A New Flavanone from Chromolaena tacotana (Klatt) R. M. King and H. Rob, Promotes Apoptosis in Human Breast Cancer Cells by Downregulating Antiapoptotic Proteins
Abstract
1. Introduction
2. Results
2.1. Structural Identification of the Flavanone
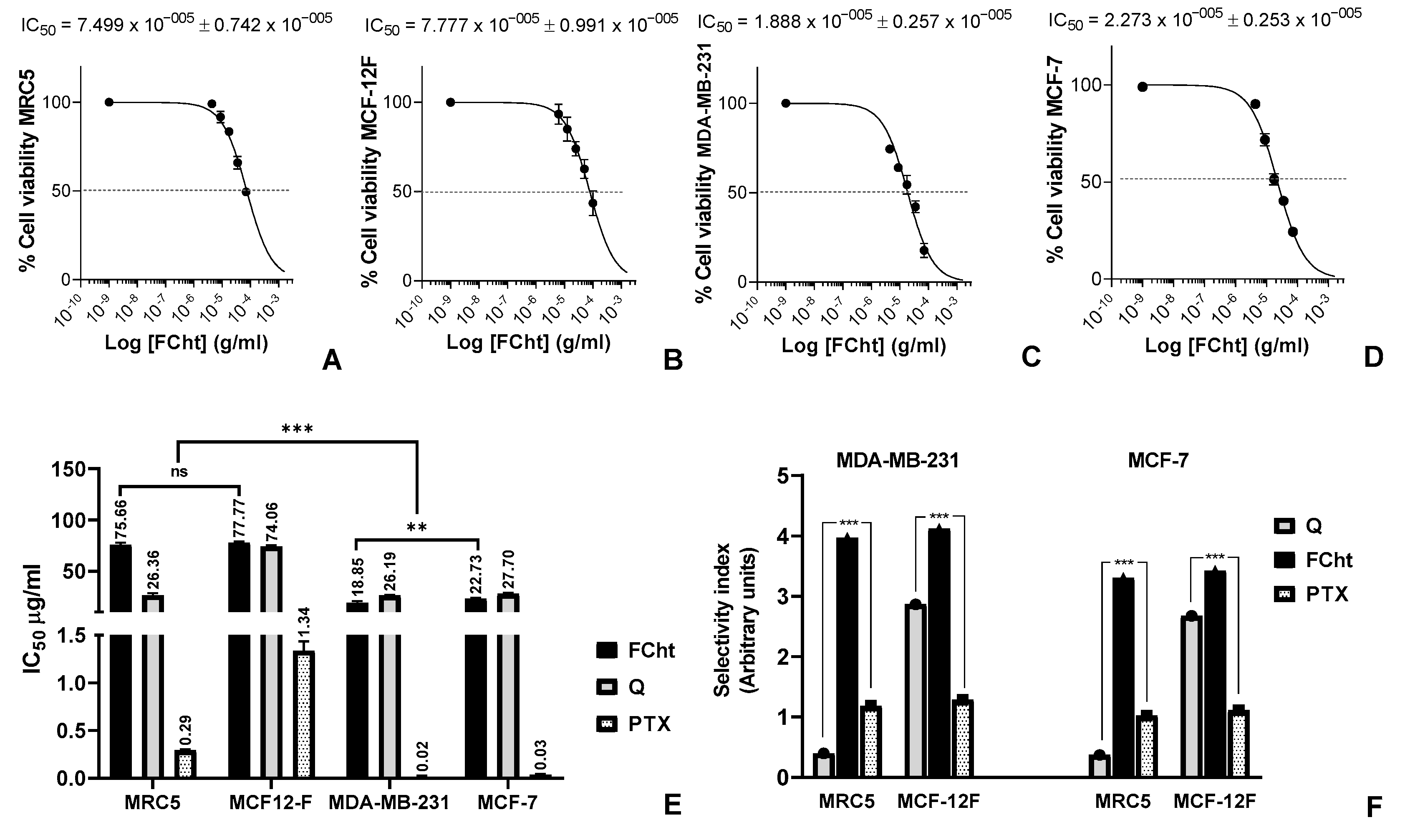
2.2. Cytotoxic Activity and Selectivity Index on BC Cells
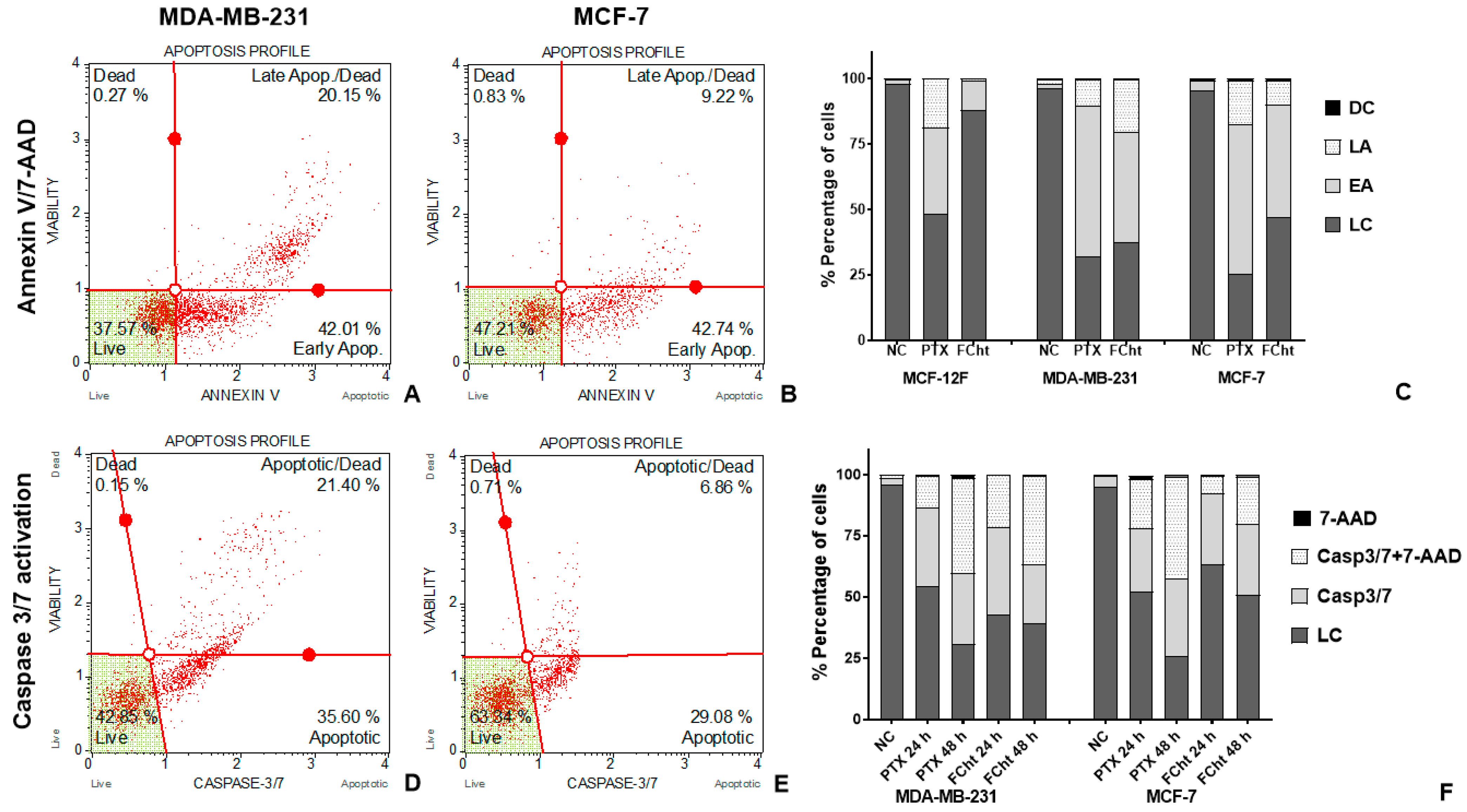
2.3. Apoptosis Induction
2.4. Morphological Analysis of Nuclei and Microtubules of MDA-MB-231
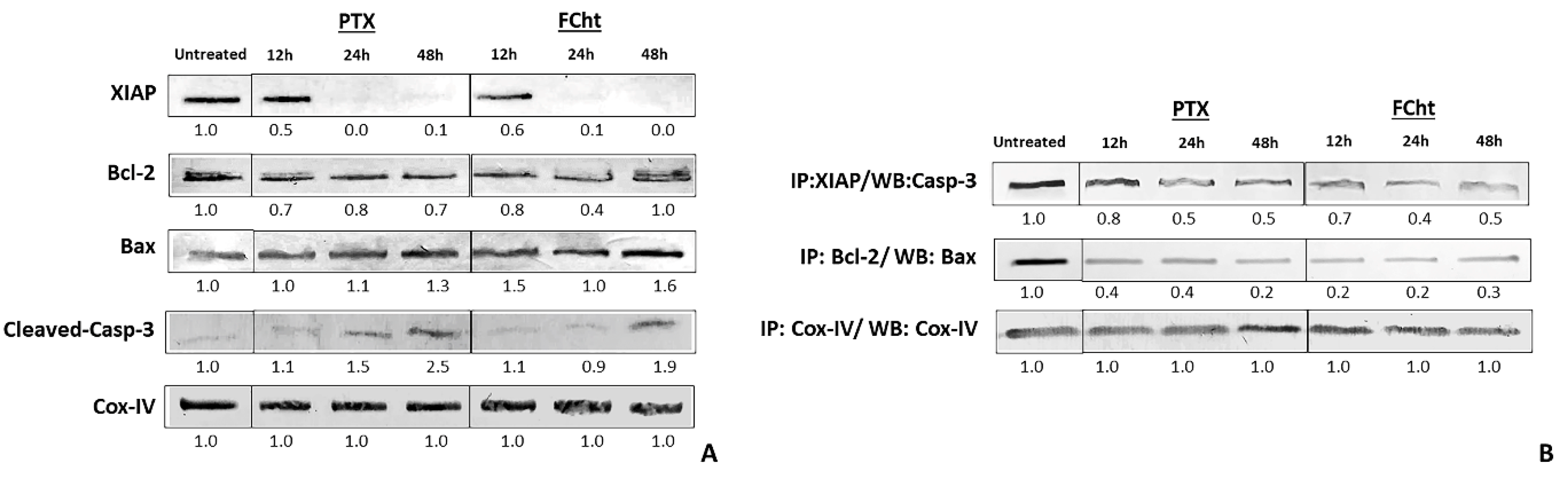
2.5. Anti- and Pro-Apoptotic Proteins Expression and Complexes Dissociation
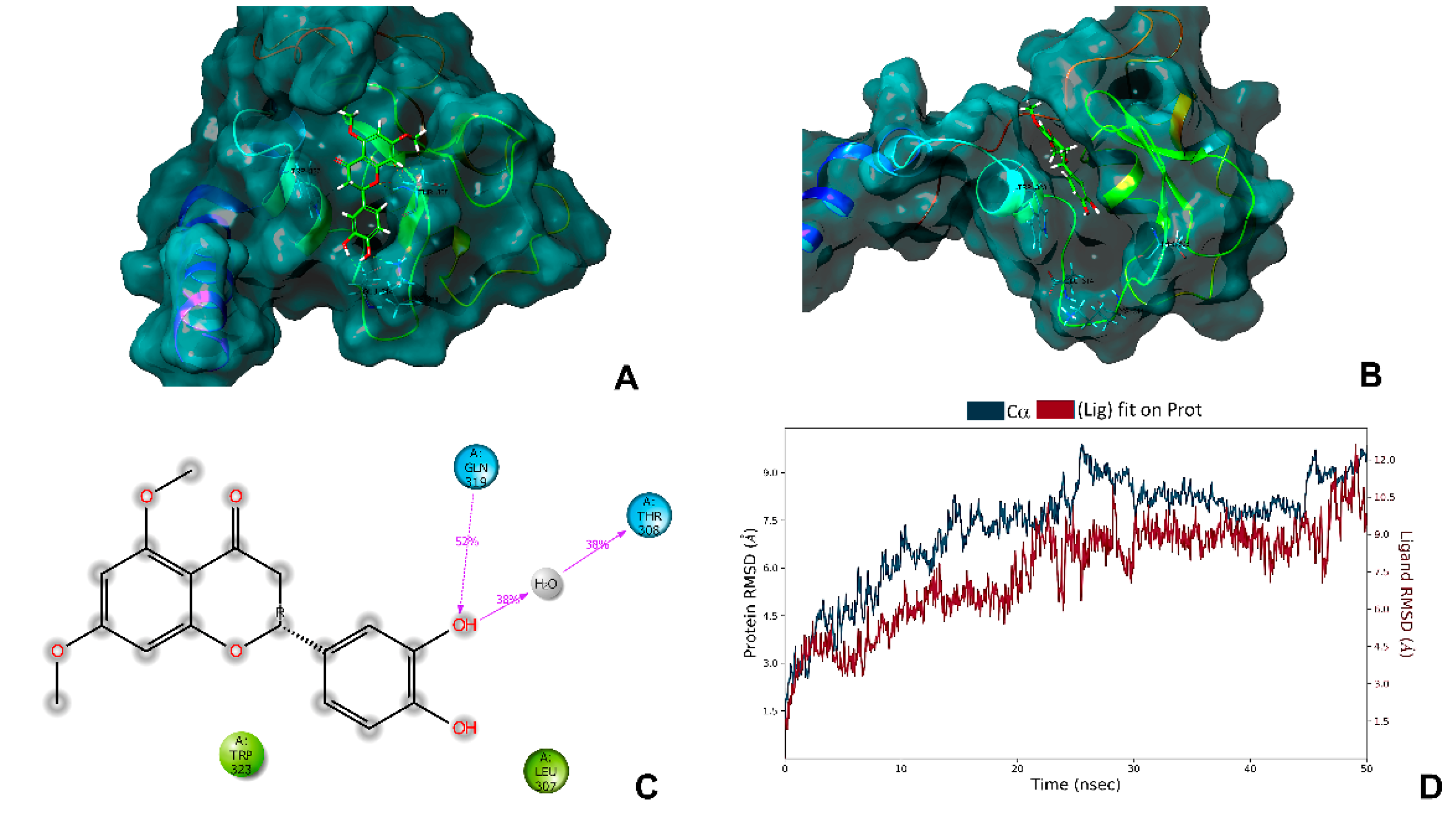
2.6. In Silico Analysis with the Antiapoptotic Protein XIAP
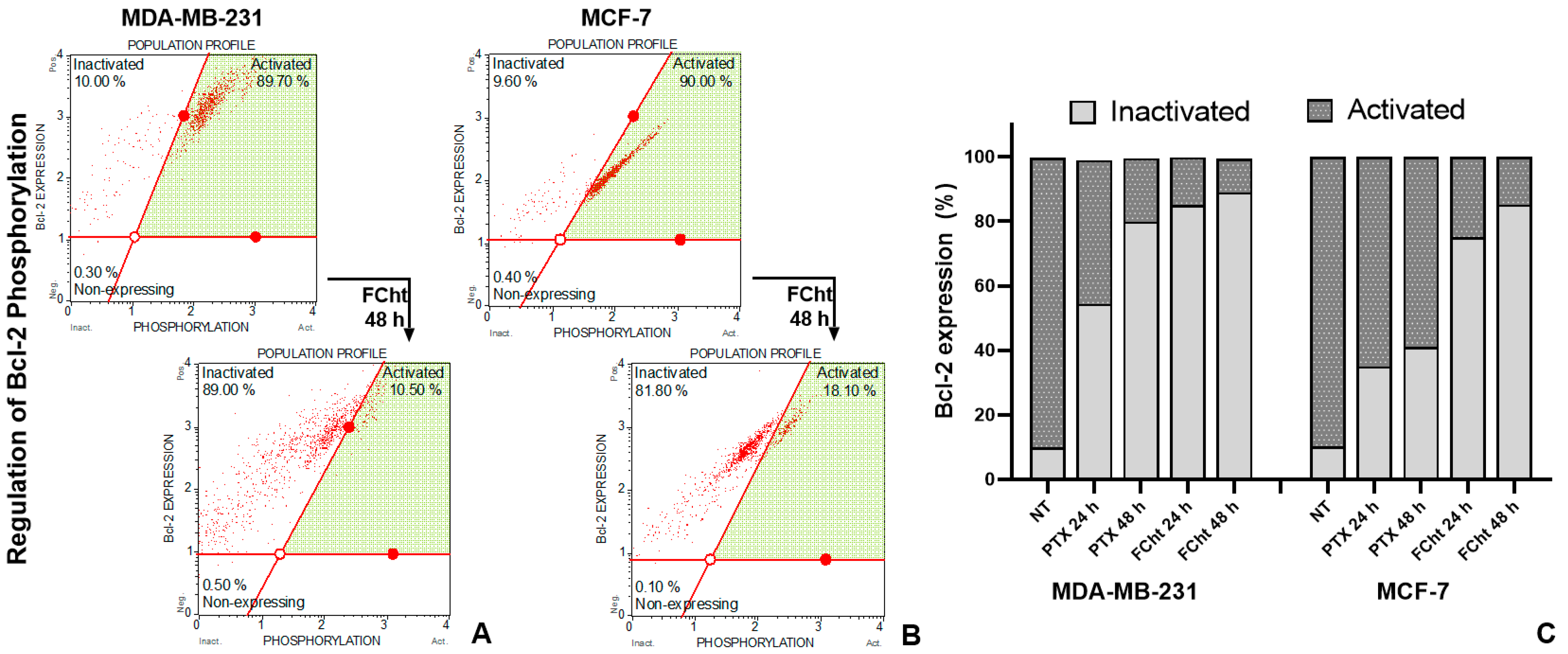
2.7. Bcl-2 Regulation
3. Discussion
4. Materials and Methods
4.1. Extraction and Isolation
4.2. Cell Lines and Culture
4.3. Cytotoxic Activity and Selectivity of Flavanone
4.4. Apoptosis Assay
4.5. Morphological Analysis in Nuclei and Microtubules by Epifluorescent Microscopy
4.6. Western Blot Analysis of Pro- and Anti-Apoptotic Proteins
4.7. Co-Immunoprecipitation of Anti and Pro-Apoptotic Proteins Complexes
4.8. Phospho-Bcl-2 Protein Detection
4.9. Geometry Optimization
4.10. Preparation of the XIAP BIR3 Domain
4.11. Molecular Docking
4.12. Molecular Dynamics
4.13. Statistical Analysis for Biological Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Chandrika, B.B.; Steephan, M.; Kumar, T.R.S.; Sabu, A.; Haridas, M. Hesperetin and Naringenin Sensitize HER2 Positive Cancer Cells to Death by Serving as HER2 Tyrosine Kinase Inhibitors. Life Sci. 2016, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, R.; Wang, C.; Zhou, S.; Fan, Y.; Ma, S.; Gao, D.; Gai, N.; Yang, J. Pinocembrin Inhibits the Proliferation and Metastasis of Breast Cancer via Suppression of the PI3K/AKT Signaling Pathway. Front. Oncol. 2021, 11, 2804. [Google Scholar] [CrossRef]
- Williams, M.M.; Cook, R.S. Bcl-2 Family Proteins in Breast Development and Cancer: Could Mcl-1 Targeting Overcome Therapeutic Resistance? Oncotarget 2015, 6, 3519. [Google Scholar] [CrossRef]
- Chong, S.J.F.; Iskandar, K.; Lai, J.X.H.; Qu, J.; Raman, D.; Valentin, R.; Herbaux, C.; Collins, M.; Low, I.C.C.; Loh, T.; et al. Serine-70 Phosphorylated Bcl-2 Prevents Oxidative Stress-Induced DNA Damage by Modulating the Mitochondrial Redox Metabolism. Nucleic Acids Res. 2020, 48, 12727. [Google Scholar] [CrossRef]
- Delbridge, A.; Strasser, A. The BCL-2 Protein Family, BH3-Mimetics and Cancer Therapy. Cell Death Differ. 2015, 22, 1071–1080. [Google Scholar] [CrossRef]
- Pluta, P.; Jeziorski, A.; Pluta, A.; Cebula-Obrzut, B.; Wierzbowska, A.; Piekarski, J.; Smolewski, P. Expression of IAP Family Proteins and Its Clinical Importance in Breast Cancer Patients. Neoplasma 2015, 62, 666–673. [Google Scholar] [CrossRef]
- Obexer, P.; Ausserlechner, M. X-Linked Inhibitor of Apoptosis Protein—A Critical Death Resistance Regulator and Therapeutic Target for Personalized Cancer Therapy. Front. Oncol. 2014, 4, 197. [Google Scholar] [CrossRef]
- Li, X.; Guo, S.; Xiong, X.K.; Peng, B.Y.; Huang, J.M.; Chen, M.F.; Wang, F.Y.; Wang, J.N. Combination of Quercetin and Cisplatin Enhances Apoptosis in OSCC Cells by Downregulating XIAP through the NF-ΚB Pathway. J. Cancer 2019, 10, 4509. [Google Scholar] [CrossRef]
- Taleb-Contini, S.H.; Schorr, K.; da Costa, F.B.; de Oliveira, D.C.R. Detection of Flavonoids in Glandular Trichomes of Chromolaena Species (Eupatorieae, Asteraceae) by Reversed-Phase High-Performance Liquid Chromatography. Rev. Bras. Ciênc. Farm. 2007, 43, 315–321. [Google Scholar] [CrossRef]
- Herrera-Calderon, O.; Arroyo-Acevedo, J.L.; Rojas-Armas, J.; Chumpitaz-Cerrate, V.; Figueroa-Salvador, L.; Enciso-Roca, E.; Tinco-Jayo, J.A. Phytochemical Screening, Total Phenolic Content, Antioxidant and Cytotoxic Activity of Chromolaena Laevigata on Human Tumor Cell Lines. Annu. Res. Rev. Biol. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Ojo, O.; Mphahlele, M.P.; Oladeji, O.S.; Mmutlane, E.M.; Ndinteh, D.T. From Wandering Weeds to Pharmacy: An Insight into Traditional Uses, Phytochemicals and Pharmacology of Genus Chromolaena (Asteraceae). J. Ethnopharmacol. 2022, 291, 115155. [Google Scholar] [CrossRef] [PubMed]
- Torrenegra-Guerrero, R.D.; Bautista-Bautista, L.; Rodríguez-Mayusa, J.; Méndez-Callejas, G.M. Nmr Spectroscopy and Antioxidant Activity of Flavanones and Flavones Isolated from Chromolaena tacotana (Klatt) r.m. King & h. Rob. Pharmacologyonline 2020, 3, 444–452. [Google Scholar]
- Rodriguez, J.; Gómez, L.M.; Gutierrez, A.C.; Mendez-Callejas, G.; Reyes, A.I.; Tellez, L.C.; Oscar, A.; Rodriguez, E.; Rubén, G.; Torrenegra, D. Chromolaena tacotana (Klatt) R. M. King and H. Rob. Source of Flavonoids with Antiproliferative and Antioxidant Activity. Indian J. Sci. Technol. 2018, 11, 1–7. [Google Scholar] [CrossRef]
- Mendez-Callejas, G.M.; Torrenegra-Guerrero, R.D.; Bayona, M.-A.; Rodríguez-Mayusa, J. Anti-Colon Cancer Potential of Two Flavone Isomers Isolated from Chromolaena tacotana (Klatt) R.M. King & H. Rob. Pharmacologyonline 2021, 2, 1033–1039. [Google Scholar]
- Nikolovska-Coleska, Z.; Xu, L.; Hu, Z.; Tomita, Y.; Li, P.; Roller, P.P.; Wang, R.; Fang, X.; Guo, R.; Zhang, M.; et al. Discovery of Embelin as a Cell-Permeable, Small-Molecular Weight Inhibitor of XIAP through Structure-Based Computational Screening of a Traditional Herbal Medicine Three-Dimensional Structure Database. J. Med. Chem. 2004, 47, 2430–2440. [Google Scholar] [CrossRef]
- Cong, H.; Xu, L.; Wu, Y.; Qu, Z.; Bian, T.; Zhang, W.; Xing, C.; Zhuang, C. Inhibitor of Apoptosis Protein (IAP) Antagonists in Anticancer Agent Discovery: Current Status and Perspectives. J. Med. Chem. 2019, 62, 5750–5772. [Google Scholar] [CrossRef]
- Jin, X.; Lee, K.; Kim, N.H.; Kim, H.S.; Yook, J.I.; Choi, J.; No, K.T. Natural Products Used as a Chemical Library for Protein-Protein Interaction Targeted Drug Discovery. J. Mol. Graph. Model. 2018, 79, 46–58. [Google Scholar] [CrossRef]
- Opo, F.A.D.M.; Rahman, M.M.; Ahammad, F.; Ahmed, I.; Bhuiyan, M.A.; Asiri, A.M. Structure Based Pharmacophore Modeling, Virtual Screening, Molecular Docking and ADMET Approaches for Identification of Natural Anti-Cancer Agents Targeting XIAP Protein. Sci. Rep. 2021, 11, 4049. [Google Scholar] [CrossRef]
- Xu, T.; Yang, M.; Li, Y.; Chen, X.; Wang, Q.; Deng, W.; Pang, X.; Yu, K.; Jiang, B.; Guan, S.; et al. An Integrated Exact Mass Spectrometric Strategy for Comprehensive and Rapid Characterization of Phenolic Compounds in Licorice. Rapid. Commun. Mass Spectrom. 2013, 27, 2297–2309. [Google Scholar] [CrossRef] [PubMed]
- Whitted, C.; Torrenegra, R.; Méndez, G.; Lejeune, T.; Rodríguez, J.; Tsui, H.; Rodríguez, O.; Street, S.; Miller, G.; Palau, V. Increased Cytotoxicity of 3,5 Dihydroxy -7- Methoxyflavone in MIA PaCa-2 and Panc28 Pancreatic Cancer Cells When Used in Conjunction with Proliferative Compound 3,5 Dihydroxy-7-Methoxyflavanone Both Derived from Chromolaena leivensis (Hieron). Pharmacologyonline 2016, 3, 80–89. [Google Scholar]
- Torrenegra, R.D.; Rodríguez, O.E. Chemical and Biological Activity of Leaf Extracts of Chromolaena leivensis. Nat. Prod. Commun. 2011, 6, 947–950. [Google Scholar] [PubMed]
- Abusoglu, G.; Ozturk, B. Effect of Static Magnetic Field with Quercetin and Hesperetin on MCF-7 and MDA MB-231 Breast Cancer Cells. Turk. J. Biochem. 2020, 45, 833–841. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Dong, T.; Shen, J.; Gao, X.; Zhou, J. Naringenin Has a Chemoprotective Effect in MDA-MB-231 Breast Cancer Cells via Inhibition of Caspase-3 and −9 Activities. Oncol. Lett. 2019, 17, 1217. [Google Scholar] [CrossRef]
- Sordon, S.; Popłoński, J.; Milczarek, M.; Stachowicz, M.; Tronina, T.; Kucharska, A.Z.; Wietrzyk, J.; Huszcza, E. Structure–Antioxidant–Antiproliferative Activity Relationships of Natural C7 and C7–C8 Hydroxylated Flavones and Flavanones. Antioxidants 2019, 8, 210. [Google Scholar] [CrossRef]
- Povea-Cabello, S.; Oropesa-Ávila, M.; de la Cruz-Ojeda, P.; Villanueva-Paz, M.; de La Mata, M.; Suárez-Rivero, J.M.; Álvarez-Córdoba, M.; Villalón-García, I.; Cotán, D.; Ybot-González, P.; et al. Dynamic Reorganization of the Cytoskeleton during Apoptosis: The Two Coffins Hypothesis. Int. J. Mol. Sci. 2017, 18, 2393. [Google Scholar] [CrossRef]
- Wordeman, L.; Vicente, J.J. Microtubule Targeting Agents in Disease: Classic Drugs, Novel Roles. Cancers 2021, 13, 5650. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2019, 11, 28. [Google Scholar] [CrossRef]
- Jänicke, R.U. MCF-7 Breast Carcinoma Cells Do Not Express Caspase-3. Breast Cancer Res. Treat. 2008, 117, 219–221. [Google Scholar] [CrossRef]
- Devi, G.R.; Finetti, P.; Morse, M.A.; Lee, S.; de Nonneville, A.; van Laere, S.; Troy, J.; Geradts, J.; McCall, S.; Bertucci, F. Expression of X-linked Inhibitor of Apoptosis Protein (Xiap) in Breast Cancer Is Associated with Shorter Survival and Resistance to Chemotherapy. Cancers 2021, 13, 2807. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Lee, S.G.; Yang, W.M.; Um, J.Y.; Sethi, G.; Mishra, S.; Shanmugam, M.K.; Ahn, K.S. The Application of Embelin for Cancer Prevention and Therapy. Molecules 2018, 23, 621. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, D.; Brucoli, M.; Zecchini, S.; Sandoval-Hernandez, A.; Arboleda, G.; Lopez-Vallejo, F.; Delgado, W.; Giovarelli, M.; Coazzoli, M.; Catalani, E.; et al. XIAP as a Target of New Small Organic Natural Molecules Inducing Human Cancer Cell Death. Cancers 2019, 11, 1336. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, P.P.; Deng, X.; May, W.S. Phosphorylation of Bcl2 and Regulation of Apoptosis. Leukemia 2001, 15, 515–522. [Google Scholar] [CrossRef]
- Delgado, C.; Mendez-Callejas, G.; Celis, C. Caryophyllene Oxide, the Active Compound Isolated from Leaves of Hymenaea courbaril L. (Fabaceae) with Antiproliferative and Apoptotic Effects on PC-3 Androgen-Independent Prostate Cancer Cell Line. Molecules 2021, 26, 6142. [Google Scholar] [CrossRef]
- Méndez-Callejas, G.M.; Leone, S.; Tanzarella, C.; Antoccia, A. Combretastatin A-4 Induces P53 Mitochondrial-Relocalisation Independent-Apoptosis in Non-Small Lung Cancer Cells. Cell Biol. Int. 2014, 38, 296–308. [Google Scholar] [CrossRef]
- Mills, N. ChemDraw Ultra 10.0 CambridgeSoft, 100 CambridgePark Drive, Cambridge, MA 02140. Www.Cambridgesoft.Com. Commercial Price: $1910 for Download, $2150 for CD-ROM.; Academic Price: $710 for Download, $800 for CD-ROM. J. Am. Chem. Soc. 2006, 128, 13649–13650. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeerschd, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785. [Google Scholar] [CrossRef]
- «AutoDock4» AutoDock v4.2.6 (Linux 64bit). Available online: https://autodock.scripps.edu/download-autodock4/ (accessed on 6 June 2022).
- «MGLTools» Mgltools_Linux-86_64_1.5.6. Available online: https://ccsb.scripps.edu/mgltools/downloads/ (accessed on 23 November 2022).
- «Desmond» D. E. Shaw Research: Desmond Registration. Available online: https://www.deshawresearch.com/downloads/download_desmond.cgi/ (accessed on 6 June 2022).
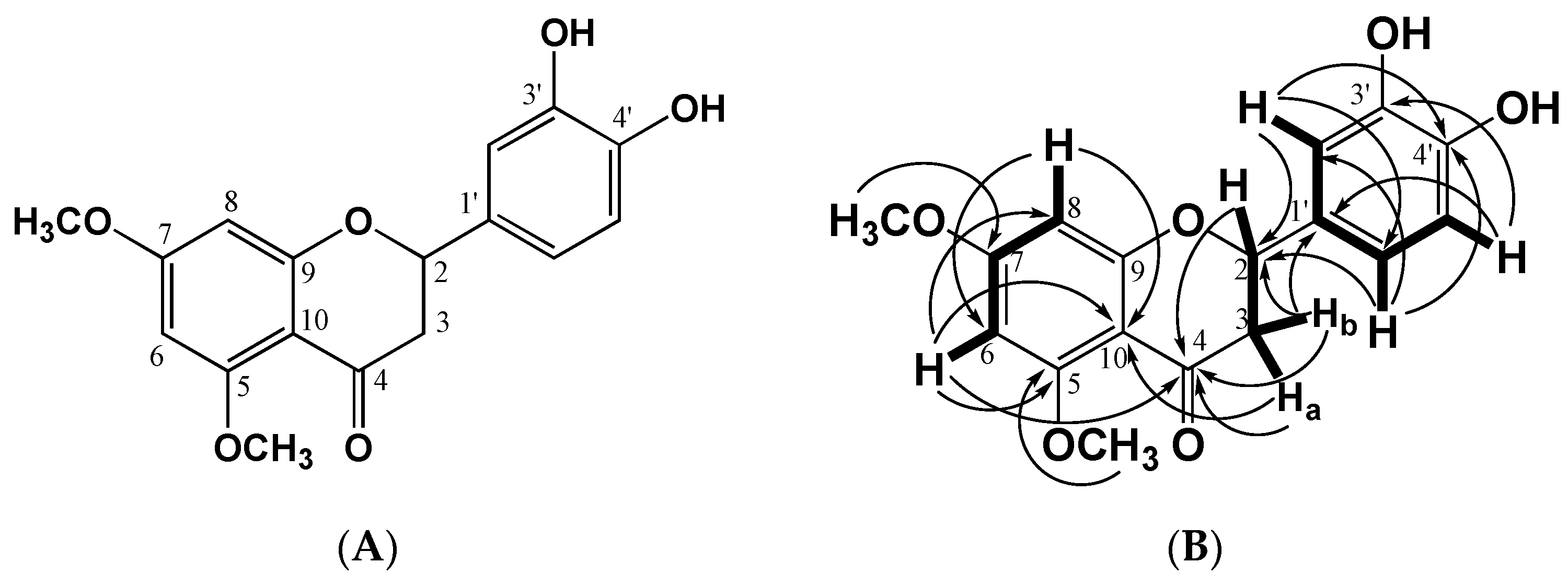

| Position | Compound Tacotanina | |
|---|---|---|
| δH (J in Hz) | δC (ppm) | |
| 2 | 5.35 (1H, dd, J = 12.7; 3.31) | 79.8 |
| 3a | 2.94 (1H, dd, J = 16.3; 12.7) | 46.2 |
| 3b | 2.59 (1H, dd, J = 16.3; 3.0) | |
| 4 | - | 188.3 |
| 5 | - | 163.2 |
| 5-OMe | 3.85 (3H, s) | 56.0 |
| 6 | 6.15 (1H, d, J = 2.3) | 94.3 |
| 7 | - | 166.6 |
| 7-OMe | 3.81 (3H, s) | 56.1 |
| 8 | 6.17 (1H, d, J = 2.3) | 93.4 |
| 9 | - | 165.2 |
| 10 | - | 106.6 |
| 1′ | - | 132.0 |
| 2′ | 7.02 (1H, brs) | 114.6 |
| 3′ | - | 145.9 |
| 4′ | - | 146.2 |
| 5′ | 6.86 (1H, brs) | 115.9 |
| 6′ | 6.86 (1H, d, J = 1.2) | 119.1 |
| Compound | UV nm | Supplementary Data |
|---|---|---|
| 3′,4′-dihydroxy-5,7-dimethoxyflavonone (Tacotanina) | In MeOH 284, 388; plus MeONa 284, 388, 419; plus AcONa 284, 388; plus, H3BO3 does not present changes, as with the other displacement reagents. | For 1H NMR, 13C NMR, COSY, HMBQC, HMBC, and HRESIMS data, see Supplementary Figure S1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendez-Callejas, G.; Torrenegra, R.; Muñoz, D.; Celis, C.; Roso, M.; Garzon, J.; Beltran, F.; Cardenas, A. A New Flavanone from Chromolaena tacotana (Klatt) R. M. King and H. Rob, Promotes Apoptosis in Human Breast Cancer Cells by Downregulating Antiapoptotic Proteins. Molecules 2023, 28, 58. https://doi.org/10.3390/molecules28010058
Mendez-Callejas G, Torrenegra R, Muñoz D, Celis C, Roso M, Garzon J, Beltran F, Cardenas A. A New Flavanone from Chromolaena tacotana (Klatt) R. M. King and H. Rob, Promotes Apoptosis in Human Breast Cancer Cells by Downregulating Antiapoptotic Proteins. Molecules. 2023; 28(1):58. https://doi.org/10.3390/molecules28010058
Chicago/Turabian StyleMendez-Callejas, Gina, Ruben Torrenegra, Diego Muñoz, Crispin Celis, Michael Roso, Jojhan Garzon, Ferney Beltran, and Andres Cardenas. 2023. "A New Flavanone from Chromolaena tacotana (Klatt) R. M. King and H. Rob, Promotes Apoptosis in Human Breast Cancer Cells by Downregulating Antiapoptotic Proteins" Molecules 28, no. 1: 58. https://doi.org/10.3390/molecules28010058
APA StyleMendez-Callejas, G., Torrenegra, R., Muñoz, D., Celis, C., Roso, M., Garzon, J., Beltran, F., & Cardenas, A. (2023). A New Flavanone from Chromolaena tacotana (Klatt) R. M. King and H. Rob, Promotes Apoptosis in Human Breast Cancer Cells by Downregulating Antiapoptotic Proteins. Molecules, 28(1), 58. https://doi.org/10.3390/molecules28010058






