Alpha Ketoglutarate Downregulates the Neutral Endopeptidase and Enhances the Growth Inhibitory Activity of Thiorphan in Highly Aggressive Osteosarcoma Cells
Abstract
1. Introduction
2. Results
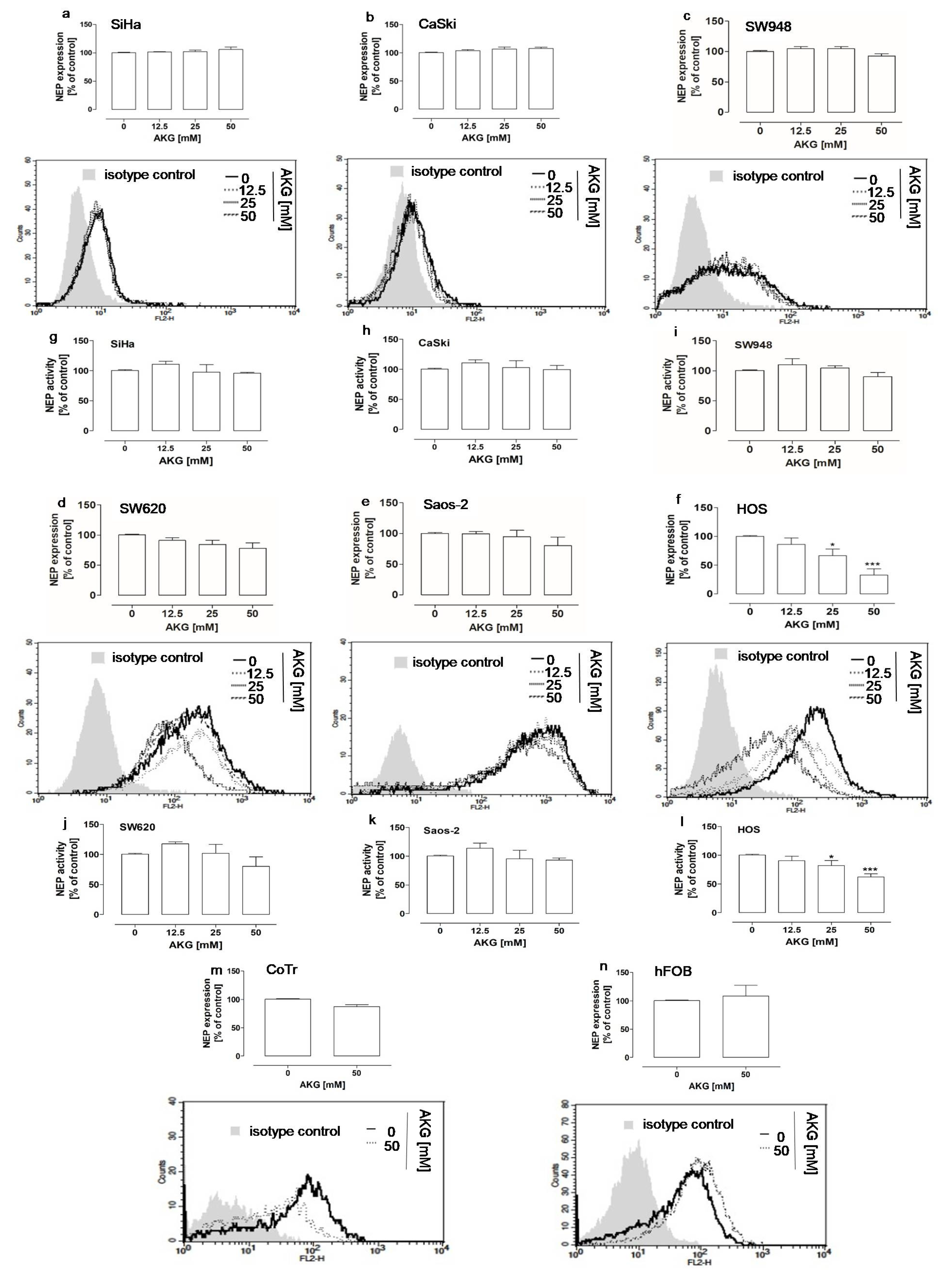
2.1. AKG Downregulated the Protein Level and Activity of NEP in Highly Aggressive Osteosarcoma Cells
2.2. The AKG-Mediated EZH2-Dependent Hypermethylation of H3K27 Was Not Implicated in the AKG-Induced NEP Downregulation in the HOS Cells
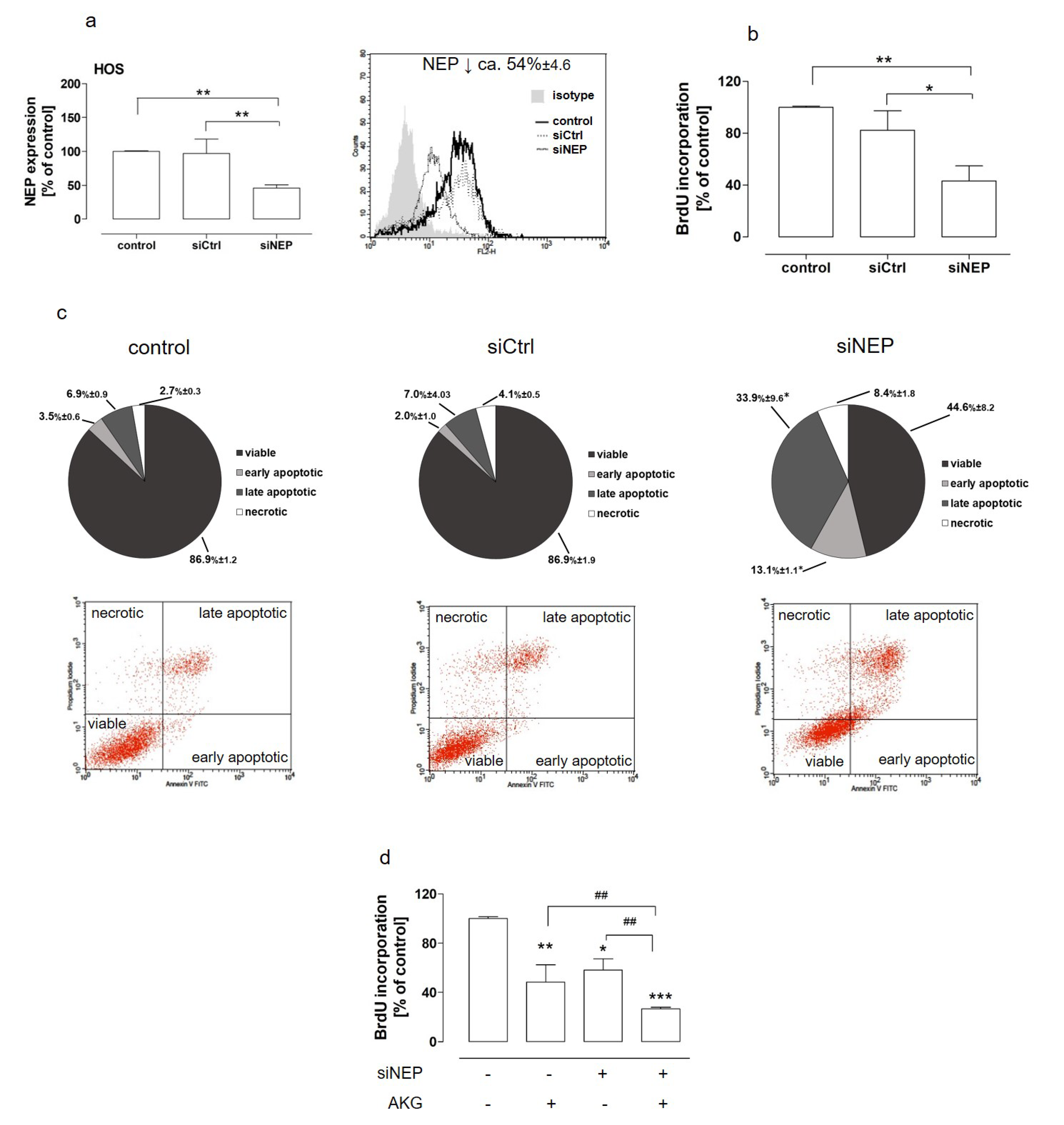
2.3. The Downregulation of NEP Inhibited Proliferation, Induced Apoptosis, and Was Involved in AKG-Induced Growth Inhibitionin the Osteosarcoma Cells
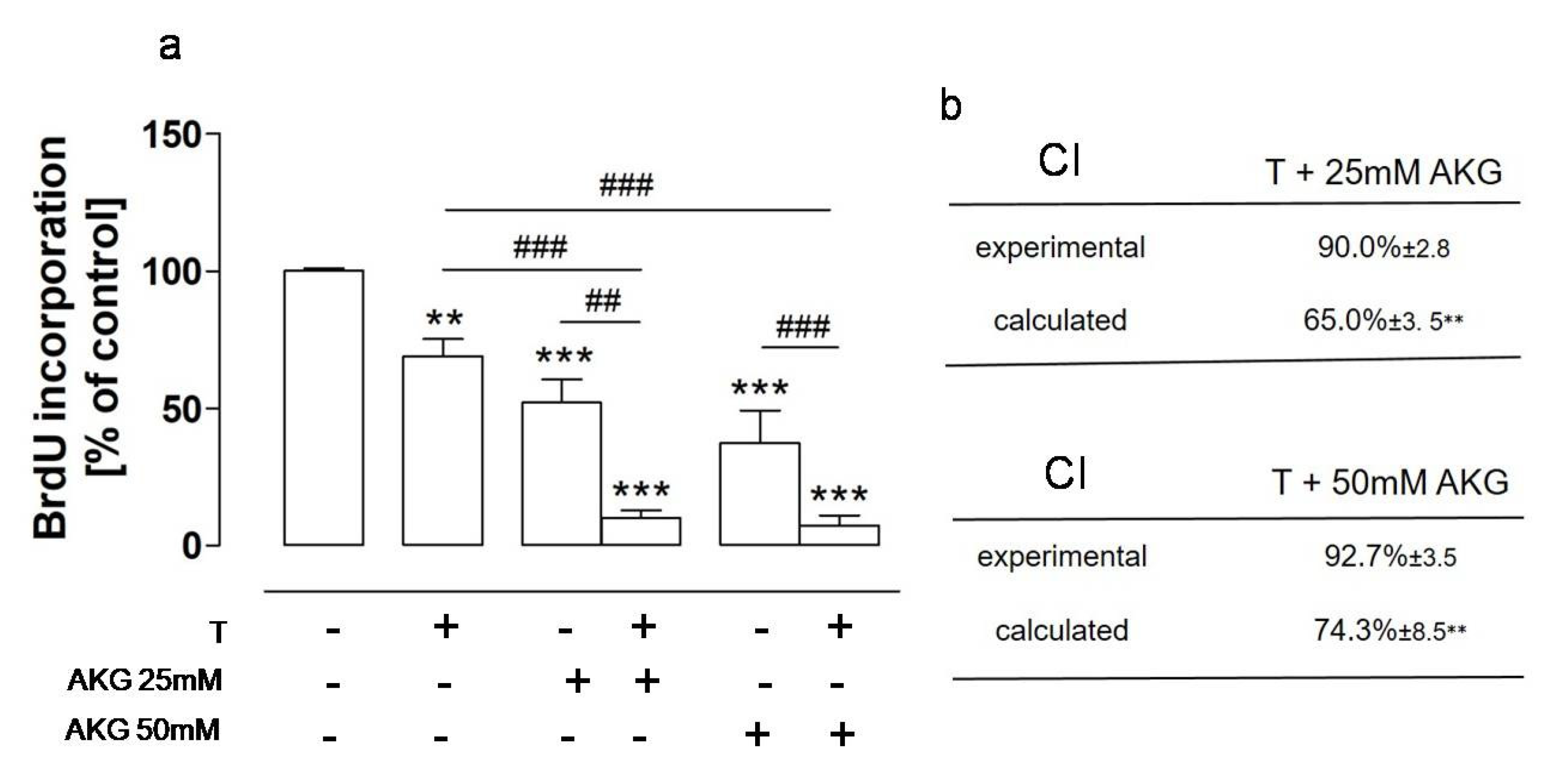
2.4. AKG and the NEP Inhibitor Thiorphan Acted Synergistically towards Osteosarcoma Cells
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Reagents
4.3. Cytotoxicity Assay
4.4. Flow Cytometry Analysis of NEP Level and Histone H3 Methylation
4.5. NEP Gene Expression Silencing
4.6. Cell Proliferation Assay
4.7. Neutral Endopeptidase Activity Assay
4.8. Apoptosis Assays
4.9. Evaluation of Combination Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CACancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Gyanwali, B.; Lim, Z.X.; Soh, J.; Lim, C.; Guan, S.P.; Goh, J.; Maier, A.B.; Kennedy, B.K. Alpha-Ketoglutarate dietary supplementation to improve health in humans. Trends Endocrinol. Metab. 2022, 33, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Zdzisińska, B.; Żurek, A.; Kandefer-Szerszeń, M. Alpha-Ketoglutarate as a Molecule with Pleiotropic Activity: Well-Known and Novel Possibilities of Therapeutic Use. Arch. Immunol. Ther. Exp. 2017, 65, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Mizerska-Kowalska, M.; Bojarska-Junak, A.; Jakubowicz-Gil, J.; Kandefer-Szerszeń, M. Neutral endopeptidase (NEP) is differentially involved in biological activities and cell signaling of colon cancer cell lines derived from various stages of tumor development. Tumor Biol. 2016, 37, 13355–13368. [Google Scholar] [CrossRef]
- Papandreou, C.N.; Usmani, B.; Geng, Y.; Bogenrieder, T.; Freeman, R.; Wilk, S.; Finstad, C.L.; Reuter, V.E.; Powell, C.T.; Scheinberg, D.; et al. Neutral endopeptidase 24.11 loss in metastatic human prostate cancer contributes to androgen-independent progression. Nat. Med. 1998, 4, 50–57. [Google Scholar] [CrossRef]
- Ganju, R.K.; Sunday, M.; Tsarwhas, D.G.; Card, A.; Shipp, M.A. CD10/NEP in non-small cell lung carcinomas. Relationship to cellular proliferation. J. Clin. Investig. 1994, 94, 1784–1791. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, H.; Mo, X.; Chen, G.; Lin, L. Synergistic relationship between dipeptidyl peptidase IV and neutral endopeptidase expression and the combined prognostic significance in osteosarcoma patients. Med. Oncol. 2013, 30, 1–8. [Google Scholar] [CrossRef]
- Torlakovic, E.; Tenstad, E.; Funderud, S.; Rian, E. CD10+ stromal cells form B-lymphocyte maturation niches in the human bone marrow. J. Pathol. 2005, 205, 311–317. [Google Scholar] [CrossRef]
- Nalivaeva, N.N.; Zhuravin, I.A.; Turner, A.J. Neprilysin expression and functions in development, ageing and disease. Mech. Ageing Dev. 2020, 192, 111363. [Google Scholar] [CrossRef]
- Nalivaeva, N.N.; Belyaev, N.D.; Zhuravin, I.A.; Turner, A.J. The Alzheimers amyloid-degrading peptidase, neprilysin: Can we control it? Int. J. Alzheimer’s Dis. 2012, 2012, 383796. [Google Scholar]
- Bayes-Genis, A.; Barallat, J.; Richards, A.M. A Test in Context: Neprilysin: Function, Inhibition, and Biomarker. J. Am. Coll. Cardiol. 2016, 68, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Sankhe, R.; Pai, S.R.K.; Kishore, A. Tumour suppression through modulation of neprilysin signaling: A comprehensive review. Eur. J. Pharmacol. 2021, 891, 173727. [Google Scholar] [CrossRef] [PubMed]
- Carl-McGrath, S.; Lendeckel, U.; Ebert, M.; Röcken, C. Ectopeptidases in tumour biology: A review. Histol. Histopathol. 2006, 21, 1339–1353. [Google Scholar]
- Maguer-Satta, V.; Besançon, R.; Bachelard-Cascales, E. Concise Review: Neutral Endopeptidase (CD10): A Multifaceted Environment Actor in Stem Cells, Physiological Mechanisms, and Cancer. Stem Cells 2011, 29, 389–396. [Google Scholar] [CrossRef]
- Sankhe, R.; Rathi, E.; Manandhar, S.; Kumar, A.; Pai, S.R.K.; Kini, S.G.; Kishore, A. Repurposing of existing FDA approved drugs for Neprilysin inhibition: An in-silico study. J. Mol. Struct. 2021, 1224, 129073. [Google Scholar] [CrossRef]
- Zhuravin, I.A.; Dubrovskaya, N.M.; Vasilev, D.S.; Tumanova, N.L.; Nalivaeva, N.N. Epigenetic and pharmacological regulation of the amyloid-degrading enzyme neprilysin results in modulation of cognitive functions in mammals. Dokl. Biol. Sci. 2011, 438, 145–148. [Google Scholar] [CrossRef]
- Stephen, H.M.; Khoury, R.J.; Majmudar, P.R.; Blaylock, T.; Hawkins, K.; Salama, M.S.; Scott, M.D.; Cosminsky, B.; Utreja, N.K.; Britt, J.; et al. Epigenetic suppression of neprilysin regulates breast cancer invasion. Title. Oncogenesis 2016, 5, e207. [Google Scholar] [CrossRef]
- Eberlin, M.; Mück, T.; Michel, M.C. A Comprehensive Review of the Pharmacodynamics, Pharmacokinetics, and Clinical Effects of the Neutral Endopeptidase Inhibitor Racecadotril. Front. Pharmacol. 2012, 3, 93. [Google Scholar] [CrossRef]
- Rougeot, C.; Messaoudi, M.; Hermitte, V.; Gaëlle Rigault, A.; Blisnick, T.; Dugave, C.; Desor, D.; Rougeon, F. Sialorphin, a natural inhibitor of rat membrane-bound neutral endopeptidase that displays analgesic activity. Proc. Natl. Acad. Sci. USA 2003, 100, 8549–8554. [Google Scholar] [CrossRef]
- Fala, L. Entresto (Sacubitril/valsartan): First-in-class angiotensin receptor neprilysin inhibitor FDA approved for patients with heart failure. Am. Health Drug Benefits 2015, 8, 330. [Google Scholar] [PubMed]
- Mizerska-Kowalska, M.; Kreczko-Kurzawa, J.; Zdzisińska, B.; Czerwonka, A.; Sławińska-Brych, A.; Maćkiewicz, Z.; Nidzworski, D.; Kandefer-Szerszeń, M. Neutral endopeptidase (NEP) inhibitors—Thiorphan, sialorphin, and its derivatives exert anti-proliferative activity towards colorectal cancer cells in vitro. Chem. Biol. Interact. 2019, 307, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Mizerska-Kowalska, M.; Sowa, S.; Donarska, B.; Płaziński, W.; Sławińska-Brych, A.; Tomasik, A.; Ziarkowska, A.; Łączkowski, K.Z.; Zdzisińska, B. New Borane-Protected Derivatives of α-Aminophosphonous Acid as Anti-Osteosarcoma Agents: ADME Analysis and Molecular Modeling, In Vitro Studies on Anti-Cancer Activities, and NEP Inhibition as a Possible Mechanism of Anti-Proliferative Activity. Int. J. Mol. Sci. 2022, 23, 6716. [Google Scholar] [CrossRef] [PubMed]
- Mizerska-Kowalska, M.; Sawa-Wejksza, K.; Sławińska-Brych, A.; Kandefer-Szerszeń, M.; Zdzisińska, B. Neutral endopeptidase depletion decreases colon cancer cell proliferation and TGF-β1 synthesis in indirect co-cultures with normal colon fibroblasts. Clin. Transl. Oncol. 2021, 23, 1405–1414. [Google Scholar] [CrossRef]
- Sobocińska, M.; Giełdoń, A.; Fichna, J.; Kamysz, E. 1-Substituted sialorphin analogues—Synthesis, molecular modelling and in vitro effect on enkephalins degradation by NEP. Amino Acids 2019, 51, 1201–1207. [Google Scholar] [CrossRef]
- Usmani, B.A.; Shen, R.; Janeczko, M.; Papandreou, C.N.; Lee, W.H.; Nelson, W.G.; Nelson, J.B.; Nanus, D.M. Methylation of the neutral endopeptidase gene promoter in human prostate cancers. Clin. Cancer Res. 2000, 6, 1664–1670. [Google Scholar]
- Kooistra, S.; Helin, K. Molecular mechanisms and potential functions of histone demethylases. Nat. Rev. Mol. Cell Biol. 2012, 13, 297–311. [Google Scholar] [CrossRef]
- Zhang, Z.; He, C.; Zhang, L.; Zhu, T.; Lv, D.; Li, G.; Song, Y.; Wang, J.; Wu, H.; Ji, P.; et al. Alpha-ketoglutarate affects murine embryo development through metabolic and epigenetic modulations. Reproduction 2019, 158, 123–133. [Google Scholar] [CrossRef]
- TeSlaa, T.; Chaikovsky, A.C.; Lipchina, I.; Escobar, S.L.; Hochedlinger, K.; Huang, J.; Graeber, T.G.; Braas, D.; Teitell, M.A. α-Ketoglutarate Accelerates the Initial Differentiation of Primed Human Pluripotent Stem Cells. Cell Metab. 2016, 24, 485–493. [Google Scholar] [CrossRef]
- Lauvrak, S.U.; Munthe, E.; Kresse, S.H.; Stratford, E.W.; Namløs, H.M.; Meza-Zepeda, L.A.; Myklebost, O. Functional characterisation of osteosarcoma cell lines and identification of mRNAs and miRNAs associated with aggressive cancer phenotypes. Br. J. Cancer 2013, 109, 2228–2236. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H.; Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; et al. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Plana, D.; Palmer, A.C.; Sorger, P.K. Independent Drug Action in Combination Therapy: Implications for Precision Oncology. Cancer Discov. 2022, 12, 606–624. [Google Scholar] [CrossRef] [PubMed]
- Kale, V.P.; Habib, H.; Chitren, R.; Patel, M.; Pramanik, K.C.; Jonnalagadda, S.C.; Challagundla, K.; Pandey, M.K. Old drugs, new uses: Drug repurposing in hematological malignancies. Semin. Cancer Biol. 2021, 68, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Sonaye, H.V.; Sheikh, R.Y.; Doifode, C.A. Drug repurposing: Iron in the fire for older drugs. Biomed. Pharmacother. 2021, 141, 111638. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Awajan, D.; Hamed, R.A.; Azzam, A.O.; Mahmod, A.I.; AL-Yasari, I.H. Combination Anticancer Therapies Using Selected Phytochemicals. Molecules 2022, 27, 5452. [Google Scholar] [CrossRef]
- Wang, H.H.; Shieh, M.J.; Liao, K.F. A blind, randomized comparison of racecadotril and loperamide for stopping acute diarrhea in adults. World J. Gastroenterol. 2005, 11, 1540–1543. [Google Scholar] [CrossRef]
- Peters, G.J.; Van Der Wilt, C.L.; Van Moorsel, C.J.A.; Kroep, J.R.; Bergman, A.M.; Ackland, S.P. Basis for effective combination cancer chemotherapy with antimetabolites. Pharmacol. Ther. 2000, 87, 227–253. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, M.; Han, X.; Zhang, R.; Chen, L.; Liu, Y.; Xiang, Y.; Xie, T. Combination of traditional Chinese medicine and epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of non-small cell lung cancer: A systematic review and meta-analysis. Medicine 2020, 99, e20683. [Google Scholar] [CrossRef]
- Ratovitski, E. Anticancer Natural Compounds as Epigenetic Modulators of Gene Expression. Curr. Genom. 2017, 18, 175–205. [Google Scholar] [CrossRef]
- Xu, W.; Yang, H.; Liu, Y.; Yang, Y.; Wang, P.; Kim, S.H.; Ito, S.; Yang, C.; Wang, P.; Xiao, M.T.; et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 2011, 19, 17–30. [Google Scholar] [CrossRef]
- Schofield, C.J.; Ratcliffe, P.J. Signalling hypoxia by HIF hydroxylases. Biochem. Biophys. Res. Commun. 2005, 338, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Klieser, E.; Swierczynski, S.; Mayr, C.; Schmidt, J.; Neureiter, D.; Kiesslich, T.; Illig, R. Role of histone deacetylases in pancreas: Implications for pathogenesis and therapy. World J. Gastrointest. Oncol. 2015, 7, 473. [Google Scholar] [CrossRef] [PubMed]
- Chin, R.M.; Fu, X.; Pai, M.Y.; Vergnes, L.; Hwang, H.; Deng, G.; Diep, S.; Lomenick, B.; Meli, V.S.; Monsalve, G.C.; et al. The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 2014, 510, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Asadi Shahmirzadi, A.; Edgar, D.; Liao, C.Y.; Hsu, Y.M.; Lucanic, M.; Asadi Shahmirzadi, A.; Wiley, C.D.; Gan, G.; Kim, D.E.; Kasler, H.G.; et al. Alpha-Ketoglutarate, an Endogenous Metabolite, Extends Lifespan and Compresses Morbidity in Aging Mice. Cell Metab. 2020, 32, 447–456.e6. [Google Scholar] [CrossRef] [PubMed]
- Rouillard, A.D.; Gundersen, G.W.; Fernandez, N.F.; Wang, Z.; Monteiro, C.D.; McDermott, M.G.; Ma’ayan, A. The harmonizome: A collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database 2016, 2016, baw100. [Google Scholar] [CrossRef] [PubMed]
- Mancarella, D.; Plass, C. Epigenetic signatures in cancer: Proper controls, current challenges and the potential for clinical translation. Genome Med. 2021, 13, 1–12. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, D.; Zhang, X.; Li, T.; Li, J.; Tang, Y.; Le, W. Hypoxia-Induced Down-Regulation of Neprilysin by Histone Modification in Mouse Primary Cortical and Hippocampal Neurons. PLoS ONE 2011, 6, e19229. [Google Scholar] [CrossRef]
- Parker, S.J.; Encarnación-Rosado, J.; Hollinshead, K.E.R.; Hollinshead, D.M.; Ash, L.J.; Rossi, J.A.K.; Lin, E.Y.; Sohn, A.S.W.; Philips, M.R.; Jones, D.R.; et al. Spontaneous hydrolysis and spurious metabolic properties of α-ketoglutarate esters. Nat. Commun. 2021, 12, 4905. [Google Scholar] [CrossRef]
- Zhang, T.; Gong, Y.; Meng, H.; Li, C.; Xue, L. Symphony of epigenetic and metabolic regulation—Interaction between the histone methyltransferase EZH2 and metabolism of tumor. Clin. Epigenetics 2020, 12, 72. [Google Scholar] [CrossRef]
- Kaławaj, K.; Sławińska-Brych, A.; Mizerska-Kowalska, M.; Żurek, A.; Bojarska-Junak, A.; Kandefer-Szerszeń, M.; Zdzisińska, B. Alpha Ketoglutarate Exerts In Vitro Anti-Osteosarcoma Effects through Inhibition of Cell Proliferation, Induction of Apoptosis via the JNK and Caspase 9-Dependent Mechanism, and Suppression of TGF-β and VEGF Production and Metastatic Potential of Cells. Int. J. Mol. Sci. 2020, 21, 9406. [Google Scholar] [CrossRef]
- Żurek, A.; Mizerska-Kowalska, M.; Sławińska-Brych, A.; Kaławaj, K.; Bojarska-Junak, A.; Kandefer-Szerszeń, M.; Zdzisińska, B. Alpha ketoglutarate exerts a pro-osteogenic effect in osteoblast cell lines through activation of JNK and mTOR/S6K1/S6 signaling pathways. Toxicol. Appl. Pharmacol. 2019, 374, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; Hedley, D. Whole Blood Measurement of Histone Modifications Linked to the Epigenetic Regulation of Gene Expression. Curr. Protoc. Cytom. 2015, 71, 6.36.1–6.36.9. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; Chow, S.; Barsyte, D.; Arrowsmith, C.; Shankey, T.V.; Minden, M.; Hedley, D. The study of epigenetic mechanisms based on the analysis of histone modification patterns by flow cytoametry. Cytom. Part A 2014, 85, 78–87. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizerska-Kowalska, M.; Sławińska-Brych, A.; Niedziela, E.; Brodovskiy, V.; Zdzisińska, B. Alpha Ketoglutarate Downregulates the Neutral Endopeptidase and Enhances the Growth Inhibitory Activity of Thiorphan in Highly Aggressive Osteosarcoma Cells. Molecules 2023, 28, 97. https://doi.org/10.3390/molecules28010097
Mizerska-Kowalska M, Sławińska-Brych A, Niedziela E, Brodovskiy V, Zdzisińska B. Alpha Ketoglutarate Downregulates the Neutral Endopeptidase and Enhances the Growth Inhibitory Activity of Thiorphan in Highly Aggressive Osteosarcoma Cells. Molecules. 2023; 28(1):97. https://doi.org/10.3390/molecules28010097
Chicago/Turabian StyleMizerska-Kowalska, Magdalena, Adrianna Sławińska-Brych, Emilia Niedziela, Viktor Brodovskiy, and Barbara Zdzisińska. 2023. "Alpha Ketoglutarate Downregulates the Neutral Endopeptidase and Enhances the Growth Inhibitory Activity of Thiorphan in Highly Aggressive Osteosarcoma Cells" Molecules 28, no. 1: 97. https://doi.org/10.3390/molecules28010097
APA StyleMizerska-Kowalska, M., Sławińska-Brych, A., Niedziela, E., Brodovskiy, V., & Zdzisińska, B. (2023). Alpha Ketoglutarate Downregulates the Neutral Endopeptidase and Enhances the Growth Inhibitory Activity of Thiorphan in Highly Aggressive Osteosarcoma Cells. Molecules, 28(1), 97. https://doi.org/10.3390/molecules28010097





