Fatty Acid-Rich Extract from Holothuria atra for Hyperuricemia via Expressions Modulation of GLUT9a and GLUT9b in Rat Model
Abstract
1. Introduction
2. Results
2.1. GC–MS Results
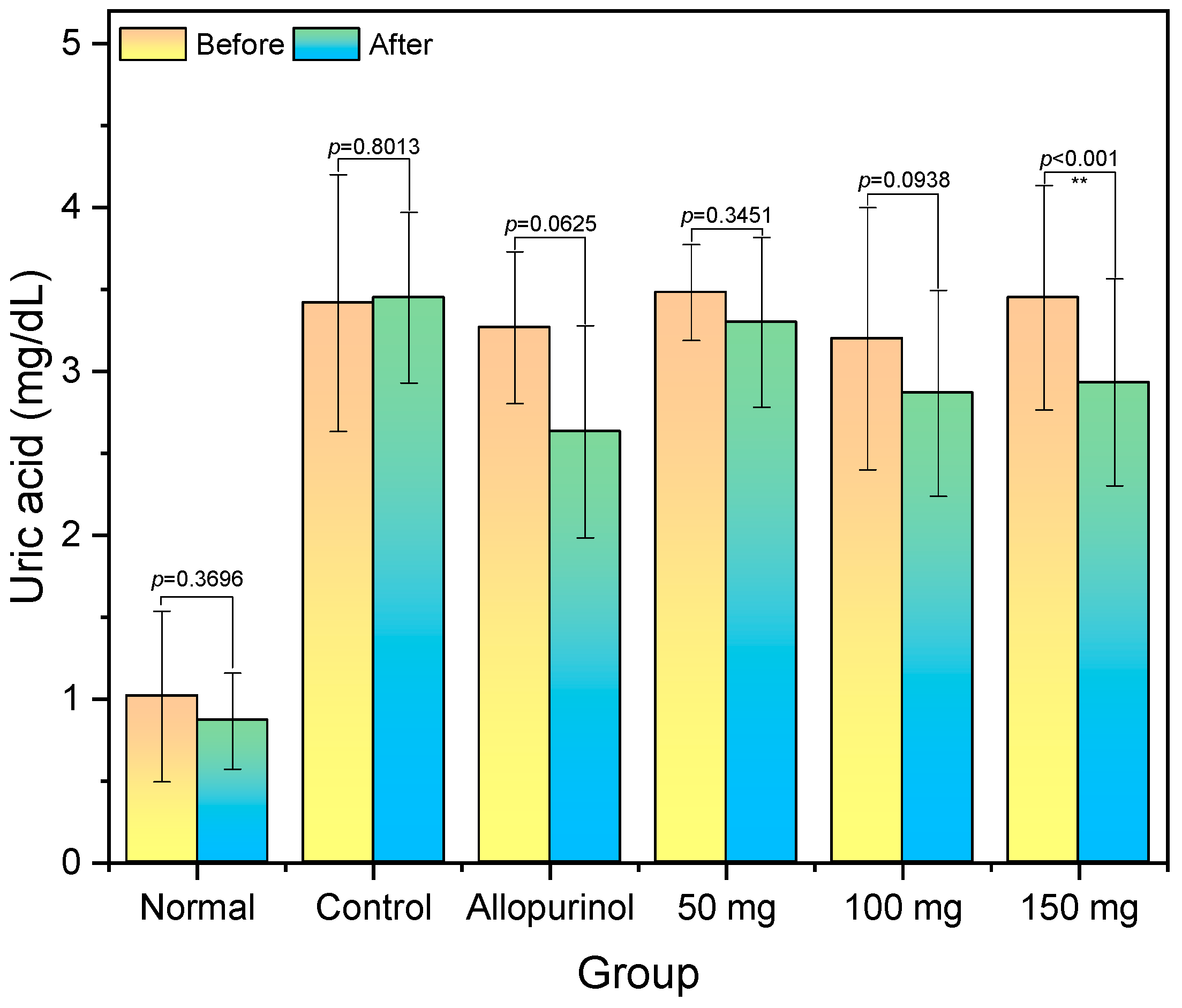
2.2. Serum Uric Acid
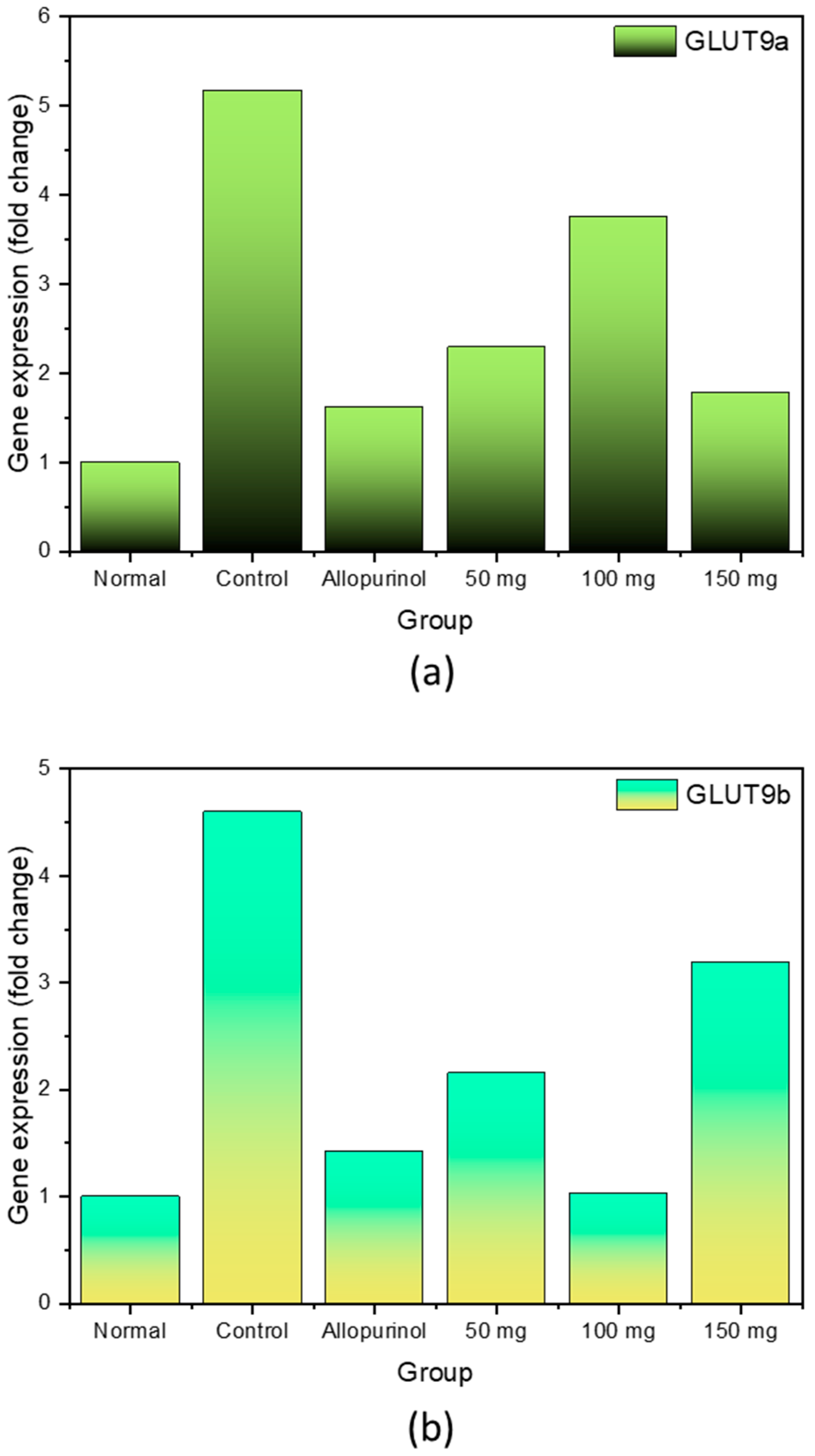
2.3. Expression of GLUT9a and GLUT9b
2.4. Liver Parameters
2.5. Kidney Parameters
2.6. BSLT Cytotoxicity
2.7. Molecular Docking Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction of H. atra
4.3. Hyperuricemic Animal Model and Treatment
4.4. Determination of Serum Parameters
4.5. Determination of Gene Expressions of GLUT9a and GLUT9b
4.6. BSLT Assay
4.7. Docking Simulations
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Syarfaini, S.; Nildawati, N.; Aeni, S.; Surahmawati, S.; Adha, A.S.; Amansyah, M. Risk factors preparation of stroke incidence in health institution employees who check up at the Health Service EXPO Event Indonesia. Gac. Sanit. 2021, 35, S49–S52. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lü, J.-M.; Yao, Q. Hyperuricemia-related diseases and xanthine oxidoreductase (XOR) inhibitors: An overview. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 2501. [Google Scholar] [CrossRef]
- Butler, F.; Alghubayshi, A.; Roman, Y. The Epidemiology and Genetics of Hyperuricemia and Gout across Major Racial Groups: A Literature Review and Population Genetics Secondary Database Analysis. J. Pers. Med. 2021, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, D. Factors Influencing Hyperuricemia: Evidence from Sukoharjo, Central Java, Indonesia. Indones. J. Med. 2019, 4, 321–328. [Google Scholar] [CrossRef]
- Jumiyati, J.; Witradharma, W.T. Factors Affecting the Incidence of Hyperuricemia on the Rejang Tribe in Bengkulu. J. Teknol. Dan Seni Kesehat. 2020, 11, 53–64. [Google Scholar] [CrossRef]
- Chen, L.; Han, S.; Liu, F.; Chen, S.; Chen, X.; Chen, H. Global prevalence of hyperuricemia in adolescents from 2000 to 2019: A meta-analysis. Res. Sq. 2020; preprint. [Google Scholar] [CrossRef]
- Lin, X.; Wang, X.; Li, X.; Song, L.; Meng, Z.; Yang, Q.; Zhang, W.; Gao, Y.; Yang, Z.; Cai, H.; et al. Gender- and Age-Specific Differences in the Association of Hyperuricemia and Hypertension: A Cross-Sectional Study. Int. J. Endocrinol. 2019, 2019, 7545137. [Google Scholar] [CrossRef]
- Song, P.; Wang, H.; Xia, W.; Chang, X.; Wang, M.; An, L. Prevalence and correlates of hyperuricemia in the middle-aged and older adults in China. Sci. Rep. 2018, 8, 4314. [Google Scholar] [CrossRef]
- Kim, A.; Kim, Y.; Kim, G.-T.; Ahn, E.; So, M.W.; Lee, S.-G. Comparison of persistence rates between allopurinol and febuxostat as first-line urate-lowering therapy in patients with gout: An 8-year retrospective cohort study. Clin. Rheumatol. 2020, 39, 3769–3776. [Google Scholar] [CrossRef]
- Stamp, L.K.; Chapman, P.T. Allopurinol hypersensitivity: Pathogenesis and prevention. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101501. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Ren, L.; Xie, Y. Advances in structures required of polyphenols for xanthine oxidase inhibition. Food Front. 2020, 1, 152–167. [Google Scholar] [CrossRef]
- Doege, H.; Bocianski, A.; Joost, H.-G.; Schürmann, A. Activity and genomic organization of human glucose transporter 9 (GLUT9), a novel member of the family of sugar-transport facilitators predominantly expressed in brain and leucocytes. Biochem. J. 2000, 350, 771–776. [Google Scholar] [CrossRef]
- Matsuo, H.; Chiba, T.; Nagamori, S.; Nakayama, A.; Domoto, H.; Phetdee, K.; Wiriyasermkul, P.; Kikuchi, Y.; Oda, T.; Nishiyama, J. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am. J. Hum. Genet. 2008, 83, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Dinour, D.; Gray, N.K.; Ganon, L.; Knox, A.J.; Shalev, H.; Sela, B.-A.; Campbell, S.; Sawyer, L.; Shu, X.; Valsamidou, E. Two novel homozygous SLC2A9 mutations cause renal hypouricemia type 2. Nephrol. Dial. Transplant. 2012, 27, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Auberson, M.; Stadelmann, S.; Stoudmann, C.; Seuwen, K.; Koesters, R.; Thorens, B.; Bonny, O. SLC2A9 (GLUT9) mediates urate reabsorption in the mouse kidney. Pflügers Arch.-Eur. J. Physiol. 2018, 470, 1739–1751. [Google Scholar] [CrossRef]
- Sun, L.; Ni, C.; Zhao, J.; Wang, G.; Chen, W. Probiotics, bioactive compounds and dietary patterns for the effective management of hyperuricemia: A review. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef]
- Oku, F.; Hara, A.; Tsujiguchi, H.; Suzuki, K.; Pham, K.-O.; Suzuki, F.; Miyagi, S.; Nakamura, M.; Takazawa, C.; Sato, K.; et al. Association between Dietary Fat Intake and Hyperuricemia in Men with Chronic Kidney Disease. Nutrients 2022, 14, 2637. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Delgado-Lista, J.; Tierney, A.C.; Perez-Martinez, P.; Garcia-Rios, A.; Alcala-Diaz, J.F.; Castaño, J.P.; Tinahones, F.J.; Drevon, C.A.; Defoort, C. Insulin resistance determines a differential response to changes in dietary fat modification on metabolic syndrome risk factors: The LIPGENE study. Am. J. Clin. Nutr. 2015, 102, 1509–1517. [Google Scholar] [CrossRef]
- Kalafati, I.-P.; Borsa, D.; Dimitriou, M.; Revenas, K.; Kokkinos, A.; Dedoussis, G.V. Dietary patterns and non-alcoholic fatty liver disease in a Greek case–control study. Nutrition 2019, 61, 105–110. [Google Scholar] [CrossRef]
- Hawas, U.W.; El-Kassem, A.; Lamia, T.; Shaher, F.M.; Ghandourah, M.; Al-Farawati, R. Sulfated triterpene glycosides from the Saudi Red Sea cucumber Holothuria atra with antioxidant and cytotoxic activities. Thalass. Int. J. Mar. Sci. 2021, 37, 817–824. [Google Scholar] [CrossRef]
- Nursid, M.; Patantis, G.; Dewi, A.S.; Achmad, M.J.; Sembodo, P.M.; Estuningsih, S. Immunnostimulatory activity of Holothuria atra sea cucumber. Pharmacia 2021, 68, 121. [Google Scholar] [CrossRef]
- Grauso, L.; Yegdaneh, A.; Sharifi, M.; Mangoni, A.; Zolfaghari, B.; Lanzotti, V. Molecular networking-based analysis of cytotoxic saponins from sea cucumber Holothuria atra. Mar. Drugs 2019, 17, 86. [Google Scholar] [CrossRef]
- Ahmed, H.; Mahdy, A.; Nasser, S.; Abd El-Wakeil, K.; Obuid-Allah, A.; Hassan, M. Biochemical composition of some Echinodermata (Holothuroidea, Echinoidea) from the Red Sea, Egypt. Braz. J. Biol. 2021, 82, e246309. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Um, B.H.; Kim, S.M. Two unsaturated fatty acids with potent α-glucosidase inhibitory activity purified from the body wall of sea cucumber (Stichopus japonicus). J. Food Sci. 2011, 76, H208–H214. [Google Scholar] [CrossRef] [PubMed]
- Hawas, U.W.; Shaher, F.; Ghandourah, M.; Abou El-Kassem, L.T.; Satheesh, S.; Al-Sofyani, A.M.A. Lipids and free fatty acids of Red Sea Avrainvillea amadelpha, Holothuria atra, and Sarcocornia fruticosa inhibit marine bacterial biofilms. Lett. Org. Chem. 2020, 17, 466–471. [Google Scholar] [CrossRef]
- Sukmiwati, M.; Ilza, M.; Putri, A.E.; Sidauruk, S.W. Antibacterial activity of sea cucumber (Holothuria atra) against Pseudomonas aeruginosa. IOP Conf. Ser. Earth Environ. Sci. 2020, 404, 012047. [Google Scholar] [CrossRef]
- Hasan, K.M.M.; Tamanna, N.; Haque, M.A. Biochemical and histopathological profiling of Wistar rat treated with Brassica napus as a supplementary feed. Food Sci. Hum. Wellness 2018, 7, 77–82. [Google Scholar] [CrossRef]
- Thammitiyagodage, M.G.; de Silva, N.R.; Rathnayake, C.; Karunakaran, R.; Wgss, K.; Gunatillka, M.M.; Ekanayaka, N.; Galhena, B.P.; Thabrew, M.I. Biochemical and histopathological changes in Wistar rats after consumption of boiled and un-boiled water from high and low disease prevalent areas for chronic kidney disease of unknown etiology (CKDu) in north Central Province (NCP) and its comparison with low disease prevalent Colombo, Sri Lanka. BMC Nephrol. 2020, 21, 38. [Google Scholar] [CrossRef]
- Ridzwan, B.; Hanita, M.; Nurzafirah, M.; Norshuhadaa, M.S.; Hanis, Z.F. Free fatty acids composition in lipid extracts of several sea cucumbers species from Malaysia. Int. J. Biosci. Biochem. Bioinform. 2014, 4, 204. [Google Scholar] [CrossRef]
- Rasyid, A.; Murniasih, T.; Putra, M.Y.; Pangestuti, R.; Harahap, I.A.; Untari, F.; Sembiring, S.B. Evaluation of nutritional value of sea cucumber Holothuria scabra cultured in Bali, Indonesia. Aquac. Aquar. Conserv. Legis. 2020, 13, 2083–2093. [Google Scholar]
- Fredalina, B.D.; Ridzwan, B.H.; Abidin, A.A.Z.; Kaswandi, M.A.; Zaiton, H.; Zali, I.; Kittakoop, P.; Jais, A.M.M. Fatty acid compositions in local sea cucumber. Gen. Pharmacol. Vasc. Syst. 1999, 33, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Xu, Q.; Zhang, X.; Peng, Q.; Yang, H. Fatty acid component in sea cucumber Apostichopus japonicus from different tissues and habitats. J. Mar. Biol. Assoc. UK 2016, 96, 197–204. [Google Scholar] [CrossRef]
- Yoon, I.S.; Park, D.H.; Kim, J.E.; Yoo, J.C.; Bae, M.S.; Oh, D.S.; Shim, J.H.; Choi, C.Y.; An, K.W.; Kim, E.I.; et al. Identification of the biologically active constituents of Camellia japonica leaf and anti-hyperuricemic effect in vitro and in vivo. Int. J. Mol. Med. 2017, 39, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, L.; Yan, H.; Jiang, X.; Hu, W.; Han, N.; Wang, D. Anti-gouty arthritis and anti-hyperuricemia properties of celery seed extracts in rodent models. Mol. Med. Rep. 2019, 20, 4623–4633. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, L.; Jiang, L.; Wu, Y.; Wei, L.; Wu, X.; Xiao, S.; Liu, Y.; Gao, C.; Cai, J.; et al. Sonneratia apetala seed oil attenuates potassium oxonate/hypoxanthine-induced hyperuricemia and renal injury in mice. Food Funct. 2021, 12, 9416–9431. [Google Scholar] [CrossRef]
- Wan, H.; Han, J.; Tang, S.; Bao, W.; Lu, C.; Zhou, J.; Ming, T.; Li, Y.; Su, X. Comparisons of protective effects between two sea cucumber hydrolysates against diet induced hyperuricemia and renal inflammation in mice. Food Funct. 2020, 11, 1074–1086. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, B.; Surbek, D.; Clemençon, B.; Huang, X.; Albrecht, C.; Marini, C.; Hediger, M.; Baumann, M. Different Pharmacological Properties of GLUT9a and GLUT9b: Potential Implications in Preeclampsia. Cell. Physiol. Biochem. 2019, 53, 508–517. [Google Scholar] [PubMed]
- Long, W.; Panigrahi, R.; Panwar, P.; Wong, K.; O′ Neill, D.; Chen, X.-Z.; Lemieux, M.J.; Cheeseman, C.I. Identification of key residues for urate specific transport in human glucose transporter 9 (hSLC2A9). Sci. Rep. 2017, 7, 41167. [Google Scholar] [CrossRef] [PubMed]
- Purnama, A.; Rizki, D.R.; Qanita, I.; Iqhrammullah, M.; Ahmad, K.; Mardina, V.; Puspita, K.; Hasballah, K. Molecular docking investigation of calotropone as a potential natural therapeutic agent against pancreatic cancer. J. Adv. Pharm. Technol. Res. 2022, 13, 44. [Google Scholar]
- Esmat, A.Y.; Said, M.M.; Soliman, A.A.; El-Masry, K.S.H.; Badiea, E.A. Bioactive compounds, antioxidant potential, and hepatoprotective activity of sea cucumber (Holothuria atra) against thioacetamide intoxication in rats. Nutrition 2013, 29, 258–267. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Antioxidant Potential of Sea Cucumbers and Their Beneficial Effects on Human Health. Mar. Drugs 2022, 20, 521. [Google Scholar] [CrossRef] [PubMed]
- Saad, D.; Soliman, M.; Mohamed, A.; Youssef, G. Protective effects of sea cucumber (Holothuria atra) extract on testicular dysfunction induced by immune suppressant drugs in Wistar rats. Andrologia 2018, 50, e13017. [Google Scholar] [CrossRef] [PubMed]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhou, W.; Chen, L.; Li, Y.; Luo, Y.; Wu, P. Phthalate esters contamination in vegetable–soil system of facility greenhouses in Jingmen, central China and the assessment of health risk. Environ. Geochem. Health 2020, 42, 2703–2721. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, X.; Vogt, R.D.; Zhou, J.; Zheng, B.; Zhang, Y.; Tu, J.; Song, Y.; Lu, X. Polyethylene terephthalate and di-(2-ethylhexyl) phthalate in surface and core sediments of Bohai Bay, China: Occurrence and ecological risk. Chemosphere 2022, 286, 131904. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhu, X.; Zhou, S.; Cheng, Z.; Shi, K.; Zhang, C.; Shao, H. Phthalic Acid Esters: Natural Sources and Biological Activities. Toxins 2021, 13, 495. [Google Scholar] [CrossRef]
- Hasballah, K.; Sarong, M.; Rusly, R.; Fitria, H.; Maida, D.R.; Iqhrammullah, M. Antiproliferative Activity of Triterpenoid and Steroid Compounds from Ethyl Acetate Extract of Calotropis gigantea Root Bark against P388 Murine Leukemia Cell Lines. Sci. Pharm. 2021, 89, 21. [Google Scholar] [CrossRef]
- Yusnaini, R.; Nasution, R.; Saidi, N.; Arabia, T.; Idroes, R.; Ikhsan, I.; Bahtiar, R.; Iqhrammullah, M. Ethanolic Extract from Limonia acidissima L. Fruit Attenuates Serum Uric Acid Level via URAT1 in Potassium Oxonate-Induced Hyperuricemic Rats. Pharmaceuticals 2023, 16, 419. [Google Scholar] [CrossRef]
- Kahn, A.M.; Weinman, E.J. Urate transport in the proximal tubule: In vivo and vesicle studies. Am. J. Physiol.-Ren. Physiol. 1985, 249, F789–F798. [Google Scholar] [CrossRef]
- McArdle, P.F.; Parsa, A.; Chang, Y.P.C.; Weir, M.R.; O’Connell, J.R.; Mitchell, B.D.; Shuldiner, A.R. Association of a common nonsynonymous variant in GLUT9 with serum uric acid levels in old order amish. Arthritis Rheum. 2008, 58, 2874–2881. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.; Xie, Y.; Liu, Z.; Zou, G. Epigallocatechin gallate reduces uric acid levels by regulating xanthine oxidase activity and uric acid excretion in vitro and in vivo. Ann. Palliat. Med. 2020, 9, 331–338. [Google Scholar] [CrossRef] [PubMed]

| No. | Compound | Retention Time (min) | Area (%) |
|---|---|---|---|
| 1 | 2-Pentadecyn-1-ol | 16.488 | 5.99 |
| 2 | 1-Octyn-3-ol, 4-ethyl- | 16.538 | 2.57 |
| 3 | 1-Dodecene | 17.571 | 1.49 |
| 4 | Cyclopropanepentanoic acid, 2-undecyl-, methyl ester, trans | 18.821 | 1.49 |
| 5 | Pentadecanoic acid, 14-methyl-, methyl ester | 19.031 | 6.62 |
| 6 | Oleic acid | 19.267 | 1.51 |
| 7 | Nonadecanoic acid | 19.452 | 2.40 |
| 8 | 1-Tetradecene | 19.754 | 1.47 |
| 9 | Heptacosanoic acid, methyl ester | 21.090 | 5.38 |
| 10 | 1-Tetradecene | 21.747 | 1.52 |
| 11 | Arachidonic acid | 22.394 | 44.61 |
| 12 | (6Z,9Z,12Z,15Z)-Methyl octadeca-6,9,12,15-tetraenoate | 22.455 | 6.35 |
| 13 | Cyclopropanepentanoic acid, 2-undecyl-, methyl ester, trans | 22.740 | 4.16 |
| 14 | 9,12,15-Octadecatrienal | 22.840 | 2.88 |
| 15 | Eicosanoic acid, methyl ester | 22.977 | 1.44 |
| 16 | Oleic acid | 23.153 | 4.37 |
| 17 | 1-Hexacosanol | 23.573 | 1.28 |
| 18 | Bis(2-ethylhexyl) phthalate | 24.801 | 2.20 |
| 19 | 11-Octadecenoic acid, methyl ester | 25.352 | 1.05 |
| 20 | 11-Octadecenoic acid, methyl ester | 26.188 | 1.21 |
| Parameters | Before | After | p-Value |
|---|---|---|---|
| AST, Mean ± SD (IU/L) | |||
| Normal | 139.2 ± 11.43 | 140.4 ± 2.074 | 0.8333 |
| Control | 208.4 ± 49.13 | 206.4 ± 55.13 | 0.7419 |
| Allopurinol | 224.4 ± 47.68 | 151.2 ± 22.07 | 0.0198 * |
| 50 mg | 214.8 ± 55.74 | 145.4 ± 15.87 | 0.0237 |
| 100 mg | 216.0 ± 44.96 | 148.8 ± 20.07 | 0.0070 ** |
| 150 mg | 224.4 ± 29.64 | 132.2 ± 32.88 | 0.0010 ** |
| ALT, Mean ± SD (IU/L) | |||
| Normal a | 80.00 ± 7.906 | 80.8 ± 5.848 | 0.7205 |
| Control a | 136.2 ± 41.57 | 133.8 ± 36.95 | 0.8750 |
| Allopurinol a | 138.6 ± 82.86 | 95.6 ± 11.48 | 0.1875 |
| 50 mg | 101.4 ± 12.76 | 94.8 ± 8.871 | 0.4353 |
| 100 mg | 124.4 ± 46.32 | 98.2 ± 21.81 | 0.0830 |
| 150 mg | 112.0 ± 14.49 | 78.2 ± 8.64 | 0.0302 * |
| Parameters a | Before | After | p-Value |
|---|---|---|---|
| BUN, Mean ± SD (mg/dL) | |||
| Normal | 23.00 ± 9.354 | 22.80 ± 8.468 | 0.7040 |
| Control | 19.80 ± 5.263 | 20.60 ± 7.162 | 0.7003 |
| Allopurinol | 22.80 ± 10.38 | 19.40 ± 4.037 | 0.4492 |
| 50 mg | 21.80 ± 13.22 | 17.40 ± 5.814 | 0.0222 * |
| 100 mg | 13.40 ± 5.60 | 18.40 ± 8.562 | 0.5392 |
| 150 mg | 14.80 ± 5.36 | 18.80 ± 8.167 | 0.0577 |
| Creatinine, Mean ± SD (mg/dL) | |||
| Normal b | 0.66 ± 0.134 | 0.68 ± 0.192 | >0.9999 |
| Control | 0.70 ± 0.235 | 0.62 ± 0.109 | 0.5122 |
| Allopurinol b | 0.68 ± 0.192 | 0.64 ± 0.089 | 0.7500 |
| 50 mg | 0.62 ± 0.217 | 0.54 ± 0.182 | 0.5769 |
| 100 mg b | 0.66 ± 0.114 | 0.62 ± 0.447 | 0.6250 |
| 150 mg | 0.60 ± 0.192 | 0.68 ± 0.0837 | 0.2420 |
| Concentration (mg/L) | Dead, Mean ± SD | Mortality (%) | Probit |
|---|---|---|---|
| 10 | 3.33 ± 2.08 | 33.33 | 4.56 |
| 25 | 4.67 ± 4.73 | 46.67 | 4.92 |
| 50 | 8.00 ± 2.00 | 80.00 | 5.84 |
| 75 | 5.33 ± 0.58 | 53.33 | 5.08 |
| 100 | 2.67 ± 2.89 | 26.67 | 4.39 |
| 250 | 7.00 ± 1.00 | 70.00 | 5.52 |
| 500 | 8.33 ± 2.08 | 83.33 | 5.95 |
| 750 | 7.33 ± 2.31 | 73.33 | 5.61 |
| 1000 | 9.00 ± 0.00 | 90.00 | 6.28 |
| Linear regression equation | y = 0.682x + 3.914 | ||
| LC50 (mg/L) | 39.12 | ||
| Compounds | Binding Energy (kcal/mol) | Hydrogen Bond | Hydrophobic Bond |
|---|---|---|---|
| 2-Pentadecyn-1-ol | −4.8 | Gln282, Trp 388 | Ile164, Phe26 |
| 1-Octyn-3-ol, 4-ethyl- | −4.2 | Gln282 | Ile164, Glu 380 |
| 1-Dodecene | −4.3 | Pro385, Phe379, Ile164, Val165, Trp388 | |
| Pentadecanoic acid, 14-methyl-, methyl ester | −5.1 | Trp388, Gln282, Asn411 | Phe378, Ile287, Ile164, Phe26 |
| Nonadecanoic acid | −5.5 | Asn411, Trp388 | Ile164, Pro385, Val165 |
| 1-Tetradecene | −4.3 | Val165, Ile164, Pro385, Trp388 | |
| Heptacosanoic acid, methyl ester | −6.0 | Trp412, Trp388, Phe26, Ile164 | |
| Arachidonic acid | −6.0 | Trp388, Gln282 | His160, Pro385 |
| (6Z,9Z,12Z,15Z)-Methyl octadeca-6,9,12,15-tetraenoate | −6.0 | Asn411, Trp388, Gln282, Gln283 | Pro385, Val165, Ile164, His160 |
| Cyclopropanepentanoic acid, 2-undecyl-, methyl ester, trans | −5.7 | Asn411, Trp388 | His160 Ile164 Val165 Pro385 |
| 9,12,15-Octadecatrienal | −5.1 | Pro385 Ile164 His160 Trp388 Val165 Phe26 | |
| Eicosanoic acid, methyl ester | −5.4 | Trp388, Asn411 | Ile164, Phe291, Val165, Pro385, Phe379 |
| Oleic acid | −5.5 | Asn411, Gln282, Trp388 | Phe26, Pro385, Ile164, Val165 |
| 1-Hexacosanol | −5.6 | Ser80 | Trp412, Trp388, Phe26, Ile164, Ile287 |
| Bis(2-ethylhexyl) phthalate | −6.1 | Gln282, Asn411 | Pro385, Trp388, Ile164, His 160 |
| 11-Octadecenoic acid, methyl ester | −5.3 | Gln282, Gln283 | Glu380, Ile164, Phe26, Pro385 |
| Native ligand | −8.6 | Gln283, Gln282, Asn288, Glu380 | Pro385, Trp388, Phe398 |
| Allopurinol | −4.9 | Asn317, Glu380 Asn288 | Ile168 |
| Molecule | Sequence |
|---|---|
| β-actin | F: 5′-CCTAAGGCCAACCGTGAAAAGATG-3′ |
| R: 5′-GTCCCGGCCAGCCAGGTCCAG-3′ | |
| GLUT9a | F: 5′-GGGTCACCAGCAGAGGAG-3′ |
| R: 5′-TGGACCAAGGCAGGGACAA-3′ | |
| GLUT9b | F: 5′-AACTCCGCAGAAACCAAGGAAAGC-3′ |
| R: 5′-TTCAAAGAGAAGGTAGCGTGGGCT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikhsan, I.; Idroes, R.; Azharuddin, A.; Nasution, R.; Yusnaini, R.; Iqhrammullah, M. Fatty Acid-Rich Extract from Holothuria atra for Hyperuricemia via Expressions Modulation of GLUT9a and GLUT9b in Rat Model. Molecules 2023, 28, 3981. https://doi.org/10.3390/molecules28103981
Ikhsan I, Idroes R, Azharuddin A, Nasution R, Yusnaini R, Iqhrammullah M. Fatty Acid-Rich Extract from Holothuria atra for Hyperuricemia via Expressions Modulation of GLUT9a and GLUT9b in Rat Model. Molecules. 2023; 28(10):3981. https://doi.org/10.3390/molecules28103981
Chicago/Turabian StyleIkhsan, Ikhsan, Rinaldi Idroes, Azharuddin Azharuddin, Rosnani Nasution, Rika Yusnaini, and Muhammad Iqhrammullah. 2023. "Fatty Acid-Rich Extract from Holothuria atra for Hyperuricemia via Expressions Modulation of GLUT9a and GLUT9b in Rat Model" Molecules 28, no. 10: 3981. https://doi.org/10.3390/molecules28103981
APA StyleIkhsan, I., Idroes, R., Azharuddin, A., Nasution, R., Yusnaini, R., & Iqhrammullah, M. (2023). Fatty Acid-Rich Extract from Holothuria atra for Hyperuricemia via Expressions Modulation of GLUT9a and GLUT9b in Rat Model. Molecules, 28(10), 3981. https://doi.org/10.3390/molecules28103981







