Chemical Constitution, Pharmacological Effects and the Underlying Mechanism of Atractylenolides: A Review
Abstract
1. Introduction
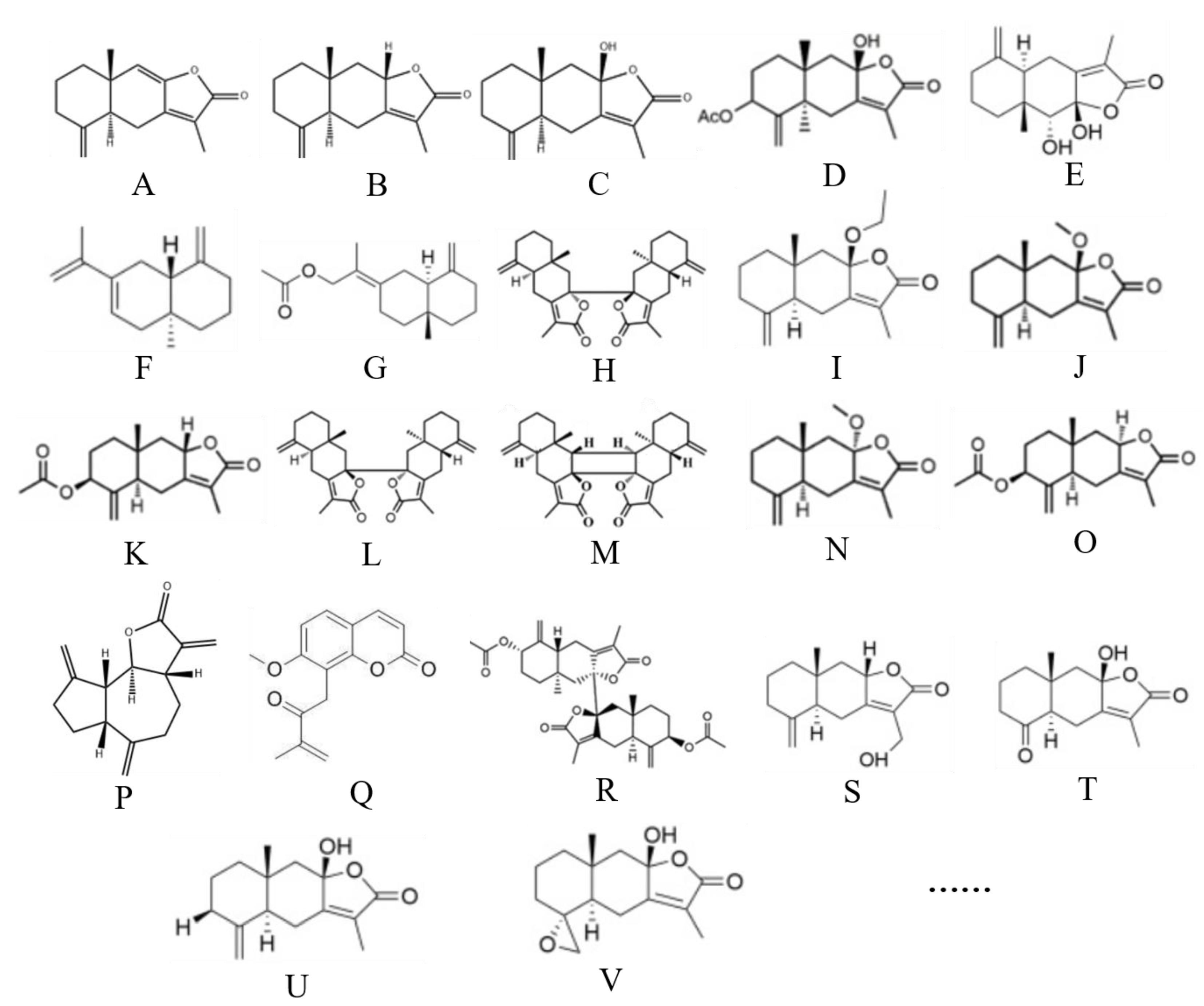
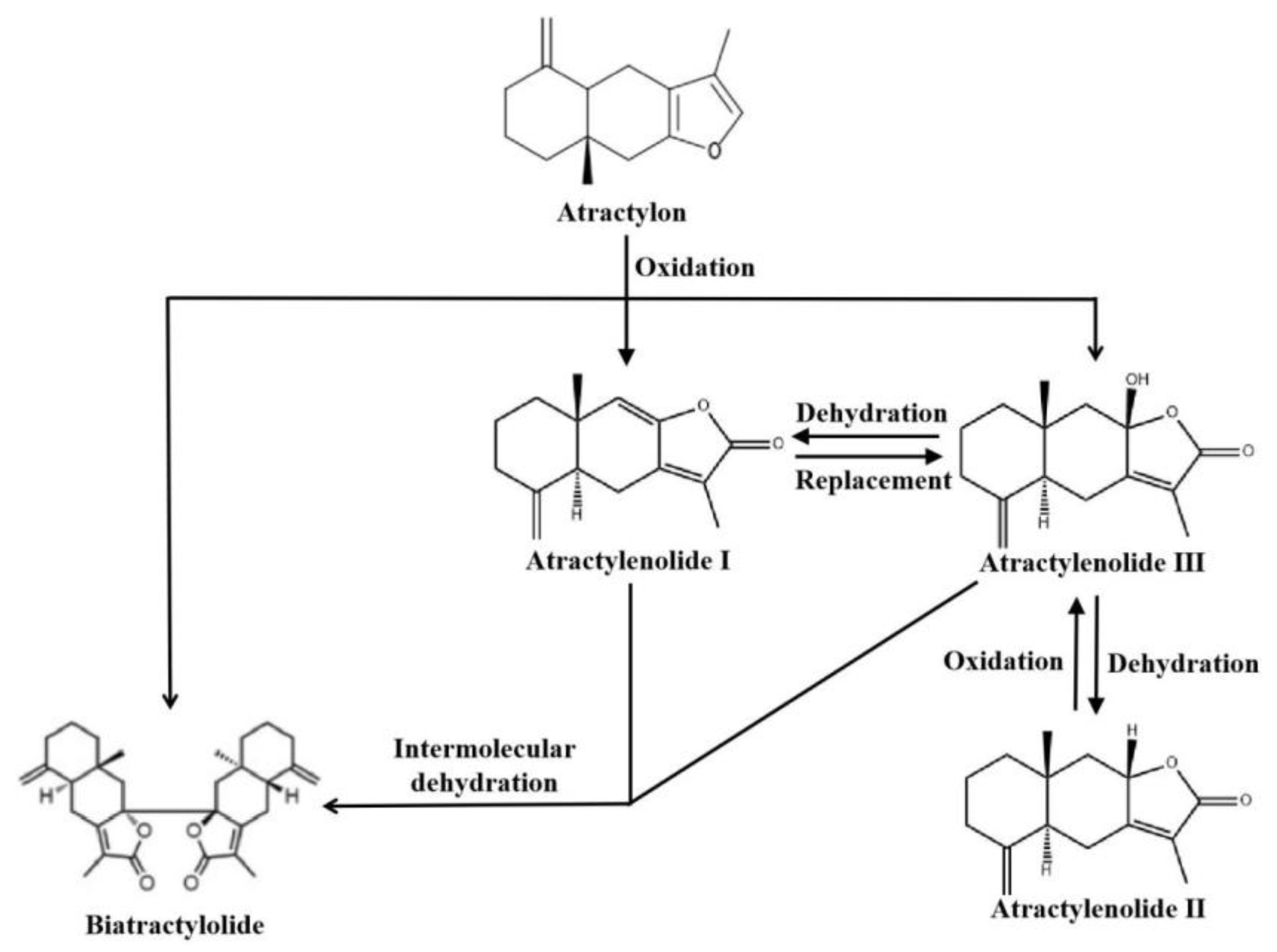
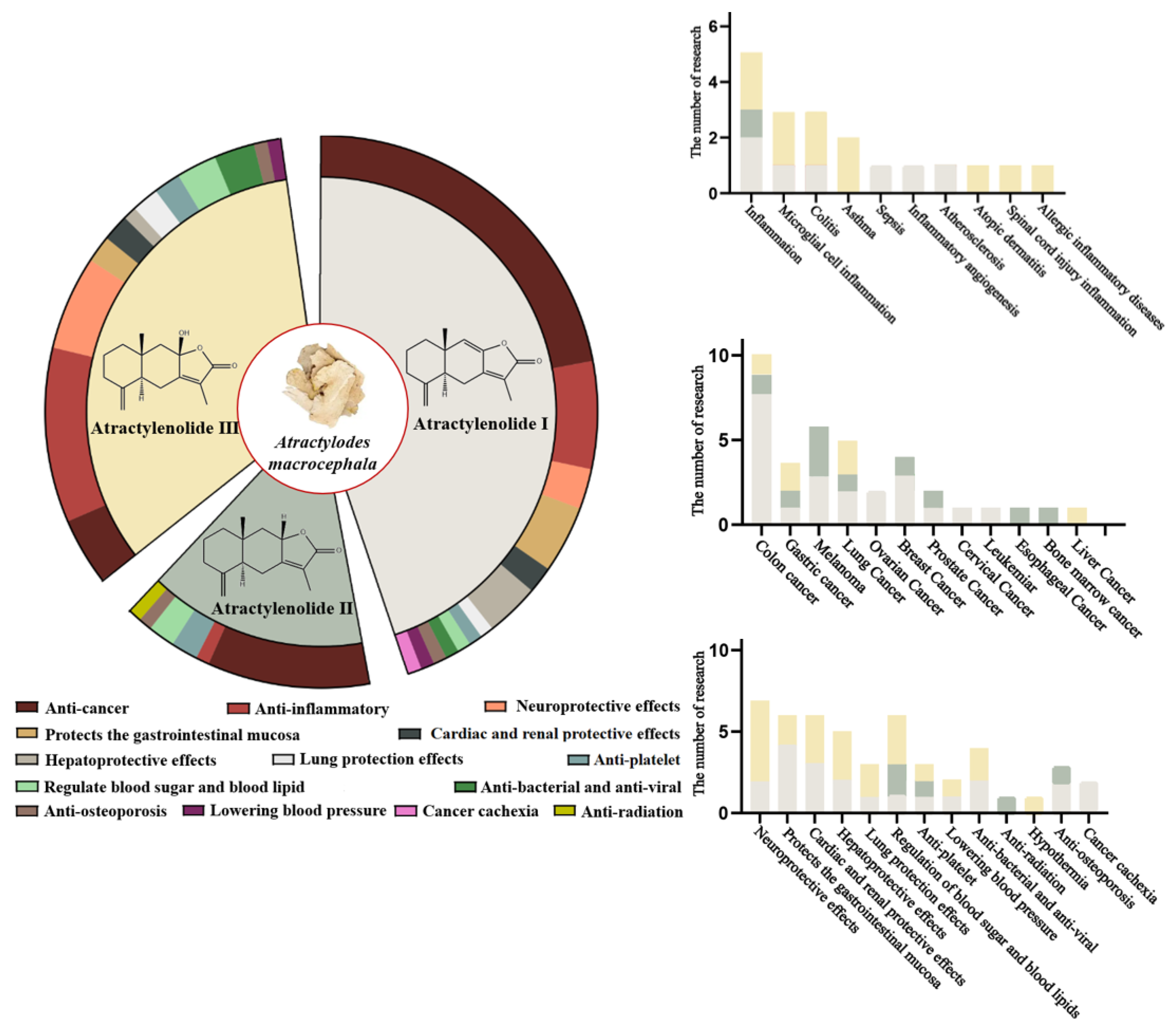
2. Overview of Atractylenolides
3. Pharmacological Effects of Atractylenolides
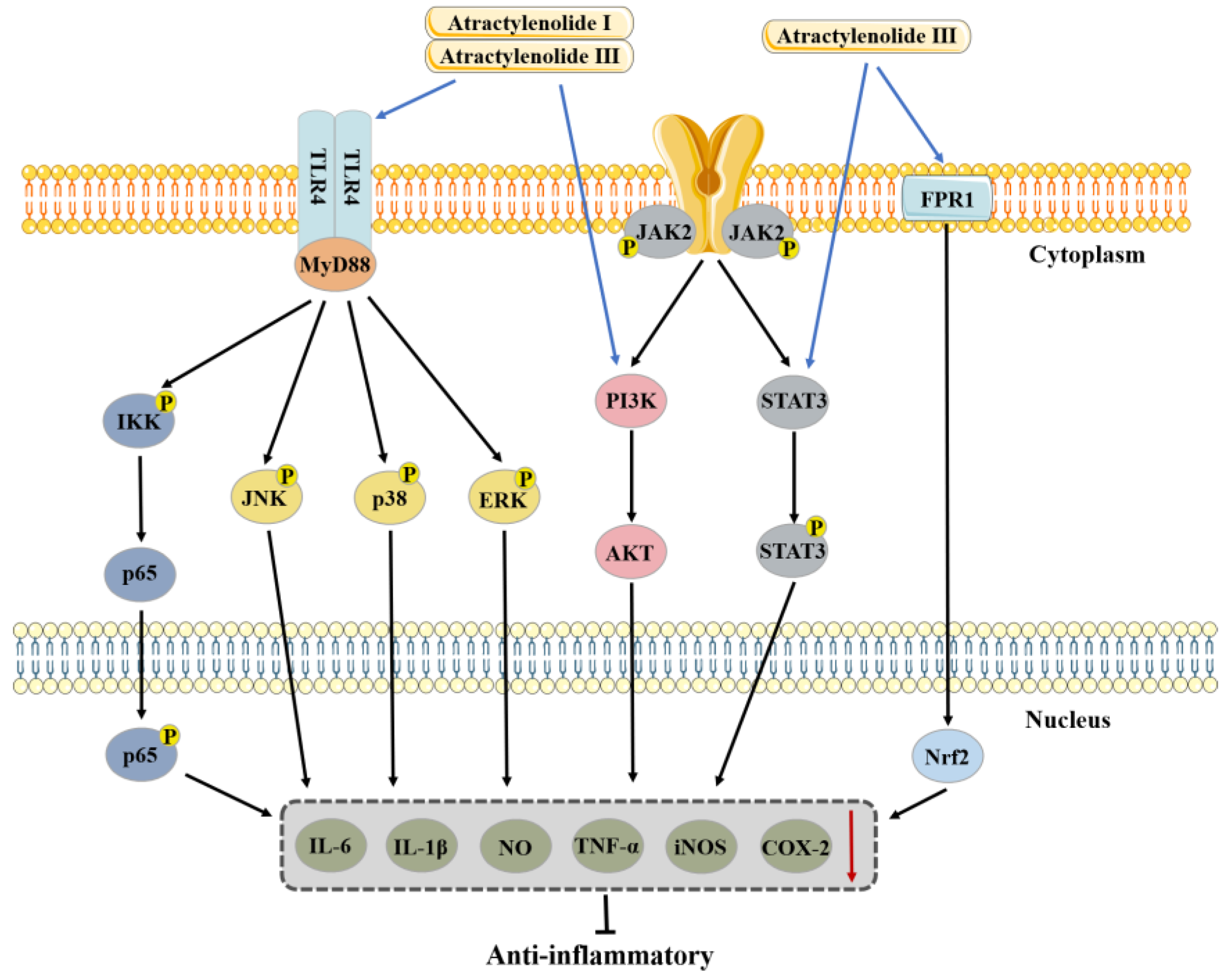
3.1. Anti-Inflammatory Effects
3.1.1. Anti-Inflammatory Effects of Atractylenolide I
3.1.2. Anti-Inflammatory Effects of Atractylenolide II
3.1.3. Anti-Inflammatory Effects of Atractylenolide III
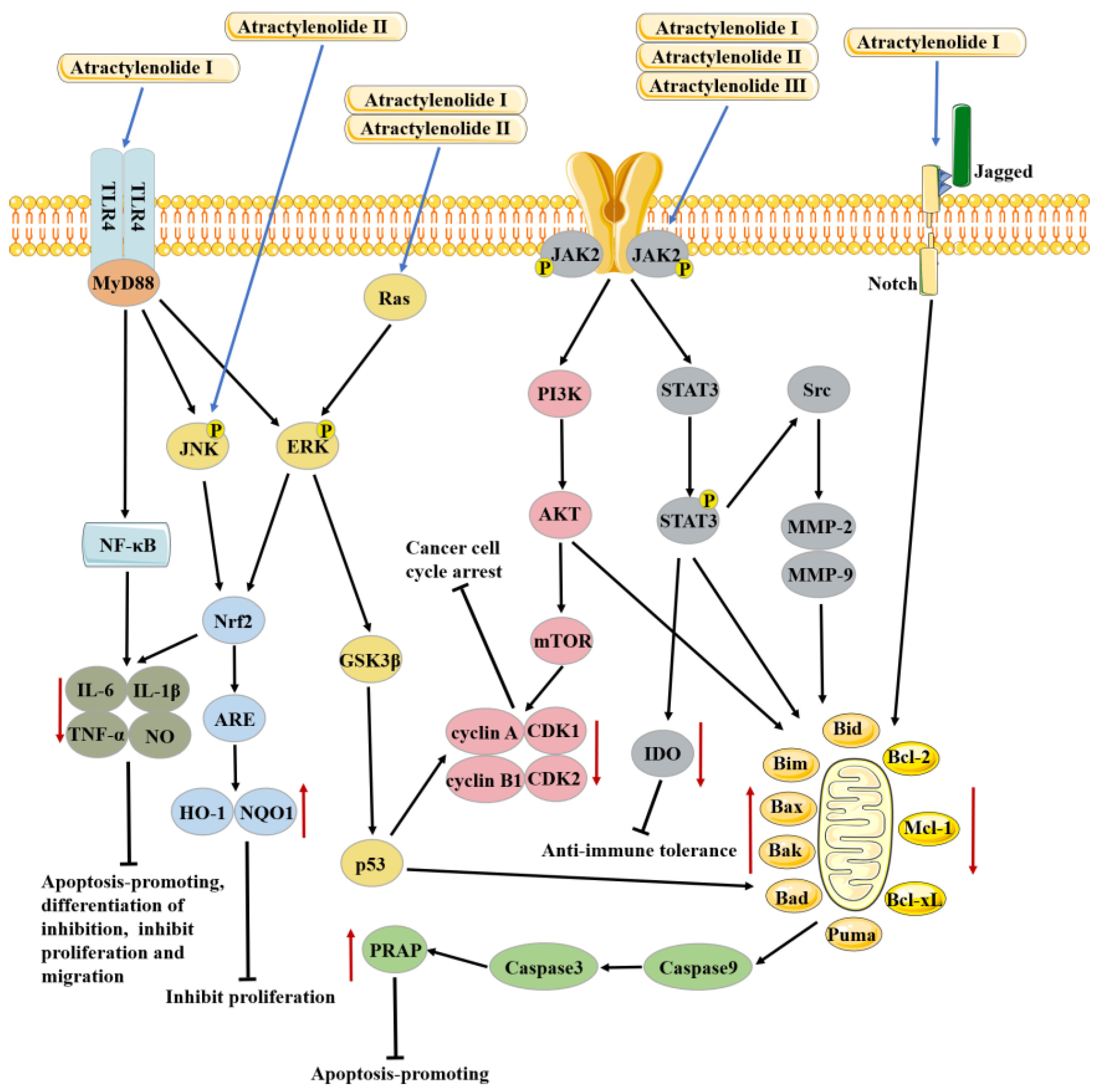
3.2. Anti-Cancer Effects
3.2.1. Anti-Cancer Effects of Atractylenolide I
3.2.2. Anti-Cancer Effects of Atractylenolide II
3.2.3. Anti-Cancer Effects of Atractylenolide III
3.3. Organ-Protective Effects
3.3.1. Protection of the Gastrointestinal Mucosa
3.3.2. Cardiac and Renal Protective Effects
Cardiac and Renal Protective Effects of Atractylenolide I
Cardiac and Renal Protective Effects of Atractylenolide III
3.3.3. Hepatoprotective Effects
Hepatoprotective Effects of Atractylenolide I
Hepatoprotective Effects of Atractylenolide III
3.3.4. Lung Protection Effects
3.3.5. Neuroprotective Effects
Neuroprotective Effects of Atractylenolide I
Neuroprotective Effects of Atractylenolide III
3.4. Regulate Blood Sugar and Blood Lipid
3.5. Other Effects
4. Safety Toxicology
5. Clinical Research
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATLs | Atractylenolides |
| LPS | Lipopolysaccharide |
| FCA | Freund’s complete adjuvant |
| DSS | Dextran sulfate sodium salt |
| TSLP | Thymic stromal lymphopoietin |
| TNBS | Trinitrobrnzen sulfonic acid |
| AOM | Azoxymethane |
| DGP | Diabetic gastroparesis |
| I/R | Ischemia/Reperfusion |
| UUO | Unilateral uretera obstruction |
| ERS | Endoplasmic reticulum stress |
| CUMS | Chronic unpredictable mild stress |
| CHO | Chinese hamster ovary |
| BMSC | Bone marrow mesenchymal stem cells |
| SOAT | Sterol O-acyltransferase |
| UGT2B7 | UDP-glucuronosyltransferase 2B7 |
References
- Zhao, J.-C.; Weng, Q.-Q.; Zhang, Y.; Zhang, W.; Peng, H.-S.; Yang, H.-J.; Zhan, Z.-L. Textual research on "Zhu" in Chinese classical prescriptions. Zhongguo Zhong Yao Za Zhi 2019, 44, 5248–5255. [Google Scholar] [CrossRef] [PubMed]
- Kulma, I.; Panrit, L.; Plengsuriyakarn, T.; Chaijaroenkul, W.; Warathumpitak, S.; Na-Bangchang, K. A randomized placebo-controlled phase I clinical trial to evaluate the immunomodulatory activities of Atractylodes lancea (Thunb) DC. in healthy Thai subjects. BMC Complement. Med. Ther. 2021, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.; Chaijaroenkul, W.; Na-Bangchang, K. Atractylodin inhibited the migration and induced autophagy in cholangiocarcinoma cells via PI3K/AKT/mTOR and p38MAPK signalling pathways. J. Pharm. Pharmacol. 2021, 73, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ning, N.; Li, Y.; Zhang, Q.-F.; Xie, Y.-C.; Irshad, M.; Feng, X.; Tao, X.-J. Biatractylolide Modulates PI3K-Akt-GSK3β-Dependent Pathways to Protect against Glutamate-Induced Cell Damage in PC12 and SH-SY5Y Cells. Evid.-Based Complement. Altern. Med. 2017, 2017, 1291458. [Google Scholar] [CrossRef]
- Zhang, B.-X.; Qi, X.-J.; Cai, Q. Metabolomic study of raw and bran-fried Atractylodis Rhizoma on rats with spleen deficiency. J. Pharm. Biomed. Anal. 2020, 182, 112927. [Google Scholar] [CrossRef]
- Li, W.; Xiang, X.; Li, B.; Wang, Y.; Qian, L.; Tian, Y.; Huang, Y.; Xu, D.; Cao, N. PAMK Relieves LPS-Induced Enteritis and Improves Intestinal Flora Disorder in Goslings. Evid.-Based Complement. Altern. Med. 2021, 2021, 9721353. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Q.-L.; Hua, J.-W.; Cheng, W.-L.; Qin, L.-P. The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: A review. J. Ethnopharmacol. 2018, 226, 143–167. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Y.-N.; Xu, R.-Z.; Zhang, X.-W.; Sun, Y.-R.; Feng, Q.-M.; Li, Z.-H.; Xu, J.-Y.; Xie, Z.-S.; Zhang, Z.-Q.; et al. Sesquiterpene Lactams and Lactones with Antioxidant Potentials from Atractylodes macrocephala Discovered by Molecular Networking Strategy. Front. Nutr. 2022, 9, 865257. [Google Scholar] [CrossRef]
- Chen, Z.-L. The Acetylenes from Atractylodes macrocephala. Planta Medica 1987, 53, 493–494. [Google Scholar] [CrossRef]
- Zhang, Y.; Bo, C.; Fan, Y.; An, R.; Chen, L.; Zhang, Y.; Jia, Y.; Wang, X. Qualitative and quantitative determination of Atractylodes rhizome using ultra-performance liquid chromatography coupled with linear ion trap–Orbitrap mass spectrometry with data-dependent processing. Biomed. Chromatogr. 2019, 33, e4443. [Google Scholar] [CrossRef]
- Li, Y.; Dai, M.; Peng, D. New bisesquiterpenoid lactone from the wild rhizome of Atractylodes macrocephala Koidz grown in Qimen. Nat. Prod. Res. 2017, 31, 2381–2386. [Google Scholar] [CrossRef]
- Zhu, Q.; Lin, M.; Zhuo, W.; Li, Y. Chemical Constituents from the Wild Atractylodes macrocephala Koidz and Acetylcholinesterase Inhibitory Activity Evaluation as Well as Molecular Docking Study. Molecules 2021, 26, 7299. [Google Scholar] [CrossRef]
- Wang, P.; Xie, Z.-S.; Song, J.-Y.; Zeng, H.-H.; Dai, L.-P.; E, H.-C.; Ye, Z.-P.; Gao, S.; Xu, J.-Y.; Zhang, Z.-Q. Four new sesquiterpene lactones from Atractylodes macrocephala and their CREB agonistic activities. Fitoterapia 2020, 147, 104730. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, C.; Sun, T.-M.; Ran, X.-K.; Kang, T.-G.; Dou, D.-Q. Two new compounds from Atractylodes macrocephala with neuroprotective activity. J. Asian Nat. Prod. Res. 2017, 19, 35–41. [Google Scholar] [CrossRef]
- Bailly, C. Atractylenolides, essential components of Atractylodes-based traditional herbal medicines: Antioxidant, anti-inflammatory and anticancer properties. Eur. J. Pharmacol. 2021, 891, 173735. [Google Scholar] [CrossRef]
- Zhan, C.; Wang, H.; Wang, Y. Quality evaluation of Atractylodis macrocephalae rhizoma through fingerprint qualitative analysis and quantitative analysis of multi-components by single marker. J. Pharm. Biomed. Anal. 2022, 219, 114899. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, Y.; Lee, G.; Doh, E.-J.; Hong, S. Quantitative Interrelation between Atractylenolide I, II, and III in Atractylodes japonica Koidzumi Rhizomes, and Evaluation of Their Oxidative Transformation Using a Biomimetic Kinetic Model. ACS Omega 2018, 3, 14833–14840. [Google Scholar] [CrossRef]
- Wang, X.F.; Wang, F.; Zhang, Y.H.; Zheng, X.H. Kinetics of oxidation of atractylodes ketone in volatile oil of Atractylodes macrocephala Koidz. Chin. J. Appl. Chem. 2007, 3, 301–305. [Google Scholar]
- Li, W.; Wen, H.-M.; Cui, X.-B.; Zhang, K.-W. Process mechanism of Atractylodes macrocephala and conversion of sesquiterpenes. Zhongguo Zhong Yao Za Zhi 2006, 31, 1600–1603. [Google Scholar]
- Wang, K.-T.; Chen, L.-G.; Yang, L.-L.; Ke, W.-M.; Chang, H.-C.; Wan, C.-C. Analysis of the Sesquiterpenoids in Processed Atractylodis Rhizoma. Chem. Pharm. Bull. 2007, 55, 50–56. [Google Scholar] [CrossRef]
- Ji, G.; Chen, R.; Zheng, J. Atractylenolide I inhibits lipopolysaccharide-induced inflammatory responses via mitogen-activated protein kinase pathways in RAW264.7 cells. Immunopharmacol. Immunotoxicol. 2014, 36, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Q.; He, L.-C.; Jin, J.-Q. Atractylenolide I and atractylenolide III inhibit Lipopolysaccharide-induced TNF-α and NO production in macrophages. Phytother. Res. 2007, 21, 347–353. [Google Scholar] [CrossRef] [PubMed]
- More, S.; Choi, D.-K. Neuroprotective Role of Atractylenolide-I in an In Vitro and In Vivo Model of Parkinson’s Disease. Nutrients 2017, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Xiao, Z.; Zhou, L.; Zhang, J.; Li, X.; He, Q. The protective effect of atractylenolide I on systemic inflammation in the mouse model of sepsis created by cecal ligation and puncture. Pharm. Biol. 2015, 54, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Duan, H.; He, L. Inhibitory effect of atractylenolide I on angiogenesis in chronic inflammation in vivo and in vitro. Eur. J. Pharmacol. 2009, 612, 143–152. [Google Scholar] [CrossRef]
- Li, W.; Zhi, W.; Liu, F.; He, Z.; Wang, X.; Niu, X. Atractylenolide I restores HO-1 expression and inhibits Ox-LDL-induced VSMCs proliferation, migration and inflammatory responses in vitro. Exp. Cell Res. 2017, 353, 26–34. [Google Scholar] [CrossRef]
- Qu, L.; Shi, K.; Xu, J.; Liu, C.; Ke, C.; Zhan, X.; Xu, K.; Liu, Y. Atractylenolide-1 targets SPHK1 and B4GALT2 to regulate intestinal metabolism and flora composition to improve inflammation in mice with colitis. Phytomedicine 2022, 98, 153945. [Google Scholar] [CrossRef]
- Hoang, L.S.; Tran, M.H.; Lee, J.-S.; Ngo, Q.M.T.; Woo, M.H.; Min, B.S. Inflammatory Inhibitory Activity of Sesquiterpenoids from Atractylodes macrocephala Rhizomes. Chem. Pharm. Bull. 2016, 64, 507–511. [Google Scholar] [CrossRef]
- Ji, G.-Q.; Chen, R.-Q.; Wang, L. Anti-inflammatory activity of atractylenolide III through inhibition of nuclear factor-κB and mitogen-activated protein kinase pathways in mouse macrophages. Immunopharmacol. Immunotoxicol. 2016, 38, 98–102. [Google Scholar] [CrossRef]
- Yoou, M.-S.; Nam, S.-Y.; Jin, M.H.; Lee, S.Y.; Kim, M.-S.; Roh, S.S.; Choi, I.H.; Woo, N.; Lim, S.; Kim, D.H.; et al. Ameliorative effect of atractylenolide III in the mast cell proliferation induced by TSLP. Food Chem. Toxicol. 2017, 106, 78–85. [Google Scholar] [CrossRef]
- Ren, Y.; Jiang, W.; Luo, C.; Zhang, X.; Huang, M. Atractylenolide III Ameliorates TNBS-Induced Intestinal Inflammation in Mice by Reducing Oxidative Stress and Regulating Intestinal Flora. Chem. Biodivers. 2021, 18, e2001001. [Google Scholar] [CrossRef]
- Han, J.; Li, W.; Shi, G.; Huang, Y.; Sun, X.; Sun, N.; Jiang, D. Atractylenolide III Improves Mitochondrial Function and Protects Against Ulcerative Colitis by Activating AMPK/SIRT1/PGC-1α. Mediat. Inflamm. 2022, 2022, 9129984. [Google Scholar] [CrossRef]
- Zhang, L.; Yi, H.; Jiang, D.; Li, Z. Protective effects of Atractylenolide III on inflammation and oxidative stress in ovalbumin-induced asthma mice and its possible mechanisms. Gen. Physiol. Biophys. 2021, 40, 137–146. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, L.; Liu, Z.; Li, C.; Bai, Y.; Wang, L. Atractylenolide III reduces NLRP3 inflammasome activation and Th1/Th2 imbalances in both in vitro and in vivo models of asthma. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1360–1367. [Google Scholar] [CrossRef]
- Zhang, N.-N.; Park, D.K.; Park, H.-J. The inhibitory activity of atractylenolide capital SHA, Cyrillic, a sesquiterpenoid, on IgE-mediated mast cell activation and passive cutaneous anaphylaxis (PCA). J. Ethnopharmacol. 2013, 145, 278–285. [Google Scholar] [CrossRef]
- Xue, M.; Sheng, W.; Song, X.; Shi, Y.; Geng, Z.; Shen, L.; Wang, R.; Lü, H.; Hu, J. Atractylenolide III ameliorates spinal cord injury in rats by modulating microglial/macrophage polarization. CNS Neurosci. Ther. 2022, 28, 1059–1071. [Google Scholar] [CrossRef]
- Novianti, E.; Katsuura, G.; Kawamura, N.; Asakawa, A.; Inui, A. Atractylenolide-III suppresses lipopolysaccharide-induced inflammation via downregulation of toll-like receptor 4 in mouse microglia. Heliyon 2021, 7, e08269. [Google Scholar] [CrossRef]
- Zhou, K.; Chen, J.; Wu, J.; Wu, Q.; Jia, C.; Xu, Y.X.Z.; Chen, L.; Tu, W.; Yang, G.; Kong, J.; et al. Atractylenolide III ameliorates cerebral ischemic injury and neuroinflammation associated with inhibiting JAK2/STAT3/Drp1-dependent mitochondrial fission in microglia. Phytomedicine 2019, 59, 152922. [Google Scholar] [CrossRef]
- Chan, K.W.K.; Chung, H.Y.; Ho, W.S. Anti-Tumor Activity of Atractylenolide I in Human Colon Adenocarcinoma In Vitro. Molecules 2020, 25, 212. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Cai, Y.; Han, P.; Hu, S.; Cao, L. Atractylenolide I inhibited the development of malignant colorectal cancer cells and enhanced oxaliplatin sensitivity through the PDK1-FoxO1 axis. J. Gastrointest. Oncol. 2022, 13, 2382–2392. [Google Scholar] [CrossRef]
- Qin, Y.; Yu, Y.; Yang, C.; Wang, Z.; Yang, Y.; Wang, C.; Zheng, Q.; Li, D.; Xu, W. Atractylenolide I Inhibits NLRP3 Inflammasome Activation in Colitis-Associated Colorectal Cancer via Suppressing Drp1-Mediated Mitochondrial Fission. Front. Pharmacol. 2021, 12, 674340. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huang, W.; Sang, X.; Wu, X.; Shan, Q.; Tang, D.; Xu, X.; Cao, G. Atractylenolide I inhibits colorectal cancer cell proliferation by affecting metabolism and stemness via AKT/mTOR signaling. Phytomedicine 2020, 68, 153191. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Xu, X.; Ying, J.; Xie, T.; Cao, G. Transfer of metastatic traits via miR-200c in extracellular vesicles derived from colorectal cancer stem cells is inhibited by atractylenolide I. Clin. Transl. Med. 2020, 10, e139. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Liu, Z.; Guo, X.; Miao, Z.; Ma, S. Atractylenolide I Induces Apoptosis and Suppresses Glycolysis by Blocking the JAK2/STAT3 Signaling Pathway in Colorectal Cancer Cells. Front. Pharmacol. 2020, 11, 273. [Google Scholar] [CrossRef]
- Xu, H.; Van der Jeught, K.; Zhou, Z.; Zhang, L.; Yu, T.; Sun, Y.; Li, Y.; Wan, C.; So, K.M.; Liu, D.; et al. Atractylenolide I enhances responsiveness to immune checkpoint blockade therapy by activating tumor antigen presentation. J. Clin. Investig. 2021, 131, 146832. [Google Scholar] [CrossRef]
- Li, L.; Jing, L.; Wang, J.; Xu, W.; Gong, X.; Zhao, Y.; Ma, Y.; Yao, X.; Sun, X. Autophagic flux is essential for the downregulation of D-dopachrome tautomerase by atractylenolide I to ameliorate intestinal adenoma formation. J. Cell Commun. Signal. 2018, 12, 689–698. [Google Scholar] [CrossRef]
- Long, F.; Wang, T.; Jia, P.; Wang, H.; Qing, Y.; Xiong, T.; He, M.; Wang, X. Anti-Tumor Effects of Atractylenolide-I on Human Ovarian Cancer Cells. Med. Sci. Monit. 2017, 23, 571–579. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, G.; Huang, J.; Ma, S.; Mi, K.; Cheng, J.; Zhu, Y.; Zha, X.; Huang, W. Atractylenolide I modulates ovarian cancer cell-mediated immunosuppression by blocking MD-2/TLR4 complex-mediated MyD88/NF-κB signaling in vitro. J. Transl. Med. 2016, 14, 104. [Google Scholar] [CrossRef]
- Xiao, Q.; Zheng, F.; Wu, J.; Tang, Q.; Wang, W.; Hann, S.S. Activation of ERK and Mutual Regulation of Stat3 and SP1 Contribute to Inhibition of PDK1 Expression by Atractylenolide-1 in Human Lung Cancer Cells. Cell Physiol. Biochem. 2017, 43, 2353–2366. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Y.; Zhang, T.; Zhao, Z.; Zhao, Y.; Cheng, P.; Li, H.; Gao, H.; Su, X. Anti-Tumor Effects of Atractylenolide I Isolated from Atractylodes macrocephala in Human Lung Carcinoma Cell Lines. Molecules 2013, 18, 13357–13368. [Google Scholar] [CrossRef]
- Ye, Y.; Chao, X.-J.; Wu, J.-F.; Cheng, B.C.-Y.; Su, T.; Fu, X.-Q.; Li, T.; Guo, H.; Tse, A.K.-W.; Kwan, H.-Y.; et al. ERK/GSK3β signaling is involved in atractylenolide I-induced apoptosis and cell cycle arrest in melanoma cells. Oncol. Rep. 2015, 34, 1543–1548. [Google Scholar] [CrossRef]
- Fu, X.-Q.; Chou, J.-Y.; Li, T.; Zhu, P.-L.; Li, J.-K.; Yin, C.-L.; Su, T.; Guo, H.; Lee, K.-W.; Hossen, M.J.; et al. The JAK2/STAT3 pathway is involved in the anti-melanoma effects of atractylenolide I. Exp. Dermatol. 2018, 27, 201–204. [Google Scholar] [CrossRef]
- Wang, M.; Li, X.-Z.; Zhang, M.-X.; Ye, Q.-Y.; Chen, Y.-X.; Chang, X. Atractylenolide-I Sensitizes Triple-Negative Breast Cancer Cells to Paclitaxel by Blocking CTGF Expression and Fibroblast Activation. Front. Oncol. 2021, 11, 738534. [Google Scholar] [CrossRef]
- Xu, H.; Li, L.; Qu, L.; Tu, J.; Sun, X.; Liu, X.; Xu, K. Atractylenolide-1 affects glycolysis/gluconeogenesis by downregulating the expression of TPI1 and GPI to inhibit the proliferation and invasion of human triple-negative breast cancer cells. Phytother. Res. 2023, 37, 820–833. [Google Scholar] [CrossRef]
- Long, F.; Lin, H.; Zhang, X.; Zhang, J.; Xiao, H.; Wang, T. Atractylenolide-I Suppresses Tumorigenesis of Breast Cancer by Inhibiting Toll-Like Receptor 4-Mediated Nuclear Factor-κB Signaling Pathway. Front. Pharmacol. 2020, 11, 598939. [Google Scholar] [CrossRef]
- Ma, L.; Mao, R.; Shen, K.; Zheng, Y.; Li, Y.; Liu, J.; Ni, L. Atractylenolide I-mediated Notch pathway inhibition attenuates gastric cancer stem cell traits. Biochem. Biophys. Res. Commun. 2014, 450, 353–359. [Google Scholar] [CrossRef]
- Huang, H.-L.; Lin, T.-W.; Huang, Y.-L.; Huang, R.-L. Induction of apoptosis and differentiation by atractylenolide-1 isolated from Atractylodes macrocephala in human leukemia cells. Bioorg. Med. Chem. Lett. 2016, 26, 1905–1909. [Google Scholar] [CrossRef]
- Yu, R.; Yu, B.-X.; Chen, J.-F.; Lv, X.-Y.; Yan, Z.-J.; Cheng, Y.; Ma, Q. Anti-tumor effects of Atractylenolide I on bladder cancer cells. J. Exp. Clin. Cancer Res. 2016, 35, 40. [Google Scholar] [CrossRef]
- Qiao, P.; Tian, Z. Atractylenolide I inhibits EMT and enhances the antitumor effect of cabozantinib in prostate cancer via targeting Hsp27. Front. Oncol. 2022, 12, 1084884. [Google Scholar] [CrossRef]
- Han, Y.; Bai, C.; He, X.-M.; Ren, Q.-L. P2X7 receptor involved in antitumor activity of atractylenolide I in human cervical cancer cells. Purinergic Signal. 2023, 19, 145–153. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Yu, Q.; Shen, J.; He, W.; Zhou, D.; Yu, Q.; Fan, J.; Gao, S.; Duan, L. Atractylenolide II reverses the influence of lncRNA XIST/miR-30a-5p/ROR1 axis on chemo-resistance of colorectal cancer cells. J. Cell Mol. Med. 2019, 23, 3151–3165. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Yu, H. Atractylenolide II Inhibits Proliferation, Motility and Induces Apoptosis in Human Gastric Carcinoma Cell Lines HGC-27 and AGS. Molecules 2017, 22, 1886. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, H.; Chu, J.-H.; Chou, G.-X.; Chen, S.-B.; Mo, H.; Fong, W.-F.; Yu, Z.-L. Atractylenolide II induces G1 cell-cycle arrest and apoptosis in B16 melanoma cells. J. Ethnopharmacol. 2011, 136, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-Q.; Chou, G.-X.; Kwan, H.Y.; Tse, A.K.-W.; Zhao, L.-H.; Yuen, T.-K.; Cao, H.-H.; Yu, H.; Chao, X.-J.; Su, T.; et al. Inhibition of STAT3 signalling contributes to the antimelanoma action of atractylenolide II. Exp. Dermatol. 2014, 23, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Chou, G.-X.; Wang, H.; Chu, J.-H.; Fong, W.-F.; Yu, Z.-L. Effects of Sesquiterpenes Isolated From Largehead Atractylodes Rhizome on Growth, Migration, and Differentiation of B16 Melanoma Cells. Integr. Cancer Ther. 2011, 10, 92–100. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Wang, J.; Jiang, Z.; Zhang, L.; Cui, Y.; Zhao, D.; Wang, Y. Atractylenolide II inhibits tumor-associated macrophages (TAMs)-induced lung cancer cell metastasis. Immunopharmacol. Immunotoxicol. 2022, 44, 227–237. [Google Scholar] [CrossRef]
- Wang, T.; Long, F.; Zhang, X.; Yang, Y.; Jiang, X.; Wang, L. Chemopreventive effects of atractylenolide II on mammary tumorigenesis via activating Nrf2-ARE pathway. Oncotarget 2017, 8, 77500–77514. [Google Scholar] [CrossRef]
- Wang, J.; Nasser, M.I.; Adlat, S.; Jiang, M.M.; Jiang, N.; Gao, L. Atractylenolide II Induces Apoptosis of Prostate Cancer Cells through Regulation of AR and JAK2/STAT3 Signaling Pathways. Molecules 2018, 23, 3298. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X.; Song, D.; Chen, S.; Zhang, Z.; Cao, S.; Liu, M. Atractylenolide III induces apoptosis by regulating the Bax/Bcl-2 signaling pathway in human colorectal cancer HCT-116 Cells in vitro and in vivo. Anti-Cancer Drugs 2022, 33, 30–47. [Google Scholar] [CrossRef]
- Kang, T.-H.; Bang, J.-Y.; Kim, M.-H.; Kang, I.-C.; Kim, H.-M.; Jeong, H.-J. Atractylenolide III, a sesquiterpenoid, induces apoptosis in human lung carcinoma A549 cells via mitochondria-mediated death pathway. Food Chem. Toxicol. 2011, 49, 514–519. [Google Scholar] [CrossRef]
- Sheng, L.; Li, J.; Li, N.; Gong, L.; Liu, L.; Zhang, Q.; Li, X.; Luo, H.; Chen, Z. Atractylenolide III predisposes miR-195-5p/FGFR1 signaling axis to exert tumor-suppressive functions in liver cancer. J. Food Biochem. 2021, 45, e13582. [Google Scholar] [CrossRef]
- Liu, J.-B.; Chen, D.; Bao, T.-T.; Fan, F.-T.; Yu, C. The Anticancer Effects of Atractylenolide III Associate With the Downregulation of Jak3/Stat3-Dependent IDO Expression. Front. Pharmacol. 2019, 10, 1505. [Google Scholar] [CrossRef]
- Ji, Y.; Kang, Z.; Kang, N.; Zhao, Y.; Guo, Q.; Chen, Y. Atractylenolide III Enhances the Anti-Neoplastic Efficacy of Docetaxel in Gastric Cancer Cell by Inhibiting Fibroblast Growth Factor Receptors 1, -2, and -4 Expression. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 217–227. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Zhao, M.; Xia, T.; Liu, Q.; Chen, N.; Liao, W.; Zeng, Z.; You, F.; Zeng, J. Atractylenolide III Attenuates Angiogenesis in Gastric Precancerous Lesions Through the Downregulation of Delta-Like Ligand 4. Front. Pharmacol. 2022, 13, 797805. [Google Scholar] [CrossRef]
- Wang, K.-T.; Chen, L.-G.; Wu, C.-H.; Chang, C.-C.; Wang, C.-C. Gastroprotective activity of atractylenolide III from Atractylodes ovata on ethanol-induced gastric ulcer in vitro and in vivo. J. Pharm. Pharmacol. 2010, 62, 381–388. [Google Scholar] [CrossRef]
- Li, H.; Cao, W.; Zhang, X.-B.; Zhang, X.-X.; Gu, C.; Gu, L.-M.; Pan, C.-Y.; Tian, Y.-Z.; Lu, M. Atractylenolide-1 alleviates gastroparesis in diabetic rats by activating the stem cell factor/c-kit signaling pathway. Mol. Med. Rep. 2021, 24, 691. [Google Scholar] [CrossRef]
- Song, H.-P.; Hou, X.-Q.; Li, R.-Y.; Yu, R.; Li, X.; Zhou, S.-N.; Huang, H.-Y.; Cai, X.; Zhou, C. Atractylenolide I stimulates intestinal epithelial repair through polyamine-mediated Ca2+ signaling pathway. Phytomedicine 2017, 28, 27–35. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, G.; Zhang, L.; Gong, Y.; Gu, Y. Atractylenolide I inhibits antibiotic-induced dysbiosis of the intestinal microbiome. Ann. Transl. Med. 2021, 9, 1539. [Google Scholar] [CrossRef]
- Xu, R.; Liu, X.; Tian, M.; Chen, D. Atractylodes-I overcomes the oxidative stress-induced colonic mucosal epithelial cells dysfunction to prevent irritable bowel syndrome via modulating the miR-34a-5p-LDHA signaling pathway. Curr. Mol. Med. 2022, 22. [Google Scholar] [CrossRef]
- Huang, M.; Jiang, W.; Luo, C.; Yang, M.; Ren, Y. Atractylenolide III inhibits epithelial-mesenchymal transition in small intestine epithelial cells by activating the AMPK signaling pathway. Mol. Med. Rep. 2022, 25, 98. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, X.; Yu, F.; Liu, C.; Hu, F.; Liu, L.; Chen, J.; Wang, J. Atractylenolide I alleviates ischemia/reperfusion injury by preserving mitochondrial function and inhibiting caspase-3 activity. J. Int. Med. Res. 2021, 49, 0300060521993315. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, P.; Zhang, J.; Ma, Z.; Tian, X.; Liu, Y.; Lv, G.; Qu, L. Atractylenolide-1 Targets FLT3 to Regulate PI3K/AKT/HIF1-α Pathway to Inhibit Osteogenic Differentiation of Human Valve Interstitial Cells. Front. Pharmacol. 2022, 13, 899775. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xiao, Y.; Zhu, H.; Guo, H.; Zhou, Y.; Shentu, Y.; Zheng, C.; Chen, C.; Bai, Y. Inhibition of proliferation-linked signaling cascades with atractylenolide I reduces myofibroblastic phenotype and renal fibrosis. Biochem. Pharmacol. 2020, 183, 114344. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.Y.; Tang, T.J.; Zhou, P.; Wang, X.; Ding, R.; Gu, J.F.; Chen, J.; Wang, L.; Yao, J.; Li, X.Y.; et al. Mechanism of atractylenolide III in alleviating H9c2 cell apoptosis through ROS/GRP78/caspase-12 signaling pathway based on molecular docking. Zhongguo Zhong Yao Za Zhi 2022, 47, 4436–4445. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.-Y.; Tang, T.-J.; Wang, X.; Gu, J.-F.; Wang, L.; Chen, J.; Yao, J.; Li, X.-Y.; Zhou, P.; Huang, J.-L. Atractylenolide III Attenuates Apoptosis in H9c2 Cells by Inhibiting Endoplasmic Reticulum Stress through the GRP78/PERK/CHOP Signaling Pathway. Evid.-Based Complement. Altern. Med. 2022, 2022, 1149231. [Google Scholar] [CrossRef]
- Wang, M.; Hu, R.; Wang, Y.; Liu, L.; You, H.; Zhang, J.; Wu, X.; Pei, T.; Wang, F.; Lu, L.; et al. Atractylenolide III Attenuates Muscle Wasting in Chronic Kidney Disease via the Oxidative Stress-Mediated PI3K/AKT/mTOR Pathway. Oxidative Med. Cell Longev. 2019, 2019, 1875471. [Google Scholar] [CrossRef]
- Du, Z.; Ma, Z.; Lai, S.; Ding, Q.; Hu, Z.; Yang, W.; Qian, Q.; Zhu, L.; Dou, X.; Li, S. Atractylenolide I Ameliorates Acetaminophen-Induced Acute Liver Injury via the TLR4/MAPKs/NF-κB Signaling Pathways. Front. Pharmacol. 2022, 13, 797499. [Google Scholar] [CrossRef]
- Wang, C.; Geng, Q.; Wang, Y. Protective effect of atractylenolide I on immunological liver injury. Zhongguo Zhong Yao Za Zhi 2012, 37, 1809–1813. [Google Scholar]
- Li, Q.; Tan, J.-X.; He, Y.; Bai, F.; Li, S.-W.; Hou, Y.-W.; Ji, L.-S.; Gao, Y.-T.; Zhang, X.; Zhou, Z.-H.; et al. Atractylenolide III ameliorates Non-Alcoholic Fatty Liver Disease by activating Hepatic Adiponectin Receptor 1-Mediated AMPK Pathway. Int. J. Biol. Sci. 2022, 18, 1594–1611. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Chen, L.; Yang, T.; Liang, B.; Zhang, Z.; Shao, J.; Xu, X.; Yin, G.; Wang, S.; et al. Inhibition of ASCT2 induces hepatic stellate cell senescence with modified proinflammatory secretome through an IL-1α/NF-κB feedback pathway to inhibit liver fibrosis. Acta Pharm. Sin. B 2022, 12, 3618–3638. [Google Scholar] [CrossRef]
- Wu, H.; Wu, L.; Xiao, L.; Gu, Y.; Liu, H.; Zhang, L.; Zhang, M.; Qi, L. Atractylenolide III suppresses senescence-associated secretome via inhibiting cGAS/NF-κB pathway in hepatic stellate cells. Clin. Exp. Pharmacol. Physiol. 2023, 50, 316–324. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Huang, W.-M.; Zeng, Q.-Y. Atractylenolide I protects mice from lipopolysaccharide-induced acute lung injury. Eur. J. Pharmacol. 2015, 765, 94–99. [Google Scholar] [CrossRef]
- Fu, J.-D.; Gao, C.-H.; Li, S.-W.; Tian, Y.; Li, S.C.; Wei, Y.-E.; Xian, L.-W. Atractylenolide III alleviates sepsis-mediated lung injury via inhibition of FoxO1 and VNN1 protein. Acta Cir. Bras. 2021, 36, e360802. [Google Scholar] [CrossRef]
- Huai, B.; Ding, J. Atractylenolide III attenuates bleomycin-induced experimental pulmonary fibrosis and oxidative stress in rat model via Nrf2/NQO1/HO-1 pathway activation. Immunopharmacol. Immunotoxicol. 2020, 42, 436–444. [Google Scholar] [CrossRef]
- More, S.V.; Choi, D.-K. Atractylenolide-I Protects Human SH-SY5Y Cells from 1-Methyl-4-Phenylpyridinium-Induced Apoptotic Cell Death. Int. J. Mol. Sci. 2017, 18, 1012. [Google Scholar] [CrossRef]
- Gao, H.; Zhu, X.; Xi, Y.; Li, Q.; Shen, Z.; Yang, Y. Anti-depressant-like effect of atractylenolide I in a mouse model of depression induced by chronic unpredictable mild stress. Exp. Ther. Med. 2018, 15, 1574–1579. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, H.; Ji, Z.-H.; Yu, X.-Y. Neuroprotection of Atractylenolide III from Atractylodis macrocephalae Against Glutamate-Induced Neuronal Apoptosis Via Inhibiting Caspase Signaling Pathway. Neurochem. Res. 2014, 39, 1753–1758. [Google Scholar] [CrossRef]
- Zhao, H.; Ji, Z.-H.; Liu, C.; Yu, X.-Y. Neuroprotection and mechanisms of atractylenolide III in preventing learning and memory impairment induced by chronic high-dose homocysteine administration in rats. Neuroscience 2015, 290, 485–491. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Z.; Yu, J.; Yin, L.; Zhu, A. Atractylenolide III alleviates isoflurane-induced injury in rat hippocampal neurons by activating the PI3K/Akt/mTOR pathway. J. Food Biochem. 2021, 45, e13892. [Google Scholar] [CrossRef]
- Gong, W.-X.; Zhou, Y.-Z.; Qin, X.-M.; DU, G.-H. Involvement of mitochondrial apoptotic pathway and MAPKs/NF-κ B inflammatory pathway in the neuroprotective effect of atractylenolide III in corticosterone-induced PC12 cells. Chin. J. Nat. Med. 2019, 17, 264–274. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, S.; Wu, F.; Zheng, Q.; Zhang, F.; Luo, Y.; Jian, X. Atractylenolide III reduces depressive- and anxiogenic-like behaviors in rat depression models. Neurosci. Lett. 2021, 759, 136050. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-L.; Huang, H.-C.; Lin, H.-C.; Chang, T.-C.; Chang, W.-L. Sesquiterpenes from Baizhu Stimulate Glucose Uptake by Activating AMPK and PI3K. Am. J. Chin. Med. 2016, 44, 963–979. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Jung, H.W.; Kang, S.Y.; Park, Y.-K. Atractylenolide III Enhances Energy Metabolism by Increasing the SIRT-1 and PGC1α Expression with AMPK Phosphorylation in C2C12 Mouse Skeletal Muscle Cells. Biol. Pharm. Bull. 2017, 40, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-J.; Liang, J.-W.; Lin, H.-R. Sesquiterpenoids from Atractylodes macrocephala act as farnesoid X receptor and progesterone receptor modulators. Bioorg. Med. Chem. Lett. 2012, 22, 2326–2329. [Google Scholar] [CrossRef] [PubMed]
- Nur, E.A.A.; Ohshiro, T.; Kobayashi, K.; Wu, J.; Wahyudin, E.; Zhang, H.; Hayashi, F.; Kawagishi, H.; Tomoda, H. Inhibition of cholesteryl ester synthesis by polyacetylenes from Atractylodes rhizome. Bioorg. Med. Chem. Lett. 2020, 30, 126997. [Google Scholar] [CrossRef]
- Kannan, M.; Ahmad, F.; Saxena, R. Platelet activation markers in evaluation of thrombotic risk factors in various clinical settings. Blood Rev. 2019, 37, 100583. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.; Guo, L.; Wu, X.; Zhang, T.; Liu, J.; Zhang, J. Atractylodes lactone compounds inhibit platelet activation. Platelets 2017, 28, 194–202. [Google Scholar] [CrossRef]
- Tang, X.-M.; Liao, Z.-K.; Huang, Y.-W.; Lin, X.; Wu, L.-C. Atractylenolide Ⅰ protects against lipopolysaccharide-induced disseminated intravascular coagulation by anti-inflammatory and anticoagulation effect. Asian Pac. J. Trop. Med. 2017, 10, 582–587. [Google Scholar] [CrossRef]
- Liu, W.; Li, Z.; Chu, S.; Ma, X.; Wang, X.; Jiang, M.; Bai, G. Atractylenolide-I covalently binds to CYP11B2, selectively inhibits aldosterone synthesis, and improves hyperaldosteronism. Acta Pharm. Sin. B 2022, 12, 135–148. [Google Scholar] [CrossRef]
- Chappell, L.C.; Cluver, C.A.; Kingdom, J.; Tong, S. Pre-eclampsia. Lancet 2021, 398, 341–354. [Google Scholar] [CrossRef]
- Liu, M.; Wang, R.-B.; Xing, J.-H.; Tang, Y.-X. Atractylenolide inhibits apoptosis and oxidative stress of HTR-8/SVneo cells by activating MAPK/ERK signalling in preeclampsia. Phytomedicine 2021, 93, 153773. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.X.; Song, L.J.; Shi, X.Y.; Wang, X.T.; Tan, W.P.; Lu, H.Q.; Zhao, W.C. Study on the anti-rotavirus effect of Atractylodes I, II and III in vivo and in vitro. Chin. Tradit. Herb. Drugs 2019, 50, 104–110. [Google Scholar]
- Jeong, S.-I.; Kim, S.-Y.; Kim, S.-J.; Hwang, B.-S.; Kwon, T.-H.; Yu, K.-Y.; Hang, S.-H.; Suzuki, K.; Kim, K.-J. Antibacterial Activity of Phytochemicals Isolated from Atractylodes japonica against Methicillin-Resistant Staphylococcus aureus. Molecules 2010, 15, 7395–7402. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, D.; Liu, W.; Xie, J.; Lu, Z.; Yang, H.; Yan, H.; Wang, L.; Che, C. Therapeutic effect of Atractylenolide I on Aspergillus fumigatus keratitis by affecting MyD88/ NF-κB pathway and IL-1β, IL-10 expression. Cytokine 2023, 162, 156112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-L.; Li, N.; Shen, Q.; Fan, M.; Guo, X.-D.; Zhang, X.-W.; Zhang, Z.; Liu, X. Establishment of a mouse model of cancer cachexia with spleen deficiency syndrome and the effects of atractylenolide I. Acta Pharmacol. Sin. 2020, 41, 237–248. [Google Scholar] [CrossRef]
- Fan, M.; Gu, X.; Zhang, W.; Shen, Q.; Zhang, R.; Fang, Q.; Wang, Y.; Guo, X.; Zhang, X.; Liu, X. Atractylenolide I ameliorates cancer cachexia through inhibiting biogenesis of IL-6 and tumour-derived extracellular vesicles. J. Cachex-Sarcopenia Muscle 2022, 13, 2724–2739. [Google Scholar] [CrossRef]
- Xiao, C.; Xu, C.; He, N.; Liu, Y.; Wang, Y.; Zhang, M.; Ji, K.; Du, L.; Wang, J.; Wang, Q.; et al. Atractylenolide II prevents radiation damage via MAPKp38/Nrf2 signaling pathway. Biochem. Pharmacol. 2020, 177, 114007. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Y.; Liang, X.; Wu, Q.; Wang, N.; Zhou, L.-J.; Liu, W.-W.; Ma, Q.; Hu, B.; Gao, H.; et al. Atractylenolide III from Atractylodes macrocephala Koidz promotes the activation of brown and white adipose tissue through SIRT1/PGC-1α signaling pathway. Phytomedicine 2022, 104, 154289. [Google Scholar] [CrossRef]
- Li, X.; Wei, G.; Wang, X.; Liu, D.-H.; Deng, R.-D.; Li, H.; Zhou, J.-H.; Li, Y.-W.; Zeng, H.-P.; Chen, D.-F. Targeting of the Sonic Hedgehog Pathway by Atractylenolides Promotes Chondrogenic Differentiation of Mesenchymal Stem Cells. Biol. Pharm. Bull. 2012, 35, 1328–1335. [Google Scholar] [CrossRef]
- Ha, H.; An, H.; Shim, K.-S.; Kim, T.; Lee, K.J.; Hwang, Y.-H.; Ma, J.Y. Ethanol Extract of Atractylodes macrocephala Protects Bone Loss by Inhibiting Osteoclast Differentiation. Molecules 2013, 18, 7376–7388. [Google Scholar] [CrossRef]
- Britza, S.M.; Musgrave, I.F.; Byard, R.W. Implications for herbal polypharmacy: Coumarin-induced hepatotoxicity increased through common herbal phytochemicals astragaloside IV and atractylenolide I. Toxicol. Mech. Methods 2022, 32, 606–615. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, Y.-F.; Ran, R.-X.; Li, R.-S.; Wu, X.; Dong, P.-P.; Zhang, Y.-Y.; Hu, C.-M.; Wang, W.-M. Strong Specific Inhibition of UDP-glucuronosyltransferase 2B7 by Atractylenolide I and III. Phytother. Res. 2016, 30, 25–30. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, L.; Yang, K.; Lei, N.; Geng, Z.; Ma, P.; Cai, Q.; Du, S.; Deng, Z. Insecticidal and Repellant Activities of Polyacetylenes and Lactones Derived from Atractylodes lancea Rhizomes. Chem. Biodivers. 2015, 12, 593–598. [Google Scholar] [CrossRef]
- Kim, H.-K.; Yun, Y.-K.; Ahn, Y.-J. Toxicity of Atractylon and Atractylenolide III Identified in Atractylodes ovata Rhizome to Dermatophagoides farinae and Dermatophagoides pteronyssinus. J. Agric. Food Chem. 2007, 55, 6027–6031. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, Z.; Dong, L.; Wang, R.; Qiu, G. A Randomized Pilot Study of Atractylenolide I on Gastric Cancer Cachexia Patients. Evid. Based Complement. Altern. Med. 2008, 5, 337–344. [Google Scholar] [CrossRef]
- Adamcakova, J.; Mokra, D. New Insights into Pathomechanisms and Treatment Possibilities for Lung Silicosis. Int. J. Mol. Sci. 2021, 22, 4162. [Google Scholar] [CrossRef]
- Chen, S.; Tang, K.; Hu, P.; Tan, S.; Yang, S.; Yang, C.; Chen, G.; Luo, Y.; Zou, H. Atractylenolide III alleviates the apoptosis through inhibition of autophagy by the mTOR-dependent pathway in alveolar macrophages of human silicosis. Mol. Cell Biochem. 2021, 476, 809–818. [Google Scholar] [CrossRef]
| Inflammatory Disorders | Active Compound | Dosage | Models | Mechanism of Action | Ref. |
|---|---|---|---|---|---|
| Inflammation | I | 1–100 μM | LPS-induced RAW264.7 cells | Suppresses MD-2, CD14, SR-A, TLR4 and MyD88 expression, reduces IL-6 and TNF-α production by inhibiting NF-κB, ERK1/2 and p38 signaling pathways | [21] |
| II | 3–100 μM | LPS-induced macrophages RAW264.7 | Reduces NO secretion | [28] | |
| III | 50–100 μM | LPS-induced macrophages RAW264.7 | Inhibits ERK1/2, p38, and JNK1/2 expression, reduces NO, PGE2, TNF-α, and IL-6 production and through inhibition of NF-κB and MAPK signaling pathways | [29] | |
| I, III | 1–100 μM | LPS-induced macrophages | Inhibits TNF-α expression and reduces NO production and iNOS activity | [22] | |
| Microglial cell inflammation | I | 25–100 μM; 3 mg/kg | LPS-induced inflammation in BV2 microglia; tetrahydropyridine poisoned mice | Reduces nuclear translocation of NF-κB and induction of HO-1 | [23] |
| III | 100 μM | Immortalized mice microglia MG6 | Inhibits p38 MAPK and JNK signaling pathways by down-regulation of TLR4 reduces TNF-α, IL-1β, IL-6, iNOS, and COX-2 production | [37] | |
| III | 10 mg/kg | Mice model of transient middle cerebral artery occlusion | Reduces expression of pro-inflammatory factors IL-1β, TNF-α, IL-6, and anti-inflammatory factors by inhibiting JAK2/STAT3/Drp1 signaling pathway | [38] | |
| Colitis | I | 50 mg/kg | DSS-induced colitis in mice | Inhibits PI3K-Akt signaling pathway through suppression of SPHK1 and B4GALT2 gene variants in colitis colon, reduction in TNF-α, IL-6, IL-1β secretion, down-regulation of MUC2 and ZO-1, ocludin expression, and regulation of intestinal flora | [27] |
| III | 10–50 mg/kg | TNBS-induced colitis in mice | Reduces inflammatory factors IL-1β and TNF-α expression, decreases MPO content, increases CAT, SOD, and GSH-Px, decreases MDA and ROS levels, and regulates intestinal flora by inhibiting FPR1 and Nrf2 signaling pathways | [31] | |
| III | 40–80 μM; 5–10 mg/kg | Lipopolysaccharide-induced IEC-6 cells; DSS-induced ulcerative colitis in mice | Alleviates mitochondrial dysfunction by activating AMPK/SIRT1/PGC-1α signaling pathway | [32] | |
| Asthma | III | 100 μg/mL; 100 mg/mL | IL-4-induced human bronchial epithelial-like cells 16HBE; ovalbumin-induced asthma in mice | Inhibits Cleaved Caspase-1, ASC, and NLRP3 expression, reduces NLRP3 inflammatory vesicle activation, and regulates Th1/Th2 balance | [34] |
| III | 0.1–10 mg/kg | Ovalbumin-induced asthma in mice | Reduces inflammation and oxidative stress by inhibiting STAT3 expression in lung tissue | [33] | |
| Sepsis | I | 10–40 mg/kg | Cecum ligation puncture in septic mice | Reduces WBC, LPS, TNF-α, IL-1β, IL-6, ALT, AST, Cre, and BUN levels | [24] |
| Inhibition of inflammatory angiogenesis | I | 5–20 mg/kg | Freund’s complete adjuvant-induced air sac mice model, mice aortic ring model co-cultured with abdominal macrophages | Reduces NO, TNF-α, IL-1β, IL-6, VEGF, and PlGF production | [25] |
| Atherosclerosis | I | 12.5–100 μg/mL | Ox-LDL-induced vascular smooth muscle cells | Reduces inflammatory cytokine production and MCP-1 expression, and inhibits p38-MAPK and NF-κB activation | [26] |
| Atopic dermatitis | III | 1–100 μM | TSLP-induced HMC-1 human mast cells | Decreases production of pro-inflammatory cytokines, decreased Bcl2 and proCaspase-3 levels and increased p53, Caspase-3, and cleaved PARP levels by downregulating pSTAT6 and MDM2 levels | [30] |
| Spinal cord injury inflammation | III | 1–100 μM; 5 mg/kg | Lipopolysaccharide-induced inflammation in BV2 microglia; rats with spinal cord injury | Promotes cellular M1 to M2 conversion by inhibiting NF-κB, MAPK/JNK, p38 MAPK, and Akt signaling pathways, further inhibiting the expression of corresponding inflammatory factors | [36] |
| Allergic inflammatory diseases | III | 4–200 μM | IgE/Ag-induced basophilic leukemia cells in rats | Reduces IL-4 and TNF-α levels, inhibits phosphorylation of Lyn, Fyn, Syk, LAT, PLCγ, Gab2, Akt, p38, and JNK kinases, and increases Ca2+ levels | [35] |
| Types of Cancers | Active Compound | Dosage | Models | Mechanism of Action | Ref |
|---|---|---|---|---|---|
| Colon cancer | I | 100–200 μM | HT-29 | Induces DNA fragmentation in cells, decreases Caspase 9, Caspase 3, Caspase 7, Caspase 8, and PRAP expression, down-regulates Bcl-2 expression, and up-regulates Bax, Bak, Bad, Bim, Bid, and Puma expression | [39] |
| I | 30–50 μM | HCT116 and MC38 | Enhances MHC-I-mediated antigen presentation by binding to the target protein PSMD4 and increases cytotoxic responses of CD8+ T cells | [45] | |
| I | 25–100 μg/mL; 25–50 mg/kg | HCT116 and SW480; azomethane- and DSS-induced colon cancer in mice | Inhibits Drp1-mediated mitochondrial division; inhibits NLRP3 inflammatory vesicle activation in colitis-associated colorectal cancer | [41] | |
| I | 80–200 μM; 25–75 mg/kg | HCT116 and COLO205; xenograft colorectal cancer nude mice | Inhibits glucose metabolism and disrupts dry maintenance through inhibition of the Akt/mTOR signaling pathway | [42] | |
| I | 100–200 μM; 50 mg/kg | HCT116 and SW480; xenograft colorectal cancer mice | Inhibits glycolysis in CRC cells through inhibition of the JAK2/STAT3 signaling pathway and reduction in HK2 expression | [44] | |
| I | 200 μM | LoVo and HT29 colorectal cancer stem cells | Inhibits the function of colorectal cancer stem cells and blocks the transfer of oncogenic miR-200c by impeding the delivery of extracellular vesicle uptake, thereby inhibiting the PI3K/Akt/mTOR signaling pathway | [43] | |
| I | 25–100 μM; 25–50 mg/kg | HCT116; APCMin/+ mice | Reduces intestinal adenoma formation by down-regulation of D-dopachrome isomerase through activation of autophagy | [46] | |
| I | 100 μM | HCT116 | Decreases PDK1 and inhibited FoxO1 phosphorylation | [40] | |
| II | 12.5–200 μg/mL | SW480, HCT116, Lovo and SW620 | Inhibits the LncRNA XIST/miR-30a-5p/ROR1 signaling pathway | [61] | |
| III | 100–200 μM | HCT-116 | Promotes Bax, Caspase-9, and Caspase-3 expression; inhibits Bcl-2 expression and regulates the Bax/Bcl-2 apoptotic signaling pathway | [69] | |
| Gastric cancer | I | 25–100 μM | MGC-803 | Inhibits proliferation through inhibition of the Notch signaling pathway | [56] |
| II | 200–400 μM | HGC-27 and AGS | Up-regulates Bax expression and down-regulates Bcl-2 expression through inhibition of Ras/ERK and PI3K/Akt signaling pathways | [62] | |
| III | 50 μM | AGS and SGC-7901 | Inhibits FGFR1, FGFR2, and FGFR4 expression | [73] | |
| III | 80–120 μM; 1.2–2.4 mg/kg | AGS and HGC-27;MNNG-induced gastric precancerous lesions in rats | Inhibits HIF-1α and VEGF-A related to angiogenesis and down-regulation of DLL4 | [74] | |
| Lung cancer | I | 50–150 μM; 25–75 mg/kg | A549 and H1299; A549 xenograft lung cancer nude mice | Inhibits PDK1 expression by activating ERK1/2 to suppress SP1 levels and reducing Stat3 levels | [49] |
| I | 10–40 μM | A549 and HCC827; A549 xenograft lung cancer nude mice | Up-regulates Caspase-3, Caspase-9, and Bax, down-regulates Bcl-2 and Bcl-xL | [50] | |
| II | 2.5–5 μM; 50 mg/kg | A549; xenograft lung cancer nude mice | Inhibits M2-like polarization | [66] | |
| III | 10–100 μM | A549 | Causes cleavage of PAPR by enhancing Caspase-3 and Caspase-9 protein expression, and activating the mitochondrial pathway | [70] | |
| III | 8–32 μM | LLC, H1703, H520, PC-9, A549 and H1299; LCC xenograft lung cancer nude mice | Down-regulates interferon-γ-induced IDO expression in lung cancer cells and activates anti-tumor immunity through inhibition of Jak3 and Stat3 phosphorylation and nuclear translocation of Stat3 | [72] | |
| Melanoma | I | 100 μM | B16 | Increases p21, Caspase 3, Caspase 8, and p53 expression and decreases CDK2, p-ERK, p-GSK3β, and c-Jun expression through inhibition of ERK/GSK3β signaling pathway | [51] |
| I | 40–150 μM | A375 | Down-regulates STAT3 target genes Bcl-xL, MMP-2, and MMP-9 levels through inhibition of the JAK2/STAT3 signaling pathway | [52] | |
| II | 100 mM | B16 | Up-regulates p38, p53, p21, p27, Caspase-8, Caspase-9, and Caspase-3, down-regulates CDK2, p-Akt, p-ERK and Bcl-2 | [63] | |
| II | 20–40 μM; 12.5–25 mg/kg | B16 and A375; Melanoma cell B16 xenografts in mice | Reduces p-STAT3, p-Src, Mcl-1, and Bcl-xL through inhibition of STAT3 signaling pathway | [64] | |
| I, II | 25–100 μM | B16 | Inhibits Ras/ERK and PI3K/Akt signaling pathways | [65] | |
| Ovarian cancer | I | 50–100 μM | A2780 | Up-regulates Bax, cleaved Caspase-9, cleaved Caspase-3, cytochrome c, and AIF expression, down-regulates B1, CDK1, and Bcl-2 expression, and inhibits PI3K/Akt/mTOR signaling pathway | [47] |
| I | 100 μM | SKOV3 EOC | Induces immunosuppressive molecules and products immunosuppressive T cells through activation of the MyD88/NF-κB signaling pathway | [48] | |
| Breast cancer | I | 50–100 μM; 50 mg/kg | MDA-MB-231 and HS578T; TNBC, breast cancer cells MDA-MB-231 xenografts in mice | Sensitizes cells to paclitaxel by blocking CTGF expression and fibroblast activation | [53] |
| I | 20–50 μM | MDA-MB-231 | Glycolysis/gluconeogenesis is affected by down-regulating the expression of TPI 1 and GPI | [54] | |
| I | 50–100 μM; 100–200 mg/kg | MCF 10A, MCF-7 and MDA-MB-231; N-Nitroso-N-methylurea-induced breast cancer in rats | Reduces NF-κB-regulated cytokines in breast cancer cells by inhibiting the TLR4/NF-κB signaling pathway | [55] | |
| II | 50 μM; 100–200 mg/kg | MCF 10A; N-Nitroso-N-methylurea-induced breast cancer in rats | Elevates Nrf2 expression, nuclear translocation and expression of its downstream detoxification enzymes through the JNK/ERK-Nrf2-ARE signaling pathway; reduces inflammation and oxidative stress | [67] | |
| Bladder Cancer | I | 10–30 μM; 25–75 mg/kg | RT4, 5637, 253J and T-24; Xenograft bladder cancer nude mice | Inhibits PI3K/Akt/mTOR signaling pathway, up-regulates p21 expression and down-regulation of cyclin B1, CDK1, and Cdc25c expression | [58] |
| Leukemia | I | 50–100 μg/mL | K562 (CML), U937 (AML) and Jurkat T | Up-regulates Caspase-3 and Caspase-9 expression, down-regulates proCaspase-3 and proCaspase-9 expression, and elevates CD14 and CD14/CD68 | [57] |
| Cervical cancer | I | 20–80 μM | Hela and SiHa | Combinates therapy with the P2X7R receptor antagonist JNJ; enhances cell growth inhibition | [60] |
| Prostate cancer | I | 10 μM | DU 145 and PC-3 | Inhibits Hsp27 expression | [59] |
| II | 50–100 μM | DU145 and LNCaP | Inhibits AR expression and activation of PIAS1 and JAK2/STAT3 signaling pathway | [68] | |
| Liver cancer | III | 10–500 μM | HepG2 and SMMC7721 | Up-regulates miR-195-5p expression and down-regulates FGFR1 expression | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Lin, M.; He, X.; Dong, Y.; Chen, Y.; Li, B.; Chen, S.; Lv, G. Chemical Constitution, Pharmacological Effects and the Underlying Mechanism of Atractylenolides: A Review. Molecules 2023, 28, 3987. https://doi.org/10.3390/molecules28103987
Xie Z, Lin M, He X, Dong Y, Chen Y, Li B, Chen S, Lv G. Chemical Constitution, Pharmacological Effects and the Underlying Mechanism of Atractylenolides: A Review. Molecules. 2023; 28(10):3987. https://doi.org/10.3390/molecules28103987
Chicago/Turabian StyleXie, Zhiyi, Minqiu Lin, Xinglishang He, Yingjie Dong, Yigong Chen, Bo Li, Suhong Chen, and Guiyuan Lv. 2023. "Chemical Constitution, Pharmacological Effects and the Underlying Mechanism of Atractylenolides: A Review" Molecules 28, no. 10: 3987. https://doi.org/10.3390/molecules28103987
APA StyleXie, Z., Lin, M., He, X., Dong, Y., Chen, Y., Li, B., Chen, S., & Lv, G. (2023). Chemical Constitution, Pharmacological Effects and the Underlying Mechanism of Atractylenolides: A Review. Molecules, 28(10), 3987. https://doi.org/10.3390/molecules28103987





