Characterization of Some Stilbenoids Extracted from Two Cultivars of Lambrusco—Vitis vinifera Species: An Opportunity to Valorize Pruning Canes for a More Sustainable Viticulture
Abstract
1. Introduction
2. Results and Discussion
2.1. Proximate Analysis
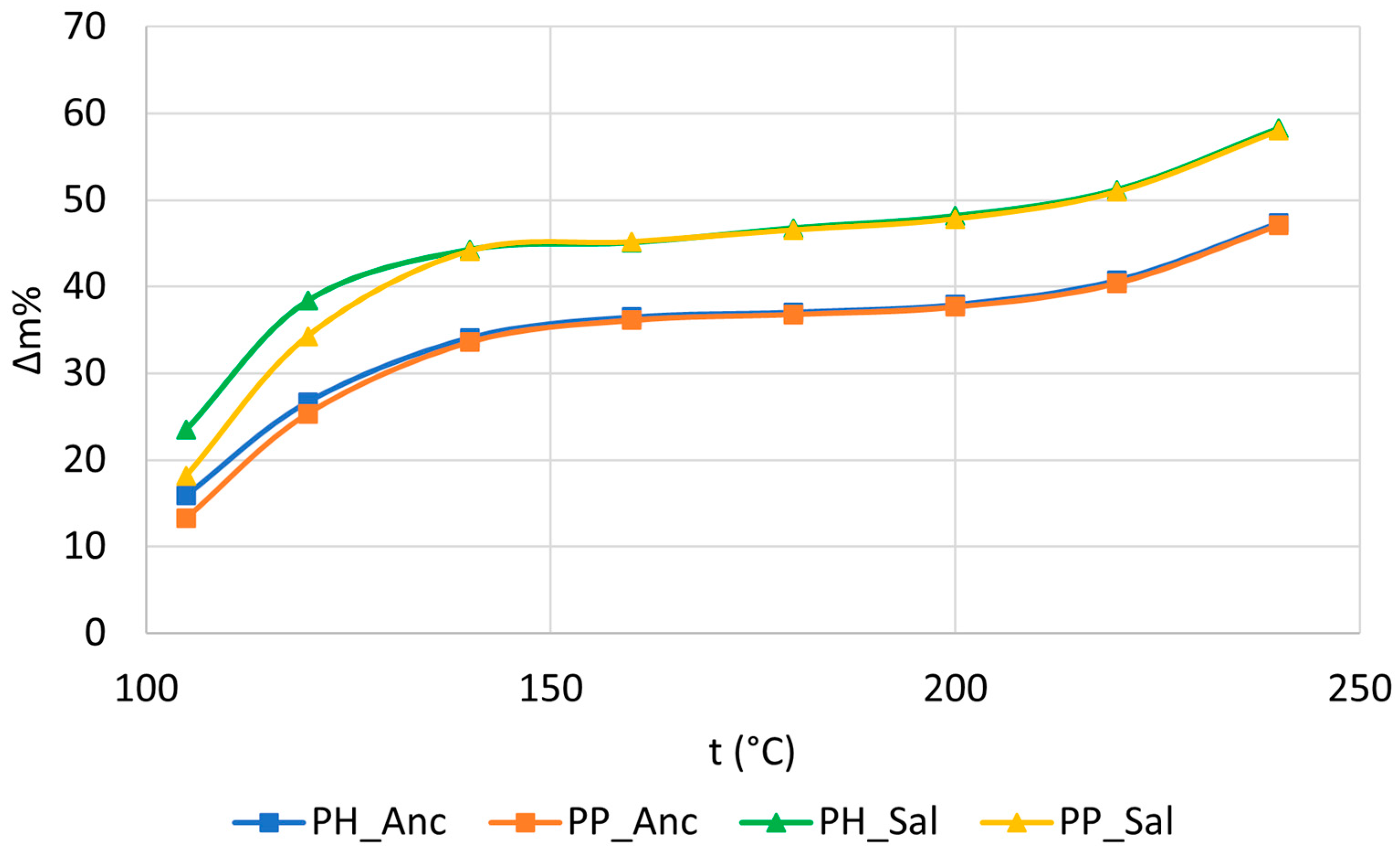
2.2. Effect of the Roasting Temperature on the Samples’ Characteristics
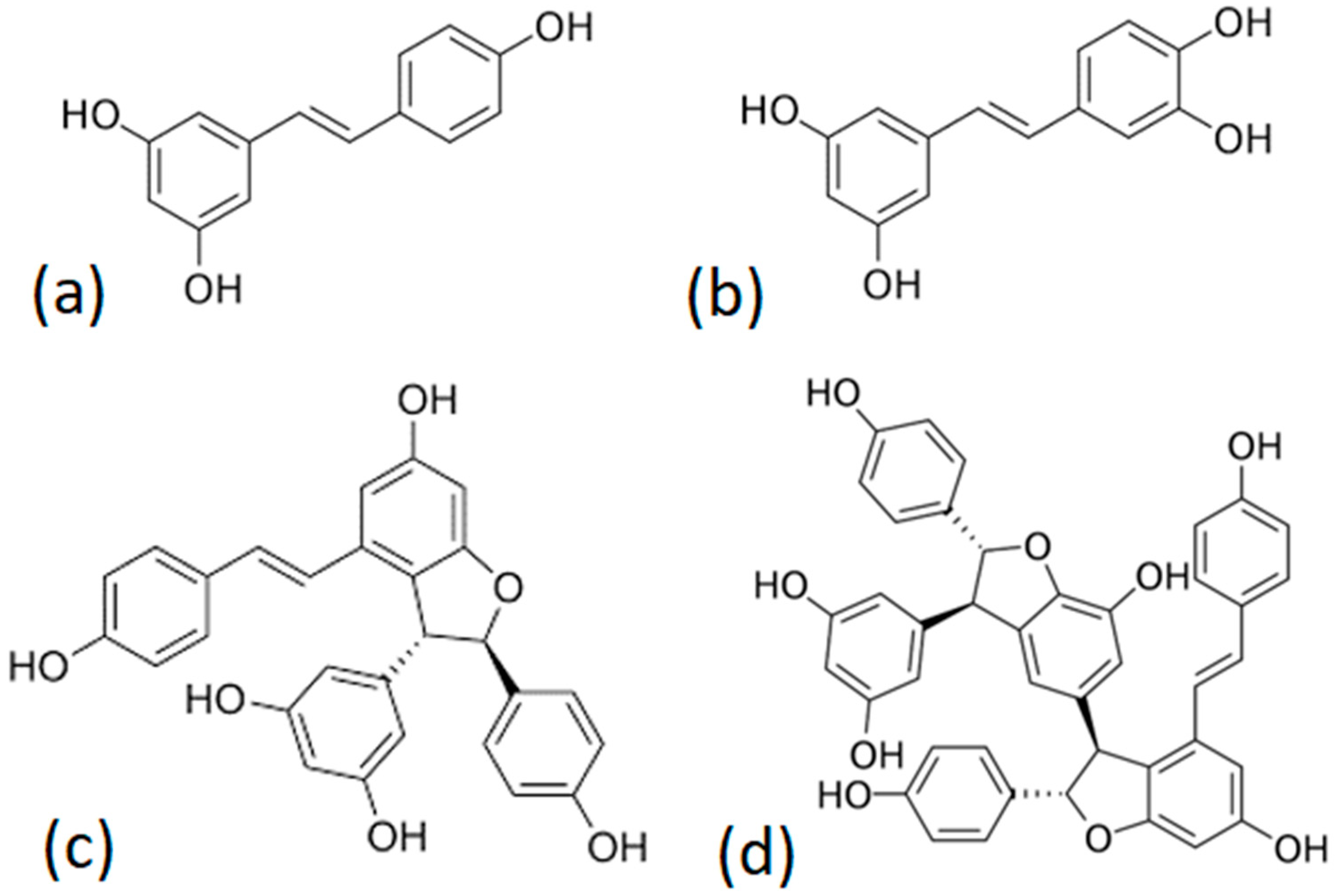
2.3. Stilbenoids Concentration in the Different Samples of Roasted Grape Pruning Canes
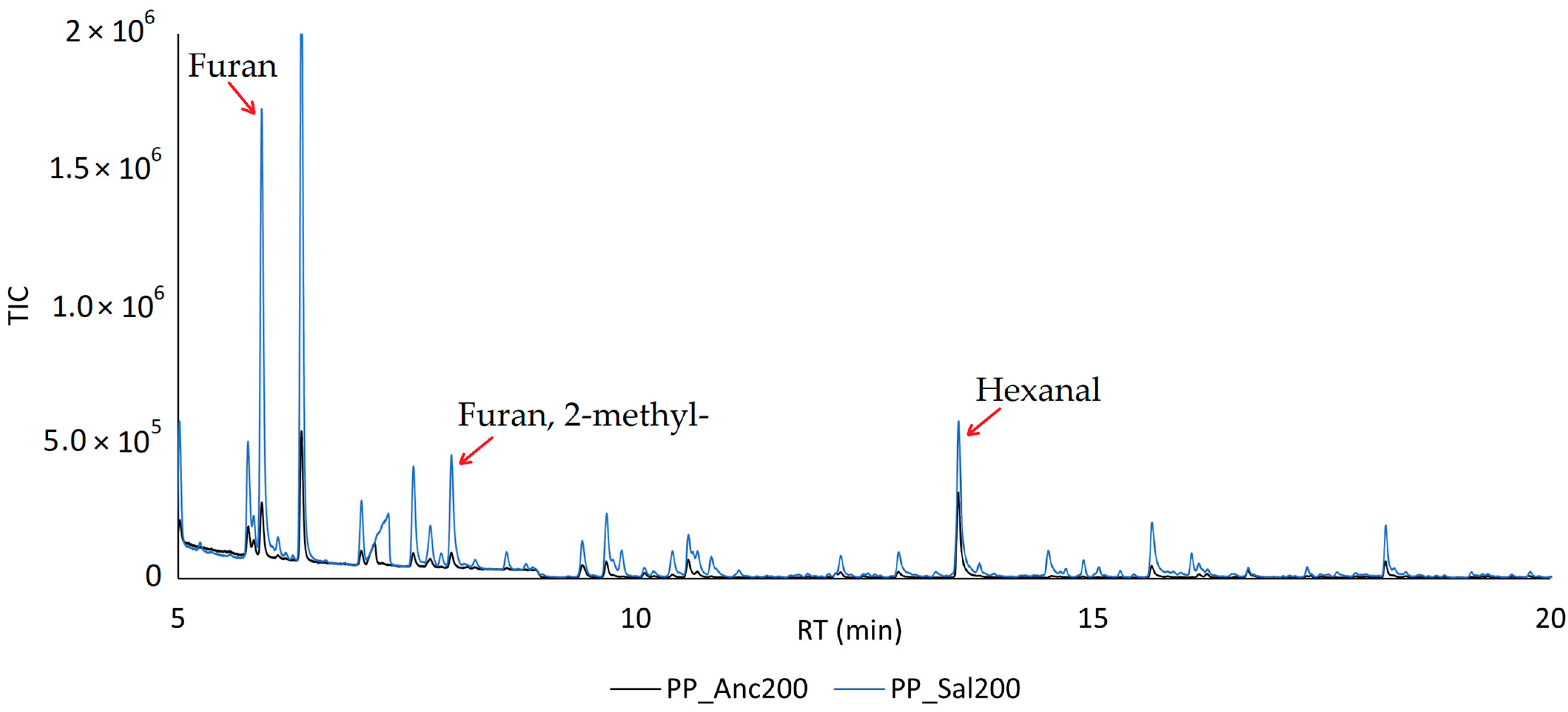
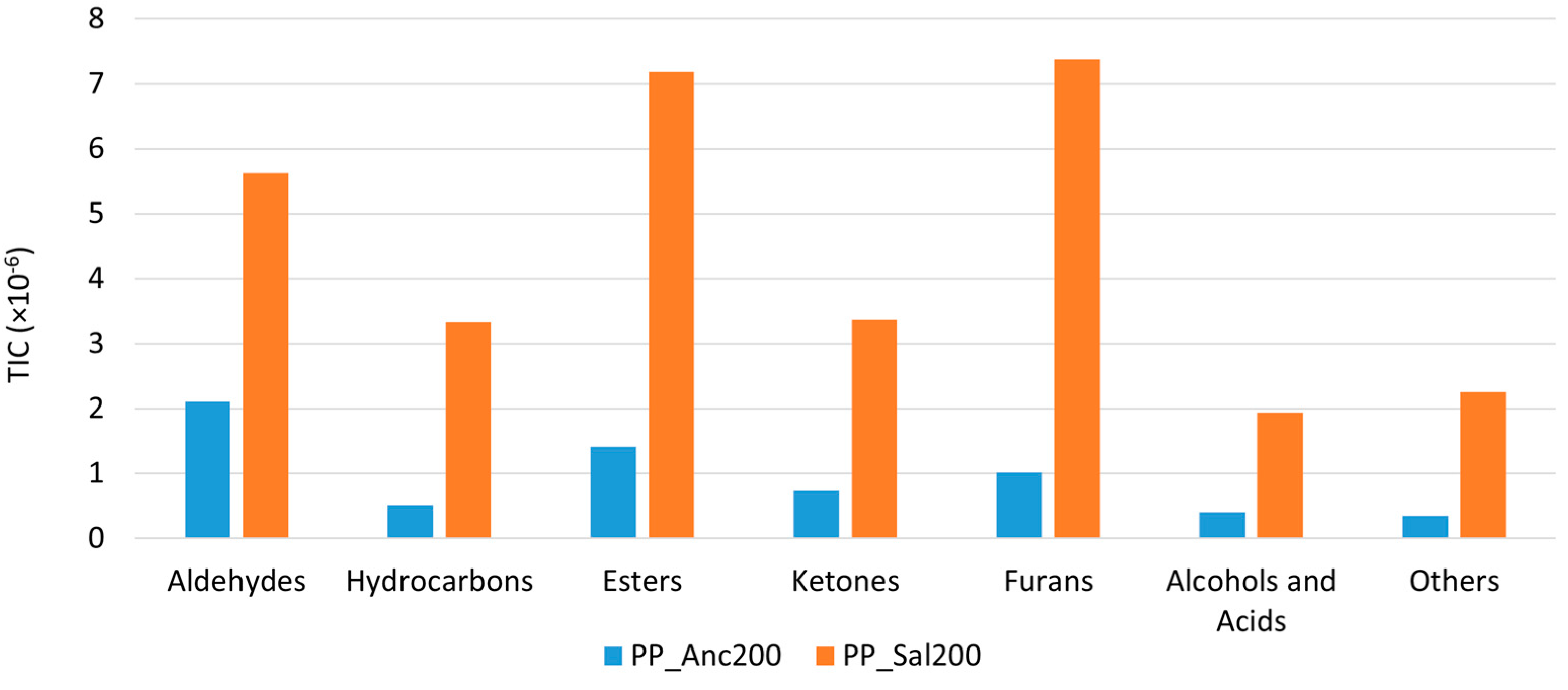
2.4. HS-SPME-GC-MS Analysis
3. Materials and Methods
3.1. Reagents and Standards
3.2. Samples Preparation
3.3. Proximate Composition
3.4. Macerative Solvent Extraction
3.5. HPLC-DAD Analysis
3.6. UHPLC-MS Analysis
3.7. HS-SPME-GC-MS
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- FAO Faostat: FAO Statistical Databases. Available online: https://www.fao.org/faostat/en/#data (accessed on 21 January 2023).
- Droulia, F.; Charalampopoulos, I. Future Climate Change Impacts on European Viticulture: A Review on Recent Scientific Advances. Atmosphere 2021, 12, 495. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Sustainability of Wine Production. Sustainability 2020, 12, 559. [Google Scholar] [CrossRef]
- D’Eusanio, V.; Malferrari, D.; Marchetti, A.; Roncaglia, F.; Tassi, L. Waste by-Product of Grape Seed Oil Production: Chemical Characterization for Use as a Food and Feed Supplement. Life 2023, 13, 326. [Google Scholar] [CrossRef]
- Cortés, A.; Moreira, M.T.; Feijoo, G. Integrated Evaluation of Wine Lees Valorization to Produce Value-Added Products. Waste Manag. 2019, 95, 70–77. [Google Scholar] [CrossRef]
- UN General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development; A/RES/70/1; UN General Assembly: New York, NY, USA, 2015. [Google Scholar]
- Murray, A.; Skene, K.; Haynes, K. The Circular Economy: An Interdisciplinary Exploration of the Concept and Application in a Global Context. J. Bus. Ethics 2017, 140, 369–380. [Google Scholar] [CrossRef]
- Cortés, A.; Oliveira, L.F.S.; Ferrari, V.; Taffarel, S.R.; Feijoo, G.; Moreira, M.T. Environmental Assessment of Viticulture Waste Valorisation through Composting as a Biofertilisation Strategy for Cereal and Fruit Crops. Environ. Pollut. 2020, 264, 114794. [Google Scholar] [CrossRef]
- D’Eusanio, V.; Maletti, L.; Marchetti, A.; Roncaglia, F.; Tassi, L. Volatile Aroma Compounds of Gavina® Watermelon (Citrullus lanatus L.) Dietary Fibers to Increase Food Sustainability. AppliedChem 2023, 3, 66–88. [Google Scholar] [CrossRef]
- Maletti, L.; D’Eusanio, V.; Durante, C.; Marchetti, A.; Tassi, L. VOCs Analysis of Three Different Cultivars of Watermelon (Citrullus lanatus L.) Whole Dietary Fiber. Molecules 2022, 27, 8747. [Google Scholar] [CrossRef]
- Rayne, S.; Karacabey, E.; Mazza, G. Grape Cane Waste as a Source of Trans-Resveratrol and Trans-Viniferin: High-Value Phytochemicals with Medicinal and Anti-Phytopathogenic Applications. Ind. Crops Prod. 2008, 27, 335–340. [Google Scholar] [CrossRef]
- Das, M.; Das, D.K. Resveratrol and Cardiovascular Health. Mol. Asp. Med. 2010, 31, 503–512. [Google Scholar] [CrossRef]
- Athar, M.; Back, J.; Tang, X.; Kim, K.; Kopelovich, L.; Bickers, D.; Kim, A. Resveratrol: A Review of Preclinical Studies for Human Cancer Prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef]
- Fu, J.; Jin, J.; Cichewicz, R.H.; Hageman, S.A.; Ellis, T.K.; Xiang, L.; Peng, Q.; Jiang, M.; Arbez, N.; Hotaling, K.; et al. Trans-(-)-ε-Viniferin Increases Mitochondrial Sirtuin 3 (SIRT3), Activates AMP-Activated Protein Kinase (AMPK), and Protects Cells in Models of Huntington Disease. J. Biol. Chem. 2012, 287, 24460–24472. [Google Scholar] [CrossRef]
- Richard, T.; Poupard, P.; Nassra, M.; Papastamoulis, Y.; Iglésias, M.-L.; Krisa, S.; Waffo-Teguo, P.; Mérillon, J.-M.; Monti, J.-P. Protective Effect of ε-Viniferin on β-Amyloid Peptide Aggregation Investigated by Electrospray Ionization Mass Spectrometry. Bioorg. Med. Chem. 2011, 19, 3152–3155. [Google Scholar] [CrossRef]
- Zghonda, N.; Yoshida, S.; Ezaki, S.; Otake, Y.; Murakami, C.; Mliki, A.; Ghorbel, A.; Miyazaki, H. ε-Viniferin Is More Effective than Its Monomer Resveratrol in Improving the Functions of Vascular Endothelial Cells and the Heart. Biosci. Biotechnol. Biochem. 2012, 76, 954–960. [Google Scholar] [CrossRef]
- Fuloria, S.; Sekar, M.; Khattulanuar, F.S.; Gan, S.H.; Rani, N.N.I.M.; Ravi, S.; Subramaniyan, V.; Jeyabalan, S.; Begum, M.Y.; Chidambaram, K.; et al. Chemistry, Biosynthesis and Pharmacology of Viniferin: Potential Resveratrol-Derived Molecules for New Drug Discovery, Development and Therapy. Molecules 2022, 27, 5072. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Zorita, S.; Milton-Laskibar, I.; Eseberri, I.; Beaumont, P.; Courtois, A.; Krisa, S.; Portillo, M.P. Beneficial Effects of ε-Viniferin on Obesity and Related Health Alterations. Nutrients 2023, 15, 928. [Google Scholar] [CrossRef]
- Lambert, C.; Richard, T.; Renouf, E.; Bisson, J.; Waffo-Téguo, P.; Bordenave, L.; Ollat, N.; Mérillon, J.-M.; Cluzet, S. Comparative Analyses of Stilbenoids in Canes of Major Vitis vinifera L. Cultivars. J. Agric. Food Chem. 2013, 61, 11392–11399. [Google Scholar] [CrossRef] [PubMed]
- Püssa, T.; Floren, J.; Kuldkepp, P.; Raal, A. Survey of Grapevine Vitis vinifera Stem Polyphenols by Liquid Chromatography-Diode Array Detection-Tandem Mass Spectrometry. J. Agric. Food Chem. 2006, 54, 7488–7494. [Google Scholar] [CrossRef] [PubMed]
- Aaviksaar, A.; Haga, M.; Püssa, T.; Roasto, M.; Tsoupras, G. Purification of Resveratrol from Vine Stems. Proc. Est. Acad. Sci. Chem. 2003, 52, 155. [Google Scholar] [CrossRef]
- Houillé, B.; Besseau, S.; Courdavault, V.; Oudin, A.; Glévarec, G.; Delanoue, G.; Guérin, L.; Simkin, A.J.; Papon, N.; Clastre, M.; et al. Biosynthetic Origin of E-Resveratrol Accumulation in Grape Canes during Postharvest Storage. J. Agric. Food Chem. 2015, 63, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Kayaoğlu, M.; Bayram, M.; Topuz, S. Effect of Oak Chips Addition on the Phenolic Composition of Grape Vinegar in Fermentation Process. J. Food Meas. Charact. 2022, 16, 3106–3116. [Google Scholar] [CrossRef]
- Lalou, S.; Mantzouridou, F.T.; Tsimidou, M.Z. Challenges in the Production Line of New-Generation Balsamic Vinegars. In Biotechnological Progress and Beverage Consumption; Elsevier: Amsterdam, The Netherlands, 2020; pp. 311–338. ISBN 978-0-12-816678-9. [Google Scholar]
- Fernández de Simón, B.; Sanz, M.; Cadahía, E.; Martínez, J.; Esteruelas, E.; Muñoz, A.M. Polyphenolic Compounds as Chemical Markers of Wine Ageing in Contact with Cherry, Chestnut, False Acacia, Ash and Oak Wood. Food Chem. 2014, 143, 66–76. [Google Scholar] [CrossRef]
- Cerezo, A.B.; Tesfaye, W.; Soria-Díaz, M.E.; Torija, M.J.; Mateo, E.; Garcia-Parrilla, M.C.; Troncoso, A.M. Effect of Wood on the Phenolic Profile and Sensory Properties of Wine Vinegars during Ageing. J. Food Compos. Anal. 2010, 23, 175–184. [Google Scholar] [CrossRef]
- Basalekou, M.; Pappas, C.; Tarantilis, P.; Kotseridis, Y.; Kallithraka, S. Wine Authentication with Fourier Transform Infrared Spectroscopy: A Feasibility Study on Variety, Type of Barrel Wood and Ageing Time Classification. Int. J. Food Sci. Technol. 2017, 52, 1307–1313. [Google Scholar] [CrossRef]
- Tesfaye, W.; Morales, M.L.; García-Parrilla, M.C.; Troncoso, A.M. Improvement of Wine Vinegar Elaboration and Quality Analysis: Instrumental and Human Sensory Evaluation. Food Rev. Int. 2009, 25, 142–156. [Google Scholar] [CrossRef]
- Delgado-González, M.J.; Sánchez-Guillén, M.M.; García-Moreno, M.V.; Rodríguez-Dodero, M.C.; García-Barroso, C.; Guillén-Sánchez, D.A. Study of a Laboratory-Scaled New Method for the Accelerated Continuous Ageing of Wine Spirits by Applying Ultrasound Energy. Ultrason. Sonochem. 2017, 36, 226–235. [Google Scholar] [CrossRef]
- Chinnici, F.; Natali, N.; Sonni, F.; Bellachioma, A.; Riponi, C. Comparative Changes in Color Features and Pigment Composition of Red Wines Aged in Oak and Cherry Wood Casks. J. Agric. Food Chem. 2011, 59, 6575–6582. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, E.; Chen, A. Volatile Production from Pyrolysis of Cellulose, Hemicellulose and Lignin. J. Energy Inst. 2017, 90, 902–913. [Google Scholar] [CrossRef]
- Şen, D.; Gökmen, V. Kinetic Modeling of Maillard and Caramelization Reactions in Sucrose-Rich and Low Moisture Foods Applied for Roasted Nuts and Seeds. Food Chem. 2022, 395, 133583. [Google Scholar] [CrossRef]
- Ojeda-Galván, H.J.; Hernández-Arteaga, A.C.; Rodríguez-Aranda, M.C.; Toro-Vazquez, J.F.; Cruz-González, N.; Ortíz-Chávez, S.; Comas-García, M.; Rodríguez, A.G.; Navarro-Contreras, H.R. Application of Raman Spectroscopy for the Determination of Proteins Denaturation and Amino Acids Decomposition Temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 285, 121941. [Google Scholar] [CrossRef]
- Johnson, C.M. Differential Scanning Calorimetry as a Tool for Protein Folding and Stability. Arch. Biochem. Biophys. 2013, 531, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic Biomass Pyrolysis Mechanism: A State-of-the-Art Review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Ding, Y.; Huang, B.; Li, K.; Du, W.; Lu, K.; Zhang, Y. Thermal Interaction Analysis of Isolated Hemicellulose and Cellulose by Kinetic Parameters during Biomass Pyrolysis. Energy 2020, 195, 117010. [Google Scholar] [CrossRef]
- Gorena, T.; Saez, V.; Mardones, C.; Vergara, C.; Winterhalter, P.; von Baer, D. Influence of Post-Pruning Storage on Stilbenoid Levels in Vitis vinifera L. Canes. Food Chem. 2014, 155, 256–263. [Google Scholar] [CrossRef]
- Decendit, A. Identification and Quantification of Stilbenes in Some Tunisian Red Wines Using UPLC-MS and HPLC-DAD. Oeno One 2017, 51, 231–236. [Google Scholar] [CrossRef]
- Stecher, G.; Huck, C.W.; Popp, M.; Bonn, G.K. Determination of Flavonoids and Stilbenes in Red Wine and Related Biological Products by HPLC and HPLC–ESI–MS–MS. Fresenius J. Anal. Chem. 2001, 371, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Vergara, C.; von Baer, D.; Mardones, C.; Wilkens, A.; Wernekinck, K.; Damm, A.; Macke, S.; Gorena, T.; Winterhalter, P. Stilbene Levels in Grape Cane of Different Cultivars in Southern Chile: Determination by HPLC-DAD-MS/MS Method. J. Agric. Food Chem. 2012, 60, 929–933. [Google Scholar] [CrossRef]
- Mattivi, F.; Vrhovsek, U.; Malacarne, G.; Masuero, D.; Zulini, L.; Stefanini, M.; Moser, C.; Velasco, R.; Guella, G. Profiling of Resveratrol Oligomers, Important Stress Metabolites, Accumulating in the Leaves of Hybrid Vitis vinifera (Merzling × Teroldego) Genotypes Infected with Plasmopara Viticola. J. Agric. Food Chem. 2011, 59, 5364–5375. [Google Scholar] [CrossRef]
- Cottart, C.-H.; Nivet-Antoine, V.; Beaudeux, J.-L. Review of Recent Data on the Metabolism, Biological Effects, and Toxicity of Resveratrol in Humans. Mol. Nutr. Food Res. 2014, 58, 7–21. [Google Scholar] [CrossRef]
- Vang, O.; Ahmad, N.; Baile, C.A.; Baur, J.A.; Brown, K.; Csiszar, A.; Das, D.K.; Delmas, D.; Gottfried, C.; Lin, H.-Y.; et al. What Is New for an Old Molecule? Systematic Review and Recommendations on the Use of Resveratrol. PLoS ONE 2011, 6, e19881. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Baur, J.A.; Hausenblas, H.A. Resveratrol and Health—A Comprehensive Review of Human Clinical Trials. Mol. Nutr. Food Res. 2011, 55, 1129–1141. [Google Scholar] [CrossRef]
- Ndiaye, M.; Kumar, R.; Ahmad, N. Resveratrol in Cancer Management: Where Are We and Where We Go from Here? Ann. N. Y. Acad. Sci. 2011, 1215, 144–149. [Google Scholar] [CrossRef]
- Csiszar, A. Anti-Inflammatory Effects of Resveratrol: Possible Role in Prevention of Age-Related Cardiovascular Disease. Ann. N. Y. Acad. Sci. 2011, 1215, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic Potential of Resveratrol: The in Vivo Evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Timmers, S.; Auwerx, J.; Schrauwen, P. The Journey of Resveratrol from Yeast to Human. Aging 2012, 4, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K.J.; Baur, J.A.; Lewis, K.N.; Peshkin, L.; Price, N.L.; Labinskyy, N.; Swindell, W.R.; Kamara, D.; Minor, R.K.; Perez, E.; et al. Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span. Cell Metab. 2008, 8, 157–168. [Google Scholar] [CrossRef]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological Activity of Piceatannol: Leaving the Shadow of Resveratrol. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Rhayem, Y.; Thérond, P.; Camont, L.; Couturier, M.; Beaudeux, J.-L.; Legrand, A.; Jore, D.; Gardés-Albert, M.; Bonnefont-Rousselot, D. Chain-Breaking Activity of Resveratrol and Piceatannol in a Linoleate Micellar Model. Chem. Phys. Lipids 2008, 155, 48–56. [Google Scholar] [CrossRef]
- Houillé, B.; Papon, N.; Boudesocque, L.; Bourdeaud, E.; Besseau, S.; Courdavault, V.; Enguehard-Gueiffier, C.; Delanoue, G.; Guérin, L.; Bouchara, J.-P.; et al. Antifungal Activity of Resveratrol Derivatives against Candida Species. J. Nat. Prod. 2014, 77, 1658–1662. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; Sui, Y.; Gao, X.; Yang, H.; Ma, T. Identification of Resveratrol Oligomers as Inhibitors of Cystic Fibrosis Transmembrane Conductance Regulator by High-Throughput Screening of Natural Products from Chinese Medicinal Plants. PLoS ONE 2014, 9, e94302. [Google Scholar] [CrossRef]
- Ku, K.L.; Chang, P.S.; Cheng, Y.C.; Lien, C.Y. Production of Stilbenoids from the Callus of Arachis Hypogaea: A Novel Source of the Anticancer Compound Piceatannol. J. Agric. Food Chem. 2005, 53, 3877–3881. [Google Scholar] [CrossRef]
- Gatto, P.; Vrhovsek, U.; Muth, J.; Segala, C.; Romualdi, C.; Fontana, P.; Pruefer, D.; Stefanini, M.; Moser, C.; Mattivi, F.; et al. Ripening and Genotype Control Stilbene Accumulation in Healthy Grapes. J. Agric. Food Chem. 2008, 56, 11773–11785. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Heene, E.; Qiao, F.; Nick, P. The Phytoalexin Resveratrol Regulates the Initiation of Hypersensitive Cell Death in Vitis Cell. PLoS ONE 2011, 6, e26405. [Google Scholar] [CrossRef] [PubMed]
- Adrian, M.; Jeandet, P.; Douillet-Breuil, A.C.; Tesson, L.; Bessis, R. Stilbene Content of Mature Vitis vinifera Berries in Response to UV-C Elicitation. J. Agric. Food Chem. 2000, 48, 6103–6105. [Google Scholar] [CrossRef]
- Jiménez, J.B.; Orea, J.M.; Ureña, A.G.; Escribano, P.; de la Osa, P.L.; Guadarrama, A. Short Anoxic Treatments to Enhance Trans-Resveratrol Content in Grapes and Wine. Eur. Food Res. Technol. 2007, 224, 373–378. [Google Scholar] [CrossRef]
- Official Journal of the European Union Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32009R1107 (accessed on 12 February 2023).
- Dorosh, O.; Fernandes, V.C.; Moreira, M.M.; Delerue-Matos, C. Occurrence of Pesticides and Environmental Contaminants in Vineyards: Case Study of Portuguese Grapevine Canes. Sci. Total Environ. 2021, 791, 148395. [Google Scholar] [CrossRef] [PubMed]
- Vichi, S.; Santini, C.; Natali, N.; Riponi, C.; López-Tamames, E.; Buxaderas, S. Volatile and Semi-Volatile Components of Oak Wood Chips Analysed by Accelerated Solvent Extraction (ASE) Coupled to Gas Chromatography–Mass Spectrometry (GC–MS). Food Chem. 2007, 102, 1260–1269. [Google Scholar] [CrossRef]
- Chatonnet, P.; Cutzach, I.; Pons, M.; Dubourdieu, D. Monitoring Toasting Intensity of Barrels by Chromatographic Analysis of Volatile Compounds from Toasted Oak Wood. J. Agric. Food Chem. 1999, 47, 4310–4318. [Google Scholar] [CrossRef]
- Cadahía, E.; Fernández de Simón, B.; Jalocha, J. Volatile Compounds in Spanish, French, and American Oak Woods after Natural Seasoning and Toasting. J. Agric. Food Chem. 2003, 51, 5923–5932. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Guchu, E.; Castro-Vázquez, L.; de Torres, C.; Pérez-Coello, M.S. Aroma-Active Compounds of American, French, Hungarian and Russian Oak Woods, Studied by GC–MS and GC–O. Flavour Fragr. J. 2008, 23, 93–98. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Sánchez-Palomo, E.; Pérez-Coello, M.S. Fast Screening Method for Volatile Compounds of Oak Wood Used for Aging Wines by Headspace SPME-GC-MS (SIM). J. Agric. Food Chem. 2004, 52, 6857–6861. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.K.; Elmore, J.S.; Methven, L.; Balagiannis, D.P. Flavour Development, Analysis and Perception in Food and Beverages; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK; Waltham, MA, USA; Kidlington, UK, 2015; ISBN 978-1-78242-103-0. [Google Scholar]
- Zou, Y.; Gaida, M.; Franchina, F.A.; Stefanuto, P.-H.; Focant, J.-F. Distinguishing between Decaffeinated and Regular Coffee by HS-SPME-GC × GC-TOFMS, Chemometrics, and Machine Learning. Molecules 2022, 27, 1806. [Google Scholar] [CrossRef] [PubMed]
- Berk, E.; Hamzalıoğlu, A.; Gökmen, V. Investigations on the Maillard Reaction in Sesame (Sesamum indicum L.) Seeds Induced by Roasting. J. Agric. Food Chem. 2019, 67, 4923–4930. [Google Scholar] [CrossRef]
- Siegmund, B.; Murkovic, M. Changes in Chemical Composition of Pumpkin Seeds during the Roasting Process for Production of Pumpkin Seed Oil (Part 2: Volatile Compounds). Food Chem. 2004, 84, 367–374. [Google Scholar] [CrossRef]
- Smit, B.A.; Engels, W.J.M.; Smit, G. Branched Chain Aldehydes: Production and Breakdown Pathways and Relevance for Flavour in Foods. Appl. Microbiol. Biotechnol. 2009, 81, 987–999. [Google Scholar] [CrossRef]
- Poisson, L.; Blank, I.; Dunkel, A.; Hofmann, T. The Chemistry of Roasting-Decoding Flavor Formation. In The Craft and Science of Coffee; Elsevier: Amsterdam, The Netherlands, 2017; pp. 273–309. ISBN 978-0-12-803558-0. [Google Scholar]
- Ridgway, K. Analysis of Taints and Off-Flavours. In Flavour Development, Analysis and Perception in Food and Beverages; Woodhead Publishing Series; Woodhead Publishing: Sawston, UK, 2015; pp. 63–79. [Google Scholar]
- Bail, S.; Stuebiger, G.; Krist, S.; Unterweger, H.; Buchbauer, G. Characterisation of Various Grape Seed Oils by Volatile Compounds, Triacylglycerol Composition, Total Phenols and Antioxidant Capacity. Food Chem. 2008, 108, 1122–1132. [Google Scholar] [CrossRef]
- Sevindik, O.; Kelebek, H.; Rombolà, A.D.; Selli, S. Grape Seed Oil Volatiles and Odour Activity Values: A Comparison with Turkish and Italian Cultivars and Extraction Methods. J. Food Sci. Technol. 2022, 59, 1968–1981. [Google Scholar] [CrossRef]
- Limacher, A.; Kerler, J.; Davidek, T.; Schmalzried, F.; Blank, I. Formation of Furan and Methylfuran by Maillard-Type Reactions in Model Systems and Food. J. Agric. Food Chem. 2008, 56, 3639–3647. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.; Lourenço, S.; Silva, C.; Lopes, A.; Andrade, C.; Perestrelo, R. Exploring the Potential of Wine Industry by-Products as Source of Additives to Improve the Quality of Aquafeed. Microchem. J. 2020, 155, 104758. [Google Scholar] [CrossRef]
- Perez Locas, C.; Yaylayan, V.A. Origin and Mechanistic Pathways of Formation of the Parent Furan A Food Toxicant. J. Agric. Food Chem. 2004, 52, 6830–6836. [Google Scholar] [CrossRef] [PubMed]
- Boss, P.K.; Pearce, A.D.; Zhao, Y.; Nicholson, E.L.; Dennis, E.G.; Jeffery, D.W. Potential Grape-Derived Contributions to Volatile Ester Concentrations in Wine. Molecules 2015, 20, 7845–7873. [Google Scholar] [CrossRef]
- Gambetta, J.M.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. Factors Influencing the Aroma Composition of Chardonnay Wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine Aroma Compounds in Grapes: A Critical Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wan, P.; Xie, C.; Chen, D.-W. Key Aroma-Active Compounds in Brown Sugar and Their Influence on Sweetness. Food Chem. 2021, 345, 128826. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of the Association of Official’s Analytical Chemists, 14th ed.; Associataion of Official Analytical Chemist: Washington, DC, USA, 1990; pp. 223–225. [Google Scholar]
- Chikwanha, O.C.; Raffrenato, E.; Muchenje, V.; Musarurwa, H.T.; Mapiye, C. Varietal Differences in Nutrient, Amino Acid and Mineral Composition and in Vitro Rumen Digestibility of Grape (Vitis vinifera) Pomace from the Cape Winelands Vineyards in South Africa and Impact of Preservation Techniques. Ind. Crops Prod. 2018, 118, 30–37. [Google Scholar] [CrossRef]
- Lumivero XLSTAT Statistical and Data Analysis Solution. Paris, France, 2023. Available online: https://www.xlstat.com (accessed on 31 January 2023).



| PH_Anc | PP_Anc | PH_Sal | PP_Sal | |
|---|---|---|---|---|
| Moisture (at 105 °C) | 15.87 ± 0.32 | 13.26 ± 0.24 | 23.52 ± 0.23 | 18.12 ± 0.25 |
| C% * | 39.64 ± 0.22 | 46.56 ± 0.36 | 37.32 ± 0.30 | 45.31± 0.40 |
| H% * | 6.51 ± 0.08 | 9.15 ± 0.09 | 3.65 ± 0.05 | 5.72 ± 0.10 |
| N% * | 0.46 ± 0.03 | 0.57 ± 0.05 | 0.38 ± 0.06 | 0.47 ± 0.04 |
| S% * | <0.1 | <0.1 | <0.1 | <0.1 |
| O% *# | 50.32 ± 0.29 | 40.49 ± 0.56 | 55.84 ± 0.39 | 45.44 ± 0.21 |
| Ash% * | 2.84 ± 0.05 | 3.23 ± 0.06 | 2.81 ± 0.08 | 3.06 ± 0.05 |
| Protein content% * | 2.87 ± 0.34 | 3.56 ± 0.31 | 2.37 ± 0.5 | 2.93 ± 0.25 |
| Roasting t/°C | PH_Anc | PP_Anc | PH_Sal | PP_Sal |
|---|---|---|---|---|
| 180 | 45.74 ± 0.43 | 36.81 ± 0.42 | 55.75 ± 0.41 | 46.55 ± 0.38 |
| 200 | 46.62 ± 0.40 | 37.68 ± 0.41 | 56.62 ± 0.39 | 47.83 ± 0.34 |
| 220 | 49.21 ± 0.44 | 40.42 ± 0.42 | 59.14 ± 0.40 | 50.96 ± 0.54 |
| 240 | 55.98 ± 0.41 | 47.13 ± 0.43 | 65.84 ± 0.37 | 58.01 ± 0.51 |
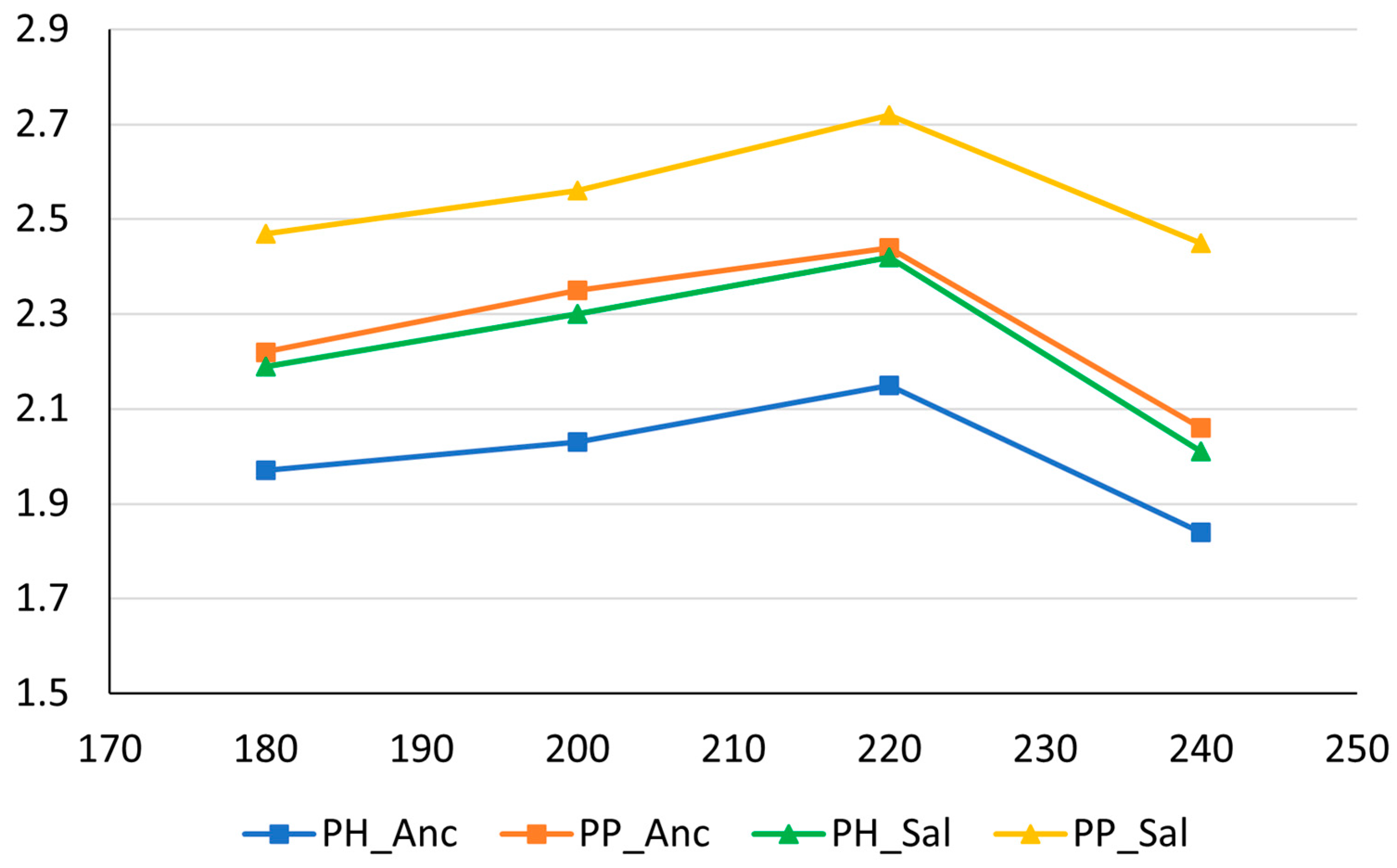
| Extraction Yield% * | ||||
|---|---|---|---|---|
| Roasting t/°C | PH_Anc | PP_Anc | PH_Sal | PP_Sal |
| 180 | 1.97 ± 0.09 | 2.22 ± 0.10 | 2.19 ± 0.08 | 2.47 ± 0.15 |
| 200 | 2.03 ± 0.10 | 2.35 ± 0.09 | 2.30 ± 0.11 | 2.56 ± 0.13 |
| 220 | 2.15 ± 0.12 | 2.44 ± 0.09 | 2.42 ± 0.07 | 2.72 ± 0.11 |
| 240 | 1.84 ± 0.09 | 2.06 ± 0.08 | 2.01 ± 0.09 | 2.45 ± 0.12 |
| Stilbenoids Concentration (mg/kg) 1,2 | |||||
|---|---|---|---|---|---|
| Sample | trans-Resveratrol | trans-Piceatannol | trans-ε-Viniferin | Main Trimer | Total |
| PH_Anc180 | 815.9 ± 153 | 153.4 ± 46.0 | 261.4 ± 60.9 | 93.0 ± 23.4 | 1323 ± 283 |
| PP_Anc180 | 2176 ± 366 | 339.8 ± 111 | 555.4 ± 114 | 152.2 ± 46.9 | 3223 ± 638 |
| PH_Sal180 | 706.4 ± 230 | 139.5 ± 61.6 | 224.1 ± 25.2 | 75.4 ± 14.8 | 1145 ± 332 |
| PP_Sal180 | 1786 ± 301 | 304.1 ± 101 | 489.8 ± 70.2 | 140.2 ± 29.8 | 2720 ± 502 |
| PH_Anc200 | 693.1 ± 134.6 | 139.0 ± 48.2 | 241.0 ± 34.7 | 59.4 ± 11.7 | 1132 ± 229 |
| PP_Anc200 | 2044 ± 427 | 275.5 ± 94.0 | 520.6 ± 80.0 | 135.7 ± 24.0 | 2976 ± 625 |
| PH_Sal200 | 508.8 ± 188.3 | 116.3 ± 38.5 | 201.4 ± 50.8 | 43.6 ± 8.5 | 870.2 ± 286.1 |
| PP_Sal200 | 1463 ± 277 | 346.4 ± 76.1 | 491.1 ± 69.9 | 129.2 ± 56.6 | 2330 ± 480 |
| PH_Anc220 | 548.8 ± 114.5 | 102.2 ± 27.5 | 180.4 ± 20.1 | NQ | 831.5 ± 162.1 |
| PP_Anc220 | 1671 ± 255 | 226.5 ± 40.5 | 439.9 ± 59.9 | 97.1 ± 22.9 | 2435 ± 378 |
| PH_Sal220 | 381.3 ± 83.9 | 93.76 ± 20.3 | 167.7 ± 30.5 | NQ | 642.8 ± 134.7 |
| PP_Sal220 | 1203 ± 112 | 201.8 ± 41.0 | 428.7 ± 68.8 | 83.7 ± 16.3 | 1917 ± 238 |
| PH_Anc240 | 195.8 ± 82.3 | NQ | 145.5 ± 45.0 | NQ | 341.3 ± 127.3 |
| PP_Anc240 | 831.3 ± 181.4 | 188.8 ± 42.9 | 359.3 ± 59.9 | NQ | 1379 ± 284 |
| PH_Sal240 | 105.9 ± 33.7 | NQ | 133.3 ± 31.8 | NQ | 239.3 ± 65.5 |
| PP_Sal240 | 667.8 ± 37.7 | 128.3 ± 28.2 | 326.9 ± 56.5 | NQ | 1123 ± 122 |
| Compound | LRI | ID # | Aroma | PP_Anc200 | PP_Sal200 |
|---|---|---|---|---|---|
| Area × 10−6 | Area × 10−6 | ||||
| Aldehydes | |||||
| Acetaldehyde | 430 | A, B | Pungent, fresh, lifting, fruity, musty | 1.75 ± 0.07 | 5.51 ± 0.12 |
| Propanal | 470 | A, B | Pungent, earthy, wine, nutty, cocoa | 1.53 ± 0.06 | 3.76 ± 0.09 |
| Propanal, 2-methyl- | 512 | A, B | Fresh, aldehydic, floral, green | 1.39 ± 0.09 | 6.25 ± 0.11 |
| Butanal | 592 | A, B | Pungent, cocoa, musty, green, malty | - | 0.186 a |
| Butanal, 3-methyl- | 597 | A, B, C | Aldehydic, chocolate, peach, fatty | 1.82 ± 0.06 | 4.65 ± 0.07 |
| Butanal, 2-methyl- | 606 | A, B | Musty, cocoa, coffee, nutty, malty | 1.70 ± 0.06 | 7.17 ± 0.19 |
| Pentanal | 638 | A, B | Fermented, bready, fruity, berry | 2.12 a | 4.70 ± 0.12 |
| Hexanal | 742 | A, B, C | Green, fatty, leafy, vegetative, fruity, clean | 10.3 ± 0.1 | 22.1 ± 0.3 |
| Heptanal | 834 | A, B | Fresh, aldehydic, fatty, green, herbal | 0.442 a | 2.02 a |
| Organic acids and alcohols | |||||
| Acetic acid | 530 | A, B, C | Sharp, pungent, sour, vinegar | 3.80 ± 0.15 | 18.0 ± 0.3 |
| 1-Penten-3-ol | 621 | A, B | Ethereal, green, radish, vegetable, fruity | - | 1.14 a |
| 1-Pentanol | 705 | A, B | Pungent, fermented, bready, yeasty | 0.260 a | 0.324 ± 0.082 |
| Esters | |||||
| Formic acid, methyl ester | 442 | A, B | Fruity, plum, ester | 1.75 ± 0.08 | 10.4 ± 0.09 |
| Formic acid, ethyl ester | 483 | A, B, C | Fruity | - | 0.902 a |
| Acetic acid, methyl ester | 488 | A, B, C | Ethereal, sweet, fruity, winey | 11.7 ± 0.08 | 56.6 ± 0.21 |
| Propanoic acid, methyl ester | 567 | A, B | Fresh, harsh, rum, fruity | 0.705 a | 3.96 ± 0.18 |
| Ketones | |||||
| Acetone | 468 | A, B, C | Solvent, ethereal, apple, pear | 2.51 ± 0.14 | 11.2 ± 0.4 |
| 2,3-Butanedione | 532 | A, B | Butter, sweet, creamy, pungent, caramel | 2.39 ± 0.08 | 12.1 ± 0.2 |
| 2-Butanone | 538 | A, B, C | Acetone, ethereal, fruity, camphor | 2.14 ± 0.12 | 6.18 ± 0.09 |
| 2,3-Pentanedione | 632 | A, B | Pungent, sweet, butter, creamy, nutty | 0.417 ± 0.071 | 3.86 ± 0.06 |
| 3-Pentanone, 2-methyl- | 692 | A, B | - | - | 0.354 ± 0.092 |
| Furan derivatives | |||||
| Furan | 473 | A, B | Ethereal | 5.51 ± 0.13 | 46.4± 0.4 |
| Furan, 2-methyl- | 546 | A, B | Ethereal, acetone, chocolate | 3.26 ± 0.17 | 14.7 ± 0.3 |
| Furan, 2-ethyl- | 641 | A, B | Beany, cocoa, bread, malty, coffee | 0.931 ± 0.105 | 3.63 ± 0.23 |
| Furan, 2,5-dimethyl- | 647 | A, B | Chemical, ethereal, meaty, gravy, roast | 0.182 a | 3.60 ± 0.11 |
| Furan, 2,4-dimethyl- | 657 | A, B | - | - | 1.23 ± 0.08 |
| Furfural | 776 | A, B, C | Sweet, woody, bready, caramel, phenolic | 0.298 ± 0.079 | 4.22 ± 0.09 |
| Hydrocarbons | |||||
| 1-Propene, 2-methyl- | 432 | A, B | - | 3.72 a | 13.4 ± 0.12 |
| Butane | 435 | A, B | - | - | 3.85 ± 0.09 |
| 1-Butene | 438 | A, B | - | - | 1.43 ± 0.08 |
| 1,3-Butadiene, 2-methyl- | 479 | A, B | - | 0.380 a | 2.74 ± 0.11 |
| 2-Butene, 2-methyl- | 486 | A, B | - | - | 0.608 ± 0.084 |
| Hexane | 542 | A, B | - | - | 2.14 ± 0.09 |
| Cyclopentane, methyl- | 575 | A, B | - | - | 1.57 ± 0.12 |
| 1,3-Pentadiene, 3-methyl- | 578 | A, B | - | - | 1.56 ± 0.08 |
| 4-Methyl-1,3-pentadiene | 581 | A, B | - | - | 0.239 a |
| Cyclohexane | 612 | A, B | - | - | 3.41 ± 0.06 |
| Nonane | 831 | A, B | - | - | 2.38 ± 0.10 |
| Cycloheptene | 853 | A, B | - | 1.09 ± 0.06 | - |
| Others | |||||
| Benzene | 608 | A, B | Aromatic | - | 1.98 a |
| 1H-Pyrrole, 1-methyl- | 684 | A, B | Woody, smoky, herbal | 0.138 a | 0.460 ± 0.081 |
| Disulfide, dimethyl- | 696 | A, B | Sulfurous, vegetable | 0.724 ± 0.095 | 3.13 ± 0.11 |
| Toluene | 719 | A, B | Sweet | 0.986 ± 0.077 | 4.20 ± 0.09 |
| 3(2H)-Furanone, dihydro-2-methyl- | 750 | A, B | Sweet, solvent, bread, buttery, nutty | - | 2.27 ± 0.09 |
| Pyridine, 2,5-dimethyl- | 790 | A, B | Roasted, green, earthy | - | 1.88 ± 0.08 |
| Ethylbenzene | 809 | A, B | - | - | 0.387 ± 0.075 |
| Xylene | 816 | A, B | - | 1.62 ± 0.09 | 8.28 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Eusanio, V.; Genua, F.; Marchetti, A.; Morelli, L.; Tassi, L. Characterization of Some Stilbenoids Extracted from Two Cultivars of Lambrusco—Vitis vinifera Species: An Opportunity to Valorize Pruning Canes for a More Sustainable Viticulture. Molecules 2023, 28, 4074. https://doi.org/10.3390/molecules28104074
D’Eusanio V, Genua F, Marchetti A, Morelli L, Tassi L. Characterization of Some Stilbenoids Extracted from Two Cultivars of Lambrusco—Vitis vinifera Species: An Opportunity to Valorize Pruning Canes for a More Sustainable Viticulture. Molecules. 2023; 28(10):4074. https://doi.org/10.3390/molecules28104074
Chicago/Turabian StyleD’Eusanio, Veronica, Francesco Genua, Andrea Marchetti, Lorenzo Morelli, and Lorenzo Tassi. 2023. "Characterization of Some Stilbenoids Extracted from Two Cultivars of Lambrusco—Vitis vinifera Species: An Opportunity to Valorize Pruning Canes for a More Sustainable Viticulture" Molecules 28, no. 10: 4074. https://doi.org/10.3390/molecules28104074
APA StyleD’Eusanio, V., Genua, F., Marchetti, A., Morelli, L., & Tassi, L. (2023). Characterization of Some Stilbenoids Extracted from Two Cultivars of Lambrusco—Vitis vinifera Species: An Opportunity to Valorize Pruning Canes for a More Sustainable Viticulture. Molecules, 28(10), 4074. https://doi.org/10.3390/molecules28104074






