ± 0.02 | 0.3 ± 0.01 | 0.3 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.01 |
| Total flavonols | 50.9 ± 0.5 | 58.1 ± 0.6 | 21.3 ± 0.2 | 35.2 ± 0.3 | 34.1 ± 0.3 |
| HCA | “Nadzeya” | “Aron” | “Cherno- Plodnaya” | “Venisa” | “Mulatka” |
|---|---|---|---|---|---|
| Neochlorogenic acid | 45.8 ± 0.2 | 31.6 ± 0.2 | 17.5 ± 0.1 | 15.0 ± 0.1 | 33.2 ± 0.2 |
| Chlorogenic acid | 81.2 ± 0.4 | 47.4 ± 0.3 | 29.2 ± 0.2 | 21.3 ± 0.2 | 43.1 ± 0.3 |
| Cryptochlorogenic acid | 9.9 ± 0.2 | 3.0 ± 0.1 | 1.3 ± 0.02 | 4.8 ± 0.1 | 6.7 ± 0.1 |
| Total HCAs | 136.9 ± 0.6 | 82.0 ± 0.5 | 48.0 ± 0.4 | 41.1 ± 0.3 | 83.0 ± 0.4 |
| Cultivar | Total Polyphenols, mg GAE */100 g FW | Antiradical Activity, mM TE **/100 g FW |
|---|---|---|
| “Nadzeya” | 1369.9 ± 27.4 | 4.00 ± 0.08 |
| “Aron” | 1594.5 ± 31.9 | 4.75 ± 0.10 |
| “Chernoplodnaya” | 747.2 ± 14.8 | 2.09 ± 0.04 |
| “Venisa” | 1651.5 ± 33.0 | 4.26 ± 0.07 |
| “Mulatka” | 1667.8 ± 33.4 | 4.31 ± 0.06 |
| Organic Acid | “Nadzeya” | “Aron” | “Cherno- Plodnaya” | “Venisa” | “Mulatka” |
|---|---|---|---|---|---|
| Malic acid | 879.4 ± 17.6 | 927.4 ± 18.5 | 517.2 ± 10.3 | 950.0 ± 19.0 | 873.4 ± 17.5 |
| Citric acid | 45.1 ± 0.9 | 43.4 ± 0.9 | 35.9 ± 0.72 | 44.7 ± 0.9 | 33.7 ± 0.7 |
| Succinic acid | 91.9 ± 1.8 | 240.8 ± 4.8 | 98.2 ± 2.0 | 124.5 ± 2.5 | 111.7 ± 2.2 |
| Quinic acid | 465.2 ± 9.3 | 482.2 ± 9.6 | 396.1 ± 7.9 | 483.7 ± 9.7 | 443.2 ± 8.9 |
| Shikimic acid | 9.1 ± 0.2 | 8.3 ± 0.2 | 6.0 ± 0.1 | 8.5 ± 0.2 | 7.5 ± 0.2 |
| Total organic acids | 1490.7 ± 29.8 | 1702.1 ± 34.0 | 1053.4 ± 21.0 | 1611.4 ± 32.2 | 1469.5 ± 29.4 |
| Sugar/Sugar Alcohol | “Nadzeya” | “Aron” | “Cherno-Plodnaya” | “Venisa” | “Mulatka” |
|---|---|---|---|---|---|
| Fructose | 4.89 ± 0.09 | 4.82 ± 0.08 | 4.71 ± 0.08 | 4.93 ± 0.09 | 5.12 ± 0.10 |
| Glucose | 5.08 ± 0.10 | 4.89 ± 0.09 | 4.85 ± 0.08 | 5.25 ± 0.10 | 5.32 ± 0.09 |
| Sucrose | 0.08 ± 0.005 | 0.01 ± 0.001 | 0.04 ± 0.002 | ND 1 | 0.02 ± 0.002 |
| Total sugars | 10.05 ± 0.11 | 9.72 ± 0.10 | 9.60 ± 0.10 | 10.18 ± 0.11 | 10.46 ± 0.12 |
| Sorbitol | 5.71 ± 0.07 | 5.69 ± 0.07 | 5.21 ± 0.05 | 5.58 ± 0.05 | 5.61 ± 0.07 |
| HPLC–DAD–MS Conditions | Anthocyanin Profile | Flavonol Profile | HCAs | |
|---|---|---|---|---|
| LC column | Phenomenex Luna C18(2) 250 mm × 4.6 mm, 5 µm | |||
| Mobile phase component A | 1% HCOOH in H2O | 0.1% HCOOH in H2O | ||
| Mobile phase component B | 1% HCOOH in acetonitrile | 0.1% HCOOH in acetonitrile | ||
| Gradient elution | 0 min: 10% B 10 min: 12% B 20 min: 15% B 30–32 min: 30% B 33–45 min: 10% B | 0 min: 15% B 35–40 min: 60% B 41–50 min: 15% B | 0 min: 10% B 18 min: 25% B 30 min: 40% B 35 min: 60% B 36–45 min: 10% B | |
| Flow rate | 0.5 mL/min | |||
| Column temperature | 40 °C | 30 °C | ||
| Injection volume | 10 µL | 5 µL | ||
| DAD wavelengths | 520 nm | 370 nm, 350 nm | 330 nm, 275 nm | |
| 200–700 nm | 200–400 nm | |||
| Ionization source | Heated electrospray ionization (HESI) | |||
| Mode | Positive | Negative | ||
| Capillary voltage | 3500 V | 2500 V | ||
| Ion source temperature | 350 °C | 325 °C | 275 °C | |
| Ion transfer tube temperature | 325 °C | 300 °C | 275 °C | |
| Sheath gas flow rate | 5.6 L/min | |||
| Auxiliary gas flow rate | 8.0 L/min | |||
| Sweep gas flow rate | 1.5 L/min | |||
| MS scanning m/z range | 150–1500 | 100–1000 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasimov, M.A.; Perova, I.B.; Eller, K.I.; Akimov, M.Y.; Sukhanova, A.M.; Rodionova, G.M.; Ramenskaya, G.V. Investigation of Polyphenolic Compounds in Different Varieties of Black Chokeberry Aronia melanocarpa. Molecules 2023, 28, 4101. https://doi.org/10.3390/molecules28104101
Gerasimov MA, Perova IB, Eller KI, Akimov MY, Sukhanova AM, Rodionova GM, Ramenskaya GV. Investigation of Polyphenolic Compounds in Different Varieties of Black Chokeberry Aronia melanocarpa. Molecules. 2023; 28(10):4101. https://doi.org/10.3390/molecules28104101
Chicago/Turabian StyleGerasimov, Makar A., Irina B. Perova, Konstantin I. Eller, Michail Y. Akimov, Anna M. Sukhanova, Galina M. Rodionova, and Galina V. Ramenskaya. 2023. "Investigation of Polyphenolic Compounds in Different Varieties of Black Chokeberry Aronia melanocarpa" Molecules 28, no. 10: 4101. https://doi.org/10.3390/molecules28104101
APA StyleGerasimov, M. A., Perova, I. B., Eller, K. I., Akimov, M. Y., Sukhanova, A. M., Rodionova, G. M., & Ramenskaya, G. V. (2023). Investigation of Polyphenolic Compounds in Different Varieties of Black Chokeberry Aronia melanocarpa. Molecules, 28(10), 4101. https://doi.org/10.3390/molecules28104101






