Synthesis of Aminoalkyl Sclareolide Derivatives and Antifungal Activity Studies
Abstract
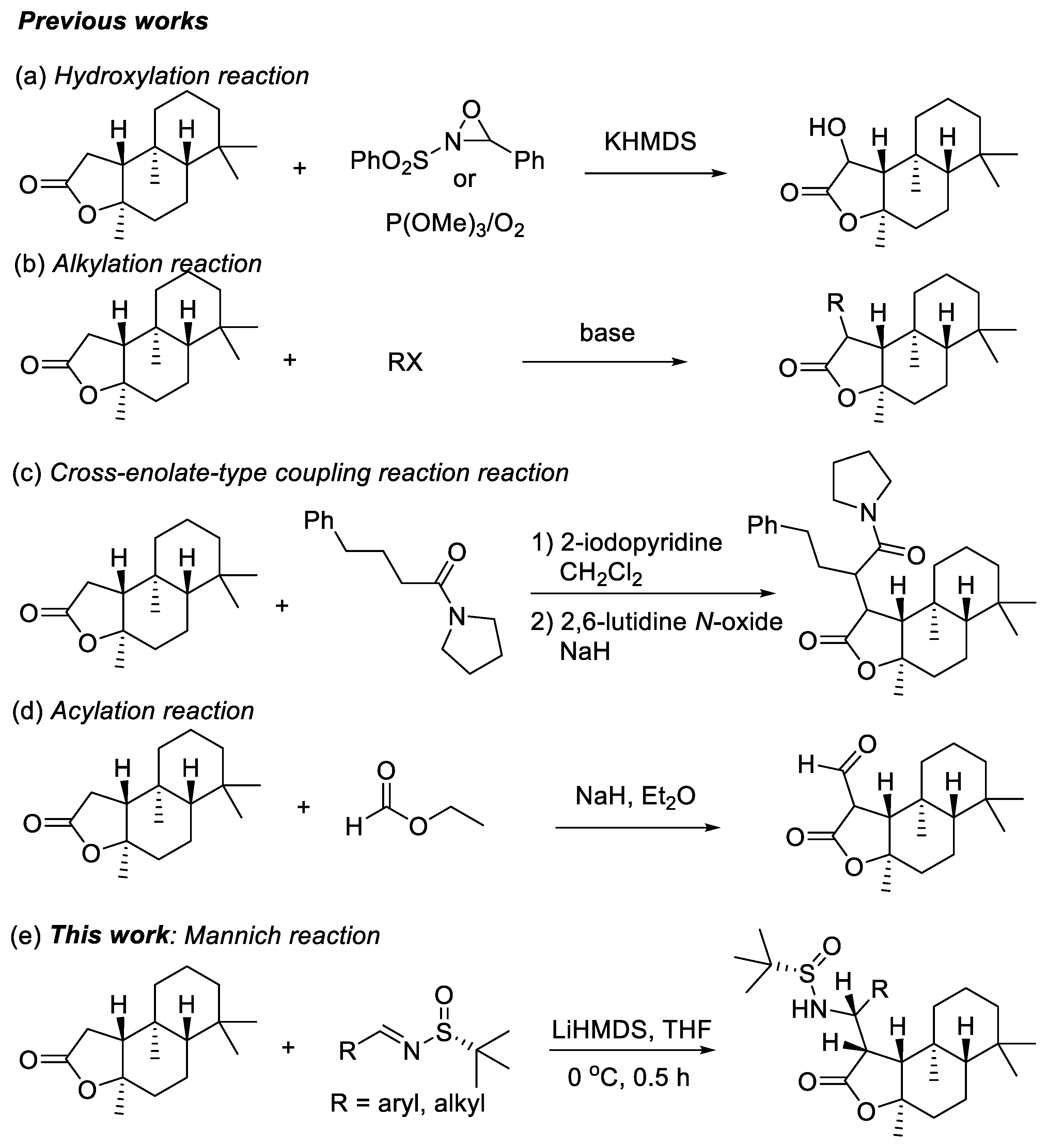
1. Introduction
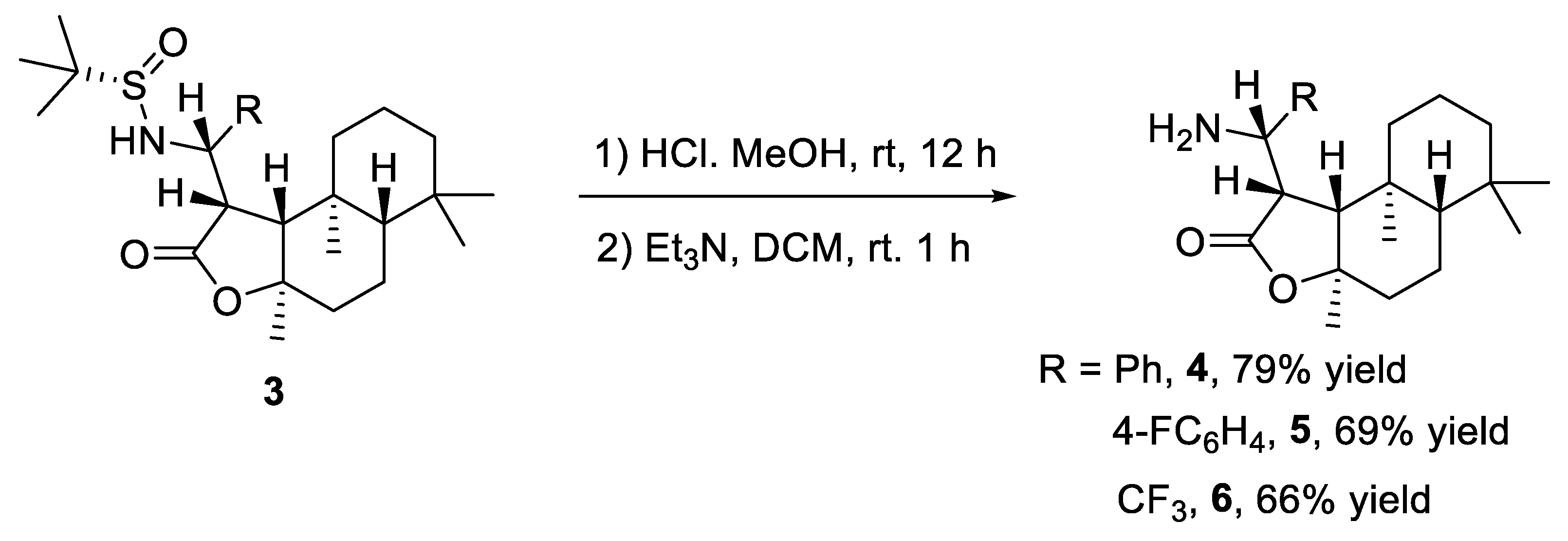
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Mannich Reaction
3.3. Procedure for the Synthesis of 4, 5, 6
3.4. Large Scale Synthesis
3.5. In Vitro Antifungal Effects Studies
3.6. Product Identification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lieshchova, M.A.; Bohomaz, A.A.; Brygadyrenko, V.V. Effect of Salvia officinalis and S. sclarea on rats with a high-fat hypercaloric diet. Regul. Mech. Biosyst. 2021, 12, 554–563. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, K.; Guo, Y. Discovery of sclareol and sclareolide as filovirus entry inhibitors. J. Asian Nat. Prod. Res. 2020, 22, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, R.; Zhang, W.; Li, A. Total Synthesis of Indotertine A and Drimentines A, F, and G. Angew. Chem. Int. Ed. 2013, 52, 9201–9204. [Google Scholar] [CrossRef]
- Zoretic, P.A.; Fang, H.; Ribeiro, A.A.; Dubay, G. Synthesis of Acuminolide and 17-O-Acetylacuminolide from (+)-Sclareolide. J. Org. Chem. 1998, 63, 1156–1161. [Google Scholar] [CrossRef]
- Rosselli, S.; Bruno, M.; Pibiri, I.; Piozzi, F. Synthesis of β-Methylfurolabdanes from (+)-Sclareolide. Eur. J. Org. Chem. 2002, 2002, 4169–4173. [Google Scholar] [CrossRef]
- Li, F.; Renata, H. A Chiral-Pool-Based Strategy to Access trans-syn-Fused Drimane Meroterpenoids: Chemoenzymatic Total Syntheses of Polysin, N-Acetyl-polyveoline and the Chrodrimanins. J. Am. Chem. Soc. 2021, 143, 18280–18286. [Google Scholar] [CrossRef]
- Li, D.; Zhang, S.; Song, Z.; Li, W.; Zhu, F.; Zhang, J.; Li, S. Synthesis and bio-inspired optimization of drimenal: Discovery of chiral drimane fused oxazinones as promising antifungal and antibacterial candidates. Eur. J. Med. Chem. 2018, 143, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Dai, A.; Zheng, Z.; Huang, Y.; Yu, L.; Wang, Z.; Wu, J. Hydrazone modification of non-food natural product sclareolide as potential agents for plant disease. Heliyon 2022, 8, e12391. [Google Scholar] [CrossRef]
- Crespi, S.; Fagnoni, M. Generation of alkyl radicals: From the tyranny of tin to the photon democracy. Chem. Rev. 2020, 120, 9790–9833. [Google Scholar] [CrossRef] [PubMed]
- Rea, P.A. Plant ATP-binding cassette transporters. Annu. Rev. Plant Biol. 2007, 58, 347–375. [Google Scholar] [CrossRef]
- Lodh, J.; Paul, S.; Sun, H.; Song, L.; Schöfberger, W.; Roy, S. Electrochemical organic reactions: A tutorial review. Front. Chem. 2023, 10, 1571. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Xu, M.; Zheng, C.; Yang, B.; Hou, M.; He, H.; Gao, S. Titanium-promoted Intramolecular Photoenolization/Diels–Alder Reaction to Construct Polycyclic Terpenoids: Formal Synthesis of Mycoleptodiscin A. Chin. J. Chem. 2019, 37, 135–139. [Google Scholar] [CrossRef]
- Dhiman, S.; Ulrich, J.F.; Wienecke, P.; Wichard, T.; Arndt, H.D. Stereoselective Total Synthesis of (−)-Thallusin for Bioactivity Profiling. Angew. Chem. Int. Ed. 2022, 61, e202206746. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.A.; Quinn, R.K.; Brusoe, A.T.; Alexanian, E.J. Site-Selective Aliphatic C–H Bromination Using N-Bromoamides and Visible Light. J. Am. Chem. Soc. 2014, 136, 14389–14392. [Google Scholar] [CrossRef]
- Mazzarella, D.; Pulcinella, A.; Bovy, L.; Broersma, R.; Noël, T. Rapid and Direct Photocatalytic C(sp3)−H Acylation and Arylation in Flow. Angew. Chem. Int. Ed. 2021, 60, 21277–21282. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Eschenmoser, A.; Baran, P.S. Strain Release in C-H Bond Activation? Angew. Chem. Int. Ed. 2009, 48, 9705–9708. [Google Scholar] [CrossRef]
- Hall, E.A.; Sarkar, R.; Lee, J.H.Z.; Munday, S.D.; Bell, S.G. Improving the Monooxygenase Activity and the Regio- and Stereoselectivity of Terpenoid Hydroxylation Using Ester Directing Groups. ACS Catal. 2016, 6, 6306–6317. [Google Scholar] [CrossRef]
- De la Torre, M.C.; García, I.; Sierra, M.A. Straightforward synthesis of the strong ambergris odorant γ-bicyclohomofarnesal and its endo-isomer from R-(+)-sclareolide. Tetrahedron Lett. 2002, 43, 6351–6353. [Google Scholar] [CrossRef]
- George, J.H.; McArdle, M.; Baldwin, J.E.; Adlington, R.M. Biomimetic rearrangements of simplified labdane diterpenoids. Tetrahedron 2010, 66, 6321–6330. [Google Scholar] [CrossRef]
- Hong, B.; Luo, T.; Lei, X. Late-stage diversification of natural products. ACS Central. Sci. 2020, 6, 622–635. [Google Scholar] [CrossRef]
- Sterckx, H.; Morel, B.; Maes, B.U.W. Catalytic aerobic oxidation of C (sp3)− H bonds. Angew. Chem. Int. Ed. 2019, 58, 7946–7970. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Xu, L.; Zhou, Z.; Zhao, S.; Yang, T.; Zhu, F. Recent advances in glycosylation involving novel anomeric radical precursors. J. Carbohyd. Chem. 2021, 40, 361–400. [Google Scholar] [CrossRef]
- Hua, D.H.; Huang, X.; Chen, Y.; Battina, S.K.; Tamura, M.; Noh, S.K.; Koo, S.I.; Namatame, I.; Tomoda, H.; Perchellet, E.M.; et al. Total Syntheses of (+)-Chloropuupehenone and (+)-Chloropuupehenol and Their Analogues and Evaluation of Their Bioactivities. J. Org. Chem. 2004, 69, 6065–6078. [Google Scholar] [CrossRef] [PubMed]
- Nuchemin, N.; Buccafusca, R.; Daumas, M.; Ferey, V.; Arseniyadis, S. A Unified Strategy for the Synthesis of Difluoromethyl- and Vinylfluoride-Containing Scaffolds. Org. Lett. 2019, 21, 8205–8210. [Google Scholar] [CrossRef]
- Quideau, S.; Lebon, M.; Lamidey, A.M. Enantiospecific Synthesis of the Antituberculosis Marine Sponge Metabolite (+)-Puupehenone. The Arenol Oxidative Activation Route. Org. Lett. 2002, 4, 3975–3978. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Li, H.J.; Wang, J.L.; Wu, Y.C. Protecting-group-free synthesis of haterumadienone- and puupehenone-type marine natural products. Green Chem. 2017, 19, 2140–2144. [Google Scholar] [CrossRef]
- Rao, Y.N.S.; Ghosh, P.; Mainkar, P.S.; Chandrasekhar, S. Access to Spiroindanolactones/lactams through an Aryne Insertion/Spirocyclization Strategy. Org. Lett. 2022, 24, 5372–5375. [Google Scholar]
- Margaros, I.; Montagnon, T.; Tofi, M.; Pavlakos, E.; Vassilikogiannakis, G. The power of singlet oxygen chemistry in biomimetic syntheses. Tetrahedron 2006, 62, 5308–5317. [Google Scholar] [CrossRef]
- Wang, H.S.; Li, H.J.; Zhang, Z.G.; Wu, Y.C. Divergent Synthesis of Bioactive Marine Meroterpenoids by Palladium-Catalyzed Tandem Carbene Migratory Insertion. Eur. J. Org. Chem. 2018, 2018, 915–925. [Google Scholar] [CrossRef]
- Guo, J.; Xu, X.; Xing, Q.; Gao, Z.; Gou, J.; Yu, B. Furfuryl Cation Induced Cascade Formal [3 + 2] Cycloaddition/Double Ring-Opening/Chlorination: An Approach to Chlorine-Containing Complex Triazoles. Org. Lett. 2018, 20, 7410–7414. [Google Scholar] [CrossRef]
- Kaiser, D.; Teskey, C.J.; Adler, P.; Maulide, N. Chemoselective Intermolecular Cross-Enolate-Type Coupling of Amides. J. Am. Chem. Soc. 2017, 139, 16040–16043. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Chen, X.; Khan, S.N.; Jia, S.; Chen, G. Synthesis and germination activity study of novel strigolactam/strigolactone analogues. Tetrahedron Lett. 2023, 115, 154315. [Google Scholar] [CrossRef]
- Riofski, M.V.; John, J.P.; Zheng, M.M.; Kirshner, J.; Colby, D.A. Exploiting the facile release of trifluoroacetate for the α-methylenation of the sterically hindered carbonyl groups on (+)-sclareolide and (−)-eburnamonine. J. Org. Chem. 2011, 76, 3676–3683. [Google Scholar] [CrossRef]
- Mei, H.; Xie, C.; Han, J.; Soloshonok, V.A. N-tert-Butylsulfinyl-3, 3, 3-trifluoroacetaldimine: Versatile Reagent for Asymmetric Synthesis of Trifluoromethyl-Containing Amines and Amino Acids of Pharmaceutical Importance. Eur. J. Org. Chem. 2016, 2016, 5917–5932. [Google Scholar] [CrossRef]
- Mei, H.; Han, J.; Fustero, S.; Román, R.; Ruzziconi, R.; Soloshonok, V.A. Recent progress in the application of fluorinated chiral sulfinimine reagents. J. Fluorine Chem. 2018, 216, 57–70. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Huang, Y.; Mei, H.; Konno, H.; Moriwaki, H.; Soloshonok, V.A.; Han, J. Asymmetric Mannich reactions of (S)-N-tert-butylsulfinyl-3, 3, 3-trifluoroacetaldimines with yne nucleophiles. Beilstein J. Org. Chem. 2020, 16, 2671–2678. [Google Scholar] [CrossRef]
- Li, Z.; Wang, N.; Mei, H.; Konno, H.; Soloshonok, V.A.; Han, J. Asymmetric Synthesis of α-Difluorinated β-Amino Sulfones through Detrifluoroacetylative Mannich Reactions. Eur. J. Org. Chem. 2021, 2021, 3035–3038. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Birolli, W.G.; Alvarenga, N.; Mouad, A.M.; Porto, A.L.M. Brazilian Marine-Derived Microorganisms: Biocatalytic Discoveries and Applications in Organic Chemistry; Marine OMICS; CRC Press: Boca Raton, FL, USA, 2016; pp. 611–660. [Google Scholar]
- Sorochinsky, A.E.; Soloshonok, V.A. Asymmetric synthesis of fluorine-containing amines, amino alcohols, α- and β-amino acids mediated by chiral sulfinyl group. J. Fluorine Chem. 2010, 131, 127–139. [Google Scholar] [CrossRef]
- Bravo, P.; Guidetti, M.; Viani, F.; Zanda, M.; Markovsky, A.L.; Sorochinsky, A.E.; Soloshonok, I.V.; Soloshonok, V.A. Chiral Sulfoxide Controlled Asymmetric Additions to C,N Double Bond. An Efficient Approach to Stereochemically Defined α-Fluoroalkyl Amino Compounds. Tetrahedron 1998, 54, 12789–12806. [Google Scholar] [CrossRef]
- Röschenthaler, G.V.; Kukhar, V.P.; Kulik, I.B.; Belik, M.Y.; Sorochinsky, A.E.; Rusanov, E.B.; Soloshonok, V.A. Asymmetric synthesis of phosphonotrifluoroalanine and its derivatives using N-tert-butanesulfinyl imine derived from fluoral. Tetrahedron Lett. 2012, 53, 539–542. [Google Scholar] [CrossRef]
- Bravo, P.; Capelli, S.; Meille, S.V.; Viani, F.; Zanda, M.; Kukhar, V.P.; Soloshonok, V.A. Synthesis of Optically Pure (R)- and (S)- α-Trifluoromethyl-Alanine. Tetrahedron Asymmetry 1994, 5, 2009–2018. [Google Scholar] [CrossRef]
- He, J.; Li, Z.; Dhawan, G.; Zhang, W.; Sorochinsky, A.E.; Butler, G.; Soloshonok, V.A.; Han, J. Fluorine-containing drugs approved by the FDA in 2021. Chin. Chem. Lett. 2023, 34, 107578. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, A.; Dhawan, G.; Mei, H.; Zhang, W.; Izawa, K.; Soloshonok, V.A.; Han, J. Fluorine-containing pharmaceuticals approved by the FDA in 2020: Synthesis and biological activity. Chin. Chem. Lett. 2021, 32, 3342–3354. [Google Scholar] [CrossRef]
- Mei, H.; Remete, A.M.; Zou, Y.; Moriwaki, H.; Fustero, S.; Kiss, L.; Soloshonok, V.A.; Han, J. Fluorine-containing drugs approved by the FDA in 2019. Chin. Chem. Lett. 2020, 31, 2401–2413. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Sun, X.; Huo, X.; Li, M.; Han, C.; Liu, A. Establishment and Application of a Multiplex PCR Assay for Detection of Sclerotium rolfsii, Lasiodiplodia theobromae, and Fusarium oxysporum in Peanut. Mol. Biotechnol. 2023, 1–9. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, Y.; Liu, Q.; Li, A.; Wang, W.; Gu, W. Design, synthesis, and antifungal activity of novel thiophene/furan-1, 3, 4-oxadiazole carboxamides as potent succinate dehydrogenase inhibitors. J. Agric. Food Chem. 2021, 69, 13373–13385. [Google Scholar] [CrossRef]
- Alforja, S.I.R.; Rico PM, B.; Caoili, B.L.; Latina, R.A. Two Philippine Photorhabdus luminescens strains inhibit the in vitro growth of Lasiodiplodia theobromae, Fusarium oxysporum f. sp. lycopersici, and Colletotrichum spp. Egypt. J. Biol. Pest Control 2021, 31, 108. [Google Scholar] [CrossRef]
- Khanzada, M.A.; Lodhi, A.M.; Shahzad, S. Pathogenicity of Lasiodiplodia theobromae and Fusarium solani on mango. Pak. J. Bot. 2004, 36, 181–190. [Google Scholar]

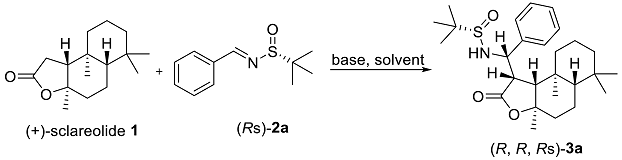 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Base | 1 (Equiv) | Solvent | Time (h) | T (°C) | Yield (%) b | Dr c |
| 1 | LiHMDS | 1.2 | THF | 4 | 0 | 69 | 92:8:0:0 |
| 2 | MeONa | 1.2 | THF | 4 | 0 | trace | - |
| 3 | LDA | 1.2 | THF | 4 | 0 | 48 | 93:7:0:0 |
| 4 | n-BuLi | 1.2 | THF | 4 | 0 | trace | - |
| 5 | LiHMDS | 1.2 | THF | 4 | rt | trace | - |
| 6 | LiHMDS | 1.2 | THF | 4 | −78 | 31 | 97:3:0:0 |
| 7 | LiHMDS | 1.2 | THF | 1 | 0 | 88 | 96:4:0:0 |
| 8 | LiHMDS | 1.2 | THF | 12 | 0 | 90 | 95:5:0:0 |
| 9 | LiHMDS | 1.2 | THF | 0.5 | 0 | 95 | 94:6:0:0 |
| 10 | LiHMDS | 1.2 | DCM | 0.5 | 0 | 20 | 94:6:0:0 |
| 11 | LiHMDS | 1.2 | MeCN | 0.5 | 0 | nd | |
| 12 | LiHMDS | 1.2 | 1,4-dioxane | 0.5 | 0 | 46 | 91:8:1:0 |
| 13 | LiHMDS | 1.5 | THF | 0.5 | 0 | 95 | 98:2:0:0 |
| Compound | Structure | Inhibition Rate (%) a | |
|---|---|---|---|
| F. oxysporum | L. theobromae | ||
| 1 | 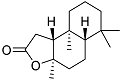 | 20 ± 4.3 | 53 ± 1.1 |
| 4 | 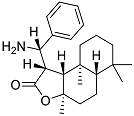 | 48 ± 2.5 | 67 ± 3.4 |
| 5 |  | 53 ± 3.2 | 61 ± 16.4 |
| 6 | 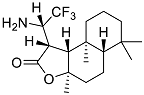 | 26 ± 1.0 | 52 ± 4.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Gao, H.; Mei, H.; Wu, G.; Soloshonok, V.A.; Han, J. Synthesis of Aminoalkyl Sclareolide Derivatives and Antifungal Activity Studies. Molecules 2023, 28, 4067. https://doi.org/10.3390/molecules28104067
Li Z, Gao H, Mei H, Wu G, Soloshonok VA, Han J. Synthesis of Aminoalkyl Sclareolide Derivatives and Antifungal Activity Studies. Molecules. 2023; 28(10):4067. https://doi.org/10.3390/molecules28104067
Chicago/Turabian StyleLi, Ziyi, Hua Gao, Haibo Mei, Guangwei Wu, Vadim A. Soloshonok, and Jianlin Han. 2023. "Synthesis of Aminoalkyl Sclareolide Derivatives and Antifungal Activity Studies" Molecules 28, no. 10: 4067. https://doi.org/10.3390/molecules28104067
APA StyleLi, Z., Gao, H., Mei, H., Wu, G., Soloshonok, V. A., & Han, J. (2023). Synthesis of Aminoalkyl Sclareolide Derivatives and Antifungal Activity Studies. Molecules, 28(10), 4067. https://doi.org/10.3390/molecules28104067








