Adsorption of Methylene Blue and Tetracycline by Zeolites Immobilized on a PBAT Electrospun Membrane
Abstract
1. Introduction
2. Results and Discussions
2.1. Morphology of Zeolites and PBAT Membranes
2.2. Physicochemical Properties of Acid-Treated Membranes
2.3. Adsorption Studies
2.3.1. Performance of Electrospun PBAT-Z Mats and Powdered Zeolite after Treatment with HCl 6 M on Adsorption of TC and MB
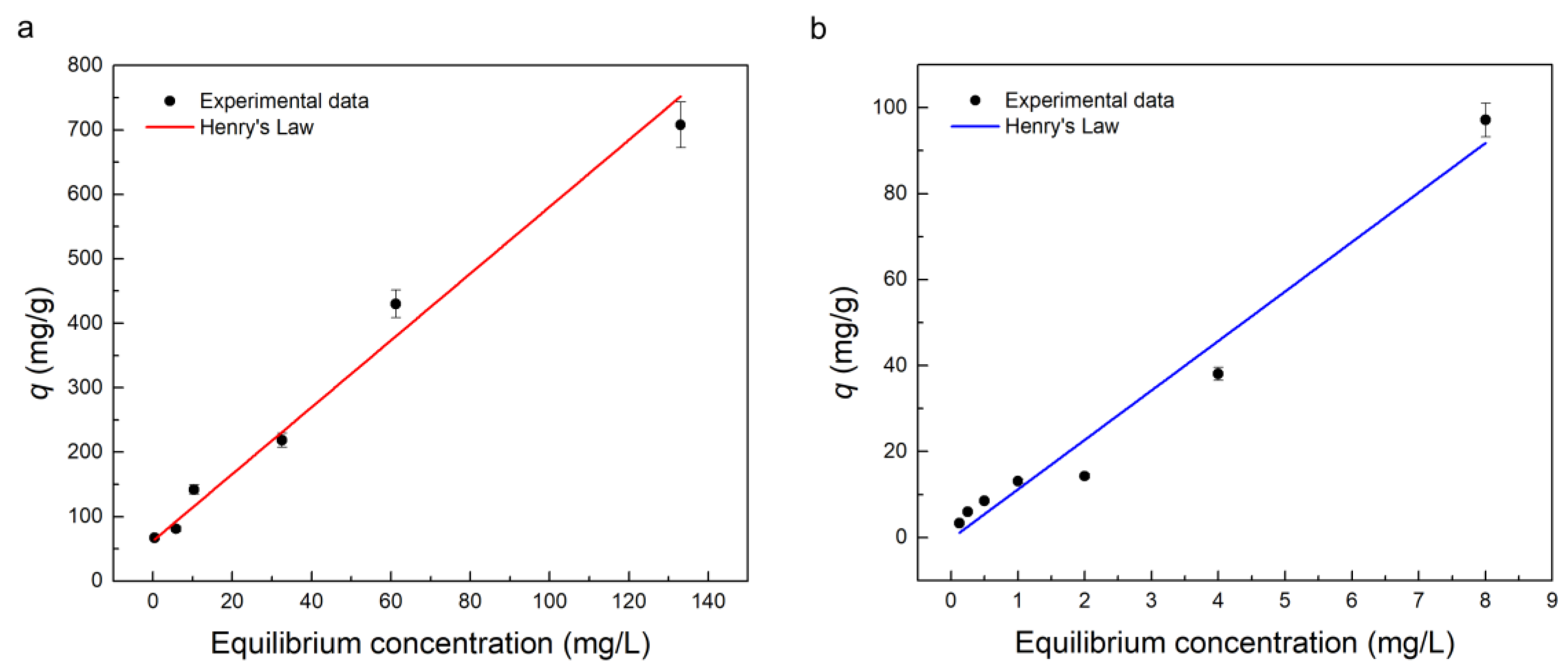
2.3.2. Adsorption Isotherm
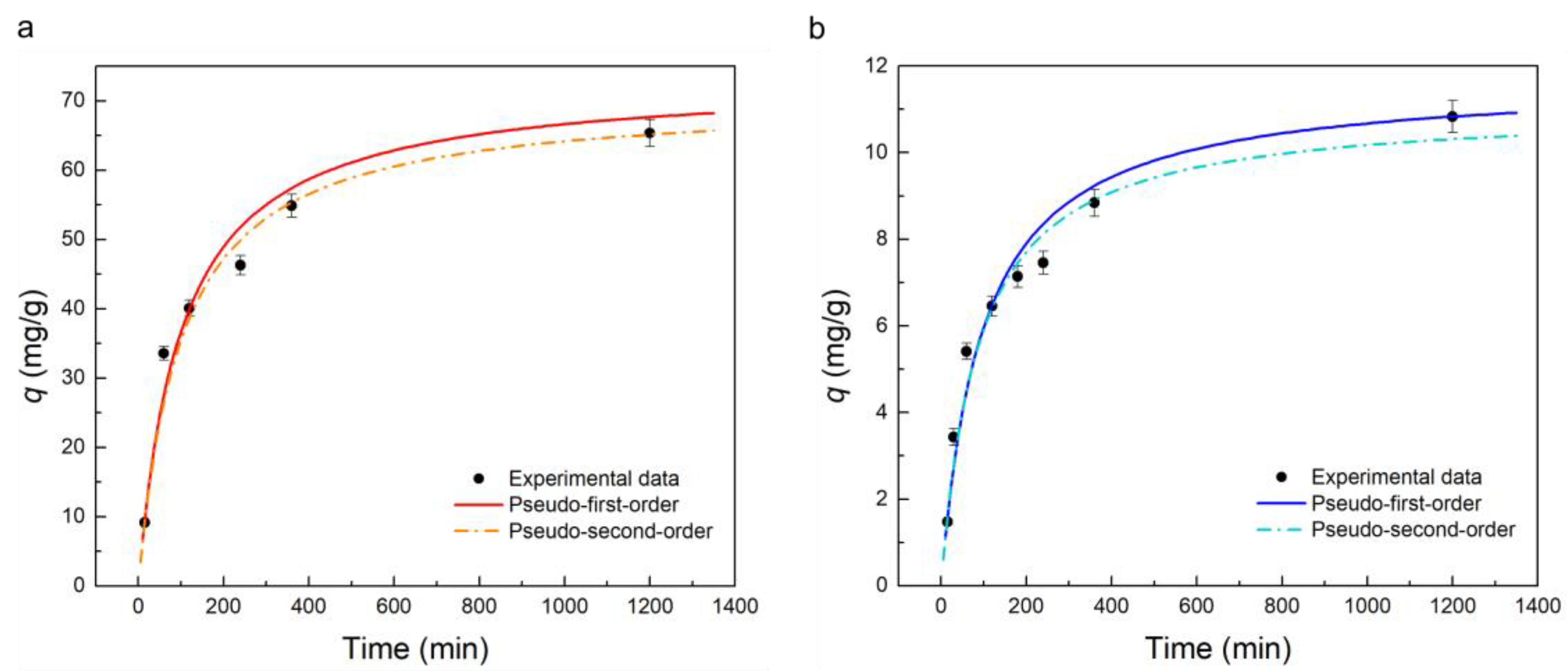
2.3.3. Adsorption Kinetics
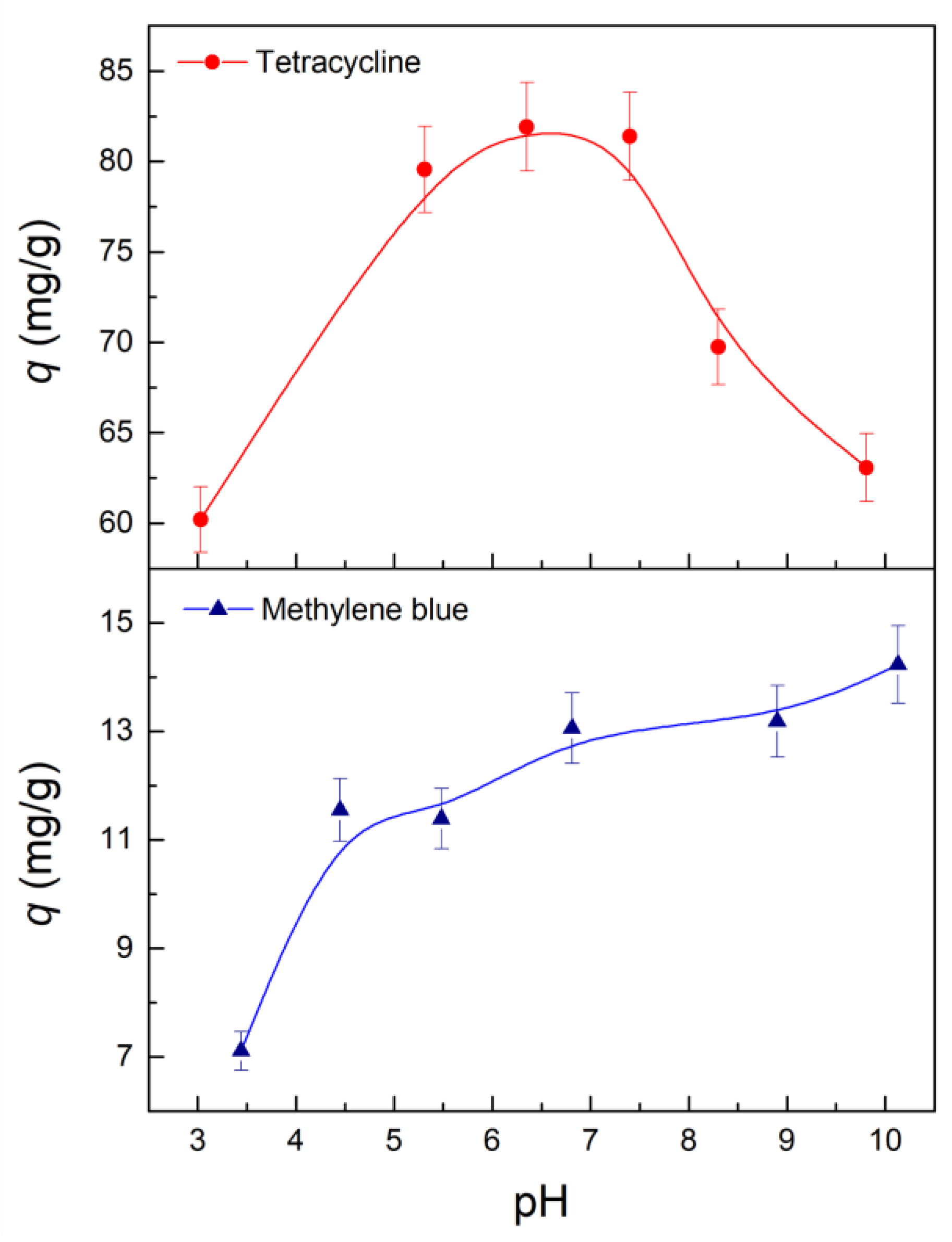
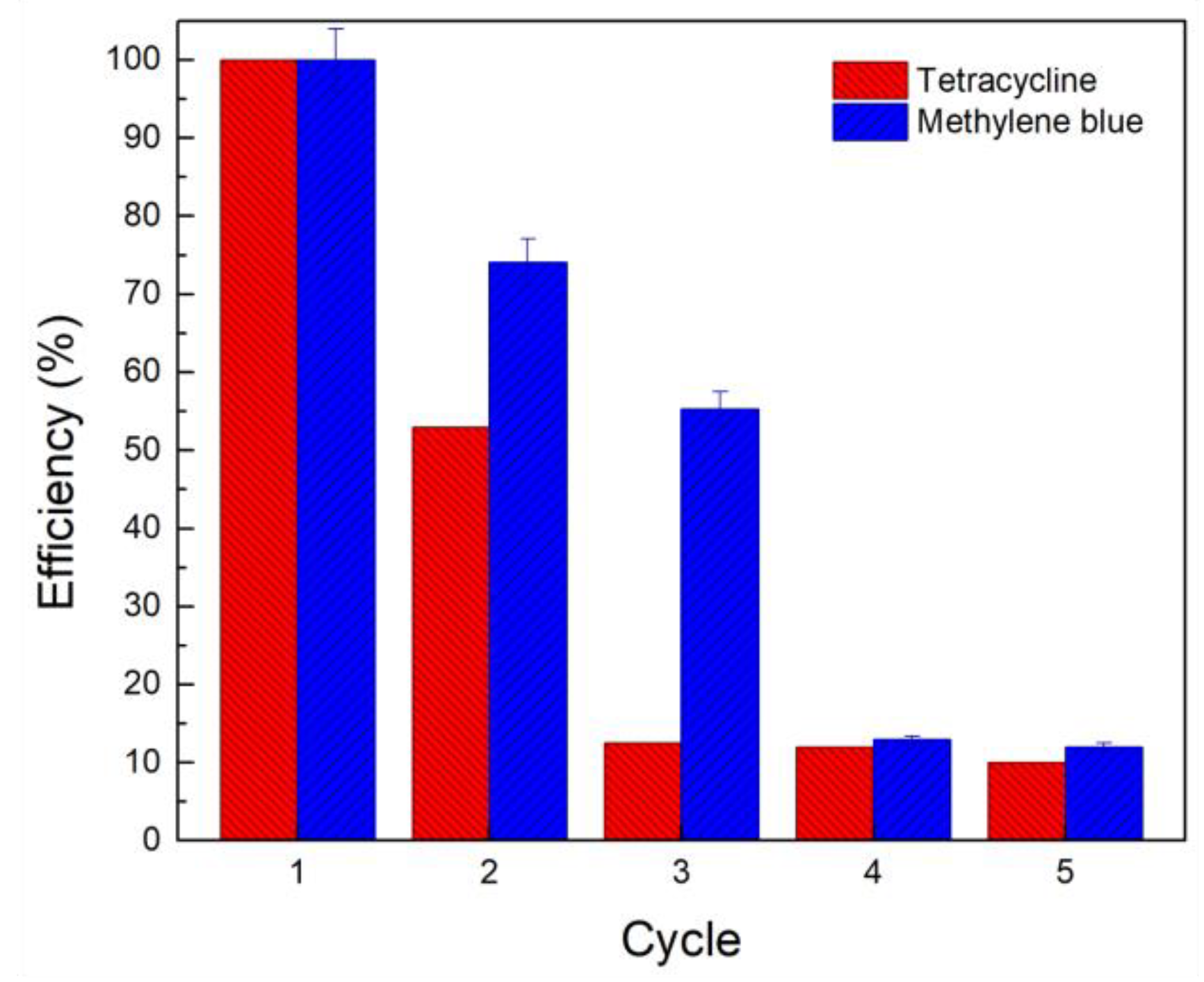
2.3.4. Effect of pH and Reusing
3. Materials and Methods
3.1. Materials
3.2. Fabrication of Membranes
3.3. Characterizations
3.3.1. Microscopy
3.3.2. Infrared Spectroscopy
3.3.3. Contact Angle
3.3.4. Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- WHO/UNICEF Joint Monitoring Program for Water Supply, Sanitation and Hygiene (JMP) Report: Progress on Household Drinking Water, Sanitation and Hygiene 2000–2020. Available online: https://www.unwater.org/publications/who/unicef-joint-monitoring-program-water-supply-sanitation-and-hygiene-jmp-progress-0 (accessed on 29 November 2022).
- Laborde, L. Safe Uses of Agricultural Water. Available online: https://extension.psu.edu/safe-uses-of-agricultural-water (accessed on 29 November 2022).
- Al-Hashimi, O.; Hashim, K.; Loffill, E.; Marolt Čebašek, T.; Nakouti, I.; Faisal, A.A.H.; Al-Ansari, N. A Comprehensive Review for Groundwater Contamination and Remediation: Occurrence, Migration and Adsorption Modelling. Molecules 2021, 26, 5913. [Google Scholar] [CrossRef]
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and Fate of Emerging Pollutants in Water Environment and Options for Their Removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Dulio, V.; van Bavel, B.; Brorström-Lundén, E.; Harmsen, J.; Hollender, J.; Schlabach, M.; Slobodnik, J.; Thomas, K.; Koschorreck, J. Emerging Pollutants in the EU: 10 Years of NORMAN in Support of Environmental Policies and Regulations. Environ. Sci. Eur. 2018, 30, 5. [Google Scholar] [CrossRef]
- Tavengwa, N.T.; Dalu, T. Introduction to Emerging Freshwater Pollutants. In Emerging Freshwater Pollutants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–6. [Google Scholar]
- Affat, S.S. Classifications, Advantages, Disadvantages, Toxicity Effects of Natural and Synthetic Dyes: A Review. Univ. Thi-Qar J. Sci. 2021, 8, 130–135. [Google Scholar]
- Li, H.; Budarin, V.L.; Clark, J.H.; North, M.; Wu, X. Rapid and Efficient Adsorption of Methylene Blue Dye from Aqueous Solution by Hierarchically Porous, Activated Starbons®: Mechanism and Porosity Dependence. J. Hazard. Mater. 2022, 436, 129174. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Magsorbents: Potential Candidates in Wastewater Treatment Technology–A Review on the Removal of Methylene Blue Dye. J. Magn. Magn. Mater. 2020, 500, 166408. [Google Scholar] [CrossRef]
- Low, C.X.; Tan, L.T.H.; Mutalib, N.S.A.; Pusparajah, P.; Goh, B.H.; Chan, K.G.; Letchumanan, V.; Lee, L.H. Unveiling the Impact of Antibiotics and Alternative Methods for Animal Husbandry: A Review. Antibiotics 2021, 10, 578. [Google Scholar] [CrossRef]
- Gothwal, R.; Shashidhar, T. Antibiotic Pollution in the Environment: A Review. Clean–Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Singh, R.; Singh, A.P.; Kumar, S.; Giri, B.S.; Kim, K.H. Antibiotic Resistance in Major Rivers in the World: A Systematic Review on Occurrence, Emergence, and Management Strategies. J. Clean. Prod. 2019, 234, 1484–1505. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline Antibiotics in the Environment: A Review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Scaria, J.; Anupama, K.V.; Nidheesh, P.V. Tetracyclines in the Environment: An Overview on the Occurrence, Fate, Toxicity, Detection, Removal Methods, and Sludge Management. Sci. Total Environ. 2021, 771, 145291. [Google Scholar] [CrossRef]
- Azizi, D.; Arif, A.; Blair, D.; Dionne, J.; Filion, Y.; Ouarda, Y.; Pazmino, A.G.; Pulicharla, R.; Rilstone, V.; Tiwari, B.; et al. A Comprehensive Review on Current Technologies for Removal of Endocrine Disrupting Chemicals from Wastewaters. Environ. Res. 2022, 207, 112196. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Qureshi, M.; Vishwakarma, D.K.; Al-Ansari, N.; Kuriqi, A.; Elbeltagi, A.; Saraswat, A. A Review on Emerging Water Contaminants and the Application of Sustainable Removal Technologies. Case Stud. Chem. Environ. Eng. 2022, 6, 100219. [Google Scholar] [CrossRef]
- Hashemi, H.; Bahrami, S.; Emadi, Z.; Shariatipor, H.; Nozari, M. Optimization of Ammonium Adsorption from Landfill Leachate Using Montmorillonite/Hematite Nanocomposite: Response Surface Method Based on Central Composite Design. Desalin. Water Treat 2021, 232, 39–54. [Google Scholar] [CrossRef]
- García-Carvajal, C.; Villarroel-Rocha, J.; de Souza, V.C.; Sapag, K. Development of Ceramic Honeycomb Monolith from Natural Zeolite Tested as Adsorbent to Remove Methylene Blue in Aqueous Media. Environ. Sci. Pollut. Res. 2022, 29, 79890–79902. [Google Scholar] [CrossRef]
- Varsha, M.; Kumar, P.S.; Rathi, B.S. A Review on Recent Trends in the Removal of Emerging Contaminants from Aquatic Environment Using Low-Cost Adsorbents. Chemosphere 2022, 287, 132270. [Google Scholar] [CrossRef]
- Parvaresh, V.; Hashemi, H.; Khodabakhshi, A.; Sedehi, M. Removal of Dye from Synthetic Textile Wastewater Using Agricultural Wastes and Determination of Adsorption Isotherm. Desalin. Water Treat. 2018, 111, 345–350. [Google Scholar] [CrossRef]
- Kalebić, B.; Pavlović, J.; Dikić, J.; Rečnik, A.; Gyergyek, S.; Škoro, N.; Rajić, N. Use of Natural Clinoptilolite in the Preparation of an Efficient Adsorbent for Ciprofloxacin Removal from Aqueous Media. Minerals 2021, 11, 518. [Google Scholar] [CrossRef]
- Sodha, V.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Bandyopadhyay, R.; Sridewi, N. Comprehensive Review on Zeolite-Based Nanocomposites for Treatment of Effluents from Wastewater. Nanomaterials 2022, 12, 3199. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.M.; Mattii, G.B. Application of Zeolites in Agriculture and Other Potential Uses: A Review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- Irannajad, M.; Kamran Haghighi, H. Removal of Heavy Metals from Polluted Solutions by Zeolitic Adsorbents: A Review. Environ. Process. 2021, 8, 7–35. [Google Scholar] [CrossRef]
- Torasso, N.; Vergara-Rubio, A.; Rivas-Rojas, P.; Huck-Iriart, C.; Larrañaga, A.; Fernández-Cirelli, A.; Cerveny, S.; Goyanes, S. Enhancing Arsenic Adsorption via Excellent Dispersion of Iron Oxide Nanoparticles inside Poly(Vinyl Alcohol) Nanofibers. J. Environ. Chem. Eng. 2020, 9, 104664. [Google Scholar] [CrossRef]
- Torasso, N.; González-Seligra, P.; Trupp, F.; Grondona, D.; Goyanes, S. Turning a Novel Janus Electrospun Mat into an Amphiphilic Membrane with High Aromatic Hydrocarbon Adsorption Capacity. Colloids Interfaces 2022, 6, 66. [Google Scholar] [CrossRef]
- Picón, D.; Torasso, N.; Baudrit, J.R.V.; Cerveny, S.; Goyanes, S. Bio-Inspired Membranes for Adsorption of Arsenic via Immobilized L-Cysteine in Highly Hydrophilic Electrospun Nanofibers. Chem. Eng. Res. Des. 2022, 185, 108–118. [Google Scholar] [CrossRef]
- Estevez-Areco, S.; Guz, L.; Candal, R.; Goyanes, S. Release Kinetics of Rosemary (Rosmarinus officinalis) Polyphenols from Polyvinyl Alcohol (PVA) Electrospun Nanofibers in Several Food Simulants. Food Packag. Shelf Life 2018, 18, 42–50. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Castro, G.R.; Goyanes, S. A Simple Green Route to Obtain Poly (Vinyl Alcohol) Electrospun Mats with Improved Water Stability for Use as Potential Carriers of Drugs. Mater. Sci. Eng. C 2016, 69, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, L.; Chen, G.; Liu, Z.; Lu, T.; Yang, Y.; Yu, J.; Kang, D.; Yan, W.; He, M. PBAT Hollow Porous Microfibers Prepared via Electrospinning and Their Functionalization for Potential Peptide Release. Mater. Des. 2021, 207, 109880. [Google Scholar] [CrossRef]
- Abdul Momin, S.; Mariatti, M.; Rusli, A.; Shafiq, M.D.; Vilay, V.; Todo, M. Development of Non-Woven Poly Butadiethylene Terephthalate (PBAT) Mats Using Electrospinning. Mater. Today Proc. 2022, 66, 2868–2872. [Google Scholar] [CrossRef]
- De, S.; Rossin, A.R.; Caetano, J.; Zanella, H.G.; Bariccatti, R.A.; Gaffo, L.; Muniz, E.C.; Caetano, W.; Stolf, S.F.; Dragunski, D.C. Obtaining and Characterization of PBAT/PLA Fibers Containing Zinc Phthalocyanine Prepared by the Electrospinning Method. J. Therm. Anal. Calorim. 2021, 147, 4579–4587. [Google Scholar] [CrossRef]
- Scheibel, J.M.; Menezes, F.C.; Reginatto, C.L.; de Brito da Silva, C.; Moura, D.J.; Rodembusch, F.; Bussamara, R.; Weibel, D.E.; Soares, R.M.D. Antibiotic-Loaded Wound Dressings Obtained from the PBAT-Gentamicin Combination. J. Appl. Polym. Sci. 2021, 138, 50633. [Google Scholar] [CrossRef]
- Threepopnatkul, P.; Wongsuton, K.; Jaiaue, C.; Rakkietwinai, N.; Stittatrakul, A.; Kulsetthanchalee, C. Effect of Zeolite on Mechanical and Barrier Properties of PBAT Films for Life Extension of Agricultural Products. Key Eng. Mater. 2020, 861, 176–181. [Google Scholar] [CrossRef]
- Correia, P.R.C.; Ramos, I.G.; Viana, A.C.; Mascarenhas, A.J.S.; Sant’ana, A.E.G.; Goulart, H.F.; Druzian, J.I. Development of Composite Membrane PBAT: Zeolite Y for Application as Rhynchophorol Release System. J. Appl. Polym. Sci. 2018, 135, 45757. [Google Scholar] [CrossRef]
- Marzano-Barreda, L.A.; Yamashita, F.; Bilck, A.P. Effect of Biodegradable Active Packaging with Zeolites on Fresh Broccoli Florets. J. Food Sci. Technol. 2020, 58, 197–204. [Google Scholar] [CrossRef]
- Westrup, J.L.; Bertoldi, C.; Cercena, R.; Dal-Bó, A.G.; Soares, R.M.D.; Fernandes, A.N. Adsorption of Endocrine Disrupting Compounds from Aqueous Solution in Poly(Butyleneadipate-Co-Terephthalate) Electrospun Microfibers. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 611, 125800. [Google Scholar] [CrossRef]
- Facchi, D.P.; Facchi, S.P.; Souza, P.R.; Bonafé, E.G.; Popat, K.C.; Kipper, M.J.; Martins, A.F. Composite Filter with Antimicrobial and Anti-Adhesive Properties Based on Electrospun Poly(Butylene Adipate-Co-Terephthalate)/Poly(Acid Lactic)/Tween 20 Fibers Associated with Silver Nanoparticles. J. Memb. Sci. 2022, 650, 120426. [Google Scholar] [CrossRef]
- Vergara-Rubio, A.; Ribba, L.; Picón, D.; Candal, R.; Goyanes, S. A Highly Efficient Nanostructured Sorbent of Sulfuric Acid from Ecofriendly Electrospun Poly(Vinyl Alcohol) Mats. Ind. Eng. Chem. Res. 2022, 61, 2091–2099. [Google Scholar] [CrossRef]
- Aghel, B.; Mohadesi, M.; Gouran, A.; Razmegir, M.H. Use of Modified Iranian Clinoptilolite Zeolite for Cadmium and Lead Removal from Oil Refinery Wastewater. Int. J. Environ. Sci. Technol. 2020, 17, 1239–1250. [Google Scholar] [CrossRef]
- Li, X.; Shao, H.; Ma, Q.; Yu, W.; Dong, X. Self-Supporting Flexible Metal-Organic Framework-Based Electrospun Nanofibers Membrane for Efficient Removal of Tetracycline from Aqueous Solutions. J. Solid State Chem. 2022, 312, 123233. [Google Scholar] [CrossRef]
- Sultana, N.; Rahman, R. Electrospun Nanofiber Composite Membranes Based on Cellulose Acetate/Nano-Zeolite for the Removal of Oil from Oily Wastewater. Emergent Mater. 2022, 5, 145–153. [Google Scholar] [CrossRef]
- Ohlin, L.; Bazin, P.; Thibault-Starzyk, F.; Hedlund, J.; Grahn, M. Adsorption of CO2, CH4, and H2O in Zeolite ZSM-5 Studied Using in Situ ATR-FTIR Spectroscopy. J. Phys. Chem. C 2013, 117, 16972–16982. [Google Scholar] [CrossRef]
- Yazdi, F.; Anbia, M.; Salehi, S. Characterization of Functionalized Chitosan-Clinoptilolite Nanocomposites for Nitrate Removal from Aqueous Media. Int. J. Biol. Macromol. 2019, 130, 545–555. [Google Scholar] [CrossRef]
- Cai, Y.; Lv, J.; Feng, J. Spectral Characterization of Four Kinds of Biodegradable Plastics: Poly (Lactic Acid), Poly (Butylenes Adipate-Co-Terephthalate), Poly (Hydroxybutyrate-Co-Hydroxyvalerate) and Poly (Butylenes Succinate) with FTIR and Raman Spectroscopy. J. Polym. Environ. 2013, 21, 108–114. [Google Scholar] [CrossRef]
- Kuzniatsova, T.; Kim, Y.; Shqau, K.; Dutta, P.K.; Verweij, H. Zeta Potential Measurements of Zeolite Y: Application in Homogeneous Deposition of Particle Coatings. Microporous Mesoporous Mater. 2007, 103, 102–107. [Google Scholar] [CrossRef]
- Valdés, H.; Tardón, R.F.; Zaror, C.A. Role of Surface Hydroxyl Groups of Acid-Treated Natural Zeolite on the Heterogeneous Catalytic Ozonation of Methylene Blue Contaminated Waters. Chem. Eng. J. 2012, 211, 388–395. [Google Scholar] [CrossRef]
- Garcia-Basabe, Y.; Rodriguez-Iznaga, I.; de Menorval, L.-C.; Llewellyn, P.; Maurin, G.; Lewis, D.W.; Binions, R.; Autie, M.; Ruiz-Salvador, A.R. Step-Wise Dealumination of Natural Clinoptilolite: Structural and Physicochemical Characterization. Microporous Mesoporous Mater. 2010, 135, 187–196. [Google Scholar] [CrossRef]
- Doula, M.K.; Ioannou, A. The Effect of Electrolyte Anion on Cu Adsorption–Desorption by Clinoptilolite. Microporous mesoporous Mater. 2003, 58, 115–130. [Google Scholar] [CrossRef]
- Ma, Y.-K.; Rigolet, S.; Michelin, L.; Paillaud, J.-L.; Mintova, S.; Khoerunnisa, F.; Daou, T.J.; Ng, E.-P. Facile and Fast Determination of Si/Al Ratio of Zeolites Using FTIR Spectroscopy Technique. Microporous Mesoporous Mater. 2021, 311, 110683. [Google Scholar] [CrossRef]
- Burris, L.E.; Juenger, M.C.G. The Effect of Acid Treatment on the Reactivity of Natural Zeolites Used as Supplementary Cementitious Materials. Cem. Concr. Res. 2016, 79, 185–193. [Google Scholar] [CrossRef]
- Thakuria, R.; Nath, N.K.; Saha, B.K. The Nature and Applications of π-π Interactions: A Perspective. Cryst. Growth Des. 2019, 19, 523–528. [Google Scholar] [CrossRef]
- Frantz, T.S.; Silveira, N.; Quadro, M.S.; Andreazza, R.; Barcelos, A.A.; Cadaval, T.R.S.; Pinto, L.A.A. Cu (II) Adsorption from Copper Mine Water by Chitosan Films and the Matrix Effects. Environ. Sci. Pollut. Res. 2017, 24, 5908–5917. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hossain, M.F.; Duan, C.; Lu, J.; Tsang, Y.F.; Islam, M.S.; Zhou, Y. Isotherm Models for Adsorption of Heavy Metals from Water-a Review. Chemosphere 2022, 307, 135545. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, Y.; Chu, W.; Chen, Z.; Wang, J. Adsorptive Removal of Antibiotics from Water Using Peanut Shells from Agricultural Waste. RSC Adv. 2018, 8, 13546–13555. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Huang, B.; Cao, C.; Liu, X.; Sun, X.; Xiao, L.; Qiu, J.; Luo, Y.; Qian, Q.; Chen, Q. Adsorption–Desorption Behavior of Methylene Blue onto Aged Polyethylene Microplastics in Aqueous Environments. Mar. Pollut. Bull. 2021, 167, 112287. [Google Scholar] [CrossRef] [PubMed]
- Lye, J.W.P.; Saman, N.; Noor, A.M.M.; Mohtar, S.S.; Othman, N.S.; Sharuddin, S.S.N.; Kong, H.; Mat, H. Application of Nanoscale Zero-Valent Iron-Loaded Natural Zeolite for Tetracycline Removal Process. Chem. Eng. Technol. 2020, 43, 1285–1296. [Google Scholar] [CrossRef]
- Jannat Abadi, M.H.; Nouri, S.M.M.; Zhiani, R.; Heydarzadeh, H.D.; Motavalizadehkakhky, A. Removal of Tetracycline from Aqueous Solution Using Fe-Doped Zeolite. Int. J. Ind. Chem. 2019, 10, 291–300. [Google Scholar] [CrossRef]
- Rouhani, M.; Ashrafi, S.D.; Taghavi, K.; Joubani, M.N.; Jaafari, J. Evaluation of Tetracycline Removal by Adsorption Method Using Magnetic Iron Oxide Nanoparticles (Fe3O4) and Clinoptilolite from Aqueous Solutions. J. Mol. Liq. 2022, 356, 119040. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, F.; Wei, W.; Gao, H.; Zhang, K.; Zhang, G.; Xu, Y.; Zhang, P. Efficient and Fast Adsorption of Methylene Blue Dye onto a Nanosheet MFI Zeolite. J. Solid State Chem. 2021, 295, 121917. [Google Scholar] [CrossRef]
- Wang, C.; Yu, J.; Feng, K.; Wang, L.; Huang, J. Synthesis of Porous Magnetic Zeolite-Based Material and Its Performance on Removal of Cd2+ Ion and Methylene Blue from Aqueous Solution. Microporous Mesoporous Mater. 2022, 345, 112256. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, W.; Chen, B.; Zhao, Y.; Liu, D.; Sun, Y.; Gong, B. Removal of Tetracycline from Aqueous Solution by MCM-41-Zeolite A Loaded Nano Zero Valent Iron: Synthesis, Characteristic, Adsorption Performance and Mechanism. J. Hazard. Mater. 2017, 339, 22–32. [Google Scholar] [CrossRef]
- de Sousa, D.N.R.; Insa, S.; Mozeto, A.A.; Petrovic, M.; Chaves, T.F.; Fadini, P.S. Equilibrium and Kinetic Studies of the Adsorption of Antibiotics from Aqueous Solutions onto Powdered Zeolites. Chemosphere 2018, 205, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, Z.H. Characterisation and Environmental Application of an Australian Natural Zeolite for Basic Dye Removal from Aqueous Solution. J. Hazard. Mater. 2006, 136, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Lye, J.W.P.; Saman, N.; Sharuddin, S.S.N.; Othman, N.S.; Mohtar, S.S.; Md Noor, A.M.; Buhari, J.; Cheu, S.C.; Kong, H.; Mat, H. Removal Performance of Tetracycline and Oxytetracycline From Aqueous Solution Via Natural Zeolites: An Equilibrium and Kinetic Study. Clean Soil Air Water 2017, 45, 1600260. [Google Scholar] [CrossRef]
- Schneider, R.; Mercante, L.A.; Andre, R.S.; de Brandão, H.M.; Mattoso, L.H.C.; Correa, D.S. Biocompatible Electrospun Nanofibers Containing Cloxacillin: Antibacterial Activity and Effect of PH on the Release Profile. React. Funct. Polym. 2018, 132, 26–35. [Google Scholar] [CrossRef]
- Ribba, L.G. Nanoestructuras Biodegradables Obtenidas Mediante Técnicas de Electroestirado. PhD Thesis, University of Buenos Aires, Buenos Aires, Argentina, 2017. [Google Scholar]
- Farias-Pineira, T.; Picazo-Mozo, O.; Concepción Rosabal, B.; Charles-de Ménorval, L. Cuban Clinoptilolite for Rhodamine B Adsorption. Characterization of the Obtained Zeolite-Dye Materials. Rev. Cub. Quim. 2018, 30, 175–190. [Google Scholar]
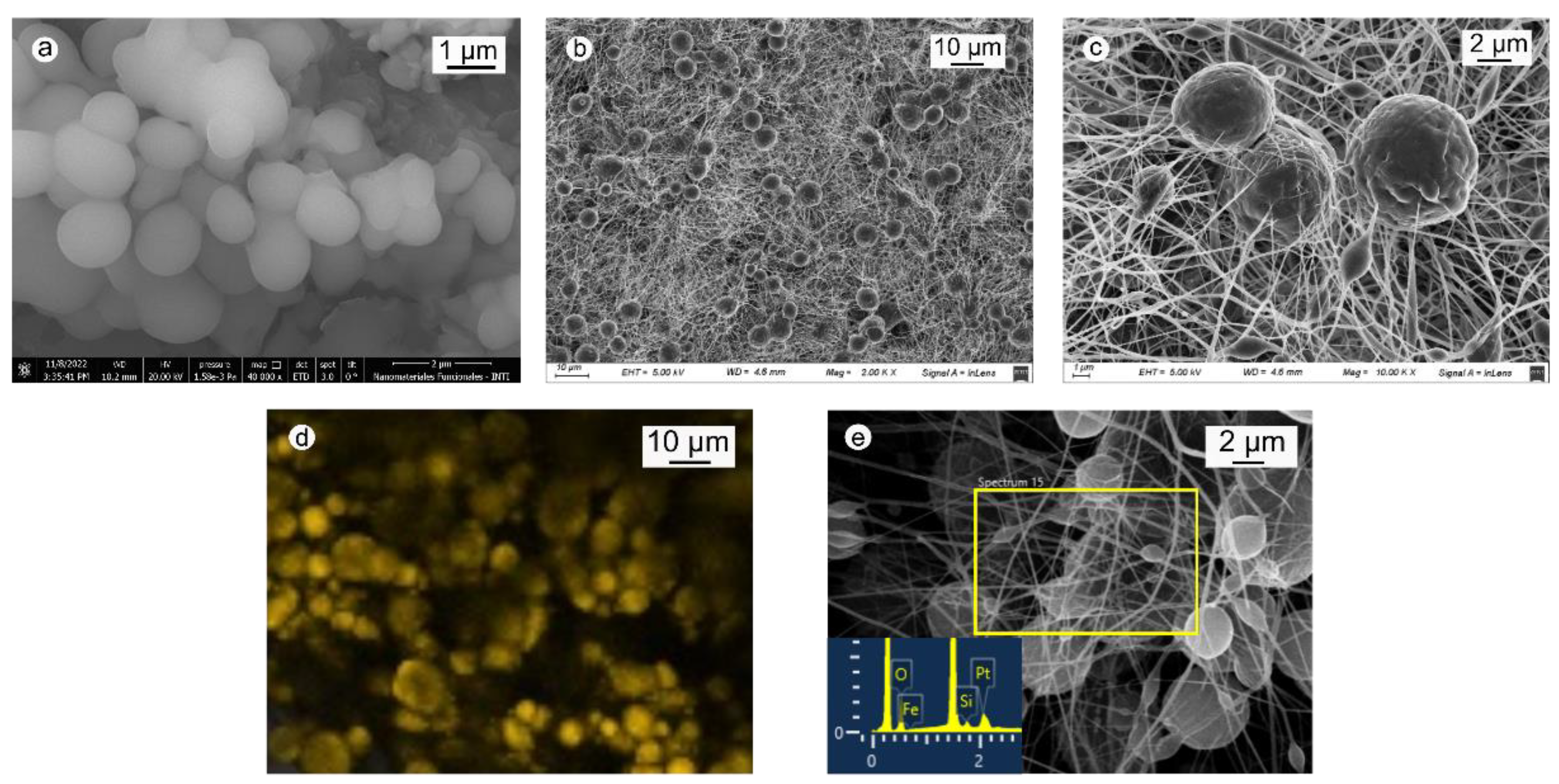
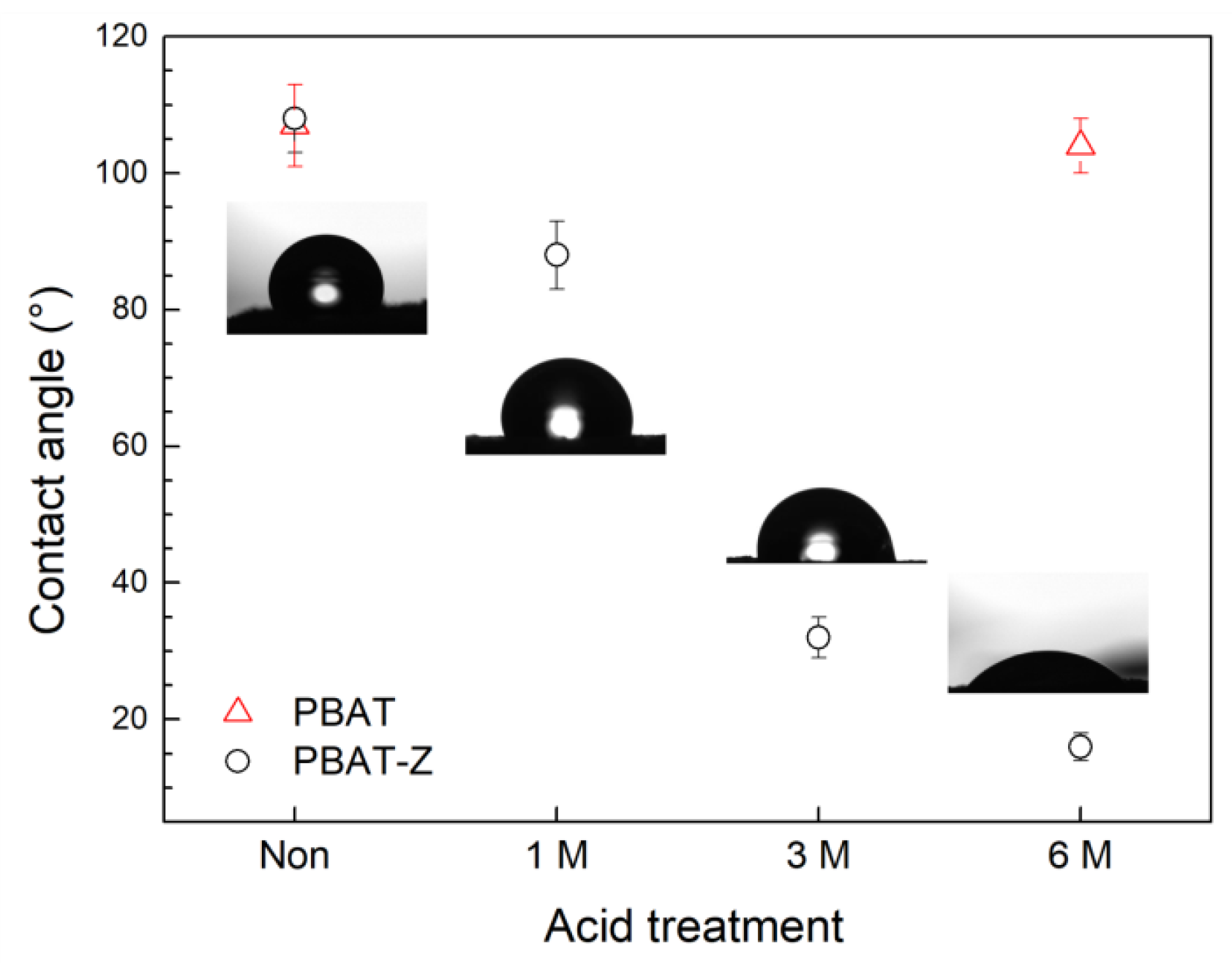

| Material | q TC (mg/g) 1 | q MB (mg/g) 2 |
|---|---|---|
| PBAT | 3.1 ± 0.5 | 0.11 ± 0.3 |
| PBAT-6M | 3.5 ± 0.7 | 0.13 ± 0.3 |
| PBAT-Z | 37.9 ± 2.7 | 8.2 ± 0.5 |
| PBAT-Z-6M | 142.4 ± 7.8 | 12.2 ± 0.7 |
| Powdered zeolite | 27.3 ± 3.8 | 3.3 ± 0.4 |
| Powdered zeolite-6M | 50.7 ± 3.0 | 4.6 ± 0.2 |
| Contaminant | Henry’s Constant (L/g) | R2 |
|---|---|---|
| TC | 5.18 ± 0.51 | 0.962 |
| MB | 11.51 ± 0.87 | 0.972 |
| Adsorbate | Pseudo First Order | Pseudo Second Order | ||||
|---|---|---|---|---|---|---|
| k1 (min−1)/(10−3) | qe (mg/g) | R2 | k2 (g/mg min)/(10−5) | qe (mg/g) | R2 | |
| TC | 12.4 ± 2.0 | 55.3 ± 4.4 | 0.911 | 14.4 ± 1.3 | 70.5 ± 2.1 | 0.998 |
| MB | 13.1 ± 2.0 | 8.68 ± 0.64 | 0.887 | 104 ± 11 | 11.06 ± 0.77 | 0.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picón, D.; Vergara-Rubio, A.; Estevez-Areco, S.; Cerveny, S.; Goyanes, S. Adsorption of Methylene Blue and Tetracycline by Zeolites Immobilized on a PBAT Electrospun Membrane. Molecules 2023, 28, 81. https://doi.org/10.3390/molecules28010081
Picón D, Vergara-Rubio A, Estevez-Areco S, Cerveny S, Goyanes S. Adsorption of Methylene Blue and Tetracycline by Zeolites Immobilized on a PBAT Electrospun Membrane. Molecules. 2023; 28(1):81. https://doi.org/10.3390/molecules28010081
Chicago/Turabian StylePicón, David, Alicia Vergara-Rubio, Santiago Estevez-Areco, Silvina Cerveny, and Silvia Goyanes. 2023. "Adsorption of Methylene Blue and Tetracycline by Zeolites Immobilized on a PBAT Electrospun Membrane" Molecules 28, no. 1: 81. https://doi.org/10.3390/molecules28010081
APA StylePicón, D., Vergara-Rubio, A., Estevez-Areco, S., Cerveny, S., & Goyanes, S. (2023). Adsorption of Methylene Blue and Tetracycline by Zeolites Immobilized on a PBAT Electrospun Membrane. Molecules, 28(1), 81. https://doi.org/10.3390/molecules28010081







