Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives
Abstract
1. Introduction
2. Nanomaterial Characterization
3. Toxicity and Stability of Nanomaterials
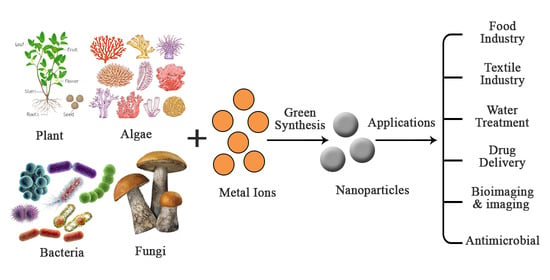
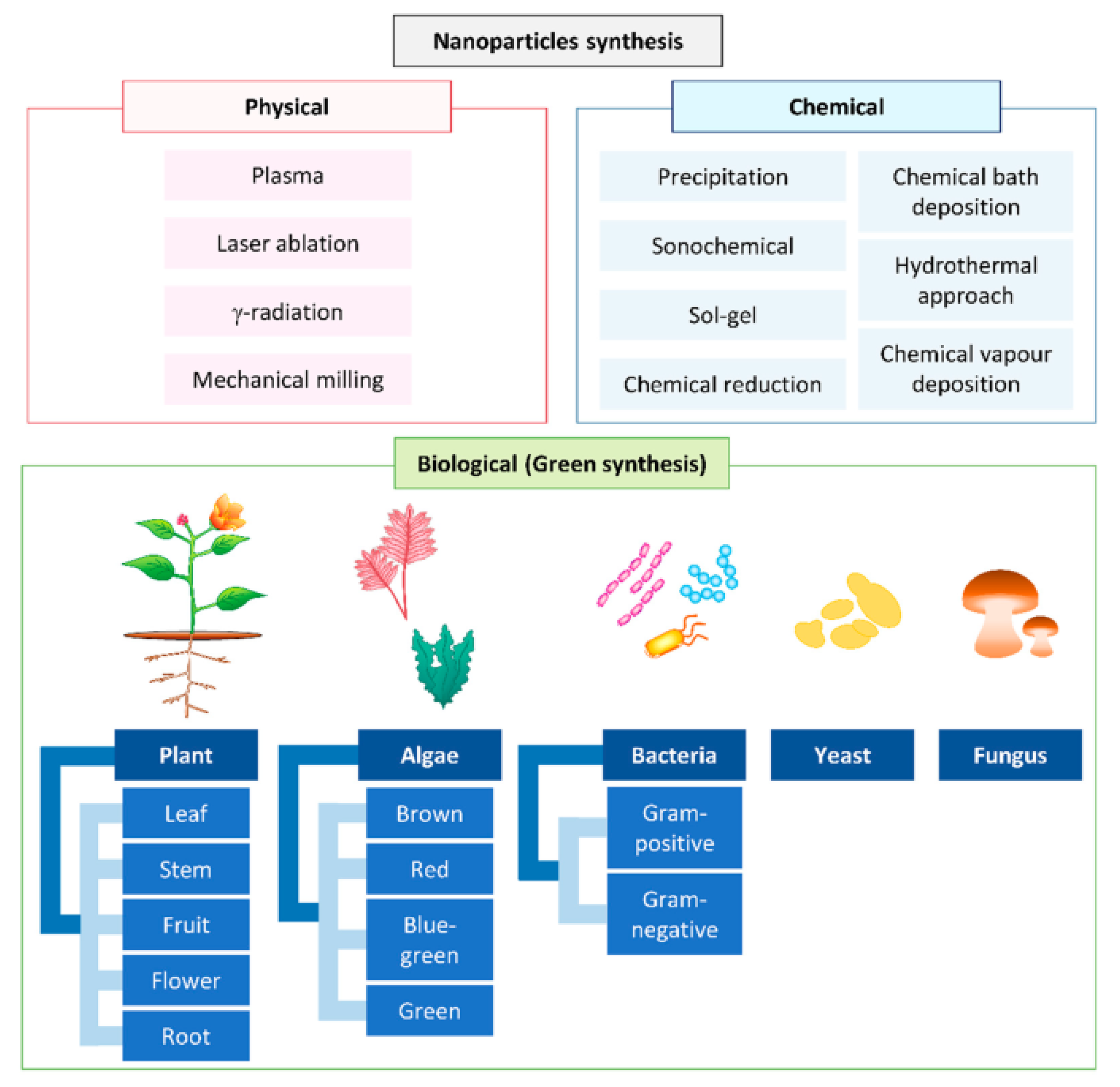
4. Green Synthesis of Nanomaterials
4.1. Actinomycetes
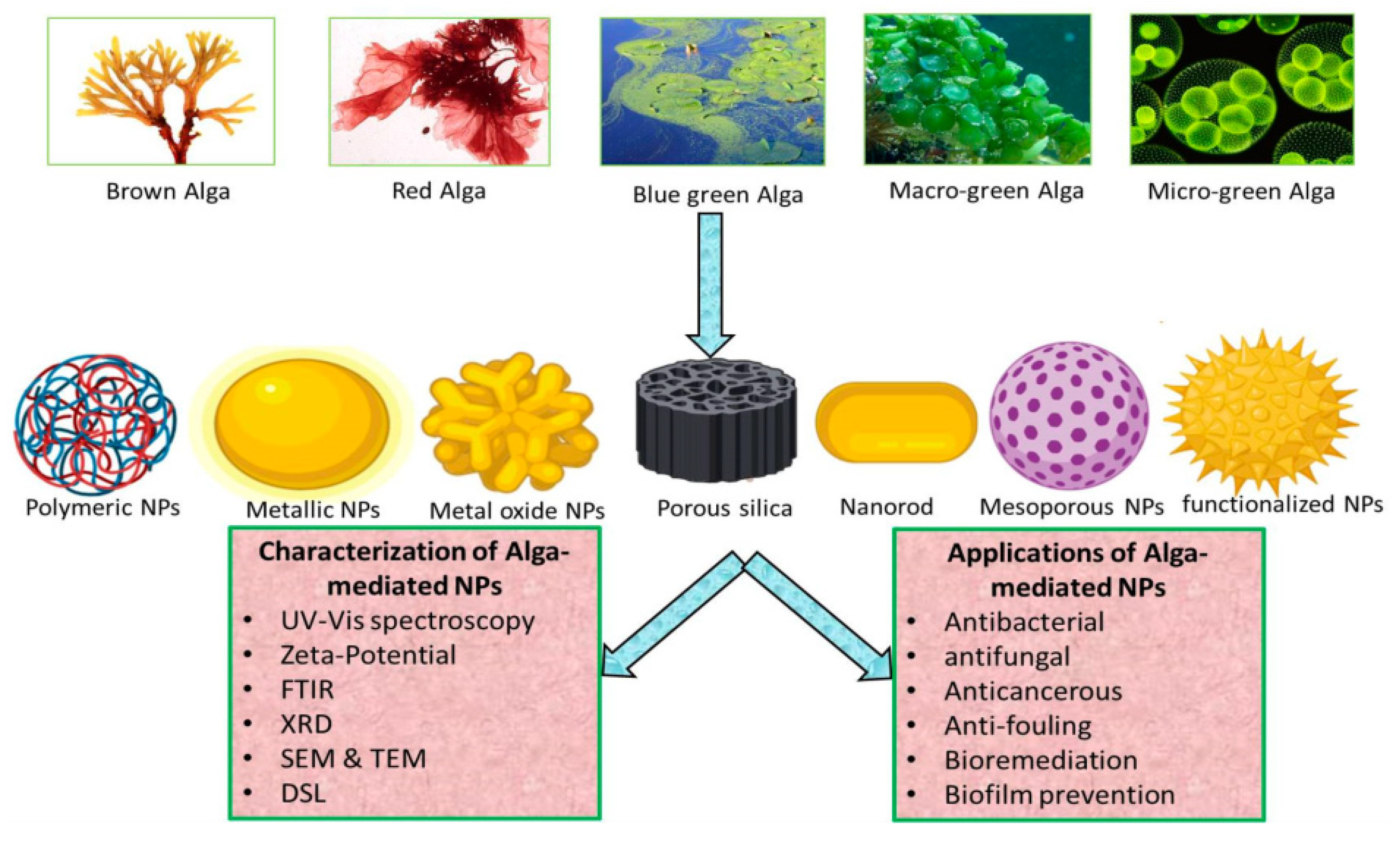
4.2. Algae
4.3. Plant-Mediated Synthesis
4.4. Viruses
4.5. Fungi
4.6. Yeast
4.7. Bacteria
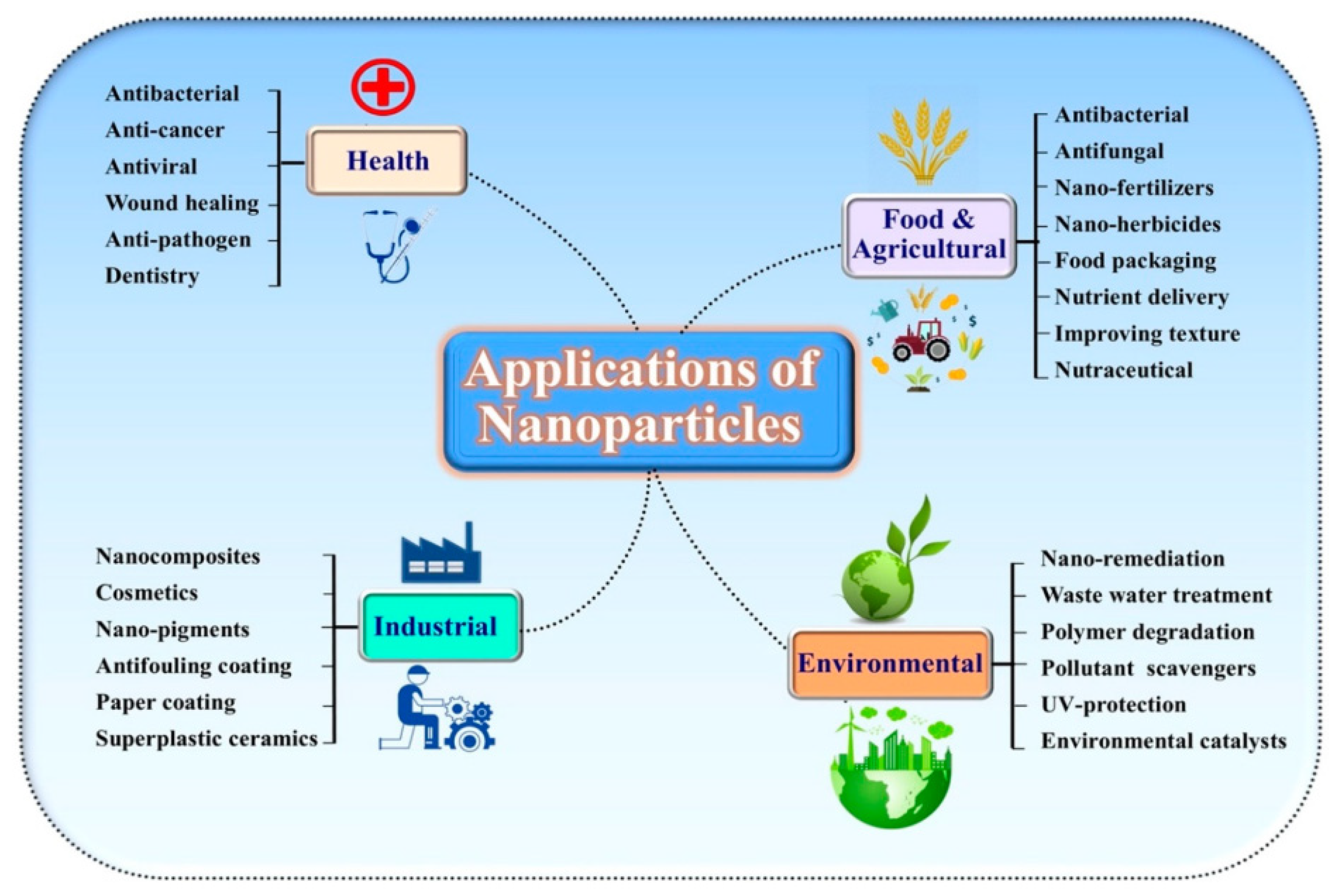
5. Different Applications of Nanomaterials
5.1. Food Industry
5.2. Water Treatment
5.3. Textile Industry
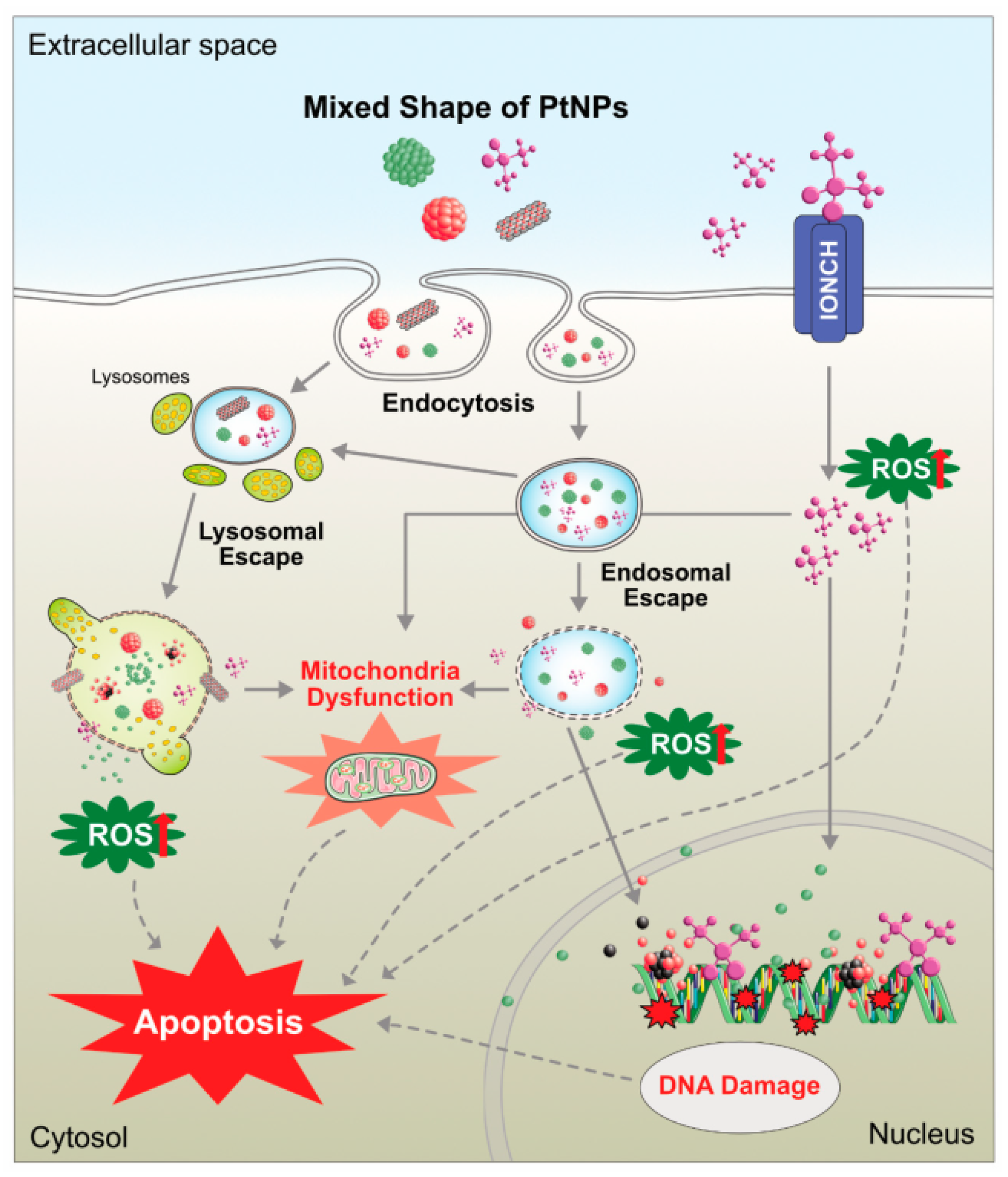
5.4. Mutagenicity, Autophagy, and Cytotoxicity
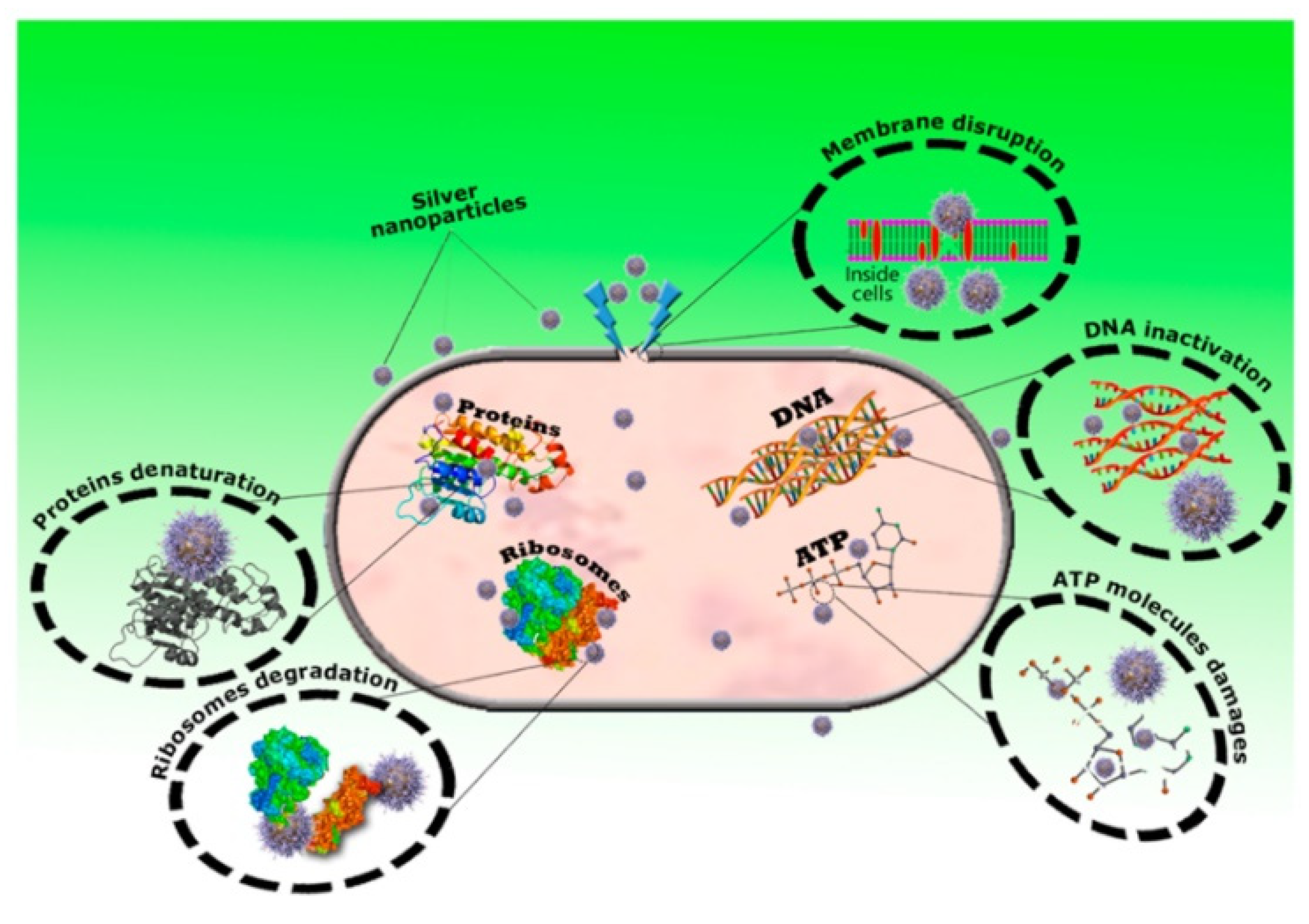
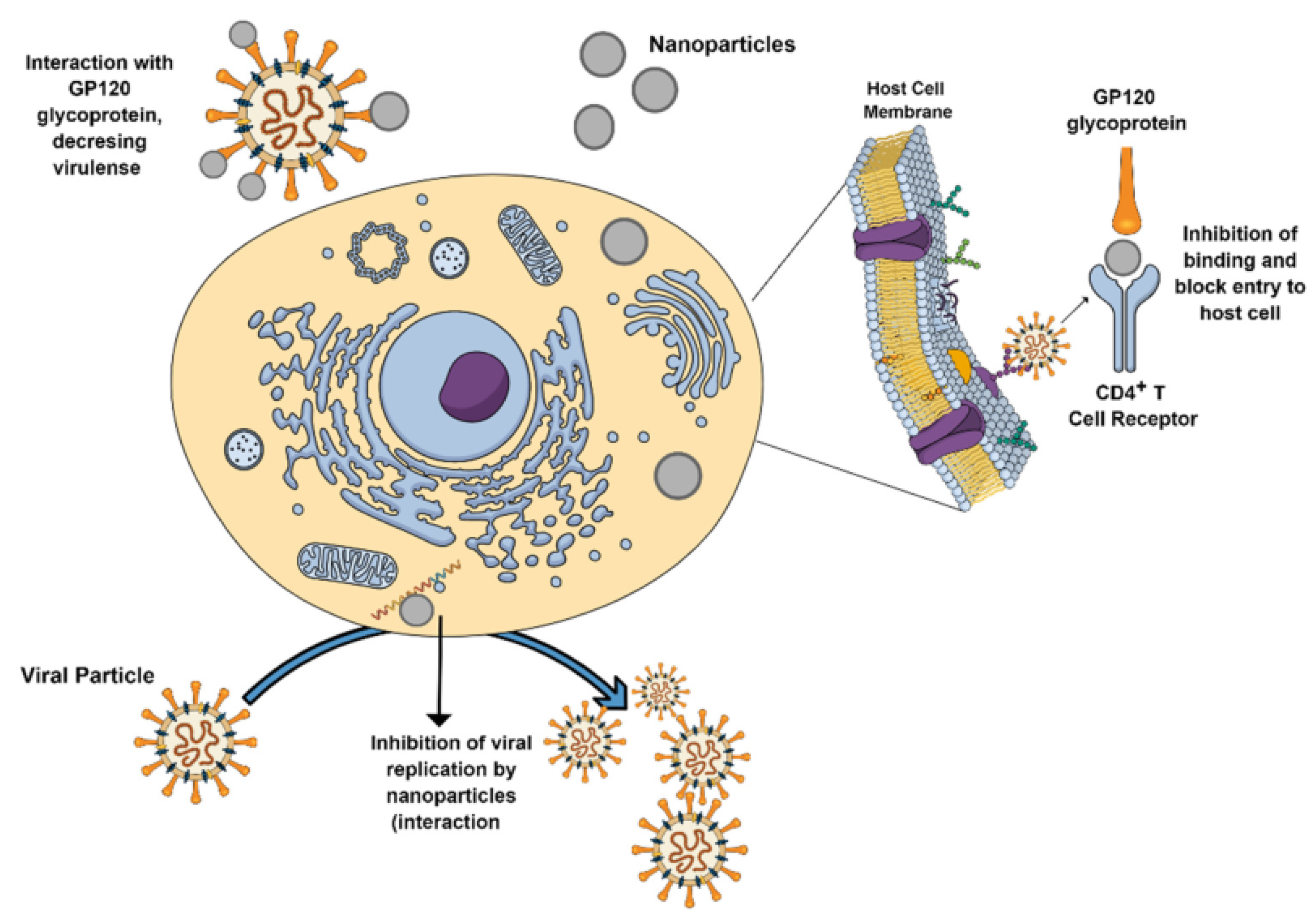
5.5. Antiviral and Antimicrobial Effect
5.6. Drug Delivery Agents
5.7. Bioimaging and Imaging Agents
6. Limitations of Green-Synthesized Nanoparticles
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Shinde, M.U.; Patwekar, M.; Patwekar, F.; Bajaber, M.A.; Medikeri, A.; Mohammad, F.S.; Mukim, M.; Soni, S.; Mallick, J.; Jawaid, T. Nanomaterials: A potential hope for life sciences from bench to bedside. J. Nanomater. 2022, 2022, 5968131. [Google Scholar] [CrossRef]
- Demir, E. A review on nanotoxicity and nanogenotoxicity of different shapes of nanomaterials. J. Appl. Toxicol. 2021, 41, 118–147. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green synthesis of metallic nanoparticles: Applications and limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Shafique, M.; Luo, X. Nanotechnology in transportation vehicles: An overview of its applications, environmental, health and safety concerns. Materials 2019, 12, 2493. [Google Scholar] [CrossRef]
- Pan, M.; Yin, Z.; Liu, K.; Du, X.; Liu, H.; Wang, S. Carbon-based nanomaterials in sensors for food safety. Nanomaterials 2019, 9, 1330. [Google Scholar] [CrossRef]
- Rawtani, D.; Tharmavaram, M.; Pandey, G.; Hussain, C.M. Functionalized nanomaterial for forensic sample analysis. TrAC Trends Anal. Chem. 2019, 120, 115661. [Google Scholar] [CrossRef]
- Mondal, P.; Anweshan, A.; Purkait, M.K. Green synthesis and environmental application of iron-based nanomaterials and nanocomposite: A review. Chemosphere 2020, 259, 127509. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostructure Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef]
- Alsaiari, N.S.; Katubi, K.M.M.; Alzahrani, F.M.; Siddeeg, S.M.; Tahoon, M.A. The application of nanomaterials for the electrochemical detection of antibiotics: A review. Micromachines 2021, 12, 308. [Google Scholar] [CrossRef]
- Feng, H.-p.; Tang, L.; Zeng, G.-m.; Zhou, Y.; Deng, Y.-C.; Ren, X.; Song, B.; Liang, C.; Wei, M.-y.; Yu, J.-f. Core-shell nanomaterials: Applications in energy storage and conversion. Adv. Colloid Interface Sci. 2019, 267, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Amari, A.; Mohammed Alzahrani, F.; Mohammedsaleh Katubi, K.; Salem Alsaiari, N.; Tahoon, M.A.; Ben Rebah, F. Clay-polymer nanocomposites: Preparations and utilization for pollutants removal. Materials 2021, 14, 1365. [Google Scholar] [CrossRef] [PubMed]
- Siddeeg, S.M.; Tahoon, M.A.; Alsaiari, N.S.; Shabbir, M.; Rebah, F.B. Application of functionalized nanomaterials as effective adsorbents for the removal of heavy metals from wastewater: A review. Curr. Anal. Chem. 2021, 17, 4–22. [Google Scholar] [CrossRef]
- Siddeeg, S.M.; Alsaiari, N.S.; Tahoon, M.A.; Rebah, F.B. The application of nanomaterials as electrode modifiers for the electrochemical detection of ascorbic acid. Int. J. Electrochem. Sci 2020, 15, 3327–3346. [Google Scholar] [CrossRef]
- Andreo, J.; Ettlinger, R.; Zaremba, O.; Peña, Q.; Lächelt, U.; de Luis, R.F.; Freund, R.; Canossa, S.; Ploetz, E.; Zhu, W. Reticular Nanoscience: Bottom-Up Assembly Nanotechnology. J. Am. Chem. Soc. 2022, 144, 7531–7550. [Google Scholar] [CrossRef]
- Sikiru, S.; Ayodele, O.A.; Sanusi, Y.; Adebukola, S.Y.; Soleimani, H.; Yekeen, N.; Haslija, A.A. A comprehensive review on nanotechnology application in wastewater treatment a case study of metal-based using green synthesis. J. Environ. Chem. Eng. 2022, 10, 108065. [Google Scholar] [CrossRef]
- Letchumanan, D.; Sok, S.P.; Ibrahim, S.; Nagoor, N.H.; Arshad, N.M. Plant-based biosynthesis of copper/copper oxide nanoparticles: An update on their applications in biomedicine, mechanisms, and toxicity. Biomolecules 2021, 11, 564. [Google Scholar] [CrossRef]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green synthesis of nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnology 2018, 16, 1–24. [Google Scholar] [CrossRef]
- Kabir, E.; Kumar, V.; Kim, K.-H.; Yip, A.C.; Sohn, J. Environmental impacts of nanomaterials. J. Environ. Manag. 2018, 225, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, C.; Pinsino, A. Nanomaterial Ecotoxicology in the Terrestrial and Aquatic Environment: A Systematic Review. Toxics 2022, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- El Shafey, A.M. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Huang, Y.; Parsons, J.G.; Zhao, L.; Lopez-Moreno, L.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Plant-based green synthesis of metallic nanoparticles: Scientific curiosity or a realistic alternative to chemical synthesis? Nanotechnol. Environ. Eng. 2016, 1, 1–29. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Mechouet, M.; Wijesekera, K.; Filipe, C.D.; Sicard, C.; Bazylinski, D.A.; Jeffryes, C. Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology–a review. Green Chem. 2017, 19, 552–587. [Google Scholar] [CrossRef]
- Ahmed, H.E.; Iqbal, Y.; Aziz, M.H.; Atif, M.; Batool, Z.; Hanif, A.; Yaqub, N.; Farooq, W.; Ahmad, S.; Fatehmulla, A. Green synthesis of CeO2 Nanoparticles from the Abelmoschus esculentus extract: Evaluation of antioxidant, anticancer, antibacterial, and wound-healing activities. Molecules 2021, 26, 4659. [Google Scholar] [CrossRef]
- Suárez-Cerda, J.; Espinoza-Gómez, H.; Alonso-Núñez, G.; Rivero, I.A.; Gochi-Ponce, Y.; Flores-López, L.Z. A green synthesis of copper nanoparticles using native cyclodextrins as stabilizing agents. J. Saudi Chem. Soc. 2017, 21, 341–348. [Google Scholar] [CrossRef]
- Gonçalves, J.P.Z.; Seraglio, J.; Macuvele, D.L.P.; Padoin, N.; Soares, C.; Riella, H.G. Green synthesis of manganese based nanoparticles mediated by Eucalyptus robusta and Corymbia citriodora for agricultural applications. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128180. [Google Scholar] [CrossRef]
- Soto-Robles, C.; Luque, P.; Gómez-Gutiérrez, C.; Nava, O.; Vilchis-Nestor, A.; Lugo-Medina, E.; Ranjithkumar, R.; Castro-Beltrán, A. Study on the effect of the concentration of Hibiscus sabdariffa extract on the green synthesis of ZnO nanoparticles. Results Phys. 2019, 15, 102807. [Google Scholar] [CrossRef]
- Veisi, H.; Farokhi, M.; Hamelian, M.; Hemmati, S. Green synthesis of Au nanoparticles using an aqueous extract of Stachys lavandulifolia and their catalytic performance for alkyne/aldehyde/amine A 3 coupling reactions. RSC Adv. 2018, 8, 38186–38195. [Google Scholar] [CrossRef]
- Mohseni, M.S.; Khalilzadeh, M.A.; Mohseni, M.; Hargalani, F.Z.; Getso, M.I.; Raissi, V.; Raiesi, O. Green synthesis of Ag nanoparticles from pomegranate seeds extract and synthesis of Ag-Starch nanocomposite and characterization of mechanical properties of the films. Biocatal. Agric. Biotechnol. 2020, 25, 101569. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Roy, A.; Wahab, M.; Ahmed, M.; Othman-Qadir, G.; Elesawy, B.H.; Khandaker, M.U.; Islam, M.N.; Emran, T.B. Applications of nanomaterials in agrifood and pharmaceutical industry. J. Nanomater. 2021, 2021, 1472096. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Sawicki, K.; Czajka, M.; Matysiak-Kucharek, M.; Fal, B.; Drop, B.; Męczyńska-Wielgosz, S.; Sikorska, K.; Kruszewski, M.; Kapka-Skrzypczak, L. Toxicity of metallic nanoparticles in the central nervous system. Nanotechnol. Rev. 2019, 8, 175–200. [Google Scholar] [CrossRef]
- Praphawatvet, T.; Peters, J.I.; Williams, R.O., III. Inhaled nanoparticles–An updated review. Int. J. Pharm. 2020, 587, 119671. [Google Scholar] [CrossRef]
- Paunovic, J.; Vucevic, D.; Radosavljevic, T.; Mandić-Rajčević, S.; Pantic, I. Iron-based nanoparticles and their potential toxicity: Focus on oxidative stress and apoptosis. Chemico-Biol. Interact. 2020, 316, 108935. [Google Scholar] [CrossRef]
- Hoet, P.H.; Brüske-Hohlfeld, I.; Salata, O.V. Nanoparticles–known and unknown health risks. J. Nanobiotechnology 2004, 2, 1–15. [Google Scholar] [CrossRef]
- Muller, J.; Huaux, F.; Fonseca, A.; Nagy, J.B.; Moreau, N.; Delos, M.; Raymundo-Piñero, E.; Béguin, F.; Kirsch-Volders, M.; Fenoglio, I. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: Toxicological aspects. Chem. Res. Toxicol. 2008, 21, 1698–1705. [Google Scholar] [CrossRef]
- Soto, K.; Carrasco, A.; Powell, T.; Garza, K.; Murr, L. Comparative in vitro cytotoxicity assessment of some manufacturednanoparticulate materials characterized by transmissionelectron microscopy. J. Nanoparticle Res. 2005, 7, 145–169. [Google Scholar] [CrossRef]
- He, D.; Garg, S.; Wang, Z.; Li, L.; Rong, H.; Ma, X.; Li, G.; An, T.; Waite, T.D. Silver sulfide nanoparticles in aqueous environments: Formation, transformation and toxicity. Environ. Sci. Nano 2019, 6, 1674–1687. [Google Scholar] [CrossRef]
- Leonard, K.; Ahmmad, B.; Okamura, H.; Kurawaki, J. In situ green synthesis of biocompatible ginseng capped gold nanoparticles with remarkable stability. Colloids Surf. B Biointerfaces 2011, 82, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhao, J.; Liu, X.; Yu, X.; Jiang, Z.; Bu, Y.; Xu, Z.; Wang, Z.; Zhu, X.; Xing, B. Transformation and species identification of CuO nanoparticles in plant cells (Nicotiana tabacum). Environ. Sci. Nano 2019, 6, 2724–2735. [Google Scholar] [CrossRef]
- Tay, C.Y.; Setyawati, M.I.; Xie, J.; Parak, W.J.; Leong, D.T. Back to basics: Exploiting the innate physico-chemical characteristics of nanomaterials for biomedical applications. Adv. Funct. Mater. 2014, 24, 5936–5955. [Google Scholar] [CrossRef]
- Singh, R.P.; Sharma, K.; Mausam, K. Dispersion and stability of metal oxide nanoparticles in aqueous suspension: A review. Mater. Today: Proc. 2020, 26, 2021–2025. [Google Scholar] [CrossRef]
- Abdelghany, T.; Al-Rajhi, A.M.; Al Abboud, M.A.; Alawlaqi, M.; Ganash Magdah, A.; Helmy, E.A.; Mabrouk, A.S. Recent advances in green synthesis of silver nanoparticles and their applications: About future directions. A review. BioNanoScience 2018, 8, 5–16. [Google Scholar] [CrossRef]
- Albeladi, S.S.R.; Malik, M.A.; Al-thabaiti, S.A. Facile biofabrication of silver nanoparticles using Salvia officinalis leaf extract and its catalytic activity towards Congo red dye degradation. J. Mater. Res. Technol. 2020, 9, 10031–10044. [Google Scholar] [CrossRef]
- Wang, J.; Koo, Y.; Alexander, A.; Yang, Y.; Westerhof, S.; Zhang, Q.; Schnoor, J.L.; Colvin, V.L.; Braam, J.; Alvarez, P.J. Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag+ at sublethal concentrations. Environ. Sci. Technol. 2013, 47, 5442–5449. [Google Scholar] [CrossRef]
- Jin, J.-C.; Xu, Z.-Q.; Dong, P.; Lai, L.; Lan, J.-Y.; Jiang, F.-L.; Liu, Y. One-step synthesis of silver nanoparticles using carbon dots as reducing and stabilizing agents and their antibacterial mechanisms. Carbon 2015, 94, 129–141. [Google Scholar] [CrossRef]
- Arif, M.; Liu, G.; Yousaf, B.; Ahmed, R.; Irshad, S.; Ashraf, A.; Zia-ur-Rehman, M.; Rashid, M.S. Synthesis, characteristics and mechanistic insight into the clays and clay minerals-biochar surface interactions for contaminants removal-A review. J. Clean. Prod. 2021, 310, 127548. [Google Scholar] [CrossRef]
- Chen, T.-L.; Kim, H.; Pan, S.-Y.; Tseng, P.-C.; Lin, Y.-P.; Chiang, P.-C. Implementation of green chemistry principles in circular economy system towards sustainable development goals: Challenges and perspectives. Sci. Total Environ. 2020, 716, 136998. [Google Scholar] [CrossRef]
- Tehri, N.; Vashishth, A.; Gahlaut, A.; Hooda, V. Biosynthesis, antimicrobial spectra and applications of silver nanoparticles: Current progress and future prospects. Inorg. Nano-Metal Chem. 2022, 52, 1–19. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahmad, R.; Zeyaullah, M.; Khare, S.K. Microbial nano-factories: Synthesis and biomedical applications. Front. Chem. 2021, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Tandon, M.; Kaur, A. Role of metallic nanoparticles in water remediation with special emphasis on sustainable synthesis: A review. Nanotechnol. Environ. Eng. 2020, 5, 1–13. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Abdelmeguid, N.E.; Al-Zaban, M.I.; Baz, L.; Bin-Meferij, M.M. Lichens—A potential source for nanoparticles fabrication: A review on nanoparticles biosynthesis and their prospective applications. J. Fungi 2021, 7, 291. [Google Scholar] [CrossRef]
- Khan, M.K.; Pandey, A.; Hamurcu, M.; Gezgin, S.; Athar, T.; Rajput, V.D.; Gupta, O.P.; Minkina, T. Insight into the prospects for nanotechnology in wheat biofortification. Biology 2021, 10, 1123. [Google Scholar] [CrossRef]
- Hassan, S.E.-D.; Fouda, A.; Radwan, A.A.; Salem, S.S.; Barghoth, M.G.; Awad, M.A.; Abdo, A.M.; El-Gamal, M.S. Endophytic actinomycetes Streptomyces spp mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. JBIC J. Biol. Inorg. Chem. 2019, 24, 377–393. [Google Scholar] [CrossRef]
- Hassan, S.E.-D.; Salem, S.S.; Fouda, A.; Awad, M.A.; El-Gamal, M.S.; Abdo, A.M. New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J. Radiat. Res. Appl. Sci. 2018, 11, 262–270. [Google Scholar] [CrossRef]
- Golinska, P.; Wypij, M.; Ingle, A.P.; Gupta, I.; Dahm, H.; Rai, M. Biogenic synthesis of metal nanoparticles from actinomycetes: Biomedical applications and cytotoxicity. Appl. Microbiol. Biotechnol. 2014, 98, 8083–8097. [Google Scholar] [CrossRef]
- Rajivgandhi, G.; Gnanamangai, B.M.; Prabha, T.H.; Poornima, S.; Maruthupandy, M.; Alharbi, N.S.; Kadaikunnan, S.; Li, W.-J. Biosynthesized zinc oxide nanoparticles (ZnO NPs) using actinomycetes enhance the anti-bacterial efficacy against K. Pneumoniae. J. King Saud Univ. -Sci. 2022, 34, 101731. [Google Scholar] [CrossRef]
- Shivaji, S.W.; Arvind, M.D.; Zygmunt, S. Biosynthesis, optimization, purification and characterization of gold nanoparticles. Afr. J. Microbiol. Res. 2014, 8, 138–146. [Google Scholar] [CrossRef]
- Balagurunathan, R.; Radhakrishnan, M.; Rajendran, R.B.; Velmurugan, D. Biosynthesis of Gold Nanoparticles by Actinomycete Streptomyces Viridogens strain HM10. 2011. Available online: http://nopr.niscpr.res.in/handle/123456789/12941 (accessed on 23 December 2022).
- Manivasagan, P.; Venkatesan, J.; Senthilkumar, K.; Sivakumar, K.; Kim, S.-K. Biosynthesis, antimicrobial and cytotoxic effect of silver nanoparticles using a novel Nocardiopsis sp. MBRC-1. BioMed Res. Int. 2013, 2013, 287638. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, Thermomonospora sp. Langmuir 2003, 19, 3550–3553. [Google Scholar] [CrossRef]
- Manimaran, M.; Kannabiran, K. Actinomycetes-mediated biogenic synthesis of metal and metal oxide nanoparticles: Progress and challenges. Lett. Appl. Microbiol. 2017, 64, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.-K. Actinobacteria mediated synthesis of nanoparticles and their biological properties: A review. Crit. Rev. Microbiol. 2016, 42, 209–221. [Google Scholar] [CrossRef]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green synthesis of metallic nanoparticles via biological entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Zhao, H.; Maruthupandy, M.; Al-mekhlafi, F.A.; Chackaravarthi, G.; Ramachandran, G.; Chelliah, C.K. Biological synthesis of copper oxide nanoparticles using marine endophytic actinomycetes and evaluation of biofilm producing bacteria and A549 lung cancer cells. J. King Saud Univ. -Sci. 2022, 34, 101866. [Google Scholar] [CrossRef]
- Hachicha, R.; Elleuch, F.; Ben Hlima, H.; Dubessay, P.; de Baynast, H.; Delattre, C.; Pierre, G.; Hachicha, R.; Abdelkafi, S.; Michaud, P. Biomolecules from microalgae and cyanobacteria: Applications and market survey. Appl. Sci. 2022, 12, 1924. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Nawaz, K.; Khan, A.K.; Hano, C.; Abbasi, B.H.; Anjum, S. An overview of the algae-mediated biosynthesis of nanoparticles and their biomedical applications. Biomolecules 2020, 10, 1498. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Kumar, P.S.; Jaikumar, V.; Vardhan, K.H.; SundarRajan, P. Microalgae for biofuel production and removal of heavy metals: A review. Environ. Chem. Lett. 2020, 18, 1905–1923. [Google Scholar] [CrossRef]
- Caliskan, G.; Mutaf, T.; Agba, H.C.; Elibol, M. Green Synthesis and Characterization of Titanium Nanoparticles Using Microalga, Phaeodactylum tricornutum. Geomicrobiol. J. 2022, 39, 83–96. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles–An eco-friendly approach. Resour. -Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Patel, V.; Berthold, D.; Puranik, P.; Gantar, M. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol. Rep. 2015, 5, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Aldayel, M.F.; Al Kuwayti, M.A.; El Semary, N.A. Investigating the production of antimicrobial nanoparticles by Chlorella vulgaris and the link to its loss of viability. Microorganisms 2022, 10, 145. [Google Scholar] [CrossRef]
- Al-Enazi, N.M.; Alwakeel, S.; Alhomaidi, E. Photocatalytic and biological activities of green synthesized SnO2 nanoparticles using Chlorella vulgaris. J. Appl. Microbiol. 2022, 133, 3265–3275. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.A.; Sundararaju, S.; Ramaraj, R.; Maniam, G.P.; Govindan, N. Synthesis of silver nanoparticles using marine macroalgae Padina sp. and its antibacterial activity towards pathogenic bacteria. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 1–15. [Google Scholar] [CrossRef]
- Nasaruddin, R.R.; Chen, T.; Yao, Q.; Zang, S.; Xie, J. Toward greener synthesis of gold nanomaterials: From biological to biomimetic synthesis. Coord. Chem. Rev. 2021, 426, 213540. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Sarkar, T.; Choudhury, B.K.; Barman, A.; Bhattacharjya, D.; Srivastava, A.; Baishya, D.; Edinur, H.A. Green synthesis of gold nanoparticles using plant extracts as beneficial prospect for cancer theranostics. Molecules 2021, 26, 6389. [Google Scholar] [CrossRef]
- Govindasamy, R.; Govindarasu, M.; Alharthi, S.S.; Mani, P.; Bernaurdshaw, N.; Gomathi, T.; Ansari, M.A.; Alomary, M.N.; Atwah, B.; Malik, M.S. Sustainable green synthesis of yttrium oxide (Y2O3) nanoparticles using Lantana camara leaf extracts: Physicochemical characterization, photocatalytic degradation, antibacterial, and anticancer potency. Nanomaterials 2022, 12, 2393. [Google Scholar] [CrossRef]
- Lomelí-Rosales, D.A.; Zamudio-Ojeda, A.; Reyes-Maldonado, O.K.; López-Reyes, M.E.; Basulto-Padilla, G.C.; Lopez-Naranjo, E.J.; Zuñiga-Mayo, V.M.; Velázquez-Juárez, G. Green Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Capsicum chinense Plant. Molecules 2022, 27, 1692. [Google Scholar] [CrossRef]
- Hamedi, S.; Shojaosadati, S.A. Rapid and green synthesis of silver nanoparticles using Diospyros lotus extract: Evaluation of their biological and catalytic activities. Polyhedron 2019, 171, 172–180. [Google Scholar] [CrossRef]
- Hawar, S.N.; Al-Shmgani, H.S.; Al-Kubaisi, Z.A.; Sulaiman, G.M.; Dewir, Y.H.; Rikisahedew, J.J. Green synthesis of silver nanoparticles from Alhagi graecorum leaf extract and evaluation of their cytotoxicity and antifungal activity. J. Nanomater. 2022, 2022. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Al-Otibi, F.O. Facile green synthesis of silver nanoparticles using aqueous leaf extract of Origanum majorana with potential bioactivity against multidrug resistant bacterial strains. Crystals 2022, 12, 603. [Google Scholar] [CrossRef]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Jain, N.; Jain, P.; Rajput, D.; Patil, U.K. Green synthesized plant-based silver nanoparticles: Therapeutic prospective for anticancer and antiviral activity. Micro Nano Syst. Lett. 2021, 9, 1–24. [Google Scholar] [CrossRef]
- Mao, C.; Flynn, C.E.; Hayhurst, A.; Sweeney, R.; Qi, J.; Georgiou, G.; Iverson, B.; Belcher, A.M. Viral assembly of oriented quantum dot nanowires. Proc. Natl. Acad. Sci. USA 2003, 100, 6946–6951. [Google Scholar] [CrossRef]
- Lee, S.-W.; Mao, C.; Flynn, C.E.; Belcher, A.M. Ordering of quantum dots using genetically engineered viruses. Science 2002, 296, 892–895. [Google Scholar] [CrossRef]
- Makarov, V.; Love, A.; Sinitsyna, O.; Makarova, S.; Yaminsky, I.; Taliansky, M.; Kalinina, N. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Naturae (англoязычная версия) 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tomita, S.; Sawada, K.; Shiba, K.; Yanagi, H.; Yamashita, I.; Uraoka, Y. Chiral meta-molecules consisting of gold nanoparticles and genetically engineered tobacco mosaic virus. Opt. Express 2012, 20, 24856–24863. [Google Scholar] [CrossRef]
- Górzny, M.Ł.; Walton, A.S.; Evans, S.D. Synthesis of high-surface-area platinum nanotubes using a viral template. Adv. Funct. Mater. 2010, 20, 1295–1300. [Google Scholar] [CrossRef]
- Love, A.J.; Makarov, V.; Yaminsky, I.; Kalinina, N.O.; Taliansky, M.E. The use of tobacco mosaic virus and cowpea mosaic virus for the production of novel metal nanomaterials. Virology 2014, 449, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, R.M.; Ganapathy, R.; Ramasamy, P.; Krishnan, K. Fabrication of virus metal hybrid nanomaterials: An ideal reference for bio semiconductor. Arab. J. Chem. 2020, 13, 2750–2765. [Google Scholar] [CrossRef]
- Merzlyak, A.; Lee, S.-W. Phage as templates for hybrid materials and mediators for nanomaterial synthesis. Curr. Opin. Chem. Biol. 2006, 10, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Shenton, W.; Douglas, T.; Young, M.; Stubbs, G.; Mann, S. Inorganic–organic nanotube composites from template mineralization of tobacco mosaic virus. Adv. Mater. 1999, 11, 253–256. [Google Scholar] [CrossRef]
- Gade, A.; Ingle, A.; Whiteley, C.; Rai, M. Mycogenic metal nanoparticles: Progress and applications. Biotechnol. Lett. 2010, 32, 593–600. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Priyadarshini, S.S.; Cousins, B.G.; Pradhan, N. Metal-Fungus interaction: Review on cellular processes underlying heavy metal detoxification and synthesis of metal nanoparticles. Chemosphere 2021, 274, 129976. [Google Scholar] [CrossRef]
- Jain, A.S.; Pawar, P.S.; Sarkar, A.; Junnuthula, V.; Dyawanapelly, S. Bionanofactories for green synthesis of silver nanoparticles: Toward antimicrobial applications. Int. J. Mol. Sci. 2021, 22, 11993. [Google Scholar] [CrossRef]
- Santos, T.S.; Silva, T.M.; Cardoso, J.C.; Albuquerque-Júnior, R.L.d.; Zielinska, A.; Souto, E.B.; Severino, P.; Mendonça, M.d.C. Biosynthesis of silver nanoparticles mediated by entomopathogenic fungi: Antimicrobial resistance, nanopesticides, and toxicity. Antibiotics 2021, 10, 852. [Google Scholar] [CrossRef]
- Pandiarajan, J. Diverse Manifolds of Biogenic Nanoparticles in Synthesis, Characterization, and Applications. In Nanotechnology Applications in Health and Environmental Sciences; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–28. [Google Scholar]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Extra-/intracellular biosynthesis of gold nanoparticles by an alkalotolerant fungus, Trichothecium sp. J. Biomed. Nanotechnol. 2005, 1, 47–53. [Google Scholar] [CrossRef]
- Kumar, A.; Mandal, S.; Selvakannan, P.; Pasricha, R.; Mandale, A.; Sastry, M. Investigation into the interaction between surface-bound alkylamines and gold nanoparticles. Langmuir 2003, 19, 6277–6282. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Green approach for nanoparticle biosynthesis by fungi: Current trends and applications. Crit. Rev. Biotechnol. 2012, 32, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Rautaray, D.; Bharde, A.; Ahire, K.; Sanyal, A.; Ahmad, A.; Sastry, M. Fungus-mediated biosynthesis of silica and titania particles. J. Mater. Chem. 2005, 15, 2583–2589. [Google Scholar] [CrossRef]
- Mosallam, F.M.; El-Sayyad, G.S.; Fathy, R.M.; El-Batal, A.I. Biomolecules-mediated synthesis of selenium nanoparticles using Aspergillus oryzae fermented Lupin extract and gamma radiation for hindering the growth of some multidrug-resistant bacteria and pathogenic fungi. Microb. Pathog. 2018, 122, 108–116. [Google Scholar] [CrossRef]
- Kadam, V.V.; Ettiyappan, J.P.; Balakrishnan, R.M. Mechanistic insight into the endophytic fungus mediated synthesis of protein capped ZnO nanoparticles. Mater. Sci. Eng. B 2019, 243, 214–221. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mahanty, S.; Das, P.; Chaudhuri, P.; Das, S. Biofabrication of iron oxide nanoparticles using manglicolous fungus Aspergillus niger BSC-1 and removal of Cr (VI) from aqueous solution. Chem. Eng. J. 2020, 385, 123790. [Google Scholar] [CrossRef]
- Shi, C.; Qi, H.; Ma, R.; Sun, Z.; Xiao, L.; Wei, G.; Huang, Z.; Liu, S.; Li, J.; Dong, M. N, S-self-doped carbon quantum dots from fungus fibers for sensing tetracyclines and for bioimaging cancer cells. Mater. Sci. Eng. C 2019, 105, 110132. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Gupta, R.K. Nanotechnology and potential of microorganisms. Crit. Rev. Biotechnol. 2005, 25, 199–204. [Google Scholar] [CrossRef]
- Rasouli, M. Biosynthesis of Selenium Nanoparticles using yeast Nematospora coryli and examination of their anti-candida and anti-oxidant activities. IET Nanobiotechnology 2019, 13, 214–218. [Google Scholar] [CrossRef]
- Wu, Z.; Ren, Y.; Liang, Y.; Huang, L.; Yang, Y.; Zafar, A.; Hasan, M.; Yang, F.; Shu, X. Synthesis, characterization, immune regulation, and antioxidative assessment of yeast-derived selenium nanoparticles in cyclophosphamide-induced rats. ACS Omega 2021, 6, 24585–24594. [Google Scholar] [CrossRef]
- Kowshik, M.; Deshmukh, N.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K. Microbial synthesis of semiconductor CdS nanoparticles, their characterization, and their use in the fabrication of an ideal diode. Biotechnol. Bioeng. 2002, 78, 583–588. [Google Scholar] [CrossRef]
- Kowshik, M.; Ashtaputre, S.; Kharrazi, S.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 2002, 14, 95. [Google Scholar] [CrossRef]
- Pimprikar, P.; Joshi, S.; Kumar, A.; Zinjarde, S.; Kulkarni, S. Influence of biomass and gold salt concentration on nanoparticle synthesis by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Colloids Surf. B Biointerfaces 2009, 74, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; He, W.; Cui, J.; Zhang, X.; Zhou, W.; Yan, S.; Sun, X.; Han, X.; Han, S.; Yue, Y. Mesoporous zirconium phosphate from yeast biotemplate. J. Colloid Interface Sci. 2010, 343, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Kowshik, M.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Microbial synthesis of semiconductor PbS nanocrystallites. Adv. Mater. 2002, 14, 815–818. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Nvkv Prasad, T.; Subba Rao Kambala, V.; Naidu, R. A critical review on biogenic silver nanoparticles and their antimicrobial activity. Curr. Nanosci. 2011, 7, 531–544. [Google Scholar] [CrossRef]
- Sunkar, S.; Nachiyar, C.V. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pac. J. Trop. Biomed. 2012, 2, 953–959. [Google Scholar] [CrossRef]
- Iravani, S. Bacteria in nanoparticle synthesis: Current status and future prospects. Int. Sch. Res. Not. 2014, 2014, 359316. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanoparticle Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Baco-Carles, V.; Datas, L.; Tailhades, P. Copper nanoparticles prepared from oxalic precursors. Int. Sch. Res. Not. 2011, 2011, 729594. [Google Scholar] [CrossRef]
- Parikh, R.Y.; Singh, S.; Prasad, B.; Patole, M.S.; Sastry, M.; Shouche, Y.S. Extracellular synthesis of crystalline silver nanoparticles and molecular evidence of silver resistance from Morganella sp.: Towards understanding biochemical synthesis mechanism. ChemBioChem 2008, 9, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Tarafdar, J.C. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Otari, S.; Patil, R.; Nadaf, N.; Ghosh, S.; Pawar, S. Green biosynthesis of silver nanoparticles from an actinobacteria Rhodococcus sp. Mater. Lett. 2012, 72, 92–94. [Google Scholar] [CrossRef]
- Tripathi, R.; Bhadwal, A.S.; Gupta, R.K.; Singh, P.; Shrivastav, A.; Shrivastav, B. ZnO nanoflowers: Novel biogenic synthesis and enhanced photocatalytic activity. J. Photochem. Photobiol. B Biol. 2014, 141, 288–295. [Google Scholar] [CrossRef]
- Mehta, S.; Kumar, S.; Chaudhary, S.; Bhasin, K. Effect of cationic surfactant head groups on synthesis, growth and agglomeration behavior of ZnS nanoparticles. Nanoscale Res. Lett. 2009, 4, 1197–1208. [Google Scholar] [CrossRef]
- Kundu, D.; Hazra, C.; Chatterjee, A.; Chaudhari, A.; Mishra, S. Extracellular biosynthesis of zinc oxide nanoparticles using Rhodococcus pyridinivorans NT2: Multifunctional textile finishing, biosafety evaluation and in vitro drug delivery in colon carcinoma. J. Photochem. Photobiol. B Biol. 2014, 140, 194–204. [Google Scholar] [CrossRef]
- Xiao, X.; Wu, Z.-C.; Chou, K.-C. A multi-label classifier for predicting the subcellular localization of gram-negative bacterial proteins with both single and multiple sites. PloS ONE 2011, 6, e20592. [Google Scholar] [CrossRef]
- Schlüter, M.; Hentzel, T.; Suarez, C.; Koch, M.; Lorenz, W.G.; Böhm, L.; Düring, R.-A.; Koinig, K.A.; Bunge, M. Synthesis of novel palladium (0) nanocatalysts by microorganisms from heavy-metal-influenced high-alpine sites for dehalogenation of polychlorinated dioxins. Chemosphere 2014, 117, 462–470. [Google Scholar] [CrossRef]
- Lloyd, J.R.; Yong, P.; Macaskie, L.E. Enzymatic recovery of elemental palladium by using sulfate-reducing bacteria. Appl. Environ. Microbiol. 1998, 64, 4607–4609. [Google Scholar] [CrossRef]
- Sintubin, L.; De Windt, W.; Dick, J.; Mast, J.; Van Der Ha, D.; Verstraete, W.; Boon, N. Lactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl. Microbiol. Biotechnol. 2009, 84, 741–749. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Babu, R.S.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B Biointerfaces 2008, 65, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Pugazhenthiran, N.; Anandan, S.; Kathiravan, G.; Udaya Prakash, N.K.; Crawford, S.; Ashokkumar, M. Microbial synthesis of silver nanoparticles by Bacillus sp. J. Nanoparticle Res. 2009, 11, 1811–1815. [Google Scholar] [CrossRef]
- Das, V.L.; Thomas, R.; Varghese, R.T.; Soniya, E.; Mathew, J.; Radhakrishnan, E. Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. 3 Biotech 2014, 4, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.P.; Kang, M.-j.; Niyonizigiye, I.; Singh, A.; Kim, J.-O.; Seo, Y.B.; Kim, G.-D. Extracellular synthesis of gold nanoparticles using the marine bacterium Paracoccus haeundaensis BC74171T and evaluation of their antioxidant activity and antiproliferative effect on normal and cancer cell lines. Colloids Surf. B Biointerfaces 2019, 183, 110455. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Rokhbakhsh-Zamin, F.; Shakibaie, M.; Moshafi, M.H.; Ameri, A.; Rahimi, H.R.; Forootanfar, H. Cytotoxic and antibacterial activities of biologically synthesized gold nanoparticles assisted by Micrococcus yunnanensis strain J2. Biocatal. Agric. Biotechnol. 2018, 15, 245–253. [Google Scholar] [CrossRef]
- Vairavel, M.; Devaraj, E.; Shanmugam, R. An eco-friendly synthesis of Enterococcus sp.–mediated gold nanoparticle induces cytotoxicity in human colorectal cancer cells. Environ. Sci. Pollut. Res. 2020, 27, 8166–8175. [Google Scholar] [CrossRef]
- Sneha, K.; Sathishkumar, M.; Mao, J.; Kwak, I.; Yun, Y.-S. Corynebacterium glutamicum-mediated crystallization of silver ions through sorption and reduction processes. Chem. Eng. J. 2010, 162, 989–996. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, J.; Chen, P.; Yu, X.; Yang, P. Studies on biosorption of Au3+ by Bacillus megaterium. Wei Sheng Wu Xue Bao = Acta Microbiol. Sin. 2000, 40, 425–429. [Google Scholar]
- Zhong-Yu, L.; Fu, J.; Jin-Kun, F.; Jian-Ming, W.; Yue-Ying, L.; Hu, C. Preliminary study on the mechanism of nonenzymatic bioreduction of precious metal ions. Acta Phys.-Chim. Sin 2001, 17, 477–480. [Google Scholar] [CrossRef]
- Jin-Zhou, F.; Yue-Ying, L.; Ping-Ying, G.; Ding-Liang, S.; Zhong-Yu, L.; Bing-Xin, Y.; Sheng-Zhou, W. Spectroscopic characterization on the biosorption and bioreduction of Ag (I) by Lactobacillus sp. A09. Acta Phys. Chim. Sin. 2000, 16, 779–782. [Google Scholar] [CrossRef]
- Ali, M.A.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Islam Masum, M.M.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogens. Nanomaterials 2020, 10, 1146. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rodriguez, P.E.; Meshulam, D.; Lesmes, U. Characterization of Pickering O/W emulsions stabilized by silica nanoparticles and their responsiveness to in vitro digestion conditions. Food Biophys. 2014, 9, 406–415. [Google Scholar] [CrossRef]
- Bredeck, G.; Kämpfer, A.A.; Sofranko, A.; Wahle, T.; Lison, D.; Ambroise, J.; Stahlmecke, B.; Albrecht, C.; Schins, R.P. Effects of dietary exposure to the engineered nanomaterials CeO2, SiO2, Ag, and TiO2 on the murine gut microbiome. Nanotoxicology 2021, 15, 934–950. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Xu, Y.; Ye, Q. Effect of nano-SiO2-LDPE packaging on biochemical, sensory, and microbiological quality of Pacific white shrimp Penaeus vannamei during chilled storage. Fish. Sci. 2015, 81, 983–993. [Google Scholar] [CrossRef]
- Rahimi Kalateh Shah Mohammad, G.; Seyedi, S.M.R.; Karimi, E.; Homayouni-Tabrizi, M. The cytotoxic properties of zinc oxide nanoparticles on the rat liver and spleen, and its anticancer impacts on human liver cancer cell lines. J. Biochem. Mol. Toxicol. 2019, 33, e22324. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Saravanan, K.; Manogar, P.; Johnson, J.; Vinoth, E.; Mayakannan, M. Green synthesis and characterization of biocompatible zinc oxide nanoparticles and evaluation of its antibacterial potential. Sens. Bio-Sens. Res. 2021, 31, 100399. [Google Scholar] [CrossRef]
- Channa, I.A.; Ashfaq, J.; Gilani, S.J.; Shah, A.A.; Chandio, A.D.; Jumah, M.N.b. UV Blocking and Oxygen Barrier Coatings Based on Polyvinyl Alcohol and Zinc Oxide Nanoparticles for Packaging Applications. Coatings 2022, 12, 897. [Google Scholar] [CrossRef]
- Zorraquín-Peña, I.; Cueva, C.; Bartolomé, B.; Moreno-Arribas, M.V. Silver nanoparticles against foodborne bacteria. Effects at intestinal level and health limitations. Microorganisms 2020, 8, 132. [Google Scholar] [CrossRef]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food packaging based on polymer nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- Damm, C.; Münstedt, H.; Rösch, A. Long-term antimicrobial polyamide 6/silver-nanocomposites. J. Mater. Sci. 2007, 42, 6067–6073. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Zhou, S.; Feng, H.; Liu, Y.; Jia, G. Review of health safety aspects of titanium dioxide nanoparticles in food application. NanoImpact 2020, 18, 100224. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Alsaiari, N.S.; Osman, H.; Amari, A.; Tahoon, M.A. The Synthesis of Metal–Organic-Framework-Based Ternary Nanocomposite for the Adsorption of Organic Dyes from Aqueous Solutions. Magnetochemistry 2022, 8, 133. [Google Scholar] [CrossRef]
- Alzahrani, F.M.; Amari, A.; Katubi, K.M.; Alsaiari, N.S.; Tahoon, M.A. The synthesis of nanocellulose-based nanocomposites for the effective removal of hexavalent chromium ions from aqueous solution. Open Chem. 2022, 20, 970–983. [Google Scholar] [CrossRef]
- Tahoon, M.A.; Siddeeg, S.M.; Salem Alsaiari, N.; Mnif, W.; Ben Rebah, F. Effective heavy metals removal from water using nanomaterials: A review. Processes 2020, 8, 645. [Google Scholar] [CrossRef]
- Bibi, I.; Nazar, N.; Ata, S.; Sultan, M.; Ali, A.; Abbas, A.; Jilani, K.; Kamal, S.; Sarim, F.M.; Khan, M.I. Green synthesis of iron oxide nanoparticles using pomegranate seeds extract and photocatalytic activity evaluation for the degradation of textile dye. J. Mater. Res. Technol. 2019, 8, 6115–6124. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.H.; Miah, M.Y.; Paul, S.C.; Aka, T.D.; Saha, O.; Rahaman, M.M.; Sharif, M.J.I.; Habiba, O.; Ashaduzzaman, M. Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: Application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon 2020, 6, e04603. [Google Scholar] [CrossRef]
- Indhira, D.; Krishnamoorthy, M.; Ameen, F.; Bhat, S.A.; Arumugam, K.; Ramalingam, S.; Priyan, S.R.; Kumar, G.S. Biomimetic facile synthesis of zinc oxide and copper oxide nanoparticles from Elaeagnus indica for enhanced photocatalytic activity. Environ. Res. 2022, 212, 113323. [Google Scholar] [CrossRef]
- Kumar, S.A.; Jarvin, M.; Inbanathan, S.; Umar, A.; Lalla, N.; Dzade, N.Y.; Algadi, H.; Rahman, Q.I.; Baskoutas, S. Facile green synthesis of magnesium oxide nanoparticles using tea (Camellia sinensis) extract for efficient photocatalytic degradation of methylene blue dye. Environ. Technol. Innov. 2022, 28, 102746. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabhu, R.; Muruganantham, K.; Shanmuganathan, R.; Natarajan, S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem. Photobiol. B Biol. 2019, 190, 86–97. [Google Scholar] [CrossRef]
- Kumar, B. Green synthesis of gold, silver, and iron nanoparticles for the degradation of organic pollutants in wastewater. J. Compos. Sci. 2021, 5, 219. [Google Scholar] [CrossRef]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process. Synth. 2020, 9, 171–181. [Google Scholar] [CrossRef]
- Goutam, S.P.; Saxena, G.; Singh, V.; Yadav, A.K.; Bharagava, R.N.; Thapa, K.B. Green synthesis of TiO2 nanoparticles using leaf extract of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem. Eng. J. 2018, 336, 386–396. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Niedbała, G.; Hassan, S.E.-D.; Salem, S.S.; Abdo, A.M.; Hetta, H.F.; Shaheen, T.I. Endophytic Streptomyces laurentii mediated green synthesis of Ag-NPs with antibacterial and anticancer properties for developing functional textile fabric properties. Antibiotics 2020, 9, 641. [Google Scholar] [CrossRef]
- Tarannum, N.; Gautam, Y.K. Facile green synthesis and applications of silver nanoparticles: A state-of-the-art review. RSC Adv. 2019, 9, 34926–34948. [Google Scholar] [CrossRef]
- Ganesan, R.; Prabu, H.G. Synthesis of gold nanoparticles using herbal Acorus calamus rhizome extract and coating on cotton fabric for antibacterial and UV blocking applications. Arab. J. Chem. 2019, 12, 2166–2174. [Google Scholar] [CrossRef]
- Karthik, S.; Siva, P.; Balu, K.S.; Suriyaprabha, R.; Rajendran, V.; Maaza, M. Acalypha indica–mediated green synthesis of ZnO nanostructures under differential thermal treatment: Effect on textile coating, hydrophobicity, UV resistance, and antibacterial activity. Adv. Powder Technol. 2017, 28, 3184–3194. [Google Scholar] [CrossRef]
- Fouda, A.; Saad, E.; Salem, S.S.; Shaheen, T.I. In-Vitro cytotoxicity, antibacterial, and UV protection properties of the biosynthesized Zinc oxide nanoparticles for medical textile applications. Microb. Pathog. 2018, 125, 252–261. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sathiyavimal, S.; Saravanan, M.; Senthilkumar, P.; Gnanasekaran, K.; Shanmugavel, M.; Manikandan, E.; Pugazhendhi, A. Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: Characterization of antibacterial activity and dye degradation potential. J. Photochem. Photobiol. B Biol. 2019, 191, 143–149. [Google Scholar] [CrossRef]
- Sharma, P.; Pant, S.; Poonia, P.; Kumari, S.; Dave, V.; Sharma, S. Green synthesis of colloidal copper nanoparticles capped with Tinospora cordifolia and its application in catalytic degradation in textile dye: An ecologically sound approach. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2463–2472. [Google Scholar] [CrossRef]
- Luque, P.; Chinchillas-Chinchillas, M.; Nava, O.; Lugo-Medina, E.; Martínez-Rosas, M.; Carrillo-Castillo, A.; Vilchis-Nestor, A.; Madrigal-Muñoz, L.; Garrafa-Gálvez, H. Green synthesis of tin dioxide nanoparticles using Camellia sinensis and its application in photocatalytic degradation of textile dyes. Optik 2021, 229, 166259. [Google Scholar] [CrossRef]
- Bogireddy, N.K.R.; Kumar, H.A.K.; Mandal, B.K. Biofabricated silver nanoparticles as green catalyst in the degradation of different textile dyes. J. Environ. Chem. Eng. 2016, 4, 56–64. [Google Scholar] [CrossRef]
- Saygi, K.O.; Usta, C. Rosa canina waste seed extract-mediated synthesis of silver nanoparticles and the evaluation of its antimutagenic action in Salmonella typhimurium. Mater. Chem. Phys. 2021, 266, 124537. [Google Scholar] [CrossRef]
- Sarac, N.; Baygar, T.; Ugur, A. In vitro mutagenic and anti-mutagenic properties of green synthesised silver nanoparticles. IET Nanobiotechnology 2018, 12, 230–233. [Google Scholar] [CrossRef]
- de Cássia Proença-Assunção, J.; Constantino, E.; Farias-de-França, A.P.; Nogueira, F.A.R.; Consonni, S.R.; Chaud, M.V.; dos Santos, C.A.; Oshima-Franco, Y. Mutagenicity of silver nanoparticles synthesized with curcumin (Cur-AgNPs). J. Saudi Chem. Soc. 2021, 25, 101321. [Google Scholar] [CrossRef]
- Fakher, S.N.; Kashi, F.J. Microbial Synthesized Ag/AgCl Nanoparticles Using Staphylococcus pasteuri sp. nov., ZAR1: Antimutagenicity, Antimicrobial Agent. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1688–1703. [Google Scholar] [CrossRef]
- Dashtizadeh, Z.; Kashi, F.J.; Ashrafi, M. Phytosynthesis of copper nanoparticles using Prunus mahaleb L. and its biological activity. Mater. Today Commun. 2021, 27, 102456. [Google Scholar] [CrossRef]
- Munir, H.; Mumtaz, A.; Rashid, R.; Najeeb, J.; Zubair, M.T.; Munir, S.; Bilal, M.; Cheng, H. Eucalyptus camaldulensis gum as a green matrix to fabrication of zinc and silver nanoparticles: Characterization and novel prospects as antimicrobial and dye-degrading agents. J. Mater. Res. Technol. 2020, 9, 15513–15524. [Google Scholar] [CrossRef]
- Khan, M.I.; Mohammad, A.; Patil, G.; Naqvi, S.; Chauhan, L.; Ahmad, I. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 2012, 33, 1477–1488. [Google Scholar] [CrossRef]
- Zhao, Y.; Howe, J.L.; Yu, Z.; Leong, D.T.; Chu, J.J.H.; Loo, J.S.C.; Ng, K.W. Exposure to titanium dioxide nanoparticles induces autophagy in primary human keratinocytes. Small 2013, 9, 387–392. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Jiao, J.; Hu, H.-M. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat. Nanotechnol. 2011, 6, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.M.; Fraietta, J.A.; Gracias, D.T.; Hope, J.L.; Stairiker, C.J.; Patel, P.R.; Mueller, Y.M.; McHugh, M.D.; Jablonowski, L.J.; Wheatley, M.A. Acute exposure to ZnO nanoparticles induces autophagic immune cell death. Nanotoxicology 2015, 9, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Farah, M.A.; Ali, M.A.; Chen, S.-M.; Li, Y.; Al-Hemaid, F.M.; Abou-Tarboush, F.M.; Al-Anazi, K.M.; Lee, J. Silver nanoparticles synthesized from Adenium obesum leaf extract induced DNA damage, apoptosis and autophagy via generation of reactive oxygen species. Colloids Surf. B Biointerfaces 2016, 141, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A comprehensive review on the synthesis, characterization, and biomedical application of platinum nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, D.; Li, W.; Yu, J.; Chen, Y. Effect of size, shape, and surface modification on cytotoxicity of gold nanoparticles to human HEp-2 and canine MDCK cells. J. Nanomater. 2012, 2012, 375496. [Google Scholar] [CrossRef]
- Hassan, D.; Khalil, A.T.; Saleem, J.; Diallo, A.; Khamlich, S.; Shinwari, Z.K.; Maaza, M. Biosynthesis of pure hematite phase magnetic iron oxide nanoparticles using floral extracts of Callistemon viminalis (bottlebrush): Their physical properties and novel biological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 693–707. [Google Scholar] [CrossRef]
- Sarani, M.; Tosan, F.; Hasani, S.A.; Barani, M.; Adeli-Sardou, M.; Khosravani, M.; Niknam, S.; Kouhbanani, M.A.J.; Beheshtkhoo, N. Study of in vitro cytotoxic performance of biosynthesized α-Bi2O3 NPs, Mn-doped and Zn-doped Bi2O3 NPs against MCF-7 and HUVEC cell lines. J. Mater. Res. Technol. 2022, 19, 140–150. [Google Scholar] [CrossRef]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; Abd El-Azim, M.H.M. Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Shankar, P.D.; Shobana, S.; Karuppusamy, I.; Pugazhendhi, A.; Ramkumar, V.S.; Arvindnarayan, S.; Kumar, G. A review on the biosynthesis of metallic nanoparticles (gold and silver) using bio-components of microalgae: Formation mechanism and applications. Enzym. Microb. Technol. 2016, 95, 28–44. [Google Scholar] [CrossRef]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef]
- Samanta, A.; Gangopadhyay, R.; Ghosh, C.K.; Ray, M. Enhanced photoluminescence from gold nanoparticle decorated polyaniline nanowire bundles. RSC Adv. 2017, 7, 27473–27479. [Google Scholar] [CrossRef]
- Geethalakshmi, R.; Sarada, D. Gold and silver nanoparticles from Trianthema decandra: Synthesis, characterization, and antimicrobial properties. Int. J. Nanomed. 2012, 7, 5375. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.A.; Caballero, S.; Díaz, L.A.; Guerrero, M.A.; Ruiz, J.; Ortiz, C.C. High antifungal activity against candida species of monometallic and bimetallic nanoparticles synthesized in nanoreactors. ACS Biomater. Sci. Eng. 2018, 4, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, J.; Zhang, Y.; Wang, Y.; Liu, L.; Li, M. Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Wang, M.; Meng, Y.; Zhu, H.; Hu, Y.; Chang-Peng, X.; Chao, X.; Li, W.; Pan, C.; Li, C. Green synthesized gold nanoparticles using Viola betonicifolia leaves extract: Characterization, antimicrobial, antioxidant, and cytobiocompatible activities. Int. J. Nanomed. 2021, 16, 7319. [Google Scholar] [CrossRef]
- Eskandari-Nojedehi, M.; Jafarizadeh-Malmiri, H.; Rahbar-Shahrouzi, J. Hydrothermal green synthesis of gold nanoparticles using mushroom (Agaricus bisporus) extract: Physico-chemical characteristics and antifungal activity studies. Green Process. Synth. 2018, 7, 38–47. [Google Scholar] [CrossRef]
- Thanighaiarassu, R.; Sivamai, P.; Devika, R.; Nambikkairaj, B. Green synthesis of gold nanoparticles characterization by using plant essential oil Menthapiperita and their antifungal activity against human pathogenic fungi. J. Nanomed. Nanotechnol. 2014, 5, 229. [Google Scholar]
- Lingaraju, K.; Raja Naika, H.; Manjunath, K.; Basavaraj, R.; Nagabhushana, H.; Nagaraju, G.; Suresh, D. Biogenic synthesis of zinc oxide nanoparticles using Ruta graveolens (L.) and their antibacterial and antioxidant activities. Appl. Nanosci. 2016, 6, 703–710. [Google Scholar] [CrossRef]
- Perveen, S.; Nadeem, R.; ur Rehman, S.; Afzal, N.; Anjum, S.; Noreen, S.; Saeed, R.; Amami, M.; Al-Mijalli, S.H.; Iqbal, M. Green synthesis of iron (Fe) nanoparticles using Plumeria obtusa extract as a reducing and stabilizing agent: Antimicrobial, antioxidant and biocompatibility studies. Arab. J. Chem. 2022, 15, 103764. [Google Scholar] [CrossRef]
- Naseem, T.; Farrukh, M.A. Antibacterial activity of green synthesis of iron nanoparticles using Lawsonia inermis and Gardenia jasminoides leaves extract. J. Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Da’na, E.; Taha, A.; Afkar, E. Green synthesis of iron nanoparticles by Acacia nilotica pods extract and its catalytic, adsorption, and antibacterial activities. Appl. Sci. 2018, 8, 1922. [Google Scholar] [CrossRef]
- Shejawal, K.P.; Randive, D.S.; Bhinge, S.D.; Bhutkar, M.A.; Wadkar, G.H.; Jadhav, N.R. Green synthesis of silver and iron nanoparticles of isolated proanthocyanidin: Its characterization, antioxidant, antimicrobial, and cytotoxic activities against COLO320DM and HT29. J. Genet. Eng. Biotechnol. 2020, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.d.; Delbem, A.C.B.; Monteiro, D.R. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.R.; Emam, A.N.; Koraney, N.F.; Hefny, E.G.; Ali, S.F. Combating the prevalence of water-borne bacterial pathogens using anisotropic structures of silver nanoparticles. J. Nanoparticle Res. 2020, 22, 1–15. [Google Scholar] [CrossRef]
- Huq, M.A.; Ashrafudoulla, M.; Rahman, M.M.; Balusamy, S.R.; Akter, S. Green synthesis and potential antibacterial applications of bioactive silver nanoparticles: A review. Polymers 2022, 14, 742. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Tortella, G.; Rubilar, O.; Fincheira, P.; Pieretti, J.C.; Duran, P.; Lourenço, I.M.; Seabra, A.B. Bactericidal and virucidal activities of biogenic metal-based nanoparticles: Advances and perspectives. Antibiotics 2021, 10, 783. [Google Scholar] [CrossRef]
- Gaikwad, S.; Ingle, A.; Gade, A.; Rai, M.; Falanga, A.; Incoronato, N.; Russo, L.; Galdiero, S.; Galdiero, M. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 2013, 8, 4303. [Google Scholar]
- El-Mohamady, R.S.; Ghattas, T.; Zawrah, M.; Abd El-Hafeiz, Y. Inhibitory effect of silver nanoparticles on bovine herpesvirus-1. Int. J. Vet. Sci. Med. 2018, 6, 296–300. [Google Scholar] [CrossRef]
- Bekele, A.Z.; Gokulan, K.; Williams, K.M.; Khare, S. Dose and size-dependent antiviral effects of silver nanoparticles on feline calicivirus, a human norovirus surrogate. Foodborne Pathog. Dis. 2016, 13, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Kaushik, S.; Pandit, P.; Dhull, D.; Yadav, J.P.; Kaushik, S. Green synthesis of silver nanoparticles from medicinal plants and evaluation of their antiviral potential against chikungunya virus. Appl. Microbiol. Biotechnol. 2019, 103, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Ga’al, H.; Fouad, H.; Mao, G.; Tian, J.; Jianchu, M. Larvicidal and pupicidal evaluation of silver nanoparticles synthesized using Aquilaria sinensis and Pogostemon cablin essential oils against dengue and zika viruses vector Aedes albopictus mosquito and its histopathological analysis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Sujitha, V.; Murugan, K.; Paulpandi, M.; Panneerselvam, C.; Suresh, U.; Roni, M.; Nicoletti, M.; Higuchi, A.; Madhiyazhagan, P.; Subramaniam, J. Green-synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitol. Res. 2015, 114, 3315–3325. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Li, S.; Kong, F.; Hou, R.; Guan, X.; Guo, F. Inhibition effect of silver nanoparticles on herpes simplex virus 2. Genet. Mol. Res. 2014, 13, 7022–7028. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zheng, Y.; Yin, J.; Li, X.; Zheng, C. Inhibitory effects of silver nanoparticles against adenovirus type 3 in vitro. J. Virol. Methods 2013, 193, 470–477. [Google Scholar] [CrossRef]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnology 2005, 3, 1–10. [Google Scholar] [CrossRef]
- Kim, M.; Nguyen, D.-V.; Heo, Y.; Park, K.H.; Paik, H.-D.; Kim, Y.B. Antiviral activity of fritillaria thunbergii extract against human influenza virus H1N1 (PR8) in vitro, in ovo and in vivo. J. Microbiol. Biotechnol. 2020, 30, 172–177. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Hsueh, Y.-H.; Hsieh, C.-T.; Tzou, D.-Y.; Chang, P.-L. Antiviral activity of graphene–silver nanocomposites against non-enveloped and enveloped viruses. Int. J. Environ. Res. Public Health 2016, 13, 430. [Google Scholar] [CrossRef]
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.; Nair, M.P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Fariq, A.; Khan, T.; Yasmin, A. Microbial synthesis of nanoparticles and their potential applications in biomedicine. J. Appl. Biomed. 2017, 15, 241–248. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Tan, T.; Liu, M.; Zeng, Z.; Zeng, Y.; Zhang, L.; Fu, C.; Chen, D.; Xie, T. Nanoparticle drug delivery system for glioma and its efficacy improvement strategies: A comprehensive review. Int. J. Nanomed. 2020, 15, 2563. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles for drug delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent advances in zinc oxide nanoparticles (Zno nps) for cancer diagnosis, target drug delivery, and treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef]
- Lekha, D.C.; Shanmugam, R.; Madhuri, K.; Dwarampudi, L.P.; Bhaskaran, M.; Kongara, D.; Tesfaye, J.L.; Nagaprasad, N.; Bhargavi, V.; Krishnaraj, R. Review on silver nanoparticle synthesis method, antibacterial activity, drug delivery vehicles, and toxicity pathways: Recent advances and future aspects. J. Nanomater. 2021, 2021, 4401829. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Ahmad, F.; Ashraf, N.; Ashraf, T.; Zhou, R.-B.; Yin, D.-C. Biological synthesis of metallic nanoparticles (MNPs) by plants and microbes: Their cellular uptake, biocompatibility, and biomedical applications. Appl. Microbiol. Biotechnol. 2019, 103, 2913–2935. [Google Scholar] [CrossRef]
- Tang, Y.S.; Wang, D.; Zhou, C.; Zhang, S. Preparation and anti-tumor efficiency evaluation of bacterial magnetosome–anti-4-1BB antibody complex: Bacterial magnetosome as antibody carriers isolated from Magnetospirillum gryphiswaldense. Biotechnol. Appl. Biochem. 2019, 66, 290–297. [Google Scholar] [CrossRef]
- Sun, J.-B.; Wang, Z.-L.; Duan, J.-H.; Ren, J.; Yang, X.-D.; Dai, S.-L.; Li, Y. Targeted distribution of bacterial magnetosomes isolated from Magnetospirillum gryphiswaldense MSR-1 in healthy Sprague-Dawley rats. J. Nanosci. Nanotechnol. 2009, 9, 1881–1885. [Google Scholar] [CrossRef]
- Tripathi, R.; Shrivastav, A.; Shrivastav, B. Biogenic gold nanoparticles: As a potential candidate for brain tumor directed drug delivery. Artif. Cells Nanomed. Biotechnol. 2015, 43, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Gambhir, S.; Ahmad, A. Extracellular biosynthesis of gadolinium oxide (Gd2O3) nanoparticles, their biodistribution and bioconjugation with the chemically modified anticancer drug taxol. Beilstein J. Nanotechnol. 2014, 5, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, M.; Topham, P.D.; Huang, Y. Fabrication of magnetic drug-loaded polymeric composite nanofibres and their drug release characteristics. RSC Adv. 2012, 2, 2433–2438. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.-j.; Guo, M.; Yan, H.-s.; Wang, C.-h.; Liu, K.-l. Cisplatin-loaded polymer/magnetite composite nanoparticles as multifunctional therapeutic nanomedicine. Chin. J. Polym. Sci. 2014, 32, 1329–1337. [Google Scholar] [CrossRef]
- Prabha, G.; Raj, V. Preparation and characterization of chitosan—Polyethylene glycol-polyvinylpyrrolidone-coated superparamagnetic iron oxide nanoparticles as carrier system: Drug loading and in vitro drug release study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 808–816. [Google Scholar] [CrossRef]
- Sreeja, S.; Nair, C.K. Tumor control by hypoxia-specific chemotargeting of iron-oxide nanoparticle–Berberine complexes in a mouse model. Life Sciences 2018, 195, 71–80. [Google Scholar] [CrossRef]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on nanoparticles and nanostructured materials: Bioimaging, biosensing, drug delivery, tissue engineering, antimicrobial, and agro-food applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Chauhan, A.; Zubair, S.; Tufail, S.; Sherwani, A.; Sajid, M.; Raman, S.C.; Azam, A.; Owais, M. Fungus-mediated biological synthesis of gold nanoparticles: Potential in detection of liver cancer. Int. J. Nanomed. 2011, 6, 2305–2319. [Google Scholar]
- Kalpana, V.; Devi Rajeswari, V. A review on green synthesis, biomedical applications, and toxicity studies of ZnO NPs. Bioinorg. Chem. Appl. 2018, 2018, 3569758. [Google Scholar] [CrossRef]
- Gun’ko, Y.K. Nanoparticles in bioimaging. Nanomaterials 2016, 6, 105. [Google Scholar] [CrossRef]
- Jaque, D.; Richard, C.; Viana, B.; Soga, K.; Liu, X.; Sole, J.G. Inorganic nanoparticles for optical bioimaging. Adv. Opt. Photonics 2016, 8, 1–103. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci. Food 2017, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-A.; Kim, M.-K.; Song, J.H.; Jo, M.-R.; Yu, J.; Kim, K.-M.; Kim, Y.-R.; Oh, J.-M.; Choi, S.-J. Biokinetics of food additive silica nanoparticles and their interactions with food components. Colloids Surf. B Biointerfaces 2017, 150, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Attia, N.F.; Moussa, M.; Sheta, A.M.; Taha, R.; Gamal, H. Synthesis of effective multifunctional textile based on silica nanoparticles. Prog. Org. Coat. 2017, 106, 41–49. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.Ö.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef]
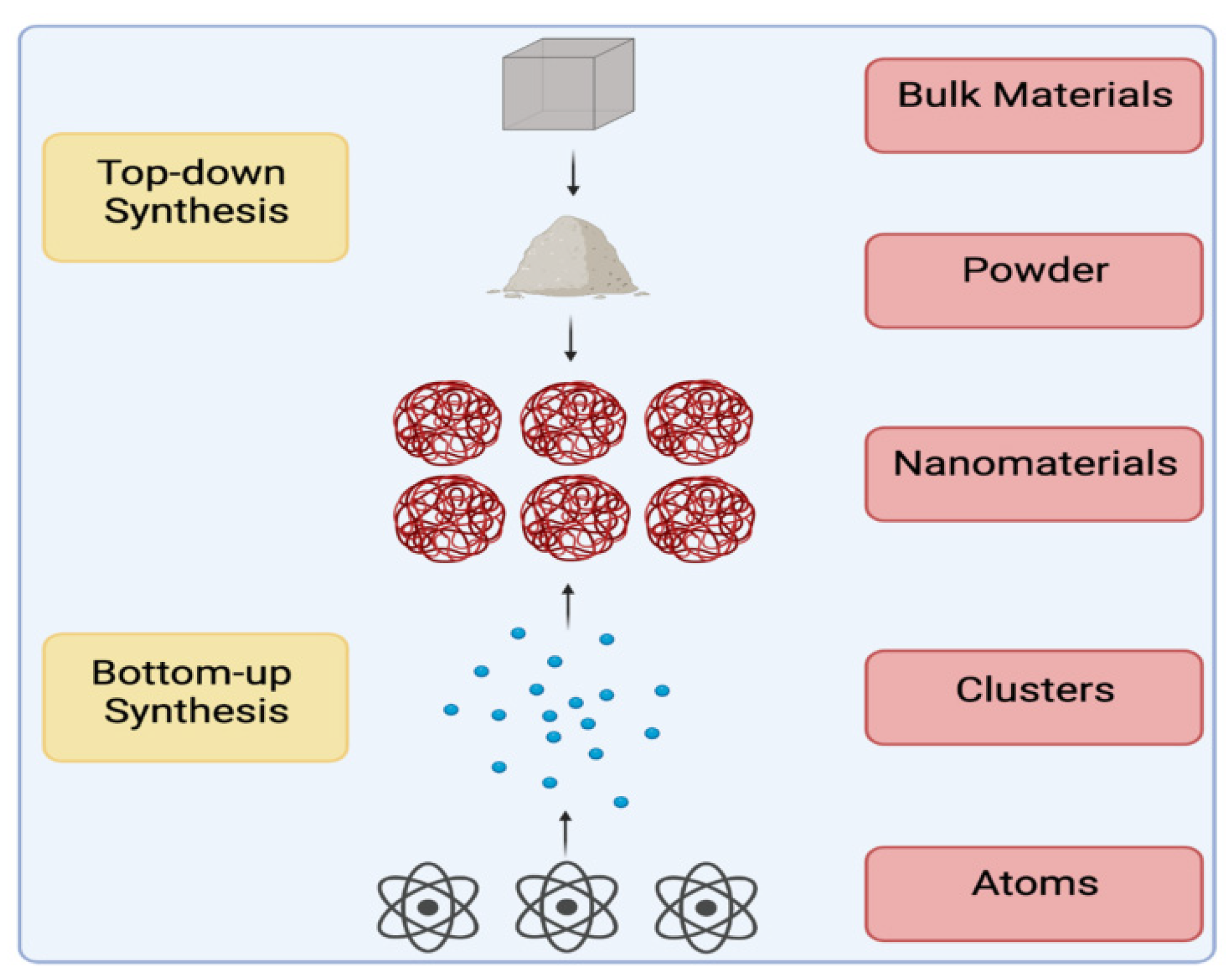


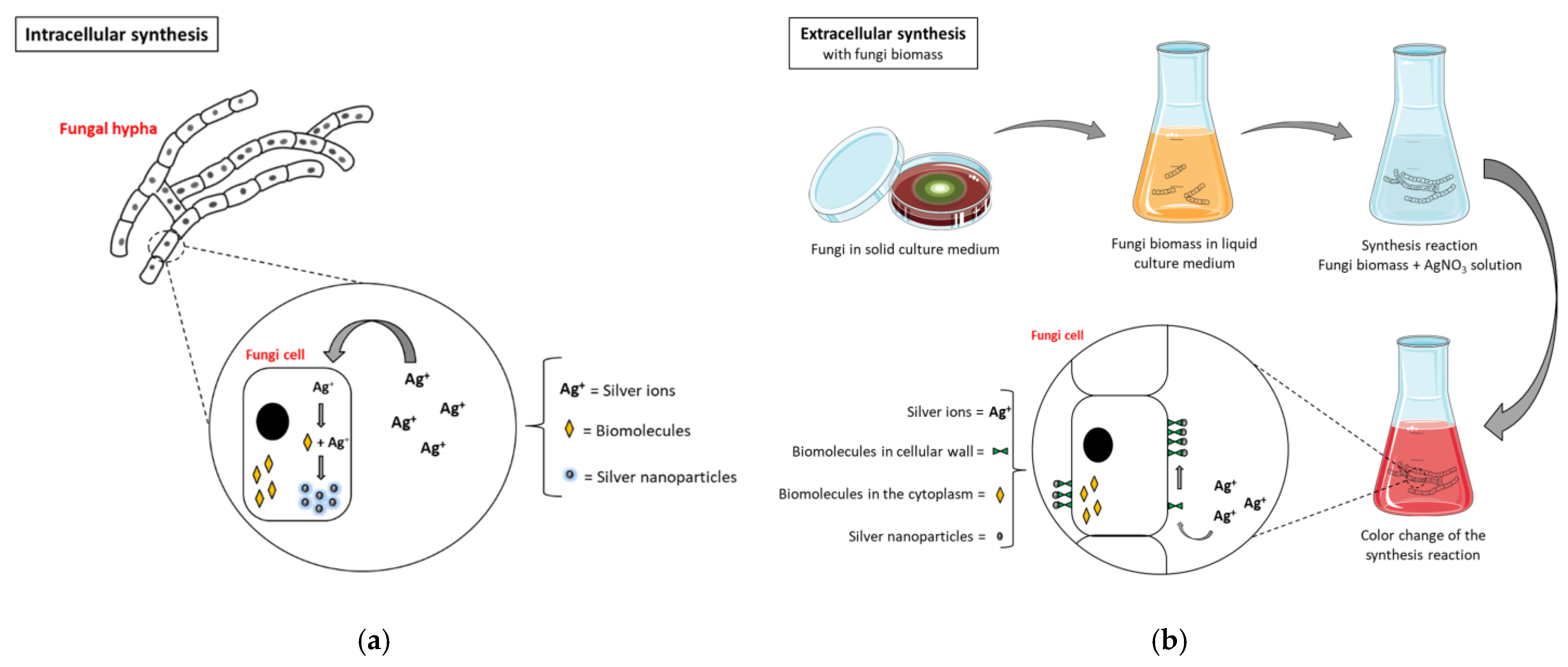
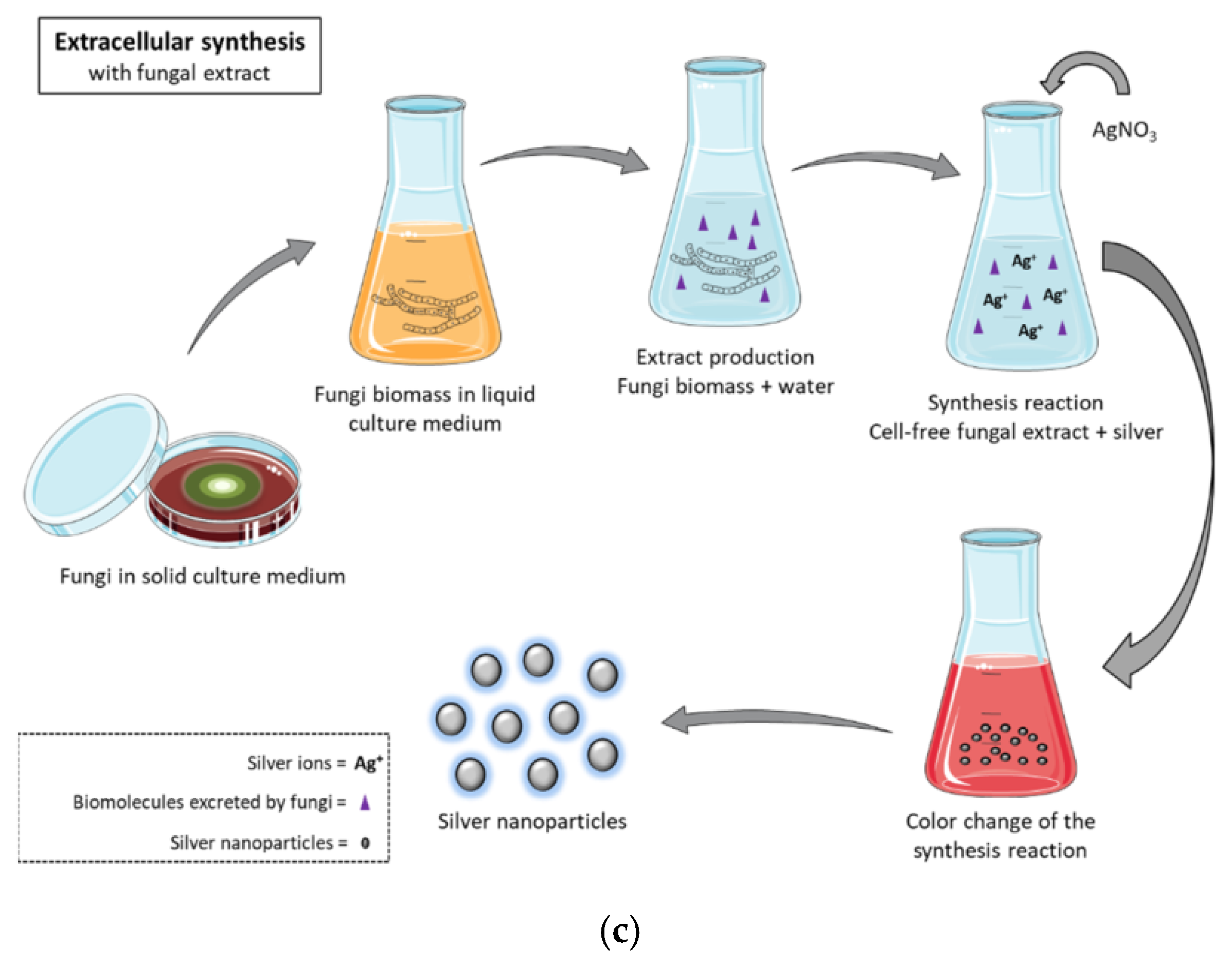

| Item | Biological Synthesis | Chemical Synthesis (Bottom-Up) | Physical Synthesis (Top-Down) |
|---|---|---|---|
| Disadvantages | -Very expensive -Need for aseptic cultivation conditions | -Stabilizing and reducing agents are toxic materials and solvents. -Requires for high energy -Produces secondary harmful products. | -Requires high energy -Very expensive |
| Advantages | -Simple and easy method -Uses eco-friendly materials -Cost-effective methods | -Possibility of large scale production. | -Associated with enhanced purity -Size is controlled. -Shape is uniform. -Crystallinity is controlled. |
| Characterization Technique | Obtained Properties |
|---|---|
| Microbial colony viability, in vivo, in vitro cell viability | Biological properties |
| High-performance liquid chromatography (HPLC) | Content |
| Mass spectrometry (MS) | Surface properties, structure, composition, molecular weight |
| Zeta potential | Stability of surface charge |
| Microscopy, Double photon correlation spectroscopy | Optical properties |
| Electrokinetics (Such as cyclic voltammetry studies) | Electrical properties |
| Dynamic light scattering (DLS) | Hydrodynamic size distribution |
| Differential scanning calorimetry (DSC) | Possible interactions of polymers and drugs, physicochemical state |
| Atomic force microscopy (AFM) Dynamic light scattering Electron microscopy (transmission/scanning) | Surface properties, aggregation, structure, shape, size, and size distribution |
| X-ray diffraction, Brunauer–Emmett–Teller (BET) | Surface and topographical properties |
| Transmission electron microscopy (TEM) | Aggregation, shape heterogeneity, size, and size distribution |
| Field emission scanning electron microscopy (FESEM) Scanning tunneling microscopy (STM) Scanning electron microscopy (SEM) | Aggregation, shape, size, and size distribution |
| Near-field scanning optical microscopy (NSOM) | Size and shape |
| X-ray photoelectron spectroscopy (XPS) | Chemical and elemental composition at the surface |
| Fourier transform infrared spectroscopy (FT-IR) Electron dispersive X-ray spectroscopy UV-visible spectroscopy | Chemical properties |
| Nuclear magnetic resonance (NMR) | Conformational change, purity, composition, structure |
| Infrared spectroscopy (IR) Raman spectroscopy Surface enhanced Raman spectroscopy (SERS) | Functional group analysis, Conformation and structure of conjugates |
| Microorganisms | Green-Synthesized Nanoparticles | Size | Applications | Ref. |
|---|---|---|---|---|
| Actinomycetes | ZnO nanoparticles | 11.57 nm | Antibacterials and anti-biofilms against pathogenic microbes | [59] |
| Au nanoparticles | 45 nm | Antimicrobial and anticancer activity | [62] | |
| CuO nanoparticles | 20 nm | Antibacterial and anticancer activity toward lung cancer cells | [67] | |
| Algae | Ti nanoparticles | 50 nm | Antistatic and anticancer activities | [72] |
| Ag nanoparticles | 13–31 nm | Antibacterial activities | [74] | |
| Ag and Au nanoparticles | 50 and 20 nm | Antimicrobial activities | [75] | |
| SnO2 nanoparticles | 32.2 nm | Photodegradation of dyes, antibacterial activity, Antioxidant activity, and cytotoxicity | [76] | |
| Ag nanoparticles | 25–60 nm | Antibacterial activity | [77] | |
| Phytosynthesis | Y2O3 nanoparticles | 20–45 nm | Photodegradation of dyes, antibacterial Activity, cytotoxicity, and drug release | [80] |
| Ag and Au nanoparticles | 20.67 and 16.76 nm | Antioxidant activity, and antimicrobial activity | [81] | |
| Ag nanoparticles | 20 nm | Antibacterial activity, preventing the coagulation of blood samples, and catalytic reduction of dyes | [82] | |
| Viruses | Au and Ag nanoparticles | 5–12 and 5–20 nm | Bio-semiconductors | [93] |
| Fe2O3, PbS, SiO2, and CdS nanoparticles | 22, 30, 24, and 5 nm | - | [95] | |
| Fungi | Au nanoparticles | 5–200 nm for extracellular synthesis and 10–25 nm for intracellular synthesis | - | [101] |
| titania and silica nanoparticles | 10.2 and 9.8 nm | - | [104] | |
| Se nanoparticles | 55 nm | Antioxidant and antimicrobial activities | [105] | |
| ZnO nanoparticles | 2–6 nm | - | [106] | |
| Fe3O4 nanoparticles | 20–40 nm | Removal of Cr(VI) ions from water | [107] | |
| Carbon quantum dots | 5.5–7.5 nm | Sensing of tetracyclines and bioimaging of cancer cells | [108] | |
| Yeast | Se0 nanoparticles | 50–250 nm | anti-candida and anti-oxidant activities | [110] |
| Se nanoparticles | 71.14 nm | Antioxidant activities, stimulated humoral immune potential, and trace element feed additive | [111] | |
| CdS nanoparticles | 1–1.5 nm | Fabrication of an ideal diode | [112] | |
| Ag nanoparticles | 2–5 nm | - | [113] | |
| Au nanoparticles and nanoplates | 7.5–27 nm | - | [114] | |
| PbS quantum dots | 2–5 nm | Semiconductors | [116] | |
| Bacteria | Ag nanoparticles | 20-40 nm | Antibacterial activities | [119] |
| Ag nanoparticles | 20 nm | - | [123] | |
| ZnO nanoflowers | 3.8 nm | Agricultural applications | [124] | |
| Spherical ZnO nanoparticles | 250 nm to 1 μm | Photocatalytic degradation of dyes | [126] | |
| ZnO nanoparticles | 100–120 nm | In textile fabrics to enhance UV-blocking, self-cleaning and antibacterial properties, photocatalytic activity, and anticancer activities | [128] | |
| Pd0 nanoparticles | 4–20 nm | Catalytic activity in dehalogenation reaction | [130] | |
| Ag nanoparticles | 42–92 nm | - | [135] | |
| Au nanoparticles | 20.93 nm | Antioxidant activity and an antiproliferative effect against cancer cells | [136] |
| Applications | Green-Synthesized Nanoparticles | Activity | Refs. |
|---|---|---|---|
| Food industry | Ag, ZnO, and Fe2O3 nanoparticles | Antimicrobial and colorant agents in the food industry | [244] |
| TiO2 nanoparticles | Used in food packaging and as additives in the food industry | [153] | |
| SiO2 nanoparticles | Used as additives in the food industry | [245] | |
| Water treatment | Fe2O3 nanoparticles | Have great potential as photocatalysts for degradation of organic dyes | [158,159] |
| MgO nanoparticles | Have great potential as photocatalysts for degradation of methylene blue dye | [161,162] | |
| TiO2 nanoparticles | Have great potential as photocatalysts for degradation of toxic heavy metals and tannery wastewater | [160,161] | |
| Textile industry | Ag, Au, and ZnO nanoparticles | Provide textile fabrics with antistatic, antimicrobial, resistance to wrinkles, UV protection, flame-retarding ability, hydrophobicity, and self-cleaning properties | [128,166,167,168,169,170] |
| CuO nanoparticles | Used for the synthesis of antimicrobial cotton fabrics and degradation of organic dyes used in textile colorization | [171,172] | |
| Silica nanoparticles | Popular for treating textile products and improving the hydrophobic qualities of the fabric surfaces | [246] | |
| Mutagenicity | Ag nanoparticle | Are non-mutagenic and showed anti-mutagenic activities | [175,176,177] |
| Ag/AgCl nanoparticles | Are non-mutagenic and showed anti-mutagenic activities | [178] | |
| Cu nanoparticles | Are non-mutagenic and have high anti-mutagenic activities | [179] | |
| Ag and Zn nanoparticles | Are non-mutagenic and non-toxic | [180] | |
| Autophagy | Ag nanoparticles | Used to promote autophagosome buildup in cancer cells | [185] |
| TiO2 and Mn nanoparticles | Can cause cellular autophagy | [247] | |
| Cytotoxicity | Ag and Au nanoparticles | Cytotoxic agents that inhibit various types of cancers | [187,188] |
| ZnO nanoparticles | Noticeably less lethal to normal cells and cytotoxic to cancer cells | [190] | |
| Antiviral and antimicrobial agents | Au nanoparticle | Have antimicrobial, antifungal, and bio-compatible properties | [194,195,196,197,198,199,200] |
| ZnO nanoparticles | Have efficient antibacterial and antifungal properties | [192,201] | |
| Fe nanoparticles | Have a notable anti-microbial properties | [202,203,204,205] | |
| Ag nanoparticles | Used as an antiviral, anti-bacterial, anti-fungal, and anti-inflammatory agents | [86,209,211,212,213,214,215,216,217,218,219,220,221] | |
| Drug delivery | Mesoporous silica, ZnO, Au, and Ag nanoparticles | Efficient drug delivery agents | [225,226,227,228] |
| polyethylene glycol nanoparticles | Increase the precision of drug delivery to target bacterial infections in the body | [229] | |
| Fe3S4 and Fe3O4 nanoparticles | Encapsulate and transport pharmaceuticals, immunotherapy against cancer, and tumor suppression agents | [230,231,232] | |
| Au nanoparticles | Delivery of medications to brain and anti-tumor drug delivery | [233,234] | |
| Fe3O4 nanoparticles | Used as a drug delivery vehicles for different medication classes | [235,236,237,238] | |
| Bioimaging and imaging | ZnO nanoparticles | Used in bioimaging systems | [241] |
| Silica, Fe3O4, Ag, Pt, Au, and Pd nanoparticles | Fluorescent silica particles are used in bioimaging and other nanoparticles are used in cancer tumor thermal imaging | [242] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaiari, N.S.; Alzahrani, F.M.; Amari, A.; Osman, H.; Harharah, H.N.; Elboughdiri, N.; Tahoon, M.A. Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives. Molecules 2023, 28, 463. https://doi.org/10.3390/molecules28010463
Alsaiari NS, Alzahrani FM, Amari A, Osman H, Harharah HN, Elboughdiri N, Tahoon MA. Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives. Molecules. 2023; 28(1):463. https://doi.org/10.3390/molecules28010463
Chicago/Turabian StyleAlsaiari, Norah Salem, Fatimah Mohammed Alzahrani, Abdelfattah Amari, Haitham Osman, Hamed N. Harharah, Noureddine Elboughdiri, and Mohamed A. Tahoon. 2023. "Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives" Molecules 28, no. 1: 463. https://doi.org/10.3390/molecules28010463
APA StyleAlsaiari, N. S., Alzahrani, F. M., Amari, A., Osman, H., Harharah, H. N., Elboughdiri, N., & Tahoon, M. A. (2023). Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives. Molecules, 28(1), 463. https://doi.org/10.3390/molecules28010463