Application of Response Surface Methodology for Optimization of the Biosorption Process from Copper-Containing Wastewater
Abstract
1. Introduction
2. Results and Discussion
2.1. Surface Morphology of Calcium Alginate Beads
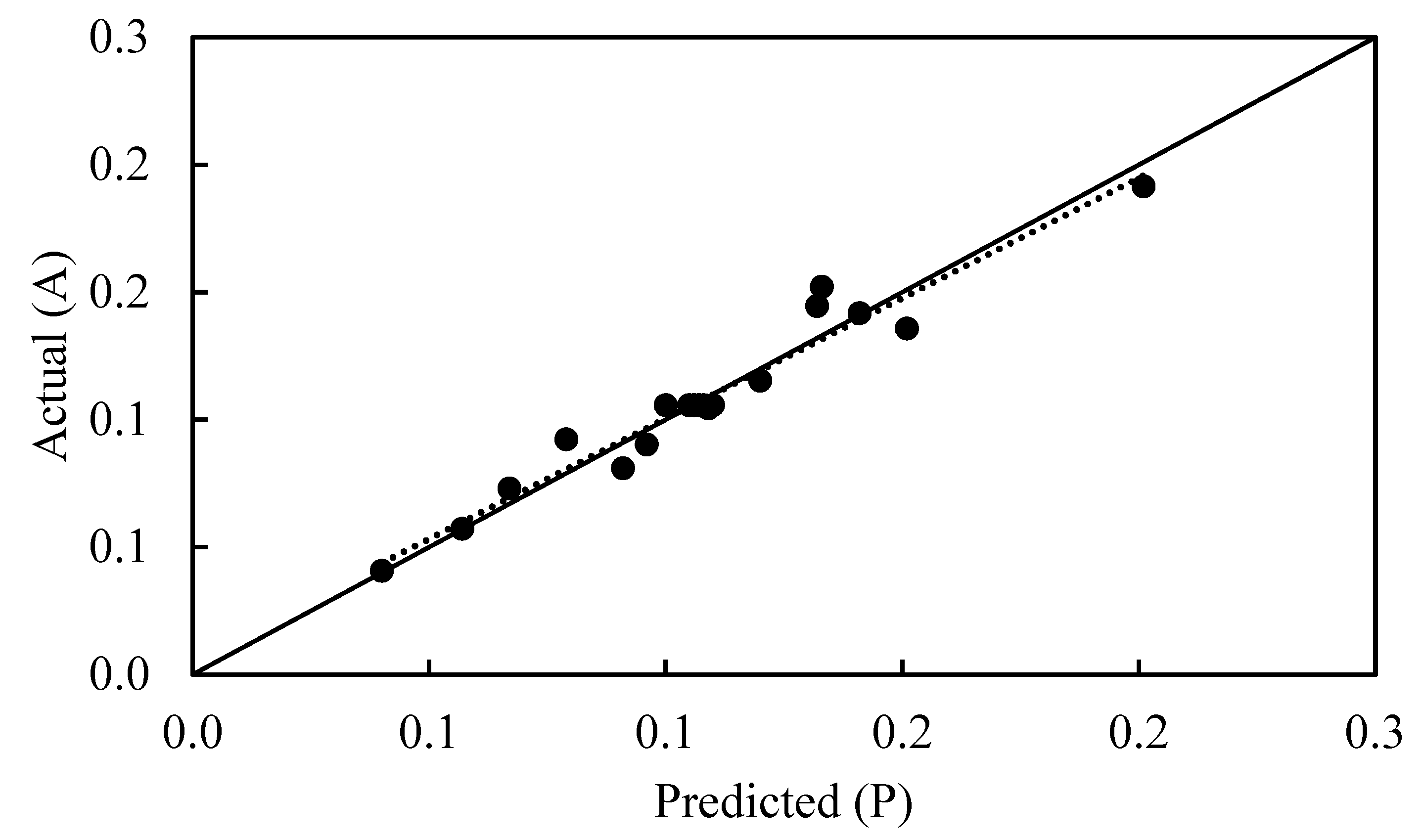
2.2. ANOVA Analysis and the Adequacy of the Mathematical Model
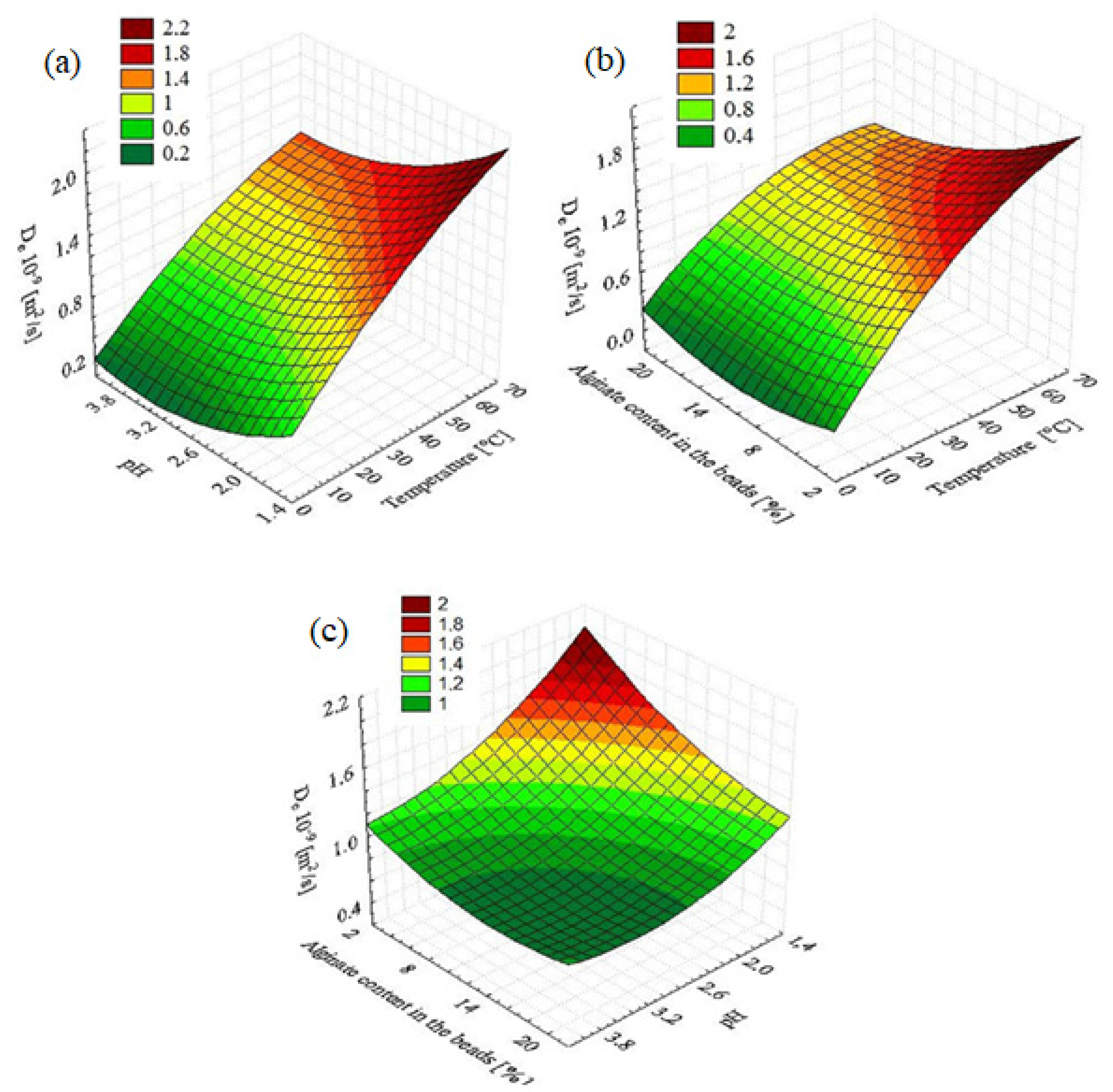
2.3. Three-Dimensional Response Surface Plots
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Calcium Alginate Beads
3.3. Conductometric Method
3.4. Experimental Design
3.5. Determination of the Point of Zero Charge (pHpzc)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maslova, M.; Mudruk, N.; Ivanets, A.; Shashkova, I.; Kitikova, N. The effect of pH on removal of toxic metal ions from aqueous solutions using composite sorbent based on Ti-Ca-Mg phosphates. J. Water Process. Eng. 2021, 40, 101830. [Google Scholar] [CrossRef]
- Ivanets, A.I.; Kitikova, N.V.; Shashkova, I.L.; Roshchina, M.Y.; Srivastava, V.; Sillanpää, M. Adsorption performance of hydroxyapatite with different crystalline and porous structure towards metal ions in multicomponent solution. J. Water Process. Eng. 2019, 32, 100963. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Yang, N.; Wang, R.; Rao, P.; Yan, L.; Zhang, W.; Wang, J.; Chai, F. The fabrication of calcium alginate beads as a green sorbent for selective recovery of Cu(II) from metal mixtures. Crystals 2019, 9, 255. [Google Scholar] [CrossRef]
- Petrovič, A.; Simonič, M. Removal of heavy metal ions from drinking water by alginate-immobilised Chlorella sorokiniana. Int. J. Environ. Sci. Technol. 2016, 13, 1761–1780. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Garcia-Diaz, I.; Lopez, F.; Rodriguez, O. Recycling of copper flue dust via leaching-solvent extraction processing. Desalin. Water Treat. 2015, 56, 1202–1207. [Google Scholar] [CrossRef]
- Pawar, R.R.; Gupta, P.; Sawant, S.Y.; Shahmoradi, B.; Lee, S.M. Porous synthetic hectorite clay-alginate composite beads for effective adsorption of methylene blue dye from aqueous solution. Int. J. Biol. Macromol. 2018, 114, 1315. [Google Scholar] [CrossRef]
- Torres-Caban, R.; Vega-Olivencia, C.A.; Alamo-Nole, L.; Morales-Irizarry, D.; Roman-Velazquez, F.R.; Mina-Camilde, N. Removal of copper from water by adsorption with calcium-alginate/spent-coffee-grounds composite beads. Materials 2019, 12, 395. [Google Scholar] [CrossRef]
- Guo, J.; Han, Y.; Mao, Y.; Wickramaratne, M.N. Influence of alginate fixation on the adsorption capacity of hydroxyapatite nanocrystals to Cu2+ ions. Colloids Surf. A 2017, 529, 801–807. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modeling simulation tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, Y.; Zhang, X.F.; Yao, J. In-situ gelation of sodium alginate supported on melamine sponge for efficient removal of copper ions. J. Colloid Interface Sci. 2018, 512, 7–13. [Google Scholar] [CrossRef]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef] [PubMed]
- Nussinovitch, A.; Dagan, O. Hydrocolloid liquid-core capsules for the removal of heavy-metal cations from water. J. Hazard. Mater. 2015, 299, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Vásquez-Quitral, P.; Butto, N.; Díaz-Soler, F.; Yazdani-Pedram, M.; Silva, J.; Neira-Carrillo, A. Effect of alginate from Chilean Lessonia nigrescens and MWCNTs on CaCO3 crystallization by classical and non-classical methods. Crystals 2018, 8, 69. [Google Scholar] [CrossRef]
- Hu, Z.H.; Omer, A.M.; Ouyang, X.K.; Yu, D. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(II) from aqueous solution. Int. J. Biol. Macromol. 2018, 108, 149–157. [Google Scholar] [CrossRef]
- Vu, H.C.; Dwivedi, A.D.; Le, T.T.; Seo, S.H.; Kim, E.J.; Chang, Y.S. Magnetite graphene oxide encapsulated in alginate beads for enhanced adsorption of Cr(VI) and As(V) from aqueous solutions: Role of crosslinking metal cations in pH control. Chem. Eng. J. 2017, 307, 220–229. [Google Scholar] [CrossRef]
- Dechojarassri, D.; Omote, S.; Nishida, K.; Omura, T.; Yamaguchi, H.; Furuike, T.; Tamura, H. Preparation of alginate fibers coagulated by calcium chloride or sulfuric acid: Application to the adsorption of Sr2+. J. Hazard. Mater. 2018, 355, 154–161. [Google Scholar] [CrossRef]
- Veglio, F.; Esposito, A.; Reverberi, A.P. Copper adsorption on calcium alginate beads: Equilibrium pH-related models. Hydrometallurgy 2002, 65, 43–57. [Google Scholar] [CrossRef]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Alginate and algal-based beads for the sorption of metal cations: Cu(II) and Pb(II). Int. J. Mol. Sci. 2016, 17, 1453. [Google Scholar] [CrossRef]
- Pandey, A.; Bera, D.; Shukla, A.; Ray, L. Studies on Cr(VI), Pb(II) and Cu(II) adsorption-desorption using calcium alginate as biopolymer. Chem. Sep. Bioavailab. 2007, 19, 17–24. [Google Scholar] [CrossRef]
- Shirzad, M.; Karimi, M. Statistical analysis and optimal design of polymer inclusion membrane for water treatment by Co(II) removal. Desalin. Water Treat. 2020, 182, 194–207. [Google Scholar] [CrossRef]
- Sarkar, M.; Majumdar, P. Application of response surface methodology for optimization of heavy metal biosorption using surfactant modified chitosan bead. Chem. Eng. J. 2011, 175, 376–387. [Google Scholar] [CrossRef]
- Tabaraki, R.; Nateghi, A.; Ahmady-Asbchin, S. Biosorption of lead (II) ions on Sargassum ilicifolium: Application of response surface methodology. Int. Biodeter. Biodegr. 2014, 93, 145–152. [Google Scholar] [CrossRef]
- Liu, W.-J.; Zeng, F.-X.; Jiang, H.; Zhang, X.-S. Adsorption of lead (Pb) from aqueous solution with Typha angustifolia biomass modified by SOCl2 activated EDTA. Chem. Eng. J. 2011, 170, 21–28. [Google Scholar] [CrossRef]
- Selvamuthu, D.; Das, D. Introduction to Statistical Methods, Design of Experiments and Statistical Quality Control; Springer: Singapore, 2018. [Google Scholar]
- Kamairudin, N.; Hoong, S.S.; Abdullah, L.C.; Ariffin, H.; Biak, D.R.A. Optimisation of epoxide ring-opening reaction for the synthesis of bio-polyol from palm oil derivative using Response Surface Methodology. Molecules 2021, 26, 648. [Google Scholar] [CrossRef] [PubMed]
- Becze, A.; Babalau-Fuss, V.L.; Varaticeanu, C.; Roman, C. Optimization of high-pressure extraction process of antioxidant compounds from Feteasca regala leaves using Response Surface Methodology. Molecules 2020, 25, 4209. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Vahabzadeh, F.; Bonakdarpour, B.; Mofarrah, E.; Mehranian, M. Application of the central composite design and Response Surface Methodology to the advanced treatment of olive oil processing wastewater using Fenton’s peroxidation. J. Hazard. Mater. 2005, 123, 187–195. [Google Scholar] [CrossRef]
- Kwiatkowska-Marks, S.; Miłek, J.; Wójcik, M. The effect of pH on the sorption of copper ions by alginates. Pol. J. Chem. Tech. 2008, 10, 28–30. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; Wiley: Toronto, ON, Canada, 1995. [Google Scholar]
- Bas, D.; Boyaci, I.H. Modeling and optimization I: Usability of Response Surface Methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Schubert, A.L.; Hagemann, D.; Voss, A.; Bergmann, K. Evaluating the model fit of diffusion models with the root mean square error of approximation. J. Math. Psychol. 2017, 77, 29–45. [Google Scholar] [CrossRef]
- Kwiatkowska-Marks, S.; Miłek, J. Diffusive properties of alginate biosorbents. Ecol. Chem. Eng. S 2019, 26, 149–163. [Google Scholar] [CrossRef]
- Gulicovski, J.J.; Čerović, L.S.; Milonjić, S.K. Point of zero charge and isoelectric point of alumina. Mater. Manuf. Process. 2008, 23, 615–619. [Google Scholar] [CrossRef]
- Kragović, M.; Stojmenović, M.; Petrović, J.; Loredo, J.; Pašalić, S.; Nedeljković, A.; Ristović, I. Influence of alginate encapsulation on point of zero charge (pHpzc) and thermodynamic properties of the natural and Fe(III)—Modified zeolite. Procedia Manuf. 2019, 32, 286–293. [Google Scholar] [CrossRef]
- Hassan, A.F.; Abdel-Mohsen, A.M.; Elhadidy, H. Adsorption of arsenic by activated carbon, calcium alginate and their composite beads. Int. J. Biol. Macromol. 2014, 68, 125–130. [Google Scholar] [CrossRef] [PubMed]

| Term | Coefficient | SE Coefficient | T, DF = 10 | p-Value |
|---|---|---|---|---|
| 1.06 | 0.04 | 21.56 | 0.00 | |
| 0.31 | 0.03 | 9.50 | 0.00 | |
| −0.18 | 0.03 | −5.66 | 0.00 | |
| −0.18 | 0.04 | −4.46 | 0.01 | |
| −0.05 | 0.03 | −1.42 | 0.19 | |
| 0.05 | 0.03 | 1.69 | 0.12 | |
| 0.06 | 0.04 | 1.43 | 0.18 | |
| −0.02 | 0.04 | −0.44 | 0.66 | |
| −0.05 | 0.04 | −1.26 | 0.23 | |
| 0.05 | 0.04 | 1.09 | 0.30 |
| Mass of Alginate [g] | Mass of Ethyl Alcohol [g] | Mass of Water [g] | Alginate Content in Beads [% wt.] | Diameter of the Beads [mm] |
|---|---|---|---|---|
| 5 | 0 | 95 | 4.5 | 2.9 |
| 4 | 12 | 36 | 10.2 | 2.4 |
| 7 | 12 | 36 | 16.0 | 2.2 |
| 10 | 12.5 | 36 | 19.9 | 2.2 |
| Variables | Decoding Value | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | +α | |
| Temperature [°C] | 9.8 | 20 | 35 | 50 | 60.2 |
| pH | 1.5 | 2 | 2.75 | 3.49 | 4 |
| Alginate content in the beads [%] | 0.57 | 4.5 | 10.25 | 16 | 19.92 |
| Run Order | Independent Variables | Y | ||
|---|---|---|---|---|
| X1 | X2 | X3 | ||
| 1 | −1 | −1 | −1 | 1.20 |
| 2 | −1 | 1 | −1 | 0.67 |
| 3 | −1 | −1 | 1 | 0.91 |
| 4 | −1 | 1 | 1 | 0.57 |
| 5 | 1 | −1 | −1 | 2.01 |
| 6 | 1 | 1 | −1 | 1.41 |
| 7 | 1 | −1 | 1 | 1.51 |
| 8 | 1 | 1 | 1 | 1.09 |
| 9 | 1.682 | 0 | 0 | 1.32 |
| 10 | −1.682 | 0 | 0 | 0.4 |
| 11 | 0 | 1.682 | 0 | 0.96 |
| 12 | 0 | −1.682 | 0 | 1.33 |
| 13 | 0 | 0 | 1.682 | 0.79 |
| 14 | 0 | 0 | −1.682 | 0.52 |
| 15 | 0 | 0 | 0 | 1.07 |
| 16 | 0 | 0 | 0 | 1.06 |
| 17 | 0 | 0 | 0 | 1.08 |
| 18 | 0 | 0 | 0 | 1.05 |
| 19 | 0 | 0 | 0 | 1.00 |
| 20 | 0 | 0 | 0 | 1.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trawczyńska, I.; Kwiatkowska-Marks, S. Application of Response Surface Methodology for Optimization of the Biosorption Process from Copper-Containing Wastewater. Molecules 2023, 28, 444. https://doi.org/10.3390/molecules28010444
Trawczyńska I, Kwiatkowska-Marks S. Application of Response Surface Methodology for Optimization of the Biosorption Process from Copper-Containing Wastewater. Molecules. 2023; 28(1):444. https://doi.org/10.3390/molecules28010444
Chicago/Turabian StyleTrawczyńska, Ilona, and Sylwia Kwiatkowska-Marks. 2023. "Application of Response Surface Methodology for Optimization of the Biosorption Process from Copper-Containing Wastewater" Molecules 28, no. 1: 444. https://doi.org/10.3390/molecules28010444
APA StyleTrawczyńska, I., & Kwiatkowska-Marks, S. (2023). Application of Response Surface Methodology for Optimization of the Biosorption Process from Copper-Containing Wastewater. Molecules, 28(1), 444. https://doi.org/10.3390/molecules28010444





